The Older Adult Patient
Overview
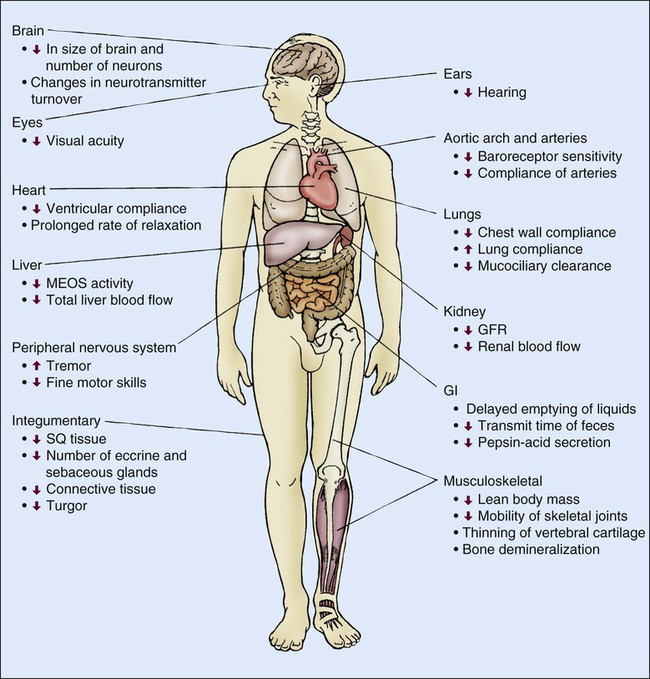
Senescence, or aging, is characterized by several physiologic changes. Incidence of disease and chronic conditions increases in older adults, although physiologic decline occurs independently of disease and is responsible for development of symptoms at an earlier stage of disease in older adults than in their younger counterparts.1 Changes in physiologic function are important to consider when caring for older adult patients. Various physiologic changes that occur with aging warrant special physical examination techniques.2 Clinicians must distinguish between changes in health caused by physiologic or pathologic processes. Comprehensive physiologic, psychologic, and environmental nursing assessment of the older adult and significant others/family members is vital in critical care and subsequent coordination of care.3 The purpose of this chapter is to familiarize the critical care nurse with literature and research on care of the critically ill older adult.
Health Care Statistics
The senescent population is increasing worldwide. In 2009 there were approximately 39.6 million American residents over the age of 65. Average life expectancy in 2009 was 84 years.4 In 2030 the number of Americans older than 65 years will be close to 70 million.2 Demographic shifts and overall population increases have resulted in changes in provision and reimbursement of health care delivery and services. Medicare and Medicaid have become major payers of hospital services, creating challenges for health care providers and organizations.5
In 2008 the Medicare program had 45 million enrollees and expenditures of $468 billion, which was a $36 billion increase over 2007.4 The Patient Protection and Affordable Care Act (PPACA) is estimated to increase coverage to an additional 32 million uninsured individuals by 2014.5 Health care services rendered to persons age 65 and over in 2007 included 289.7 million ambulatory visits, 13.9 million hospital in-patient care discharges, 1.3 million nursing home residents, 1 million home health patients, and 868,100 hospice discharges.4 Patients older than 65 years currently account for 42% to 52% of admissions to critical care units and almost 60% of all critical care unit days.2 Recent estimates of critical care costs account for 4% of national health expenditures, or approximately $81.7 billion.6
Health Conditions and the Older Adult
Health conditions experienced by older persons include hypertension (64% to 80%), heart disease (31% to 46%), cancer (17% to 23%), and diabetes (27%). Causes of mortality in persons over age 65 include heart disease (28%), cancer (22%), stroke (7%), chronic lower respiratory diseases (6%), and Alzheimer’s disease (4%).4 All mortality rates declined between 1997 and 2007 except Alzheimer’s disease, which increased more than 50%.7 Many of these conditions result in admission to critical care units; therefore critical care nurses need to understand key components in caring for older adult patients.
Cardiovascular System
Age-Related Changes of the Cardiovascular System
Advancing age has many effects on the cardiovascular system (Table 41-1). Age-related anatomic and cellular changes in the myocardium and peripheral vasculature have significant impact on the critically ill older adult.2 Age is a major risk factor for cardiovascular disease in older adults, leading to more cardiovascular events in this population when admitted to the critical care unit.8 Cardiac and arterial system changes include atherosclerosis, hypertension, myocardial infarction, and stroke. Pathologic alterations of aging include hypertrophy, altered left ventricular (LV) function, increased arterial stiffness, and impaired endothelial function.8
TABLE 41-1
AGE-RELATED CARDIOVASCULAR CHANGES
| PHYSIOLOGIC ALTERATION | MECHANISM | PATHOLOGIC CHANGE |
| Cardiovascular Structural Remodeling | ||
| ↑ Vascular intimal thickness | ||
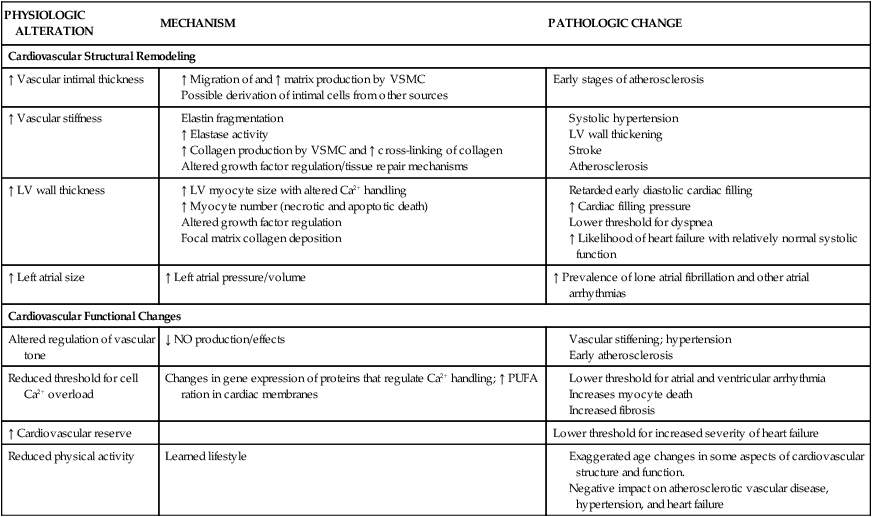
LV, Left ventricular; PUFA, polyunsaturated fatty acids; VSMC, vascular smooth muscle cells.
Adapted from Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Failures Clinic. 2012;8(1):143.
Age-Related Changes in Electrocardiogram
Changes in the electrocardiogram (ECG) include decreased R-wave and S-wave amplitude, increased P-R interval, and increased Q-T duration reflective of prolonged rate of relaxation (Table 41-2).9 The QRS axis shifts leftward with age, perhaps due to increased LV wall thickness or hypertrophy.9 Intrinsic heart rate decreases with age in the deficiency of parasympathetic influences; however, increases in atrial and ventricular arrhythmias increase in prevalence.9 Cardiac dysrhythmias include atrial fibrillation, paroxysmal supraventricular tachycardia, with the most common dysrhythmia of premature ventricular contraction (PVC).9 Reports show that 3% to 4% of patients older than 60 years of age experience atrial fibrillation9; incidence may be as high 10% in octogenarians.8 A study of healthy geriatric patients with 24-hour ambulatory ECG recordings showed that 13% to 50% experienced paroxysmal supraventricular tachycardia in frequency with exercise.9 Older individuals also have a high prevalence of left ventricular hypertrophy (LVH), which predisposes them to ventricular arrhythmias and sudden death. Atrial fibrillation is thought to be related to increased arterial stiffness, reduced LV compliance and rate control, and anticoagulation is recommended for most patients.10 Age-associated cardiac conduction changes (even if asymptomatic) are predictive of future cardiac morbidity and mortality older adults.9
TABLE 41-2
AGE-RELATED CHANGES IN RESTING ELECTROCARDIOGRAPHIC VARIABLES
| ECG VARIABLE | CHANGE WITH AGE |
| R–R internal | No change |
| P-wave duration | Minor increase |
| PR interval | Increase |
| QRS duration | No change |
| QRS axis | Leftward shift |
| QRS voltage | Decrease |
| QT interval | Minor increase |
| T-wave voltage | Decrease |
Modified from Strait JB, Lakatta EG. Aging-associated cardiovascular changes. Heart Failure Clin. 2012;8:143.
Age-Related Changes in Myocardial Structure and Function
Collagen is the principal noncontractile protein occupying the cardiac interstitium. Since myocardial collagen content increases with age, increased myocardial collagen content renders the myocardium less compliant and may be responsible for increased loading of blood vessels.11 Slipping of myocytes can adversely affect diastolic filling, and structural changes that occur in the walls of blood vessels can lead to increased systolic blood pressure (SBP).11 Collagen type I is more plentiful in the aging heart than the young heart, which contains more collagen type III. Collagen type I is less distensible than type III collagen, and both have different physical and mechanical properties.11 Consequently, the left ventricle must develop a higher filling pressure for a given increase in ventricular volume. Decreased LV compliance may be evident in the older adult by the presence of an S4 heart sound.12
Myocardial Infarction and Heart Failure
Chronic heart failure (CHF) affects approximately 5.7 million people in the United States of America and may be a contributing cause of death in 282,000 individuals per year, at a predicted cost of $39 billion annually.13 Approximately 50% of heart failure (HF) cases are found within the 6% of U.S. population older than age 75.9 Incidence of myocardial infarction (MI) in the Framingham Heart Study demonstrated that the rate of MI doubles for men and increases more than five-fold in women from the age group of 55 to 64 years to the age group of 85 to 94 years.13 Similar results were seen in the Atherosclerosis Risk in Communities (ARIC) surveillance study.13 Critical care nurses should recognize that older adult patients are not only more likely than young patients to experience an MI, but are more likely to die from an MI.
MI is one of the most common causes of CHF leading to HF via an intricate process known as left ventricular (LV) remodeling.13 Age-associated changes in arterial structure and function cause large vessels to become less distensible, leading to prolonged systolic contraction, lengthened diastolic relaxation, increased myocardial oxygen demand, and diminished organ perfusion.9 Additionally, atherosclerotic coronary arteries may limit blood flow to the myocardium, creating a higher risk of developing myocardial ischemia or infarction. MI in older adults is often associated with ST-segment depression rather than ST elevation. Sensation of chest pain may be altered and may be less intense and of shorter duration.2 Other atypical symptomatology may include dyspnea, confusion, and failure to thrive, which results in unrecognized signs and symptoms of cardiac problems and delays in diagnosis and treatment.2
The aging heart undergoes a modest degree of hypertrophy and thickening of the LV wall without significant changes in LV cavity size.9 Increase in LV wall thickness is primarily due to growth in muscle cell size, which can lead to delayed early diastolic filling and increased cardiac pressures.9 The early diastolic filling period and isovolumic phase of myocardial relaxation are prolonged in the older adult human myocardium. These changes may suggest diastolic dysfunction, but do not translate into decreases in end-diastolic volume or stroke volume.9 Myocardial contraction is dependent on intracellular levels of free calcium and the sensitivity of the contractile proteins for calcium.9 The senescent myocardium may have a lower threshold for atrial and ventricular arrhythmias due to changes in gene expression of proteins that regulate calcium.9 Prolonged duration of contraction (systole) is caused in part by a slowed or delayed rate of myocardial relaxation. This may be an adaptive mechanism to preserve contractile function compromised by age-related increases in afterload.9
Age-Related Changes in Left Ventricular Function During Exercise
Aging is associated with a decline in exercise performance due to many physiologic variables. Cardiac performance is dependent on the heart’s ability to increase and maintain cardiac output (CO), allowing oxygen delivery to the peripheral tissues. During exercise CO is increased by several mechanisms, the most important of which are increased heart rate, increased inotropic state of the myocardium, and decreased aortic impedance.9 The Baltimore Longitudinal Study of Aging reported that older adults’ diminished ability to exercise was not related to a difference in CO response.9 Maximal heart rate achieved during exercise is attenuated in older adults; however, decreased heart rate response is accompanied by increased LV end-diastolic volume. As adults age, there is a progressive decline in VO2max, which begins in the second decade of life and falls by roughly 10% per decade.
