Sedation, Agitation, Delirium
Assessment and Management
One of the challenges facing clinicians is how to provide a therapeutic healing environment for patients in the alarm-filled, emergency-focused critical care unit. Many critical care patients demonstrate agitation and discomfort caused by painful procedures, invasive tubes, sleep deprivation, fear, anxiety, and physiologic stress. Clinical practice guidelines were developed by the Society of Critical Care Medicine (SCCM) to increase awareness of these issues in the critically ill. The initial guidelines were released in 20021 and substantially revised in 2013.2
Sedation
Sedation and Agitation Assessment Scales
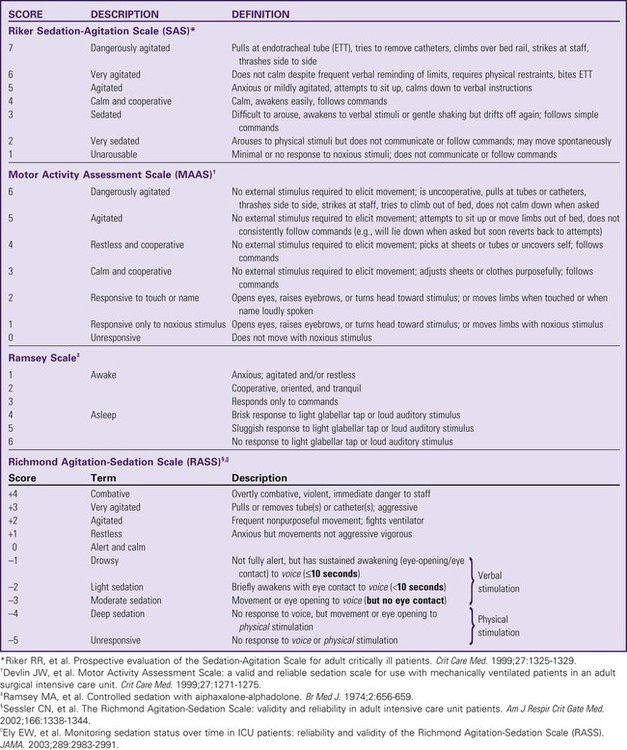
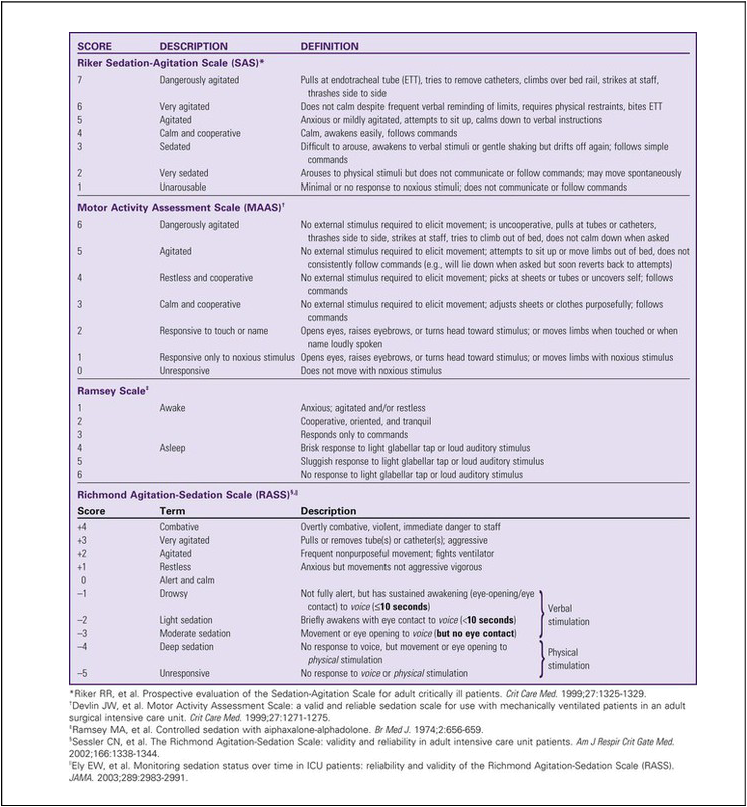
The use of scoring systems to assess and record levels of sedation and agitation is now strongly recommended.1,2 Four frequently used scales are the Ramsay Scale,3 the Riker Sedation-Agitation Scale (SAS),4 the Motor Activity Assessment Scale (MAAS),5 and the Richmond Agitation-Sedation Scale (RASS)6,7 (Table 10-1). The two scales that are recommended for assessment of agitation and sedation in the adult critically ill patient are the SAS and the RASS.2 Because individuals do not metabolize sedative medications at the same rate, the use of a standardized scale will ensure that continuous infusions of sedatives such as propofol or dexmedetomidine are titrated to a specific goal. Collaboratively, the critical care team must determine which level of sedation is most appropriate for each individual patient.
