Chapter 35 The Patient with a Full Stomach
II. Definition of Pulmonary Aspiration
III. Timing of Pulmonary Aspiration
IV. Physiologic Risk Factors in the Perioperative Patient
V. Patients at Risk for Pulmonary Aspiration
VI. Perioperative Anesthesia Considerations in Full-Stomach Patients
VII. General Anesthesia Management
VIII. Management of the Difficult Airway in the Full-Stomach Patient
I Introduction
Anesthetic administration in a patient with a full stomach poses three challenges to the anesthesia provider: prevention of gastric regurgitation, prevention of pulmonary aspiration, and institution of appropriate airway management. The accepted safe anesthesia management plan for reducing pulmonary aspiration of gastric contents in these patients involves identifying those at risk for gastric regurgitation and pulmonary aspiration by using practice guidelines for preoperative fasting, implementing prophylactic pharmacologic therapies, and applying appropriate airway management techniques that may reduce the risk of pulmonary aspiration. A review of the relevant literature suggests that the incidence of perioperative pulmonary aspiration is declining.1 Mortality rates for this dreaded complication have dramatically decreased over the past few decades.1
II Definition of Pulmonary Aspiration
Perioperative pulmonary aspiration of regurgitant gastric contents is defined as the presence of bilious secretions or particulate matter in the tracheobronchial tree. The term bronchoaspiration has been used to describe the same phenomenon, but the previous nomenclature for pulmonary aspiration is used in this chapter.2 Pulmonary aspiration can occur at any time preoperatively until 2 hours after terminating anesthesia. Diagnosis is made by direct examination of the airway, bronchoscopic assessment of the tracheobronchial tree, or postoperative imaging that demonstrates lung infiltrates not previously identified on the preoperative radiograph.3 The incidence, morbidity, and mortality from pulmonary aspiration are considered in Chapter 12.
III Timing of Pulmonary Aspiration
Patients with a full stomach are considered to be at high risk for pulmonary aspiration, which may occur before or during induction of anesthesia or at emergence from anesthesia. Most adult cases of pulmonary aspiration occur during induction of anesthesia (i.e., before laryngoscopy and tracheal intubation) (50%), during laryngoscopy (29%), or during and after emergence from anesthesia.1,4 In the pediatric population, most cases of pulmonary aspiration occur during induction with an inhalation anesthetic or tracheal intubation without a muscle relaxant, and more than 30% take place during emergence and extubation.3
Although most instances of pulmonary aspiration coincide with anesthetic induction, laryngoscopy, or surgery, they can also occur postoperatively.5 Patients who are at risk before surgery are also at risk during the postoperative period because the residual effects of anesthetic agents, muscle relaxants, and narcotic analgesics decrease protective airway reflexes.
IV Physiologic Risk Factors in the Perioperative Patient
Several physiologic risk factors predispose to aspiration pneumonitis (Table 35-1):
1. Increased gastric fluid volume (GFV) with acidic pH, increased bacterial count, or solid material
3. Impaired protective physiologic mechanisms, which include reduction in lower esophageal sphincter (LES) and upper esophageal sphincter (UES) pressure
4. Loss of protective airway (laryngeal-pharyngeal) reflexes
TABLE 35-1 Physiologic Risk Factors for Regurgitation and Aspiration of Gastric Contents
| Physiologic Effect | Risk Factors |
|---|---|
| Increased gastric volume, pressure, and acidity |
CNS, Central nervous system; LES, lower esophageal sphincter; NPO, nil per os (no oral intake); UES, upper esophageal sphincter.
A Gastric Volume and pH
The widely cited criteria for aspiration pneumonitis (GFV = 0.4 mL/kg and pH < 2.5), which were generated from the study of a single Rhesus monkey and extrapolated to humans, have been refuted.6 Current evidence supports a dose-response relationship for gastric volume instilled directly into the lung and gastric acidity. The lethal dose for acid pulmonary aspiration has been studied in numerous animal models.5,7,8 James and colleagues demonstrated that hydrochloric (HCl) acid instilled directly into rat tracheas resulted in a high mortality rate.9 Late mortality rates were 90% with a volume of 0.3 mL/kg at a pH of 1.0 and 14% with a volume of 1 to 2 mL/kg at a pH less than 1.8. An investigation involving primates demonstrated that aspiration of large volumes (0.8 to 1.0 mL/kg) of acid at a pH of 1 was associated with severe pneumonitis. Instillation of smaller volumes (0.4 to 0.6 mL/kg) produced mild radiologic and clinical changes but no deaths. The median lethal dose for acid aspiration into the lungs was 1.0 mL/kg. Extrapolation of these data to humans provides a critical volume of approximately 50 mL for severe pulmonary aspiration in adults.10
The effect of aspiration of milky products into the lungs has also been studied in animals.11 Acidification of human milk to a pH of 1.8 with HCl acid increased the severity of aspiration pneumonitis compared with 5% dextrose acidified to a pH of 1.8 with HCl acid. Acidification of human breast milk with gastric juice instead of HCl acid did not increase the severity of lung injury. Instillation of soy-based formula or other dairy milk formula into the lungs caused a less severe form of acute injury.12 Human milk is particularly noxious when aspirated compared with other types of milk.
In normal, healthy children, the range of residual gastric volume at the time of anesthetic induction is broad. Cote and coauthors reported values of 0.11 to 4.72 mL/kg, which were higher than the values (0.01 to 4.08 mL/kg) reported by Splinter and associates.13,14 Children in clinical studies typically have average gastric volumes greater than 0.4 mL/kg and acidic pH levels,14–22 but they rarely have (1 in 10,000 anesthetics) aspiration pneumonia associated with anesthesia.23 Instead of focusing on GFV at induction, the emphasis to prevent pulmonary aspiration should be on patients’ comorbidities, their risk factors, type of anesthesia, and characteristics of the aspirate.
C Impaired Protective Physiologic Mechanisms
1 Lower Esophageal Sphincter Tone
The LES forms a border between the stomach and the esophagus, with the lower esophagus creating a sling around the abdominal esophagus.24 Barrier pressure is the difference between LES pressure and gastric pressure. Intragastric pressure is normally less than 7 mm Hg. Normal resting LES pressure in conscious individuals is 15 to 25 mm Hg higher than intragastric pressure. An incompetent LES reduces barrier pressure and increases the risk of regurgitation of gastric contents.
LES tone reduction is the major problem in patients with gastroesophageal reflux (GER) during anesthesia and in disease states. In patients presenting with hiatal hernia, the maximum pressure at the gastroesophageal junction was lower (17.1 mm Hg) than the pressure in healthy volunteers (28 mm Hg).25 Intragastric pressure increases to 35 mm Hg when the stomach is distended.26 Gastric distention with increased intragastric pressure and reflex relaxation of the LES causes spontaneous GER. When esophageal pressure equals gastric pressure, the development of a common cavity leads to spontaneous GER.27
Patients with coexisting gastroesophageal pathology presenting for anesthesia are susceptible to GER. The mechanism for reflux is a transient relaxation of the LES.27,28 Anesthetic agents and techniques relax the LES, reduce barrier pressure, and predispose the patient to GER.29 Cricoid pressure application and laryngoscopy during anesthesia also lower LES tone.30 Drugs that lower LES tone include anticholinergics, benzodiazepines, dopamine, sodium nitroprusside, ganglion blockers, thiopental, tricyclic antidepressants, β-adrenergic stimulants, halothane, enflurane, opioids, and propofol.29 Inhalation agents can reduce the LES pressure below the intragastric pressure, depending on the degree of relaxation.29,31 Propofol has no effect on barrier pressure except for a transient decrease in LES tone and gastric pressure at 1 minute, which return to baseline values later.32 Drugs that increase LES pressure include antiemetics, cholinergic drugs, succinylcholine, pancuronium, metoclopramide, domperidone, edrophonium, neostigmine, metoprolol, metoprolol, α-adrenergic stimulants, and antacids.29,32
2 Upper Esophageal Sphincter Tone
The cricopharyngeus muscle acts as the functional UES. It is one of the two inferior constrictor muscles of the pharynx. It extends around the pharynx from one end of the cricoid arch to the other and is continuous with the circular, muscular coat of the esophagus.24 In the conscious, healthy patient, the UES helps to prevent pulmonary aspiration by sealing off the upper esophagus from the hypopharynx.33
Various anesthetic techniques and agents (except ketamine) reduce the UES tone and predispose the patient to regurgitation of material from the esophagus into the hypopharynx. Residual neuromuscular blockade (even with train-of-four ratio of 0.7) puts the patient at risk for pulmonary aspiration because of a reduction in UES tone and impaired swallowing.34–38
D Loss of Protective Laryngeal-Pharyngeal Airway Reflexes
Four well-defined reflexes in the upper airway protect the lungs from aspiration. They are apnea with laryngospasm, coughing, expiration, and spasmodic panting that involves the glottic area and the true or false vocal cords.39 Impaired laryngeal-pharyngeal function usually results from an altered consciousness level. Patients with central nervous system disorders, cerebrovascular injuries, head trauma, alcohol intoxication, and neuromuscular disorders (particularly myotonia dystrophica and scleroderma) have an increased pulmonary aspiration risk due to diminished pharyngeal sensation and diminished protective airway reflexes.40
Anesthetic agents that result in the loss of UES tone impair protective reflexes.41 Prevention of protective laryngeal closure permits entry of this foreign matter into the tracheobronchial passages, causing regurgitation of gastroesophageal contents into the pharynx.
V Patients at Risk for Pulmonary Aspiration
Recognition of patients who have any of the aforementioned risk factors for pulmonary aspiration is the first step toward minimizing the incidence of perioperative pulmonary aspiration. These patients can be categorized in two groups: those with a full stomach (i.e., history of ingestion of a meal with less than 6 hours fasting time)42 and those designated as having a full stomach despite a prolonged preoperative fast.
Patients who are at high risk for pulmonary aspiration are at the extremes of age, have altered consciousness, have ingested solids or liquids despite orders to take nothing by mouth (nil per os [NPO]), are pregnant (particularly those in labor), or have sustained trauma. Trauma, even if not abdominal, delays gastric emptying.43,44 Trauma patients, especially those in acute pain who are scheduled for emergency surgery, have decreased gastrointestinal motility and increased gastrointestinal secretion despite fasting preoperatively.45 The incidence of pulmonary aspiration increases markedly after trauma because of recent ingestion of food, depressed consciousness, diminished or absent airway reflexes, or gastric stasis induced by raised sympathoadrenal influx of catecholamines. In 53 adults with Glasgow Coma Scale scores less than 8 who were intubated by the London Helicopter Emergency Medical Service, the incidence the of gross pulmonary aspiration was 38%.46,47
Other factors increase the risk of pulmonary aspiration. Medications that delay gastric emptying affect the protective physiologic mechanisms. Patients receiving narcotics are expected to have delayed gastric emptying,48,49 but it has also been demonstrated that administration of opioids in a single dose to healthy patients did not delay gastric emptying or affect acidity.50–52
Long-standing diabetes and gastroparesis are risk factors. Diabetic gastroparesis impairs gastric emptying and may compromise LES function.1,53–55 Patients who are morbidly obese can have delayed gastric emptying, increased abdominal pressure, and a difficult airway, all of which are potential risks for pulmonary aspiration. The gastric volumes in 71% of these patients are in the at-risk range compared with the levels found in normal subjects.56 The risk of aspiration also is increased for patients with stress and acute pain, raised intracranial pressure, and neuromuscular disorders.
Certain types of surgery are associated with pulmonary aspiration. The incidence of silent gastric regurgitation is higher in esophageal,1,4 upper abdominal,57 and emergency laparoscopic operations.58,59 Comorbidities are more likely in patients with American Society of Anesthesiologists (ASA) IV or V physical status. Emergency surgery, especially when performed at night, is a risk factor for aspiration.
Failed tracheal intubation of a patient with a difficult airway can lead to hypoxia associated with gastric regurgitation and pulmonary aspiration. General anesthesia administration in patients with a full stomach necessitates tracheal intubation to protect the airway from pulmonary aspiration. If tracheal intubation that is undertaken to isolate the airway and avoid pulmonary aspiration proves to be difficult, the procedure itself can lead to pulmonary aspiration. A difficult intubation can be predicted in only two thirds of cases. Problematic anesthetic inductions compounded by difficult tracheal intubation, difficult mask ventilation, inadequate anesthesia with coughing or straining, difficult emergence, or difficult extubation constitute prime conditions for gastric regurgitation and pulmonary aspiration, and they have been reported in up to 77% of these cases.60–63
Regional anesthesia may be followed by complications. Regional anesthesia is usually considered safe in patients at risk for pulmonary aspiration because they are awake and have intact protective airway reflexes.64 However, after administration of spinal anesthesia, some patients develop extensive sympathetic block followed by hypotension, vomiting, difficulty swallowing, or impaired cough reflex.1 The risk of pulmonary aspiration is exacerbated by difficulty in swallowing because of a high level of sympathetic block. Concomitant administration of narcotics or sedatives can obtund the protective airway reflexes, leading to gastric regurgitation and pulmonary aspiration.39,65,66 Vomiting associated with sympathetic blockade–induced hypotension requires turning the patient’s head to the lateral position and placing the patient in the Trendelenburg position to avoid pulmonary aspiration of gastric contents.
