CHAPTER 6 THE NEGLECT SYNDROME
There are few more dramatic sights in clinical neurology than a patient ignoring one half of his or her environment, including one half of a meal, or insisting that a paretic left arm is entirely normal, while that held by the examiner must belong to someone else. These examples of severe neglect syndromes, manifest by spatial neglect, and personal neglect with anosognosia (denial of deficit), respectively, are uncommon and a suitable subject for medical literature.1 However, lesser degrees of the neglect syndrome are common, especially in right hemisphere stroke, and have an adverse effect on prospects for rehabilitation and function. Therefore, any practicing neurologist likely to encounter patients with acute or subacute hemispherical lesions—the typical cause of the neglect syndrome—must be aware of its possible manifestations and must be able to confirm their presence at the patient’s bedside.
The neglect syndrome is a constellation of related lateralized deficits, including neglect of sensory stimuli; extinction of awareness of one sensory stimulus by another when both are delivered simultaneously; neglect of one half of an object or of space (recognizing that different reference points for “left” exist in this context); neglect of part of a person’s own body; failure to move a (nonparetic) body part as rapidly or persistently as its contralateral equivalent; and failure to recognize that the function of one part of the body is, indeed impaired.2 Although many of these features tend to occur together in individual patients, they are potentially dissociable, and each patient must have his or her own distinctive pattern of impaired and retained abilities elucidated and documented, to facilitate further monitoring, care, and rehabilitation.
TERMINOLOGY AND PHENOMENOLOGY
The various components of the neglect syndrome each have their own descriptive terms; because patients may exhibit a wide range of combinations of these individual deficits, it would be as inappropriate to lump these together as “neglect” as it would be to refer to all disorders of central language processes as “aphasia” without further qualification. The reader interested in a more detailed exposition of each of the elements of the neglect syndrome is referred to Heilman and colleagues (2003). The following classification is somewhat arbitrary—for example, neglect dyslexia might conceivably also be regarded as a motor disorder, and spatial neglect might legitimately be considered “sensory”—but some form of organization, even imperfect, is probably useful in considering the wide range of deficits subsumed under “neglect”.
Sensory Aspects of the Neglect Syndrome
Sensory Neglect
Sensory neglect is said to exist when the patient is not consciously aware of or able to respond to a sensory stimulus contralateral to the lesion, in the absence of a deficit in the relevant sensory pathways or its cortical projections sufficient to prevent apprehension of the stimulus. This defect can be unimodal, but it may affect vision, touch, hearing, and even olfaction together. It can, of course, be difficult to determine whether a patient has neglect or a primary sensory disturbance. However, as pointed out by Heilman and colleagues (2003),2 the bilateral nature of the central auditory pathways makes the diagnosis of auditory neglect easy: A patient with unilateral deafness will hear a sound applied to their deaf side in their good ear if the sound is loud enough, and unilateral cortical lesions typically do not cause deafness. Complete hemianesthesia is uncommon with hemispherical lesions, apart from those involving the thalamus. A patient with a thalamus-sparing cortical lesion who has hemianesthesia probably actually has sensory neglect. The olfactory pathways are uncrossed. Hemianopia, particularly hemianopia plus neglect, is the most difficult to distinguish from hemineglect alone.2,3 Patients with hemianopia without neglect are often aware of and compensate for their deficit, deliberately scanning into their area of field loss, but even the use of examination techniques such as supramaximal stimuli (e.g., bright torch in a dark room) may leave room for doubt.
Extinction
Extinction (or sensory extinction to double simultaneous stimulation) is said to be present when the patient does respond to sensory stimulation on the contralesional side but then fails to do so when another stimulus is applied simultaneously. The extinguishing stimulus is typically similar to that being extinguished and is usually applied to the corresponding contralateral area, but transmodal extinction (e.g., of a left-sided tactile stimulus by a right-sided visual stimulus) can occur, as can extinction of one stimulus by a second ipsilateral stimulus. When this occurs, the rightward stimulus typically extinguishes that further to the left: this allocentric effect can be seen in the ipsilesional as well as the contralesional receptive field. Extinction, too, may be unimodal or multimodal.