In summary, an older individual’s ability to exercise may be limited by reduction in cardiac reserve, increased vascular afterload, arterial-ventricular load mismatching, pulmonary function, reduced intrinsic myocardial contractility, impaired autonomic regulation, and physical deconditioning.9
Peripheral Vascular System
The aging effects on the peripheral vascular system are revealed in a gradual but linear rise in SBP up until age 80 years when values tend to plateau.2 Diastolic blood pressure (BP) is less affected by age and generally remains the same or decreases.2 Important determinants of SBP include vascular compliance and blood volume within the system. Endothelial function and compliance of the vasculature is determined by cell type and tissue composition. In older adults, the intimal layer of the large and distal arteries thickens due to an increase in smooth muscle cells and connective tissue.8 This gradual decrease in arterial compliance, or “stiffening of the arteries,” is sometimes referred to as arteriosclerosis. Arteriosclerotic changes are also accompanied by changes caused by atherosclerosis. Atherosclerosis is the accumulation of lipoproteins and fibrinous products such as platelets, macrophages, and leukocytes within a vessel.8 Arteriosclerotic and atherosclerotic processes cause arteries to become progressively less distensible and alter the vascular pressure-volume relationship. These changes are clinically significant because small changes in intravascular volume are accompanied by disproportionate increases in SBP, leading to increases in afterload and development of concentric (pressure-induced) ventricular hypertrophy in the older adult.8
Improved cardiovascular care has led to delay of cardiovascular disease; however, monitoring of cardiovascular risk factors such as cholesterol, BP, and obesity is now needed in the older adult population.14 Lipoprotein levels increase with advancing age, adding to risk factors for development and progression of atherosclerosis. Serum lipoproteins are particles that contain various amounts of cholesterol, triglycerides, phospholipids, and apoproteins. The classification of lipoproteins is based on their size and relative concentration of cholesterol, triglycerides, and apoproteins. The five principal serum lipoproteins are chylomicrons, low-density lipoproteins (LDLs), very-low-density lipoproteins (VLDLs), intermediate-density lipoproteins (IDLs), and high-density lipoproteins (HDLs). Lipoprotein (Lp) (a) is composed of an LDL-like particle that in elevated levels has been implicated as a strong risk factor for coronary heart disease (CHD) and stroke.15 Timely identification of high-risk individuals with high Lp(a) may allow for therapy such as statins to reduce LDL cholesterol to guideline-recommended levels.15
Another condition that may warrant attention in persons older than 65 years is metabolic syndrome. Metabolic syndrome is characterized by a group of risk factors, including abdominal obesity, high triglycerides, low HDLs, small LDLs, elevated BP, proinflammatory, prothrombotic states, and high plasma insulin (insulin resistance).14 Metabolic syndrome has been reported to increase cardiovascular and mortality risk in middle-aged populations due to glucose metabolism and insulin resistance that is linked to cholesterol metabolism.14 Reduction of risk associated with diabetes and metabolic syndrome may be modulated by manipulation of cholesterol absorption, leading to fewer events and improved survival in older adult cardiovascular patients.14
Hypertension
The population is aging, with hypertension affecting most individuals older than 65 years of age.10 Pathophysiology of hypertension in older adults results from changes in arterial structure and function that decrease distensibility of large vessels, reduce forward circulation flow, increase pulse wave velocity, cause late SBP augmentation, and increase myocardial oxygen demand, all of which limit organ perfusion.10 Older adults with poor BP control have an increased risk of cerebrovascular disease (CVD), coronary artery disease (CAD), disorders of LV structure and function, aortic and peripheral arterial disease, chronic kidney disease (CKD), ophthalmologic disorders, and quality of life (QOL) issues.10 Secondary issues related to hypertension include renal artery stenosis, obstructive sleep apnea, primary aldosteronism, and thyroid disorders. Older adult patients with higher baseline SBP or longer duration of hypertension often experience autonomic dysfunction, microvascular damage, and CKD related to reduced renal tubular mass, fewer transport pathways for potassium excretion, and hyperkalemia.10 Glomerulosclerosis and interstitial fibrosis lead to progressive renal dysfunction and reduction in GFR, increased intracellular sodium, reduced sodium–calcium exchange, and volume expansion.10 Older adults generally have contracted intravascular volumes and impaired baroreflexes, which may be exacerbated by diuretics, sodium, and water depletion, causing orthostatic hypotension. Volume overload is commonly due to excessive salt intake, inadequate kidney function, or insufficient diuretic therapy. An age-related decline in plasma renin activity, tubular function, and glomerular filtration rate affects overall sodium and water homeostasis.2
Management Recommendations.
Risk stratification tools like the Framingham Risk Score can be used to predict MI, stroke, or CVD.10 Evaluation of older adult patients with known or suspected hypertension should include identification of reversible and/or treatable causes, evaluation of organ damage, assessment for other risk factors, and identification of treatment barriers. Recommended laboratory testing for hypertensive patients includes urinalysis, serum chemistries, lipid profile, blood glucose testing (including hemoglobin A1c), and ECG/echocardiography.10
Treatment of cardiovascular risk factors in older adults includes aggressive treatment of dyslipidemia with lipid-lowering medication, control of blood glucose, and lifestyle modification. There should be consideration that QOL issues such as cognitive function, physical activity, and sexual function are reduced by aging and disease.10 Drug treatment is recommended for older adult hypertensive patients with attention to alterations in medication distribution and disposal, changes in homeostatic CV control, and QOL factors. Lifestyle modification may be the only treatment necessary for milder forms of hypertension in older adults. Smoking cessation, weight reduction, increased physical activity, and sodium restriction result in greater benefit in older individuals than in young adults.10
Pharmacologic Management of Uncomplicated Hypertension.
Antihypertensive treatment in older adults should be started at the lowest dose and gradually increased to the maximum dose needed to achieve an SBP 140 mm Hg. A second medication from another class should be added if the initial therapeutic response does not achieve an SBP 140 mm Hg. A third medication from another class can be added after maximum doses of two classes of medications are reached. Recommended initial medications include thiazide diuretics (hydrochlorothiazide), calcium antagonists (diltiazem, nicardipine), angiotensin-converting enzyme inhibitors (captopril, lisinopril), angiotensin-receptor blockers (losartan), and beta-blockers (metoprolol, carvedilol).10
Refer to cardiovascular chapter for more information on cardiac medications.
Pharmacologic Management of Complicated Hypertension.
Older adult patients who have CAD, hypertension, stable angina, or previous MI should be prescribed a beta-blocker such as metroprolol for initial therapy. A long-acting calcium antagonist (CA) such as verapamil may be added to initial therapy if the BP remains elevated or if angina persists. An angiotensin-converting enzyme inhibitor (ACEI) such as benzapril is indicated if LV ejection fraction is reduced and/or if HF is present. Verapamil and diltiazem are not recommended if significant LV systolic dysfunction or conduction system disease is present.10
A BP of 130/80 mm Hg should be targeted in patients with HF and CAD.10 Older adult patients with hypertension and systolic HF should receive a diuretic, beta-blocker, ACEI, and aldosterone antagonist. Limited studies support use of ARBs and ACEIs in the presence of chronic renal dysfunction. Older, black hypertensive patients with HF may benefit from a regimen including isosorbide dinitrate and hydralazine. Older adult patients with hypertension and asymptomatic LV systolic dysfunction should be treated with ACEIs and beta-blockers. Because HF may improve in hypertensive older adult patients with renal artery stenosis after renal revascularization, this should be considered when HF patients are refractory to conventional management of hypertension. Older adults with diabetes mellitus, hypertension, and nephropathy should be treated initially with ACEIs or ARBs. In older adults with prediabetes/metabolic syndrome, attempts should be made to reduce BP using lifestyle modification. If medications are needed, thiazide diuretics increase risk for incident diabetes mellitus and hyperglycemia.10
Medication regimens for hypertensive older adult patients with CKD include angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers (ARBs). ACEIs should be considered for patients with nondiabetic nephropathy and ACEIs or ARBs are indicated if proteinuria is present. Older adult patients with hypertension and diabetes mellitus have a higher mortality risk than similarly aged nondiabetic controls.10 Hypertension and HF are both associated with a more pronounced decline in renal function in older age. With the recognition of early renal dysfunction, more patients should benefit from aggressive therapy.10
Age-Related Changes in Baroreceptor Function
Baroreceptors are mechanoreceptors that respond to stretch and other changes in the blood vessel wall. They are located at the bifurcation of the common carotid artery and the aortic arch.16 Impulses arising in the baroreceptor region project to the vasomotor center (nucleus of tractus solitarius) in the medulla. Abrupt changes in BP caused by increases in peripheral resistance, CO, or blood volume are sensed by baroreceptors, resulting in an increase in impulse frequency to the vasomotor center within the medulla. This increase inhibits vasoconstrictor impulses arising from the vasoconstrictor region within the medulla, resulting in a decrease in heart rate and peripheral vasodilation returning BP to within normal limits.16 The baroreflex can be tested by measuring heart rate response (i.e., increase or decrease in heart rate) after administration of a pressor or a depressor agent and by changing the patient’s position from lying to standing. Baroreflex-mediated tachycardia response to depressor agents is also attenuated in older adults. There are several reports of attenuation in heart rate response of older adults after changes in position.16 When an individual changes position from supine to standing, distribution of blood volume changes, resulting in a reduction in CO and BP. This is known as orthostatic hypotension.16 Simultaneously baroreceptors increase heart rate and maintain BP by increasing CO. The baroreceptor-reflex response also mediates changes in peripheral resistance and force of myocardial contraction, which offsets the drop in BP. Prevalence of orthostatic hypotension is greater in geriatric patients; therefore, judicious use of antihypertensive medications is recommended.16
Cardiac Medication Considerations in Older Adults
Treatment with medication should be considered when nonpharmacologic interventions are unsuccessful. Medication therapy for hypertension should target a SBP of 140 mm Hg to 145 mm Hg and diastolic of greater than 95 mm Hg in patients older than 80 years of age.10 Heart rate control can be managed with beta-blockers and calcium channel blockers. Amiodarone can be used for conversion and maintenance of sinus rhythm with atrial fibrillation.10 Aging increases the risk of major hemorrhage in patients with atrial fibrillation, with or without warfarin therapy.10 Anticoagulation therapy can be complicated by polypharmacy, simultaneous use of antiplatelet medications, uncontrolled hypertension, and poorly controlled anticoagulation therapy.17 Ventricular dysrhythmias are best managed with cardiac-resynchronization therapy and pharmacologic therapy in patients with advanced HF.18 Cardiac resynchronization therapy (CRT) is a type of pacemaker or pacemaker/defibrillator that resynchronizes the contractions of the heart’s ventricles by sending electrical impulses to the heart muscle, assisting blood flow and vascular remodeling. CRT has become an established therapy for advanced heart failure LV reverse remodeling, with significant reduction in LV volumes and improvement in LVEF.18
Combination pharmacologic therapy in older adult patients provides opportunity for enhanced efficacy, avoidance of adverse effects, enhanced convenience, and compliance.17 Older adult patients will often discontinue or take medications inappropriately, resulting in failure to reach recommended targets and outcomes.17 The average older adult patient takes six prescription medications; therefore, daily polypharmacy, nonadherence, and potential medication interactions are important concerns.17 Nonadherence to medication regimens may be due to competing health problems, socioeconomic status, treatment complexity, side effects, and cost of medications.19 Clinical and fiscal consequences of medication nonadherence can lead to increased hospital readmissions and adverse medical events.19 Assessing factors that affect medication adherence such as literacy, eyesight, understanding of directions, education level, severity of disease, and comorbid conditions may assist in understanding barriers to compliance.17 Patient management is most effective when intraprofessional health care teams collaborate to achieve and maintain goals. Novel opportunities for therapeutic management and compliance include smart phones, telemedicine, and computerized technologies. Other collaborative efforts include effective communication with local pharmacies, medication reconciliation at discharge, follow-up discharge phone calls, and timely provider handoff.
