The Obstetric Patient
In 2010, the maternal mortality rate of the United States was 21 deaths per 100,000 live births, compared with 12 per 100,000 in 1990. This represents a total of about 840 women who died from maternal causes in 2010.1 Some of this increase observed over the past decades may have been caused by changes in the coding and classification of maternal deaths. An alarming trend in maternal mortality in the United States is race-specific pregnancy-related mortality rates. Analysis of rates has demonstrated that compared with white women, African American women have 2.7 times the risk of dying as a result of childbirth and that the maternal mortality rate among Hispanic women was about 0.8% less than that of white women.2 At the global level, the World Health Organization (WHO), The United Nations Children’s Fund, the United Nations Population Fund and the World Bank assessed trends in maternal mortality rate from the period 1990-2010 and revealed that the number of women dying of pregnancy- and childbirth-related complications has almost halved in 20 years. The WHO’s fifth Millennium Development Goal (MDG) aims to improve maternal health with a target of reducing maternal death by 75% in the period 1990-2015. The resulting findings were that the percentage reductions for 10 countries have already achieved the MDG by 2010, 11 countries are “on track” of achieving this goal, 50 countries are “making progress,” 14 countries have made “insufficient progress,” and 11 countries are characterized as having made “no progress” and are likely to miss the MDG target unless accelerated interventions are put in place.1
Risks to Fetal Development
Factors that influence embryonic and fetal development may be intrinsic or extrinsic in nature. Intrinsic factors such as chromosomal abnormalities and congenital anomalies account for 25% of all birth defects. Extrinsic factors, also known as teratogens, account for those remaining.3 A teratogen is any chemical, substance, or exposure that may cause any form of birth defects in a developing fetus. This would include medicines, radiation, and agents of infection. It is important to remember that the effects of teratogens depend on maternal and fetal genotypes, the stage of development when exposure occurs, and the dose and duration of the exposure of the agent.3 Exposure to ionizing radiation is usually not a concern until more than a cumulative 100 to 200 milligrays (mGy; 10 to 20 rads) have been exceeded, but some experts recommend caution in the first 25 weeks because of fetal organogenesis and central nervous system development.4–5 At doses less than 0.05 gray (Gy), no evidence of an increased risk of fetal anomalies, intellectual disability, growth restriction, or pregnancy loss from ionizing radiation is present. A small increased risk of childhood cancer, 1 in 2000 versus the 1 in 3000 background rate, may exist.4
Medication use in critically ill obstetric patients requires analysis of the risk–benefit ratio. It is important to consider the influence that drug exposure could have on the developing fetus, but often, the benefit may outweigh the potential fetal risk when all factors are considered.6 Also, fetal and newborn outcomes may be dependent on timely maternal stabilization on potential harmful medication. Critical ill obstetric patients may be reluctant to take medication during pregnancy because of the possibility or perceived possibility of the adverse effects on the fetus. The U.S. Food and Drug Administration (FDA) placed medications into risk categories regarding use during pregnancy along with potential effects on the growing fetus. Box 39-1 describes the FDA labeling with regard to a drug’s risk to a fetus.6–7 (As of February 2011, the FDA proposed major revisions to prescription drug labeling to more completely inform the use of medicines during pregnancy and breastfeeding. They are presently in the writing and clearance process.8)
Perinatal Infection
Group B Streptococcal Infection
During the 1970s, group B Streptococcus (GBS) was recognized as the leading infectious cause of neonatal sepsis and mortality and remains so today.9 The prevalence of neonatal infection is approximately 0.4 per 1000 live births, and approximately 1300 cases of neonatal streptococcal septicemia occur each year in the United States.10 Mortality is higher among preterm infants, with case fatality rates of approximately 20% and as high as 30% among those less than 33 weeks’ gestation, compared with 2% to 3% among full-term infants.11
Maternal colonization may be intermittent, transient, or chronic, and it is likely that nearly every woman is colonized by GBS at some time. Most women are asymptomatic, but in symptomatic women, GBS is responsible for considerable maternal morbidity from infections such as pyelonephritis, chorioamnionitis, postpartum endometrioses, sepsis, wound infections and, in rare instances, meninigitis.9
In the infant, GBS may result in unexpected intrapartum stillbirth.12 Early-onset neonatal infection results almost exclusively from vertical transmission from a colonized mother to her infant and is often characterized by signs of serious illness, including respiratory distress, apnea, and shock. Late-onset disease occurs 1 week or more after birth. These infants often develop meningitis. Long-term neurologic complications are common in survivors of both types of GBS.9
At the present time, the standard for diagnosis of GBS infection is bacteriologic culture using the Todd-Hewitt broth or selective blood agar. The specimen for culture should be obtained from the lower vagina, perineum, and anus using a simple cotton swab.13 Prevention of early onset neonatal GBS infection is based on the guidelines from the Centers for Disease Control and Prevention (CDC), which were published in 1996 and updated in 2010.14
Cytomegalovirus Infection
Cytomegalovirus (CMV) is a deoxyribonucleic acid (DNA) virus belonging to the herpes simplex virus (HSV) group. It causes both congenital and acquired disorders. The significance of this virus in pregnancy is related to its ability to be transmitted by asymptomatic women across the placenta to the fetus or by the cervical route during birth.15 Although the virus is usually innocuous in adults and children, it may be fatal to the fetus. The virus is passed between humans by close contact such as during kissing, breastfeeding, and sexual intercourse.15 The diagnosis of CMV infection can be confirmed by isolation of the virus in tissue culture, with the highest concentrations of virus typically in the urine, seminal fluid, saliva, and breast milk. About 50% to 80% of adult women in the United States have serologic evidence of past CMV infection. The overall risk of congenital infection is greatest when maternal infection occurs in the third trimester, but the probability of severe fetal injury is highest when maternal infection develops in the first trimester.16
Although 85% to 90% of infected fetuses will be asymptomatic at birth, the remaining 10% to 15% will have abnormalities of varying severity.16 Mortality rate among the symptomatic infants is 20% to 30%, and 90% of the these survivors have significant neurologic complications. The most common severe neonatal infections are hepatosplenomegaly, intracranial calcification, growth restriction, microcephaly, chorioretinitis, hearing loss, thrombocytopenia, hyperbilirubinemia, and intellectual disability. The virus may be identified in amniotic fluid by culture or polymerase chain reaction (PCR); however, mere identification of the virus does not necessarily delineate the severity of the fetal injury.17 Ultrasonographic findings may include fetal hydrops, growth restriction, hydramnios, cardiomegaly, and fetal ascites.
Currently, no effective therapies are available to manage this infection. A recent placebo-controlled, randomized, double-blind trail evaluated a CMV vaccine and showed that it has the potential to decrease cases of maternal and congenital CMV infection.18 Ideally, preventive measures should be employed to ensure that women do not contract CMV infection during pregnancy. One simple measure is encouraging women to use careful hand-washing techniques.
Toxoplasmosis
Toxoplasmosis is caused by the protozoan Toxoplasma gondii (T. gondii), with both farm animals, especially cattle, pigs, and sheep, and domestic cats playing an important role in the life cycle of the Toxoplasma organism. Cats are the usual host for the protozoan, and the infective oocytes are passed in feces and subsequently ingested by grazing farm animals. The organism disseminates throughout the animal’s body, ultimately forming cysts in brain tissue and muscle. Cats acquire the organism through ingestion of undercooked or uncooked meat, possibly infected rodents. Human infection occurs when infected, undercooked meat is ingested or when food has been contaminated by cat feces.19
In the United States, congenital toxoplasmosis is found to occur in 0.8 per 10,000 live births annually, and in Europe, it is estimated to be 10 cases per 10,000 live births.12 It is innocuous in most adults, resembling a minor viral illness, but it affects the fetus profoundly, creating long-term sequelae if the mother contracted the disease shortly before or during pregnancy.19
Maternal infection occurring in the first trimester typically results in more severe fetal damage and often ends in spontaneous abortion. The fetal infection occurring during the last month of pregnancy results in infants being born without any clinical signs of infections, although 50% will become symptomatic if left untreated.12,16 Severe neonatal disorders associated with congenital infection include convulsions, coma, microcephaly, and hydrocephalus, causing many infant to die soon after birth. Survivors often have visual, hearing, and intellectual impairment.20
The goal is to identify the woman at risk and to treat the disease promptly if diagnosed. Diagnosis is made by serologic testing, including the immunoglobulin M (IgM) and IgG fluorescent antibody test.20 PCR for T. gondii DNA in amniotic fluid is the best way to diagnose fetal infection, with ultrasonography revealing findings such as ascites, ventriculomegaly, microcephaly, and growth restriction.20 If fetal infection is suspected, pyrimethamine, sulfadiazine, or folinic acid should be given to the mother after the 18th week of pregnancy.12
Rubella
Rubella is one of the most teratogenic of all viruses. Although it presents as a mild illness in most children and adults, rubella infection in the fetus can have overwhelming consequences. Estimates suggest that in the United States up to 10% of women are susceptible to rubella.12
The period of greatest risk for the teratogenic effects of rubella on the fetus is during the first trimester, when maternal infection results in up to 80% of maternal–fetal transmission.12 Defects are rare when infection develops after 20 weeks’ gestation.21 Infants born with congenital rubella syndrome are infectious and should be isolated at birth. Prognosis of these infants is poor, with 10% to 20% of the affected infants dying during the first year of life. Rubella syndrome manifests in the newborn most commonly as congenital cataract, sensorineural deafness, and congenial heart defects. Mental retardation and cerebral palsy may become evident in infancy. Diagnosis is made when these conditions and an elevated rubella IgM antibody titer are present at birth.21
The best therapy for rubella is prevention. Vaccination with live, attenuated vaccines should be given to all women of childbearing age who are susceptible and prior to pregnancy. Although no fetal infection has resulted from immunization of a pregnant woman, pregnancy should be avoided for 1 month after immunization. Testing for immunity involves the serology test of hemagglutination inhibition (HAI); the presence of a 1 : 18 titer or greater is evidence of immunity, and less than 1 : 8 indicates susceptibility to rubella. If pregnant when diagnosed as being “nonimmune,” the immunization should be administered in the postpartum period.22
Herpes Simplex Virus
HSV is a DNA virus with two principle strains: 1) HSV-I and 2) HSV-II. HSV is estimated to infect 1 in 6 people between the ages of 14 and 49 years (16.2%) in the United States.23 The incidence of neonatal herpes infection is 1 per 3500 live births.22 A primary herpes simplex infection may increase the risk of spontaneous abortion when infection occurs in the first trimester, whereas preterm labor, intrauterine growth restriction (IUGR), and neonatal infection are greater risks if the infection occurs late in the second trimester or early in the third trimester. If a primary lesion develops close to the time of labor, the risk of transmission is 30% to 60% for a vaginal birth.21 Exposure of the newborn to a recurrent lesion drops the risk of transmission to between 2% and 5%.24 The most likely mechanism of infection is exposure of the neonate to the viruses in the lower genital tract during the process of vaginal delivery; therefore, in a woman with either a primary or secondary outbreak of genital herpes during labor, the preferred method of delivery is by cesarean section. An estimated number of 1500 to 2000 newborns contact herpes each year, with 85% resulting from viral transmission near the time of birth from asymptomatic women.21
Neonatal HSV infection may take the form of disseminated mucocutaneous eruption, central nervous system (CNS) infection, or disseminated visceral infection. Approximately 30% of infants with disseminated disease die despite antiviral therapy, and 40% of the survivors have severe neurologic damage. Many times, the infected infant is asymptomatic at birth, with symptoms occurring any time after birth and up to 4 weeks of age.16 These symptoms include jaundice, fever, seizures, vesicular skin lesion, and poor feeding. CNS symptoms generally occur during the second or third week. All infants who have neonatal herpes should be evaluated and treated with acyclovir.25
Any woman who is planning a pregnancy and who might have been exposed to the herpesvirus should have type specific serology testing to determine her risk of acquiring HSV. If she has HSV and has experienced recurrent outbreaks during pregnancy, antiviral therapy (with acyclovir, famciclovir, and valacyclovir) is recommended after 36 weeks’ gestation to reduce the need for a cesarean section.24 Currently, no evidence of any adverse fetal effects exists in relation to exposure to these antiviral drugs used in HSV treatment during any trimester.26
Parvovirus is caused by the DNA organism, the B19 parvovirus. It causes erythema infectiosum, or “fifth disease,” in children and a mild disease in adults that produces a characteristic “slapped cheek” rash but a potential extremely serious fetal outcome. Although the risk of fetal morbidity is low, fetal infection is associated with spontaneous abortion, fetal hydrops, and stillbirth. Severe effects occur most frequently with maternal infection before 20 weeks’ gestation.27 The major concern for the fetus is nonimmune hydrops and fetal anemia, which, if left untreated, may result in death. If hydrops and fetal anemia are diagnosed, intrauterine fetal transfusion may reduce the mortality from about 50% to 18%.27 Fetal death may occur at 4 to 12 weeks after infection; therefore, fetal surveillance should be maintained from 8 to 12 weeks. In fetuses who survive the infection, long-term development appears to be normal. Nonimmune women with school-age children are more likely to acquire parvovirus, and serologic evaluation should be performed if the pregnant woman has been exposed to a child diagnosed with fifth disease.28
Prematurity
The condition or illness in a critically ill mother may justify an early termination of pregnancy to prevent serious complications in or death of the patient. Depending on the duration of gestation, a severely premature infant may result. Technologic advances, improvements in maternal–fetal diagnostics, and aggressive neonatal interventions have improved the survival of extremely low-birth-weight infants. Research has placed minimal viability parameters between 23 and 24 weeks’ gestation and fetal weight between 500 and 1000 g (0.5 and 1 kg).22 Critical care clinicians may encounter situations in which extrauterine viability, fetal outcomes, and maternal stability are uncertain. Clinical decisions must be made in light of the maternal–fetal risk–benefit ratio. Personal, cultural, spiritual, and social beliefs regarding viability may affect the clinical decision-making process. Parental and family beliefs and desires may conflict with those of the health care team. When confronting the dilemma of viability, the parameters of gestational age, fetal weight, parental desires, and maternal–fetal mortality must be considered.