Pain Assessment Scales
The first step in assessing the agitated patient is to rule out any sensations of pain.1,2 Clinical assessment is more challenging when the patient is obtunded or has an artificial airway in place. If the patient can communicate, the verbal pain scale of 0 to 10 is very useful. If the patient is intubated and cannot vocalize, pain assessment becomes considerably more complex. The Behavioral Pain Scale (BPS) and the Critical-Care Pain Observation Scale (CPOT) are the most reliable behavioral pain scales for monitoring of pain in critically ill adults.2 These pain scales are shown in Table 9-1 (BPS) and Table 9-2 (CPOT) in Chapter 9. Pre-emptive analgesia should be provided before painful procedures.2 The next step is to determine the minimum level of sedation required1,2 (Box 10-1). The SCCM guidelines recommend that all critically ill, intubated, mechanically ventilated patients have stated goals for analgesia and sedation.2 Assessment, treatment and prevention strategies for the management of pain are listed in Figure 9-6 in Chapter 9.
Pharmacologic Management of Sedation
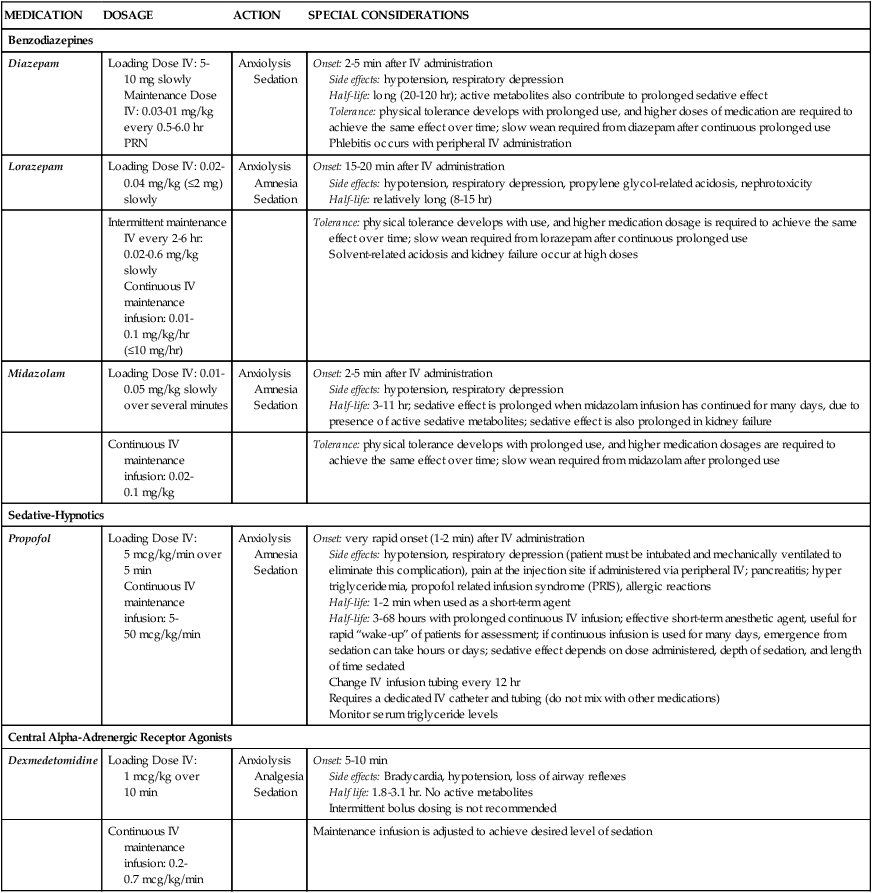
Several categories of sedatives are available. If the patient is experiencing pain, analgesia must be administered in addition to any sedative agents. Sedative agents include the benzodiazepines, sedative-hypnotic agents such as propofol, and the central alpha agonist dexmedetomidine (Table 10-2).1 Currently dexmedetomidine-based and propofol-based sedative regimens are recommended for routine sedation of mechanically ventilated adult patients.2
TABLE 10-2
PHARMACOLOGIC MANAGEMENT
Sedation
| MEDICATION | DOSAGE | ACTION | SPECIAL CONSIDERATIONS |
| Benzodiazepines | |||
| Diazepam | Loading Dose IV: 5-10 mg slowly Maintenance Dose IV: 0.03-01 mg/kg every 0.5-6.0 hr PRN |
Anxiolysis Sedation |
Onset: 2-5 min after IV administration Side effects: hypotension, respiratory depression Half-life: long (20-120 hr); active metabolites also contribute to prolonged sedative effect Tolerance: physical tolerance develops with prolonged use, and higher doses of medication are required to achieve the same effect over time; slow wean required from diazepam after continuous prolonged use Phlebitis occurs with peripheral IV administration |
| Lorazepam | Loading Dose IV: 0.02-0.04 mg/kg (≤2 mg) slowly | Anxiolysis Amnesia Sedation |
Onset: 15-20 min after IV administration Side effects: hypotension, respiratory depression, propylene glycol-related acidosis, nephrotoxicity Half-life: relatively long (8-15 hr) |