Pulmonary System
Many changes in the pulmonary system that occur with aging are reflected in tests of pulmonary function and include changes in compliance of the chest wall, in static elastic recoil of the lung, and in strength of respiratory muscles.20 Progressive changes due to age should not alter the older adult’s ability to breathe effortlessly; however, factors such as repeated exposure to environmental pollutants and frequent pulmonary infections may accelerate age-related changes. Changes in tests of pulmonary function are listed in Tables 41-3 and 41-4.
TABLE 41-3
AGE-RELATED CHANGES IN COMMONLY PERFORMED PULMONARY FUNCTION TESTS
| PULMONARY FUNCTION TEST | DESCRIPTION | STANDARD LUNG VOLUME AND CAPACITY (mL) | AGE-RELATED CHANGE (mL) |
| Total lung capacity (TLC) | Vital capacity plus residual volume | 6000 | No change |
| Vital capacity (VC) | Amount of air exhaled after a maximal inspiration | 5000 | ↓ 3750 |
| Tidal volume (VT) | Amount of air inhaled or exhaled with each breath | 500 | No change |
| Residual volume (RV) | Amount of air left in lungs after forced exhalation | 1200 | ↓ 1800 |
| Inspiratory reserve volume (IRV) | Amount of air that can be forcefully inhaled after inspiring a normal VT | 3100 | ↓ 2800 |
| Expiratory reserve volume (ERV) | Amount of air that can be forcefully exhaled after expiring a normal VT | 1200 | ↓ 1000 |
| Forced expiratory volume in 1 second (FEV1) | Volume exhaled in the first second of a single forced expiratory volume; expressed as a percentage of the forced vital capacity | 80% | ↓ 75% |
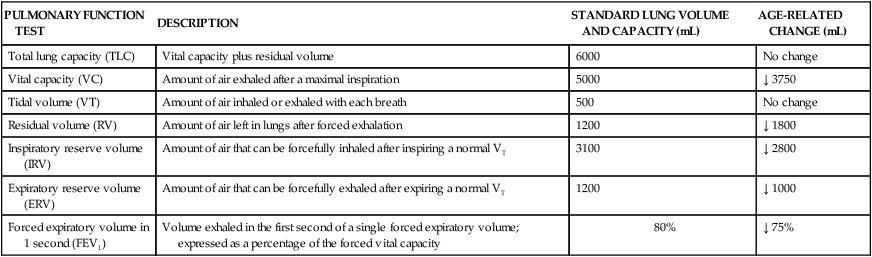
TABLE 41-4
PROGRESSIVE CHANGES IN ARTERIAL OXYGEN TENSION AND CARBON DIOXIDE TENSION
| AGE GROUP (YR) | Pao2 (mm Hg) | Paco2 (mm Hg) |
| ≤30 | 94 | 39 |
| 31-40 | 87 | 38 |
| 41-50 | 84 | 40 |
| 51-60 | 81 | 39 |
| >60 | 74 | 40 |
Paco2, Carbon dioxide tension; Pao2, arterial oxygen tension.
Data from Sorbini CA, et al. Arterial oxygen tension in relation to age in healthy subjects. Respiration. 1968;25:3.
Thoracic Wall and Respiratory Muscles
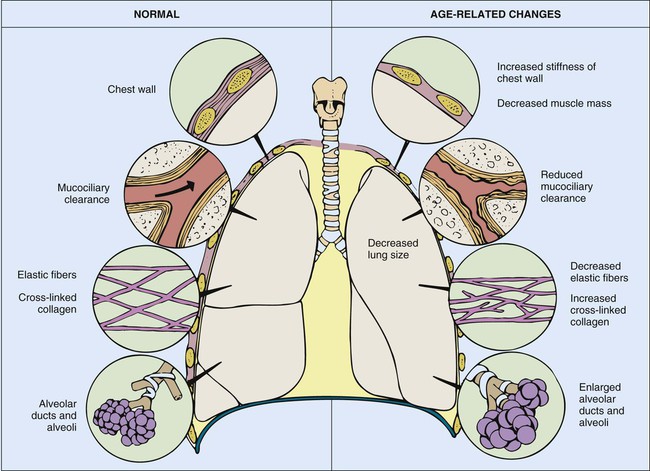
The aging thorax has a greater anterior–posterior diameter than in younger adults and there is some degree of dorsal kyphosis due to osteoporosis.20 Rib mobility declines because of contractures of intercostal muscles and calcification of costal cartilage. Progressive decreases in chest wall compliance and changes in the shape of the thorax change chest wall mechanics and lead to deterioration in respiratory function (Fig. 41-1).20 Strength of the diaphragm and intercostal muscles decreases with age. The diaphragm is the most important inspiratory muscle because its movement accounts for 75% of change in intrathoracic volume during quiet respiration. Other factors, such as an increase in abdominal girth and change in posture, also decrease thoracic excursion. Respiratory muscle function is affected by skeletal muscle and peripheral muscle strength.20 During aging, skeletal muscle progressively atrophies and its energy metabolism decreases, which may partially explain the declining strength of the respiratory muscles.20 Handgrip strength testing can be a simple and useful measurement in assessment of muscle function.20 Respiratory muscle performance is impaired concomitantly by the age-related geometric modifications of the rib cage, decreased chest wall compliance, and increase in functional residual capacity (FRC) resulting from decreased elastic recoil of the lung. Changes in chest wall compliance lead to a greater contribution to breathing from the diaphragm and abdominal muscles and a lesser contribution from thoracic muscles.20 These anatomic changes are reflected by an increase in residual volume and decrease in vital capacity (see Table 41-3). Age-related decline in chest wall compliance of the respiratory system is 20% less in a 60-year-old subject compared with a 20-year-old.20 Table 41-5 summarizes age-related changes in the chest.
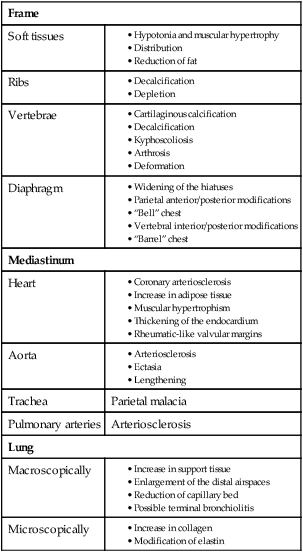
Adapted from Bonomo L, et al. Aging and the respiratory system. Radiologic Clin North Am. 2008;46(4).
Maximum inspiratory and expiratory pressures may decrease by as much as 50% because of decline in respiratory muscle strength, resulting in a decrease in thoracic wall excursion.2 The age-related reductions in maximal inspiratory pressure and maximal expiratory pressure are likely a consequence of impaired respiratory mechanics and sarcopenia.21 Sarcopenia describes reduced muscle mass and function, which may be related to decreased muscle protein synthesis, increased muscle proteolysis, motor neuron loss, and increased muscle fat content.21 Respiratory muscle strength is related to nutritional status, often deficient in older adults.20 Additionally, prevalence of vertebral fractures increases with age, particularly in women.20 The accessory inspiratory muscles (sternocleidomastoid, scalene, and trapezius) facilitate inspiration during exercise. Reports have described that ventilatory muscle strength improved after older adult men and women received ventilatory muscle training.2
There is an age-associated decrease in effectiveness of the cough reflex and decrease in ciliary responsiveness and motion that predisposes older adult patients to aspiration and hospital-acquired infections.2 These changes emphasize the importance of dysphagia screening, deep breathing, and coughing for the older patient in the critical care unit.
Age-associated changes in pulmonary function do not alter the older adult’s ability to breathe effortlessly, although a decrease in respiratory muscle strength may be a limiting factor during exercise. The application of conventional quality control standards to objective assessment of pulmonary function in older subjects may prove difficult because of mood alterations, fatigability, lack of cooperation, or cognitive impairment.21
Pulmonary Gas Exchange
A diminished recoil of the lung occurs with aging, causing increased lung volume, distention of the alveolar spaces, and a decrease in surface area of airspace occurs starting in the third decade of life.20 Reduced lung elasticity results from changes in the ratio of elastic to support tissue that occur with advancing age.22
Ventilation and diffusion depend on numerous factors, including lung surface area. Displacement of inhaled air volume away from the alveoli limits the surface area available for gas exchange. This may in part explain the progressive and linear decrease in pulmonary diffusion capacity, which depends on surface area and capillary blood volume.22 There are reports that capillary blood volume and surface area also decrease with advancing age. Changes in pulmonary circulation result in a ventilation/perfusion (V/Q) mismatch.21 V/Q mismatch leads to a decline in arterial oxygen tension of approximately 0.3 mm Hg per year from the age of 30 years.2 The typical Pao2 for healthy persons older than 65 years is approximately 89 mm Hg, compared with 100 mm Hg for younger adults aged 18 to 24 years.21 Age-related changes in ventilation and arterial tension for carbon dioxide (Paco2) occur across the adult life span. Total minute ventilation (VE) must increase with advancing age to maintain arterial tension for carbon dioxide (Paco2). Paco2 is largely dependent on the VE, which is the sum of alveolar ventilation (VA) and dead space ventilation. V/Q inequality may exist with decreased diffusion capacity of the lung for carbon monoxide and the transfer capacity of oxygen across the alveolar–capillary interface.21 The impact of exposure to air and inhaled pollutants creates a challenge in differentiating the true impact of normal physiologic aging from that of environmental exposure.20
Control of ventilatory responses to hypoxia and hypercapnia falls by 50% and 40%, respectively, in the older adult.2 This may be due to declining chemoreceptor function.5 Decreases in Pao2 could be the result of an increase in closing volume in dependent lung zones during resting tidal breathing in older subjects.22a,22b Consequently, dependent lung zones may be ventilated intermittently, leading to regional differences. Alterations in blood volume and vascular resistance within the pulmonary circulation may also contribute to V/Q mismatching. Important considerations for critical care nurses with regard to older adult patients includes recognition of decreased respiratory reserve and more rapid decompensation than in younger patients.2 Other factors, such as exposure to environmental pollutants and chronic pulmonary disease, have an impact on the ability to compensate for respiratory conditions or wean from mechanical ventilation.2
Lung Volumes and Capacities
Pulmonary function studies may be the best way to assess respiratory impairment in older adults, because muscle strength, ventilator control, and gaseous exchange may impair respiratory mechanics.21 Dynamic lung volumes and flow rates depend on resistance of airways and chest wall compliance and are limited by collapse of small airways during forced expiration. Age-related changes in respiratory mechanics lead to airflow restrictions, which are reflected in decreased 1-second forced expiratory volume (FEV1).21 Maximal expiratory flow rate and maximal midexpiratory flow rate are also decreased.21 Age-related decrease in dynamic lung volume is probably caused by decreased chest wall compliance, small airways closure during forced expiration, and decreased strength of expiratory muscles. Breathing exercises generate lung volumes of forced vital capacity (FVC), which is an untimed lung volume and FEV1, which is a timed lung volume. These spirometry tests can assist in assessing restrictive respiratory patterns.21 Reduced timed lung volume is exhibited in chronic obstructive pulmonary disease (COPD), asthma, bronchiectasis, and cystic fibrosis.21 Other conditions that display reductions in the timed and untimed lung volumes include kyphosis, scoliosis, myasthenia gravis, diaphragmatic paralysis, pleural effusions or fibrosis, and pulmonary hypertension.21
Older individuals are less able to protect against environmental injury and infection of the respiratory system. Decreases in T-cell function, decline in mucociliary clearance, and a decrease swallowing ability with loss of cough reflex can increase frequency and severity of pneumonia in older adults. Poor dentition, nutrition, and oral hygiene also play a role in the incidence of oropharyngeal colonization with gram-negative bacteria and aspiration pneumonia.2 Noncritical conditions of the respiratory system such as bronchitis, emphysema, COPD, and lung cancer should also be assessed in older adults, as pathology and normal aging processes are often difficult to differentiate.21
Renal System
Acute kidney injury (AKI) is common in critically ill patients, with a prevalence ranging from 2% to 25%.23 Age places patients at a higher risk of developing AKI and end-stage renal disease. Aging produces changes in renal structure and function that begin at approximately 25 years of age.2 Between the ages of 25 and 85 years, approximately 40% of nephrons become sclerotic and others hypertrophy.2 Sclerosis of the glomeruli is accompanied by atrophy of afferent and efferent arterioles, leading to a fall in renal blood flow of approximately 50%.2 Decrease in number and size of nephrons begins in the cortical regions and progresses toward the medullary portions of the kidney.2 This decrease in number of nephrons corresponds to a 20% decrease in weight of the kidney between 40 and 80 years of age. Initially, this loss of nephrons does not appreciably alter renal function because of the large renal reserve and simultaneous decrease in lean muscle mass.2 The kidney contains approximately 2 to 3 million nephrons, more than is needed to maintain adequate fluid and acid–base homeostasis. Over time, the geriatric patient also loses this renal reserve.2
The RIFLE (risk, injury, failure, loss, and end-stage) classification has been used to detect changes and predict prognosis of renal function.23 The age of 76 years has been found to be an important marker for RIFLE predictability and mortality in geriatric patients.23
Fluid Filtration
Glomerular filtration rate (GFR) is the volume of fluid traversing the glomerular membrane in a given period. GFR declines approximately 45% by age 80 years and is reflected as decreased creatinine clearance (CrCl).2 GFR is usually estimated by collecting a 24-hour urine sample to measure creatinine excretion. Endogenous creatinine is a metabolic byproduct of muscle metabolism that is excreted by the kidney and is not reabsorbed. With advancing age, muscle mass decreases, thereby reducing the renal load of serum concentration of creatinine. In the geriatric patient, neither the creatinine excreted nor the plasma creatinine level may reflect changes in GFR. In older adults, the Cockcroft-Gault equation often is used to assess CrCl and GFR (in milliliters per minute), because it incorporates the serum creatinine concentration, body weight (in kilograms), age (in years), and gender as variables.2 The Cockcroft-Gault equation for males is CrCl = (140 −Age)/72 × Serum creatinine × Weight. For females, this calculated value is multiplied by 0.85.
