Chapter 16 The Management of Epilepsy in Pregnancy
Introduction
Women with epilepsy have been reported to account for 0.3 to 0.4% of all pregnancies, although some population-based studies suggest a prevalence of epilepsy among pregnant women of up to 0.7%.1,2 The proportion of pregnancies with exposure to antiepileptic drugs (AEDs) is probably even higher, considering the increasing use of AEDs for other indications than epilepsy.3 The vast majority of these women will have uneventful pregnancies and give birth to perfectly normal children. However, medical management during pregnancy is a matter of special concern because maternal epilepsy and AED treatment are associated with an increased risk for an abnormal pregnancy outcome. The maternal and fetal risks associated with uncontrolled seizures generally necessitate continued drug treatment during pregnancy, but these seizure-related risks need to be weighed against the potential adverse outcomes in the offspring due to maternal use of AEDs. Further important issues to be considered are the effect of pregnancy on seizure control and gestation-induced alterations in the disposition of AEDs, as well as matters of relevance immediately after delivery such as breast-feeding.
Prepregnancy Counseling
To be effective, most actions that can be taken to optimize the management of epilepsy in pregnancy need to be completed or initiated before conception. Prepregnancy counseling is therefore essential. Issues that should be addressed are summarized in Table 16-1. Given that, in general, approximately half of all pregnancies are unplanned, information of relevance for future pregnancies should be the responsibility of all physicians treating young women with epilepsy. Such information should be given repeatedly and be brought up well before pregnancy is contemplated.
TABLE 16–1 Issues to be Addressed in Prepregnancy Counseling
| Genetic counseling concerning the risk of inheriting epilepsy |
| Interactions between hormonal contraceptives and antiepileptic drugs |
| The expected course of epilepsy during pregnancy and delivery |
| Fetal and maternal risks with uncontrolled seizures |
| Fetal risks associated with use of antiepileptic drugs during pregnancy |
| Possibilities and limitations of prenatal diagnostic screening tests |
| Principles of treatment of epilepsy during pregnancy |
| Folate supplementation |
Contraception
Considering the value of planned pregnancies in women with epilepsy, the importance of effective contraception cannot be overestimated. Enzyme-inducing AEDs may reduce the effectiveness of oral contraceptives by induction of the metabolism of estradiol and progesterone and possibly also by increasing the hepatic synthesis of sex-hormone-binding globulin (SHBG). The contraceptive failure rate has thus been estimated to increase several times among women on AEDs.4 AEDs with and without known inducing effects on oral contraceptives are listed in Table 16-2. These AEDs affect combined oral contraceptive pills, combined contraceptive patches, and progestogen-only pills, as well as progestogen implants.5 In women requiring enzyme-inducing AEDs, long-acting methods (e.g., medroxyprogesterone depot injection, hormone releasing or other intrauterine contraceptive devices, or barrier methods) should be considered, which in fact have been proposed as good options for all women with epilepsy. Should this not be acceptable, oral contraceptives containing at least 50 µg of estrogen could be considered.5
TABLE 16–2 Interactions Between Antiepileptic Drugs (AEDs) and Oral Contraceptives (OCs)
| AEDs Known to Induce the Metabolism of OCs | AEDs Known to be Induced by OCs |
|---|---|
| Phenobarbital | Lamotrigine |
| Primidone | Valproate* |
| Phenytoin | |
| Carbamazepine | |
| Oxcarbazepine | |
| Topiramate (at doses >200 mg/day) | |
| Lamotrigine (modest effect on norgestrel component) |
* Tentative, based only on one small study.9
The interaction between oral contraceptives and AEDs can be bidirectional. Estrogen-containing contraceptives reduce plasma concentrations of lamotrigine by at least 50%.6–8 This induction is rapidly reverted, and lamotrigine levels rise significantly during the pill-free week, when sequential pills are used.8 One small study suggests similar effects of combined contraceptives on valproate plasma concentrations.9 Concomitant use of valproate and lamotrigine, however, seems to block the effects of contraceptives on lamotrigine plasma concentrations.10
Maternal and Fetal Hazards with Seizures During Pregnancy
Epilepsy is a condition with potentially serious psychosocial and medical consequences for the patient. Seizures may cause physical injuries and occasionally even death and are thus good reasons to treat people with active epilepsy. These concerns related to the well-being of the patient with epilepsy in general are equally relevant during pregnancy. The importance of maintained seizure control during pregnancy is highlighted in a recent review of all maternal deaths in the U.K. during 1985–1999.11 Women with epilepsy were reported to account for 3.8% of all maternal deaths, considerably more than expected from the prevalence of epilepsy in pregnancy. The mortality was partly related to seizure occurrence after stopping AED treatment.11 Although the absolute risk is very low, the data underline the importance of seizure control for maternal health.
The fetal effects of maternal seizures depend on the type of seizures. Although other seizure types have negligible effects, generalized tonic-clonic seizures increase the pressure in the pregnant uterus and may lead to a trauma if the patient falls. Generalized tonic-clonic seizures also induce lactic acidosis,12 and this has been shown to transfer to the fetus.13 Convulsive seizures may also cause fetal bradycardia,14 and status epilepticus can result in intrauterine death.15,16 However, the large prospective EURAP antiepileptic drugs and pregnancy registry reported only one case of intrauterine death16 and no maternal mortality among 36 cases with status epilepticus. Furthermore, recent data suggest that the number of stillbirths is not increased among women that are adequately treated for their epilepsy during pregnancy.17,18 Occurrence of seizures during the first trimester does not seem to increase the risk of malformations in the offspring.2,18–33
Seizure Control in Pregnancy
So far, the largest prospective study on seizure control in pregnant women with epilepsy concluded that the majority, 58%, remained seizure free throughout pregnancy, and that 18% had convulsive seizures on some occasion during pregnancy (EURAP).16 Whether pregnancy in itself affects the course of epilepsy remains a partly controversial issue, with variable observations in the literature. Pooling data (altogether 2249 pregnancies) from studies published after 1980, seizure control was the same during pregnancy as before in 62.5%, improved in 11.4%, and deteriorated in 24.6%.21,25,34–51 These comparisons are, however, hampered by several methodological problems, including a different type of management during pregnancy compared to before and prospective follow-up during pregnancy compared to a retrospective prepregnancy baseline. Nevertheless, these data demonstrate that seizure control will be unaffected by pregnancy in most cases and that the majority of women with epilepsy will remain seizure free throughout. Some of the reported changes in seizure control may also be explained by the normal random fluctuations in seizure occurrence in epilepsy and thus be unrelated to pregnancy. However, women with localization-related epilepsy,16,37,46,49 epilepsy of long duration,34,39,43,46,47,49,50 and in particular poor seizure control before pregnancy46,50 are more likely to deteriorate. The effect of pregnancy may vary between patients with similar types of seizure disorders, but also in different pregnancies of the same patient,50,53 and is thus difficult to predict.
Pharmacokinetic, metabolic, hormonal, physiological, and psychological factors have all been suggested as contributing causes to gestational changes in seizure control. In some cases, changes in seizure frequency may be related to lack of compliance, not seldom due to maternal fear of teratogenic effects of AEDs,36,37 or for other reasons of decreased plasma AED levels.36,37,54,55
Recent reports from prospective pregnancy registers indicate that poor seizure control, increased seizure frequency, or need for increased dosage may be more common with lamotrigine and oxcarbazepine than with other AEDs.16,56 This might be related to pharmacokinetic changes, which will be discussed separately in the following section.
Although the data on general changes in seizure control during pregnancy are conflicting, the findings are consistent concerning an increased risk of seizures during labor and delivery. Seizures occur at labor and during delivery in approximately 2.5% of the cases,16,18,37,39,44,46 an almost 10-fold greater risk than otherwise during pregnancy. The risk is higher for patients with seizures earlier during pregnancy.16
Status epilepticus occurs in about 1% of the cases.16,34–37,39,40,43,44,46,49,50,57 The incidence of status epilepticus is probably not higher in pregnancy than otherwise. It requires prompt attention and should be treated according to the same principles as otherwise. Refractory status epilepticus in the third trimester of pregnancy could also be an indication for a caesarean.58
Effects of Pregnancy on Pharmacokinetics of Antiepileptic Drugs
Pregnancy is associated with several physiological changes that may affect drug disposition and thus maternal plasma concentrations and fetal exposure to AEDs.59,60 Decreased protein binding is relevant for highly bound drugs. It will result in reduced total plasma concentrations, but unaffected unbound drug levels. Because the unbound concentrations determine the pharmacological effects in the mother and exposure to the fetus, alterations in protein binding will not have any clinical consequences. However, total plasma concentrations can be misleading in pregnancy for highly protein-bound AEDs, such as phenytoin and valproic acid.61,62 Enhanced drug elimination due to induction of metabolizing enzymes is clinically the most important mechanism for gestation-induced alterations in AED kinetics. This occurs with drugs metabolized through the cytochrome P450 system (e.g., phenytoin and phenobarbital), but is even more pronounced for lamotrigine and possibly oxcarbazepine, drugs eliminated through glucuronidation.63–65
Effects of pregnancy on plasma concentrations of AEDs are summarized in Table 16-3. The decline in AED concentrations generally begins during the first trimester. In late pregnancy, the decrease is on average 55 to 61% for phenytoin total and 18 to 31% for unbound concentrations;61,66–69 0 to 42% for carbamazepine total and 0 to 28% for unbound concentrations; 50 to 55% for phenobarbital,62,73 55% for primidone; 50% for valproic acid total and 0 to 29% for unbound concentrations.61,74
TABLE 16–3 Effects of Pregnancy on Plasma Concentrations of Antiepileptic Drugs
| AED | Approximate Average Total Plasma Concentration in Late Pregnancy Compared to Before | Approximate Average Unbound Plasma Concentration in Late Pregnancy Compared to Before |
|---|---|---|
| Phenobarbital | 50% | Probably same as total concentration* |
| Phenytoin | 40–45% | 70–80% |
| Carbamazepine | 60–100% | 75–100% |
| Valproate | 50% | 75–100% |
| Lamotrigine | 25–40% | 30–40% |
| Oxcarbazepine | 40–50% | Probably same as total concentration* |
| Levetiracetam | 40–50% | Probably same as total concentration* |
| Topiramate | No data available | No data available |
| Tiagabine | No data available | No data available |
| Gabapentin | No data available | No data available |
| Pregabalin | No data available | No data available |
| Zonisamide | No data available | No data available |
* Data on unbound concentrations during pregnancy are lacking, but decline can be assumed to be the same as for total concentrations based on the comparatively low-binding to plasma proteins for this drug in general.