Spatial Aspects of the Neglect Syndrome
Spatial Neglect
Spatial neglect refers to decreased awareness of contralesional hemispace. However, what constitutes “hemispace” depends on the frame of reference. Patients may neglect the left side of retinocentric space (i.e., the left side of wherever they are looking), the left side of cephalocentric or somatocentric space (referable to the direction of the head or body), or the left side of environmental space (in relation to an environmental fixed point). For example, patients with neglect of somatocentric hemispace who turn their heads and eyes to the right bring their left visual field into the right side of space, as defined in relation to a somatocentric reference point. This improves their detection of left-sided visual stimuli (defined in relation to a retinocentric reference point) and may help the clinical distinction from hemianopia. A patient with left environmental-centered neglect who lies on his or her left side would neglect stimuli toward the feet. Spatial neglect also occurs for only peripersonal (near) space (i.e., what can be reached) and, less commonly, for far space, or for both together. A related concept is that of spatial neglect for object-centered (allocentric) space. This refers to a tendency to ignore the left sides of objects, regardless of where they are in retinocentric, somatocentric, or environmental space (Fig. 6-1).4 In addition to neglecting half their environment, such patients may also display neglect dyslexia, in which there is a tendency to ignore the left parts of words (e.g., misreading “consequence” as “sequence”) or of lines. This is an unlikely occurrence in hemianopia without neglect.
Representational Neglect
Representational neglect refers to the neglect of the left side of mental images. This disorder first became widely recognized as a result of a thought experiment in which patients imagined themselves standing in the Piazza del Duomo in Milan.5 If they imagined themselves standing on the steps of the cathedral, they could recall the buildings on their right in greater detail than those on the left. If they then imagined themselves standing at the opposite end of the square looking back at the cathedral, they could now recall those buildings previously on their left (but now on their right) in greater detail than those previously on their right (but now on their left).
Body Image Aspects of the Neglect Syndrome
Anosognosia and Anosodiaphoria
The reader might intuitively suspect that anosognosia (denial of deficit, such as hemiparesis), or the less severe but similar anosodiaphoria (lack of appropriate concern regarding an admitted deficit) is related to personal neglect; however, anosognosia can certainly exist in the absence of personal neglect.6 Of course, neither anosognosia alone nor anisodiaphoria alone is necessarily part of a neglect syndrome: both can occur in other circumstances (e.g., denial of cortical blindness in Anton’s syndrome, la belle indifference in conversion disorders).
Motor Aspects of the Neglect Syndrome
Motor neglect, in a broad sense, refers to a situation in which patients fail to perform an appropriate movement, despite awareness of the imperative stimulus and preservation of the requisite power. It is usually implicit that the disorder does not just affect skilled movements, inasmuch as this could then be classed as an apraxia. Heilman and colleagues classified these deficits as action-intentional disorders and recognize four types: akinesia, motor extinction, hypokinesia, and motor impersistence.2 Akinesia refers to failure of initiation of movement. If this failure of initiation is in response to an external stimulus, it may be also be termed motor neglect (in a narrower sense). Akinesia may vary, depending on in which part of peripersonal space the movement occurs and on in which direction the movement is made. For example, akinesia of the left hand may be less severe if movements are attempted in right hemispace (e.g., with the hands crossed), and the ipsilesional (right) hand may move less freely to the left side. Ingenious experiments, designed to separate motor from sensory/hemispatial neglect, have been reported. Perhaps one of the simplest is the crossed response task, in which a stimulus in the right hemifield requires movement of the left arm, and that in the left hemifield requires movement of the right arm.2 Motor extinction is analogous to sensory extinction: a limb that can move normally in isolation moves less well when the opposite limb is moved at the same time. Hypokinesia refers to a normally executed movement with an abnormally long delay from stimulus to movement onset (reaction time). This delay may be long enough to be obvious clinically. Inability to sustain a motor act constitutes motor impersistence. This may be directional (e.g., inability to keep looking in the contralesional but not ipsilesional direction) or may affect the contralesional arm or whichever arm is in contralesional hemispace.
How Separable Are the Various Components of the Neglect Syndrome?
The neglect syndrome has long been recognized to consist of various combinations of the constituent deficits outlined previously. Formal double dissociations have been recorded between a number of the components (e.g., different measures of hemispatial neglect; hemispatial neglect and extinction).7,8 The best evidence for the heterogeneity of the syndrome probably comes from the large study of Buxbaum and coworkers, who found most possible combinations of personal neglect, peripersonal spatial neglect, sensory neglect/extinction (“perceptual neglect”), and motor neglect in their sample of 166 patients with right hemisphere stroke.9 Pure personal neglect was rare, but pure peripersonal spatial neglect, pure sensory neglect, and pure motor neglect were each not uncommon. Of course, their “purity” depends on which tests are chosen for each, together with their psychometric characteristics, but the double dissociations observed still stand.
EXAMINATION FOR THE NEGLECT SYNDROME
It is apparent from the preceding section that no one test is adequate for ruling out the neglect syndrome. At the same time, a relatively brief bedside assessment readily detects most clinically significant neglect syndromes. Several batteries of appropriate tests have been developed.10,11 The following outline is condensed predominantly from Heilman and colleagues (2003), to which the reader is referred for further details.