Physiologic Alterations in Pregnancy
During pregnancy, the woman’s body undergoes profound physiologic changes. These changes are necessary to maintain the pregnancy and to allow for fetal growth and development. The changes are so dramatic that they would probably be considered pathologic in the nonpregnant woman. Adaptations occur in nearly every organ system, beginning during the first week of gestation and continuing until up to 6 weeks after delivery. The only system in which no documented characteristic changes occur is the nervous system. Boxes 39-2 and 39-3 and Tables 39-1 to 39-4 summarize the various systems and alterations during pregnancy.12,22,23,29 Understanding the physiologic adaptations is important to the management of the critically ill pregnant woman. More detailed information can be found in textbooks dedicated to obstetric issues.
TABLE 39-1
POSITIONAL CARDIAC OUTPUT CHANGES IN PREGNANCY
| MATERNAL POSITION | CARDIAC OUTPUT (L/min) |
| Knee-chest | 6.9 (± 2.1) |
| Right lateral | 6.8 (± 1.3) |
| Left lateral | 6.6 (± 1.4) |
| Sitting | 6.2 (± 0.0) |
| Supine | 6.0 (± 0.4) |
| Standing | 5.4 (± 2.0) |
TABLE 39-2
HEMODYNAMIC CHANGES ASSOCIATED WITH TERM PREGNANCY
| PARAMETER | PREGNANCY NORMAL VALUE | CHANGE |
| Mean arterial pressure (mm Hg) | 90 ± 6 | No significant change |
| Central venous pressure (mm Hg) | 8 ± 2 | No significant change |
| Pulmonary artery occlusion pressure (mm Hg) | 4 ± 3 | No significant change |
| Heart rate (beats/min) | 83 ± 10 | Increase 17% |
| Cardiac output (L/min) | 6.2 ± 1.0 | Increase 43% |
| Systemic vascular resistance (dyn · sec · cm-5) | 1210 ± 266 | Decrease 21% |
| Pulmonary vascular resistance (dyn · sec · cm-5) | 78 ± 22 | Decrease 34% |
| Serum colloid oncotic pressure (mm Hg) | 18 ± 1.5 | Decrease 14% |
| Left ventricular stroke work index (g-m/m2) | 48 ± 6 | No significant change |
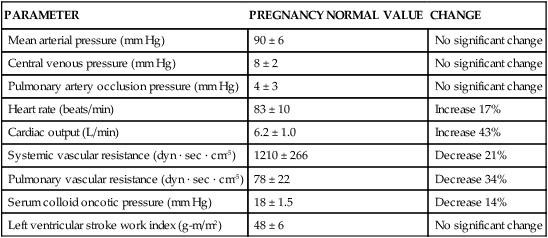
TABLE 39-3
PHYSIOLOGIC ADAPTATION OF THE GASTROINTESTINAL SYSTEM DURING PREGNANCY
| GASTROINTESTINAL FUNCTION CHANGE | PRESUMED CAUSE |
| Heartburn | Progesterone and estrogen; size of gravid uterus impeding gastroesophageal junction |
| Bleeding gums | Hyperemia |
| Constipation | Progesterone, causing decreased motility and intestinal secretion, enhanced water absorption |
| Hemorrhoids | Hyperemia, pelvic congestion, obstruction of venous return |
| “Morning sickness” or nausea | Increased levels of estrogen and human chorionic gonadotropin (hCG) |
| Risk for aspiration | Displacement of lower esophageal sphincter and reduced gastric motility |
| Gallstones | Decreased gallbladder activity, impaired emptying |
TABLE 39-4
RENAL PHYSIOLOGIC CHANGES IN PREGNANCY
| PARAMETER | PERCENT CHANGE | NORMAL LEVELS IN PREGNANCY |
| Renal blood flow | Increase 25%-50% | 1250-1500 mL/min |
| Glomerular filtration rate | Increase 50% | 140-170 mL/min |
| Renal plasma flow | Increase 35% | 700-900 mL/min |
Physiologic Changes during Labor and Delivery
Labor and delivery bring additional stresses to the maternal system, especially as a result of the pain and anxiety associated with labor. The most dramatic requirements are for the cardiopulmonary system. During labor, uterine contractions produce cyclic auto-transfusions of approximately 300 to 500 milliliters (mL). Immediately after birth, cardiac output peaks, with an 80% increase over prelabor values.12 This occurs because of the contracted uterus shunting its blood, sudden removal of fetal supply demands, and resolution of vena cava compression. Table 39-5 summarizes the cardiac output changes in labor and delivery.12,23,29
TABLE 39-5
CARDIAC OUTPUT CHANGES IN LABOR AND DELIVERY
| STAGE OF LABOR OR DELIVERY | CHANGE IN CARDIAC OUTPUT |
| Early first stage of labor | ↑ 15% plus additional 15% with each contraction |
| Last first state of labor | ↑ 30% plus additional 15% with each contraction |
| Second stage of labor | ↑ 45% plus additional 15% with each contraction |
| First 5 minutes postpartum | ↑ 80% secondary to auto-transfusion |
| First hour postpartum | ↑ 25% |
Normal blood loss from a vaginal birth is typically 500 mL; blood loss from cesarean deliveries usually is 1000 mL. Clinical estimates tend to underestimate actual blood loss by up to 50%.30 The cardiopulmonary changes occurring during labor and delivery are of significant concern because they occur over a short period and because maternal decompensation may occur.
Cardiac Disorders in Pregnancy
Cardiac disease ranks fourth after pregnancy-induced hypertension, hemorrhage, and infection as a cause of maternal mortality.31 Several factors have to be taken into consideration in the care of the pregnant woman with cardiac disease. Cardiac disease during pregnancy may be a result of pre-existing conditions such as congenital diseases, or it may be a result of primary cardiac disease arising prior to or during pregnancy. The woman with heart disease has decreased cardiac reserve, making it more difficulty for her heart to accommodate the higher workload of pregnancy. Thus, prepregnancy counseling is highly recommended for women with known cardiac disease. Counseling would include determining the New York Heart Association (NYHA) functional class, which is especially useful for pregnant women with structural heart disease, and determining the maternal and fetal risks associated with the pregnancy (Box 39-4).29,32 Major fetal risks include fetal development of congenital heart disease, prematurity, IUGR, and intrauterine fetal demise (IUFD).
The method and timing of delivery are decided primarily by obstetric considerations, taking into account the woman’s ability to tolerate the labor process and associated physiologic changes. Selection of anesthesia techniques involves weighing the risks and benefits of the procedures. As a general rule, most patients tolerate epidural anesthesia more favorably than they tolerate general anesthesia (see Chapter 42).
Congenital Cardiac Disorders
Congenital heart defects have become a more common finding in pregnant women, as improved surgical techniques have enabled females born with heart defects to live to childbearing age. When surgical repair can be accomplished with no remaining evidence of organic heart disease, pregnancy may be undertaken with confidence. When congenital heart disease is associated with cyanosis, whether the defect was originally uncorrected or the correction failed to relieve the cyanosis, the woman should be counseled about risks to both herself and to her fetus. Of equal concern in pregnant women with congenital heart disease is the risk of fetal congenital cardiac anomalies, which is approximately 5%. During the antepartum period, serial ultrasonography should be performed to access the fetus for appropriate interval growth.33
Atrial Septal Defect
Atrial septal defect (ASD) is the most common congenital anomaly seen during pregnancy, and most women with ASD tolerate pregnancy, labor, and delivery without complications. The decrease in systemic vascular resistance (SVR) lessens the degree of left-to-right shunt, whereas the hypervolemic state may slightly worsen the shunt and increase right ventricular workload. The most common complications seen with ASD are dysrhythmias, heart failure, and thromboembolism.34
Ventricular Septal Defect
The outcome for the pregnant woman with ventricular septal defect (VSD) and resultant left-to-right shunt depends on the size of the defect, with larger defects producing a less favorable prognosis. The majority of VSDs are diagnosed and repaired before the women reaches childbearing age. In the absence of significant symptoms and pulmonary hypertension, pregnancy is typically well tolerated. Therapy is aimed at early recognition and treatments of signs of heart failure.35 Common complications include tachycardia, heart failure, and pulmonary hypertension.
Patent Ductus Arteriosus
During pregnancy patent ductus arteriosus (PDA) is an unusual finding, as it is generally detected and closed during the newborn period. Patients who present with a PDA during pregnancy, usually tolerate the hemodynamic stress of labor and delivery without difficulty. Precautions against the risks of infective endocarditis and thromboembolism may be taken. Severe PDA may produce large left-to-right shunts, causing acute heart failure or pulmonary hypertension that is associated with significant maternal mortality.30
Tetralogy of Fallot
Tetralogy of Fallot (ToF) is the most common cyanotic heart defect in individuals who survive to adulthood.36 The four primary lesions associated with ToF include: 1) VSD, 2) overriding aorta, 3) right ventricular hypertrophy, and 4) pulmonary stenosis. Women with corrected ToF generally can tolerate pregnancy well. Although rare, if the congenital anomalies are not corrected, the maternal mortality rate and fetal complications increase significantly.36 Cardiopulmonary function must be maximized by measures that include treatment of dysrhythmias and use of prophylaxis for endocarditis. Considerations during labor and delivery include maintenance of adequate preload and blood pressure.