Lamotrigine plasma concentrations decrease by an average of 68% during pregnancy, with a wide interindividual variability and sometimes with deterioration of seizure control.75–79 The decrease of lamotrigine plasma levels is markedly reduced10 when lamotrigine is taken in combination with valproic acid.
More limited data indicate a decrease of a similar magnitude for the active moiety of oxcarbazepine, its monohydroxy derivative.80,81 A 50% decrease in levetiracetam concentrations has been reported in late pregnancy.82–84 Very limited or no information is available on possible changes in disposition of the other newer-generation AEDs.63
Most studies involving older-generation AEDs have failed to demonstrate a relationship between seizure control and alterations in AED plasma concentrations.35,45,49 However, with lamotrigine monotherapy breakthrough, seizures have been linked to the marked decline in plasma concentrations in a relatively high proportion of patients.78,79 Prospective pregnancy register data also indicate that patients on lamotrigine more often need either dosage adjustments during pregnancy16,56 or additional AEDs.16
Oxcarbazepine has been reported to be associated with a poorer seizure control during pregnancy compared to other monotherapies.16 This could also be explained by pharmacokinetic alterations because the pronounced decline in plasma concentrations of the active moiety of oxcarbazepine was associated with breakthrough seizures in a small case series.80
Developmental Toxicity
The most important among the effects that have been attributed to developmental toxicity of AEDs are intrauterine growth retardation, increased prevalence of minor anomalies and major congenital malformations, impaired postnatal cognitive development, and more or less specific fetal AED syndromes. Some of these effects are well documented, whereas the occurrence of others is more controversial.
INTRAUTERINE GROWTH RETARDATION
Reductions in body dimensions, in particular head circumference, have been reported in several cohorts of children exposed to AEDs.23,32,85–90 This has been associated with polytherapy,85–87,89,90 and some investigators have also found associations to monotherapy with phenobarbital, primidone, or carbamazepine. Wide et al.89 studied body dimensions in infants exposed to AEDs in utero in a Swedish population over a period of 25 years, comparing data to the general population. There was a clear trend toward normalization of the head circumference over the time period in parallel with a shift from polytherapy to monotherapy, despite an increasing use of carbamazepine. Other more recent studies also suggest that, with present treatment strategies where monotherapy prevails, microcephaly may no longer be more common among infants of mothers treated for epilepsy during pregnancy.91,92
MINOR ANOMALIES AND FETAL AED SYNDROMES
Minor anomalies are structural variations without medical, surgical, or cosmetic importance. These frequently occur in normal infants, but combinations of several anomalies can form a dysmorphic syndrome, which may indicate a more severe underlying dysfunction.93 Minor anomalies and dysmorphic syndromes have been reported more frequently in infants of mothers treated for epilepsy during pregnancy. Facial features such as hypertelorism, depressed nasal bridge, low-set ears, micrognathia, and distal digital hypoplasia, sometimes in combination with growth retardation and developmental delay, were first reported in association with exposure to phenytoin.94 Subsequently, however, similar patterns have been associated with exposure to carbamazepine.95 Valproate exposure has been claimed to cause a somewhat different dysmorphic syndrome characterized by thin arched eyebrows with medial deficiency, broad nasal bridge, short anteverted nose, and a smooth long filtrum with thin upper lip.93 However, there is a considerable overlap in the various dysmorphisms, and their drug specificity has been questioned. A more general term, fetal or prenatal AED syndrome, has therefore been suggested.96 In addition, the pathogenesis is still somewhat controversial, and some investigators have attributed most of the minor anomalies to genetic factors rather than drug exposure.97 It should, however, be underlined that minor anomalies are much more difficult to assess objectively than major malformations, and that the incidence of minor anomalies in exposed infants varies markedly between studies.98
MAJOR CONGENITAL MALFORMATIONS
The rate of major congenital malformations is two to three times higher among children of mothers with epilepsy compared to the general population. The reason for this risk increase is probably multifactorial, including effects of seizures and epilepsy, genetic and socioeconomic factors, and teratogenic effects of AEDs. However, the available evidence strongly suggests that exposure to AEDs is the major cause. Pooling data from 26 controlled studies,2,19,21,22,24,26,30,32,50,91,97,99–113 the malformation rate was 6.1% in offspring that had been exposed to AEDs (n = 4630), compared to 2.8% in children of untreated women with epilepsy (n = 1292) and 2.2% in offspring of mothers without epilepsy (n = 40, 221). Nevertheless, a genetic influence is shown by the increased risk of malformations when there is a family history of malformations.19,25,27,31,107,114–134 The importance of genetic susceptibility to teratogenic effects of AEDs is further supported by case-control studies reporting a higher proportion of relatives with epilepsy in patients with cleft palate or lip135–139 or neural tube defects.140,141
The fact that most studies report higher malformation rates in association with polytherapy than monotherapy with AEDs, and a dose-effect relationship for teratogenic risks with some AEDs, lends further support to the importance of AEDs for adverse pregnancy outcome among women with epilepsy. The rate of major malformations was 6.8% among children exposed to AED polytherapy (n = 4253), compared to 4.0% after monotherapy (n = 8339) when data from 74 studies were pooled.2,18,19,21–24,26,28,31,32,42,46,50,56,95,97,100–102,104–106,108–111,119,122,142–183
Several studies have observed a dose-related risk of birth defects in association with valproate exposure.25,152,153,158,160,184–186 Although some studies have failed to demonstrate this relationship,24,28,187 it appears that the risk is greater with dosages above 800 to 1000 mg/day. Recently a dose-related effect was reported also for lamotrigine,150 although this was not confirmed in another study.188 The severity of epilepsy may of course confound the association between doses and the risk of malformations, as well as the association between polytherapy and the risk of malformations, but it has shown beyond any reasonable doubt that exposure to AEDs is the major cause of increased birth defect rates among children of women with epilepsy. It is furthermore reasonable to assume that, in general, risks are lower with monotherapy than polytherapy and with lower compared to higher dosages.
The types of malformations reported at higher rates in the offspring of epileptic mothers are mainly heart defects, neural tube defects, facial clefts, hypospadias, and limb reduction defects. A specific association has been reported between neural tube defects and valproate114,140,153,189–194 and, to a lesser extent, between barbiturates and heart defects.114,140,153,189–194 Some studies also reported a higher risk of limb reduction defects associated with valproate exposure190,196 or hypospadias153,190 and a higher risk of oral cleft with barbiturates.27,190,192 A recent study also suggests an increased risk for oral clefts associated with lamotrigine.197
A major issue with profound implications for the management of epilepsy in pregnancy is whether AEDs differ in their overall teratogenic potential. Earlier studies have largely failed to address this because of methodological shortcomings, in particular insufficient numbers of pregnancies and low statistical power.198 For this reason, large prospective epilepsy and pregnancy registries have been established in recent years, each enrolling thousands of pregnancies with AED exposure. Some of these registries have published pregnancy outcome data in association with individual types of AED exposures. A summary of recent studies is presented in Table 16-4.