VI Perioperative Anesthesia Considerations in Full-Stomach Patients
A Preoperative Fasting
Historically, adult patients have fasted 8 to 12 hours before surgery to reduce the volume of gastric contents and the aspiration pneumonitis risk. The National Confidential Enquiry into Perioperative Deaths highlighted the issue of preoperative starvation.67 NPO after midnight is an accepted preoperative order. Long fasting before an elective operation is uncomfortable and creates detrimental effects by causing thirst, hunger, irritability, noncompliance, and resentment in adult patients.57 Prolonged fasting is especially deleterious in children because it produces dehydration or hypoglycemia.21,68 During the past 20 years, several scientific papers have challenged the traditional practice of preoperative fasting for more than 8 hours. The current understanding of gastric emptying physiology has generated revised preoperative fasting policies.3,21,50,69–77
Despite the knowledge that the stomach handles emptying solids and liquids differently, physicians traditionally lumped consideration of them together in the standard preoperative order: NPO after midnight the day before surgery. After an extensive review, the ASA task force revised policies and published specific practice guidelines for preoperative fasting.42,78
The Fourth National Audit Project (NAP4) was conducted by the Royal College of Anaesthetists and the Difficult Airway Society. The large-scale review of airway-related complications was published in 2011 after analyzing the events included in NAP4 from September 1, 2008, to August 31, 2009.63 The NAP4 examined records for major complications among the 2.9 million general anesthetics in the operating rooms, airway management procedures in intensive care units (ICUs), and airway management techniques in the emergency departments across the United Kingdom.63 The data revealed 184 serious airway-related complications that led to death, brain damage, emergency invasive airway access through the neck, unanticipated admission to the ICU, or prolonged ICU stay.63 The most frequent fatal complication from general anesthesia was pulmonary aspiration of regurgitated gastric contents, which was implicated in 50% of the deaths.63 Cook and colleagues thought that pulmonary aspiration was a well-recognized problem for patients under general anesthesia and that it should be preventable in most cases. However, in some cases reported in the study, proper precautions were not taken.63 The incidence in NAP4 was three to five times higher than that found in the analysis of the ASA Closed Claims Project database reported from the United States.79
1 Liquids
Residual gastric volume is directly related to regurgitation and pulmonary aspiration. However, the ASA Task Force on Preoperative Fasting, despite extensive scrutiny of the existing data, has been unable to establish a link between residual gastric volume and pulmonary aspiration.78,80
a Clear Liquids
Clear liquids in healthy patients empty exponentially. The gastric emptying half-life (t1/2) of clear liquids is 12 minutes, which is considerably faster than for solids. Theoretically, for a patient who consumes 500 mL of clear liquid, almost 97% of the liquid is eliminated from the stomach after five half-lives (i.e., 60 minutes).81–83 The rate of gastric emptying after a liquid meal has been well studied in adults.74,76,80 The studies demonstrated that after drinking 750 mL of pulp-free orange drink, the mean t1/2 ranged from 10 ± 7 to 20 ± 11 minutes. The fastest t1/2 for an individual subject was 2.9 minutes, and the slowest t1/2 was 41.6 minutes, indicating that almost complete gastric emptying could be accomplished in 2 hours (approximately five half-lives) after a clear liquid drink was consumed. Even in individuals with the slowest emptying rates, only 10% or less of the original liquid was retained in the stomach after 2 hours.72,75,84–86
The ASA Committee on Standards and Practice Parameters supports a fasting period of 2 hours after the ingestion of clear liquids for all patients.78 Clear liquids include water, fruit juices without pulp, carbonated beverages, clear tea, and black coffee. The clear liquid category does not include alcohol. The volume of liquid ingested is less important than the type of liquid.
b Milk
In term and preterm infants, breast milk leaves the stomach more rapidly than formula milk. The gastric emptying t1/2 for breast milk is approximately 25 minutes; for formula milk, it is twice as long.87,88 Consultants and the ASA Committee on Standards and Practice Parameters recommend fasting 4 hours after breast milk and 6 hours after infant formula.78
2 Solids and Nonhuman Milk
Gastric emptying for solids occurs in a linear pattern with time, and 10% to 30% of ingested solids may remain in a patient’s stomach after 6 hours.89 Gastric emptying is inhibited when the duodenum is distended; when the chyme contains a high concentration of acid, proteins, or fats; or when the osmolarity is not iso-osmolar. Gastric emptying after solid ingestion also depends on body posture after intake, exercise, meal weight, size of food particles, amount of food, and the type of food.90,91 Lack of readily available, appropriate methodology makes the assessment of stomach contents in the perioperative period difficult.
Miller and coworkers investigated patients who ate a light breakfast of a slice of buttered toast and a cup of tea or coffee with milk 2 to 4 hours before surgery.92 Gastric contents were measured by inserting a gastric tube after anesthetic induction. There was no significant difference in gastric volume or pH between the control group (fasting) and the study group. Soreide and associates investigated a group of healthy, female volunteers who ingested a standard hospital breakfast of one slice of white bread with butter or jam, one cup (150 mL) of coffee without milk or sugar, and one glass (150 mL) of pulp-free orange juice.93 Gastric contents were measured by repeated ultrasonography and paracetamol absorption techniques. No solid food was detected in any volunteer 240 minutes after breakfast. The latter test determined that at least 4 hours between eating and surgery are needed for solid foods to empty from the stomach.
It is appropriate to fast at least 6 hours after intake of a light meal or nonhuman milk before elective procedures. The ASA Committee on Standards and Practice Parameters revised the guidelines because the members found that intake of fried or fatty foods or meat could prolong gastric emptying time. In these situations, 8 hours or more may be needed for preoperative fasting.78
3 Patients with Diabetes
Radioisotopic techniques and electrical impedance tomography have been used to show that type 1 diabetic patients have delayed gastric emptying.55 After ingestion of a semisolid meal, the mean t1/2 of gastric emptying was 54.8 ± 26.6 minutes in diabetic patients compared with 40.4 ± 8.6 minutes in nondiabetic control subjects. Diabetic patients require a longer fasting period (8 hours) than nondiabetic patients.
4 Ambulatory Patients and Anxiety
Anxiety delays gastric emptying and increases acid secretion.94,95 Ambulatory surgery increases anxiety. A background study showed that the mean gastric volume was 69 mL in outpatients and 33 mL in inpatients, with an average pH of less than 2.5 in both groups.96
To test the hypothesis that preoperative stress affects residual GFV and pH in pediatric patients, children between the ages of 3 and 17 years were randomly assigned to three groups: outpatients, inpatients, and patients who had multiple operations.13 There were no differences in the residual GFV between the three groups and no differences in gastric contents between inpatients and outpatients. The relationships among oral premedication, preoperative anxiety, and gastric contents showed that premedication reduces anxiety. However, there were no correlations among the type of premedication, level of anxiety, gastric volume, and gastric pH.97
B Fasting Guidelines Summary
Almost 80% of elective operations are scheduled for ambulatory patients or those with same-day admittance. The ASA Task Force on Preoperative Fasting recommends avoiding anesthetizing a patient with a full stomach to reduce the risk of pulmonary aspiration.80 The ASA practice guidelines about minimum fasting period for ingested material include the following recommendations: clear liquids, 2 hours; breast milk, 4 hours; infant formula, nonhuman milk, and a light meal, 6 hours. A fast should precede elective procedures requiring general anesthesia, regional anesthesia, or sedation or analgesia (i.e., monitored anesthesia care). The guidelines also recommend a fasting period for a meal that includes fried foods, fatty foods, or meat of 8 hours or more before elective procedures. Diabetic patients need 8 hours or more for gastric emptying after ingesting semisolid material.
Most anesthesiologists practicing outpatient anesthesia in the United States have conformed to the recommendation of the ASA practice guidelines for preoperative fasting time.77 Similar opinions from associations linked to the World Federation of Societies of Anesthesiologists and from the current literature have led to changes in preoperative fasting guidelines worldwide.69–71,98,99
C Role of Preoperative Ultrasonography
Ultrasonography has been proposed as a useful, noninvasive, bedside tool to determine gastric content and volume in the perioperative period based on studies in parturients,100 a pilot phase study enrolling 18 healthy adult volunteers,101 and a follow-up phase II study enrolling 36 adult volunteers that suggested the gastric antral cross-sectional area was a reliable indicator of the quantitative assessment of gastric volume in the right lateral decubitus position.101 Another prospective trial performed an ultrasonographic qualitative and quantitative analysis of the gastric antrum in 200 fasted patients undergoing elective surgery by assigning a grade to each patient on a 3-point grading scale. Eighty-six patients were categorized as grade 0, suggesting an empty antrum (0 mL); 107 patients as grade 1, suggesting minimal fluid volume (16 ± 36 mL) detected only in the right lateral decubitus position; and 7 patients as grade 2, suggesting a distended gastric antrum with fluid 180 ± 83 mL visible in supine and right lateral decubitus positions.102 Results of this study of elective surgical patients indicates that preoperative ultrasonography may be able to help identify patients who are at higher risk of pulmonary aspiration. Because certain patient populations, such as trauma patients undergoing surgery, are known to be at high risk for pulmonary aspiration with associated morbidity and mortality,46 it seems prudent to use ultrasonography as a screening tool preoperatively for these patients.
D Pharmacotherapy
The ASA practice guidelines do not recommend the routine preoperative use of gastrointestinal stimulants, medications that block gastric acid secretion, antacids, prokinetics, antiemetics, or anticholinergics to reduce the risk of pulmonary aspiration in patients who have no apparent increased risk.78,80
Proton pump inhibitors (PPIs) reduce intragastric acidity and gastric juice volume, and they have been advocated for preanesthetic use to prevent pulmonary aspiration.103 Peptic ulcer bleeding is common, and recurrent bleeding is an independent risk factor for mortality. A PPI infusion prevents recurrent upper gastrointestinal bleeding after endoscopic therapy.104 A study from Japan showed that rabeprazole may be a suitable alternative to standard H2-blocker prophylaxis against acid aspiration pneumonia.105
Surveys on pulmonary aspiration prophylaxis in the full-stomach, nonobstetric population are rare. The accepted pharmacologic regimens include an attempt to manipulate the gastric pH, volume, and barrier pressure.106–114 Other surveys of antacid prophylaxis are limited to obstetric anesthesia.115–120 Most studies suggest improved safety from reduced gastric volume or an increase in gastric pH, or both.1,121–131 However, no data show evidence of improved outcome after the use of antacids, H2-receptor blockers, PPIs, or prokinetics. Because of the paucity of data and a lack of evidence to prove the value of pharmacologic therapies for preventing pulmonary aspiration, it is not possible to provide a cost-benefit ratio.78,80
E Preoperative Gastric Emptying
1 Preoperative Gastric Tube Insertion Before Emergency Surgery
Preoperative gastric emptying is not without hazards. Preoperative insertion of a gastric tube and subsequent gastric emptying in an awake, unsedated patient elicits a profound sympathetic response and oxygen desaturation.132 This response, which is similar to the cardiovascular response after tracheal intubation without analgesia,133,134 puts the patient with cardiac problems at risk for cardiac ischemia and dysrhythmias. Routine preoperative nasogastric tube placement is not recommended except in selected patients with small bowel or gastric outlet obstruction. Although the gastric tube helps to reduce intragastric volume and pressure, it does not guarantee that the stomach is completely empty. Preoperative nasogastric tube insertion and stomach decompression are recommended only in patients with a distended stomach (e.g., bowel obstruction).
2 In Situ Gastric Tube During Induction
The disposition of an existing gastric tube during induction of general anesthesia is a debatable issue. After the stomach is decompressed, the gastric tube can be removed or left in situ during a rapid-sequence induction (RSI). Although the presence of the gastric tube may impair the function of the UES and LES,135 studies with cadavers have shown that the efficacy of CP application during induction of anesthesia is not impaired.136,137 If the patient has a nasogastric tube, it need not be withdrawn before induction of anesthesia because it acts as an overflow valve and prevents pressure buildup in the stomach; it also allows drainage during anesthesia induction.138
3 Effects of Gastric Tube Placement in Mechanically Ventilated Patients
The third concern was tested in mechanically ventilated ICU patients to determine the effect of the nasogastric tube size on the incidence of GER.139 The concern about GER is that it can result in pulmonary aspiration and bacterial pneumonitis. Investigators found no significant difference in GER and pulmonary aspiration with the use of small-gauge and large-gauge nasogastric tubes.
4 Sealing the Esophagus by Inflatable Cuffs
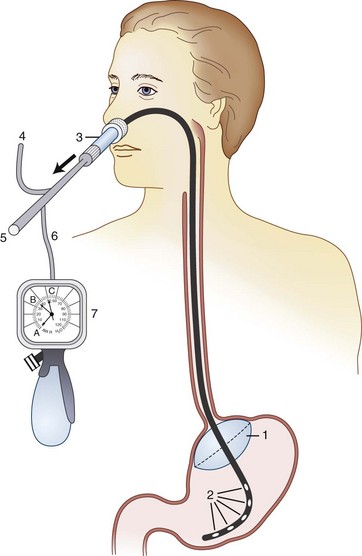
Inflating a cuff at the gastroesophageal junction to prevent gastric reflux is considered to be unsafe. A newly designed, but similar nasogastric device with an inflatable balloon to occlude the gastric cardia (Aspisafe, Braun, Melsungen, Germany) was first studied in pigs (Fig. 35-1).140 After gastric filling with large volumes, despite maneuvers and drugs used to promote regurgitation, the new nasogastric device did not produce GER.140 The same experiment was duplicated in healthy volunteers and surgical patients considered to be at risk for pulmonary aspiration, and findings were similar.140 Use of this device is considered safe because there was no test evidence of GER after RSI.39
The device was subsequently studied in conjunction with the use of a laryngeal mask airway (LMA). A dye indicator injected into the stomach revealed that the balloon tube prevented GER.141 Future clinical studies should decidedly prove balloon tube safety and usefulness in preventing regurgitation during induction and emergence.
VII General Anesthesia Management
A Awake Tracheal Intubation in Patients at High Risk for Pulmonary Aspiration
A full-stomach patient with a difficult airway warrants an awake tracheal intubation. The potential for managing a difficult airway (i.e., difficult intubation or difficult mask ventilation) may be self-evident because of a preexisting or acquired condition. However, normal individual anatomic variation may contribute to the difficulty with tracheal intubation or mask ventilation. Various physical characteristics are associated with a difficult airway, including a small mouth, limited mouth opening, short interincisor distance, prominent upper incisors with overriding maxilla, short neck, limited neck mobility, receding mandible or mandibular hypoplasia, high-arched and narrow palate, temporomandibular joint dysfunction, rigid cervical spine, obesity, and congenital anomalies found in infancy. Morbidly obese patients and infants particularly are at risk for a difficult airway and pulmonary aspiration. No single feature on physical examination accurately predicts a difficult intubation, but a variety of simple diagnostic tests have been suggested to identify patients with difficult airways.142–154
Intubation of the trachea while the patient is awake is a useful technique with a high degree of acceptance by patients, and it is considered a fail-safe method of choice when gastric regurgitation and pulmonary aspiration are likely.155 Awake intubation is useful in situations of anticipated difficulties in tracheal intubation and in patients with intestinal obstruction, gastrointestinal hemorrhage, or upper airway obstruction; in seriously ill or moribund patients; and in those with respiratory failure.156
Efficacy and safety of awake intubation in full-stomach patients can be accomplished with the use of minimal sedation, administration of oxygen by nasal cannula during intubation, and application of local anesthetic to the pharynx, larynx, and trachea. The premise of conscious sedation is to balance the comfort of the patient and tolerance of the procedure and still have a patient responsive to commands. Judicious sedation with small amounts of short-acting benzodiazepines and narcotics helps allay anxiety and helps the patient who is awake tolerate any discomfort that occurs during tracheal intubation. Ovassapian and colleagues presented the concept of awake fiberoptic intubation using judicious sedation and topicalization of upper and lower airways in patients at high risk for pulmonary aspiration.157 An accompanying editorial stated that this study of 121 patients was too small to accept the safety of the technique of awake intubation, sedation, and topicalization of the airway.