Coarctation of the Aorta
Coarctation of the aorta may occur in isolation or, most often, in combination with valvular or septal anomalies. Patients with uncomplicated coarctation of the aorta who are relatively asymptomatic (NYHA class I or II) have demonstrated good prognosis and minimal risk of complications or death.12 Assessment of the aortic gradient may also be useful in predicting pregnancy outcome in patients with coarctation of the aorta. In general, aortic gradients across the site of coarctation that are less than 20 mm Hg are associated with good maternal and fetal outcomes.34 Intrapartum management focuses on the prevention of hypertension to avoid aortic wall stress. Careful management of fluid balance and left ventricular function must occur to prevent CHF and to promote adequate perfusion.
Eisenmenger Syndrome
Eisenmenger syndrome is not a single congenital defect but a complication that may be the result of other cardiac lesions that cause left-to-right shunting. This syndrome is more likely to occur with VDS or ASD because of the high pressure and high flow associated with these defects. This shunting may result in progressive pulmonary hypertension, leading to shunt reversal or bidirectional shunting.37 Regardless of the cause, the risk of sudden death because of pulmonary hypertension in pregnancy is 40%, which has remained unchanged for the past 50 years. Avoidance or termination of pregnancy is commonly recommended. If pregnancy is continued, therapeutic management is directed at avoidance of pulmonary vasoconstrictors, thromboembolism, and hypotension; maintenance of adequate preload and oxygenation; fetal surveillance; and reduction of stress at the time of delivery.37
Acquired Cardiac Disorders
Mitral Stenosis
The presence of a stenotic mitral valve is the most common rheumatic valve disease of pregnancy. The primary concern with mitral stenosis during pregnancy is the impedance to ventricular filling, which produces a relatively fixed cardiac output. Additional risks include thromboembolism, heart failure, and arrhythmias, especially atrial fibrillation. Cardiac output in the face of mitral stenosis is determined by two primary factors: 1) length of diastolic filling and 2) left ventricular preload. The length of diastolic filling may be negatively affected because of the increased pulse rate during a normal pregnancy. Discomfort or anxiety associated with labor may produce a tachycardic state, which may drastically impede ventricular filling, producing an even lower cardiac output, with resultant heart failure and pulmonary edema. As pregnancy is a hypercoagulable state, thrombi may rapidly form, and fibrillation may dislodge the thrombi and cause arterial embolism. Thus, prophylactic anticoagulation should be considered in this subset of women.38,39
Maintenance and management of left ventricular preload is the second important consideration in mitral stenosis. Patients may require high-normal or slightly elevated left ventricular filling pressures to maintain adequate flow across the stenotic mitral valve. It is especially important to assess the patient’s fluid status. Caution must be exercised when employing therapies that decrease preload, for example, diuresis or epidural anesthesia.38 Invasive hemodynamic monitoring may be indicated to carefully tailor therapy.
In the immediate postpartum period, careful monitoring is essential because of the massive fluid shifts and large increases in cardiac output. Authorities recommend that optimal predelivery pulmonary artery occlusion pressures be maintained at 14 mm Hg or less to accommodate the increase in occlusion pressure of up to 16 mm Hg that may be associated in the immediate postpartum period.38
Aortic Stenosis
Aortic stenosis often is accompanied by other valvular disease, especially disease affecting the mitral valve. The hallmark of aortic stenosis is decreased left ventricular ejection.12 Mild aortic stenosis is usually well tolerated during pregnancy because of the natural hypervolemic state. Significant aortic stenosis can produce left ventricular hypertrophy and dilation. Thromboembolic prophylaxis is recommended. Critical to successful management is maintenance of cardiac output through prevention of hypovolemia, especially at the time of delivery. The maintenance of adequate cardiac output and oxygen transport is vital in the clinical management of pregnant women with aortic stenosis. Any factor that diminishes venous return or produces hypotension worsens the effects of aortic stenosis and significantly reduces cardiac output. Heart failure occurs in less than 10% of patients with severe aortic stenosis and arrhythmias in 3% to 25%.40 Mortality is now rare if careful management is provided.
Marfan Syndrome
Marfan syndrome is an autosomal dominant disorder of connective tissue, in which serious cardiovascular involvement, usually dissection or rupture of the aorta, may occur. Prognosis is based on aortic root and wall involvement, with most authorities citing 40 millimeters (mm) as maximal root diameter, after which significant increases in mortality occur.39,41 Prevention of tachydysrhythmias and hypertension is recommended, along with endocarditis prophylaxis. Beta-blockade therapy may be initiated for cardiac rate control and to decrease pressure on the weakened aortic wall. Goals of management include maintenance of cardiac output to meet physiologic needs without producing undue stress on the aortic wall, use of regional anesthesia, and avoidance of Valsalva maneuver by shortening the second stage of labor. Careful blood pressure maintenance is essential. Differential diagnosis of chest and back pain is essential, along with recognition of other signs of aortic dissection. Because of its inheritance pattern there is a 50% risk that the disease will be transmitted to the infant.41
Peripartum Cardiomyopathy
Peripartum cardiomyopathy (PPCM) is a type left ventricle dysfunction of unknown origin that occurs in the last month of pregnancy and up to 5 months after delivery in women with no previous history of heart disease. Thus, PPCM is a diagnosis of exclusion. Occurring in 1 in 3000 to 4000 live births, it is a relatively rare but serious condition. Early report suggested a mortality rate of nearly 50%, but more recent studies indicate a 0% to 5% rate in the United States.42,43 Controversy regarding exact causes of peripartum cardiomyopathy continues, as symptoms that are often attributable to viral and immune sources, chronic hypertension, mitral stenosis, obesity, and myocarditis have all been proposed.43 The definitive diagnosis of PPCM depends on the echocardiographic identification of new-onset heart failure during a limited period toward the end of pregnancy or in the months following delivery.40,43,44
Symptoms are identical to those of classic heart failure, but treatment depends on the pregnancy status of the patient. Women who present with PPCM during pregnancy require joint cardiac and obstetric care, but as soon as the baby is born and the patient is hemodynamically stable, standard therapy for heart failure may be applied.40 This would include treatment of diuretics, digoxin, beta-blockade, and afterload reduction. Angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and renin inhibitors are contraindicated during pregnancy because of fetotoxicity but may be used once the infant is born.40,44 Once the bleeding has been stopped after delivery, anticoagulation is commonly employed to prevent thromboembolism and the formation of left ventricular thrombus, which is associated with a worse prognosis.44 The clinical course of peripartum cardiomyopathy is quite variable, but 50% to 60% of patients show clinical recovery within the first 6 months after delivery.45 The prognosis of PPCM is positively related to the recovery of ventricular function. Medical therapy as outlined in the American College of Cardiology Foundation and American Heart Association (ACCF/AHA) guidelines should be continued when a woman does not recover function.44 Subsequent pregnancy carries a recurrence risk for PPCM of 30% to 50%.40
Ischemic Cardiac Disease
Acute Myocardial Infarction
Although acute myocardial infarction (AMI) during pregnancy is rare, pregnancy has been shown to increase the risk of AMI three- to fourfold.46 With the continuing trend of childbearing at older ages and advances in reproductive technology enabling many older women to conceive, it may be expected that the occurrence of AMI will increase. Mortality rates from an AMI during pregnancy range from 37% to 50%, depending on the timing of the myocardial event.47 However, the majority of AMI in pregnancy is not related to atherosclerosis; about 50% of ischemic heart disease in pregnancy is linked to idiopathic dissections of the coronary artieries.40 Other reported conditions that contribute to AMI risk are eclampsia, thrombophilia, postpartum infections, and severe postpartum hemorrhage.40 Increased mortality is associated with many factors, including occurrence of the event during the third trimester, multigravidas older than 33 years, cesarean section, and delivery occurring within 2 weeks of infarction.47
Clinical diagnostics are similar to those for standard AMI detection, although diagnosis must be made with consideration of the normal physiologic cardiovascular changes. Coronary angiography with the possibility of coronary intervention is preferred to thrombolysis, as it will also diagnose coronary artery dissection.47
Treatment of AMI during pregnancy is focused on restoring myocardial blood flow and balancing myocardial oxygen supply and demand.47 Management may include percutaneous coronary intervention; nitrate therapy, beta-blockade therapy, or both; cardiac monitoring; oxygen therapy; management of pain and anxiety; and afterload reduction. Again ACE inhibitors, ARBs, and renin inhibitors are not indicated during pregnancy.40 Coronary angiograms, angioplasty, bypass surgery, and intra-aortic balloon pumps all have been successfully used during pregnancy. Special consideration is given to the maternal physiologic demands required during the labor and delivery processes. Operative delivery interventions, such as forceps or cesarean section, may be necessary (see “Myocardial Infarction” in Chapter 15).
Prior Myocardial Infarction
The outcome in the pregnant woman with prior myocardial damage depends on many factors. The length of time between the myocardial event and delivery is especially important.47 Increased myocardial oxygen demands during pregnancy must be considered, and therapy is usually supportive in nature. Careful attention to preload is essential to prevent burdening the heart and producing congestive failure (see “Heart Failure” in Chapter 15).
Cardiac Arrest in Pregnancy
The occurrence of cardiopulmonary arrest during pregnancy is uncommon. Successful management of the pregnant patient in cardiac arrest requires integration of physiologic changes present during pregnancy and adaptations for those from standard resuscitative guidelines. Fetal outcomes are directly related to the mother’s condition and well-being. The interrelationship between fetal and maternal well-being may present unique ethical dilemmas for health care providers and family members. Causes of obstetric and nonobstetric cardiopulmonary arrest during pregnancy are summarized in Table 39-6.
TABLE 39-6
OBSTETRIC AND NONOBSTETRIC CAUSES OF CARDIAC ARREST IN PREGNANCY
| OBSTETRIC CAUSES | NONOBSTETRIC CAUSES |
| Hemorrhage (17%) | Pulmonary embolism (19%) |
| Pregnancy-induced hypertension (16%) | Infection or sepsis (13%) |
| Idiopathic peripartum cardiomyopathy (8%) | Stroke (5%) |
| Anesthetic complications (2%) | Myocardial infarction |
| Amniotic fluid embolism | Trauma |
From Campbell T, Sanson T. Cardiac arrest and pregnancy. J Emerg Trauma Shock. 2009;2(1):34.
Basic Cardiac Life Support.
The AHA recommendations include only minor deviations from usual life support procedures.48 The facilitation of venous return is critically important. This is accomplished by performing chest compressions slightly above the center of the sternum and lateral displacement of the uterus through manual manipulation or through the use of a wedge under the woman’s hip, as this will improve venous return by decreasing compression of the vena cava by the gravid uterus.48 Physiologic adaptations in pregnancy place the woman at greater risk for complications from cardiopulmonary resuscitation (CPR) such as fractured ribs and sternum, hemothorax, hemopericardium, and internal organ damage. Specific organs of concern include the uterus, spleen, and liver.
Advanced Cardiac Life Support.
The AHA recommends that the standard resuscitation algorithm be followed for advanced cardiac life support (ACLS), with a few modifications to compensate for the altered anatomy and physiology of pregnancy.49 The airway should be secured early with effective preoxygenation, and cricoid pressure should be applied both to aid in intubation and to prevent aspiration. As pregnancy progresses, edema of the oropharynx increases, thus necessitating the use of a smaller endotracheal tube than in a nonpregnant woman.49 Careful attention to confirmation of tube placement and oxygenation is important because of the enhanced oxygen demands during pregnancy.