Renal tubular function is an important regulator of water and solute excretion and depends on permeability of glomerular capillaries, surface area available for filtration, and balance of pressure gradients between the glomerular capillaries and Bowman space.2 Decreased GFR in older adults is most likely caused by decreased nephron number and reduced renal blood flow. Even though remaining nephrons adapt to loss of nephrons by glomerular hyperfiltration and increased solute load per nephron, the reduced GFR predisposes older adults to dehydration, adverse drug reactions, drug-induced renal failure, and acid–base imbalance.2 Some medications are excreted unchanged in the urine, whereas other medications have active or nephrotoxic metabolites.
The senescent kidney is more susceptible to injury by hypotensive episodes because of age-related decreases in renal blood flow and reduced pressure gradient across afferent arterioles. Blood urea nitrogen (BUN) and serum creatinine levels can be normal or decreased in older adults (Box 41-1). The ability of the renal tubules to regulate fluid and acid–base balance decreases with age.2 These functions are governed by the amount of sodium and water delivered to the tubules and overall acid–base balance. Age-related changes in tubular function become apparent when extreme changes occur in body fluid composition or acid–base balance. For example, with systemic acidosis the rate and amount of total acid excretion (i.e., bicarbonate, titratable acid, and ammonium) are reduced. This predisposes the older adult patient to metabolic acidosis, volume depletion, and hyperchloremia.
There is a diminished ability of the senescent kidney to excrete free water load, conserve water during periods of dehydration, and conserve sodium during periods of low salt intake.2 Dehydration becomes a problem as the aging kidney’s capacity to conserve sodium and excrete hydrogen ions decreases. The body does not compensate for nonrenal losses of sodium and water by the usual mechanisms of sodium retention, urinary concentration, and thirst. This may be due to decline in activity of the reticular activating system (RAS), decreased end-organ responsiveness to antidiuretic hormone, and changes in osmoreceptor function in the hypothalamus. Other extrarenal mechanisms such as the sympathetic nervous system are also important in homeostasis and maintaining BP in response to changes in body position. Decreased activity and responsiveness of the senescent kidney to the sympathetic nervous system and renin–angiotensin–aldosterone system are important in integrating overall fluid homeostasis and maintaining BP in response to changes in body position.2
Gastrointestinal System
The gastrointestinal tract is made up of an epithelium, mucosal immune system, numerous bacteria, and the enteric nervous system.24 The gut is complex and plays a key role in homeostasis. Age-related gastrointestinal changes occur in the processes of swallowing, motility, and absorption.24 Swallowing may be difficult for older adults because of incomplete mastication of food within the oral cavity.24 The result of deteriorating dentition, diminished lubrication (from salivary dysfunction), ill-fitting dentures, and incomplete mastication can put the older adult patient at risk for aspiration.2 The number and velocity of peristaltic contractions in the older adult’s esophagus decreases, and the number of nonperistaltic contractions increases.24 These changes in esophageal motility are referred to as presbyesophagus. The changes may predispose patients to erosion of the esophageal wall (i.e., recurrent esophagitis), because food remains in the esophagus longer. Bed rest and reclining in a supine position for prolonged periods can cause esophageal reflux, which also can lead to esophagitis. The aging process also produces thinning of smooth muscle within the gastric mucosa.24
The epithelial layer of gastric mucosa, which contains the chief and parietal cells, undergoes a modest degree of atrophy resulting in hyposecretion of pepsin and acid, respectively.24 Gastritis-induced achlorhydria (decreased acid secretion) is prevalent in older adults. Mucin secretion from mucus cells decreases, thereby altering the protective function of the gastric mucosal (bicarbonate) barrier. Because of this, the stomach wall is more susceptible to acid injury, increasing incidence of gastric ulcerations.24 It remains unknown whether changes in gastric acid secretion are a result of age-related changes or a disease process such as gastritis. Most duodenal and gastric ulcers arise because of the presence of Helicobacter pylori. Ulcers can also be promoted by the use of nonsteroidal anti-inflammatory drugs. The combination of H. pylori and medication effects can be a significant risk for gastrointestinal bleeding in critically ill older adults.24
Aging alters taste, smell, and gastric motility. Dysphagia and alterations in motor and sensory function can lead to silent aspiration and delay in gastric emptying that may contribute to postprandial hypotension and maldigestion. The gag reflex has been reported to be absent in 40% of healthy older adults and reports have revealed a reduction in esophageal peristalsis, increase in nonpropulsive contractions, and reduction in lower esophageal sphincter pressure.25
Few age-related changes have been noted in sensory and motor function of the small bowel; however, aging is linked to weakening of colonic muscles and altered rectal sensation. Structure and function of the pelvic floor and anorectum may contribute to constipation, fecal incontinence, and diverticulosis encountered in older adults.25
Although most vitamins and minerals are normally absorbed, calcium absorption is reduced, fat-soluble vitamin A is increased, and there is the potential for impaired absorption of vitamin D, B12, and folic acid.26 Thinning of intestinal lining, decreased mucus production, weaker intestinal muscles, and medications mobility may increase risks of constipation and fecal impaction in older adult patients.26
Pre-existing malnutrition can be problematic in the older hospitalized critically ill patient but can be avoided by early management of nutrition and dietary consultation during the critical care admission process. The problem of malnutrition may be undetected, which increases the risk of adverse outcomes such as increased morbidity and mortality.2 Careful nutritional assessment is essential for determining older patients’ nutritional status and planning adequate nutritional care to prevent malnutrition and propagation of critical illness in older adults.25
Age-Related Changes in the Liver
Mortality ascribed to liver disease may increase four-fold in adults aged 45 to 85 years.27 Liver disease can also shorten potential lifespan in individuals under 75 years of age with a similar magnitude to that of COPD.27 Compromised hepatic function in older adults decreases hepatocyte numbers, liver weight, hepatic volume, and perfusion.27
Structural changes in liver morphology and cell structure include increased volume of the dense body compartment (secondary lysosomes, residual bodies, or lipofuscin), loss of smooth-surfaced endoplasmic reticulum, diminished bile acid secretion, and increased biliary cholesterol.27 Another age-related alteration in hepatocellular structure includes increased hepatocyte polyploidy (organisms containing more than two paired sets of chromosomes).27 Effects of aging on hepatocyte structure may be due to increased oxidative stress and older adults’ reduced capacity to eliminate superoxide radicals as efficiently as younger individuals. The liver has many complex functions, including carbohydrate storage, ketone body formation, reduction and conjugation of adrenal and gonadal steroid hormones, synthesis of plasma proteins, deamination of amino acids, synthesis and storage of cholesterol, urea formation, and detoxification of toxins and medications. Age-related changes in hepatic function include a reduction in synthesis of cholesterol, total bile acid pool, and bile acid from cholesterol. There is also a reduced capacity of the liver for regeneration in response to injury compared with a younger population. Despite these age-related changes, liver function is not appreciably affected.27
Tests of liver function, such as serum bilirubin, alkaline phosphatase, and aspartate aminotransferase (AST) levels are unaltered with advancing age. First-pass clearance of medications may be reduced due to decreases in total liver blood flow. The most important age-related change in liver function decreases the liver’s capacity to metabolize medications.27 Although clinical tests of liver function do not reflect this change in metabolism, it is well recognized that medication side effects and toxic effects occur more frequently in older adults than in young adults. Polypharmacy is cited as a common cause of adverse medication reactions in the older adult population.27 Reduced medication-metabolizing capacity is caused by a decline in activity of the medication-metabolizing enzyme system, microsomal ethanol oxidizing system, and decrease in total liver blood flow.27 Medications that depend on the cytochrome P450 group of liver enzymes are most affected because age-associated changes cause as much as a 50% decline in enzymatic function.27
A clinically significant age-related change is decline in the rate of hepatic regeneration following partial liver resection (hepatectomy) or injury.28 The rate of liver regeneration (hepatocyte proliferation) following injury is decreased in older adults, which may enhance the progress of hepatic diseases and compromise liver transplantation in older adults.27 Liver transplantation is a complex issue, since some evidence reports that livers from older donors may be less viable than those from young donors. Recipient age should also be a consideration, since post-transplant mortality increases by 15% between 55 and 75 years of age. The impact of age is modest with respect to recipient and graft survival, at least during the first few post-transplantation years, as both recipient and graft survival rates decline in older adult patients receiving livers from older donors by only 10% to 15% over the first 3 post-transplant years.28
Age, Disease, and the Liver
Although there are no specific age-related liver diseases, the percentage of older adults will increase over the next twenty years, leading to development of new conditions and options for treatment.29 Some liver diseases are more frequently seen in older adults, including chronic hepatitis C virus (HCV) and hepatocellular carcinoma. Clinical management of liver disease in older adults may differ in several aspects from those of younger adults, for example, offering antiviral treatment to patients with chronic HCV to decrease risk of hepatocellular carcinoma.29 End-stage liver disease will increase over the coming years with augmented numbers of individuals with decompensated liver disease and hepatocellular carcinomas.