B Rapid-Sequence Induction
RSI of anesthesia to protect the airway from pulmonary aspiration of gastric contents has evolved since the introduction of succinylcholine in 1951 and the first description of CP by Sellick in 1961.135 The primary objective of RSI is to minimize the time interval between loss of consciousness and tracheal intubation. The essential features of RSI include preoxygenation with 100% oxygen, administration of a predetermined induction dose, application of effective CP, and avoidance of positive-pressure ventilation until the airway is secured with a cuffed tracheal tube.158,159 Many emergency physicians use RSI of anesthesia as their technique of choice to facilitate orotracheal intubation in patients presenting to the emergency room.160–165 Having specialized equipment for management of failed tracheal intubation is an integral part of RSI.42,166–168
RSI plays a major role in emergency anesthesia and is used almost universally for obstetric general anesthesia in the United Kingdom and United States158; however, it is practiced less widely in mainland Europe.169 A survey of French anesthetists showed that only 23% used a full complement of measures to prevent pulmonary aspiration, and CP was rarely used,1,170 but the incidence of fatal aspiration in France is lower than in other countries (1.4 per 10,000 versus 4.7 per 10,000 episodes of anesthesia).1,170
Preoxygenation is a standard component of RSI.159 Preoxygenation with fresh gas flows of 100% oxygen through a mask with a good fit for 3 to 5 minutes is recommended. Alternatively, a series of four vital capacity breaths of 100% may be used in an emergency.159 Thiopental remains the most popular induction agent for RSI,159 although propofol also is used extensively. Other intravenous induction agents include ketamine and etomidate.171,172
Succinylcholine remains the muscle relaxant of choice for use in RSI.159 Certain conditions may preclude the use of succinylcholine, as in burns and spinal cord injuries, because administration of succinylcholine can result in adverse effects such as hyperkalemia and dysrhythmias. Several studies have demonstrated that rocuronium in adequate doses (0.8 to 1.2 mg/kg) produces intubation conditions rapidly comparable to those with succinylcholine 1 mg/kg.173,174 However, this large dose leads to prolonged duration of action of up to 1 hour.175 In a patient with a difficult airway, this drug may allow less margin for error than succinylcholine.176 Fifty percent of cases of difficult intubation occur without preoperative predictive signs and can increase the risks of gastric regurgitation and pulmonary aspiration in full-stomach patients.177
1 Modified Rapid-Sequence Induction
RSI is a well-established technique in anesthesia practice. During standard RSI, patients are made apneic and unconscious without establishing the ability to ventilate the lungs. Positive pressure usually is avoided to prevent gaseous distention of the stomach and subsequent pulmonary aspiration in RSI. However, avoidance of ventilation of the lungs during RSI precludes the ability to test the airway and verify that a patient’s lungs can be ventilated by mask before the administration of a muscle relaxant. The technique of RSI therefore involves inherent risks to the patient, including the possible inability to secure an airway or to ventilate the lungs and oxygenate the patient who is unconscious and apneic. During RSI, failure to secure the airway can lead to many attempts at tracheal intubation with subsequent trauma to the airway, and failure to ventilate the lungs can lead to hypoxia, hypercarbia, and alterations in heart rate and blood pressure, resulting in significant morbidity. These risks may not be warranted or appropriate, particularly in patients who are at risk for gastric regurgitation and pulmonary aspiration. For them, appropriate management can include modification of the standard RSI technique consisting of preoxygenation, laryngoscopy using an elevated-head position,178 induction of anesthesia, application of CP, apneic diffusion oxygenation,179 and the added step of gentle positive-pressure ventilation of the lungs before tracheal intubation.135,180
2 Cricoid Pressure
CP is an integral component of RSI and is an accepted standard of care during anesthesia and to a lesser extent during resuscitation in patients with a full stomach.159,181–183 As early as the 1770s, CP was recognized as an important method to occlude the esophagus and prevent gastric distention during lung ventilation of drowning victims.184 However, it was almost 200 years later that Sellick introduced CP into clinical anesthesia practice.135 Although studies cite increasing concerns regarding the safety and efficacy of CP, it is still widely practiced.185–187
The CP, a force measured in newtons (N), must be of sufficient amount applied during induction of general anesthesia to prevent regurgitation from the esophagus and stomach. Regurgitation of stomach contents depends on esophageal pressure, gastrointestinal pathology, intragastric pressure, UES and LES pressures, and the effectiveness of occluding the esophageal lumen. On the basis of several studies,136,188–192 a force (CP) of 44 N (9.8 N = 1 kg = 2.2 lb) was accepted as the gold standard for prevention of gastric regurgitation in adults when applied in the standard or tonsillectomy position.192 CP greater than 44 N prevents gastric regurgitation. When appropriate force is applied, the upper end of the esophagus is occluded and compressed against the C6 vertebra. In paralyzed intubated patients, lowering the CP to 40 N increased esophageal pressure to more than 38 mm Hg.193 In a study using the double-lumen Salem sump tube in 20 women undergoing emergency cesarean delivery under general anesthesia,194 the mean gastric pressure was 11 mm Hg (range, 4 to 19 mm Hg). It was predicted that 99% of women undergoing emergency cesarean delivery having a gastric pressure of 25 mm Hg were unlikely to have regurgitation of fluid. Evidence from a cadaver study showed that a CP of 30 N prevents regurgitation of esophageal fluid even with gastric pressure at 42 mm Hg. Anatomic studies suggest that a CP of 30 N is adequate and should reduce the risk of esophageal rupture.137,193
The CP necessary to provide an adequate occlusive effect in children of different age groups is debatable. No data are available to clarify this issue. An observational study using the bench model indicated that the mean cricoid force applied clinically to a 5-year-old child should be 22.4 to 25.1 N.195
a Head and Neck Position
Sellick made the original recommendation to use the tonsillectomy position, without a pillow, with CP applied at the C5 vertebra.135 The rationale for the tonsillar position is that it increases the concavity of the cervical spine, stretches the esophagus, and prevents lateral displacement of the esophagus. Unfortunately, this mode of CP application often worsens the view at laryngoscopy.196 The head position must be ideal for intubating with head extension on the neck, neck flexion, and a pillow beneath the occiput.197
b Timing of Cricoid Pressure Application
The timing and amount of CP applied during induction of general anesthesia are important. The original recommendation was synchronous CP application; moderate pressure in an awake, conscious individual; and a gradual increase to a full force of 44 N immediately on loss of consciousness. Applying a full force of 44 N before induction in a conscious individual is detrimental; it produces significant discomfort, complete airway obstruction,198 retching, and vomiting.181 Vomiting with continued CP application can cause death from pulmonary aspiration or a ruptured esophagus.199,200
A cricoid yoke has been used to apply pressure in awake volunteers without considerable pain or coughing.189 The accepted practice is to apply a lower CP (20 N) before induction of anesthesia and increase it to 30 N as the patient loses consciousness.201
Reluctance to use CP stems from faulty technique of its application and its effect on airway management, reported cases of pulmonary aspiration, and esophageal rupture. CP application causes anatomic distortion of the upper airway, making airway management more difficult. Most data were collected with a CP of 40 N. Studies show that a CP of 30 N applied in an upward and backward manner improves the laryngoscopic view during intubation.201 Similarly, Randell and colleagues showed that CP,202 modified to backward, upward, rightward pressure (BURP) on the thyroid cartilage, improved the view in 57 of 68 cases. The thyroid cartilage is the surface marking for the glottic opening, and application of BURP on the thyroid cartilage therefore improves the glottic view at laryngoscopy.203
c Single-Handed Cricoid Pressure
For single-handed CP, the thumb and middle finger are placed on either side of the cricoid cartilage, and the index finger is placed above to prevent lateral movement of the cricoid.135 The laryngoscopic view is better with the head in the Magill position and better with single-handed CP than with double-handed CP.204
Another single-handed method is to place the palm of the hand on the sternum, applying pressure with only the index and middle fingers.205 This technique improved the laryngoscopic view of the glottis. In infants and children, the single-handed technique compressing the cricoid cartilage is performed with the little or middle finger while the same hand holds the face mask.206
d Double-Handed Cricoid Pressure
The bimanual or two-handed CP technique uses the single-handed technique in addition to using the assistant’s right hand to provide counterpressure beneath the cervical vertebra for neck support. This maneuver provides support to the hyperextended arch of the vertebral column to maintain the efficacy of CP and to optimize the laryngoscopic view. Variations of this technique include placing the left hand behind the head and holding the extended head to maintain the Magill intubating position.207
e Cricoid Pressure in Clinical Practice
In clinical practice, application of a predetermined CP can be sustained by the assistant for only a short period. Application of CP with a flexed arm can be sustained only for a mean time of 3.7 minutes at 40 N, with considerable onset of pain at 2.3 minutes. This limitation has important clinical implications in cases of failed tracheal intubation in full-stomach patients. Application of CP in clinical practice may interfere with airway management (i.e., tracheal intubation, face mask ventilation, and LMA placement), and failure to manage the airway appropriately is a more frequent cause of morbidity and mortality than pulmonary aspiration.208
VIII Management of the Difficult Airway in the Full-Stomach Patient
Management of difficult RSIs in full-stomach patients is little studied. Airway catastrophes occur predominantly when airway difficulty is not recognized before anesthesia is administered.209,210 Although airway difficulty should be anticipated in 90% of cases, a prospective study showed that only approximately 51% of difficult intubations were recognized and expected.211 Difficult and failed tracheal intubations occur more frequently during an emergency than during elective surgery, during nights than during days, and during weekends than during weekdays.97,212,213 Unanticipated difficult intubations in full-stomach patients undergoing emergency surgery poses additional problems. There is no option for anesthesiologists to postpone surgery, and the risk of pulmonary aspiration of gastric contents is much greater.
In full-stomach patients, repeated attempts to intubate the trachea increase the risk of airway trauma, bleeding, and laryngeal edema, and failure to secure the airway can increase the incidence of pulmonary aspiration.214 Hypoxia and death can result from failure to oxygenate the patient and ventilate the patient’s lungs or from unrecognized intubation of the esophagus, neither of which is caused solely by failed tracheal intubation.142
Significant advances in airway management have been made in the past 2 decades. New airway devices and adjuncts to assist in the tracheal intubation of difficult airways are available. In most cases of difficult intubation, a partial view of the glottis is possible (i.e., grade III laryngoscopic view).97,167,212 Of the many airway devices available to physicians in the United Kingdom and Canada, the Eschmann stylet (gum elastic bougie) is used to facilitate tracheal intubation in more than 95% of grade III laryngoscopic views.215 Another commonly used device is the McCoy laryngoscope (CLM, Mercury Medical, Clearwater, FL), which can improve visualization of the larynx in up to 50% of cases with difficult laryngoscopic views.216,217
Additional levering laryngoscopes include the Flipper (Rusch, Research Triangle Park, NC) and the Heine Flex Tip (Heine USA, Dover, NH). These laryngoscopes were designed to provide greater flexibility and improved visualization of the larynx in patients with a difficult airway, smaller mouth opening, or restricted head and neck movement.218
A practical classification proposes subdividing the grade 3 Cormack-Lehane laryngoscopic view into two grades: grade 3A, in which the epiglottis is visible and can be elevated with the laryngoscope blade, and grade 3B, in which the epiglottis is visible but cannot be elevated.219 This new classification is as sensitive and more specific than the Cormack-Lehane classification in predicting difficult tracheal intubation, but it is more sensitive and more specific in predicting easy tracheal intubation.219
A manikin-simulated difficult airway study showed that the Eschmann stylet and a fiberoptic stylet were effective in facilitating tracheal intubation in a simulated grade 3A Cormack-Lehane view, with success rates of 100% and 98%, respectively.220 The mean times to successful intubation were similar between groups: 31 seconds for the Eschmann stylet and 29.2 seconds for the fiberoptic stylet.220 However, in the simulated grade 3B Cormack-Lehane view, use of the fiberoptic stylet significantly improved the success rate (98% for the fiberoptic stylet and 9% for the Eschmann stylet) and reduced the mean time to successful tracheal intubation (31 seconds for the fiberoptic stylet and 45.6 seconds for the Eschmann stylet).220
A Difficult Airway Management Using the Laryngeal Mask Airway
1 Classic Laryngeal Mask Airway
The use of the LMA Classic is contraindicated in full-stomach patients and patients at high risk for pulmonary aspiration because of gastrointestinal pathology, obesity, GER, or emergency surgery. The main disadvantage of using the LMA Classic in patients with a full stomach or obstetric patients is an increased risk of gastric regurgitation and pulmonary aspiration. The LMA Classic design is such that even when correctly placed, its tip lies against the UES, and the device does not isolate the respiratory tract from the gastrointestinal tract. Because the esophagus is included in the rim of the LMA Classic, it does not protect the lungs from regurgitated gastric contents. Full-stomach and pregnant patients may be vulnerable to gastric regurgitation and pulmonary aspiration with the use of this device.221
Studies of gastric regurgitation and pulmonary aspiration rates associated with using these supraglottic devices have mainly focused on the LMA Classic. A meta-analysis of 547 publications suggested that the overall incidence of pulmonary aspiration associated with the LMA Classic is about 2 cases per 10,000 patients,222 a figure corroborated by a large number of studies. This rate is comparable to that of outpatient anesthesia administered with a face mask and tracheal tube (Table 35-2).53 Studies also have demonstrated that the use of the LMA Classic is associated with a reduction in barrier pressure at the LES.223 In comparing the cuffed tracheal tube with the LMA Classic during general anesthesia with positive-pressure ventilation, Valentine and colleagues (using a pH electrode placed in the midesophageal zone) determined that there were significantly more episodes of reflux among the LMA Classic patients.224 In contrast, Agro and associates found no episodes of reflux with the use of LMA Classic in patients undergoing elective orthopedic surgery.225 The association between GER and the LMA Classic is not clear. However, there is insufficient evidence to support the hypothesis that the reduction in lower esophageal pH is influenced directly by the pressure or the volume in the cuff of the LMA Classic.226 Application of CP reduces LES pressure30; a similar reflux may occur because of the increased pharyngeal pressure exerted by the LMA Classic. There is controversy about the effect of the LMA Classic on LES tone, with some studies reporting a reduction in tone and others reporting no change.223,227 However, it is accepted that UES function is relatively unimpaired by the LMA Classic. Some anesthesiologists believe that the LMA Classic is contraindicated in patients with a full stomach and in obstetric patients.