TABLE 16–4 Malformation Rates with Exposure to Different Antiepileptic Drugs in Monotherapy (N = Offspring with Malformation)
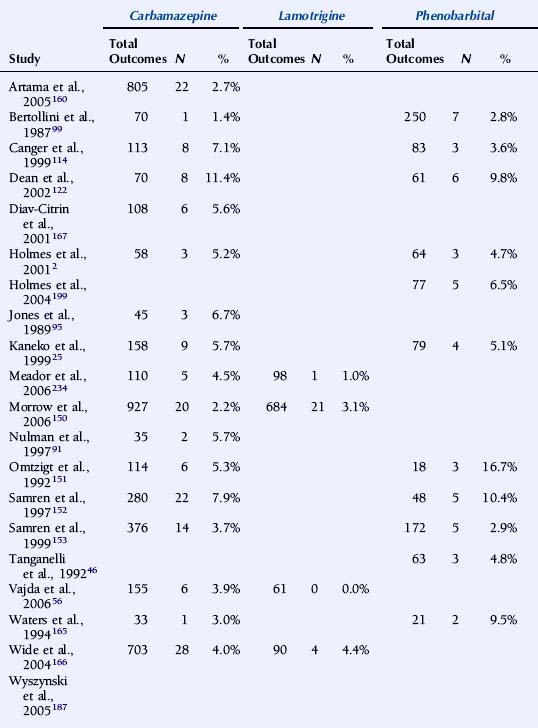
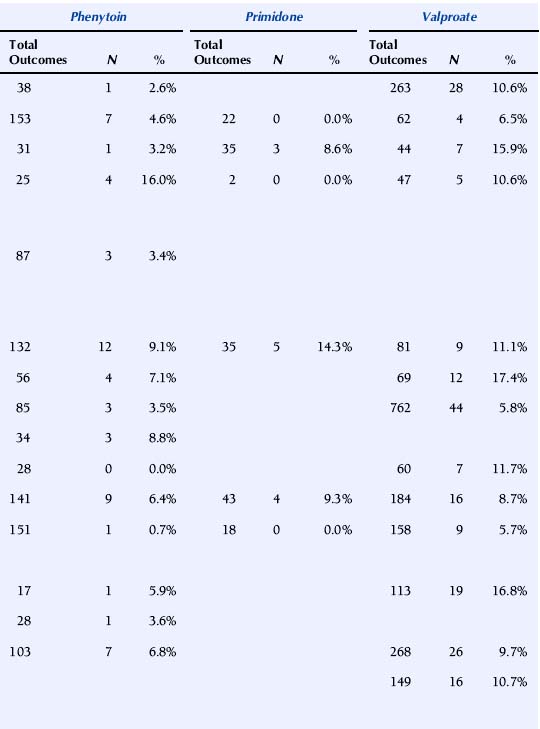
The U.K. Epilepsy and Pregnancy Register reported malformation rates based on 3607 prospective pregnancies.150 The overall prevalence of major congenital malformations for all cases exposed to AEDs was 4.2% (95% CI: 3.6% to 5.0%), slightly higher, but interestingly not significantly so, compared with 3.5% (1.8% to 6.8%) for infants of women with epilepsy who had not taken AEDs during pregnancy. The rate of birth defects was greater for pregnancies exposed to valproate in monotherapy, 6.2% (4.6% to 8.2%; n = 715) than to carbamazepine monotherapy, 2.2% (1.4% to 3.4%; n = 900). The malformation rate for those exposed to lamotrigine monotherapy was 3.2% (2.1% to 4.9%; n = 647). A correlation between major congenital malformations and drug dosage was found for lamotrigine.150
The North American AED Pregnancy Registry has released outcome data for four AEDs.187,197,199,200 With exposure to phenobarbital monotherapy 6.5% (95% CI: 2.1 to 14.5%; n = 77) had major malformations. The relative risk (RR) compared with the external background rate (infants of mothers without epilepsy from a Boston Hospital with a malformation rate of 1.62%) was 4.2 (95% CI: 1.5% to 9.4%), and compared with three other AEDs combined in monotherapy from the same registry 2.0 (95% CI: 0.9 to 4.5%).199
The rate of major malformations with exposure to valproate monotherapy was 10.7% (95% CI: 6.3 to 16.9%; n = 149). RR compared with background rate was 7.3 (95% CI: 4.4 to 12.2%), and OR compared with an internal comparison group (infants exposed to all other AEDs as monotherapy in the same registry) was 4.0 (95% CI: 2.1 to 7.4%).187 The prevalence of major malformations among infants born to women taking lamotrigine monotherapy was 2.7% (95% CI: 1.5 to 4.3%; n = 564). RR compared with the background rate was 1.7 (95% CI: 1.0 to 2.7%). An increased risk was found for orofacial clefts with exposure to lamotrigine, RR being 32.8 (95% CI: 10.6 to 101.3%) compared with background rate.197 Among infants exposed to carbamazepine as monotherapy in the first trimester, 2.6% (95% CI: 1.5 to 4.3%; n = 873) had major malformations. RR compared to the background rate was 1.6 (95% CI: 0.9 to 2.8%).200
The Australian AED and Pregnancy Register recently released outcome data based on 555 pregnancies, of which 485 were prospective.56 Valproate at doses above 1100 mg/day was associated with significantly higher risk of fetal malformations than other AEDs.
Comparatively high malformation rates with valproate have been reported in two additional national register studies. The nationwide population-based Swedish Medical Birth Registry was published based on 1398 pregnancies with exposure to AEDs.166 The OR for severe malformation after exposure to valproate monotherapy (n = 268) compared with carbamazepine monotherapy (n = 703) was 2.59 (95% CI: 1.43 to 4.68%). In a study based on the Finnish drug prescription database and the National Medical Birth Registry, (pregnancies with AED exposure (n = 1411), the risk of malformations was higher in fetuses exposed to valproate monotherapy (malformation rate 10.7%; OR = 4.18%; 2.31% to 7.57%) than of untreated patients. In contrast, the risk of malformations was not elevated in association with exposure to carbamazepine, oxcarbazepine, or phenytoin monotherapy.160
It is difficult to compare rates of birth defects between registries because of significant differences in methodology, including methods for enrollment and inclusion, exclusion criteria, and duration of follow-up, as well as criteria for teratogenic outcome. Nevertheless, a higher malformation rate in association with valproate compared with some other AEDs, in particular carbamazepine, has been a consistent finding. Recent studies indicate that the prevalence of birth defects with lamotrigine is similar to that with carbamazepine. The manufacturer’s International Lamotrigine Pregnancy Registry thus reported major birth defects in 22 of 802 first trimester monotherapy exposures (2.7%; 1.8% to 4.2%).188 Unfortunately, prospective data on teratogenic outcome in association with exposure to other newer generation AEDs is very scarce, and no firm conclusions can be drawn about their relative safety in pregnancy.
POSTNATAL DEVELOPMENT
Studies on potential adverse effects of prenatal exposure to AEDs on long-term postnatal development are scarcer and have come to partly conflicting conclusions. Such studies are difficult to perform and also complicated to interpret because of several confounding factors and because environmental factors become more important with increasing age of the child. In a prospective population-based study, Gaily et al.201 found no influence on global IQ, and the observed cognitive dysfunction in exposed children, mainly phenytoin and carbamazepine, was attributed to maternal seizures and educational level of the parents, rather than to the treatment. In another population-based prospective study, Wide et al.,202 found no difference in psychomotor development in children exposed to carbamazepine compared to control children of healthy mothers. Scolnik et al.203 reported lower global IQ in children exposed to phenytoin but not in those exposed to carbamazepine. Another study found normal intellectual capacity in most of 170 individuals exposed to phenobarbital and phenytoin, but 12% of the exposed subjects versus 1% of unexposed controls had persistent learning problems.90 A Cochrane Review concluded that the majority of studies on developmental effects of AEDs are of limited quality and that there is little evidence about which drugs carry more risks than others to the development of children exposed.204 Some studies in recent years, however, have suggested that exposure to valproate might be associated with a particular risk of adverse developmental effects.205,206 A retrospective survey from the U.K. found additional educational needs to be considerably more common among children that had been exposed to valproate monotherapy than in those exposed to carbamazepine or in unexposed children.205 A more thorough investigation of partly the same cohort of children revealed significantly lower verbal IQ in children exposed to valproate monotherapy (mean 83.6%, 95% CI: 78.2 to 89.0%, n = 41) than in unexposed children (90.9% CI: 87.2 to 94.6%, n = 80) and children exposed to carbamazepine (94.1%, CI: 89.6 to 98.5%, n = 52) or phenytoin (98.5% CI: 90.6 to 106.4%, n = 21).11,206 Exposure to valproate remained associated with lower verbal IQ after adjustment for confounding factors. Valproate doses above 800 mg/day were associated with lower verbal IQ than lower doses.
These results still need to be interpreted with some caution given the small numbers, the retrospective nature of the study, and the fact that only 40% of eligible mothers agreed to participate. The important signals from this report need to be confirmed or refuted in well-designed prospective studies. A recent small population-based prospective study from Finland found a lower verbal IQ in children exposed in utero to valproic acid and to polytherapy in general compared with nonexposed children or children exposed to carbamazepine.207 However, this study could not demonstrate an independent effect of valproate because of small numbers and because the results were confounded by low maternal education and polytherapy. Another small prospective population-based Finnish study signals a similar trend for worse outcome in children exposed to valproate but also points to the problem of confounding factors, as the mothers using valproate in pregnancy scored lower on IQ than other groups.208
Obstetrical Complications and Delivery
With modern management, epilepsy does not seem to be associated with a significant increase in obstetrical complications, such as preeclampsia, premature delivery, or placental abruption.17,18,209 However, as the risk of seizures is increased during labor and delivery, delivery should take place in appropriately equipped units. A caesarean delivery might be necessary if a generalized tonic-clonic seizure occurs during labor. These are rare occurrences, and the vast majority of women with epilepsy proceed with deliveries that are in line with those of women in general.
Folate Supplementation
Low maternal levels of folate have been associated with an increased risk of neural tube defects in the general population,210–213 and some studies have also reported an increased risk of adverse pregnancy outcome with lower folate levels in women on AEDs.214–216 Although periconceptional folate supplementation has been shown to significantly reduce the risk of birth defects, and in particular that of neural tube defects in the general population,217–219 evidence is still lacking for the effectiveness of folic acid in the prevention of AED-induced teratogenicity, and the appropriate supplementation dosage is still debated.220 Nevertheless periconceptional supplementation with folic acid is usually recommended for women exposed to AEDs,220–222 but the women need to be informed about the lack of solid evidence documenting the efficacy. Suggested doses range from 0.4 to 0.5 mg/day to 5 mg/day.