Sensory Aspects
The patient should be stimulated on the left side, right side, and on both sides together, in random order, with visual, tactile, and auditory stimuli and asked to state or indicate on which side or sides the stimulus occurred. Verbal misreporting of left as right, despite absence of left-right confusion on other measures (e.g., the patient can point to and/or move the appropriate side as requested), suggests allesthesia. As pointed out previously, auditory neglect or extinction is clearly separable from unilateral deafness, or unilateral involvement of auditory cortex, and nonthalamic lesions typically do not cause complete hemianesthesia, but it can be more difficult to separate visual hemineglect from hemianopia. Confrontational field testing should then be performed with the head turned to the right and to the left: a true hemianopic defect should remain retinocentric, but visual neglect may improve with the head’s turning to the right. Stronger stimuli should also be employed (e.g., a torch, the spot of a laser pointer on the wall in a darkened room, or, at least, targets larger than a hat pin). Sometimes, however, it is difficult to be certain of with what the clinician is dealing.3 Visual extinction of the more central stimulus by that further to the right may be demonstrable in the intact field.3
Spatial Aspects
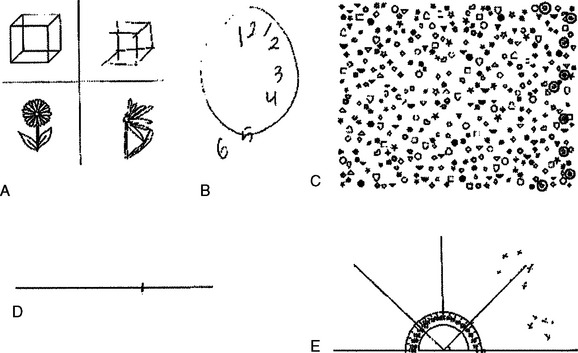
Gross degrees of spatial neglect are evident on observation: the patient disregards the left side of the environment. Asking the patient to report 10 objects around their room (provided that the bed is not against the left wall) and marking these with regard to a patient-centered reference frame can be a useful measure of less severe spatial neglect (Fig. 6-2E).3 Persistent head and/or eye deviation to the right may also offer a clue to lesser degrees of neglect, which may be elicited by a number of pencil and paper tests. Object drawing/copying tests (see Fig. 6-2A and B) are traditionally used but are not particularly sensitive by themselves,12 are difficult to quantify, and may be confounded by other visuoconstructional problems. A number of cancellation tests have been devised. Those with dense, random arrays with many different types of distractor items, such as the Bells Test,13 or Mesulam’s shape cancellation test (Fig. 6-3; see also Fig. 6-2C),14 seem to be the most sensitive.2,3 A starting point toward the right of the array is probably the most sensitive measure,12 and the location of the first item canceled should be noted. The left-minus-right omission score is also noted: a cutoff point of greater than 2 has been used on the Bells Test, for example.12
Line bisection tests are also easily carried out at the bedside; normal subjects tend to bisect a 20-cm line slightly toward the left, whereas those with left spatial neglect tend to bisect toward the right (see Fig. 6-2D). (They bisect short lines [e.g., 2 to 5 cm] toward the left—the so-called crossover effect—but although this is of great theoretical interest, it is not of practical importance to the clinician, provided that these short lines are avoided.) If the average rightward deviation from the true center points of two successively centrally presented horizontal 20-cm lines is used, a cutoff point of greater than 6.5 mm has been suggested.12 Occasional patients (actually almost 25% in the study of Azouvi et al)12 bisect even the longer lines toward the left: so-called ipsilateral neglect. Although line bisection tasks are less sensitive than cancellation tasks, patients with neglect may show abnormalities on just one or the other, which makes assessment with both necessary. In fact, line bisection may be more related to extinction than to the form of neglect elicited by cancellation tasks, and they may have different anatomical substrates (see “Anatomy of the Neglect Syndrome” section).15
Body Image Aspects
Personal neglect, if severe, may be evident in the patient’s failure to dress or groom one side, in fact, or after they are asked to demonstrate how they would do so. This has been formalized as the comb and razor/compact test.16 Personal neglect may also be detected by asking the patient to reach his or her left arm with the right arm. This may be rated from 0 (normal) to 3 (no attempt to reach the left arm) according to a published scale.17 Another graded test (the Fluff Test) involves placement of Velcro-backed stickers or cotton balls on a blindfolded patient, and asking the patient to find and remove them.9,18 In the author’s opinion, severe directional akinesia might confound these tests, and should be ruled out separately.