TABLE 35-2 Incidence of Aspiration with the Laryngeal Mask Airway
| Study | Cases per Patient Population |
|---|---|
| Haden, et al, 1994309 | 1 : 3500 |
| Wainwright, 1995 310 | 0 : 1877 |
| Verghese and Brimacombe, 1996228 | 1 : 11910 |
| Brimacombe, 1996311 | 0 : 1500 |
| Lopez-Gil, et al, 1996312 | 0 : 2000 |
Despite the pulmonary aspiration risks with an LMA Classic (with or without CP) being the same as those encountered with a face mask,222,228 particularly in patients with a full stomach, there are several case reports documenting the use of the LMA Classic after failed tracheal intubation attempts during emergency cesarean section. In these cases, the LMA Classic device provided a clear airway and was useful in rapidly providing oxygenation and relieving hypoxia. There were no reports of pulmonary aspiration.222,229–233 In 1346 elective cesarean sections, the LMA Classic was used as an alternative to tracheal intubation to ventilate lungs. The tracheal tube was blindly inserted with minimal cardiovascular responses, and there was no incidence of pulmonary aspiration in 98% of cases.234 In another study, the LMA Classic was used in 1067 patients undergoing elective cesarean sections.98 After RSI with CP, the LMA Classic was inserted, and an effective airway was obtained in 1060 patients (99%). There were no episodes of pulmonary aspiration, hypoxia, laryngospasm, or gastric insufflation.235
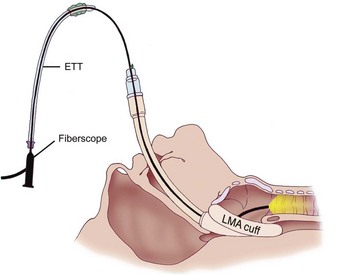
The major benefit of using the LMA Classic after failed tracheal intubation attempts in a patient with a full stomach is that it serves as a rescue device and provides oxygenation.236 However, because of the potential for gastric regurgitation and the risk of pulmonary aspiration, a choice must be made about whether to use the LMA Classic as a definitive airway or as a conduit for tracheal intubation. Technical problems associated with the LMA Classic include the less than ideal position of the device in relation to the glottic opening and downfolding of epiglottis in the LMA Classic aperture bars. The blind passage of a tracheal tube through the LMA Classic has an unacceptably low degree of success. Difficulties include catching the tracheal tube tip on the aperture bars or the anterior commissure because of the natural bend in a standard polyvinyl chloride tracheal tube and the natural bend where the tube exits the LMA. When CP was not applied, Heath and Allagain could intubate the trachea blindly through the LMA Classic in 72% of patients at first attempt and in most patients when time was not limited.237 However, the blind technique of tracheal intubation through the LMA Classic often requires significant manipulation of the head and neck to accomplish the intubation. An Eschmann stylet can be used to aid tracheal intubation. A high success rate was obtained for patients in whom difficult intubation was not anticipated by inserting the Eschmann stylet with its angulated end pointing anteriorly until it passed through the grille of the LMA Classic and then rotating the Eschmann stylet by 180 degrees.238 Failures, however, have been reported with this visually unassisted guided technique of endotracheal intubation through the LMA Classic.239 Because of the potential problems, in an urgent situation, blind tracheal intubation through the LMA Classic may not be advisable. Fiberoptic-guided tracheal intubation through the LMA Classic has been shown to be the most reliable technique, which has a much higher success rate than other approaches (Fig. 35-2).213–237
If the LMA Classic is used as a definitive airway without the passage of a tracheal tube, timing of the removal of the device in a patient with a full stomach becomes crucial. A randomized, controlled trial demonstrated that the pH in the lower esophagus was significantly higher in patients in whom the LMA Classic remained in situ until the end of the case, at which point the patient was able to follow commands. It is suggested that the LMA Classic should be removed only when the patient has fully regained consciousness at the end of the anesthetic.240
2 Fastrach Intubating Laryngeal Mask Airway
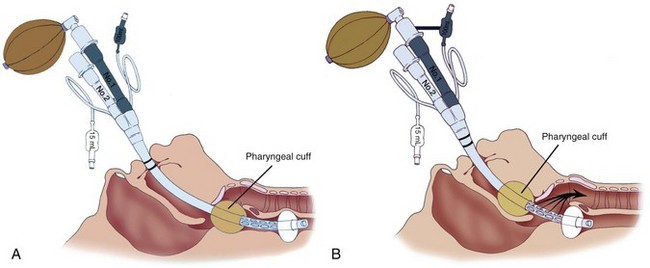
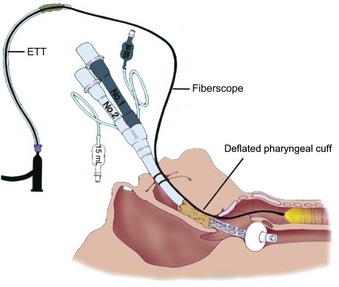
The highest degree of success in intubating the trachea blindly through the ILMA-Fastrach can be achieved by using the special tracheal tube (Euromedical ILM Tracheal Tube, Euromedical, Sungai Petani, Kedah, Malaysia) supplied with the device. This silicon tube is soft tipped, straight, wire reinforced, and cuffed. Ferson and colleagues reported their clinical experience with the ILMA in 254 patients with difficult airways,241 including patients with Cormack-Lehane grade 4 views; patients with immobilized cervical spines; those with airways distorted by tumors, surgery, or radiation therapy; and those wearing stereotactic frames. The device was particularly useful in patients undergoing emergency or elective surgery for whom tracheal intubation with a rigid laryngoscope had failed.242–244 Similar multicenter trials and reports have described the use of an ILMA-Fastrach in patients with difficult airways and failed tracheal intubation (including emergency cases at risk for aspiration).245–251 The ILMA-Fastrach has been used as a rescue device by emergency room physicians after failed RSI.252 Our institution has reported cases in which the device proved to be a life-saving rescue device after failed tracheal intubation during emergency cesarean section.253,254
3 ProSeal Laryngeal Mask Airway
The ProSeal laryngeal mask airway (PLMA) is a complex and potentially useful device for patients particularly at risk for gastric regurgitation and pulmonary aspiration. Some anesthesiologists refuse to use the LMA Classic because of concerns regarding gastric distention with positive-pressure ventilation and the potential for pulmonary aspiration. To address these issues, the primary goal of designing the PLMA was to construct a device with improved ventilatory characteristics that also offered protection against gastric insufflation and regurgitation. The principal feature of the PLMA is a double mask forming two end-to-end junctions: one with the respiratory tract and the other with the gastrointestinal tract. In contrast, the original LMA Classic forms a single end-to-end junction with the respiratory tract. The PLMA has a modified posterior cuff that provides high seal pressure—up to 30 cm H2O—providing a tighter seal against the glottic opening with no increase in mucosal pressure. The PLMA also offers a better airway seal at a given pressure than the LMA Classic.255 When properly positioned, the distal orifice of the PLMA lies against the UES and separates the esophagus and stomach from the glottic area. It has an integral gastric access and venting port that allows passage of a 14-F gastric tube, allowing suctioning of gastric contents.
In awake volunteers, neither the PLMA nor the LMA Classic appeared to interfere with UES or LES tone.256 No manometric studies have been performed to assess LES function in anesthetized patients, but FOB inspection of the PLMA drainage tube showed that the UES was completely open in 3% to 7% of paralyzed patients and in 9% of nonparalyzed patients.257,258 This effect, which may have implications for the frequency of regurgitation into the drainage tube, may be mechanical, a local reflex, or result from direct exposure to atmosphere through the drainage tube.
The PLMA is easy to insert and is a more effective ventilatory device when using positive-pressure ventilation. A meta-analysis using Fisher’s method has shown that the PLMA forms a more effective seal with the respiratory tract than the LMA Classic.259 The efficacy of the seal with the gastrointestinal tract has been determined by measuring the airway pressure at which air leaks into the drainage tube in anesthetized adults. Depending on cuff volume,260 the efficacy of the seal for air is at least 27 to 29 cm H2O,255,261 and for fluid, the efficacy is 19 to 73 cm H2O, with a similar seal mechanism for the respiratory tract.
The drainage tube provides a conduit to the gastrointestinal tract. It is also possible to insert a gastric balloon tube to further reduce the risk of pulmonary aspiration.141 The success rate for gastric tube insertion is 88% to 100%.258,261 Inserting a gastric tube has several advantages. The tube allows removal of gas or fluid from the stomach, the process of insertion provides information about the position or patency of the drainage tube, and the tube can function as a guide for PLMA reinsertion if accidental displacement occurs.262
The incidence of gastric insufflation seems to be low, even at high airway pressures.255,263 Gastric insufflation has been detected in only 1 of 572 patients (data from seven studies).255,257,258,261,263–265 Gastric insufflation is less common with the PLMA than with the LMA Classic when high positive-pressure ventilation is used.255
There are at least two reports of the PLMA being used in the difficult airway scenario. Keller and colleagues,263 in a prospective study of morbidly obese patients (i.e., patients at risk for gastric regurgitation and pulmonary aspiration), found that the PLMA was successful in 11 of 11 patients who were difficult or impossible to intubate with a laryngoscope. However, there are two case reports of pulmonary aspiration of gastric contents using the PLMA. In the first patient, brownish fluid was seen ejecting from the drainage tube of PLMA after administration of reversal agents at the end of surgery. There were clinical signs of pulmonary aspiration without any radiologic changes.266 In the second patient, foldover malposition of the distal cuff against the posterior oropharyngeal wall (confirmed later by FOB) during insertion was cited as the reason for pulmonary aspiration.267
In two reports, the PLMA was used successfully in obstetric patients after failed tracheal intubation.268 Keller and colleagues showed that the PLMA was a rescue device after failed tracheal intubation in an obstetric patient with hemolysis, elevated liver enzymes, and low platelet count (HELLP syndrome) and proved to be useful for postoperative respiratory support for 8 hours until the platelet count had increased and the patient was hemodynamically stable.268
Emergence characteristics for the PLMA seem to be similar to those for the LMA Classic.257 Practical considerations include suctioning the gastric tube and reversing any residual neuromuscular blockade before beginning of emergence. As with the LMA Classic, the patient should not be disturbed, and the PLMA should be removed only when the patient is conscious and obeys commands. When the patient is fully awake and responsive to verbal commands, the device is removed with the cuff inflated along with the gastric tube on continuous suction to prevent any oropharyngeal secretions from entering the laryngopharynx.
B Esophageal-Tracheal Combitube in the Full-Stomach Patient
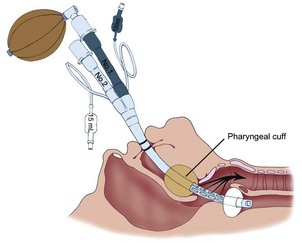
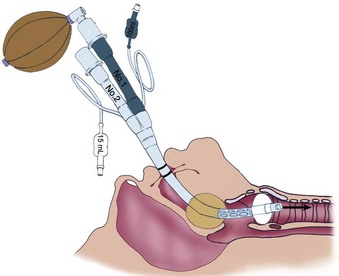
The Combitube, developed by Frass and colleagues, is a disposable, polyvinyl chloride, double-lumen airway device that combines the features of an esophageal obturator airway and a tracheal tube.269 It functions well in both the esophageal and tracheal positions.270 Using the Lipp maneuver,271 the Combitube is inserted blindly. Insertion usually results in esophageal placement (Fig. 35-3), and ventilation is initiated through the gastric lumen.272 With both cuffs inflated, ventilation through the proximal lumen allows airflow through eight oval fenestrations from the hypopharynx into the trachea. An effective seal of the latex oropharyngeal cuff prevents the escape of air from the mouth and nose. Instead, air is forced into the trachea and lungs. If the Combitube is placed in the esophagus, gastric fluids can be aspirated through the gastric lumen. If breath sounds are not heard, the Combitube has more than likely been placed in the trachea (Fig. 35-4). Without removing the Combitube, ventilation is switched to the tracheoesophageal lumen and confirmed with auscultation and capnography.
Unsatisfactory ventilation through either lumen indicates that the Combitube is too far advanced into the esophagus to produce airway obstruction by the oropharyngeal cuff (Fig. 35-5A). In this situation, the distal esophageal cuff and the oropharyngeal cuff are deflated, and the Combitube is withdrawn approximately 2 to 3 cm (see Fig. 35-5B). The esophageal cuff and the oropharyngeal cuff are then reinflated, and pulmonary ventilation is resumed.119
The Combitube serves as a rescue device for establishing an airway in the “cannot intubate, can ventilate” and “cannot intubate, cannot ventilate” situations, and it is particularly useful in the full-stomach patient. It is inserted blindly or under direct laryngoscopy,273 can secure the airway easily and rapidly, and requires minimal preparation (i.e., can be properly used with relatively little formal training).60 Combitube placement by inexperienced personnel (e.g., ICU nurses) under medical supervision was found to be as safe and successful in providing effective oxygenation and ventilation of the lungs as tracheal intubation performed by ICU physicians during cardiopulmonary resuscitation.274 As a rescue device, it is helpful in securing the airway if vocal cord visualization is compromised or if oropharyngeal bleeding has occurred.
The Combitube prevents pulmonary aspiration during cardiopulmonary resuscitation,270 especially when protective airway reflexes are absent, protecting the airway from pulmonary aspiration of gastric contents.275 Frass and coworkers found that the tracheoesophageal lumen served as a conduit for decompression of gastric contents and allowed gastric suctioning.270 Urtubia and associates administered methylene blue orally to 25 patients before induction of anesthesia and found no evidence of methylene blue in the hypopharynx during laryngoscopy before Combitube placement or after its removal.275 Hagberg and colleagues determined that the incidence of pulmonary aspiration is comparable for the Combitube and the LMA.276
C Esophageal-Tracheal Combitube for Prevention of Pulmonary Aspiration
Many case reports attest to the safety of the Combitube and the use of the tracheoesophageal lumen as an effective decompression channel for regurgitated gastric contents, which prevents pulmonary aspiration during cardiopulmonary resuscitation or during anesthesia.276–278 The 10-F orogastric tube, available in the prepackaged Combitube kit, can be readily used for esophageal and gastric suctioning. The Combitube has been used successfully as a primary airway device in seven obstetric patients (R. M. Urtubia, personal communication, 2001), two of whom were reported.279 They did not have a history of difficult tracheal intubation, and they underwent general anesthesia for emergency cesarean section. The Combitube was used as the initial airway device, and it allowed adequate oxygenation, ventilation, and protection from pulmonary aspiration of gastric contents.279 Wissler reported the use of the Combitube under direct laryngoscopy during obstetric anesthesia.280 The Combitube has become his primary choice for managing an anesthetized parturient whose trachea cannot be intubated or whose lungs cannot be mask ventilated while applying CP.280
If tracheal intubation becomes necessary, it can be achieved using the FOB with the Combitube in place. The fiberscope is passed alongside the Combitube, the oropharyngeal balloon is partially deflated, and tracheal intubation is achieved visually and is followed by removal of the Combitube without interruption of airway control, oxygenation, or ventilation (Fig. 35-6). This technique has been used for spontaneously breathing and mechanically ventilated patients.44,281 One of the authors (AW) managed a morbidly obese, 550-pound woman with obstructive sleep apnea, who had been in the medical intensive care unit (MICU) with a Combitube in situ. Tracheal intubation was requested by the MICU team to help with weaning the patient from the ventilator. Using the hybrid technique of direct laryngoscopy and fiberoptic bronchoscopy, tracheal intubation was successfully accomplished, and was followed by Combitube removal.
Video film recordings of 25 patients undergoing laparoscopic cholecystectomy under general anesthesia and mechanical ventilation using the Combitube revealed no gastric insufflation at the beginning or end of surgery.282 A large, retrospective survey of 12,020 cases of nontraumatic cardiac arrest in Japan revealed 1594 instances of Combitube use without any evidence of pulmonary aspiration of gastric contents.277
The Combitube has several advantages compared with the LMA:
1. Preparation time is minimal.
2. The device is ideal for full-stomach patients and pregnant patients (especially after failed tracheal intubation), for whom oxygenation, avoidance of arterial desaturation, and pulmonary aspiration are important factors.
3. Lubrication is not required.
4. No special method is required for cuff inflation or deflation.
The few case reports of complications related to the use of the Combitube primarily involve the original version (41 F) of the device and are attributed to its stiffer, rather large, and potentially traumatic design.283 These complications have included esophageal rupture, subcutaneous emphysema, piriform sinus rupture, pneumomediastinum, and pneumoperitoneum.284,285 Common factors in these cases included difficult ambient conditions in the field or prehospital environment, multiple attempts to insert the device, resistance with insertion, and the use of excessive force.286
1. Use the smaller Combitube SA universally for patients between 4 and 6 ft tall.
2. Briefly dip the device in warm saline before use.
3. Use the Lipp maneuver (i.e., holding the two ends of the Combitube together to bend it into a semicircle for a few seconds before insertion) during placement of the device.