| Intermittent maintenance IV every 2-6 hr: 0.02-0.6 mg/kg slowly Continuous IV maintenance infusion: 0.01-0.1 mg/kg/hr (≤10 mg/hr) |
Tolerance: physical tolerance develops with use, and higher medication dosage is required to achieve the same effect over time; slow wean required from lorazepam after continuous prolonged use Solvent-related acidosis and kidney failure occur at high doses |
||
| Midazolam | Loading Dose IV: 0.01-0.05 mg/kg slowly over several minutes | Anxiolysis Amnesia Sedation |
Onset: 2-5 min after IV administration Side effects: hypotension, respiratory depression Half-life: 3-11 hr; sedative effect is prolonged when midazolam infusion has continued for many days, due to presence of active sedative metabolites; sedative effect is also prolonged in kidney failure |
| Continuous IV maintenance infusion: 0.02-0.1 mg/kg | Tolerance: physical tolerance develops with prolonged use, and higher medication dosages are required to achieve the same effect over time; slow wean required from midazolam after prolonged use | ||
| Sedative-Hypnotics | |||
| Propofol | Loading Dose IV: 5 mcg/kg/min over 5 min Continuous IV maintenance infusion: 5-50 mcg/kg/min |
Anxiolysis Amnesia Sedation |
Onset: very rapid onset (1-2 min) after IV administration Side effects: hypotension, respiratory depression (patient must be intubated and mechanically ventilated to eliminate this complication), pain at the injection site if administered via peripheral IV; pancreatitis; hyper triglyceridemia, propofol related infusion syndrome (PRIS), allergic reactions Half-life: 1-2 min when used as a short-term agent Half-life: 3-68 hours with prolonged continuous IV infusion; effective short-term anesthetic agent, useful for rapid “wake-up” of patients for assessment; if continuous infusion is used for many days, emergence from sedation can take hours or days; sedative effect depends on dose administered, depth of sedation, and length of time sedated Change IV infusion tubing every 12 hr Requires a dedicated IV catheter and tubing (do not mix with other medications) Monitor serum triglyceride levels |
| Central Alpha-Adrenergic Receptor Agonists | |||
| Dexmedetomidine | Loading Dose IV: 1 mcg/kg over 10 min | Anxiolysis Analgesia Sedation |
Onset: 5-10 min Side effects: Bradycardia, hypotension, loss of airway reflexes Half life: 1.8-3.1 hr. No active metabolites Intermittent bolus dosing is not recommended |
| Continuous IV maintenance infusion: 0.2-0.7 mcg/kg/min | Maintenance infusion is adjusted to achieve desired level of sedation | ||
Barr J, et al. Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit. Crit Care Med. 2013;41(1):278.