Older adults infected with hepatitis B virus (HBV) often develop a subclinical hepatitis with a low rate of HBV clearance, perhaps due to impaired immunologic status.29 These individuals appear healthy, but are highly infective chronic HBV surface antigen carriers. Persons with chronic hepatitis and/or cirrhosis are usually inactive, progress slowly, and do not reach old age, due to progressive disease.29
HCV infection is the most important clinical form of chronic hepatitis in older adults. Prevalence of antibodies against HCV increases with advancing age. Prior to this decade, estimates of subjects over 65 years of age infected with HCV ranged from 5.2% to 42.1%.29 HCV infection has been linked to blood transfusions and nondisposable syringes. Advancing age is associated with more severe histologic damage and increased risk for progression to cirrhosis. Additionally, factors such as alcohol, medications, or metabolic alteration can affect the severity of hepatic damage and disease in older adults.29
Alcohol consumption and subsequent liver disease is common in old age. Reports suggest that 5% to 15% of older adults over 65 years of age have alcohol-related problems.29 Individuals with alcoholic hepatitis and underlying alcoholic cirrhosis have the highest mortality. Hepatocellular carcinoma is the most common complication of liver cirrhosis affecting older adults, with incidence rates tripling between 1975 and 2005.29
In conclusion, aging and liver impairment may decrease liver regeneration related to hepatocyte proliferation, increase residual bodies or lipofuscin in hepatocytes, and create inability to eliminate cellular waste products that compromise normal cell activity. Furthermore, lifestyle, age, hepatocyte telomere shortening, and hepatic injury and disease can affect individual outcomes.28
Central Nervous System
Cognitive Functioning and Aging
Cognitive functioning has become one of the greatest threats of old age, with more than 50% of older adults over the age of 85 years affected by Alzheimer’s disease.30 Structural and neurophysiologic changes occur in the brain with aging; however, it is not clear which changes are a normal process of aging or pathology of neurogenic disorders.30 Cognitive function involves transforming, synthesizing, storing, and retrieving sensory input in addition to perception, attention, thinking, memory, and problem solving. Neuropsychologic assessment of older adults can be achieved with psychometric and clinical neuropsychologic assessment and standardized testing. Cognitive profile information can be helpful in differentiating between normal aging, mild cognitive impairment, early dementia, depression, mental health issues, and progressive neurologic disease. An assessment may also be useful following a stroke—or in conditions such as Parkinson disease—to assist in decision making about ability to drive or about the extent to which the individual has capacity to make specific decisions (medical treatment, financial affairs, or self-care).31 The rate at which complex tasks are completed may diminish; however, this is not synonymous with cognitive impairment, as deterioration of cognitive functioning is not a normal expectation of the aging process. Some slight memory dysfunction is common with increasing age, but significant decline may represent a change in individual need and may be a result of acute or chronic conditions. Acute mental status changes due to infection, metabolic imbalances, or medications are usually reversible after identification and treatment.
Delirium is a frequent complication in critical care but is often unrecognized in the hospitalized older adult.32 Delirium is characterized by acute changes in mental status from baseline, inattention, and disorganized thinking or changes in level of consciousness. Cost estimates for care associated with delirium including postcare range from $38 to $152 billion annually.32 Delirium is discussed further in another section of this book.
Postoperative cognitive dysfunction (POCD) is an acute and short-term disorder of cognition, memory, and attention that may be seen in critical care. Distinction should be made between etiologic factors and this syndrome. A study of Long-term Postoperative Cognitive Dysfunction in the Elderly (ISPOCD1) found that POCD was present in 26% of patients 1 week after surgery and in 9.9% 3 months after surgery, compared with 3.4% and 2.8% of controls.2 Increasing age, duration of anesthesia, postoperative infections, and respiratory complications were risk factors for early POCD. Only age was a risk factor for prolonged POCD. POCD after cardiac surgery in older adults, with an incidence of up to 80% at discharge and 50% at 6 weeks. In some cases, neurocognitive decline lasted up to 5 years following some cardiac surgeries. Cardiopulmonary bypass appears to be a factor in short- and long-term POCD.2
Delusions and hallucinations may be present in delirium but are also evident in other psychiatric disorders. Long-term chronic impairment develops from more organic causes, such as those associated with neurodegenerative dementias (e.g., Alzheimer’s disease) or nonneurodegenerative types (e.g., multi-infarct dementia, traumatic brain injury). Although dementia is pathology-based there is an increased incidence associated with advanced age, especially in those older than 85 years.30 Alzheimer’s disease is identified by amyloid-containing neuritic plaques and intraneuronal neurofibrillary tangles in various areas of the cortex.30 Alzheimer’s disease is characterized initially by progressive short-term memory loss, and later by long-term memory loss. Marked decline in memory leaves individuals functionally impaired and physically dependent. In contrast, non-neurodegenerative dementias, such as multi-infarct types, present as fixed deficits associated with the area of brain injury. In contrast to Alzheimer’s type of dementia, cognitive impairment may be associated with significant impairment following stroke rather than over time.31
Baseline dementia, stress of surgery or acute illness, and hospitalization can cause cognitive decline and significantly increase risk for delirium in older adults. Sensory perception decreases with age, resulting in difficulties for the older patient in unfamiliar surroundings such as hospitals. Immobility and deprivation of vision and hearing increase the likelihood of dehydration, anorexia, confusion, depression, and disoriention.2
Structure and Morphology
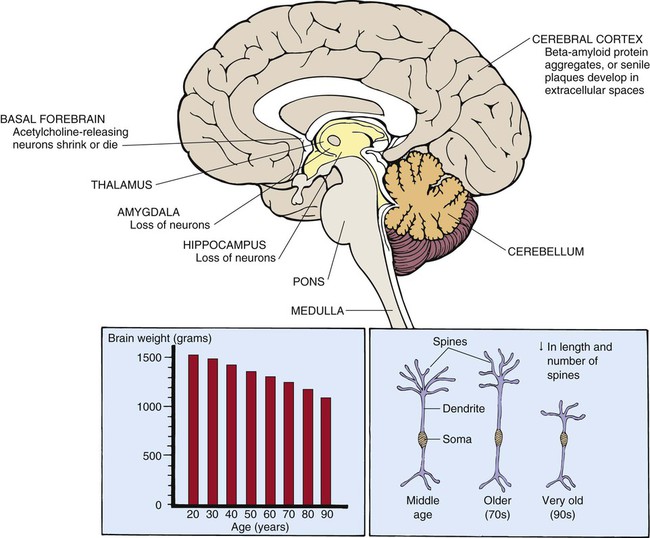
The brain decreases approximately 20% in size between 25 and 95 years of age (Fig. 41-2).33 Reduced brain weight may be related to the overall decrease in the number of neurons that occurs with advancing age. Neurons are lost from the hippocampus, amygdala, cerebellum, and from areas of the brainstem such as the locus ceruleus, dorsal motor nucleus of the vagus, and substantia nigra. Conversely, very few neurons disappear from the hypothalamus with advancing age.30 Portions of the cerebral cortex atrophy, predominantly the frontal and temporal cortical association areas (the superior frontal gyrus and superior temporal gyrus, respectively).30 Cerebral blood flow (CBF) and brain weight decrease with advancing age, most likely due to reduction in neuron number and metabolic needs of cerebral tissue.33 CBF is also influenced by age-related changes in BP, barometric response to positional change, and severity of CVD. Accompanying the loss of neurons are changes in ultrastructure and intracellular structures of the neuron. The neuron is composed of a cell body, dendrite, and axon. Dendrites are long, spiny processes that extend out from the cell body. Dendrite spines decrease in number with age; the length of dendritic spines increases in middle and late old age, then decreases after late old age (older than 90 years).33 Shrinking of large neurons and degenerative changes occurring in cell bodies and axons of some acetylcholine-secreting neurons have been reported. These changes may explain alterations in processing and receiving of information.33
Lipofuscins, neuritic plaques, and neurofibrillary bodies appear within the cytoplasm of the neuron.34 Lipofuscins, or age pigments, are granules containing a dark, fluorescent pigment and derive from lipid-rich membranes that have been partially disintegrated and oxidized. It is still not clear whether lipofuscin accumulation is harmful to the brain.34 Neuritic, or senile, plaques are aggregates of beta-amyloid protein that accumulate in the brain of normal senescent persons. Neuritic plaques are found in the hippocampus, cerebral cortex, and other brain regions.34 Neurofibrillary tangles, which are bundles of helically wound protein filaments, occur in the hippocampus with advancing age in healthy persons. They are present in larger numbers in persons with neuropathologic disorders such as Alzheimer’s disease and may affect neuronal signaling.34 Synaptogenesis or synaptic regeneration still occurs after partial nerve degeneration but at a slower rate in the older brain. After a nerve fiber is damaged, neighboring undamaged neurons often sprout new fibers and form new connections.35
Cognitive Function
Cognitive and brain reserve are well studied in the context of age-associated cognitive impairment and dementia. However, there is a paucity of research that examines the role of cognitive or brain reserve in delirium.36 Brain and cognitive reserve concepts developed from observations that some individuals demonstrate less cognitive impairment than others with comparable brain injury or neuropathology. Most research on reserve is based on studies in chronic progressive disorders such as Alzheimer’s disease. Brain and cognitive reserve describes variability across persons in the relationship of pathologic changes with clinical expression of disease. Brain reserve refers to structural aspects of the brain, and cognitive reserve relates to how cognitive tasks are initiated and coordinated. Cognitive reserve comprises neural reserve and neural compensation. Neural reserve describes the efficiency, capacity, or flexibility of brain networks or cognitive paradigms that underlie task performance in the healthy brain. Neural compensation refers to the ability to function optimally when pathology disrupts standard processing networks. Studies are ongoing to assess if these processes are modifiable and may offer benefit to older populations for the prevention of delirium and long-term cognitive decline.36
Cognitive impairment is a core element shared by a large number of different neurologic and neuropsychiatric diseases. Some form of cognitive impairment appears to be a factor in many neuropsychiatric and neurologic disorders (bipolar disease, stroke, epilepsy, Parkinson disease, multiple sclerosis, Alzheimer’s disease, and other neurodegenerative brain disorders).37 Cognitive decline seems to be linked to temporofrontal functional deficiency, which is expressed as deficient auditory discrimination and orienting.37 New electrophysiologic technology is known as mismatch negativity (MMN). MMN abnormality is closely associated with cognitive change and decline occurring in a number of different neurologic and neuropsychiatric illnesses as well as in normal aging, providing ability to measure cognitive decline.37
Central Nervous System Assessment
Neurologic examination of the geriatric patient always includes assessment of muscle strength, reflexes, sensation, and cranial nerves.30 There may be changes in fine and gross motor skills and handgrip strength, leading to decreased ability to perform fine motor activities. Age diminishes the geriatric patient’s vibratory sense in the lower extremities. Reflexes are slowed as a result of neuronal loss. Neurologic deficits may alter the patient’s ability to perform self-care, including ability to follow instructions and interpret patient-teaching instructions.30
Several standardized neurologic assessments are available for use in critical care.38 Most can be used for all critical care patients to accurately and consistently define patient status. Examples of scales that should be used routinely in critical care—particularly in care of the older adult patient—include the Glasgow Coma Scale (GCS), National Institutes of Health Stroke Scale (NIHSS), International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI), Mini-Cog Assessment Instrument for Dementia, and the Confusion Assessment Method for the ICU (CAM-ICU). Assessment of older adults in critical care should also include consideration of age-related changes such as vision impairment,38 hearing deficits (particularly low- and high-pitched sounds), slight loss of taste/dentition, and diminished sense of smell.
Recent studies have identified a relationship between critical illness and long-term cognitive impairment.39 Executive function, attention, and memory appear to be affected for up to 6 years after a critical illness. Associations between acute care and critical illness hospitalizations permit greater cognitive decline and risk for incident dementia.39 Unfortunately, most studies have not been able to objectively demonstrate cognitive function prior to critical illness, and therefore the magnitude of the problem is unclear. The ability to prognosticate risk of cognitive impairment would be useful in providing better information about long-term outcomes for patients and families and allow them to make difficult decisions surrounding critical illness.39
Immune System
Infections in older adults are usually more severe and frequent than those in younger adults. Infections in the geriatric population are associated with higher rates of mortality and hospital admissions, especially in persons older than age 85.40 Persons older than age 65 are hospitalized at more than three times the rate of persons of all ages.40 Since persons older than 65 years are the fastest growing segment of the population, more critical care services will be required during the next decade if treatment patterns remain unchanged. Alterations in immune function, increase in comorbidities, and greater frailty render older adults more susceptible to infections. Common infections in the older adult are associated with bacterial pneumonia, urinary tract infection, intra-abdominal infections, gram-negative bacteremia, and decubitus ulcers.40 Increased susceptibility to infection may be due to cell-mediated and humoral-mediated immunity and breakdown in physical barriers, such as the skin and oral mucosa.