4. Place the device under direct laryngoscopy.273
5. Use a gentle technique to reduce the hemodynamic response from stimulation of the proprioceptors at the base of the tongue.283
6. Inflate the oropharyngeal balloon slowly to the minimum volume necessary for an airtight seal to reduce soft tissue injury.283
7. Employ a reliable technique, using direct laryngoscopy as an aid to Combitube placement if necessary.273
D King Laryngeal Tube Suction Airways in the Full-Stomach Patient
The King LTS and its disposable version, the King LTS-D, are second-generation supraglottic airways that are modifications of the Combitube. They are slightly shorter, softer, and smaller than the Combitube and may have a lower complication profile.287 These devices are placed using gentle, blind insertion along the surface of the tongue from the mouth into the upper esophagus (see Fig. 37-7A in Chapter 37) . They have twin lumens, one for ventilation and the other for gastric suctioning with an 18-F orogastric tube and passive venting, thereby isolating the trachea from the esophagus (see Fig. 37-7B in Chapter 37). In contrast to the Combitube, they have a single pilot balloon for the proximal oropharyngeal nonlatex cuff and the distal esophageal cuff, preventing confusion regarding the choice of pilot balloon required for a particular cuff. They are easily placed,288 use low-pressure cuffs,289 allow exchange for a tracheal tube by passing an FOB preloaded with an Aintree airway exchange catheter290 (see Fig. 37-7C in Chapter 37), and have been used during controlled ventilation and during spontaneous respiration.288,291 Based on the literature, The device has potential for successful use in the full-stomach patient. A study comparing the LMA Classic and the King LT in 22 patients showed that the mean leak pressure was significantly higher for the King LT and that gastric insufflation was not observed with King LT.289 A second study enrolling 30 patients showed that a ventilatory seal of 40 cm H2O was easily obtained without gastric insufflation.287 A case report of an obstetric patient demonstrated the successful use of King LTS in providing access for oxygenation and ventilation after failed tracheal intubation during an emergency cesarean section.292
IX Extubation
When an awake intubation or RSI is indicated to prevent pulmonary aspiration, an awake tracheal extubation should be considered to prevent airway complications and to enhance patient safety during emergence from general anesthesia, at extubation, and in the postanesthesia care unit.293 The patient should be awake, conscious, and appropriately responding to commands before extubation. Daley and colleagues performed a survey of tracheal extubation of adult surgical patients who were still deeply anesthetized.294 Most respondents in the survey, who otherwise used the technique for extubation, considered the risk of pulmonary aspiration a contraindication to deep tracheal extubation.
X Management of Pulmonary Aspiration
When a fully conscious person aspirates foreign substances into the tracheobronchial tree, a brief but effective bout of coughing can clear the aspirate. Patients with observed pulmonary aspiration under sedation should have the mouth and pharynx suctioned immediately so that the patency of the upper airway is restored. Suctioning solid or liquid material allows recovery of the aspirated material so that it is not absorbed by the lungs, and it stimulates coughing to further expel the aspirate. After asphyxiation has been averted, bronchoscopy can be performed to clear the obstructing material from the lower airways. Bronchoscopy should not be routinely performed; it should be reserved for patients who have aspirated sufficient solid material to cause significant airway obstruction.293 Damage to the mucosa of the tracheobronchial tree occurs within seconds, but the bronchial secretions neutralize the aspirated acid within minutes.295 Attempts to neutralize the acid aspirate with saline or bicarbonate lavage have proved to be futile and may increase the damage.296
The treatment of pulmonary aspiration of gastric contents is aimed at restoring pulmonary function to normal as soon as possible. If the patient is awake and able to maintain a reasonable arterial oxygen tension (PaO2), a conservative approach is to provide supplemental oxygen through a nasal cannula or a face mask. The inspired oxygen concentration (FIO2) can be increased to maintain PaO2 at approximately 60 to 70 mm Hg. This may suffice in a patient with a mild condition, but more aggressive therapy is indicated if aspiration is more severe. Severe bronchospasm may be treated by aminophylline infusion or the inhalation of a β-adrenergic bronchodilator.296,297
When severe pulmonary aspiration is suspected, early ventilatory support is the mainstay of treatment. Early continuous positive airway pressure (CPAP) is indicated in awake and alert patients who do not respond to a face mask. CPAP up to 12 to 14 mm Hg can be administered through a tight-fitting mask. If higher levels are required, mechanical ventilation should be considered. The level of CPAP can be reduced as the patient improves, but it should not be completely withdrawn before the alveoli can maintain stability. A high FIO2 level can be used initially but should be decreased as soon as possible.298
If the patient is obtunded, a tracheal tube should be placed and mechanical ventilation initiated. Positive end-expiratory pressure (PEEP) should be applied, and FIO2 should be decreased as soon as possible while adequate oxygenation is maintained. PEEP is commonly used to elevate functional residual capacity and prevent atelectasis resulting from poor ventilatory efforts.297 It also improves the ventilation-perfusion (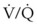 ) ratio and allows the use of less toxic levels of oxygen to be administered, giving the lungs a chance to recover.295 Caution should be exercised when using PEEP because high levels can worsen pulmonary damage by causing increased transudation of fluid through injured capillary beds.299 Cereda and coworkers investigated the effect of PEEP in patients with acute lung injury and found that a PEEP of at least 15 cm H2O was needed to prevent a decay in the respiratory system compliance.300
) ratio and allows the use of less toxic levels of oxygen to be administered, giving the lungs a chance to recover.295 Caution should be exercised when using PEEP because high levels can worsen pulmonary damage by causing increased transudation of fluid through injured capillary beds.299 Cereda and coworkers investigated the effect of PEEP in patients with acute lung injury and found that a PEEP of at least 15 cm H2O was needed to prevent a decay in the respiratory system compliance.300
Despite these measures, if hypoxemia persists along with bilateral lung infiltrates and poor lung compliance, management should be similar to that for the acute respiratory distress syndrome (ARDS). A large, multicenter, randomized trial sponsored by the National Institutes of Health compared ventilation with lower versus traditional tidal volumes in patients with ARDS.301 Smaller tidal volumes (6 mL/kg predicted body weight) and hypoventilation with permissive hypercapnia were associated with a 10% reduction in mortality, along with a shorter period using mechanical ventilation. The lower tidal volume ventilation approach protected the lungs from excessive stretch, improving several important clinical indicators of outcome in patients with ARDS.301 An alveolar recruitment maneuver using a CPAP of 40 cm H2O for 40 seconds improved oxygenation in patients with early ARDS who did not have impairment of the chest wall.302 A recruitment maneuver with a smaller tidal volume was associated with a survival rate of 62%, compared with a rate of 29% when using conventional ventilation without the recruitment maneuver.303
Use of prophylactic antibiotics is not recommended.304 Antibiotics alter the normal flora of the respiratory tract, which predisposes the susceptible patient to secondary infection with resistant organisms. Mitsushima and colleagues demonstrated that acid aspiration–induced epithelial injury led to subsequent bacterial infection in mice.305 Approximately 20% to 30% of patients who manifest initial gastric content aspiration eventually develop a secondary infection.202 Antibiotics should be reserved for patients who show signs of clinical infection and for patients who have aspirated grossly contaminated material into their lungs.
Wolfe and coworkers found that pneumonia resulting from gram-negative bacteria was more common after pulmonary aspiration among patients treated with corticosteroids than those who were not treated.306 Corticosteroids interfered with the healing of granulomatous lesions in rabbit models.307 The consensus appears to be that corticosteroids play no role in the treatment of aspiration pneumonitis.308
XII Clinical Pearls
• The incidence of pulmonary aspiration in the general surgical population is low, but it is slightly increased among obstetric, pediatric, and trauma patients.
• Recommendations for preoperative fasting times are 2 hours for clear liquids and 6 hours for solids.
• Preoperative ultrasonography of the gastric antrum may be used to screen patients at risk for perioperative pulmonary aspiration of regurgitant gastric contents.
• Routine preoperative use of gastrointestinal stimulants, H2-receptor antagonists, and proton pump inhibitors (PPIs) is not recommended in patients with no increased risk of pulmonary aspiration.
• Meticulous attention to airway management during induction, through emergence, and after extubation is crucial for patients at risk for pulmonary aspiration.
• Awake tracheal intubation and dealing with a difficult or failed airway in a patient with a full stomach requires expertise in advanced airway management.
• Airway management skills should be advanced by participating in workshops and by frequently using them in routine clinical practice.
All references can be found online at expertconsult.com.
2 Beck-Schimmer B, Bonvini JM. Bronchoaspiration: Incidence, consequences and management. Eur J Anaesthesiol. 2011;28:78–84.
62 Mhyre JM, Riesner MN, Polley LS, Naughton NN. A series of anesthesia-related maternal deaths in Michigan, 1985-2003. Anesthesiology. 2007;106:1096–1104.
63 Cook TM, Woodall N, Frerk C, for the Fourth National Audit Project. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anesthesia. Br J Anaesth. 2011;106:617–631.
78 American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011;114:495–511.
102 Perlas A, Davis L, Khan M, et al. Gastric sonography in the fasted surgical patient: A prospective descriptive study. Anesth Analg. 2011;113:93–97.
110 Nishina K, Mikawa K, Maekawa N, et al. A comparison of lansoprazole, omeprazole, and ranitidine for reducing preoperative gastric secretion in adult patients undergoing elective surgery. Anesth Analg. 1996;82:832–836.
135 Sellick BA. Cricoid pressure to prevent regurgitation of stomach contents during induction of anaesthesia. Lancet. 1961;2:404–406.
203 Roth JV. Cricoid pressure is for full stomachs, thyroid pressure is for assisting intubations [letter]. Anesth Analg. 2007;104:219.
214 Mort TC. Emergency tracheal intubation: Complications associated with repeated laryngoscopic attempts. Anesth Analg. 2004;99:607–613.
301 Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308.
1 Olsson GL, Hallen B, Hambraeus-Jonzon K. Aspiration during anaesthesia: A computer-aided study of 185,358 anaesthetics. Acta Anaesthesiol Scand. 1986;30:84–92.
2 Beck-Schimmer B, Bonvini JM. Bronchoaspiration: Incidence, consequences and management. Eur J Anaesthesiol. 2011;28:78–84.
3 Warner MA, Warner ME, Warner DO, et al. Perioperative pulmonary aspiration in infants and children. Anesthesiology. 1999;90:66–71.
4 Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78:56–62.
5 Leigh JM, Tytler JA. Admissions to the intensive care unit after complications of anaesthetic techniques over 10 years. 2. The second 5 years. Anaesthesia. 1990;45:814–820.
6 Roberts RB, Shirley MA. Reducing the risk of acid aspiration during cesarean section. Anesth Analg. 1974;53:859–868.
7 O’Hare B, Lerman J, Endo J. Acute lung injury after instillation of human breast milk or infant formula into rabbit’s lungs. Anesthesiology. 1996;90:1112–1118.
8 Plourde G, Hardy JF. Aspiration pneumonia: Assessing the risk of regurgitation in the cat. Can Anaesth Soc J. 1986;33:345–348.
9 James CF, Modell JH, Gibbs CP, et al. Pulmonary aspiration—Effects of volume and pH in the rat. Anesth Analg. 1984;63:665–668.
10 Raidoo DM, Rocke DA, Brock-Utne JG, et al. Critical volume for pulmonary acid aspiration: Reappraisal in a primate model. Br J Anaesth. 1990;65:248–250.
11 Brodsky JB, Brock-Utne AJ, Levi DC, et al. Pulmonary aspiration of a milk/cream mixture. Anesthesiology. 1999;91:1533–1534.
12 Chin C, Lerman J, Endo J. Acute lung injury after tracheal instillation of acidified soya-based or Enfalac formula or human breast milk in rabbits. Can J Anaesth. 1999;46:282–286.
13 Cote CJ, Goudsouzian NG, Liu LM, et al. Assessment of risk factors related to the acid aspiration syndrome in pediatric patients—Gastric pH and residual volume. Anesthesiology. 1982;56:70–72.
14 Splinter WM, Stewart JA, Muir JG. Large volumes of apple juice preoperatively do not affect gastric pH and volume in children. Can J Anaesth. 1990;37:36–39.
15 Crawford M. Fasting in children prior to surgery. Anesth Analg. 1990;71:402–403.
16 Ingebo KR, Rayhorn NJ, Hecht RM, et al. Sedation in children: Adequacy of two-hour fasting. J Pediatr. 1997;131:155–158.
17 Litman RS, Wu CL, Quinlivan JK. Gastric volume and pH in infants fed clear liquids and breast milk prior to surgery. Anesth Analg. 1994;79:482–485.
18 Maekawa N, Mikawa K, Yaku H, et al. Effects of 2-, 4- and 12-hour fasting intervals on preoperative gastric fluid pH and volume, and plasma glucose and lipid homeostasis in children. Acta Anaesthesiol Scand. 1993;37:783–787.
19 Meakin G, Dingwall AE, Addison GM. Effects of fasting and oral premedication on the pH and volume of gastric aspirate in children. Br J Anaesth. 1987;59:678–682.
20 Sandhar BK, Goresky GD, Maltby JR, et al. Effects of oral liquids and ranitidine on gastric fluid volume and pH in children undergoing outpatient surgery. Anesthesiology. 1989;71:402–403.
21 Schreiner MS, Triebwasser A, Keon TP. Ingestion of liquids compared with preoperative fasting in pediatric outpatients. Anesthesiology. 1990;72:593–597.
22 Splinter WM, Schaefer JD. Ingestion of clear fluids is safe for adolescents up to 3 h before anaesthesia. Br J Anaesth. 1991;66:48–52.
23 Schreiner M. The preop fast: Not quite so fast. Contemp Pediatr. 1992;6:45–52.
24 Sinnatamby CS. Abdomen. In Last’s anatomy: Regional and applied, ed 10, Edinburgh: Churchill Livingstone; 1999:215–320.
25 Kahrilas PJ, Lin S, Chen J, Manka M. The effect of hiatus hernia on gastro-oesophageal junction pressure. Gut. 1999;44:476–482.
26 Broussard CN, Richter JE. Treating gastro-oesophageal reflux disease during pregnancy and lactation: What are the safest therapy options? Drug Saf. 1998;19:325–337.
27 Dent J, Dodds WJ, Friedman RH, et al. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest. 1980;65:256–267.
28 Schoeman MN, Tippett MD, Akkermans LM, et al. Mechanisms of gastroesophageal reflux in ambulant healthy human subjects. Gastroenterology. 1995;108:289–291.
29 Cotton BR, Smith G. The lower oesophageal sphincter and anaesthesia. Br J Anaesth. 1984;56:37–46.
30 Tournadre JP, Chassard D, Berrada KR, Bouletreau P. Cricoid cartilage pressure decreases lower esophageal sphincter tone. Anesthesiology. 1997;86:7–9.
31 Chassard D, Tournadre JP, Berrada KR, et al. Effect of halothane, isoflurane and desflurane on lower oesophageal sphincter tone. Br J Anaesth. 1996;77:781–783.
32 Gardaz JP, Theumann N, Guyot J, et al. Effects of propofol on lower oesophageal sphincter pressure and barrier pressure. Eur J Anaesthesiol. 1996;13:203.
33 Kahrilas PJ, Dodds WJ, Dent J, et al. Effect of sleep, spontaneous gastroesophageal reflux, and a meal on upper esophageal sphincter pressure in normal human volunteers. Gastroenterology. 1987;92:466–471.
34 Berg H. Is residual neuromuscular block following pancuronium a risk factor for postoperative pulmonary complications? Acta Anaesthesiol Scand Suppl. 1997;110:156–158.
35 McGrath JP, McCaul C, Byrne PJ, et al. Upper oesophageal sphincter function during general anaesthesia. Br J Surg. 1996;83:1276–1278.
36 Sundman E, Witt H, Olsson R, et al. The incidence and mechanisms of pharyngeal and upper esophageal dysfunction in partially paralyzed humans: Pharyngeal videoradiography and simultaneous manometry after atracurium. Anesthesiology. 2000;92:977–984.
37 Tagaito Y, Isono S, Nishino T. Upper airway reflexes during a combination of propofol and fentanyl anesthesia. Anesthesiology. 1998;88:1459–1466.
38 Vanner RG, Pryle BJ, O’Dwyer JP, Reynolds F. Upper oesophageal sphincter pressure and the intravenous induction of anaesthesia. Anaesthesia. 1992;47:371–375.
39 Ng A, Smith G. Gastroesophageal reflux and aspiration of gastric contents in anesthetic practice. Anesth Analg. 2001;93:494–513.