Standard ACLS recommendations should be followed for administration of resuscitation medications. Use of lower extremity sites or the femoral vein for venous access should be avoided because of the potential of the gravid uterus to impede venous return.49 Epinephrine may decrease uteroplacental perfusion because of its vasoconstrictive nature; however, the benefits outweigh the risks of administration. Lidocaine crosses the placenta but in therapeutic levels does not have adverse fetal or uteroplacental effects. If maternal toxicity occurs, fetal cardiac and CNS depression may occur. No contraindications exist for use of atropine in pregnancy. Administration of sodium bicarbonate is to be undertaken cautiously. Maternal acidosis increases uteroplacental adrenergic reactivity and must be avoided, although maternal alkalosis may impair oxygen exchange to the fetus. Electrical therapies such as defibrillation, cardioversion, and pacing are not contraindicated in pregnancy, but all fetal monitoring devices should be removed because a theoretical risk of the scalp electrode arcing and electrocuting the infant does exist.
Evaluation of fetal tolerance of the mother’s condition is essential during cardiopulmonary arrest. Fetal hypoxia may develop because of decreased uteroplacental perfusion. Fetal gestational age is a prime consideration when determining course of action. Before 24 weeks’ gestation, resuscitative efforts are focused primarily on maternal outcome. After the 24th week of gestation, evaluation includes maternal and fetal responses to resuscitative efforts. Emergent cesarean section may be undertaken for fetal distress or to improve maternal status, although consideration also must be given to the stress that cesarean section produces. In late pregnancy, survival of the infant is directly proportional to the time interval between death of the mother and delivery of the infant. Infant viability is best if delivery occurs within 5 minutes of cardiac arrest.47, 49
Hypertensive Disease
Hypertensive disease is present in up to 5% of pregnant women.50 Hypertension is defined as systolic blood pressure of 140 mm Hg or greater, or diastolic blood pressure of 90 mm Hg or greater, or both.50 It is the second leading cause of death in childbearing women, and it contributes to high rates of newborn morbidity and mortality.12,51,52 Maternal complications include pathologic compromise of the cardiovascular, pulmonary, renal, neurologic, and hepatic systems (Table 39-7).52–54 Understanding of hypertensive disease in pregnancy is based on: 1) a classification according to manifestations and time of onset in relation to gestation; 2) the fact that pregnancy could induce hypertension in women without a history of high blood pressure; and 3) the fact that elevated blood pressure in pregnancy could occur without the presence of generalized edema or proteinuria (transient hypertension).53 Proper diagnosis of hypertensive complications is critical and requires in-depth knowledge of disease pathophysiology to prevent or decrease the risk of maternal or fetal compromise.
TABLE 39-7
COMPLICATIONS OF HYPERTENSIVE DISEASE IN PREGNANCY
| BODY SYSTEM | COMPLICATION |
| Cardiovascular | Dysrhythmias, acute heart failure, severe hypertension |
| Pulmonary | Pulmonary edema, acute airway obstruction |
| Renal | Oliguria, renal failure, acute kidney injury |
| Neurologic | Cerebral edema, eclampsia, cerebral hemorrhage, coma |
| Hepatic | Necrosis, rupture, periportal and subcapsular hemorrhage |
| Hematologic | Disseminated intravascular coagulation (DIC), hemolysis thrombocytopenia |
Classification of Hypertension
The National Institutes of Health Working Group on High Blood Pressure has endorsed the classification and terminology of the American Congress of Obstetricians and Gynecologists (ACOG; 2012) for hypertensive disease in pregnancy.50,52 These definitions have proved useful in establishing consistent guidelines for pregnancy management:55
I Chronic hypertension is hypertension before conception or diagnosed before 20 weeks’ gestation.
II Preeclampsia and eclampsia constitute a systemic syndrome of hypertensive disease, with proteinuria diagnosed after 20 weeks’ gestation. Eclampsia indicates the additional presence of convulsions.
III Preeclampsia superimposed on chronic hypertension may occur before 20 weeks’ gestation or have a sudden onset.
IV Gestational hypertension is hypertension without proteinuria after 20 weeks’ gestation.
Preeclampsia and Eclampsia
Preeclampsia is the most common hypertensive disorder of pregnancy. It is estimated that 50,000 women die from preeclampsia each year worldwide. Among women with chronic hypertension, 22% to 25% will develop this complication.56,57 Preeclampsia is a syndrome that affects both mother and fetus. It is clinically defined as an increase in blood pressure after 20 weeks’ gestation, accompanied by proteinuria in a previously normotensive woman.57,58 Eclampsia is the occurrence of a seizure in a woman with preeclampsia who has no other cause for seizure.
Preeclampsia has been called a “disease of theories” because true mechanisms behind the pathogenesis remain unclear. It is a multisystem disorder unique to humans and therefore difficult to study in laboratory animals, which creates an obstacle to discovering its exact etiology.58
One of the key features of preeclampsia involves the failure of uterine spiral arteries to transform from thick-walled muscular vessels to saclike flaccid vessels, exaggerated inflammatory response, and inappropriate endothelial-cell activation.59 Characteristics of preeclampsia include widespread arteriolar vasospasms resulting in decreased perfusion to virtually all organs, including the placenta; a decrease in plasma volume; activation of coagulation cascade; and alteration in glomerular capillary endothelium. These generalized cyclic vasospasms lead to tissue ischemia and eventually end-organ dysfunction (Fig. 39-1).54
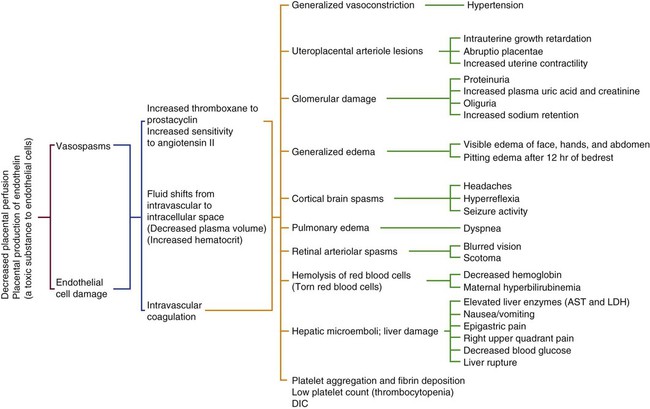
AST, Aspartate aminotransferase (SGOT); DIC, disseminated intravascular coagulation; LDH, lactate dehydrogenase. (From Gilbert E. Manual of High Risk Pregnancy and Delivery. 5th ed. St. Louis: Mosby; 2011.)
One of the more common problems related to preeclampsia is pulmonary edema caused by increased capillary permeability. Thrombocytopenia complicates severe preeclampsia in about 7% to 11% of women.59,60 Abruptio placentae and the release of procoagulants such as thromboplastin may result in acute disseminated intravascular coagulation (DIC). Women who develop preeclampsia are more sensitive to pressor agents, and this response has been linked to the ratio between prostaglandins, prostacyclin, and thromboxane. Prostacyclin is decreased in preeclampsia, allowing the potent vasoconstrictor and platelet-aggregating effects of thromboxane to dominate. No effective intervention seems to be available, although low-dose aspirin may lead to modest risk reduction of the disease.59
New research suggests pathogenesis related to imbalance between circulating antiangiogenesis-related factors.61,62 These antiangiogenic factors produce systemic endothelial dysfunction, resulting in hypertension, proteinuria, and other systemic expressions of preeclampsia. The molecular basis for placental misuse of these pathogenic factors remains unknown, and the role of angiogenic proteins in early placental vascular development and trophoblast invasion is just beginning to be explored. Widespread systemic vascular dysfunction and microangiopathy is demonstrated in the mother but not in the fetus.61,62 With preeclampsia, decreased production of nitric oxide, which is a potent vasodilator and important regulator of maternal blood pressure, also occurs.
Hyperhomocysteinemia may play role in the etiology or pathophysiology of preeclampsia.63 Mild elevations of homocysteine have been found in women with normal blood pressures who go on to develop preeclampsia. Once preeclampsia is established, homocysteine levels are considerably increased. It is unclear whether the increased levels of homocysteine caused the disease or if they reflect the metabolic alterations resulting from preeclampsia. Deficiencies of vitamin B6, vitamin B12, or folic acid may cause a rise in homocysteine. In one study in which approximately 4000 women received folic acid supplements, the results demonstrated a decrease in plasma homocysteine and a reduced risk of preeclampsia.63
Intracerebral hemorrhage is a rare complication, but it is the most common cause of death in women with severe preeclampsia and eclampsia, being fatal in 50% to 60% of the cases. Infants of women with preeclampsia tend to be small for gestational age because of IUGR. In addition, the maternal condition may warrant early termination of pregnancy, which leads to a severely premature infant. Placental abruption secondary to hypertension may result in fetal hypoxia or death.57,58
The treatment goals of severe preeclampsia are to prevent seizures, decrease arterial spasms, and effect prompt delivery of the fetus. Magnesium sulfate (MgSO4) is the standard treatment for the prevention and control of seizures in women with preeclampsia or eclampsia. Serum magnesium levels of 4 to 7 milliequivalent per liter (mEq/L) are thought to be therapeutic for prevention of seizure activity. A loading dose of 4 to 6 grams (g) is given by infusion pump over 15 to 20 minutes, followed by a maintenance infusion of 2 to 3 gram per hour (g/hr).12
Control of eclamptic seizures is accomplished through administration of 4 to 6 g of intravenous MgSO4 over 5 to 10 minutes. This bolus is followed by a continuous infusion of 2 to 3 g/hr. If a patient has a recurrent seizure, another bolus of 2 to 4 g may be given over 3 to 5 minutes. Sodium amobarbitol I, benzodiazepines, or phenytoin may be used for treating seizures that are not responsive to MgSO4.51,64 The use of multiple agents to decrease eclamptic seizures should be avoided, unless necessary.
Severe hypertension must be addressed after magnesium infusions. Antihypertensive agents need to be used to keep diastolic blood pressure between 90 and 100 mm Hg. The main drugs used to achieve this are hydralazine hydrochloride or labetalol.51,64 Diuretics are used only in the setting of pulmonary edema. The placenta plays a central role in the development of the disease, for which the only known cure is delivery of the fetus and removal of the placenta.
Hemolysis, Elevated Liver Enzymes, and Low Platelet Syndrome
Hemolysis, elevated liver enzymes, and low platelet (HELLP) syndrome affects 2% to 20% of patients with severe preeclampsia or eclampsia.56,65 Maternal mortality ranges from 3.5% to 24%, whereas perinatal mortality ranges from 10% to 60%.53 Approximately 10% to 15% of pregnant patients with HELLP syndrome do not have elevated proteinuria of hypertensive disease.58,66,67 The clinical manifestations of HELLP syndrome, which include nausea, vomiting, malaise, flulike symptoms, and epigastric pain, may suggest a multitude of other clinical diagnoses. Misdiagnosis is common and may result in a delay of correct treatment. HELLP syndrome may be confused with acute renal disease, gastroenteritis, hepatitis, gallbladder disease, pyelonephritis, or thrombotic thrombocytopenic purpura.23 Any pregnant woman demonstrating clinical manifestations and showing hemolysis, elevated liver enzymes, and low platelets must be diagnosed with HELLP syndrome. Complications of HELLP syndrome include abruptio placentae, liver hematoma, DIC, pulmonary edema, liver rupture, and acute kidney injury.12,58
Disseminated Intravascular Coagulation
DIC is not a separate clinical entity; rather, it is an effect of other disease processes. The obstetric causes of DIC include abruptio placentae, preeclampsia or eclampsia, dead fetus syndrome, septic abortion, and amniotic fluid embolus. In DIC, fibrinogen levels and platelet counts are usually decreased, whereas prothrombin time (PT) and partial thromboplastin time (PTT) are normal to prolonged.68 The pathophysiologic mechanisms of DIC are summarized in Table 39-8.12,53 Primary treatment goals include identification of the underlying disorder, removal of the trigger or initiating event, and volume replacement, including blood component therapy. Secondary treatment may include anticoagulation therapy. Heparin therapy remains controversial.