Targeted Sedation
All sedatives are to be administered to a specific target level identified by a RASS or SAS level appropriate to the patient’s clinical condition (Figure 10-1).2 A target level is specified every day and reevaluated whenever there is a change in sedation dosage or patient condition. One likely sedation goal is that the patient is awake and calm, specified as a target sedation level of RASS = 0, or SAS = 4. This describes a patient who follows simple verbal commands without agitation and who can sustain eye contact for at least 10 seconds, as described in Table 10-1. When the intent is to provide deep sedation, the target sedation level is specified as RASS = -3 to -5 or SAS = 1 to 2.2 This describes a mechanically-ventilated patient who is not responsive to voice, but who may respond to physical stimulation (see Table 10-1).
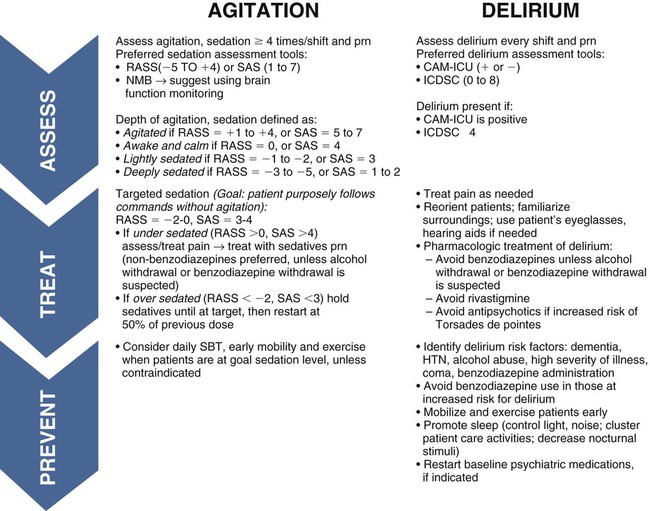
CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; HTN, hypertension; ICDSC, Intensive Care Delirium Screening Checklist; NMB, neuromuscular blockade; RASS, Richmond Agitation-Sedation Scale; SAS, Riker Sedation-Agitation Scale; SBT, spontaneous breathing trial. (From Barr J, Fraser GL, Puntillo KA, Ely WE, Gelinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Critical Care Medicine. 2013, in press.)
Neuromuscular Blockade
Pharmacologic neuromuscular blockade (NMB) induces a state of physical paralysis. NMB is administered to achieve ventilator synchrony and improve oxygenation when this cannot be achieved by sedation and analgesia alone. Table 21-10 in Chapter 21 lists NMB medications, dosages, and management.
Brain Function Monitoring.
Brain function monitoring is recommended to assess level of consciousness following NMB administration (see Figure 10-1).2 In the operating room, brain function or activity is routinely monitored during anesthesia to ensure the patient does not have awareness during surgery. Similar monitoring technologies are now recommended for critically ill patients receiving NMB in addition to standard sedation assessments with RASS or SAS.2 Various technologies are available that monitor the electrical activity of the brain frontal lobes and include electromyelogram (EMG) sensors to record forehead muscle movements.
Benzodiazepines
Benzodiazepines have powerful amnesic properties that inhibit reception of new sensory information.1,7 Benzodiazepines do not confer analgesia. The most frequently used benzodiazepines are diazepam (Valium), midazolam (Versed), and lorazepam (Ativan). Once a mainstay of sedation, benzodiazepines are no longer recommended for sedation of the mechanically ventilated critically ill adult.2 This is because benzodiazepine-based sedative regimens are associated with worse patient outcomes, including longer duration of mechanical ventilation and delirium.8–10
Short-acting benzodiazepines (midazolam) are still helpful for the treatment of acute short-term agitation,2 because the intravenous (IV) onset of action is less than 3 minutes. However, when midazolam is administered for longer than 24 hours as a continuous infusion, the sedative effect is prolonged by its active metabolites.1 Longer acting benzodiazepines (lorazepam and diazepam) also have a role in the management of delirium tremens (described later in the chapter) and seizures,2 but these are different indications from routine sedation in critical illness.