40 Engelhardt T, Webster NR. Pulmonary aspiration of gastric contents in anaesthesia. Br J Anaesth. 1999;83:453–460.
41 Caranza R, Nandwani N, Tring JP, et al. Upper airway reflex sensitivity following general anaesthesia for day-case surgery. Anaesthesia. 2000;55:367–370.
42 Adams AP. General principles of emergency anaesthesia. In: Adams AP, Hewitt PB, Rogers MC. Emergency anaesthesia. London: Edward Arnold; 1986:1–13.
43 Bricker SR, McLuckie A, Nightingale DA. Gastric aspirates after trauma in children. Anaesthesia. 1989;44:721–724.
44 Ovassapian A, Liu S, Krejcie T. Fiberoptic tracheal intubation with Combitube in place. Anesth Analg. 1993;76(Suppl):S315.
45 Jorgensen NH, Byer DE, Gould AB, Jr. Aspiration pneumonitis. Prevention and treatment. Minn Med. 1989;72:517–519. 530
46 Lockey DJ, Coats T, Parr MJ. Aspiration in severe trauma: A prospective study. Anaesthesia. 1999;54:1097–1098.
47 Moulton C, Pennycook AG. Relation between Glasgow coma score and cough reflex. Lancet. 1994;343:1261–1262.
48 Nimmo WS. Aspiration of gastric contents. Br J Hosp Med. 1985;34:176–179.
49 Nimmo WS, Heading RC, Wilson J, et al. Inhibition of gastric emptying and drug absorption by narcotic analgesics. Br J Clin Pharmacol. 1975;2:509–513.
50 Maltby JR, Reid CR, Hutchinson A. Gastric fluid volume and pH in elective inpatients. Part II: Coffee or orange juice with ranitidine. Can J Anaesth. 1988;35:16–19.
51 Manchikanti L, Canella MG, Hohlbein LJ, Colliver JA. Assessment of effect of various modes of premedication on acid aspiration risk factors in outpatient surgery. Anesth Analg. 1987;66:81–84.
52 Salem MR, Wong AY, Mani M, et al. Premedicant drugs and gastric juice pH and volume in pediatric patients. Anesthesiology. 1976;44:216–219.
53 Kallar SK, Everett LL. Potential risks and preventive measures for pulmonary aspiration: New concepts in preoperative fasting guidelines. Anesth Analg. 1993;77:171–182.
54 Merio R, Festa A, Bergmann H, et al. Slow gastric emptying in type I diabetes: Relation to autonomic and peripheral neuropathy, blood glucose, and glycemic control. Diabetes Care. 1997;20:419–423.
55 Vaisman N, Weintrob N, Blumental A, et al. Gastric emptying in patients with type I diabetes mellitus. Ann N Y Acad Sci. 1999;873:506–511.
56 Vaughan RW, Bauer S, Wise L. Volume and pH of gastric juice in obese patients. Anesthesiology. 1975;43:686–689.
57 Agarwal A, Chari P, Singh H. Fluid deprivation before operation. The effect of a small drink. Anaesthesia. 1989;44:632–634.
58 Blitt CD, Gutman HL, Cohen DD, et al. “Silent” regurgitation and aspiration during general anesthesia. Anesth Analg. 1970;49:707–713.
59 Carlsson C, Islander G. Silent gastropharyngeal regurgitation during anesthesia. Anesth Analg. 1981;60:655–657.
60 Bishop MJ, Kharasch ED. Is the Combitube a useful emergency airway device for anesthesiologists? Anesth Analg. 1998;86:1141–1142.
61 Kluger MT, Short TG. Aspiration during anaesthesia: A review of 133 cases from the Australian Anaesthetic Incident Monitoring Study (AIMS). Anaesthesia. 1999;54:19–26.
62 Mhyre JM, Riesner MN, Polley LS, Naughton NN. A series of anesthesia-related maternal deaths in Michigan, 1985-2003. Anesthesiology. 2007;106:1096–1104.
63 Cook TM, Woodall N, Frerk C, for the National Audit Project. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anesthesia. Br J Anaesth. 2011;106:617–631.
64 Mellin-Olsen J, Fasting S, Gisvold SE. Routine preoperative gastric emptying is seldom indicated. A study of 85,594 anaesthetics with special focus on aspiration pneumonia. Acta Anaesthesiol Scand. 1996;40:1184–1188.
65 Murphy PJ, Langton JA, Barker P, Smith G. Effect of oral diazepam on the sensitivity of upper airway reflexes. Br J Anaesth. 1993;70:131–134.
66 Tweedle D, Nightingale P. Anesthesia and gastrointestinal surgery. Acta Chir Scand Suppl. 1989;550:131–139.
67 Strunin L. Report of the National Confidential Enquiry into Perioperative Deaths (NCEPOD) conference. London: NCEPOD; 1995. pp 76–78
68 O’Flynn PE, Milford CA. Fasting in children for day case surgery. Ann R Coll Surg Engl. 1989;71:218–219.
69 Eriksson LI, Sandin R. Fasting guidelines in different countries. Acta Anaesthesiol Scand. 1996;40:971–974.
70 Fasting S, Soreide E, Raeder JC. Changing preoperative fasting policies. Impact of a national consensus. Acta Anaesthesiol Scand. 1998;42:1188–1191.
71 Green CR, Pandit SK, Schork MA. Preoperative fasting time: Is the traditional policy changing? Results of a national survey. Anesth Analg. 1996;83:123–128.
72 Hutchinson A, Maltby JR, Reid CR. Gastric fluid volume and pH in elective inpatients. Part I: Coffee or orange juice versus overnight fast. Can J Anaesth. 1988;35:12–15.
73 Ljungqvist O, Soreide E. Preoperative fasting. Br J Surg. 2003;90:400–406.
74 Maltby JR, Lewis P, Martin A, Sutherland LR. Gastric fluid volume and pH in elective patients following unrestricted oral fluid until three hours before surgery. Can J Anaesth. 1991;38:425–429.
75 Maltby JR, Sutherland AD, Sale JP, Shaffer EA. Preoperative oral fluids: Is a five-hour fast justified prior to elective surgery? Anesth Analg. 1986;65:1112–1116.
76 Minami H, McCallum RW. The physiology and pathophysiology of gastric emptying in humans. Gastroenterology. 1984;86:1592–1610.
77 Pandit SK, Loberg KW, Pandit UA. Toast and tea before elective surgery? A national survey on current practice. Anesth Analg. 2000;90:1348–1351.
78 American Society of Anesthesiologists. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011;114:495–511.
79 Peterson GN, Domino KB, Caplan RA, et al. Management of the Difficult Airway: A closed claims analysis. Anesthesiology. 2005;103:33–39.
80 American Society of Anesthesiologists. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: A report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology. 1999;90:896–905.
81 Beaumont W. Experiments and observation on the gastric juice and physiology of digestion. Boston: Lily, Wait; 1834. pp 159–160
82 Horowitz M, Pounder DJ. Is the stomach a useful forensic clock? Aust N Z J Med. 1985;15:73–76.
83 Hunt JN. Some properties of an alimentary osmoreceptor mechanism. J Physiol. 1956;132:267–288.
84 McGrady EM, Macdonald AG. Effect of the preoperative administration of water on gastric volume and pH. Br J Anaesth. 1988;60:803–805.
85 Scarr M, Maltby JR, Jani K, Sutherland LR. Volume and acidity of residual gastric fluid after oral fluid ingestion before elective ambulatory surgery. CMAJ. 1989;141:1151–1154.
86 Shevde K, Trivedi N. Effects of clear liquids on gastric volume and pH in healthy volunteers. Anesth Analg. 1991;72:528–531.
87 Cavell B. Gastric emptying in preterm infants. Acta Paediatr Scand. 1979;68:527–531.
88 Cavell B. Gastric emptying in infants fed human milk or infant formula. Acta Paediatr Scand. 1981;70:639–641.
89 Heading RC. Gastric motility and emptying. In: Sireus W, Smith AN. Scientific foundations of gastroenterology. London: Heinemann; 2003:287–296.
90 Moore JG, Christian PE, Coleman RE. Gastric emptying of varying meal weight and composition in man. Evaluation by dual liquid- and solid-phase isotopic method. Dig Dis Sci. 1981;26:16–22.
91 Moukarzel AA, Sabri MT. Gastric physiology and function: Effects of fruit juices. J Am Coll Nutr. 1996;15(Suppl):18S–25S.
92 Miller M, Wishart HY, Nimmo WS. Gastric contents at induction of anaesthesia. Is a 4-hour fast necessary? Br J Anaesth. 1983;55:1185–1188.
93 Soreide E, Hausken T, Soreide JA, Steen PA. Gastric emptying of a light hospital breakfast. A study using real time ultrasonography. Acta Anaesthesiol Scand. 1996;40:549–553.
94 Haavik PE, Soreide E, Hofstad B, Steen PA. Does preoperative anxiety influence gastric fluid volume and acidity? Anesth Analg. 1992;75:91–94.
95 Simpson KH, Stakes AF. Effect of anxiety on gastric emptying in preoperative patients. Br J Anaesth. 1987;59:540–544.
96 Ong BY, Palahniuk RJ, Cumming M. Gastric volume and pH in out-patients. Can Anaesth Soc J. 1978;25:36–39.
97 Barnard P, Jenkins J. Failed tracheal intubation in obstetrics: A 6-year review in a UK hospital. Anaesthesia. 2000;55:685–694.
98 Emerson BM, Wrigley SR, Newton M. Pre-operative fasting for paediatric anaesthesia. A survey of current practice. Anaesthesia. 1998;53:326–330.
99 Phillips S, Daborn AK, Hatch DJ. Preoperative fasting for paediatric anaesthesia. Br J Anaesth. 1994;73:529–536.
100 Carp H, Jayaram A, Stoll M. Ultrasound examination of the stomach contents of parturients. Anesth Analg. 1992;74:683–687.
101 Perlas A, Chan VWS, Lupu CM, et al. Ultrasound assessment of gastric contents and volume. Anesthesiology. 2009;111:82–89.
102 Perlas A, Davis L, Khan M, et al. Gastric sonography in the fasted surgical patient: A prospective descriptive study. Anesth Analg. 2011;113:93–97.
103 Raeder J, Dahl V, Bjoernestad E, Edlund G, et al. Does esomeprazole prevent postoperative nausea and vomiting? Acta Anaesthesiol Scand. 2007;51:217–225.
104 Cheng HC, Sheu BS. Intravenous proton pump inhibitors for peptic ulcer bleeding: Clinical benefits and limits. World J Gastrointest Endosc. 2011;16:49–56.
105 Hirota K, Imai A, Kudo M, et al. Efficacy of a single 24-hour pre-anesthetic dose of proton pump inhibitors. J Gastroenterol Hepatol. 2009;24:1244–1247.
106 Escolano F, Castano J, Lopez R, et al. Effects of omeprazole, ranitidine, famotidine and placebo on gastric secretion in patients undergoing elective surgery. Br J Anaesth. 1992;69:404–406.
107 Kluger MT, Willemsen G. Anti-aspiration prophylaxis in New Zealand: A national survey. Anaesth Intensive Care. 1998;26:70–77.
108 Manchikanti L, Colliver JA, Marrero TC, Roush JR. Assessment of age-related acid aspiration risk factors in pediatric, adult, and geriatric patients. Anesth Analg. 1985;64:11–17.
109 Morison DH, Dunn GL, Fargas-Babjak AM, et al. A double-blind comparison of cimetidine and ranitidine as prophylaxis against gastric aspiration syndrome. Anesth Analg. 1982;61:988–992.
110 Nishina K, Mikawa K, Maekawa N, et al. A comparison of lansoprazole, omeprazole, and ranitidine for reducing preoperative gastric secretion in adult patients undergoing elective surgery. Anesth Analg. 1996;82:832–836.
111 Nishina K, Mikawa K, Takao Y, et al. A comparison of rabeprazole, lansoprazole, and ranitidine for improving preoperative gastric fluid property in adults undergoing elective surgery. Anesth Analg. 2000;90:717–721.
112 Olsson GL, Hallen B. Pharmacological evacuation of the stomach with metoclopramide. Acta Anaesthesiol Scand. 1982;26:417–420.
113 Tryba M, Yildiz F, Zenz M, Schwerdt M. Prevention of aspiration pneumonia with cimetidine [in German]. Anaesthesist. 1982;31:584–587.
114 Viegas OJ, Ravindran RS, Shumacker CA. Gastric fluid pH in patients receiving sodium citrate. Anesth Analg. 1981;60:521–523.
115 Cohen SE, Jasson J, Talafre ML, et al. Does metoclopramide decrease the volume of gastric contents in patients undergoing cesarean section? Anesthesiology. 1984;61:604–607.
116 Ewart MC, Yau G, Gin T, et al. A comparison of the effects of omeprazole and ranitidine on gastric secretion in women undergoing elective caesarean section. Anaesthesia. 1990;45:527–530.
117 Gibbs CP, Banner TC. Effectiveness of Bicitra as a preoperative antacid. Anesthesiology. 1984;61:97–99.
118 Solanki DR, Suresh M, Ethridge HC. The effects of intravenous cimetidine and metoclopramide on gastric volume and pH. Anesth Analg. 1984;63:599–602.
119 Stuart JC, Kan AF, Rowbottom SJ, et al. Acid aspiration prophylaxis for emergency caesarean section. Anaesthesia. 1996;51:415–421.
120 Wyner J, Cohen SE. Gastric volume in early pregnancy: Effect of metoclopramide. Anesthesiology. 1982;57:209–212.
121 Brock-Utne JG, Downing JW, O’Keefe SJ, Gjessing J. Protection against acid pulmonary aspiration with cimetidine. Anaesth Intensive Care. 1983;11:138–140.
122 Colman RD, Frank M, Loughnan BA, et al. Use of i.m. ranitidine for the prophylaxis of aspiration pneumonitis in obstetrics. Br J Anaesth. 1988;61:720–729.
123 Dive A, Miesse C, Galanti L, et al. Effect of erythromycin on gastric motility in mechanically ventilated critically ill patients: A double-blind, randomized, placebo-controlled study. Crit Care Med. 1995;23:1356–1362.
124 Jahr JS, Burckart G, Smith SS, et al. Effects of famotidine on gastric pH and residual volume in pediatric surgery. Acta Anaesthesiol Scand. 1991;35:457–460.
125 Momose K, Shima T, Haga S, et al. The effect of preoperative oral administration of ranitidine on pH and volume of gastric juice [in Japanese]. Masui. 1992;41:1482–1485.
126 Ng WL, Glomaud D, Hardy F, Phil S. Omeprazole for prophylaxis of acid aspiration in elective surgery. Anaesthesia. 1990;45:436–438.
127 O’Connor TA, Basak J, Parker S. The effect of three different ranitidine dosage regimens on reducing gastric acidity and volume in ambulatory surgical patients. Pharmacotherapy. 1995;15:170–175.
128 Ormezzano X, Ganansia MF, Arnould JF, et al. Prevention of aspiration pneumonia in obstetrical anesthesia with the effervescent combination of cimetidine and sodium citrate [in French]. Ann Fr Anesth Reanim. 1990;9:285–288.
129 Strain JD, Moore EE, Markovchick VJ, Duzer-Moore S. Cimetidine for the prophylaxis of potential gastric acid aspiration pneumonitis in trauma patients. J Trauma. 1981;21:49–51.