Breast-Feeding
Data on excretion of AEDs in breast milk and serum concentrations in suckling infants is summarized in Table 16-5. Data are fairly extensive for the older-generation AEDs. Low milk/maternal plasma concentration ratios and low plasma concentrations in suckling infants have been documented with phenytoin223 and valproate224 and in general also for carbamazepine.225 Drug transfer to breast milk appears to be more extensive for ethosuximide226 and phenobarbital,227 and the risk for accumulation in the suckling infant has been discussed in particular for phenobarbital. For lamotrigine, the milk/maternal plasma concentration ratio has been <1, but lamotrigine levels as high as 11 µmol/L have been reported in suckling infants.75 The levetiracetam milk/maternal plasma concentration ratio is close to unity, whereas plasma concentrations in the nursed infants are generally low.84,228 The published data is more limited for other newer-generation AEDs. The mean topiramate milk/maternal plasma-concentration ratio was 0.86 in five mothers under treatment with topiramate, and drug levels in the nursed infants were very low (below the level of quantification) 2 to 3 weeks after delivery.229 Preliminary data from five mothers treated with gabapentin demonstrate a similarly extensive transfer to breast milk with a mean milk/plasma-concentration ratio of 1.0 and very low gabapentin levels in the suckling infants 2 to 3 weeks after birth.230 There is only one published case with breast milk data on oxcarbazepine, reporting a milk/plasma ratio of 0.5 for the active monohydroxy derivative but no information on serum concentrations in the infant.231 Information on zonisamide is also limited to a single case with a milk/plasma-level ratio of 0.9 and no data on drug levels in the nursed infant.232 To our knowledge, there are no published reports on breast-feeding under treatment with tiagabine or pregabalin.
TABLE 16–5 Transfer into Breast Milk of Antiepileptic Drugs and Plasma Concentrations in Breast-Fed Infants
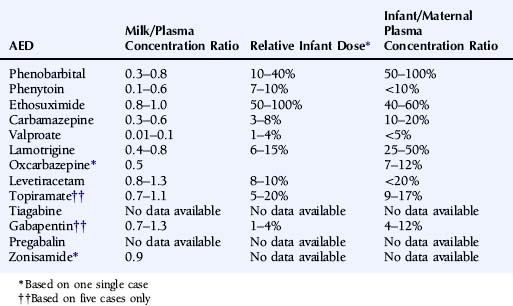
Although the numbers on some of the newer AEDs in particular are small, it is worth noting that no adverse effects related to breast-feeding have been reported with these drugs. In general, breast-feeding is thus encouraged,221 although considerable drug concentrations have been reported occasionally in children of mothers treated with some AEDs, such as phenobarbital, ethosuximide, and lamotrigine. Mothers using these AEDs should be informed on the possibility of drug effects on the neonate but not generally advised against breast-feeding.233
Practical Management
The management of epilepsy in pregnancy is based on the assumption that generalized tonic-clonic seizures are more harmful to the fetus than AEDs. From this it follows that AEDs are indicated also during pregnancy in patients who otherwise would be likely to have such convulsive seizures. Other types of maternal seizures are probably rarely harmful to the fetus. Frequent partial or myoclonic seizures might, however, indicate an increased risk of a generalized tonic-clonic seizure. Therefore, it has to be decided individually whether AEDs are justified during pregnancy in women with such seizures. Additionally, frequent partial seizures may be disabling for the mother and thus constitute an indication for treatment, whether the patient is pregnant or not.
This should be tried before pregnancy irrespective of the type of AED, although the most convincing documentation of a dose-effect relationship is with valproate. Available data indicate that valproate at < 800 mg/day might not be associated with greater risks for adverse pregnancy outcome than other AEDs at ordinary doses.56
In women who already are pregnant, there is probably very little to gain, and much to risk, by switching AEDs if the seizures are well controlled. However, if seizures persist, efforts should be made to optimize the treatment.
1. Gaily E. Development and growth in children of epileptic mothers. A prospective controlled study. Acta Obstet Gynecol Scand. 1991;70(7-8):631-632.
2. Holmes LB, Harvey EA, Coull BA, et al. The teratogenicity of anticonvulsant drugs. N Engl J Med. 2001;344(15):1132-1138.
3. Spina E, Perugi G. Antiepileptic drugs: indications other than epilepsy. Epileptic Disord. 2004;6(2):57-75.
4. Coulam CB, Annegers JF. Do anticonvulsants reduce the efficacy of oral contraceptives? Epilepsia. 1979;20(5):519-525.
5. O’Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47(9):1419-1422.
6. Sabers A, Ohman I, Christensen J, Tomson T. Oral contraceptives reduce lamotrigine plasma levels. Neurology. 2003;61(4):570-571.
7. Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46(9):1414-1417.
8. Sidhu J, Job S, Singh S, Philipson R. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61(2):191-199.
9. Galimberti CA, Mazzucchelli I, Arbasino C, Canevini MP, Fattore C, Perucca E. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47(9):1569-1572.
10. Tomson T, Luef G, Sabers A, Pittschieler S, Ohman I. Valproate effects on kinetics of lamotrigine in pregnancy and treatment with oral contraceptives. Neurology. 2006;67(7):1297-1299.
11. Adab N, Kini U, Vinten J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004;75(11):1575-1583.
12. Lipka K, Bulow HH. Lactic acidosis following convulsions. Acta Anaesthesiol Scand. 2003;47(5):616-618.
13. Hiilesmaa VK, Bardy A, Teramo K. Obstetric outcome in women with epilepsy. Am J Obstet Gynecol. 1985;152(5):499-504.
14. Teramo K, Hiilesmaa V, Bardy A, Saarikoski S. Fetal heart rate during a maternal grand mal epileptic seizure. J Perinat Med. 1979;7(1):3-6.
15. Teramo K, Hiilesmaa V. Pregnancy and fetal complications in epileptic pregnancies. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, Pregnancy, and the Child. New York: Raven Press; 1982:53-59.
16. Seizure control and treatment in pregnancy: observations from the EURAP epilepsy pregnancy registry. Neurology. 2006;66(3):354-360.
17. Katz O, Levy A, Wiznitzer A, Sheiner E. Pregnancy and perinatal outcome in epileptic women: a population-based study. J Matern Fetal Neonatal Med. 2006;19(1):21-25.
18. Richmond JR, Krishnamoorthy P, Andermann E, Benjamin A. Epilepsy and pregnancy: an obstetric perspective. Am J Obstet Gynecol. 2004;190(2):371-379.
19. Annegers JF, Hauser I. The frequency of malformations in relatives of patients with epilepsy. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, Pregnancy, and the Child. New York: Raven Press; 1982:267-273.
20. Beck-Mannagetta G, Drees B, Janz D. Malformations and minor anomalies in the offspring of epileptic parents: a retrospective study. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, Pregnancy, and the Child. New York: Raven Press; 1982:317-323.
21. Dravet C, Julian C, Legras C, et al. Epilepsy, antiepileptic drugs, and malformations in children of women with epilepsy: a French prospective cohort study. Neurology. 1992;42(4 Suppl 5):75-82.
22. Fedrick J. Epilepsy and pregnancy: a report from the Oxford Record Linkage Study. Br Med J. 1973;2(5864):442-448.
23. Fonager K, Larsen H, Pedersen L, Sorensen HT. Birth outcomes in women exposed to anticonvulsant drugs. Acta Neurol Scand. 2000;101(5):289-294.
24. Kaaja E, Kaaja R, Hiilesmaa V. Major malformations in offspring of women with epilepsy. Neurology. 2003;60(4):575-579.
25. Kaneko S, Battino D, Andermann E, et al. Congenital malformations due to antiepileptic drugs. Epilepsy Res. 1999;33(2-3):145-158.
26. Koch S, Losche G, Jager-Roman E, et al. Major and minor birth malformations and antiepileptic drugs. Neurology. 1992;42(4 Suppl 5):83-88.
27. Nakane Y, Okuma T, Takahashi R, et al. Multi-institutional study on the teratogenicity and fetal toxicity of antiepileptic drugs: a report of a collaborative study group in Japan. Epilepsia. 1980;21(6):663-680.
28. Sabers A, Dam M, A-Rogvi-Hansen B, et al. Epilepsy and pregnancy: lamotrigine as main drug used. Acta Neurol Scand. 2004;109(1):9-13.
29. Shapiro S, Hartz SC, Siskind V, et al. Anticonvulsants and parental epilepsy in the development of birth defects (prospective study). Lancet. 1976;1(7954):272-275.
30. Speidel BD, Meadow SR. Maternal epilepsy and abnormalities of fetus and the newborn. Lancet. 1972;2:839-843.
31. Starreveld-Zimmerman AA, van der Kolk WJ, Meinardi H, Elshove J. Are anticonvulsants teratogenic? Lancet. 1973;2(819):48-49.
32. Steegers-Theunissen RP, Renier WO, Borm GF, et al. Factors influencing the risk of abnormal pregnancy outcome in epileptic women: a multi-centre prospective study. Epilepsy Res. 1994;18(3):261-269.
33. Yerby MS, Leavitt A, Erickson DM, et al. Antiepileptics and the development of congenital anomalies. Neurology. 1992;42(4 Suppl 5):132-140.