The major unwanted side effects associated with benzodiazepines are dose-related respiratory depression and hypotension. If needed, flumazenil (Romazicon) is the antidote used to reverse benzodiazepine overdose in symptomatic patients. Flumazenil should be avoided in patients with benzodiazepine dependence, because rapid withdrawal can induce seizures.11
Sedative-Hypnotic Agents
Propofol is a powerful sedative and respiratory depressant used for sedation in mechanically ventilated patients in critical care. It is immediately identifiable by its white milky appearance, always in a glass container. At high doses (>100 to 200 mcg/kg/min), propofol is intended to produce a state of general anesthesia in the operating room.12 In the critical care unit, propofol is prescribed as a continuous infusion at lower doses (5 to 50 mcg/kg/min) to induce a state of deep sedation.12 Because propofol is lipid soluble it quickly crosses cell membranes, including the cells that comprise the blood-brain barrier. This allows rapid onset of sedation (about 30 seconds), with immediate loss of consciousness. In addition to a rapid onset of action propofol has very short half-life with initial use (2 to 4 minutes), is rapidly eliminated from the body (30 to 60 minutes), and does not have metabolites.12,13 This makes it an ideal sedative for use when a patient needs to be quickly awakened for a spontaneous awakening trial (SAT) and spontaneous breathing trial (SBT), or to assess neurologic status. This feature makes propofol an ideal medication to manage rapid ventilator weaning postsurgery. Propofol is both clinically effective and cost effective because it shortens time to extubation. It is important to add an opiate to ensure adequate pain control and amnesia. Propofol is not a reliable amnesic, and patients sedated with only propofol can have vivid recollections of their experiences.
Risks of unwanted complications increase when propofol is administered long term at high doses (>4.00 mg/kg/hr for longer than 48 hours). Propofol is delivered in a fat-based emulsion, and side effects may be related to disruption of fatty acid metabolism, muscle injury, and release of toxic intracellular contents. Complications have been collectively grouped under the term Propofol Related Infusion Syndrome (PRIS) including: metabolic acidosis, muscular weakness, rhabdomyolysis, myoglobinuria, acute kidney injury, and cardiovascular dysrhythmias. PRIS affects about 1% of all patients who receive propofol; almost one third of those affected will not survive.8,13,14 Other secondary side effects related to the fat-emulsion carrier include hyperlipidemia, hypertriglyceridemia, and acute pancreatitis. Nursing vigilance is required to monitor sedation levels, and to be alert for the rare but significant risk of propofol-related complications. Serum triglycerides should be measured on all patients who receive propofol for longer than 48 hours. For additional information see Table 10-2.
Central Alpha Agonists
Dexmedetomidine is prescribed with a loading dose of 1.0 mcg/kg over 10 minutes, followed by a continuous infusion of 0.4 mcg/kg (range 0.2 to 0.7 mcg/kg/hr).15 Dexmedetomidine has a short half-life (6 minutes) and is eliminated from the body in about 2 hours.15 Elimination from the body is dramatically slowed if the patient has liver failure (see Table 10-2). Complications include bradycardia and hypotension.16
Many patients will have other sedatives or opiates infusing in addition to dexmedetomidine, and the combination may potentiate the overall sedative effect. Monitoring of sedation level, blood pressure, heart rate, respiratory rate, and pulse oximetry are mandatory. Dexmedetomidine confers sedation and analgesic effects without respiratory depression.15 Consequently patients can be extubated while still on a dexmedetomidine infusion. This can be helpful for patients who are anxious during ventilator weaning. Dexmedetomidine has also been used for patients on noninvasive mask ventilation.17 Because of this favorable profile many clinicians now use dexmedetomidine as a first-line sedative agent.18
Daily Sedation Interruption
One innovative strategy to avoid the pitfalls of sedative dependence and withdrawal is a planned strategy to turn off the sedative infusions once each day.2 This intervention has been given several names, including sedation vacation and spontaneous awakening trial (SAT). At a scheduled time, all continuously infusing sedatives are stopped. Sometimes analgesics are also stopped, depending on the hospital’s protocol. The patient is allowed to regain consciousness for clinical assessment using a standardized instrument such as the RASS or SAS (see Table 10-1).19,20 The patient is carefully monitored, and when awareness is attained, an assessment of level of consciousness and neurologic function is performed. If the patient becomes agitated, it is essential that a protocol be in place for the nurse to restart the sedatives. One protocol scheduled the daily interruption of sedatives in the morning and recommended, after a full assessment, restarting the sedative and opiate infusions at 50% of the previous morning dose and adjusting upward until the patient was comfortable.19
An important nursing responsibility is to prevent the patient from coming to harm during sedative or analgesic medication withdrawal (Table 10-3). If the patient is seriously agitated, it is vital to consult with the physician and pharmacist to establish an effective treatment plan that will allow safe weaning from sedative medications (see Table 10-1).