Cell-Mediated and Humoral-Mediated Immunity
Immune system function depends on many cell types with distinct functions. T cells are the primary effector of cell-mediated immunity, whereas bone marrow-derived B cells produce antibodies that are effector cells of humoral-mediated immunity. Cell-mediated immunity declines and T-cell function decreases with aging despite the total number of T cells remaining unchanged.41 Older adults show deficiencies in the ability to produce appropriate defensive immune responses to pathogens and vaccines, contributing to increased vulnerability to infections and bacterial pathogens. Defects in T-cell function are often found in protective immunity at the cellular and humoral levels. Furthermore, evidence suggests that T cells from older adult donors are generally slower and of lower amplitude than responses of T cells from younger individuals. Defects in cytoskeletal motors, hyperglycosylation of T-cell surface macromolecules, and changes in dynamics of membrane microdomains may offer opportunity for effective methods to rectify the age-related defects in protective cellular immunity, and those affect responses to vaccines, infectious agents, and perhaps neoplasia.41
Older patients may not be more likely to contract an infectious illness, but impaired ability of bone marrow to increase neutrophil production in response to infection may cause eradication to be slower. Older adult patients with major infections often have normal white blood cell counts, but the differential count usually shows a large proportion of immature forms. Infections in the older adult may present as acute mental status changes, anorexia, urinary incontinence, falls, or generalized weakness.40 These may be signs of a urinary tract infection or pneumonia—two common infections in older adults—or may be signs of severe sepsis and septic shock.
Integumentary and Musculoskeletal Systems
Skin disorders are common in older adults and prevalence is dependent on the patient’s clinical environment.42 Older adult conditions of the skin include seborrheic keratosis, xerosis, and Campbell de Morgan spots.42 Since the aging population is increasing, incidences of skin diseases will also increase. Older adults are vulnerable to a wide variety of dermatologic conditions resulting from degenerative and metabolic changes occurring throughout the skin layers.42 Dermatologic changes can be considered in the categories of intrinsic and extrinsic aging.42 Intrinsic aging refers to the skin’s natural, metabolic aging process where the skin’s upper layers become thin and blood flow is decreased, which leads to a reduced ability to nourish and repair cells. Extrinsic influences describe metabolic reactions to environmental factors such as solar radiation/sun exposure that leads to a decline in dermatologic integrity and skin that easily sags, breaks, bruises, and itches.42 Seborrheic keratosis and xerosis are common skin diseases affecting older adults; however, infectious skin conditions such as scabies are also frequently seen. Melanoma or skin cancer is a concern in older adult populations.42
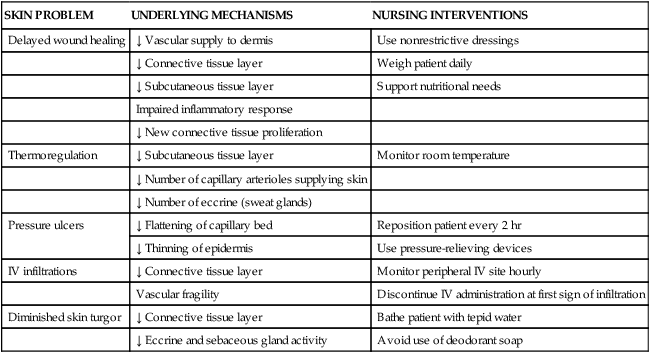
Alterations in collagen structure reduce overall skin elasticity and reductions in immune function degrade the skin’s ability to protect against bacterial assault.42 The loss of elastic and connective tissue causes skin to wrinkle. The underlying structures such as veins and muscles are more visible because of skin transparency. Table 41-6 summarizes age-related changes in the skin and associated nursing interventions. Ecchymotic areas may be seen because of decreased protective subcutaneous tissue layers, increased capillary fragility, and flattening of the capillary bed, predisposing older adults to developing ecchymoses. Medications and physiologic factors may result in augmented bleeding tendency and appearance of ecchymotic areas; nevertheless, consideration should be given to the possibility of older adult abuse if ecchymosis is widespread or in unusual areas.
TABLE 41-6
AGE-RELATED CHANGES IN THE INTEGUMENTARY SYSTEM
| SKIN PROBLEM | UNDERLYING MECHANISMS | NURSING INTERVENTIONS |
| Delayed wound healing | ↓ Vascular supply to dermis | Use nonrestrictive dressings |
| ↓ Connective tissue layer | Weigh patient daily | |
| ↓ Subcutaneous tissue layer | Support nutritional needs | |
| Impaired inflammatory response | ||
| ↓ New connective tissue proliferation | ||
| Thermoregulation | ↓ Subcutaneous tissue layer | Monitor room temperature |
| ↓ Number of capillary arterioles supplying skin | ||
| ↓ Number of eccrine (sweat glands) | ||
| Pressure ulcers | ↓ Flattening of capillary bed | Reposition patient every 2 hr |
| ↓ Thinning of epidermis | Use pressure-relieving devices | |
| IV infiltrations | ↓ Connective tissue layer | Monitor peripheral IV site hourly |
| Vascular fragility | Discontinue IV administration at first sign of infiltration | |
| Diminished skin turgor | ↓ Connective tissue layer | Bathe patient with tepid water |
| ↓ Eccrine and sebaceous gland activity | Avoid use of deodorant soap |
The skin is the largest organ of the body and is constantly exposed to environmental insult, which affects healing potential and leads to acute and chronic wounds. Nearly 1 million Americans develop chronic wounds, with annual prevalence of 10% to 35% in frail older adults.43 Partial thickness skin injuries (skin tears) are caused by flattening and loss of cohesiveness of rete ridges and rete pegs of intact skin to the dermal layer. Decreased cytokine and growth factor production, reduced cytokine receptors, and increased number of senescent cells result in a diminished inflammatory response, when compared to skin of younger persons.43 Cellular senescence is characterized by reduction of cell proliferative ability by shortening of telomeres (specialized structures on the ends of chromosomes) and increase in auto-fluorescent deposits, resulting in termination of cell division (also called replicative senescence).43 Cellular senescence is particularly important with breaks in skin integrity/wounds. Critical care nurses should recognize these issues related to wound healing when caring for patients with pressure ulcers, venous ulcers, and surgical wounds. Additionally, impaired wound healing is affected by disease conditions, comorbidities, environment, genetic factors, and the aging process.43
Musculoskeletal changes in the older adult include a decrease in lean body mass, body composition, and energy expenditure.2 There is an increase in body fat, a decrease in lean muscle mass, and a decline in muscle strength due to selective loss of muscle fibers by up to 40% at age 80 years. Inadequate nutrition, particularly high-quality protein, also affects muscle mass in older adults. Energy expenditure decreases with age, with resting energy expenditure decreasing by as much as 15%.2 Oxygen consumption and energy expenditure after acute illness or injury are approximately 20% to 25% less in patients over 65 years than in younger patients. In light of this, it is recommended that nutritional support should begin within 24 hours of admission to the critical care unit.2
Low bone mineral density (BMD) is a risk factor for osteoporotic fractures.44 Dual-energy x-ray absorptiometry (DXA) is the “gold standard” for measuring BMD and fracture prediction. The fracture risk assessment tool (FRAX) is also used as a gained estimator of hip fracture probability.44 Bone demineralization affects both men and women as they age but occurs more often in women than in men. Bone demineralization refers to an increase in osteoblast and osteoclast activity, which decreases calcium absorption into bone. Mineral loss (calcium and phosphorus) with a decrease in bone mass is referred to as osteoporosis.44 Osteoporosis produces bones that are more “porous” or fragile. With extensive bone demineralization, an older adult patient may sustain multiple fractures. There is an accelerated incidence of osteoporosis in women, which occurs after onset of menopause (possibly due to decreased estrogen). Decreased intake of dietary calcium, immobility, excess glucocorticoid secretion, and smoking all contribute to development of osteoporosis. Physical signs of deformities associated with osteoporosis, such as kyphosis or scoliosis, may place limitations on physical mobility or lead to gait instability. Fractures, particularly hip fractures, are especially devastating in older adults, leading to diminished QOL and increase morbidity and mortality. Risk assessment in critical care should consider these musculoskeletal issues when caring for and mobilizing patients.
Other complications of critical illness include polyneuropathy and myopathy. Many critical care unit survivors report limitations in physical function and activities of daily living (ADLs).45 Impairment in ADLs was reported in virtually all critical care unit survivors assessed in the first week after discharge from the critical care unit. Functionality may improve with time; however impairments still occur in 50% of critical care unit survivors in the first year after illness and are more prevalent in mechanically ventilated patients.45
Older adults have an increased likelihood of developing new or worsening disability after hospitalization.46 Hospitalization-associated disability occurs in almost 30% of older adults for up to 1 year after discharge, with less than 50% regaining pre-illness functional ability.45 The number of older adults affected by hospitalization-associated disability increases to more than 70% in those ventilated for more than 48 hours. Many older adults who require nursing home placement do not experience long-term survival.45 Hospital-associated disability is usually due to a combination of factors, including comorbid diseases, cognitive impairment, depression, immobility, polypharmacy, and lack of social support.45
Pharmacologic Therapy in Older Adults
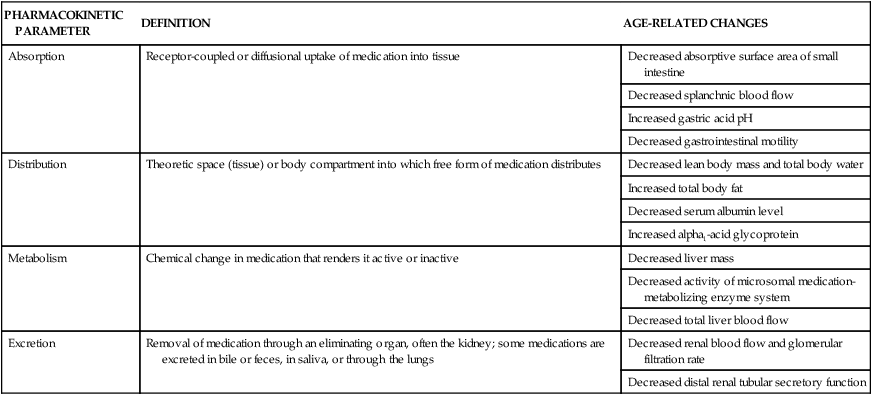
Many benefits of modern advancements in pharmacologic therapy are offset by adverse medication effects, interactions, and therapeutic failure.48 Adverse drug effects and medication interactions may be related to pharmacokinetics or the manner in which the body absorbs, distributes, metabolizes, and excretes a medication.48 The aging process is associated with changes in gastric acid secretion, which can alter ionization or solubility of a medication and hence its absorption48a,48b (Table 41-7). Medication distribution depends on body composition and on physiochemical medication properties. With advancing age, fat content increases, lean body mass decreases, and total body water decreases, which can alter medication disposition.43 For example, because of the increase in the ratio of body fat content to body weight, lipophilic medication have a greater volume of distribution per body weight in older adults compared with younger adults. Other age-related factors affecting medication disposition are listed in Table 41-7.
TABLE 41-7
AGE-RELATED CHANGES IN PHARMACOKINETICS
| PHARMACOKINETIC PARAMETER | DEFINITION | AGE-RELATED CHANGES |
| Absorption | Receptor-coupled or diffusional uptake of medication into tissue | Decreased absorptive surface area of small intestine |
| Decreased splanchnic blood flow | ||
| Increased gastric acid pH | ||
| Decreased gastrointestinal motility | ||
| Distribution | Theoretic space (tissue) or body compartment into which free form of medication distributes | Decreased lean body mass and total body water |
| Increased total body fat | ||
| Decreased serum albumin level | ||
| Increased alpha1-acid glycoprotein | ||
| Metabolism | Chemical change in medication that renders it active or inactive | Decreased liver mass |
| Decreased activity of microsomal medication-metabolizing enzyme system | ||
| Decreased total liver blood flow | ||
| Excretion | Removal of medication through an eliminating organ, often the kidney; some medications are excreted in bile or feces, in saliva, or through the lungs | Decreased renal blood flow and glomerular filtration rate |
| Decreased distal renal tubular secretory function |
Data from Gilman AG, et al, eds. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 8th ed. London: Pergamon Press; 1990; Vestal RE, Cusack BJ. Pharmacology and aging. In: Schneider EL, Rowe JW, eds. Handbook of the Biology of Aging, San Diego: Academic Press; 1990.
