Chapter 670 The Hip
Growth and Development
The hip joint begins to develop at about the 7th week of gestation, when a cleft appears in the mesenchyme of the primitive limb bud. These precartilaginous cells differentiate into a fully formed cartilaginous femoral head and acetabulum by the 11th week of gestation (Chapter 6.1). At birth, the neonatal acetabulum is completely composed of cartilage, with a thin rim of fibrocartilage called the labrum.
Vascular Supply
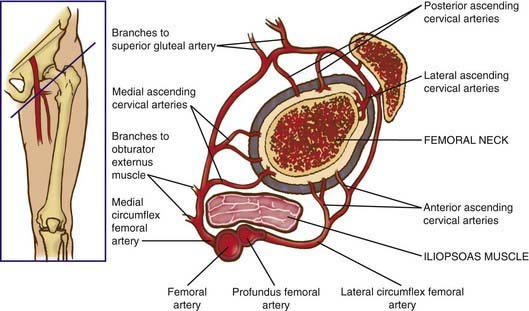
The blood supply to the capital femoral epiphysis is complex and changes with growth of the proximal femur. The proximal femur receives its arterial supply from intraosseous (primarily the medial femoral circumflex artery) and extraosseous vessels (Fig. 670-1). The retinacular vessels (extraosseous) lie on the surface of the femoral neck but are intracapsular because they enter the epiphysis from the periphery. This makes the blood supply vulnerable to damage from septic arthritis, trauma, thrombosis, and other vascular insults. Interruption of this tenuous blood supply can lead to avascular necrosis of the femoral head and permanent deformity of the hip.
670.1 Developmental Dysplasia of the Hip
Clinical Findings
The Neonate
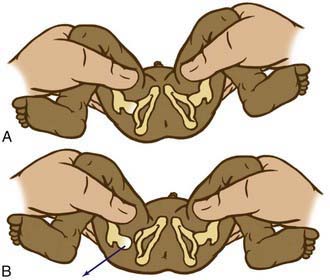
The Barlow provocative maneuver assesses the potential for dislocation of a nondisplaced hip. The examiner adducts the flexed hip and gently pushes the thigh posteriorly in an effort to dislocate the femoral head (Fig. 670-2). In a positive test, the hip is felt to slide out of the acetabulum. As the examiner relaxes the proximal push, the hip can be felt to slip back into the acetabulum.
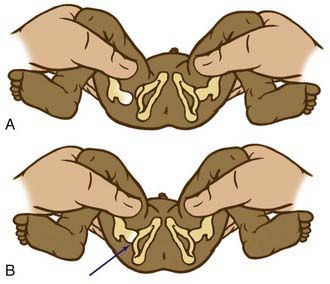
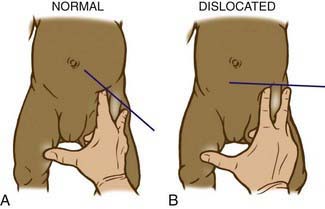
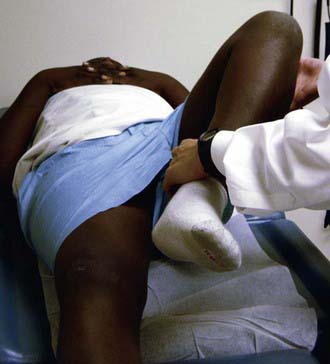
The Ortolani test is the reverse of Barlow test: The examiner attempts to reduce a dislocated hip (Fig. 670-3). The examiner grasps the child’s thigh between the thumb and index finger and, with the 4th and 5th fingers, lifts the greater trochanter while simultaneously abducting the hip. When the test is positive, the femoral head will slip into the socket with a delicate clunk that is palpable but usually not audible. It should be a gentle, nonforced maneuver.
The Infant
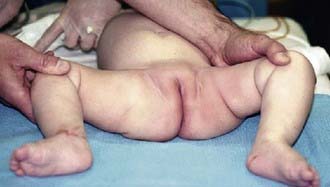
As the baby enters the 2nd and 3rd months of life, the soft tissues begin to tighten and the Ortolani and Barlow tests are no longer reliable. In this age group, the examiner must look for other specific physical findings including limited hip abduction, apparent shortening of the thigh, proximal location of the greater trochanter, asymmetry of the gluteal or thigh folds (Fig. 670-4), and pistoning of the hip. Limitation of abduction is the most reliable sign of a dislocated hip in this age group.