TABLE 10-3
SIGNS AND SYMPTOMS OF SEDATIVE OR ANALGESIC MEDICATION WITHDRAWAL*
| SYSTEM | OPIATE WITHDRAWAL | BENZODIAZEPINE WITHDRAWAL† |
| Neurologic | Delirium, tremors, seizures | Agitation, anxiety, delirium, tremors, myoclonus, headache, seizures, fatigue, paresthesias, sleep disturbances |
| Sensory | Dilation of pupils, teary eyes, irritability, increased sensitivity to pain, sweating, yawning | Increased sensitivity to light/sound, sweating |
| Musculoskeletal | Cramps, muscle aches | Muscle cramps |
| Gastrointestinal | Vomiting, diarrhea | Nausea, diarrhea |
| Respiratory | Tachypnea | Tachypnea |
*Data on propofol is limited, but withdrawal symptoms after prolonged use are similar to those of the benzodiazepines.1
Agitation
Agitation describes hyperactive patient movements that range in intensity from slight restless hand and body movements, to pulling out lines and tubes, or physical aggression and self-harm. Agitation can be caused by, pain, anxiety, delirium, hypoxia, ventilator dyssynchrony, neurological injury, uncomfortable position, full bladder, sleep deprivation, alcohol withdrawal, sepsis, medication reaction, or organ failure, to name but a few frequently encountered causes. In the past, when a patient showed physical signs of agitation, a benzodiazepine sedative (lorazepam or midazolam) was quickly administered to reduce the patient’s mental awareness (see Table 10-2). However, because benzodiazepines have been shown to induce delirium, it is now recommended that proactive assessments be conducted at least 4 times in a nursing shift (see Figure 10-1).2
Agitation is assessed using a validated scale such as the SAS or RASS (see Table 10-1).2 Standardized assessment scales allow clinicians to identify agitation in its milder forms and to potentially ameliorate the patient’s symptoms. The goal is to treat the cause of the agitation rather than to over-medicate. Obviously, when a patient is dangerously agitated (SAS +7), is combative (RASS +4), or could endanger themselves or others, immediate sedation is warranted. In these extreme situations, a benzodiazepine is administered.2
Delirium
Delirium represents a global impairment of cognitive processes, usually of sudden onset, coupled with disorientation, impaired short-term memory, altered sensory perceptions (i.e., hallucinations), abnormal thought processes, and inappropriate behavior. Routine monitoring for delirium is recommended.2 Delirium is more prevalent than generally recognized; it is difficult to diagnose in the critically ill patient and represents acute brain dysfunction caused by sepsis, critical illness, or dysfunction of other vital organs (Box 10-2). Between 60% and 85% of mechanically ventilated patients experience delirium.21 Delirium increases hospital stay and mortality rates for patients who are mechanically ventilated.22 The increased mortality remains even after controlling for associated variables such as coma and administration of sedatives and analgesics.22
When patients are agitated, restless, and pulling at tubes and lines, they are often identified as being delirious. In this scenario, delirium may be described as “ICU psychosis” or “sundowner syndrome.” However, the delirious patient is not always agitated, and it is much more difficult to detect delirium when the patient is physically calm.1,23 Provision of adequate analgesia is an essential component of delirium prevention.2
Specific scoring instruments are available to assess delirium, and two have been validated for use with mechanically ventilated critical care patients.2,24 They are the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) (Fig. 10-2 and Fig. 10-3)25–27 and the Intensive Care Delirium Screening Checklist (ICDSC) (Fig. 10-4).28,29 Both instruments are used in tandem with the RASS to exclude patients in coma and identify delirium. Coma is a known risk factor for development of delirium.2 Both the CAM-ICU and ICDSC provide a structured format to evaluate delirium both for verbal patients and for nonverbal and mechanically ventilated patients.
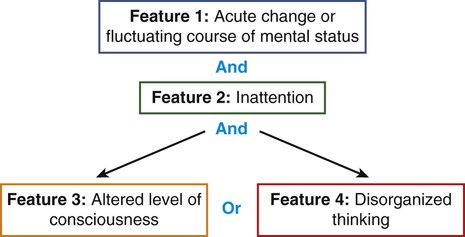
Delirium is defined as positive in Feature 1 AND Feature 2 and EITHER Feature 3 OR Feature 4.