34. Remillard G, Dansky L, Andermann E, Andermann F. Seizure frequency during pregnancy and puerperium. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, Pregnancy, and the Child. New York: Raven Press; 1982:15-25.
35. Canger R, Avanzini G, Battino D, Bossi L, Franceschetti S, Spina S. Modifications of seizure frequency in pregnant patients with epilepsy: a prospective study. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, Pregnancy, and the Child. New York: Raven Press; 1982:33-38.
36. Schmidt D, Canger R, Avanzini G, et al. Change of seizure frequency in pregnant epileptic women. J Neurol Neurosurg Psychiatry. 1983;46(8):751-755.
37. Otani K. Risk factors for the increased seizure frequency during pregnancy and puerperium. Folia Psychiatr Neurol Jpn. 1985;39(1):33-41.
38. Nogales Gaete J. [The frequency of crises in epileptic women during pregnancy: a prospective study]. Rev Med Chil. 1986;114(11):1052-1057.
39. Bardy AH. Incidence of seizures during pregnancy, labor and puerperium in epileptic women: a prospective study. Acta Neurol Scand. 1987;75(5):356-360.
40. Gjerde IO, Strandjord RE, Ulstein M. The course of epilepsy during pregnancy: a study of 78 cases. Acta Neurol Scand. 1988;78(3):198-205.
41. Kaneko S. A rational antiepileptic drug therapy of epileptic women in child bearing age. Jpn J Psychiatry Neurol. 1988;42(3):473-482.
42. Bag S, Behari M, Ahuja GK, Karmarkar MG. Pregnancy and epilepsy. J Neurol. 1989;236(5):311-313.
43. Specchio LM, La Neve A, Ostillio G, Lanzi C, Fanizza G. Frequenza delle crisi epilettiche durante la gravidanza in un’osservazione prospettica. Boll Lega It Epil. 1989;66(67):299-301.
44. Wilhelm J, Morris D, Hotham N. Epilepsy and pregnancy—a review of 98 pregnancies. Aust N Z J Obstet Gynaecol. 1990;30(4):290-295.
45. Lander CM, Eadie MJ. Plasma antiepileptic drug concentrations during pregnancy. Epilepsia. 1991;32(2):257-266.
46. Tanganelli P, Regesta G. Epilepsy, pregnancy, and major birth anomalies: an Italian prospective, controlled study. Neurology. 1992;42(4 Suppl 5):89-93.
47. Kilpatrick CJ, Hopper JL. The effect of pregnancy on the epilepsies: a study of 37 pregnancies. Aust N Z J Med. 1993;23(4):370-373.
48. Vidovic MI, Della Marina BM. Trimestral changes of seizure frequency in pregnant epileptic women. Acta Med Croatica. 1994;48(2):85-87.
49. Tomson T, Lindbom U, Ekqvist B, Sundqvist A. Epilepsy and pregnancy: a prospective study of seizure control in relation to free and total plasma concentrations of carbamazepine and phenytoin. Epilepsia. 1994;35(1):122-130.
50. Sabers A, Rogvi-Hansen B, Dam M, et al. Pregnancy and epilepsy: a retrospective study of 151 pregnancies. Acta Neurol Scand. 1998;97(3):164-170.
51. Thomas SV, Devi GC, Radhakrishnan K, Joshua C. Seizure pattern during pregnancy and puerperium among women with epilepsy [Abstract]. Epilepsia. 2001;41(Suppl 7):98.
52. Remilliard G, Dansky L, Andermann E, Andermann F. Seizure frequency during pregnancy and the puerperium. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, Pregnancy, and the Child. New York: Raven Press; 1982:15-26.
53. Schmidt D. The effect of pregnancy on the natural history of epilepsy. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, Pregnancy, and the Child. New York: Raven Press; 1982:3-14.
54. Lander CM, Edwards VE, Eadie MJ, Tyrer JH. Plasma anticonvulsant concentrations during pregnancy. Neurology. 1977;27(2):128-131.
55. Krishnamurthy K, Sundstrom D, Beaudoin J, Kiriakopoulos E. Pregnant women with epilepsy taking older anticonvulsants must have their drug levels checked frequently to avoid seizures [Abstract]. Epilepsia. 2002;43(Suppl 7):232-233.
56. Vajda FJ, Hitchcock A, Graham J, et al. Foetal malformations and seizure control: 52 months data of the Australian Pregnancy Registry. Eur J Neurol. 2006;13(6):645-654.
57. Schmidt D. The effect of pregnancy on the course of epilepsy: a prospective study. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, Pregnancy, and the Child. New York: Raven Press; 1982:39-49.
58. Tomson T, Hiilesmaa V. Epilepsy in pregnancy. BMJ. 2007;335:769-773.
59. Perucca E. Drug metabolism in pregnancy, infancy and childhood. Pharmacol Ther. 1987;34(1):129-143.
60. Krauer B, Krauer F. Drug kinetics in pregnancy. Clin Pharmacokinet. 1977;2(3):167-181.
61. Yerby MS, Friel PN, McCormick K. Antiepileptic drug disposition during pregnancy. Neurology. 1992;42(4 Suppl 5):12-16.
62. Yerby MS, Friel PN, McCormick K, et al. Pharmacokinetics of anticonvulsants in pregnancy: alterations in plasma protein binding. Epilepsy Res. 1990;5(3):223-228.
63. Tomson T, Battino D. Pharmacokinetics and therapeutic drug monitoring of newer antiepileptic drugs during pregnancy and puerperium. Clin Pharmacokinet. 2007;46(3):209-219.
64. Garnett WR. Lamotrigine: pharmacokinetics. J Child Neurol. 1997;12(Suppl 1):S10-S15.
65. May TW, Korn-Merker E, Rambeck B. Clinical pharmacokinetics of oxcarbazepine. Clin Pharmacokinet. 2003;42(12):1023-1042.
66. Battino D, Avanzini G, Bossi L, et al. Monitoring of antiepileptic drug plasma levels during pregnancy and puerperium. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, Pregnancy and the Child. New York: Raven Press; 1982:147-154.
67. Bardy AH, Hiilesmaa VK, Teramo KA. Serum phenytoin during pregnancy, labor and puerperium. Acta Neurol Scand. 1987;75(6):374-375.
68. Tomson T, Lindbom U, Ekqvist B, Sundqvist A. Disposition of carbamazepine and phenytoin in pregnancy. Epilepsia. 1994;35(1):131-135.
69. Dansky L, Andermann E, Shervin A, Andermann F. Plasma levels of phenytoin during pregnancy ant the puerperium. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, Pregnancy, and the Child. New York: Raven Press; 1982:155-162.
70. Battino D, Binelli S, Bossi L, et al. Plasma concentrations of carbamazepine and carbamazepine 10,11-epoxide during pregnancy and after delivery. Clin Pharmacokinet. 1985;10(3):279-284.
71. Yerby MS, Friel PN, Miller DQ. Carbamazepine protein binding and disposition in pregnancy. Ther Drug Monit. 1985;7(3):269-273.
72. Lander CM, Livingstone I, Tyrer JH, Eadie MJ. The clearance of anticonvulsant drugs in pregnancy. Clin Exp Neurol. 1981;17:71-78.
73. Battino D, Binelli S, Bossi L, et al. Changes in primidone/phenobarbitone ratio during pregnancy and the puerperium. Clin Pharmacokinet. 1984;9(3):252-260.
74. Philbert A, Pedersen B, Dam M. Concentration of valproate during pregnancy, in the newborn and in breast milk. Acta Neurol Scand. 1985;72(5):460-463.
75. Öhman I, Vitols S, Tomson T. Lamotrigine in pregnancy: pharmacokinetics during delivery, in the neonate, and during lactation. Epilepsia. 2000;41(6):709-713.
76. Tran TA, Leppik IE, Blesi K, Sathanandan ST, Remmel R. Lamotrigine clearance during pregnancy. Neurology. 2002;59(2):251-255.
77. Pennell PB, Newport DJ, Stowe ZN, Helmers SL, Montgomery JQ, Henry TR. The impact of pregnancy and childbirth on the metabolism of lamotrigine. Neurology. 2004;62(2):292-295.
78. de Haan GJ, Edelbroek P, Segers J, et al. Gestation-induced changes in lamotrigine pharmacokinetics: a monotherapy study. Neurology. 2004;63(3):571-573.
79. Petrenaite V, Sabers A, Hansen-Schwartz J. Individual changes in lamotrigine plasma concentrations during pregnancy. Epilepsy Res. 2005;65(3):185-188.
80. Mazzucchelli I, Onat FY, Ozkara C, et al. Changes in the disposition of oxcarbazepine and its metabolites during pregnancy and the puerperium. Epilepsia. 2006;47(3):504-509.
81. Christensen J, Sabers A, Sidenius P. Oxcarbazepine concentrations during pregnancy: a retrospective study in patients with epilepsy. Neurology. 2006;67(8):1497-1499.
82. Gramstrom ML. Development of the children of epileptic mothers: preliminary results from the prospective Helsinki study. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, Pregnancy, and the Child. New York: Raven Press; 1982:403-408.
83. Pennell PB, Koganti A, Helmers S, Beach A, Newman M, Newport DJ, Stowe ZN. The impact of pregnancy and childbirth on the elimination of levetiracetam [abstract]. Epilepsia. 2005;46(Suppl 8):89.