130 Wilson SL, Mantena NR, Halverson JD. Effects of atropine, glycopyrrolate, and cimetidine on gastric secretions in morbidly obese patients. Anesth Analg. 1981;60:37–40.
131 Wrobel J, Koh TC, Saunders JM. Sodium citrate: An alternative antacid for prophylaxis against aspiration pneumonitis. Anaesth Intensive Care. 1982;10:116–119.
132 Kristensen MS, Gellett S, Bach AB, Jensen TK. Hemodynamics and arterial oxygen saturation during preoperative emptying of the stomach. Acta Anaesthesiol Scand. 1991;35:342–344.
133 Fox EJ, Sklar GS, Hill CH, et al. Complications related to the pressor response to endotracheal intubation. Anesthesiology. 1977;47:524–525.
134 Katz RL, Bigger JT, Jr. Cardiac arrhythmias during anesthesia and operation. Anesthesiology. 1970;33:193–213.
135 Sellick BA. Cricoid pressure to prevent regurgitation of stomach contents during induction of anaesthesia. Lancet. 1961;2:404–406.
136 Salem MR, Joseph NJ, Heyman HJ, et al. Cricoid compression is effective in obliterating the esophageal lumen in the presence of a nasogastric tube. Anesthesiology. 1985;63:443–446.
137 Vanner RG, Pryle BJ. Regurgitation and oesophageal rupture with cricoid pressure: A cadaver study. Anaesthesia. 1992;47:732–735.
138 Hardy JF, Lepage Y, Bonneville-Chouinard N. Occurrence of gastroesophageal reflux on induction of anaesthesia does not correlate with the volume of gastric contents. Can J Anaesth. 1990;37:502–508.
139 Ferrer M, Bauer TT, Torres A, et al. Effect of nasogastric tube size on gastroesophageal reflux and microaspiration in intubated patients. Ann Intern Med. 1999;130:991–994.
140 Roewer N. Can pulmonary aspiration of gastric contents be prevented by balloon occlusion of the cardia? A study with a new nasogastric tube. Anesth Analg. 1995;80:378–383.
141 Schwarzmann G, Wurmb T, Greim C, Roewer N. Difficult airway management: Combination of the laryngeal mask airway with a new gastric balloon tube [abstract]. Anesthesiology. 1998;89:A1237.
142 Benumof JL. Management of the difficult adult airway. With special emphasis on awake tracheal intubation. Anesthesiology. 1991;75:1087–1110.
143 Butler PJ, Dhara SS. Prediction of difficult laryngoscopy: An assessment of the thyromental distance and Mallampati predictive tests. Anaesth Intensive Care. 1992;20:139–142.
144 el Ganzouri AR, McCarthy RJ, Tuman KJ, et al. Preoperative airway assessment: Predictive value of a multivariate risk index. Anesth Analg. 1996;82:1197–1204.
145 Frerk CM. Predicting difficult intubation. Anaesthesia. 1991;46:1005–1008.
146 Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: A prospective study. Can Anaesth Soc J. 1985;32:429–434.
147 Nichol HC, Zuck D. Difficult laryngoscopy—The “anterior” larynx and the atlanto-occipital gap. Br J Anaesth. 1983;55:141–144.
148 Oates JD, Macleod AD, Oates PD, et al. Comparison of two methods for predicting difficult intubation. Br J Anaesth. 1991;66:305–309.
149 Rocke DA, Murray WB, Rout CC, Gouws E. Relative risk analysis of factors associated with difficult intubation in obstetric anesthesia. Anesthesiology. 1992;77:67–73.
150 Rose DK, Cohen MM. The airway: Problems and predictions in 18,500 patients. Can J Anaesth. 1994;41:372–383.
151 Samsoon GL, Young JR. Difficult tracheal intubation: A retrospective study. Anaesthesia. 1987;42:487–490.
152 Savva D. Prediction of difficult tracheal intubation. Br J Anaesth. 1994;73:149–153.
153 Tse JC, Rimm EB, Hussain A. Predicting difficult endotracheal intubation in surgical patients scheduled for general anesthesia: A prospective blind study. Anesth Analg. 1995;81:254–258.
154 Wilson ME, Spiegelhalter D, Robertson JA, Lesser P. Predicting difficult intubation. Br J Anaesth. 1988;61:211–216.
155 Bonica JJ. Transtracheal anaesthesia for endotracheal intubation. Anesthesiology. 1949;10:736–738.
156 Duncan JA. Intubation of the trachea in the conscious patient. Br J Anaesth. 1977;49:619–623.
157 Ovassapian A, Krejcie TC, Yelich SJ, et al. Awake fiberoptic intubation in the patient at high risk of aspiration. Br J Anaesth. 1989;62:13–16.
158 Cook TM, McCrirrick A. A survey of airway management during induction of general anaesthesia in obstetrics. Int J Obstet Anesth. 1994;3:143–145.
159 Thwaites AJ, Rice CP, Smith I. Rapid sequence induction: A questionnaire survey of its routine conduct and continued management during a failed intubation. Anaesthesia. 1999;54:376–381.
160 Gerardi MJ, Sacchetti AD, Cantor RM, et al. Rapid-sequence intubation of the pediatric patient. Pediatric Emergency Medicine Committee of the American College of Emergency Physicians. Ann Emerg Med. 1996;28:55–74.
161 Knopp RK. Rapid sequence intubation revisited. Ann Emerg Med. 1998;31:398–400.
162 Ma OJ, Bentley B, Debehnke DJ. Airway management practices in emergency medicine residencies. Am J Emerg Med. 1995;13:501–504.
163 McAllister JD, Gnauck KA. Rapid-sequence intubation of the pediatric patient. Fundamentals of practice. Pediatr Clin North Am. 1999;46:1–32.
164 Nakayama DK, Waggoner T, Venkataraman ST, et al. The use of drugs in emergency airway management in pediatric trauma. Ann Surg. 1992;216:205–211.
165 Sakles JC, Laurin EG, Rantapaa AA, Panacek EA. Airway management in the emergency department: A one-year study of 610 tracheal intubations. Ann Emerg Med. 1998;31:325–332.
166 Aitkenhead AR, Smith G. Textbook of anaesthesia. Edinburgh: Churchill Livingstone; 1996. pp 523–525
167 Morris J, Cook TM. Rapid sequence induction: A national survey of practice. Anaesthesia. 2001;56:1090–1097.
168 Van Maren GA. Emergency anaesthesia in the unprepared patient. In: Prys-Roberts C, Brown BR. International practice of anaesthesia. Oxford: Butterworth-Heinemann; 1996:1291–1297.
169 Benhamou D. French obstetric anaesthetists and acid aspiration prophylaxis. Eur J Anaesthesiol. 1993;10:27–32.
170 Benhamou D. Controversies in obstetric anaesthesia. Cricoid pressure is unnecessary in obstetric general anaesthesia. Int J Obstet Anaesth. 1995;4:30–33.
171 Baraka AS, Sayyid SS, Assaf BA. Thiopental-rocuronium versus ketamine-rocuronium for rapid-sequence intubation in parturients undergoing cesarean section. Anesth Analg. 1997;84:1104–1107.
172 Fuchs-Buder T, Sparr HJ, Ziegenfuss T. Thiopental or etomidate for rapid sequence induction with rocuronium. Br J Anaesth. 1998;80:504–506.
173 Engbaek J, Viby-Mogensen J. Can rocuronium replace succinylcholine in a rapid induction of anaesthesia? Acta Anaesthesiol Scand. 1993;43:1–3.
174 Wright PM, Caldwell JE, Miller RD. Onset and duration of rocuronium and succinylcholine at the adductor pollicis and laryngeal adductor muscles in anesthetized humans. Anesthesiology. 1994;81:1110–1115.
175 Booth MG, Marsh B, Bryden FM, et al. A comparison of the pharmacodynamics of rocuronium and vecuronium during halothane anaesthesia. Anaesthesia. 1992;47:832–834.
176 Cadamy AJ, Booth MG, Cademy AJ. Rapid sequence induction. Anaesthesia. 1999;54:817.
177 Wilson ME. Predicting difficult intubation. Br J Anaesth. 1993;71:333–334.
178 Levitan RM, Mechem CC, Ochroch EA, et al. Head-elevated laryngoscopy position: Improving laryngeal exposure during laryngoscopy by increasing head elevation. Ann Emerg Med. 2003;41:322–330.
179 Smith RB, Sjostrand UH. Apneic diffusion oxygenation and continuous flow apneic ventilation. A review. Acta Anaesth Scand. 1985;29:101–105.
180 Schlesinger S, Blanchfield D. Modified rapid sequence induction of anesthesia: A survey of current clinical practice. AANA J. 2001;69:291–298.
181 Brimacombe JR, Berry AM. Cricoid pressure. Can J Anaesth. 1997;44:414–425.
182 Rosen M. Anesthesia for obstetrics [editorial]. Anaesthesia. 1981;36:145–146.
183 National Academy of Sciences–National Research Council. Standards and guidelines for cardiopulmonary resuscitation [CPR] and emergency cardiac care (ECC). JAMA. 1985;255:2905–2985.
184 Salem MR, Sellick BA, Elam JO. The historical background of cricoid pressure in anesthesia and resuscitation. Anesth Analg. 1974;53:230–232.
185 Kron SS. Questionable effectiveness of cricoid pressure in preventing aspiration. Anesthesiology. 1995;83:431–432.
186 Schwartz DE, Cohen NH. Questionable effectiveness of cricoid pressure in preventing aspiration [letter]. Anesthesiology. 1995;83:432.
187 Tournadre JP, Chassard D, Berrada K, et al. Cricoid cartilage pressure decreases lower oesophageal sphincter tone. Anesthesiology. 1996;86:7–9.
188 Fanning GL. The efficacy of cricoid pressure in preventing regurgitation of gastric contents. Anesthesiology. 1970;32:553–555.
189 Lawes EG, Duncan PW, Bland B, et al. The cricoid yoke—A device for providing consistent and reproducible cricoid pressure. Br J Anaesth. 1986;58:925–931.
190 Salem MR, Wong AY, Fizzotti GF. Efficacy of cricoid pressure in preventing aspiration of gastric contents in paediatric patients. Br J Anaesth. 1972;44:401–404.
191 Vanner RG, Pryle BJ. Nasogastric tubes and cricoid pressure. Anaesthesia. 1993;48:1112–1113.
192 Wraight WJ, Chamney AR, Howells TH. The determination of an effective cricoid pressure. Anaesthesia. 1983;38:461–466.
193 Vanner RG, Pryle BJ, O’Dwyer JP, Reynolds F. Upper oesophageal sphincter pressure during inhalational anaesthesia. Anaesthesia. 1992;47:950–954.
194 Hartsilver EL, Vanner RG, Bewley J, Clayton T. Gastric pressure during emergency caesarean section under general anaesthesia. Br J Anaesth. 1999;82:752–754.
195 Francis S, Enani S, Shah J, et al. Simulated cricoid force in paediatric anaesthesia. Br J Anaesth. 2000;85:164.
196 Benumof JL. Difficult laryngoscopy: Obtaining the best view. Can J Anaesth. 1994;41:361–365.
197 Baxter AD. Cricoid pressure in the sniffing position. Anaesthesia. 1991;46:327.
198 Vanner RG. Tolerance of cricoid pressure by conscious volunteers. Int J Obstet Anaesth. 1992;1:195–198.
199 Whittington RM, Robinson JS, Thompson JM. Fatal aspiration (Mendelson’s) syndrome despite antacids and cricoid pressure. Lancet. 1979;2:228–230.
200 Ralph SJ, Wareham CA. Rupture of the oesophagus during cricoid pressure. Anaesthesia. 1991;46:40–41.
201 Vanner RG. Mechanisms of regurgitation and its prevention with cricoid pressure. Int J Obstet Anaesth. 1993;2:207–215.
202 Randell T, Maattanen M, Kytta J. The best view at laryngoscopy using the McCoy laryngoscope with and without cricoid pressure. Anaesthesia. 1998;53:536–539.
203 Roth JV. Cricoid pressure is for full stomachs, thyroid pressure is for assisting intubations [letter]. Anesth Analg. 2007;104:219.
204 Cook TM. Cricoid pressure: Are two hands better than one? Anaesthesia. 1996;51:365–368.
205 Cowling J. Cricoid pressure—A more comfortable technique. Anaesth Intensive Care. 1982;10:93–94.
206 Salem MR, Wong AY, Mani M, Sellick BA. Efficacy of cricoid pressure in preventing gastric inflation during bag-mask ventilation in pediatric patients. Anesthesiology. 1974;40:96–98.
207 Crowley DS, Giesecke AH. Bimanual cricoid pressure. Anaesthesia. 1990;45:588–589.
208 Caplan RA, Posner KL, Ward RJ, Cheney FW. Adverse respiratory events in anesthesia: A closed claims analysis. Anesthesiology. 1990;72:828–833.
209 Lunn JN, Devlin HB. Lessons from the confidential enquiry into perioperative deaths in three NHS regions. Lancet. 1987;2:1384–1386.
210 King TA, Adams AP. Failed tracheal intubation. Br J Anaesth. 1990;65:400–414.
211 Latto I. Management of difficult intubation. In: Latto I, Rosen M. Difficulties in tracheal intubation. London: Baillirre Tindall; 1987:99–141.
212 Hawthorne L, Wilson R, Lyons G, Dresner M. Failed intubation revisited: 17-yr experience in a teaching maternity unit. Br J Anaesth. 1996;76:680–684.
213 Suresh M, Wali A. Failed intubation in obstetrics—Airway management strategies. Anesthesiol Clin North Am. 1998;16:477–498.
214 Mort TC. Emergency tracheal intubation: Complications associated with repeated laryngoscopic attempts. Anesth Analg. 2004;99:607–613.
215 Gataure PS, Vaughan RS, Latto IP. Simulated difficult intubation. Comparison of the gum elastic bougie and the stylet. Anaesthesia. 1996;51:935–938.
216 Chisholm DG, Calder I. Experience with the McCoy laryngoscope in difficult laryngoscopy. Anaesthesia. 1997;52:906–908.
217 Tuckey JP, Cook TM, Render CA. Forum. An evaluation of the levering laryngoscope. Anaesthesia. 1996;51:71–73.
218 Hagberg CA. Special devices and techniques. Anesthesiol Clin North Am. 2002;20:907–932.
219 Cook TM. A new practical classification of laryngeal view. Anaesthesia. 2000;55:274–279.
220 Kovacs G, Law JA, McCrossin C, et al. A comparison of a fiberoptic stylet and a bougie as adjuncts to direct laryngoscopy in a manikin-simulated difficult airway. Ann Emerg Med. 2007;50:676–685.
221 Nanji GM, Maltby JR. Vomiting and aspiration pneumonitis with the laryngeal mask airway. Can J Anaesth. 1992;39:69–70.
222 Brimacombe JR, Berry A. The incidence of aspiration associated with the laryngeal mask airway: A meta-analysis of published literature. J Clin Anesth. 1995;7:297–305.
223 Rabey PG, Murphy PJ, Langton JA, et al. Effect of the laryngeal mask airway on lower oesophageal sphincter pressure in patients during general anaesthesia. Br J Anaesth. 1992;69:346–348.
224 Valentine J, Stakes AF, Bellamy MC. Reflux during positive pressure ventilation through the laryngeal mask. Br J Anaesth. 1994;73:543–544.
225 Agro F, Brimacombe J, Verghese C, et al. Laryngeal mask airway and incidence of gastro-oesophageal reflux in paralysed patients undergoing ventilation for elective orthopaedic surgery. Br J Anaesth. 1998;81:537–539.