TABLE 39-8
CONDITIONS ASSOCIATED WITH DISSEMINATED INTRAVASCULAR COAGULATION
| OBSTETRIC | OBSTETRIC OR NONOBSTETRIC | NONOBSTETRIC |
| Abruptio placentae | Prolonged shock, any cause | Malignancy |
| Amniotic fluid embolism | Transfusion-incompatible blood | Extensive surgery |
| Eclampsia, preeclampsia | Septicemia: bacterial fungal, viral | Collagen vascular disease |
| Abortion | Septic abortion | Central nervous system trauma |
| Dead fetus syndrome | Severe chorioamnionitis | Allergic reactions |
| Hydatidiform mole | Burns | |
| Retained placenta | Vascular malformations | |
| Uterine rupture | Pancreatitis | |
| Maternal hemorrhage |
Abruptio Placentae
Because abruptio placentae is the most common obstetric cause of DIC, evaluating the results of coagulation tests is imperative. Of mothers experiencing abruption, 20% have a significant clotting defect, with 25% of this group experiencing postpartum hemorrhage.66 Supportive treatment to decrease risk of DIC includes a type and cross-matching for blood transfusions, clotting mechanism evaluation, and the administration of intravenous fluids.68 If DIC is established, the basic element of treatment is the removal of blood clots from the uterus, blood component therapy, and fluid volume resuscitation.
Dead Fetus Syndrome
Prolonged retention of a dead fetus may lead to development of DIC, also called consumption coagulopathy, in the mother. After the release of thromboplastin from the degenerating fetal tissues into the maternal bloodstream, the extrinsic clotting system is activated, triggering the formation of multiple tiny blood clots. Fibrinogen and factor V and VII are subsequently depleted and the woman begins to display symptoms of DIC. Fibrinogen levels begin a linear descent 3 to 4 weeks after the death of the fetus and continue to decrease in the absence of appropriate medical intervention.23 Because of the risk of DIC, the once common practice of waiting for the onset of labor has largely been abandoned in recent years.69
Septic Abortion
Although septic abortion is a well-documented cause of obstetric DIC, because of the legalization of abortion, a large reduction in the number of cases of septic abortion has occurred.23 Bacterial endotoxins are the most likely initiating mechanism. The clinical findings of gram-negative septic shock are applicable to this condition. The severity of the disease correlates well with the degree of coagulopathy. Aggressive antibiotic therapy and evacuation of the uterus are the frontline therapies for patients who are hemodynamically stable.
Shock
Causes of hemorrhagic shock unique to pregnancy include abruptio placentae, ectopic pregnancy, placenta previa, and postpartum hemorrhage.12,23 Postpartum hemorrhage may be attributed to uterine atony, genital tract lacerations, hematoma formation, retained placenta, and uterine prolapse. Unique causes of septic shock in the pregnant patient include chorioamnionitis, septic abortion, and postpartum pyelonephritis.12 Cardiogenic shock is most frequently a result of the presence of severe valve disease. Regardless of the cause, whether specific to pregnancy or not, the occurrence of shock requires aggressive intervention with treatment of the underlying cause.
Pulmonary Dysfunction
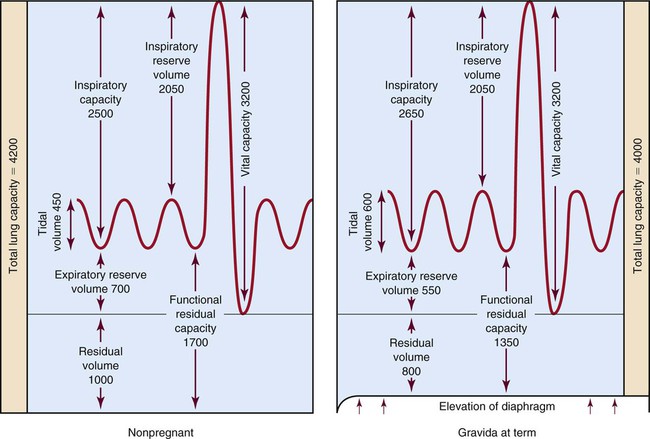
Oxygen consumption increases by approximately 15% to 25% throughout pregnancy.23 To meet these needs for additional oxygen, ventilatory changes (Fig. 39-2) must occur. Pulmonary dysfunction carries clinical significance because of the normally slightly hyperoxygenated condition associated with the physiologic changes in pregnancy. As a result of normal cardiorespiratory changes that occur during pregnancy, the pregnant woman has very limited cardiopulmonary reserve. It is, therefore, relatively easy for her to demonstrate pulmonary decompensation in the presence of any respiratory compromise.
Compromise of respiratory function places the mother and the fetus at risk. Maternal hypoxia may be the end result of several conditions, including pneumonia, asthma, cystic fibrosis, trauma, acute respiratory distress syndrome (ARDS), and pulmonary embolism. Contributing factors (e.g., smoking, drug use, pre-existing disease states), manifestations, and management differ very little from those seen in the nonpregnant individual. Common to these respiratory disorders is the issue of maternal–fetal hypoxia. Sufficient fetal oxygenation requires a maternal arterial oxygen tension (Pao2) greater than 70 mm Hg, which corresponds to an oxygen saturation of 95%.70 Hyperventilation, shortness of breath, and dyspnea are commonly seen in pregnancy and must be differentiated from the usual maternal complaints.
Asthma
Although asthma is an increasingly common chronic pulmonary complication in women in the reproductive age group, the management strategies do not differ significantly in pregnant women with asthma from women who are not.71 Patients who may not be compliant with medical regimens while pregnant for fear of fetal harm should be reassured that, although poorly controlled asthma greatly increases pregnancy risk, well-controlled asthma does not appear to affect pregnancy adversely.72,73 Poorly controlled asthma during pregnancy has been associated with an increased incidence of pneumonia (more than 60% of pneumonias that arise during pregnancy occur in women with asthma), hyperemesis gravidarum, gastroesophageal reflux disease, preeclampsia, chronic hypertension, preterm labor or birth, perinatal mortality, spontaneous abortion, complicated labor, and low birth weight.74,75 The literature indicates that maternal and fetal outcomes are related to the severity of the disease and the degree of control achieved with medical management.
It is estimated that approximately one third of patients will experience no change in asthma symptoms, one third will see improvement, and one third will have worsening of symptoms. The peak incidence of asthma exacerbation occurs during the second and early third trimesters; exacerbations during labor are rare because of the natural occurring increase of endogenous epinephrine and steroids.73,75 The lessening of asthma symptoms during pregnancy is the result of smooth muscle relaxation caused by progesterone. Decreased cell-mediated immunity and an increased level of corticosteroids may assist in diminishing the inflammatory response. Cyclic adenosine monophosphate (cAMP) levels are increased and aid in maintaining an ongoing energy supply to cells.
Factors that may contribute to exacerbation of asthma symptoms include nasal congestion, decreased functional residual volume, anxiety, noncompliance with medical regimens, stress, exercise, exposure to allergens, environmental irritants, respiratory infections, sinusitis, and smoking.73–75 The decrease in cell-mediated immunity may predispose the mother to viral infections.
Management recommendations include use of a peak flow meter twice per day to assist in objectively measuring maternal pulmonary function. A decrease in peak expiratory flow rate (PEFR) of more than 20% of the patient’s personal best requires a call the to the physician; a decrease to of greater than 50% of the patient’s personal best signals the need for a visit to the emergency department and the need for rapid assessment and intervention.73,75 Pharmacologic agents used to treat asthma in pregnancy fall into two principal categories: 1) maintenance agents and 2) rescue agents. The most commonly used agents in this category that are safe for use during pregnancy are the inhaled steroids, systemic steroids, mast cell stabilizers, methylxanthines, and leukotriene antagonist. Rescue agents, used to provide immediate symptomatic relief, include inhaled beta-agonists and inhaled anticholinergics.6,72–74
Cystic Fibrosis
Cystic fibrosis (CF) is an autosomal recessive, multisystem disease that affects the exocrine glands and epithelial tissues of the pancreas, sweat glands, and mucous glands of the respiratory, digestive, and reproductive tracts.74,76 Pulmonary disease is the leading cause of morbidity and mortality in patients with CF. More women with cystic fibrosis are living to reproductive age and becoming pregnant because of improved pulmonary and pharmacologic therapies and because of the opportunity of lung transplantation. The normal pulmonary changes of pregnancy (e.g., increased resting minute ventilation; upward displacement of the diaphragm, with resultant decrease in functional residual volume; widened alveolar-arterial oxygen gradient) lead to pulmonary decompensation and increased morbidity and mortality for the mother and the fetus. In advanced lung disease, pulmonary hypertension may be present. Pulmonary hypertension, combined with the normal pregnancy-related increase in blood volume and the consequent inability to increase cardiac output, may lead to uteroplacental insufficiency and cardiovascular collapse, especially during labor and delivery.74 Management recommendations include ongoing cardiopulmonary assessment (chest radiography, pulmonary function studies, saturation of peripheral oxygen [Spo2] monitoring, arterial blood gas determinations, pulmonary artery pressure monitoring), bronchial drainage and chest physiotherapy, ongoing replacement of pancreatic enzymes and supplementation of nutrition, and antibiotic administration for persistently present organisms such as Pseudomonas aeruginosa and Burkholderia cepacia.66,74–75 Historically, mothers with CF have poor pregnancy outcomes; however, more recent reports suggest that in women with milder disease, pregnancy outcome may be favorable. Additionally, the patient should receive genetic counseling on the risk of her children being affected by CF.77
Pneumonia
Pneumonia is one of the leading causes of infection-related death in the United States and remains a leading cause of maternal and fetal morbidity and mortality.78,79 Although data suggest that infants born to mother whose pregnancies have been complicated by pneumonia are more likely to be born preterm and to have a lower birth weight, care must be taken to balance treatment to serve both the mother and the fetus.80 Exacerbations are more common in the second and third trimesters, and they are often associated with other maternal disease processes. Prior respiratory disease, concurrent illness, human immunodeficiency virus (HIV) infection, drug or tobacco use, aspiration, and anemia have been linked with an increased maternal risk of pneumonia.78,79
Pregnancy itself appears to be an independent risk factor for major complications of pneumonia. Of pregnant women suffering from pneumonia, up to 40% may undergo major complications such as empyema or pneumothorax. Physiologic changes of pregnancy decrease the mother’s ability to clear secretions and place her at increased risk for gastric aspiration. In the fetus, growth restriction, which leads to small-for-gestational-age neonates, occurs in approximately 12% of the cases; and intrauterine and neonatal death rates have been reported to be as high as 12%.78
Typically, pneumonia is of bacterial origin; however, a variety of organisms may be seen. Some of the more common pathogens identified are Streptococcus pneumoniae, responsible for about 50% of bacterial pneumonias, with Haemophilus influenzae being the second most common causative organism. Mycoplasma pneumoniae and Chlamydia pneumoniae may account for a substantial number of cases and should be considered when selecting empiric treatment.12,66 Pregnant women may be uniquely susceptible to viral pneumonia secondary to the reduction in cell-mediated immunity that is associated with pregnancy.80
Clinicians may be reluctant to obtain chest radiographs; however, the hazard, to both mother and fetus, of delaying the diagnosis is far greater than the small dose (approximately 300 microrads) to the fetus from standard chest radiography. Pneumocystis jirovecii (formerly Pneumocystis carinii) pneumonia and resistant strains of tuberculosis are a growing concern, because many women who are HIV positive or have acquired immunodeficiency syndrome (AIDS) are in their reproductive years.78,81
Acute Respiratory Distress Syndrome
An important presenting symptom is dyspnea, especially if it is associated with a degree of anxiety or tachypnea. As this evolves, pulmonary function rapidly deteriorates over a 24-hour period. Symptoms include coughing, wheezing, diffuse crackles, pulmonary infiltrates or pleural effusion on the chest radiograph, and a deteriorating Pao2 with increasing fractional inspired oxygen (Fio2) demands and declining PF ratio.81 Pulmonary compliance may decrease from the normal average of 75 milliliters per centimeters of water (mL/cm H2O) to 20 mL/cm H2O in severe ARDS.12 In all suspected cases of ARDS, additional laboratory tests and examinations must be completed to differentiate this syndrome from other causes such as fluid overload and heart failure, which may sometimes be seen in the last trimester.