Severe anosognosia may be evident in general conversation with the patient, but milder forms are best detected with simple structured scales. In one such test,2 the hemiparetic patient is first asked, “Why did you come to the hospital?” If the patient does not mention the hemiparesis, he or she is asked whether he or she has any other problems. Failure to mention hemiparesis at this stage constitutes grade 1. Such patients are then asked, “Are you weak anywhere?” Failure to acknowledge their hemiparesis at this stage constitutes grade 2. The examiner then picks up the hemiparetic arm and moves it into the ipsilesional space. Denial of weakness when the patient is asked constitutes grade 3. Such a patient is then asked to move the arm. Continued denial of weakness under these circumstances constitutes grade 4.2 Other scales are similar in concept.9
Motor Aspects
Spontaneous (endokinetic) akinesia of the contralesional side, out of proportion to weakness, may be evident on observation. The oculomotor equivalent—a gaze preference toward the ipsilesional side—should also be noted if present. Testing arm movements with the patient’s arms crossed and uncrossed, in response to visual cues in the right hemifield (e.g., downward movement of the examiner’s finger triggers movement of the patient’s limb on the left; upward movement, of that on the right) allows distinction of sensory from motor neglect and differentiation of hemispatial from contralateral exogenously evoked akinesia. Visual saccades toward or away from the examiner’s finger in the right hemifield may be tested similarly. Marked hypokinesia, if present, can be observed during this testing as well. Motor extinction can be elicited with an adaptation of the sensory extinction test, in which the patient has to report which side was stimulated and must move that side. Patients with motor extinction, unlike those with sensory extinction, are able to report simultaneous bilateral stimulation but are able to move appropriately after only a unilateral stimulus. Motor impersistence can be tested by asking the patient to hold a limb posture for 20 seconds. This should be checked in both arms, each tested in both contralesional and ipsilesional space. A more formalized motor impersistence battery is available, if required.19
ANATOMICAL SUBSTRATE, AND THEORIES OF CAUSATION
Anatomy of the Neglect Syndrome
It is well known that in right-handed individuals, left-sided neglect is more frequent and severe with right hemisphere lesions than is right-sided neglect with left hemisphere lesions. Most left-handed patients also display this pattern; only rare left-handed patients showing severe right-sided neglect with left hemisphere lesions: so-called crossed neglect. However, severe right-sided neglect may also occur with bilateral lesions.20 In keeping with this, the author has encountered right-sided neglect in patients with probable Alzheimer’s disease.
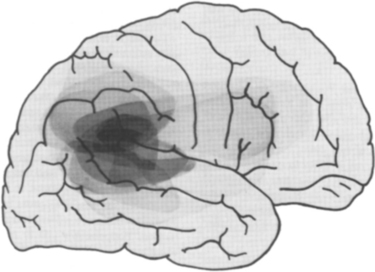
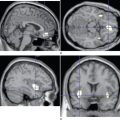
Neglect has traditionally been considered to arise from right parietal damage, and lesions of the inferior parietal lobule and the adjacent section of the superior temporal gyrus are indeed most commonly implicated in modern imaging studies (Fig. 6-4).8 However, it is now clear from both animal and human studies that neglect can arise from lesions of the inferolateral frontal lobe, cingulate gyrus, thalamus, neostriatum, (unilateral) mesencephalic reticular formation,2,21 and even the posterior limb of the internal capsule or the parahippocampal gyrus8 on occasions. It is reasonable to consider these areas as forming an attentional network, disruption of any component of which might result in the neglect syndrome (see Heilman et al, 2003, and Mesulam, 2000, for detailed discussions).
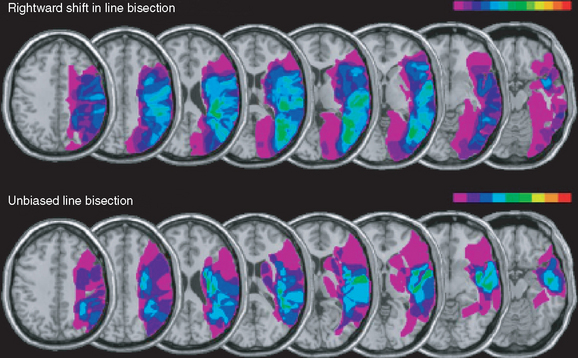
Because the neglect syndrome has a number of possible component deficits, some of which have been shown to be doubly dissociable (able to occur independently of each other), the question arises as to whether the various components might have different anatomical substrates. This issue remains unsettled, but there is at least some evidence that visual extinction (and the possibly related impairment of line bisection) is correlated with damage to the inferior parietal lobule or even the parieto-occipital junction, whereas spatial neglect as defined by abnormal performance on cancellation tasks correlated with more anterior lesions, involving the posterior portion of the superior temporal gyrus, or the parietotemporal junction (Fig. 6-5).7,8 Studies of transcranial magnetic stimulation to produce transient focal deficits in normal subjects have supported this view (see Milner and McIntosh, 2005).