226 Roux M, Drolet P, Girard M, et al. Effect of the laryngeal mask airway on oesophageal pH: Influence of the volume and pressure inside the cuff. Br J Anaesth. 1999;82:566–569.
227 Bunchungmongkol N, Chumpathong S, Catto-Smith AG, et al. Effects of the laryngeal mask airway on the lower oesophageal barrier pressure in children. Anaesth Intensive Care. 2000;28:543–546.
228 Verghese C, Brimacombe JR. Survey of laryngeal mask airway usage in 11,910 patients: Safety and efficacy for conventional and nonconventional usage. Anesth Analg. 1996;82:129–133.
229 Chadwick IS, Vohra A. Anaesthesia for emergency caesarean section using the Brain laryngeal airway. Anaesthesia. 1989;44:261–262.
230 Gataure PS, Hughes JA. The laryngeal mask airway in obstetrical anaesthesia. Can J Anaesth. 1995;42:130–133.
231 McClure S, Regan M, Moore J. Laryngeal mask airway for caesarean section. Anaesthesia. 1990;45:227–228.
232 McFarlane C. Failed intubation in an obese obstetric patient and the laryngeal mask airway. Int J Obstet Anaesth. 1992;2:183–185.
233 Priscu V, Priscu L, Soroker D. Laryngeal mask for failed intubation in emergency caesarean section [letter]. Can J Anaesth. 1992;39:893.
234 Martinez EJ: Use of classic laryngeal mask in obstetrics and gynecology [abstract]. Proceedings of the 26th Argentinian Congress of Anaesthesiology, 247[I, II], 1997.
235 Han TH, Brimacombe J, Lee EJ, Yang HS. The laryngeal mask airway is effective (and probably safe) in selected healthy parturients for elective cesarean section: A prospective study of 1067 cases. Can J Anaesth. 2001;48:1117–1121.
236 DeMello WF, Kocan M. The laryngeal mask in failed intubation [letter]. Anaesthesia. 1990;45:689–690.
237 Heath ML, Allagain J. Intubation through the laryngeal mask. A technique for unexpected difficult intubation. Anaesthesia. 1991;46:545–548.
238 Allison A, McCrory J. Tracheal placement of a gum elastic bougie using the laryngeal mask airways. Anaesthesia. 1990;45:419–420.
239 White A, Sinclair M, Pillai R. Laryngeal mask airway for coronary artery bypass grafting [letter]. Anaesthesia. 1991;46:234.
240 Cheong YP, Park SK, Son Y, et al. Comparison of incidence of gastroesophageal reflux and regurgitation associated with timing of removal of the laryngeal mask airway: On appearance of signs of rejection versus after recovery of consciousness. J Clin Anesth. 1999;11:657–662.
241 Ferson DZ, Rosenblatt WH, Johansen MJ, et al. Use of the intubating LMA-Fastrach in 254 patients with difficult-to-manage airways. Anesthesiology. 2001;95:1175–1181.
242 Asai T, Hirose T, Shingu K. Failed tracheal intubation using a laryngoscope and intubating laryngeal mask. Can J Anaesth. 2000;47:325–328.
243 Lu PP, Brimacombe J, Ho AC, et al. The intubating laryngeal mask airway in severe ankylosing spondylitis. Can J Anaesth. 2001;48:1015–1019.
244 Watson NC, Hokanson M, Maltby JR, Todesco JM. The intubating laryngeal mask airway in failed fibreoptic intubation. Can J Anaesth. 1999;46:376–378.
245 Baskett PJ, Parr MJ, Nolan JP. The intubating laryngeal mask. Results of a multicentre trial with experience of 500 cases. Anaesthesia. 1998;53:1174–1179.
246 Joo H, Rose K. Fastrach—A new intubating laryngeal mask airway: Successful use in patients with difficult airways. Can J Anaesth. 1998;45:253–256.
247 Lim CL, Hawthorne L, Ip-Yam PC. The intubating laryngeal mask airway (ILMA) in failed and difficult intubation. Anaesthesia. 1998;53:929–930.
248 Parr MJ, Gregory M, Baskett PJ. The intubating laryngeal mask. Use in failed and difficult intubation. Anaesthesia. 1998;53:343–348.
249 Skinner HJ, Ho BY, Mahajan RP. Gastro-oesophageal reflux with the laryngeal mask during day-case gynaecological laparoscopy. Br J Anaesth. 1998;80:675–676.
250 Smart NG, Varveris DA, Jacobs P. Use of the intubating laryngeal mask in failed intubation associated with amyloid macroglossia. Br J Anaesth. 2002;89:186–187.
251 Wakeling HG, Bagwell A. The intubating laryngeal mask (ILMA) in an emergency failed intubation. Anaesthesia. 1999;54:305–306.
252 Rosenblatt WH, Murphy M. The intubating laryngeal mask: Use of a new ventilating-intubating device in the emergency department. Ann Emerg Med. 1999;33:234–238.
253 Suresh M, Gardner M, Key E. Intubating Laryngeal Mask Airway (ILMA): A life saving rescue device following failed tracheal intubation during cesarean section (CS) [abstract]. Presented at the 36th Annual Meeting of the Society for Obstetric Anesthesia and Perinatology (SOAP), Fort Myers, FL. May 12-16, 2004.
254 Suresh MS, Chandrasekhar S, Munnur U. Management of unanticipated difficult intubation with intubating LMA (Fastrach LMA) and review of literature with comparison to classic LMA. Presented at the 42nd annual meeting of the Society for Obstetric Anesthesia and Perinatology, San Antonio, TX. May 12-16, 2010.
255 Lu P, Brimacombe J, Yang C, et al. The ProSeal versus the Classic laryngeal mask airway for positive pressure ventilation during laparoscopic cholecystectomy. Br J Anaesth. 2002;88:824–827.
256 Keller C, Brimacombe J. Resting esophageal sphincter pressures and deglutition frequency in awake subjects after oropharyngeal topical anesthesia and laryngeal mask device insertion. Anesth Analg. 2001;93:226–229.
257 Brimacombe J, Keller C. Stability of the LMA-ProSeal and standard laryngeal mask airway in different head and neck positions: A randomised crossover study. Eur J Anaesthesiol. 2003;20:65–69.
258 Brimacombe J, Keller C, Fullekrug B, et al. A multicenter study comparing the ProSeal and Classic laryngeal mask airway in anesthetized, nonparalyzed patients. Anesthesiology. 2002;96:289–295.
259 Rosenthal R. Combining results from independent studies. Psychol Bull. 1978;85:185–193.
260 Brimacombe J, Keller C. The ProSeal laryngeal mask airway. Anesthesiol Clin North Am. 2002;20:871–891.
261 Brimacombe J, Keller C. The ProSeal laryngeal mask airway: A randomized, crossover study with the standard laryngeal mask airway in paralyzed, anesthetized patients. Anesthesiology. 2000;93:104–109.
262 Drolet P, Girard M. An aid to correct positioning of the ProSeal laryngeal mask. Can J Anaesth. 2001;48:718–719.
263 Keller C, Brimacombe J, Kleinsasser A, Brimacombe L. The laryngeal mask airway ProSeal as a temporary ventilatory device in grossly and morbidly obese patients before laryngoscope-guided tracheal intubation. Anesth Analg. 2002;94:737–740.
264 Brain AI, Verghese C, Strube PJ. The LMA ProSeal—A laryngeal mask with an oesophageal vent. Br J Anaesth. 2000;84:650–654.
265 Howath A, Brimacombe J, Keller C. Gum-elastic bougie–guided insertion of the ProSeal laryngeal mask airway: A new technique. Anaesth Intensive Care. 2002;30:818.
266 Koay CK. A case of aspiration using the ProSeal LMA [letter]. Anaesth Intensive Care. 2003;31:123.
267 Brimacombe J, Keller C. Aspiration of gastric contents during use of a ProSeal laryngeal mask airway secondary to unidentified fold over malposition. Anesth Analg. 2003;97:1192–1194.
268 Keller C, Brimacombe J, Lirk P, Puhringer F. Failed obstetric tracheal intubation and postoperative respiratory support with the ProSeal laryngeal mask airway. Anesth Analg. 2004;98:1467–1470.
269 Gaitini LA, Vaida SJ, Agro F. The Esophageal-Tracheal Combitube. Anesthesiol Clin North Am. 2002;20:893–906.
270 Frass M, Frenzer R, Rauscha F, et al. Evaluation of esophageal tracheal Combitube in cardiopulmonary resuscitation. Crit Care Med. 1987;15:609–611.
271 Lipp M, Thierbach A, Daublander M, Dick W. Clinical evaluation of the Combitube [abstract 43]. Presented at the 18th annual meeting of the European Academy of Anaesthesiology, Copenhagen, Denmark. 1996.
272 Urtubia R, Aguila C. Combitube: A new proposal for a confusing nomenclature. Anesth Analg. 1999;89:803.
273 Frass M. The Combitube: Esophageal/tracheal double lumen airway. In: Benumof JL, ed. Airway management—Principles and practice. St. Louis: Mosby; 1996:444–455.
274 Staudinger T, Brugger S, Watschinger B, et al. Emergency intubation with the Combitube: Comparison with the endotracheal airway. Ann Emerg Med. 1993;22:1573–1575.
275 Urtubia RM, Aguila CM, Cumsille MA. Combitube: A study for proper use. Anesth Analg. 2000;90:958–962.
276 Hagberg CA, Vartazarian TN, Chelly JE, Ovassapian A. The incidence of gastroesophageal reflux and tracheal aspiration detected with pH electrodes is similar with the Laryngeal Mask Airway and Esophageal Tracheal Combitube—A pilot study. Can J Anaesth. 2004;51:243–249.
277 Tanigawa K, Shigematsu A. Choice of airway devices for 12,020 cases of nontraumatic cardiac arrest in Japan. Prehosp Emerg Care. 1998;2:96–100.
278 Deroy R, Ghoris M. The Combitube elective anesthetic airway management in a patient with cervical spine fracture. Anesth Analg. 1998;87:1441–1442.
279 Urtubia R, Medina J, Alzamora R. Combitube for emergency cesarean section under general anesthesia. Case reports. Difficult Airway. 2001;4:78–83.
280 Wissler RN. The esophageal-tracheal Combitube. Anesthesiol Rev. 1993;20:147–152.
281 Gaitini LA, Vaida SJ, Somri M, et al. Fiberoptic-guided airway exchange of the esophageal-tracheal Combitube in spontaneously breathing versus mechanically ventilated patients. Anesth Analg. 1999;88:193–196.
282 Vaida S. The effectiveness of the esophageal tracheal Combitube in mechanically ventilated patients undergoing laparoscopic cholecystectomy [abstract]. Anaesthesiol Intensivmed. 1999;34:S121.
283 Hartmann T, Krenn CG, Zoeggeler A, et al. The oesophageal-tracheal Combitube Small Adult. Anaesthesia. 2000;55:670–675.
284 Richards CF. Piriform sinus perforation during Esophageal-Tracheal Combitube placement. J Emerg Med. 1998;16:37–39.
285 Vezina D, Lessard MR, Bussieres J, et al. Complications associated with the use of the Esophageal-Tracheal Combitube. Can J Anaesth. 1998;45:76–80.
286 Vezina MC, Trepanier CA, Nicole PC, Lessard MR. Complications associated with the Esophageal-Tracheal Combitube in the pre-hospital setting. Can J Anaesth. 2007;54:124–128.
287 Dorges V, Ocker H, Wenzel V, Schmucker P. The laryngeal tube: A new simple airway device. Anesth Analg. 2000;90:1220–1222.
288 Hagberg C, Bogomolny Y, Gilmore V, et al. An evaluation of the insertion and function of a new supraglottic device, the King LT, during spontaneous ventilation. Anesth Analg. 2006;102:621–625.
289 Asai T, Shingu K. The laryngeal tube. Br J Anaesth. 2005;95:729–736.
290 Genzwuerker HV, Vollmer T, Ellinger K. Fiberoptic tracheal intubation after placement of the laryngeal tube. Br J Anaesth. 2002;89:733–738.
291 Gaitini LA, Vaida SJ, Somri M, et al. An evaluation of the laryngeal tube during general anesthesia using mechanical ventilation. Anesth Analg. 2003;96:1750–1756.
292 Zand F, Amini A. Use of the laryngeal tube-S for airway management and prevention of aspiration after a failed tracheal intubation in a parturient. Anesthesiology. 2005;102:481–483.
293 Gibbs CP, Modell JH. Pulmonary aspiration of gastric contents: Pathophysiology, prevention, and management. In: Miller RD, ed. Anesthesia. New York: Churchill Livingstone; 1994:1437–1464.
294 Daley MD, Norman PH, Coveler LA. Tracheal extubation of adult surgical patients while deeply anesthetized: A survey of United States anesthesiologists. J Clin Anesth. 1999;11:445–452.
295 Wynne JW, Modell JH. Respiratory aspiration of stomach contents. Ann Intern Med. 1977;87:466–474.
296 Hupp JR, Peterson LJ. Aspiration pneumonitis: Etiology, therapy, and prevention. J Oral Surg. 1981;39:430–435.
297 Vaughan GG, Grycko RJ, Montgomery MT. The prevention and treatment of aspiration of vomitus during pharmacosedation and general anesthesia. J Oral Maxillofac Surg. 1992;50:874–879.
298 Nader-Djalal N, Knight PR, Davidson BA, Johnson K. Hyperoxia exacerbates microvascular lung injury following acid aspiration. Chest. 1997;112:1607–1614.
299 Toung T, Saharia P, Permutt S, et al. Aspiration pneumonia: Beneficial and harmful effects of positive end-expiratory pressure. Surgery. 1977;82:279–283.
300 Cereda M, Foti G, Musch G, et al. Positive end-expiratory pressure prevents the loss of respiratory compliance during low tidal volume ventilation in acute lung injury patients. Chest. 1996;109:480–485.
301 Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308.
302 Valente Barbas CS. Lung recruitment maneuvers in acute respiratory distress syndrome and facilitating resolution. Crit Care Med. 2003;31(Suppl):S265–S271.
303 Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354.
304 Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344:665–671.
305 Mitsushima H, Oishi K, Nagao T, et al. Acid aspiration induces bacterial pneumonia by enhanced bacterial adherence in mice. Microb Pathog. 2002;33:203–210.
306 Wolfe JE, Bone RC, Ruth WE. Effects of corticosteroids in the treatment of patients with gastric aspiration. Am J Med. 1977;63:719–722.
307 Wynne JW, Reynolds JC, Hood I, et al. Steroid therapy for pneumonitis induced in rabbits by aspiration of foodstuff. Anesthesiology. 1979;51:11–19.
308 DePaso WJ. Aspiration pneumonia. Clin Chest Med. 1991;12:269–284.
309 Haden RM, Pinnock CA, Scott PV. Incidence of aspiration with the laryngeal mask airway [letter]. Br J Anaesth. 1994;72:496.
310 Wainwright AC. Positive pressure ventilation and the laryngeal mask airway in ophthalmic anaesthesia [letter]. Br J Anaesth. 1995;75:249–250.
311 Brimacombe J. Analysis of 1500 laryngeal mask uses by one anaesthetist in adults undergoing routine anaesthesia. Anaesthesia. 1996;51:76–80.
312 Lopez-Gil M, Brimacombe J, Alvarez M. Safety and efficacy of the laryngeal mask airway. A prospective survey of 1400 children. Anaesthesia. 1996;51:969–972.