Shortening of the thigh, the Galeazzi sign, is best appreciated by placing both hips in 90 degrees of flexion and comparing the height of the knees, looking for asymmetry (Fig. 670-5). Asymmetry of thigh and gluteal skin folds may be present in 10% of normal infants but suggests DDH. Another helpful test is the Klisic test, in which the examiner places the 3rd finger over the greater trochanter and the index finger of the same hand on the anterior superior iliac spine. In a normal hip, an imaginary line drawn between the two fingers points to the umbilicus. In the dislocated hip, the trochanter is elevated, and the line projects halfway between the umbilicus and the pubis (Fig. 670-6).
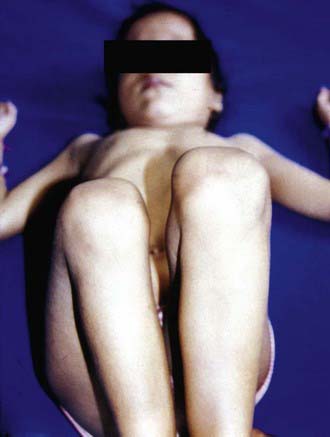
Figure 670-5 Positive Galeazzi sign noted in a case of untreated developmental dysplasia of the hip.
Diagnostic Testing
Ultrasonography
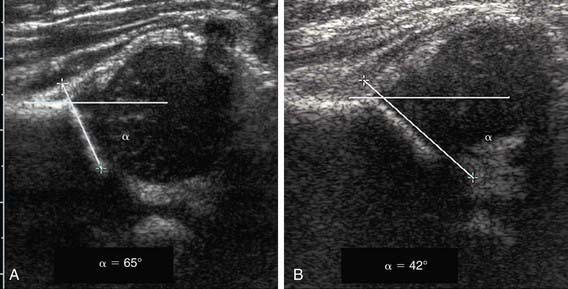
In the Graf technique, the transducer is placed over the greater trochanter, which allows visualization of the ilium, the bony acetabulum, the labrum, and the femoral epiphysis (Fig. 670-7). The angle formed by the line of the ilium and a line tangential to the boney roof of the acetabulum is termed the α angle and represents the depth of the acetabulum. Values >60 degrees are considered normal, and those <60 degrees imply acetabular dysplasia. The β angle is formed by a line drawn tangential to the labrum and the line of the ilium; this represents the cartilaginous roof of the acetabulum. A normal β angle is <55 degrees; as the femoral head subluxates, the β angle increases. Another useful test is to evaluate the position of the center of the head compared to the vertical line of the ilium. If the line of the ilium falls lateral to the center of the head, the epiphysis is considered reduced. If the line falls medial to the center of the head, the epiphysis is undercovered and is either subluxated or dislocated.
Radiography
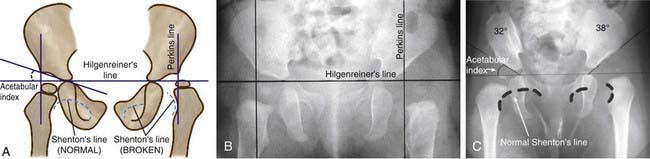
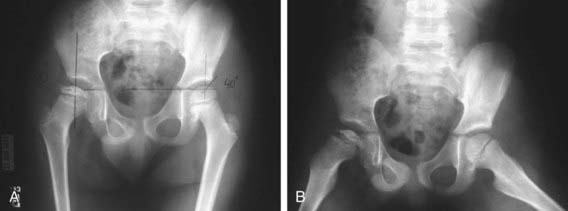
Radiographs are recommended for an infant once the proximal femoral epiphysis ossifies, usually by 4-6 mo. In infants of this age, the radiographs have proved to be more effective, less costly, and less operator dependent than an ultrasound examination. An anteroposterior (AP) view of the pelvis can be interpreted through several classic lines drawn on it (Fig. 670-8).