It is also, at first sight, appealing to speculate that motor aspects of the neglect syndrome, such as hypokinesia, might relate to damage to frontal or striatal components of the attentional network, whereas sensory aspects, such as sensory neglect and extinction, might arise particularly from parietotemporal damage. However, patients with neglect caused by frontal lesions may be indistinguishable from those with parietal lesions on standard tests of sensory neglect,22 and one transcranial magnetic stimulation study confirmed the importance of sensory aspects to the neglect syndrome observed with frontal damage.23 As both areas are strongly reciprocally connected, and subserve sensory-motor integration, it is not surprising that a strict sensory/motor dichotomy has not been confirmed and that attempts to separate frontal from parietal neglect syndromes on the basis of clinical phenomena at the bedside are likely to be unavailing.
Pathophysiology of Sensory Neglect
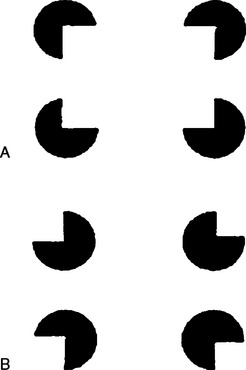
An obvious suggestion is that sensory neglect actually arises from impaired sensory input. However, patients with obviously impaired sensory input (e.g., hemianesthesia) are often only too well aware of their deficit, whereas unattended stimuli in patients with sensory neglect still elucidate cortical evoked potentials. Perhaps most convincingly, there is now overwhelming evidence for preconscious (implicit) sensory processing of the neglected stimuli. For example, a subject asked to count four indented circles could see only the two on the right, unless the indentations were oriented to form an implied rectangle, in which case all four were reported (Fig. 6-6).24 Furthermore, patients with visual neglect are still able to use left-sided visual information at a preconscious level to guide reaching and grasping movements (see Milner and McIntosh, 2005).
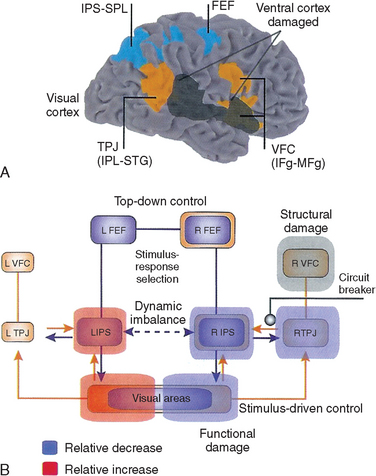
Another explanation relates to the role of attention in determining which of the many stimuli with which humans are constantly bombarded are noticed and which are deemed irrelevant and screened out before they reach consciousness. Mesulam proposed that the left hemisphere tends to endow the right side of the environment, or the right side of whatever is being attended (i.e., egocentric or object-centered right), with salience and is biased toward producing rightward-directed attentional shifts, whereas the effects of the unopposed right hemisphere are larger and more symmetrical (slightly favoring the left side).21 Posner and associates had emphasized the role of the right parietal region in disengaging attention from right-sided objects to enable a subsequent leftward shift.25 There is no fundamental conflict between the directional bias and impaired disengagement hypotheses, although there is some evidence that impaired disengagement is not solely responsible for the observed deficits.26 More recently, the attentional network concept has been elaborated to encompass different roles for roles of a ventral network—composed of the temporoparietal junction and ventrolateral frontal lobe—whose role is to detect novel sensory stimuli, and a dorsal network—composed of the superior parietal lobule and the frontal eye fields—that is responsible for goal-directed stimulus and response selection. Only lesions of the ventral network typically result in neglect, elaborated in part through functional disruption of the dorsal network (Fig. 6-7). Recovery is associated with increased activity in the dorsal network, suggesting a “top-down” rather than “bottom-up” compensatory strategy (i.e., a redirection of attention rather than a recapturing of attention by exogenous stimuli).27
THE FREQUENCY AND IMPORTANCE OF NEGLECT
The neglect syndrome is typically a result of a focal, lateralized brain lesion. Such lesions are typically structural and acute or subacute (e.g., strokes or rapidly growing tumors), but the syndrome may also occur with focal epilepsy. Diffuse processes, such as those occurring with toxic-metabolic encephalopathies or diffuse axonal injury from trauma, rarely cause neglect.21
In view of the heterogeneity of stroke topographies, the multifaceted nature of the neglect syndrome, the varying methods (with varying sensitivities) used for its detection, and the varying times after stroke at which studies have been conducted, it is not surprising that the literature contains widely differing estimates of the prevalence of the neglect syndrome in stroke. Indeed, a systematic review in 1999 concluded that although the greater frequency of the neglect syndrome after right than after left hemisphere lesions was supported, an accurate estimate of the frequency and recovery rates of the neglect syndrome could not be reached.28 Since then, several large studies have addressed these issues.