The senescent liver and kidneys are less able to metabolize and excrete medications, leading to changes in absorption rates, time to peak plasma concentration, and clearance. Examples include high dosing regimens of diuretics to facilitate diuresis.10 Increased risk of metabolic acidosis then occurs, since high diuretic doses increase competition for organic acid transport pathways at the proximal tubule. Using the example of diuretics, bioavailability between agents may also be variable. For instance, bumetanide has a fairly consistent bioavailability in advanced age, whereas that of furosemide varies from 20% to 80%.10
Similarly, other medications associated with management of common disorders seen in critically ill patients—such as digoxin, angiotensin II-converting enzyme (ACE) inhibitors, and angiotensin II-receptor blockers (ARBs)10—have delayed excretion, increased serum concentration, and more prolonged duration of action because their excretion parallels GFR, which decreases with age. Table 41-7 describes age-related changes in medication pharmacokinetics.
Pharmacodynamics refers to the pharmacologic or physiologic response to a medication that occurs after the medication interacts with its receptor on the plasma membrane. Chronotropic and inotropic effects of beta-adrenergic agonists appear to decrease in older adults.10 Age may produce no change in heparin-stimulated increases in partial thromboplastin time, whereas the effects of warfarin (Coumadin) are very susceptible to medication interactions. Table 41-8 presents cardiac medication considerations for older adults.
TABLE 41-8
CARDIAC MEDICATION CONSIDERATIONS IN OLDER ADULTS
| MEDICATION | CLINICAL CONSIDERATIONS |
| Thiazide diuretics |
• Variable effects on heart muscle, sinus node function, atrioventricular conduction, peripheral arteries, and coronary circulation
• Effective in older adult patients with hypertension due to increasing arterial stiffness, decreased vascular angina and supraventricular arrhythmias, compliance, and diastolic dysfunction
• Lowers peripheral vascular resistance and BP without reflex stimulation of heart rate and contractility
ACEIs reduce morbidity and mortality in patients with
ARBs are considered first line and as an alternative to ACEI in older adult hypertensive patients with diabetes mellitus, hypertension, and HF who cannot tolerate ACEIs
• Fluid accumulation and atrial arrhythmias (minoxidil) used as part of combination regimens
• Centrally acting agents (e.g., clonidine) are not first-line treatments in older adults because of sedation and/or bradycardia
• Abrupt discontinuation leads to increased BP and heart rate, which may aggravate ischemia and/or HF
• These agents should not be considered in noncompliant patients but may be used as part of a combination regimen if needed after several other agents are deployed
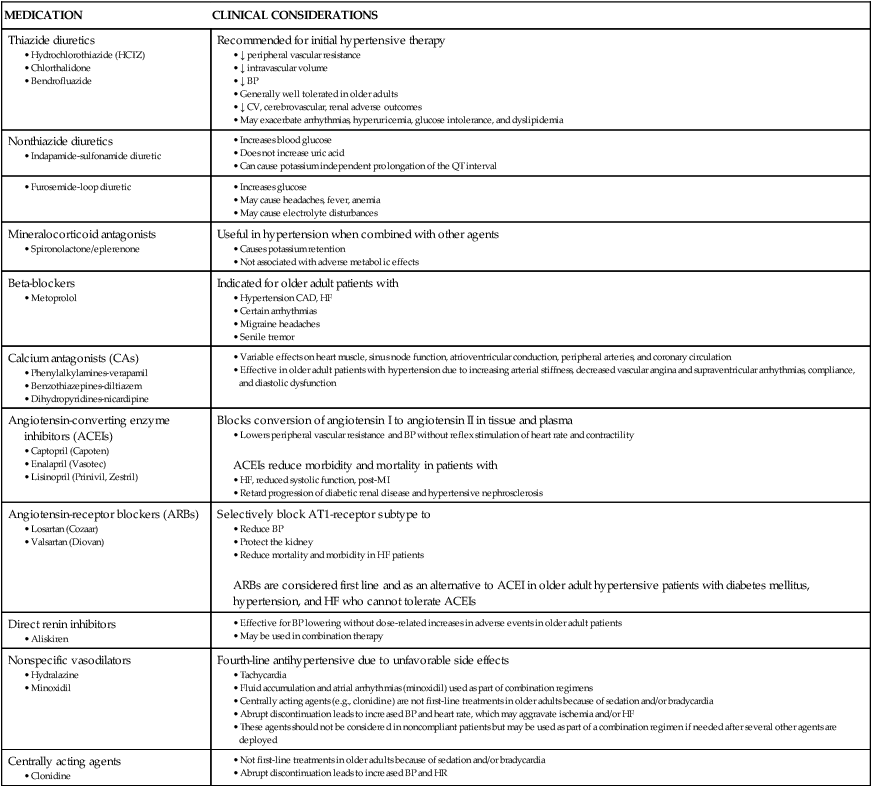
Adapted from Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2011;57:2037.
The Critical Care Safety Study found that 61% of adverse events in the critical care unit were associated with medications.49 Annual costs related to adverse pharmacologic events have been estimated to be $76.6 billion to ambulatory care, $20 billion to hospitals, and $4 billion to nursing home facilities.48 Medications most often associated with errors were cardiovascular medications (24%), anticoagulants (20%), and anti-infective agents (13%).48 Insulin and hypoglycemic agents are also responsible for hospital readmissions.48 The Beers Criteria is a list of medications that was developed to avoid inappropriate medication prescribing in adults aged 65 years and over. (Visit this website for more information: http://www.dcri.duke.edu/ccge/curtis/beers.html.)48 Table 41-9 lists potentially inappropriate symptom management medications for older adults.
TABLE 41-9
POTENTIALLY INAPPROPRIATE SYMPTOM MANAGEMENT MEDICATIONS FOR OLDER ADULTS
| MEDICATION | POSSIBLE SIDE EFFECT |
| Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)‡ | |
| Indomethacin | Central nervous system (CNS) effects (highest of all NSAIDs)* |
| Ketorolac | Asymptomatic gastrointestinal conditions (ulcers*) |
| Aspirin (>325 mg) | Asymptomatic gastrointestinal conditions (ulcers*) |
| Naproxen | Gastrointestinal bleeding*; renal failure*; high blood pressure*; heart failure* |
| Opioids | |
| Meperidine | Intense side-effect profile for adverse effects, especially CNS effects (seizures) |
| Most critical in individuals with renal compromise* | |
| Morphine, hydromorphone, fentanyl | Intense side-effect profile at higher doses, especially CNS effects (such as somnolence, respiratory depression, and delirium)* |
| Adjuvant Medications | |
| Muscle relaxants (methocarbamol, carisoprodol, chlorzoxazone, metaxalone, cyclobenzaprine, baclofen) | Anticholinergic effects*†; sedation*; weakness*; cognitive impairment* |
| Tricyclic antidepressants (amitriptyline and amitriptyline compounds) | Strong anticholinergic effects*†; may lead to ataxia, impaired psychomotor function, syncope, falls; cardiac arrhythmias (QT interval changes)*; may produce polyuria or lead to urinary incontinence*; may exacerbate chronic constipation |
| Doxepin | Cardiac arrhythmias*; may produce polyuria or lead to urinary incontinence*; may exacerbate chronic constipation |
| Antihistamines (diphenhydramine, hydroxyzine, promethazine) | Potent anticholinergic properties*† |
| May lead to confusion and sedation | |
| Benzodiazepines | Increased sensitivity at higher doses with prolonged sedation and increased risk for falls |
| Short acting (lorazepam ≥3 mg, oxazepam ≥60 mg, alprazolam ≥2 mg) | May produce or exacerbate depression; smaller doses may be both effective and safer |
| Long acting (diazepam) | CNS effects*; may cause or exacerbate respiratory depression in chronic obstructive pulmonary disease*; may produce polyuria or lead to urinary incontinence* |
| Selective serotonin reuptake inhibitor antidepressants (fluoxetine, citalopram, paroxetine, sertraline) | May produce CNS stimulation, sleep disturbances, and increasing agitation*; may exacerbate or cause syndrome of inappropriate secretion of antidiuretic hormone or hyponatremia |
| Decongestants | High level of CNS stimulation, which may lead to insomnia* |
| CNS stimulants (methylphenidate) | Altered CNS function, leading to cognitive impairment*; appetite-suppressing effect* |
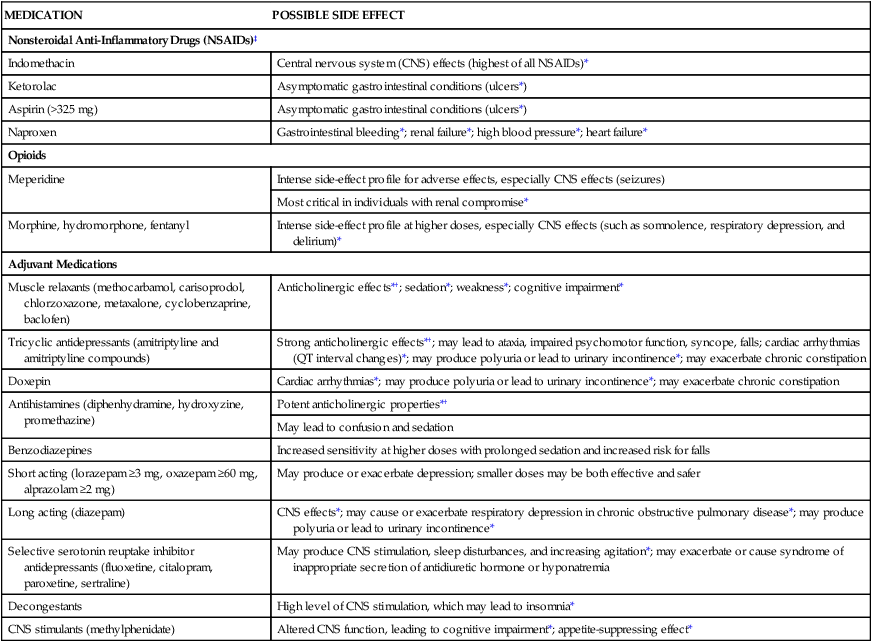
†Anticholinergic effects include some of the following symptoms: blurred vision, constipation, drowsiness, sedation, dry mouth, tachycardia, urinary retention, confusion, disorientation, memory impairment, dizziness, nausea, nervousness, agitation, anxiety, facial flushing, weakness, and delirium.
‡May cause or exacerbate gastric or duodenal ulcers, prolonged clotting time and international normalized ratio, and decreased platelet function.
Hospital-Associated Risk Factors for Older Adults
Multiple concurrent chronic illnesses produce systemic stressors that diminish immune function. Critical care nurses must be aware that exacerbation of pre-existing illness such as diabetes or emphysema may manifest before infection is suspected. Invasive devices such as central lines or chest tubes may threaten suppressed immune systems. Nutritional deficiencies are a common problem in older adults. Inadequate protein intake can develop from prolonged anorexia and cognitive impairment associated with a shrinkage of lymphoid tissue, diminishing T-cell functioning and decreased cell-mediated immunity.49 Inadequate emptying of urine because of bed rest, obstruction, or side effects from anticholinergic medications can result in stagnation of urine and recurrent urinary tract infections. Long-term placement of urinary catheters is a significant source of bacteria; however, treatment with antibiotic therapy is not indicated unless the patient becomes symptomatic with anorexia or cognitive impairment or has a history of a chronic illness, such as diabetes or COPD.
Long-Term Complications of Critical Care
Improved critical care outcomes have led to an increase in survivors of critical illness and additional challenges related to long-term complications of critical care. Mortality post-critical care ranges from 26% to 63% in the first year, with mortality risk factors dependent on age, comorbidity, and critical care severity of illness.45 Medical costs are incurred during initial hospitalizations with 40% requiring ongoing medical care for up to 2 years related to rehabilitation and readmissions increasing utilization of long-term acute care three-fold between 1997 and 2006.45 Post-critical care unit conditions create an additional burden for patients and their families for years after critical care unit discharge.45 Post-critical care unit issues include depression, lifestyle disruption, employment problems, mobility dysfunction, and cognitive impairment.46
Critical care nursing now involves prevention and management of long-term complications of critical care.47 Critical care nurses have an essential role in multidisciplinary collaboration and assessment of older patients. Nurses gather vital information about patient needs, implement key nursing interventions, and recommend specialty service consults to improve long-term clinical outcomes.