84. Tomson T, Palm R, Källén K, et al. Pharmacokinetics of levetiracetam during pregnancy, delivery, in the neonatal period, and lactation. Epilepsia. 2007;3(10):1111-1116.
85. Hiilesmaa VK, Teramo K, Granstrom ML, Bardy AH. Fetal head growth retardation associated with maternal antiepileptic drugs. Lancet. 1981;2(8239):165-167.
86. Battino D, Granata T, Binelli S, et al. Intrauterine growth in the offspring of epileptic mothers. Acta Neurol Scand. 1992;86(6):555-557.
87. Battino D, Kaneko S, Andermann E, et al. Intrauterine growth in the offspring of epileptic women: a prospective multicenter study. Epilepsy Res. 1999;36(1):53-60.
88. Bertollini R, Kallen B, Mastroiacovo P, Robert E. Anticonvulsant drugs in monotherapy. Effect on the fetus. Eur J Epidemiol. 1987;3(2):164-171.
89. Wide K, Winbladh B, Tomson T, Kallen B. Body dimensions of infants exposed to antiepileptic drugs in utero: observations spanning 25 years. Epilepsia. 2000;41(7):854-861.
90. Dessens AB, Cohen-Kettenis PT, Mellenbergh GJ, Koppe JG, Poll NE, Boer K. Association of prenatal phenobarbital and phenytoin exposure with genital anomalies and menstrual disorders. Teratology. 2001;64(4):181-188.
91. Nulman I, Scolnik D, Chitayat D, Farkas LD, Koren G. Findings in children exposed in utero to phenytoin and carbamazepine monotherapy: independent effects of epilepsy and medications. Am J Med Genet. 1997;68(1):18-24.
92. Choulika E, Harvey, Holmes. Effect of antiepileptic drugs (AED) on fetal growth: assessment at birth. Teratology. 1999;59:388.
93. Kini U, Adab N, Vinten J, Fryer A, Clayton-Smith J. Dysmorphic features: an important clue to the diagnosis and severity of fetal anticonvulsant syndromes. Arch Dis Child Fetal Neonatal Ed. 2006;91(2):F90-F95.
94. Hanson JW, Smith DW. The fetal hydantoin syndrome. J Pediatr. 1975;87(2):285-290.
95. Jones KL, Lacro RV, Johnson KA, Adams J. Pattern of malformations in the children of women treated with carbamazepine during pregnancy [see comments]. N Engl J Med. 1989;320(25):1661-1666.
96. Zahn C. Neurologic care of pregnant women with epilepsy. Epilepsia. 1998;39(Suppl 8):S26-S31.
97. Gaily E, Granstrom ML, Hiilesmaa V, Bardy A. Minor anomalies in offspring of epileptic mothers. J Pediatr. 1988;112(4):520-529.
98. Perucca E, Tomson T. Prenatal exposure to antiepileptic drugs. Lancet. 2006;367(9521):1467-1469.
99. Akhtar N, Millac P. Epilepsy and pregnancy: a study of 188 pregnancies in 92 patients. Br J Clin Pract. 1987;41(8):862-864.
100. Dansky L, Anderman E, Anderman F. Major congenital malformation in the offspring of epileptic patients. Genetic and environmental risk factors. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, Pregnancy, and the Child. New York: Raven Press; 1982:223-234.
101. Fabris C, Licata D, Stasiowska B, Tanzilli S, Bertolini R. [Newborn infants from epileptic mothers: malformation and auxonologic risk]. Pediatr Med Chir. 1989;11(1):27-31.
102. Fairgrieve SD, Jackson M, Jonas P, et al. Population based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321(7262):674-675.
103. Goujard J, Huel G, Rumeau-Rouquette C. [Anti-epileptics and congenital malformations]. J Gynecol Obstet Biol Reprod (Paris). 1974;3(6):831-842.
104. Hvas CL, Henriksen TB, Ostergaard JR. Birth weight in offspring of women with epilepsy. Epidemiol Rev. 2000;22(2):275-282.
105. Jick SS, Terris BZ. Anticonvulsants and congenital malformations. Pharmacotherapy. 1997;17(3):561-564.
106. Kallen B. Maternal epilepsy, antiepileptic drugs and birth defects. Pathologica. 1986;78(1058):757-768.
107. Knight AH, Rhind EG. Epilepsy and pregnancy: a study of 153 pregnancies in 59 patients. Epilepsia. 1975;16(1):99-110.
108. Laskowska M, Leszczynska-Gorzelak B, Oleszczuk J. Pregnancy in women with epilepsy. Gynecol Obstet Invest. 2001;51(2):99-102.
109. Lowe CR. Congenital malformations among infants born to epileptic women. Lancet. 1973;1(7793):9-10.
110. Olafsson E, Hallgrimsson JT, Hauser WA, Ludvigsson P, Gudmundsson G. Pregnancies of women with epilepsy: a population-based study in Iceland. Epilepsia. 1998;39(8):887-892.
111. Sonneveld SW, Correy JF. Outcome of pregnancies complicated by epilepsy in Tasmania 1981-1988. Aust N Z J Obstet Gynaecol. 1990;30(4):286-289.
112. South J. Teratogenic effect of anticonvulsants. Lancet. 1972;2(7787):1154.
113. Svigos JM. Epilepsy and pregnancy. Aust N Z J Obstet Gynaecol. 1984;24(3):182-185.
114. Canger R, Battino D, Canevini MP, et al. Malformations in offspring of women with epilepsy: a prospective study. Epilepsia. 1999;40(9):1231-1236.
115. Nakane Y. Factors influencing the risk of malformations among infants born to epileptic mothers. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, Pregnancy, and the Child. New York: Raven Press; 1982:259-265.
116. Ornoy A, Cohen E. Outcome of children born to epileptic mothers treated with carbamazepine during pregnancy. Arch Dis Child. 1996;75(6):517-520.
117. Annegers JF, Elveback LR, Hauser WA, Kurland LT. Do anticonvulsants have a teratogenic effect? Arch Neurol. 1974;31(6):364-373.
118. Elshove J, van Eck JH. [Congenital abnormalities, cleft lip and cleft palate in particular, in children of epileptic mothers]. Ned Tijdschr Geneeskd. 1971;115(33):1371-1375.
119. Oguni M, Dansky L, Andermann E, Sherwin A, Andermann F. Improved pregnancy outcome in epileptic women in the last decade: relationship to maternal anticonvulsant therapy. Brain Dev. 1992;14(6):371-380.
120. Dansky L. Outcome of pregnancy in epileptic women [PhD thesis]. Montreal: McGill University, 1989.
121. Weber M, Schweitzer M, Andre JM, Tridon P, Vert P. [Epilepsy, anticonvulsants and pregnancy]. Arch Fr Pediatr. 1977;34(4):374-383.
122. Dean JC, Hailey H, Moore SJ, Lloyd DJ, Turnpenny PD, Little J. Long term health and neurodevelopment in children exposed to antiepileptic drugs before birth. J Med Genet. 2002;39(4):251-259.
123. Meadow SR. Congenital abnormalities and anticonvulsant drugs. Proc R Soc Med. 1970;63(1):48-49.
124. Van Dyke DC, Hodge SE, Heide F, Hill LR. Family studies in fetal phenytoin exposure. J Pediatr. 1988;113(2):301-306.
125. Duncan S, Mercho S, Lopes-Cendes I, et al. Repeated neural tube defects and valproate monotherapy suggest a pharmacogenetic abnormality. Epilepsia. 2001;42(6):750-753.
126. Lindhout D, Omtzigt JG, Cornel MC. Spectrum of neural-tube defects in 34 infants prenatally exposed to antiepileptic drugs. Neurology. 1992;42(4 suppl 5):111-118.
127. Waziri M, Ionasescu V, Zellweger H. Teratogenic effect of anticonvulsant drugs. Am J Dis Child. 1976;130(9):1022-1023.
128. Kozma C. Valproic acid embryopathy: report of two siblings with further expansion of the phenotypic abnormalities and a review of the literature. Am J Med Genet. 2001;98(2):168-175.
129. Ozkinay F, Yenigun A, Kantar M, Ozkinay C, Avanoglu A, Ulman I. Two siblings with fetal hydantoin syndrome. Turk J Pediatr. 1998;40(2):273-278.
130. Karpathios T, Zervoudakis A, Venieris F, Parchas S, Youroukos S. Genetics and fetal hydantoin syndrome. Acta Paediatr Scand. 1989;78(1):125-126.
131. Gardner RJ, Savarirayan R, Dunne KB, McLellan JA, Coleman LT, Suthers GK. Microlissencephaly with cardiac, spinal and urogenital defects. Clin Dysmorphol. 2001;10(3):203-208.
132. Erickson JD. Facial and oral form in sibs of children with cleft lip with or without cleft palate. Ann Hum Genet. 1974;38(1):77-88.
133. Malm H, Kajantie E, Kivirikko S, Kaariainen H, Peippo M, Somer M. Valproate embryopathy in three sets of siblings: further proof of hereditary susceptibility. Neurology. 2002;59(4):630-633.
134. Pashayan H, Pruzansky D, Qruzansky S. Are anticonvulsants teratogenic? Lancet. 1971;2(7726):702-703.
135. Dronamraju KR. Epilepsy and cleft lip and palate. Lancet. 1970;2(7678):876-877.
136. Abrishamchian AR, Khoury MJ, Calle EE. The contribution of maternal epilepsy and its treatment to the etiology of oral clefts: a population based case-control study. Genet Epidemiol. 1994;11(4):343-351.