Management of Respiratory Failure
Management of maternal hypoxia includes restoration and maintenance of the hypervolemic state without inducing fluid overload and further compromising cardiopulmonary function. Because colloidal osmotic pressure is decreased, great care must be taken to prevent the development of pulmonary edema when providing fluid replacement therapy. Oxygen is administered at a high flow rate by mask to achieve an optimal Pao2 greater than 100 mm Hg and an Spo2 greater than 95%. Noninvasive mechanical ventilation should be used with caution because of the risk of gastric aspiration. If intubation is required, placement of an orotracheal tube is more desirable than a nasotracheal tube because of hyperemic nasal passageways. If nasotracheal intubation is required, the smallest tube possible that still allows adequate ventilation is used. Gastric decompression is instituted with a small-bore nasogastric or orogastric tube.66,79 Precautions must be taken to maintain the maternal Paco2 of 28 to 32 mm Hg because respiratory alkalosis may lead to decreased uterine blood flow. Pulmonary compliance should be routinely assessed to evaluate the effectiveness of interventions. Pharmacologic therapies must consider maternal–fetal risk and benefits. Table 39-9 summarizes common medications used in pulmonary dysfunction and obstetric concerns regarding their use.71,74,75
TABLE 39-9
COMMON MEDICATIONS USED IN PULMONARY DYSFUNCTION DURING PREGNANCY
| MEDICATION | CONSIDERATIONS |
| Antibiotics | Cephalosporins, erythromycin are generally well tolerated. |
| Tetracyclines and quinolones should be avoided. | |
| Vancomycin and aminoglycosides may lead to fetal toxicity. | |
| Acyclovir improves maternal morbidity and appears to improve fetal outcomes. | |
| Inhaled corticosteroids | Beclomethasone generally considered safe. |
| Systemic corticosteroids | Documentation of decreased birth weights and increase in small-for-date babies. |
| Most drug is metabolized by placental enzymes before entering the fetal circulation. | |
| Small amount of steroid may cross into breast milk, but breastfeeding is still considered safe. | |
| Mast cell stabilizers | Cromolyn and nedocromil are generally considered safe. |
| They are not associated with increased risks to the fetus. | |
| Beta-adrenergic agonists | Epinephrine and isoproterenol may contribute to maternal–fetal tachycardia. |
| Albuterol and terbutaline have fewer side effects and may have the additional benefit of tocolytic actions. | |
| Leukotriene receptor antagonists | They appear to be safe for the management of chronic, mild to moderate asthma. |
| Inhaled anticholinergics | Ipratropium is beneficial for acute asthma attack. |
| They probably are safe for the fetus because they are poorly absorbed by bronchial mucosa, decreasing the risk of fetal exposure. | |
| Theophylline | It is not commonly used because of maternal side effects. |
| It is recommended that serum levels be maintained between 5 and 12 mg/mL to prevent complications of toxicity. | |
| Analgesics | Morphine and meperidine should be avoided in active labor because they can worsen bronchospasm |
| They will cross the placenta and should be considered when completing a fetal assessment | |
| Fentanyl may be a better agent to use. | |
| Epidural analgesia is considered safe. | |
| Beta-mimetic tocolytics | These are contraindicated because they may worsen maternal lung damage. |
| Labor induction | Oxytocin is the drug of choice. |
| Prostaglandin F2 should be avoided because it is a bronchoconstrictor. | |
| Neuromuscular blockade | It can be administered safely as long as peripheral nerve testing is conducted to monitor drug dosages. |
| These agents will cross the placenta, which should be considered when assessing fetal activity. |
Pulmonary Embolism
Venous thromboembolism (VTE), including pulmonary embolism (PE), results from a variety of causes, many of which are related to the physiologic changes in pregnancy. Deep vein thrombosis (DVT) during pregnancy is far more common in the left leg than in the right leg. In one study of 60 pregnant women with a first episode of VTE, 58 had isolated left lower extremity DVTs, 2 had bilateral DVTs, and no isolated right lower extremity DVTs occurred.82 This unusual distribution has been attributed to increased venous stasis in the left leg related to compression of the left iliac vein by the right iliac artery, combined with compression of the inferior vena cava by the gravid uterus itself. Pelvic vein DVT is also more likely in pregnancy, making the diagnosis more difficult.
Other factors to be considered include the increasing frequency of long-term bed rest for high-risk multiple pregnancies, preterm labor, maternal age older than 35 years, obesity, smoking, cancer, surgery, and a history of VTE or PE.83–86
Pulmonary embolism remains a leading cause of maternal mortality and accounts for 20% of pregnancy-related deaths.82 The greatest risk for developing PE is in the immediate postpartum period, especially if a cesarean section was performed. Two thirds of the patients who die of PE do so within 30 minutes of the initial event. Unfortunately, physiologic changes associated with pregnancy may obscure the diagnosis of VTE during pregnancy. Assessment, management, and complications associated with PE are essentially no different from those in the nonpregnant woman; however, differential diagnosis of amniotic fluid embolism (AFE) must be considered.83 If DVT or PE is diagnosed, anticoagulation should be initiated. Either (unfractionated) heparin or low–molecular-weight heparin is an acceptable treatment for acute VTE. Both have risks and benefits, but both may be used safely during pregnancy. Intravenous heparin is the treatment of choice surrounding delivery because of its short half-life. Because of the risk of adverse effects on the fetus, warfarin is not generally used until the postpartum period.87,88 A vena cava filter may be implanted, but a suprarenal position is selected to prevent restriction of venous blood coming from the left ovary and draining into the left renal vein.
Amniotic Fluid Embolism
A potentially disastrous complication involving cardiopulmonary collapse in the pregnant woman is amniotic fluid embolism (AFE), also known as anaphylactoid syndrome of pregnancy (ASP).89 ASP, or AFE, is rare. Most studies indicate that the incidence rate is between 1 and 12 cases per 100,000 deliveries.89,90 The maternal mortality rate from AFE has been reported to be anywhere from 10% to 90%, although more recent data from large unselected populations suggest that overall mortality rates may be closer to 20%.91 Those who survive generally have a poor outcome, with as many as 85% suffering significant neurologic injury from cerebral hypoxia.89,90 It is one of the leading causes of maternal mortality.
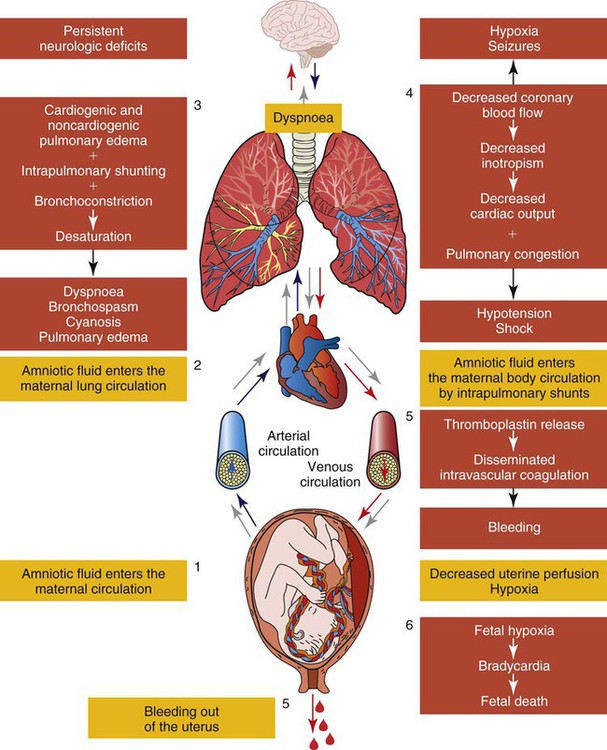
The most common precipitating factors associated with the syndrome are a large fetus, multiparity, premature separation of the placenta, induction of labor, tumultuous labor, and small tears in the endocervical veins that may occur during normal labor.79,89,91,92 An increased risk also was noted in older, ethnic-minority women.91 Placental abruption is seen in almost half the cases, with fetal death having occurred before the event.92 Figure 39-3 presents the pathophysiology of AFE.93
A patient with AFE may present with sudden onset of agitation and dyspnea, followed by symptomatic hypoxia, hypotension, and later DIC. Signs and symptoms of shock out of proportion to blood loss and altered mental status may also be observed. Chest pain and bronchospasm are uncommon. In a small percentage of patients, a grand mal seizure may be the initial symptom. More than 25% to 50% of patients die within the first hour, 80% die within the first 9 hours, and 40% of survivors develop DIC.79,92 The fetal mortality rate is 21%.92
No specific diagnostic test exists for ASP, although the presence of fetal squamous cells, lanugo, vernix caseosa, meconium, and mucin in blood aspirated from the pulmonary artery indicates a possibility of its occurrence; however, this debris has been reported even in blood samples obtained from normal pregnant women.79,91 Testing should be done to rule out DIC. Chest radiography may show pulmonary edema, effusions, and cardiac enlargement. Electrocardiography (ECG) may show tachycardia, nonspecific ST-wave changes, and right ventricular strain. Lung scans may indicate perfusion defects.
Management consists of maintaining oxygenation and supporting cardiac function. Supportive therapy consists of maintenance of blood pressure through aggressive volume replacement and inotropic support, intubation and mechanical ventilation, and blood component therapy for hemorrhage.79,89 Other therapies such as low-dose heparin, bronchodilators, and steroids may be used.
Trauma
Trauma is the leading nonobstetric cause of death among pregnant women; the rate has been estimated to be about 6% to 7%, although a precise number is difficult to obtain because injuries may range from minor to life threatening.94,95 Motor vehicle accidents (MVAs) are the leading cause of maternal blunt trauma and account for 55% to 82% of maternal trauma.96 Blunt abdominal trauma is associated with a 3% to 38% incidence of fetal mortality. Direct fetal injury is not usually seen with blunt trauma because of the absorption of forces by the uterus, placenta, and amniotic fluid. Fetal injury and death are more indirect results of maternal shock and death. When the mother survives, abruption is the next leading cause of fetal mortality, followed by uterine rupture.96
With domestic violence or intimate partner violence (IPV), rates are estimated to be 4% to 17%; exact rates are unknown because of underreporting and differences in methods of data collection. Injuries associated with domestic violence include defensive wounds; multiple blunt trauma injuries; penetrating injuries: bruises in various stages of healing; injuries to the face, neck, and especially the abdomen; and uterine rupture.97–100 It may be difficult to differentiate between damage occurring as a result of an accidental fall and that resulting from being pushed or struck; therefore, the patient should be carefully interviewed. Reports of violence were more likely to be associated with alcohol consumption, drugs, inadequate prenatal care (contributing to low birth weight), and a history of a fetal death and previous induced or spontaneous abortion.101 The attacks may also become increasingly violent and include knife wounds, gunshot wounds, and homicide.102 Additionally, if IPV is suspected as the cause of maternal injury, appropriate reporting and referrals should be initiated without delay.