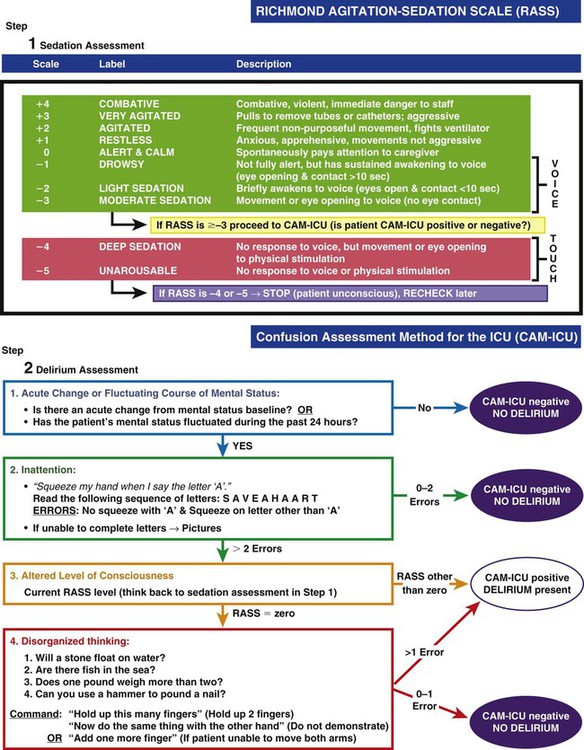
Step 1—Sedation Assessment; Step 2—Delirium Assessment. (Copyright 2002, E. Wesley Ely, MD, MPH and Vanderbilt University, all rights reserved.)
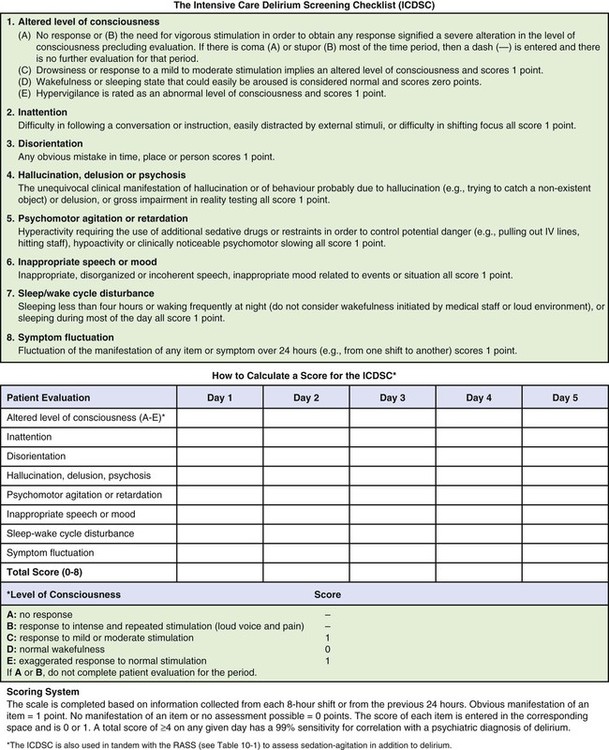
The ICDSC is also used in tandem with the RASS scale (see Table 10-1) to assess sedation-agitation in addition to delirium. (From Bergeron N, et al. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859.)