Treatment
Newborns and Infants Younger Than 6 Months
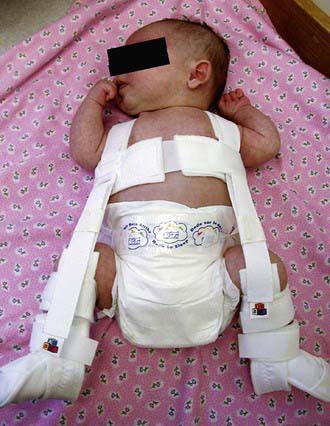
Triple diapers or abduction diapers have no place in the treatment of DDH in the newborn; they are usually ineffective and give the family a false sense of security. Acetabular dysplasia, subluxation, or dislocation can all be readily managed with the Pavlik harness. Although other braces are available (von Rosen splint, Frejka pillow), the Pavlik harness remains the most commonly used device worldwide (Fig. 670-9). By maintaining the Ortolani-positive hip in a Pavlik harness on a full-time basis for 6 wk, hip instability resolves in 95% of cases. After 6 mo of age, the failure rate for the Pavlik harness is >50% because it is difficult to maintain the increasingly active and crawling child in the harness. Frequent examinations and readjustments are necessary to ensure that the harness is fitting correctly. The anterior straps of the harness should be set to maintain the hips in flexion (usually ~100 degrees); excessive flexion is discouraged because of the risk of femoral nerve palsy. The posterior straps are designed to encourage abduction. These are generally set to allow adduction just to neutral, as forced abduction by the harness can lead to avascular necrosis of the femoral epiphysis.
Children 6 Months to 2 Years of Age
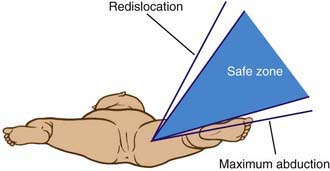
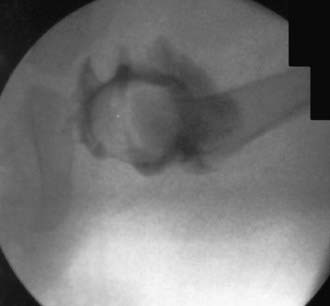
The principal goals in the treatment of the late-diagnosed dysplasia are to obtain and maintain reduction of the hip without damaging the femoral head. Closed reductions are performed in the operating room under general anesthesia. The hip is moved to determine the range of motion in which it remains reduced. This is compared to the maximal range of motion to construct a “safe zone” (Fig. 670-10). An arthrogram obtained at the time of reduction is very helpful for evaluating the depth and stability of the reduction (Fig. 670-11). The reduction is maintained in a well-molded spica cast, with the “human position” of moderate flexion and abduction being the preferred position. After the procedure, single-cut CT or MRI may be used to confirm the reduction. Twelve weeks after closed reduction, the plaster cast is removed; an abduction orthosis is often used at this point to encourage further remodeling of the acetabulum. Failure to obtain a stable hip with a closed reduction indicates the need for an open reduction. In patients <2 yr of age, a secondary acetabular or femoral procedure is rarely required. The potential for acetabular development after closed or open reduction is excellent and continues for 4-8 yr after the procedure.
Dezateux C, Rosendahl K. Developmental dysplasia of the hip. Lancet. 2007;369:1541-1552.
Guille JT, Pizzutillo PD, MacEwen GD. Developmental dysplasia of the hip from birth to six months. J Am Acad Orthop Surg. 2000;8:232-242.
Haynes DJ. Developmental dysplasia of the hip: etiology, pathogenesis and examination and physical findings in the newborn. Instr Course Lect. 2001;50:535-540.
Hennrikus WL. Developmental dysplasia of the hip: diagnosis and treatment in children younger than 6 months. Pediatr Ann. 1999;28:740-746.
Lehmann HP, Hinton R, Morello P, et al. American Academy of Pediatrics. Developmental dysplasia of the hip practice guideline: technical report. Pediatrics. 2000;105:1-25.
Lowry CA, Donoghue VB, Murphy JF. Auditing hip ultrasound screening of infants at increased risk of developmental dysplasia of the hip. Arch Dis Child. 2005;90:579-581.
Mahan ST, Katz JN, Kim YJ. To screen or not to screen? A decision analysis of the utility of screening for developmental dysplasia of the hip. J Bone Joint Surg Am. 2009;91:1705-1719.