Using a standardized (but not exhaustive) test battery, Azouvi and associates found moderate to severe behavioral neglect in 25 (36%) of 69 subacute patients (an average of about 3 months after right hemisphere stroke onset), whereas 177 (86%) of 206 such patients displayed some degree of neglect on at least one pencil and paper measure.12 A study by the same group of 78 additional patients with subacute left hemisphere lesions (but without major impairment of comprehension) showed that for each test in the battery, right-sided neglect was less severe. Than was left-sided neglect after right-sided lesions, and only 25-50% as frequent.29 Patients with left hemisphere lesions also tended to show abnormalities on fewer tests within the battery; 44% displayed abnormalities on at least one measure.29 One report of 1281 patients with acute stroke, who were assessed only for tactile extinction and “visual inattention” on describing a standard scene (the Cookie Theft picture from the Boston Diagnostic Aphasia Examination) revealed that 43% of patients with right hemisphere lesions and 20% of those with left hemisphere lesions displayed abnormalities on one or both measures.30 Subacutely (at 3 months), the respective figures were 17% and 5%. (These percentages are doubtless lower than those reported by the other cited studies as a result of the limited assessment of the neglect syndrome in this study.) Further analysis showed that cortical involvement, right-sided involvement, and increasing age were associated with lesser degrees of improvement by 3 months, whereas handedness and gender had no effect.30 A similar estimate of recovery was derived from an earlier study employing life table analyses, which showed that only 7 (21%) of 34 patients with obvious behavioral neglect in the acute period still displayed such neglect at 3 months.31 The median duration of obvious left-sided sensory neglect was 9 weeks; less florid features such as extinction (median, 43 weeks) and motor impersistence (median, 54 weeks) improved more gradually.31
The presence of the neglect syndrome is an adverse factor for recovery. Patients with right hemisphere strokes recover more slowly than those with left hemisphere strokes; the difference appears to relate to the presence of neglect rather than to poorer motor strength recovery.32 Although this study did not demonstrate the intuitively expected correlation of outcome with anosognosia, others have done so since.33,34 Indeed, one of the study groups found that limited recovery and failure to regain functional independence were correlated with the severity of neglect and the presence of anosognosia in the acute period, as well as with increasing age,34 whereas the other group found no effect of hemispatial neglect (defined on a cancellation task) and personal neglect once anosognosia was allowed for.33 The adverse effect of anosognosia is hardly surprising: It is difficult for patients to cooperate enthusiastically with a rehabilitation program or use strategies to overcome a deficit that they do not believe they have. Chronic neglect increases burden of care; one study of 80 patients with subacute or chronic right hemisphere lesions showed that 37 (48%) displayed neglect on at least one of five measures and that neglect severity rather than lesion size was predictive of increased functional impairment and caregiver burden.9 Neglect can also pose safety concerns, such as in crossing roads or standing next to a hot stove in the kitchen. Education of caregivers is therefore an important aspect of rehabilitation for persistent neglect.
TREATMENT OF THE NEGLECT SYNDROME
Various rehabilitation strategies have been tried for the neglect syndrome, and the existing literature has been the subject of two 2002 reviews.35,36 Treatment strategies may be divided into those targeting arousal deficits, those directed at deficient visual attention, and those seeking to improve spatial representation deficits.35 (See Pierce and Buxbaum, 2002, for further details of the treatments outlined as follows, together with their outcomes.)
Treatment of Arousal Deficits
Dopaminergic agonists (e.g., bromocriptine) have been used to overcome arousal deficits, with contradictory results: Some studies reported improvements, whereas others reported worsening of neglect. It has been postulated that the effects of dopaminergic agonists depend on whether the lesion includes damage to the striatum, with the unintended effect of increased disparity in striatal activation in such patients, resulting in increased neglect. (See also Heilman et al, 2003, for additional discussion of dopaminergic agonist treatment.)
Cognitive Training for Remediation of Visual Attention Deficits
Remediation of visual attention deficits through a cognitive training (“top-down”) approach has resulted in improvement in some aspects of the neglect syndrome. For example, subjects may be taught to scan back to a red line at the left margin of a text, in order not to ignore the start of each line. Unfortunately, the benefits do not seem to generalize readily to other activities, and maintenance of improvement has not been adequately addressed.35
Treatment of Spatial Representation Deficits
Treatments targeting spatial representation deficits include hemispherical activation approaches (e.g., moving the contralesional limb) and constraint approaches (e.g., immobilizing the unaffected ipsilesional limb or obscuring the ipsilesional visual field in each eye with hemifield patches on glasses). There is some evidence in favor of these approaches, but it is hardly overwhelming.35 A number of other “bottom-up” approaches, aimed at inducing preconscious shifts in spatial representations, have also been explored. An example is leftward trunk rotation therapy, in which more of the left visual field is brought within right-sided peripersonal space. Application of vibration to the left posterior side of the neck produces a similar illusion. Cold water caloric irrigation of the contralesional ear might be thought to act by producing the illusion of head rotation to the right (with compensatory slow eye movement to the left and nystagmus toward the right) but may actually act through the vestibular system’s contribution to spatial representations. Unfortunately, the treatment is uncomfortable, and the effects last only 10 to 15 minutes. Similarly, opticokinetic nystagmus with quick phases induced to the right (i.e., stripes moving to the left) might be considered to produce the illusion of rightward movement. The benefits, however, are reported not to outlast the stimulus.