137. Kelly TE, Rein M, Edwards P. Teratogenicity of anticonvulsant drugs. IV: the association of clefting and epilepsy. Am J Med Genet. 1984;19(3):451-458.
138. Friis ML. Epilepsy among parents of children with facial clefts. Epilepsia. 1979;20(1):69-76.
139. Erickson JD, Oakley GP. Seizure disorder in mothers of children with orofacial clefts: a case-control study. J Pediatr. 1974;84(2):244-246.
140. Robert E, Guibaud P. Maternal valproic acid and congenital neural tube defects. Lancet. 1982;2(8304):937.
141. Lindhout D, Meinardi H. Spina bifida and in-utero exposure to valproate. Lancet. 1984;2(8399):396.
142. Lindhout D, Meinardi H, Barth P. Major malformation in children of epileptic mothers—due to epilepsy or its therapy? In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, Pregnancy, and the Child. New York: Raven Press, 1982.
143. Lindhout D, Meinardi H, Meijer JW, Nau H. Antiepileptic drugs and teratogenesis in two consecutive cohorts: changes in prescription policy paralleled by changes in pattern of malformations. Neurology. 1992;42(4 Suppl 5):94-110.
144. Martin F. [Pregnancy and epilepsy]. Rev Med Suisse Romande. 1978;98(4):199-208.
145. Martin PJ, Millac PA. Pregnancy, epilepsy, management and outcome: a 10-year perspective. Seizure. 1993;2(4):277-280.
146. Meischenguiser R, D’Giano CH, Ferraro SM. Oxcarbazepine in pregnancy: clinical experience in Argentina. Epilepsy Behav. 2004;5(2):163-167.
147. Melchior JC, Svensmark O, Trolle D. Placental transfer of phenobarbitone in epileptic women, and elimination in newborns. Lancet. 1967;2(7521):860-861.
148. Montouris G, Creasy G, Khan A, Neto W. Pregnancy outcome in topiramate-treated pregnancy [Abstract]. Epilepsia. 2003;44(suppl 9):290.
149. Montouris G. Gabapentin exposure in human pregnancy: results from the Gabapentin Pregnancy Registry. Epilepsy Behav. 2003;4(3):310-317.
150. Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77(2):193-198.
151. Omtzigt JG, Los FJ, Hagenaars AM, Stewart PA, Sachs ES, Lindhout D. Prenatal diagnosis of spina bifida aperta after first-trimester valproate exposure. Prenat Diagn. 1992;12(11):893-897.
152. Samren EB, van Duijn CM, Koch S, et al. Maternal use of antiepileptic drugs and the risk of major congenital malformations: a joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia. 1997;38(9):981-990.
153. Samren EB, van Duijn CM, Christiaens GC, Hofman A, Lindhout D. Antiepileptic drug regimens and major congenital abnormalities in the offspring. Ann Neurol. 1999;46(5):739-746.
154. Viinikainen K, Heinonen S, Eriksson K, Kalviainen R. Community-based, prospective, controlled study of obstetric and neonatal outcome of 179 Pregnancies in Women with epilepsy. Epilepsia. 2006;47(1):186-192.
155. Watson JD, Spellacy WN. Neonatal effects of maternal treatment with the anticonvulsant drug diphenylhydantoin. Obstet Gynecol. 1971;37(6):881-885.
156. Deblay MF, Vert P, Andre M. [Children of epileptic mothers (author’s transl)]. Nouv Presse Med. 1982;11(3):173-176.
157. Kelly TE, Edwards P, Rein M, Miller JQ, Dreifuss FE. Teratogenicity of anticonvulsant drugs. II: A prospective study. Am J Med Genet. 1984;19(3):435-443.
158. Mawer G, Clayton-Smith J, Coyle H, Kini U. Outcome of pregnancy in women attending an outpatient epilepsy clinic: adverse features associated with higher doses of sodium valproate. Seizure. 2002;11(8):512-518.
159. Thomas D, Buchanan N. Teratogenic effects of anticonvulsants [letter]. J Pediatr. 1981;99(1):163.
160. Artama M, Auvinen A, Raudaskoski T, Isojarvi I, Isojarvi J. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology. 2005;64(11):1874-1878.
161. Burja S, Rakovec-Felser Z, Treiber M, Hajdinjak D, Gajsek-Marchetti M. The frequency of neonatal morbidity after exposure to antiepileptic drugs in utero: a retrospective population-based study. Wien Klin Wochenschr. 2006;118(Suppl 2):12-16.
162. Kaneko S, Otani K, Kondo T, et al. Malformation in infants of mothers with epilepsy receiving antiepileptic drugs. Neurology. 1992;42(4 Suppl 5):68-74.
163. Laine-Cessac P, Le Jaoen S, Rosenau L, Gamelin L, Allain P, Grosieux P. [Uncontrolled retrospective study of 75 pregnancies in women treated for epilepsy]. J Gynecol Obstet Biol Reprod (Paris). 24(5):537-542.
164. Millar JH, Nevin NC. Congenital malformations and anticonvulsant drugs. Lancet. 1973;1(7798):328.
165. Waters CH, Belai Y, Gott PS, Shen P, De Giorgio CM. Outcomes of pregnancy associated with antiepileptic drugs. Arch Neurol. 1994;51(3):250-253.
166. Wide K, Winbladh B, Kallen B. Major malformations in infants exposed to antiepileptic drugs in utero, with emphasis on carbamazepine and valproic acid: a nation-wide, population-based register study. Acta Paediatr. 2004;93(2):174-176.
167. Diav-Citrin O, Shechtman S, Arnon J, Ornoy A. Is carbamazepine teratogenic? A prospective controlled study of 210 pregnancies. Neurology. 2001;57(2):321-324.
168. D’Souza SW, Robertson IG, Donnai D, Mawer G. Fetal phenytoin exposure, hypoplastic nails, and jitteriness. Arch Dis Child. 1991;66(3):320-324.
169. Hill BC, Verniaud WM, Rettig GM, et al. Relation between antiepileptic drug exposure of the infant and developmental potential. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, Pregnancy, and the Child. New York: Raven Press; 1982:409-417.
170. Kaneko S, Otani K, Fukushima Y, et al. Teratogenicity of antiepileptic drugs: analysis of possible risk factors. Epilepsia. 1988;29(4):459-467.
171. Meyer JG. The teratological effects of anticonvulsants and the effects on pregnancy and birth. Eur Neurol. 1973;10(3):179-190.
172. Nakane Y. The teratological problem of antiepileptic drugs. Folia Psychiatr Neurol Jpn. 1980;34(3):277-287.
173. van der Pol MC, Hadders-Algra M, Huisjes HJ, Touwen BC. Antiepileptic medication in pregnancy: late effects on the children’s central nervous system development. Am J Obstet Gynecol. 1991;164(1 Pt 1):121-128.
174. Al Bunyan M, Abo-Talib Z. Outcome of pregnancies in epileptic women: a study in Saudi Arabia. Seizure. 1999;8(1):26-29.
175. Anderman E, Duncan S, Mercho S, et al. Safety of carbamazepine exposure during pregnancy [Abstract]. Epilepsia. 2003;44(Suppl 8):35.
176. Barry JE, Danks DM. Letter: Anticonvulsants and congenital abnormalities. Lancet. 1974;2(7871):48-49.
177. Cunnington M, Tennis P. Lamotrigine and the risk of malformations in pregnancy. Neurology. 2005;64(6):955-960.
178. Eskazan E, Aslan S. Antiepileptic therapy and teratogenicity in Turkey. Int J Clin Pharmacol Ther Toxicol. 1992;30(8):261-264.
179. Hunt SJ, Morrow JI. Safety of antiepileptic drugs during pregnancy. Expert Opin Drug Saf. 2005;4(5):869-877.
180. Katz JM, Pacia SV, Devinsky O. Current management of epilepsy and pregnancy: fetal outcome, congenital malformations, and developmental delay. Epilepsy Behav. 2001;2(2):119-123.
181. Kondo T, Kaneko S, Amano Y, Egawa I. Preliminary report on teratogenic effects of zonisamide in the offspring of treated women with epilepsy. Epilepsia. 1996;37(12):1242-1244.
182. Lander CM, Eadie MJ. Antiepileptic drug intake during pregnancy and malformed offspring. Epilepsy Res. 1990;7(1):77-82.
183. Lekwuwa GU, Adewole IF, Thompson MO. Antiepileptic drugs and teratogenicity in Nigerians. Trans R Soc Trop Med Hyg. 1995;89(2):227.
184. Jager-Roman E, Deichl A, Jakob S, et al. Fetal growth, major malformations, and minor anomalies in infants born to women receiving valproic acid. J Pediatr. 1986;108(6):997-1004.
185. Vajda FJ, O’Brien TJ, Hitchcock A, Graham J, Lander C. The Australian registry of anti-epileptic drugs in pregnancy: experience after 30 months. J Clin Neurosci. 2003;10(5):543-549.
186. Duncan S, Mercho S, Lopes-Cendas I, et al. The effects of valproic acid on the outcome of pregnancy: a prospective study [Abstract]. Epilepsia. 2003;44(Suppl 8):59.