Burn injuries during pregnancy may be caused by exposure to a thermal, chemical, or electrical source. The severity of total body surface area (TBSA) of thermal injury in the mother correlates with deteriorating fetal outcomes related to prolonged hypotension, inadequate volume resuscitation, sepsis, hypernatremia, and carbon monoxide exposure.94,103,104 The best chance of fetal survival is maternal survival.
Approximately 10% of traumatic injuries occur in the first trimester, 40% in the second trimester, and 50% in the third trimester.103 As women continue to work late into their pregnancies and become involved in higher-risk activities, the type and severity of injuries may begin to increase. Most mechanisms that produce injuries in pregnant patients are no different from those that injure nonpregnant women.
Some types of fall injuries may be associated with the stage of pregnancy. In the first trimester, fall injuries may result from fainting from fatigue, hypoglycemia, and normal physiologic changes. At this stage, the fetus is usually well protected from external trauma because the uterus is located within the pelvis and is protected by bony structures. In the second and third trimesters, as the enlarging uterus expands out of the pelvis, maternal abdominal organs are displaced upward and laterally. Some protection is provided to the maternal abdominal organs because the uterus and fetus now occupy much of the abdominal cavity; however, the growing fetus becomes more vulnerable to injury. Hyperventilation and fainting are common as the fetus grows and places greater metabolic demands on the mother. By the third trimester, the fetus has begun to settle into the pelvis in preparation for birth. As relaxin secretion increases, the end effect of relaxed pelvis ligaments may lead to lordosis and pelvic tilt. This produces a change in gait and in the center of gravity and balance, which, again, may increase the risk of falls.12 Normal physiologic changes associated with pregnancy may mask assessment findings and thus affect decisions about interventions that are provided (Table 39-10).101,103
TABLE 39-10
INITIAL ASSESSMENT AND MANAGEMENT OF OBSTETRIC TRAUMA
| ASSESSMENT | MANAGEMENT, RATIONALE, OR PURPOSE |
| Primary Survey | |
| Airway | |
| Signs of Obstruction | |
| Same as for nonpregnant patient | Remove visible debris with caution because of increased hyperemia of nasal or oral area. |
| Decrease risk of aspiration (because of enlarged uterus and effects of progesterone) by lateral tilt or by inserting an NGT or OGT. | |
| Emergency cricoid thyrotomy or tracheotomy, if performed, is done above usual site because of upward displacement of thoracic structures. | |
| Breathing | |
| Signs of Ineffective Respiration | |
| Same as for nonpregnant patient with exception of the following: Spo2 <95 Pao2 <100 mm Hg Paco2 >30 mm Hg pH <7.40 Tidal volume <800 mL Labored respirations RR <20 breaths/min or >24 beats/min |
100% oxygen by mask because passages tend to be hyperemic and a tendency for breathing through mouth exists. Elevate head of bed, if possible, to decrease pressure on thoracic structures caused by elevated diaphragm. Oral intubation using smaller size (5.5 to 7.0 French) endotracheal tubes to reduce risk of bleeding caused by increased vascularity of area. Gastric decompression with OGT (or small NGT if patient does not tolerate OGT placement) because prone to ileus and gastric reflux. Physiologic monitoring (RR, Spo2, ETco2). |
| Obtain ABGs, chest radiograph. | |
| Emergent needle decompression or chest tube insertion for hemopneumothorax; may need to reassess entry point because of upward or outward displacement of thorax. | |
| Circulation | |
| Signs of Ineffective Circulation | |
| Same as for nonpregnant patient with exception of the following: Systolic blood pressure <110 mm Hg |
Diagnostic peritoneal lavage may be performed; open technique is usually preferred. Assess for possible placental abruption. Normally hypervolemic skin is warm and slightly moist because of progesterone and may mask signs of hypoperfusion. |
| Mean arterial pressure <80 mm Hg Central venous pressure <6 mm Hg Pale, moist, cool skin |
Central venous catheter or large-bore IV access to upper extremity sites because of potential for impeded venous return from lower extremities. Fluid resuscitation. LR is recommended because it has the potential to metabolize into bicarbonate through intrinsic body pathways; do not administer blood products through same IV line. |
| With 0.9 NS, can administer blood products through line; may decrease risk of developing maternal alkalosis (compared with LR). | |
| O-negative blood may be given until type and cross-matching are done. | |
| Replace 3 mL per 1 mL of blood lost to compensate for hypervolemia associated with pregnancy. | |
| Use caution because of increased risk of pulmonary edema caused by decreased colloidal osmotic pressure. | |
| May apply MAST, but do not inflate abdominal compartment. | |
| Tilt the patient to her side, even if on a back board, to maximize venous return. | |
| Secondary Survey | |
| Focused Obstetric History | |
| Gestational age | Keep the mother alive. |
| Single versus multiple pregnancy | Keep the baby where it is. |
| Number of pregnancies, live births, abortions | Perform vaginal examination to determine fetal presentation, status of amniotic membrane, and presence of fetal parts or umbilical cord in vagina. |
| Name of obstetrician | Avoid manipulating cord because of risk of spasms. |
| Rh factor | Emergent delivery; vaginal versus cesarean; live versus perimortem. |
| Prenatal complications | |
| Past vaginal drainage, bleeding, clots | |
| Past abdominal pain or uterine contractions | |
| Maternal Studies | |
| Unexpected Diagnostic Study Results | |
| CBC WBC >18,000/mm3 |
Insert a urinary Foley catheter with caution because of the risk of bleeding caused by increased pelvic vascularity; consider using a smaller size of catheter. |
| RBC <6,500,000/mm3 Hg <12 g/dL |
Consider significance of positive toxicity results. Is the fetus at risk for issues such as drug withdrawal or fetal alcohol syndrome? |
| Hct <32% Platelets <200,000/mm3 |
Hct and Hg values that are below those identified may put the mother and fetus at risk for hypoxia. |
| Fibrinogen >400 mg/dL Chemistries: BUN <9 mg/dL Creatinine >0.5 mg/dL Na+ slightly increased Glucose slightly decreased |
Use caution when performing a rectal examination to assist in determining fetal position or traumatic damage; pelvic vascular congestion contributes to development of hemorrhoids and predisposes the mother to bleeding from this site. Obtain radiologic studies, as needed; however, implement measures to decrease risk to fetus. Left axis deviation may be seen on the 12-lead electrocardiogram as a normal variant. |
| Fetal Studies | |
| Signs of Fetal Distress | |
| Fetal heart rate <100-110 | Ultrasound to evaluate fetus. |
| Nonreassuring patterns on fetal monitor Fundal height not appropriate for gestational age |
Assess fundal height, firmness, contractions every 30 min. Fetal monitoring (with pocket Doppler, ultrasonography, or fetal monitor) as allowed based on interventions provided to mother. Differentiate uterine pain or contractions from other sources of abdominal pain. |
| Vaginal drainage, bleeding, clots present | Administer tocolytics, as needed. L/S ratio and presence of PG to determine fetal lung maturity. |
| Abdominal pain, uterine firmness or contractions present | Kleihauer-Betke result indicates a break in the integrity of placental circulation if fetal cells are present. |
| Amniocentesis: | |
| Presence of RBCs | |
| L/S ratio and presence of PG | |
| Kleihauer-Betke test to detect presence of fetal blood in maternal bloodstream | |
| Tertiary Survey | |
| Aspect of Care | |
| Administer “follow-up meds” | Prophylactic measures: Choose broad-spectrum antibiotics, nonteratogenic agents. Tetanus toxoid does not cross placenta. |
| Rh-negative mothers: | |
| May become Rh immunized within 72 hr. | |
| Administer Rh immune globulin (300 mcg/15 mL of fetal blood or 30 mL of whole blood) if possibility of mother having received Rh-positive blood. | |
| Breach in integrity of the placenta of an Rh-positive fetus as evidenced by positive Kleihauer-Betke test result. |
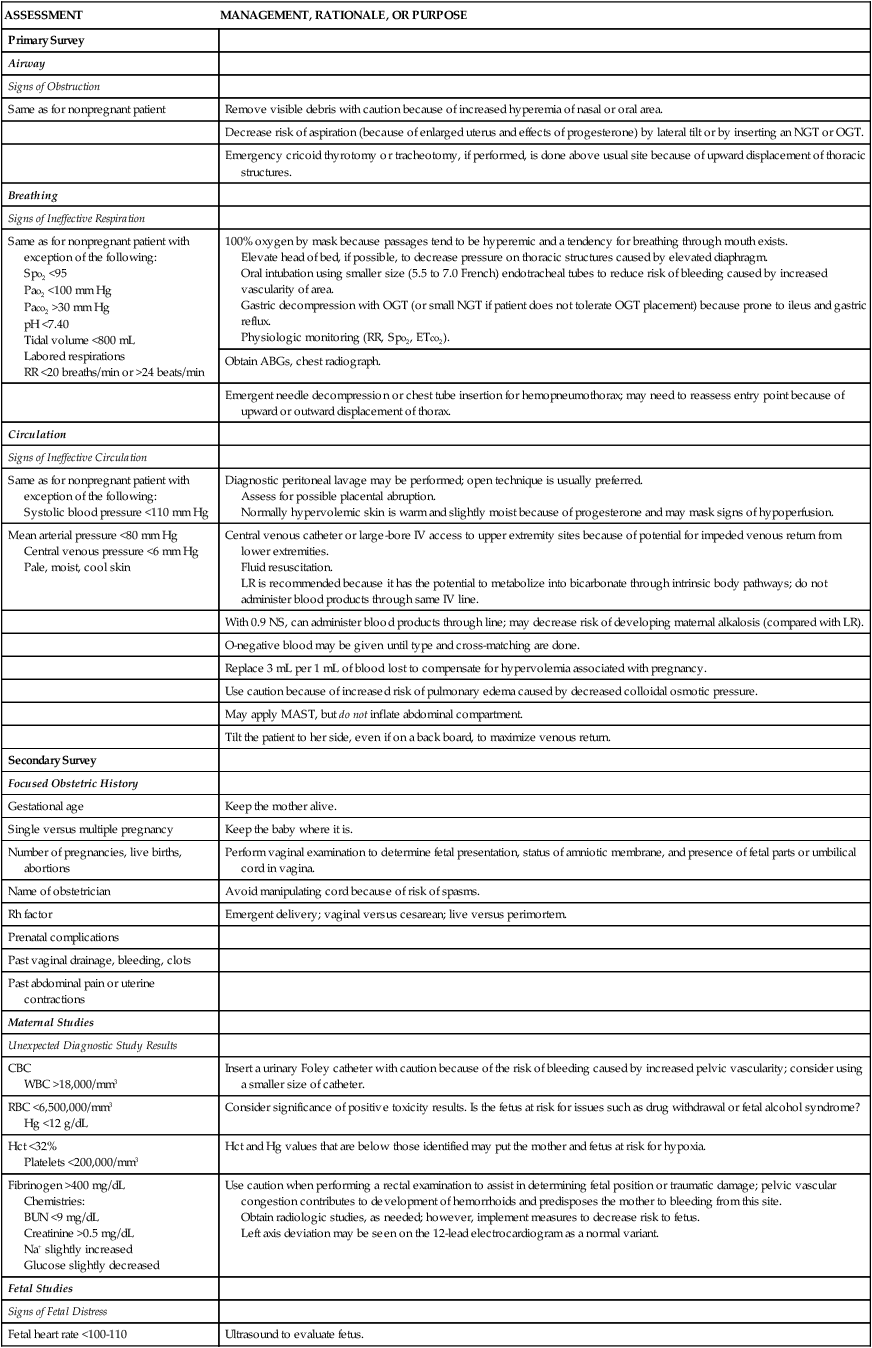
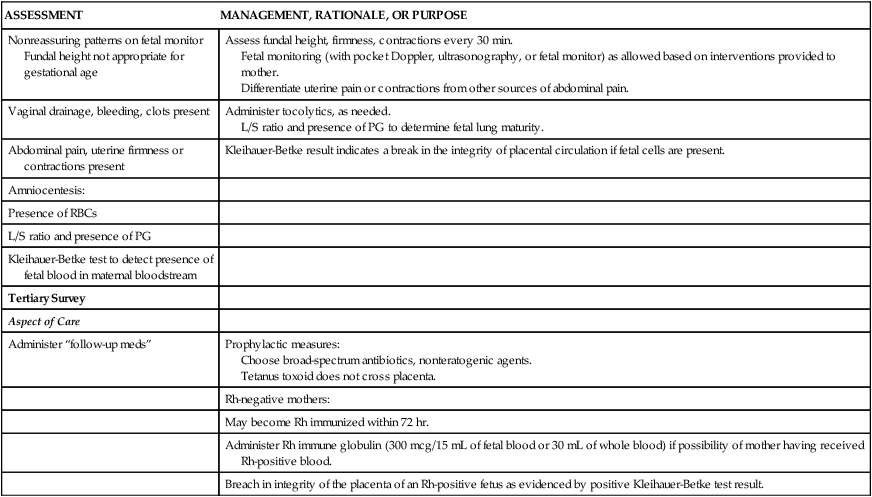
Types of Injuries
Cardiovascular Injuries
Manifestations related to cardiovascular injury may be masked because of normal physiologic changes in pregnancy. The hypervolemic, low systemic-resistance state and increased heart rate that occur during pregnancy may mask shock because significant blood volume may have been lost before the classic manifestations are seen.12 Therefore, tachycardia and hypotension in the pregnant trauma patient are clinically significant findings that require careful evaluation and should not be solely attributed to pregnancy. Because of venous congestion of the lower extremities, leg wounds may bleed more vigorously than expected.105
Pulmonary Injuries
The pregnant patient has diminished oxygen reserves and decreased blood buffering capacity. This leaves the pregnant trauma patient vulnerable to hypoxemia and less able to compensate when acidemia ensues. During pregnancy, as the uterus grows, the maternal diaphragm is displaced 4 cm above its normal location. This change in location must be considered if placement of a chest tube is necessary to avoid injury to the diaphragm. The insertion point during pregnancy is usually between the third and fourth intercostal spaces.106
Neurologic Injuries