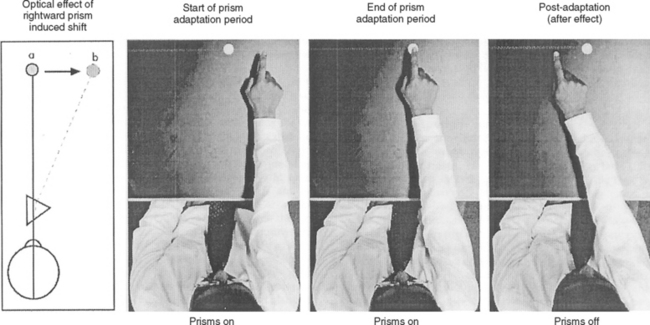
Perhaps the most exciting therapy is prism adaptation, a cheap and noninvasive treatment in which the patient wears Fresnel prisms, which cause a 10-degree apparent rightward shift of viewed objects (Fig. 6-8).37 The benefits generalize to nonvisual aspects of the neglect syndrome, such as tactile extinction38 and, in an initial study, were apparently long-lasting (at least weeks).39 The effects may also extend to patients with chronic neglect syndromes.40 This is potentially an exciting advance in neurorehabilitation, and the results of the first randomized controlled trials should be available in the near future.15
CONCLUSION
The neglect syndrome is common, readily detectable at the patient’s bedside, and has an important effect on rehabilitation and recovery of function. However, it is probably underrecognized, at least in its less florid forms, partly because of its diverse manifestations. Examination for these disorders should form part of the assessment of any patient with an acute or subacute hemispherical lesion, especially if it is right-sided. An impressive body of experimental work is now beginning to generate ideas for rationally based rehabilitative therapies, of which prism adaptation shows considerable early promise.
Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. 4th ed. New York: Oxford University Press; 2003:296-346.
Mesulam M-M. Attentional networks, confusional states and neglect syndromes. In: Mesulam M-M, editor. Principles of Behavioral and Cognitive Neurology. 2nd ed. New York: Oxford University Press; 2000:193-256.
Milner AD, McIntosh RD. The neurological basis of visual neglect. Curr Opin Neurol. 2005;18:748-753.
Parton A, Malhotra P, Husain M. Hemispatial neglect [Review]. J Neurol Neurosurg Psychiatry. 2004;75:13-21.
Pierce SR, Buxbaum LJ. Treatments of unilateral neglect: A review. Arch Phys Med Rehabil. 2002;83:256-268.
1 Sacks O. The Man Who Mistook His Wife for a Hat. London: Duckworth, 1985.
2 Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. 4th ed. New York: Oxford University Press; 2003:296-346.
3 Parton A, Malhotra P, Husain M. Hemispatial neglect [Review]. J Neurol Neurosurg Psychiatry. 2004;75:13-21.
4 Driver J, Halligan PW. Can visual neglect operate in object-centered coordinates? An affirmative single case study. Cogn Neuropsychol. 1991;8:475-496.
5 Bisiach E, Luzzatti C. Unilateral neglect of representational space. Cortex. 1978;14:129-133.
6 Adair JC, Na DL, Schwartz RL, et al. Anosognosia for hemiplegia: test of the personal neglect hypothesis. Neurology. 1995;45:2195-2199.
7 Mort DJ, Malhotra P, Mannan SK, et al. The anatomy of visual neglect. Brain. 2003;126:1986-1997.
8 Rorden C, Fruhmann Berger M, Karnath H-O. Disturbed line bisection is associated with posterior brain lesions. Brain Res Cogn Brain Res. 2005. [ePublication available ahead of print].
9 Buxbaum LJ, Ferraro MK, Veramonti T, et al. Hemispatial neglect: subtypes, neuroanatomy and disability. Neurology. 2004;62:749-756.
10 Azouvi P, Marchal F, Samuel C, et al. Functional consequences and awareness of unilateral neglect: study of an evaluation scale. Neuropsychol Rehab. 1996;6:133-150.