Pharmacologic Management of Delirium
Pharmacologic treatment of delirium remains challenging.2 The neuroleptic medication haloperidol (Haldol) has traditionally been administered to treat hyperactive delirium.30 This antipsychotic agent stabilizes cerebral function by blocking dopamine-mediated neurotransmission at the cerebral synapses and in the basal ganglia. Electrocardiographic (ECG) monitoring is recommended, because haloperidol prolongs the QTc-interval, increasing the risk of ventricular dysrhythmias. Haloperidol and other antipsychotics should be avoided in patients who have a prolonged QTc or a history of torsades de pointes.2
Atypical antipsychotics are sometimes administered to treat delirium associated with critical illness. These are medications that were developed to treat another condition, such as schizophrenia or bipolar disorder, which are administered to shorten the duration of delirium in critical illness. In a small clinical trial of mechanically ventilated patients, quetiapine was associated with a faster resolution of delirium compared with placebo.31,32 Other antipsychotics need to be subjected to the rigors of a clinical trial. This is needed because there is limited evidence on the efficacy and safety of atypical antipsychotics used to treat delirium in critical illness. The cholinesterase inhibitor medication rivastigmine should be avoided as it is associated with higher mortality.33
Haloperidol or other atypical antipsychotics are not to be administered prophylactically as prevention against delirium because there is no evidence that this reduces the incidence of delirium.2
Nonpharmacologic Interventions to Prevent Delirium
Delirium is frequently associated with critical illness.2 Provision of adequate sleep and early mobilization are recommended to reduce the incidence of delirium.2 The nonpharmacologic strategies used to prevent agitation and delirium are similar to those used as adjuncts to minimize pain.2 These methods include back massage, music therapy, noise reduction, decreasing lights at night to promote sleep, clustering nursing care interventions to provide some uninterrupted rest, and speaking in a calm and gentle voice. Some pre-existing conditions increase the likelihood that a patient will experience delirium, including dementia, alcohol use disorder, and prior sedative/opiate dependence.
Alcohol Withdrawal Syndrome and Delirium Tremens
Critically ill patients who are alcohol dependent and were drinking prior to hospital admission are at risk of alcohol withdrawal syndrome (AWS).34 AWS is associated with an increased risk of delirium, hallucinations, seizures, increased need for mechanical ventilation, and death. When hyperactive agitated delirium is caused by alcohol withdrawal, it is termed delirium tremens, often verbally described as DTs.34 Following hospital admission, as the alcohol-dependent patient’s blood-alcohol concentration (BAC) falls, about 50% of patients will have AWS-related symptoms.35 Fewer than 5% will experience severe complications such as DTs or a seizure.34,35 Screening tools to identify alcohol dependence such as the Alcohol Use Disorders Identification Test (AUDIT) shown in Table 34-1 in the Trauma chapter are very helpful. Management of alcohol withdrawal involves close monitoring of AWS-related agitation, and administration of IV benzodiazepines, generally diazepam (Valium) or lorazepam (Ativan). Diazepam has the advantage of a longer half-life and high lipid solubility.34 Lipid-soluble medications quickly cross the blood-brain barrier and enter the central nervous system to rapidly produce a sedative effect.34,36 Benzodiazepines should be administered in response to increased signs of agitation associated with DTs, with dosage guided by a clinical protocol. This is described as an AWS symptom-triggered approach.34 The severity of alcohol withdrawal can be assessed with a scale such as the Clinical Institute Withdrawal Assessment of Alcohol Scale (revised) (CIWA-Ar).34,36 Multivitamins, including thiamine (Vitamin B1), are administered prophylactically to prevent additional neurologic sequelae.34–37 Delirium related to alcohol withdrawal is pharmacologically managed very differently than delirium from other causes. Long-acting benzodiazepines are the medications of choice in AWS.34 In contrast; benzodiazepines are contraindicated for treatment of delirium from non–alcohol-related causes.38
Collaborative Management
Collaborative management of anxiety, agitation, sedation, and delirium is a responsibility shared by all members of the health care team, as indicated by the clinical practice guidelines summarized in Box 10-3. Recognition of the problem is the first step toward a solution to establish a more effective standard of patient care in sedation, analgesia, and delirium management.
Summary
• Use a validated sedation instrument (RASS or SAS) and titrate sedation medications to achieve the lightest possible level of sedation to prevent immobility-associated problems and delirium.
• Benzodiazepines are not recommended for routine sedation of mechanically ventilated adult patients.
• Dexmedetomidine and propofol are recommended for sedation of mechanically ventilated adult patients.
• Daily interruption of sedation and a spontaneous breathing trial are recommended for patients who are mechanically ventilated.
• Delirium is a frequent complication of critical illness. Delirium can be hypoactive (withdrawn) or hyperactive (with agitation).
• Use a validated delirium assessment instrument (CAM-ICU or ICDSC) to identify delirium.
• Pharmacologic management of delirium includes treatment with haloperidol and sometimes atypical antipsychotics.
• Non-pharmacologic interventions to prevent delirium include provision of adequate sleep and early mobility.
• Delirium tremens is a complication of alcohol withdrawal syndrome.
• The benzodiazepines, diazepam (Valium) or lorazepam (Ativan) are used in the treatment of delirium tremens.