Coordination of critical care interventions can decrease negative physical, functional, and cognitive long-term outcomes associated with critical illness.47 Noise reduction and medication management can enhance orientation, decrease delirium, and improve long-term consequences of critical care. Early mobility protocols, avoidance of restraints, and removal of indwelling catheters can decrease infection risks and improve rehabilitative care. Physical and occupational therapy can assist with mobility and ADLs. Daily reviews of medical care, medications, nutrition, and emphasis on discharge needs of patients and caregivers can expedite recovery and improve outcomes.50 Evidence supports utilization of care bundles to improve process of care.50 Bundles such as Awakening and Breathing Coordination, Delirium Monitoring and Management, and Early Mobility (ABCDE) standardize care through algorithms.50 Intraprofessional teams pair sedation protocols and daily awakening for ventilator management; use the Confusion Assessment Method-ICU (CAM-ICU) or the Intensive Care Delirium Screening Checklist (ICDSC) for delirium screening, and mobilize patients based on individual physiologic stability and readiness.51 These strategies allow for optimal patient progression along the continuum of care.
Care Transitions
Transitions of care and provider hand-off within one episode of care are difficult for older adults, resulting in multiple touch points and increasing fragmentation of care due to incomplete transfer of information between health care providers.52 Families and informal caregivers of vulnerable older adults often become the messengers between providers. Older adults without family frequently remain in higher levels of care for extended time periods, reducing bed availability for others. Several models exist for a new nursing role known as a navigator. Navigators combine the role of nurse, social worker, and case manager to assist high-risk older patients through transitions of care. The navigation role may offer opportunity for vulnerable populations and older adults to safely navigate the complex continuum of care while assisting with economic and clinical outcomes.52
Palliative Care and End of Life in Critical Care
In 2001, 17% of U.S. deaths occurred in critical care units, with 47% of hospital deaths preceded by a critical care unit stay.53 Almost three-quarters of all deaths in the United States occur among persons 65 years of age and over, accounting for about 1.8 million deaths in 2007.53 During the past decade, overall death rates have declined by 8% for this age group. Older adult deaths account for 42% to 52% of admissions to critical care units and for almost 60% of all critical care unit days.2 Because of this, palliative care is a necessary part of critical care nursing.
Palliative care is frequently misconstrued as synonymous with withdrawal of life support in the critical care unit. Palliative care is focused on the relief of suffering, in all of its dimensions, throughout the course of a patient’s illness. Palliative care teams offer specialized medical care for people with serious illnesses. Palliative care teams focus on providing patients with relief from symptoms, pain, and stress of serious illness irrespective of diagnosis. The goal is to improve QOL for both the patient and the family. (Visit this website for more information: http://www.getpalliativecare.org.) Palliative care teams can assist with pain/symptom assessment, social/spiritual assessment, understanding of illness/prognosis, treatment options, identification of patient-centered goals of care, and transition of care postdischarge.54
Compassionate end-of-life care in accordance with patient wishes is an essential component of critical care. Studies have found that performance of specific evidence-based care processes are essential components of high-quality critical care but these specific practices are inconsistent and infrequent.55 Recommendations include use of care and communication bundles. These bundles provide daily goals of care to be performed on specified days. For example, day 1 interventions include identification of a decision-maker, advance directives, and resuscitation preferences. On day 1 families are also provided with information and patients symptoms are managed. Day 3 interventions include support from social work or spiritual support and consideration of plans for a family meeting. Critical care nurses must be skilled in end of-life care just as in clinical competency. Team collaboration promotes clear communication and goal alignment for patients and families.56
Conclusion
Older adults require more intense observation and consideration in critical care units because they are less adaptable to stress and illness. Table 41-10 summarizes the major changes in various systems with clinical considerations.2 As shown in Figure 41-3
TABLE 41-10
PHYSIOLOGIC CHANGES OF AGING AND THEIR RELATIONSHIP TO SYMPTOM MANAGEMENT CHANGE
| AGE-RELATED EFFECT | CLINICAL CONSIDERATIONS |
| Skin | |
| Decreased cutaneous layers and thinned subcutaneous tissue | Can increase risk for effects of anorexia and cachexia |
| Decreased blood vessels | May alter absorption of transdermal medications |
| May decrease ability to use intravenous route for symptom management | |
| Decreased nerves | May alter pain sensation |
| Decreased elasticity | Increases risk for skin tears |
| Bones | |
| Altered calcium metabolism leading to bone loss | Increases risk for bone instability with metastatic bone disease |
| Tooth loss | Increases risk for malnutrition during therapy and subsequent nutrition-related symptoms such as anemia, mucous membrane and skin breakdown, and electrolyte disturbances |
| Soft Tissue | |
| Muscle atrophy | Decreases strength and endurance, which may increase fatigue |
| Nervous system slowing | Decreases fine motor control, which may have an impact on implementing symptom management strategies |
| Increased body fat | May have an impact on medication metabolism |
| Sensory Loss | |
| Hearing | May have an impact on communication of patient education information for symptom management |
| Vision | May have an impact on communication of patient education information for symptom management |
| Smell | May have an impact on successful treatment of anorexia or cachexia |
| Hematology and Immunology | |
| Decreased bone marrow reserve | May have delayed response to infection and anemia |
| Anemia related to decreased intrinsic factor production and decreased iron metabolism | May contribute to cancer-related fatigue |
| Increased clotting caused by increased platelet adhesion | May contribute to perfusion issues and lead to vague, non–cancer-related symptoms |
| Circulation | |
| Enlargement of heart; slowing of electrical activity; and changes in collagen in arteries, causing stiffness and thickening | Caution should be used with symptom management medications that may affect cardiac function, such as medications used for treating neuropathic pain |
| Pulmonary | |
| Decreased oxygen and carbon dioxide exchange because of decreased elasticity of lung tissue and alveoli enlargement | Caution should be used with medications that have a direct effect on pulmonary functioning, such as benzodiazepines or opioids |
| Decreased cough reflex and ciliary function | May increase risk of decreased airway clearance |
| Gastrointestinal | |
| Decline in small intestinal absorption of vitamins D and B12 and folic acid | Increases risk for developing anemia and bone loss |
| Thinning of intestinal lining, decreased mucus production, and weaker intestinal muscles | Increases risk for constipation |
| Diminished liver function because of circulatory and metabolic changes | May have an impact on medication metabolism by slowing drug metabolism and leading to increased medication toxicity |
| Urinary | |
| Decreased renal perfusion beginning at age 40 years | Increases risk for developing drug toxicity, especially with nonsteroidal anti-inflammatory medications and diuretics |
| Decreased number of nephrons and glomeruli with decreased glomerular filtration rate | May increase risk for dehydration or fluid overload |
| Decreased adaptability of kidneys to handle stress | Altered potassium regulation |
| Decreased bladder capacity and tone Decreased tone of pelvic floor |
May lead to urinary incontinence |
| Enlargement of prostate | Eventually leads to lower urinary tract symptoms |
| Cognitive | |
| Decrease in short-term memory | May decrease ability to remember details of symptom onset, duration, and treatment |
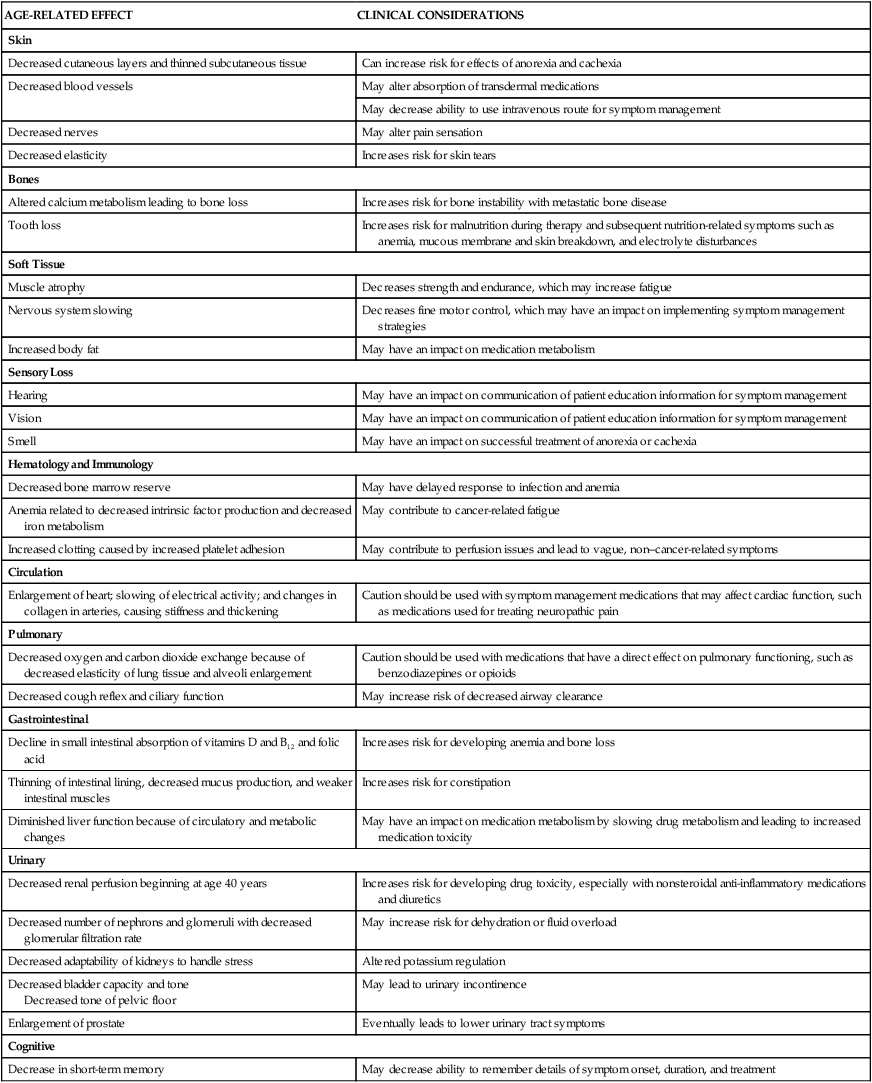
Based on data from Lacasse C. Polypharmacy and symptom management in older adults. Clin J Oncology Nurs. 2010;15(1):27. doi: 10.1188/11. CJON. 27-30; Aldwin CM, Gilmer DF. Health, Illness, and Optimal Aging. Thousand Oaks, CA: Sage; 2004; Tabloski PA. Gerontological Nursing. Upper Saddle River, NJ: Pearson Prentice Hall; 2006.
, many physiologic changes occur with advancing age, and each change may render a particular system less adaptable to stress. Changes in one system may affect another system in the presence of disease. The inability to adapt poses a significant risk for functional decline after discharge. Older adults at greatest risk include those with poor nutritional status or cognitive dysfunction and those who required assistance with at least two ADLs before admission. To provide the best care and prevent iatrogenic complications, the critical care nurse must consider all physiologic and psychologic factors that affect the older adult patient.
Summary
Cardiovascular System
• Aorta and other arteries become stiff and less pliable, leading to increased workload on the heart to perfuse tissue.
• Systolic and diastolic pressures increase, along with an increase in systemic vascular resistance and a decrease in cardiac output.
• There is a loss of capacity in the myocardium and arterial system and decreased ability to respond and recover from periods of physiologic and psychologic stress.
Renal System
• Decreased renal blood flow leads to a decrease in glomerular filtration and decreased renal tubule function.
• There is decreased elimination of physiologic substances (i.e., BUN and creatinine).
• The older adult is predisposed to developing metabolic acidosis, volume depletion, and hyperchloremia.
Gastrointestinal System
Central Nervous System
• There are changes in gyral function, formation of neurofibrillary tangles, a decrease in brain volume, and increase in size of cerebral ventricles.
• Senile plaque formation increases.
• There are changes in neurotransmitter synthesis and function, and there is degeneration of the blood–brain barrier.
Pharmacokinetics and Pharmacodynamics
• Loading and maintenance doses of certain medications should be reduced.
• Reduced clearance of medications by the kidneys from dehydration may be reversible by rehydration.
• Liver and kidney function tests need to be repeated at regular intervals or when new medications are added.
• Nonessential medications should be limited, and patients should be monitored for toxic effects associated with new medications.