11 Wilson B, Cockburn J, Halligan P. Behavioural Inattention Test. Bury St. Edmunds, UK: Thames Valley Test Co., 1987.
12 Azouvi P, Samuel C, Louis-Dreyfus A, et al. Sensitivity of clinical and behavioral tests of spatial neglect after right hemisphere stroke. J Neurol Neurosurg Psychiatry. 2002;73:160-166.
13 Gauthier L, Dehaut F, Joanette Y. The Bells Test: a quantitative and qualitative test for visual neglect. Int J Clin Neuropsychol. 1989;11:49-54.
14 Mesulam M-M. Principles of Behavioral Neurology: Tests of Directed Attention and Memory. Philadelphia: FA Davis, 1985.
15 Milner AD, McIntosh RD. The neurological basis of visual neglect. Curr Opin Neurol. 2005;18:748-753.
16 Breschin N, Robertson IH. Personal versus extrapersonal neglect: a group study of their dissociation using a reliable clinical test. Cortex. 1997;33:379-384.
17 Bisiach E, Perani D, Vallar G, et al. Unilateral neglect: personal and extrapersonal. Neuropsychologia. 1986;24:759-767.
18 Cocchini G, Beschin N, Jehkonen M. The Fluff Test: a simple task to assess body representational neglect. Neuropsychol Rehab. 2001;11:17-31.
19 Benton AL, Sivan AB, Hamsher K de S, et al. Contributions to Neuropsychological Assessment: A Clinical Manual, 2nd ed. New York: Oxford University Press, 1994.
20 Weintraub S, Daffner KR, Ahern G, et al. Right-sided hemispatial neglect and bilateral cerebral lesions. J Neurol Neurosurg Psychiatry. 1996;60:342-344.
21 Mesulam M-M. Attentional networks, confusional states and neglect syndromes. In Mesulam M-M, editor: Principles of Behavioral and Cognitive Neurology, 2nd ed., New York: Oxford University Press, 2000.
22 Husain M, Mattingley JB, Rorden C, et al. Distinguishing sensory and motor biases in parietal and frontal neglect. Brain. 2000;123:1643-1659.
23 Brighina F, Bisiach E, Piazza A, et al. Perceptual and response bias in visuospatial neglect due to frontal and parietal repetitive transcranial magnetic stimulation in normal subjects. Neuroreport. 2002;13:2571-2575.
24 Mattingley JB, Davis G, Driver J. Preattentive filling-in of visual surfaces in parietal extinction. Science. 1997;275:671-674.
25 Posner MI, Walker JA, Friedrich JF, et al. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4:1863-1874.
26 Mark VW, Kooistra CA, Heilman KM. Hemispatial neglect affected by non-neglected stimuli. Neurology. 1988;38:1207-1211.
27 Corbetta M, Kincade MJ, Lewis C, et al. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603-1610.
28 Bowen A, McKenna K, Tallis RC. Reasons for variability in the reported rate of occurrence of unilateral spatial neglect after stroke. Stroke. 1999;30:1196-1202.
29 Beis J-M, Keller C, Morin ST, et al. Right spatial neglect after left hemisphere stroke: qualitative and quantitative study. Neurology. 2004;63:1600-1605.
30 Ringman JM, Saver JL, Woolson RF, et al. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology. 2004;63:468-474.
31 Hier DB, Mondlock J, Caplan LR. Recovery of behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33:345-350.
32 Denes G, Semenza C, Stoppa E, et al. Unilateral spatial neglect and recovery from hemiplegia. Brain. 1982;105:543-552.
33 Pedersen PM, Jørgenson HS, Nakayama H, et al. Hemineglect in acute stroke—incidence and prognostic implications: the Copenhagen Stroke Study. Am J Phys Med Rehab. 1997;76:122-127.
34 Stone SP, Patel P, Greenwood RJ, et al. Measuring visual neglect in acute stroke and predicting its recovery: the visual neglect recovery index. J Neurol Neurosurg Psychiatry. 1992;55:431-436.
35 Pierce SR, Buxbaum LJ. Treatments of unilateral neglect: a review. Arch Phys Med Rehabil. 2002;83:256-268.
36 Bowen A, Lincoln NB, Dewey M. Cognitive rehabilitation for spatial neglect following stroke. Cochrane Database Syst Rev. (2):2002. CD003586.
37 Rossetti Y, Rode G, Pisella L, et al. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature. 1998;395:166-169.
38 Maravita A, McNeil J, Malhotra P, et al. Prism adaptation can improve contralesional tactile perception in neglect. Neurology. 2003;60:1829-1831.
39 Frassinetti F, Angeli V, Meneghello F, et al. Long-lasting amelioration of visuospatial neglect by prism adaptation. Brain. 2002;125:608-623.
40 McIntosh RD, Rossetti Y, Milner AD. Prism adaptation improves chronic visual and haptic neglect: a single case study. Cortex. 2002;38:309-320.