187. Wyszynski DF, Nambisan M, Surve T, Alsdorf RM, Smith CR, Holmes LB. Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology. 2005;64(6):961-965.
188. Cunnington M, Ferber S, Quartey G. Effect of dose on the frequency of major birth defects following fetal exposure to lamotrigine monotherapy in an international observational study. Epilepsia. 2007;48(6):1207-1210.
189. Bertollini R, Mastroiacovo P, Segni G. Maternal epilepsy and birth defects: a case-control study in the Italian Multicentric Registry of Birth Defects (IPIMC). Eur J Epidemiol. 1985;1(1):67-72.
190. Arpino C, Brescianini S, Robert E, et al. Teratogenic effects of antiepileptic drugs: use of an international database on malformations and drug exposure (MADRE). Epilepsia. 2000;41(11):1436-1443.
191. Hernandez-Diaz S, Werler MM, Walker AM, Mitchell AA. Neural tube defects in relation to use of folic acid antagonists during pregnancy. Am J Epidemiol. 2001;153(10):961-968.
192. Kallen B, Robert E, Mastroiacovo P, Martinez-Frias ML, Castilla EE, Cocchi G. Anticonvulsant drugs and malformations; is there a drug specificity? Eur J Epidemiol. 1989;5(1):31-36.
193. Omtzigt JG, Los FJ, Grobbee DE, et al. The risk of spina bifida aperta after first-trimester exposure to valproate in a prenatal cohort. Neurology. 1992;42(4 Suppl 5):119-125.
194. Lindhout D, Schmidt D. In-utero exposure to valproate and neural tube defects. Lancet. 1986;1(8494):1392-1393.
195. Annegers JF, Hauser WA, Elveback LR, Anderson VE, Kurland LI. Congenital malformations and seizure disorders in the offspring of parents with epilepsy. Int J Epidemiol. 1978;7(3):241-247.
196. Rodriguez-Pinilla E, Arroyo I, Fondevilla J, Garcia MJ, Martinez-Frias ML. Prenatal exposure to valproic acid during pregnancy and limb deficiencies: a case-control study. Am J Med Genet. 2000;90(5):376-381.
197. Holmes LB, Wyszynski DF, Baldwin EJ, Habecker E, Glassman LH, Smith CR. Increased risk for non-syndromic cleft palate among infants exposed to lamotrigine during pregnancy [Abstract]. Birth Def Res (Part A): Clin Mol Teratol. 2006;78:318.
198. Tomson T, Perucca E, Battino D. Navigating toward fetal and maternal health: the challenge of treating epilepsy in pregnancy. Epilepsia. 2004;45(10):1171-1175.
199. Holmes LB, Wyszynski DF, Lieberman E. The AED (antiepileptic drug) pregnancy registry: a 6-year experience. Arch Neurol. 2004;61(5):673-678.
200. Hernandez-Diaz S, Smith CR, Wyszynski DF, Holmes LB. Risk of major malformations among infants exposed to carbamazepine during pregnancy. Birth Def Res (Part A): Clin Mol Teratol. 2007;79:357.
201. Gaily E, Kantola-Sorsa E, Granstrom ML. Specific cognitive dysfunction in children with epileptic mothers. Dev Med Child Neurol. 1990;32(5):403-414.
202. Wide K, Henning E, Tomson T, Winbladh B. Psychomotor development in preschool children exposed to antiepileptic drugs in utero. Acta Paediatr. 2002;91(4):409-414.
203. Scolnik D, Nulman I, Rovet J, et al. Neurodevelopment of children exposed in utero to phenytoin and carbamazepine monotherapy. JAMA. 1994;271(10):767-770.
204. Adab N, Tudur SC, Vinten J, Williamson P, Winterbottom J. Common Antiepileptic Drugs in Pregnancy in Women with Epilepsy (Cochrane Review). The Cochrane Library. Chichester, UK: John Wiley & Sons, Ltd, 2004.
205. Adab N, Jacoby A, Smith D, Chadwick D. Additional educational needs in children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2001;70(1):15-21.
206. Vinten J, Adab N, Kini U, Gorry J, Gregg J, Baker GA. Neuropsychological effects of exposure to anticonvulsant medication in utero. Neurology. 2005;64(6):949-954.
207. Gaily E, Kantola-Sorsa E, Hiilesmaa V, et al. Normal intelligence in children with prenatal exposure to carbamazepine. Neurology. 2004;62(1):28-32.
208. Eriksson K, Viinikainen K, Monkkonen A, et al. Children exposed to valproate in utero—population based evaluation of risks and confounding factors for long-term neurocognitive development. Epilepsy Res. 2005;65(3):189-200.
209. Hiilesmaa VK. Pregnancy and birth in women with epilepsy. Neurology. 1992;42(4 suppl 5):8-11.
210. Daly LE, Kirke PN, Molloy A, Weir DG, Scott JM. Folate levels and neural tube defects. Implications for prevention. JAMA. 1995;274(21):1698-1702.
211. Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med. 1993;86(11):703-708.
212. Mills JL, McPartlin JM, Kirke PN, et al. Homocysteine metabolism in pregnancies complicated by neural-tube defects. Lancet. 1995;345(8943):149-151.
213. Yates JR, Ferguson-Smith MA, Shenkin A, Guzman-Rodriguez R, White M, Clark BJ. Is disordered folate metabolism the basis for the genetic predisposition to neural tube defects? Clin Genet. 1987;31(5):279-287.
214. Dansky LV, Andermann E, Rosenblatt D, Sherwin AL, Andermann F. Anticonvulsants, folate levels, and pregnancy outcome: a prospective study. Ann Neurol. 1987;21(2):176-182.
215. Dansky L, Wolfson C, Anderman E, Andermann F, Sherwin A. A multivariate analysis of risk factors for major congenital malformations in offspring of epileptic women [Abstract]. Epilepsia. 1989;30:678.
216. Ogawa Y, Kaneko S, Otani K, Fukushima Y. Serum folic acid levels in epileptic mothers and their relationship to congenital malformations. Epilepsy Res. 1991;8(1):75-78.
217. Botto LD, Moore CA, Khoury MJ, Erickson JD. Neural-tube defects. N Engl J Med. 1999;341(20):1509-1519.
218. Czeizel AE, Bod M, Halasz P. Evaluation of anticonvulsant drugs during pregnancy in a population-based Hungarian study. Eur J Epidemiol. 1992;8(1):122-127.
219. Hernandez-Diaz S, Werler MM, Walker AM, Mitchell AA. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med. 2000;343(22):1608-1614.
220. Moore JL. The significance of folic acid for epilepsy patients. Epilepsy Behav. 2005;7(2):172-181.
221. Stokes T, Shaw EJ, Juarez-Garcia A, Camosso-Stefinovic J, Baker R. Clinical Guidelines and Evidence Review for the Epilepsies: Diagnosis and Management in Adults and Children in Primary and Secondary Care. London: Royal College of General Practitioners, 2004.
222. Crawford P. Best practice guidelines for the management of women with epilepsy. Epilepsia. 2005;46(Suppl 9):117-124.
223. Nau H, Kuhnz W, Egger HJ, Rating D, Helge H. Anticonvulsants during pregnancy and lactation. Transplacental, maternal and neonatal pharmacokinetics. Clin Pharmacokinet. 1982;7(6):508-543.
224. von Unruh GE, Froescher W, Hoffmann F, Niesen M. Valproic acid in breast milk: how much is really there? Ther Drug Monit. 1984;6(3):272-276.
225. Froescher W, Eichelbaum M, Niesen M, Dietrich K, Rausch P. Carbamazepine levels in breast milk. Ther Drug Monit. 1984;6(3):266-271.
226. Kuhnz W, Koch S, Jakob S, Hartmann A, Helge H, Nau H. Ethosuximide in epileptic women during pregnancy and lactation period. Placental transfer, serum concentrations in nursed infants and clinical status. Br J Clin Pharmacol. 1984;18(5):671-677.
227. Kuhnz W, Koch S, Helge H, Nau H. Primidone and phenobarbital during lactation period in epileptic women: total and free drug serum levels in the nursed infants and their effects on neonatal behavior. Dev Pharmacol Ther. 1988;11(3):147-154.
228. Johannessen SI, Helde G, Brodtkorb E. Levetiracetam concentrations in serum and in breast milk at birth and during lactation. Epilepsia. 2005;46(5):775-777.
229. Öhman I, Vitols S, Luef G, Soderfeldt B, Tomson T. Topiramate kinetics during delivery, lactation, and in the neonate: preliminary observations. Epilepsia. 2002;43(10):1157-1160.
230. Öhman I, Vitols S, Tomson T. Pharmacokinetics of gabapentin during delivery, in the neonatal period, and lactation: does a fetal accumulation occur during pregnancy? Epilepsia. 2005;46(10):1621-1624.
231. Myllynen P, Pienimaki P, Jouppila P, Vahakangas K. Transplacental passage of oxcarbazepine and its metabolites in vivo. Epilepsia. 2001;42(11):1482-1485.
232. Shimoyama R, Ohkubo T, Sugawara K. Monitoring of zonisamide in human breast milk and maternal plasma by solid-phase extraction HPLC method. Biomed Chromatogr. 1999;13(5):370-372.
233. Tomson T. Gender aspects of pharmacokinetics of new and old AEDs: pregnancy and breast-feeding. Ther Drug Monit. 2005;27(6):718-721.
234. Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67(3):407-412.