Spinal cord injury (SCI) in pregnancy may be acute (result of recent trauma) or may be chronic (from pre-existing damage). Initial management of acute SCI is the same as for nonpregnant patients, including the administration of steroids. Adequate uterine perfusion may be evaluated by observing for a reassuring fetal heart rate tracing and lack of uterine contractions.107 Patients with SCI, similar to pregnant women, are at high risk for DVT, which necessitates the provision of prophylactic anticoagulation. Disruption of autonomic nervous system activity is not associated with labor dysfunction; therefore, vaginal delivery with a SCI is possible. Patients with injuries above T6 are at risk to developing autonomic dysreflexia reaction (ADR) and may require assisted vaginal delivery or a cesarean section.107–109 Uterine sensory nerves enter the spinal cord at T11 to L1; women with SCI above T10 are, therefore, unable to feel uterine contractions.108 They must rely on fetal monitoring of contractions or uterine palpation, and symptoms such as shortness of breath and abdominal spasms to determine labor. Of most importance is that autonomic dysreflexia often occurs with uterine contractions at time of labor and delivery. Proper anesthesia or antihypertensives may treat the problem but immediate delivery of the baby and placenta is vital.109
Abdominal and Pelvic Injuries
As stated earlier, MVAs are the primary cause of blunt trauma during pregnancy. Failure to wear seat belts or the improper use of seat belts increases the likelihood of abdominal injury, causes excessive maternal bleeding, and thus increases the risk of fetal death. The engorgement of pelvic vasculature increases the risk of retroperitoneal hemorrhage after lower abdominal trauma.96 Because the uterus displaces maternal abdominal structures, the traditional assessment findings and sites of referred pain may be altered. Peritoneal signs such as tenderness, rigidity, and rebound tenderness are unreliable indicators because of stretching of the abdominal wall. All of these factors may contribute to delayed detection of abdominal injuries. Splenic injuries are seen more commonly in the third trimester, and damage with blood loss may be seen after relatively mild trauma from vascular engorgement.
Penetrating abdominal injuries are also seen, with gunshot and stab wounds being the most common causes of a penetrating injury.102 Mortality rates following gunshot or stab wounds are lower in pregnant women because of the anatomic changes that occur related to the growing uterus. In contrast, the fetal mortality rate ranges from 40% to 70% and generally results from either direct fetal injury from the object of penetration or from preterm delivery following the injury.102
Blunt abdominal trauma may also be one of the more common types of injury from IPV during pregnancy. The abuser tends to focus the attacks on the abdomen, breasts, and genitalia, the “swim suit” area. The effects of this violence may include placental abruption and uterine injury.102
Reproductive System Injuries
Until 12 weeks’ gestation, the uterus is a pelvic organ. As the uterus enlarges, it may assist in protecting other organs, but it becomes more vulnerable to injury. Direct trauma to the uterus or placenta reverses protective hemostasis by releasing an increased concentration of placental thromboplastin, a plasminogen activator, from the myometrium. The uteroplacental bed functions as a dilated, passive, low-resistance system that lacks autoregulation and, therefore, has few or no compensatory mechanisms.12
Abruptio placentae, commonly seen with blunt abdominal trauma and pelvic fractures, may occur immediately, within 48 hours, or up to 5 days after injury.102 Uterine damage or rupture is rare, but if it occurs, it is commonly at the fundus or the site of a previous cesarean section and usually results in fetal death. Even if no identifiable damage is present after trauma, the risk for premature rupture of membranes (PROM), premature labor, fetal or maternal hemorrhage, or fetal damage or demise is increased. It is recommended that pregnant patients with trauma be admitted to the hospital for 24 to 48 hours of fetal monitoring because latent injuries or the need for tocolytics may be present.105,106
Fetal Injuries
The fetus is usually well cushioned by the amniotic fluid, the gravid uterus, and the abdominal wall, which distribute the force of injury. In most cases of minor trauma, the fetus survives intact. The most common cause of fetal death in maternal trauma is maternal death. The second leading cause of fetal death is placental abruption.12 Although not common, fetal death may occur even in the absence of significant maternal injury. During the third trimester, direct fetal injury is more common, as the uterine wall has thinned and the amniotic fluid may have decreased and is thus unable to absorb the impact. Additionally, the fetal head may be engaged in the pelvis; a resulting pelvic trauma may lead to a skull fracture in the fetus. Potential predictors of fetal demise vary, depending on the study. Increased maternal injury severity score (ISS), increased maternal hypoxia and acidemia, need for blood replacement, and presence of DIC have been associated with increased fetal mortality rates.96
Postpartum Hemorrhage
Postpartum hemorrhage (PPH) is the leading cause of maternal mortality. All women beyond 20 weeks’ pregnancy are at risk for PPH and its complications. The direct pregnancy-related maternal mortality rate in the United States is approximately 7 to 10 women per 100,000 live births. National statistics suggest that approximately 8% of these deaths are caused by PPH.110
Risk Factors and Causes
PPH is best defined, and diagnosed clinically, as excessive bleeding that makes the patient symptomatic (pallor, lightheadedness, weakness, palpitations, diaphoresis, restlessness, confusion, air hunger, syncope), results in signs of hypovolemia (hypotension, tachycardia, oliguria, low oxygen saturation of less than 95%), or both.111 Other definitions that have been suggested have been problematic. The most common definition of PPH is estimated blood loss of greater than 500 mL after vaginal birth or greater than 1000 mL after cesarean delivery. The inadequacy of this definition was illustrated in studies that assessed blood loss using various objective methods; also, clinicians were likely to underestimate the volume of blood lost.112
Another classic definition of PPH is a 10% decline in postpartum hemoglobin concentration from antepartum levels. However, this is not a clinically useful definition, as rapid blood loss may trigger a medical emergency prior to observation of a fall in hemoglobin concentration; also, laboratory changes that are not correlated with events that endanger the patient should not be used to define a medical emergency.112 In a recent randomized trial, increased risk of PPH was associated with infant’s birth weight, labor induction and augmentation, chorioamnionitis, MgSO4 use, and previous PPH.111
PPH has many potential causes. As a way of remembering the causes of PPH, several sources have suggested using the “4 T’s” as a mnemonic: 1) tone, 2) tissue, 3) trauma, and 4) thrombosis.113 Tone refers to uterine atony, which is, by far, the most common cause of PPH. Overdistension of the uterus, fatigue caused by prolonged labor or rapid forceful labor, and inhibition of contractions by drugs constitute major risk factor for atony. Tissue would include retained placenta or failure of complete separation of the placenta, which occurs with placenta accreta. Any damage to the genital tract during delivery would constitute trauma. Trauma would also include cesarean delivery, uterine rupture, and cervical or vaginal sidewall lacerations. With thrombosis, the abnormities may be pre-existing or acquired. Thrombocytopenia may be related to a pre-existing disease such as idiopathic thrombocytopenic purpura or acquired secondary to HELLP syndrome, abruptio placentae, DIC, or sepsis.114
Prevention
Research suggests that active management of the third stage of labor reduces the incidence and severity of PPH.115 Active management consist of a uterine-contracting medication (preferably oxytocin) immediately on delivery of the baby, early cord clamping and cutting, and gentle cord traction with uterine countertraction when the uterus is well contracted (Brandt-Andrews maneuver). One research demonstrated that early administration of oxytocin (before placental delivery) did not increase the rate of retained placenta, as initially suspected. Plus, the trial showed trends toward a benefit for early administration of oxytocin, including a 25% reduction in PPH and a 50% reduction in the need for transfusion.115
Summary
• Optimal care for the critically ill obstetric patient or one who has sustained injuries requires persistent application of physiologic changes of pregnancy to normal adult values.
• Symptoms of serious disorders may be altered because of the pregnant state.
• Understanding the intricate maternal–fetal relationship provides practitioners with a focus of maintaining maternal stability to enhance uteroplacental perfusion and an optimal in utero environment.
• All potential therapeutic and pharmacologic interventions must be weighed in light of the risk–benefit ratio to maternal–infant status.
• Critical situations may bring personal, cultural, social, spiritual, and ethical values into conflict.
• Determining the appropriate environment may be challenging. Some institutions have created dedicated obstetric intensive care units, whereas others provide care in the obstetric care unit or critical care unit.
• Decision making must be done in collaboration with family and health care team members, and all options should be considered.
• The key factor is collaboration among specialties to optimize maternal–fetal outcomes and facilitate the family experience.