Moseley CF. Developmental hip dysplasia and dislocation: management of the older child. Instr Course Lect. 2001;50:547-553.
Roovers EA, Boere-Boonekamp MM, Castelein RM, et al. Effectiveness of ultrasound screening for developmental dysplasia of the hip. Arch Dis Child Fetal Neonatal Ed. 2005;90:F25-F30.
Roy DR. Current concepts in Legg-Calvé-Perthes disease. Pediatr Ann. 1999;28:748-752.
Sewell MD, Rosendahl K, Eastwood DM. Developmental dysplasia of the hip. BMJ. 2009;339:1242-1248.
US Preventive Services Task Force. Screening for developmental dysplasia of the hip: recommendation statement. Pediatrics. 2006;117:898-902.
Willis RB. Developmental dysplasia of the hip: assessment and treatment before walking age. Am Acad Orthop Surg Instr Course Lect. 2001;50:541-545.
Woolacott NF, Puhan MA, Steurer J, et al. Ultrasonography in screening for developmental dysplasia of the hip in newborns: systematic review. Br Med J. 2005;330:1413-1415.
670.2 Transient Monoarticular Synovitis (Toxic Synovitis)
Diagnosis
The most important condition to exclude before confirming a diagnosis of toxic synovitis is septic arthritis. Children with septic arthritis usually appear more systemically ill than those with transient synovitis. The pain associated with septic arthritis is more severe, and children often refuse to walk or move their hip at all. High fever, refusal to walk, and elevations of the ESR, serum CRP, and WBC all point toward a diagnosis of septic arthritis. If the clinical scenario is suspicious for septic arthritis, an ultrasonography-guided aspiration of the hip joint should be performed to make the definitive diagnosis (Chapter 677).
Gough-Palmer A, McHugh K. Investigating hip pain in a well child. BMJ. 2007;334:1216-1220.
Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81:1662-1670.
Perry DC, Bruce C. Evaluating the child who presents with an acute limp. BMJ. 2010;341:444-449.
Taekema HC, Landham PR, Maconochine I. Distinguishing between transient synovitis and septic arthritis in the limping child: how useful are clinical prediction tools? Arch Dis Child. 2009;94:167-168.
670.3 Legg-Calvé-Perthes Disease
Diagnosis
Routine plain radiographs are the primary diagnostic tool for LCPD. AP and Lauenstein (frog-leg) lateral views are used to diagnose, stage, provide prognosis for, and follow the course of the disease and to assess results (Fig. 670-12). It is most important in following the course of the disease that all radiographs be viewed sequentially and compared with previous radiographs to assess the stage of the disease and to determine the true extent of epiphyseal involvement.
Classification
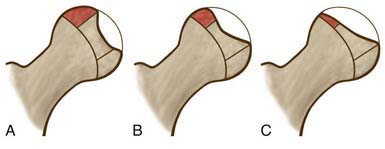
The Herring lateral pillar classification is the most widely used radiographic classification system for determining treatment and prognosis during the active stage of the disease (Fig. 670-13). Unlike the Catterral system, the Herring classification has been shown to have a high degree of interobserver reliability. Classification is based on several radiographs taken during the early fragmentation stage. The lateral pillar classification system for LCPD evaluates the shape of the femoral head epiphysis on AP radiograph of the hip. The head is divided into three sections or pillars. The lateral pillar occupies the lateral 15-30% of the head width, the central pillar is about 50% of the head width, and the medial pillar is 20-35% of the head width. The degree of involvement of the lateral pillar can be subdivided into three groups. In group A, the lateral pillar is radiographically normal. In group B, the lateral pillar has some lucency but >50% of the lateral pillar height is maintained. In group C, the lateral pillar is more lucent than in group B and <50% of the pillar height remains. Herring has added a B/C border group to the classification system to describe patients with ~50% collapse of the lateral pillar.
Catterall A. The natural history of Perthes disease. J Bone Joint Surg Br. 1971;53:37-53.
Hamer AJ. Pain in the hip and knee. Br Med J. 2004;328:1067-1069.
Herring JA, Neustadt JB, Williams JJ, et al. The lateral pillar classification of Legg-Calvé-Perthes disease. J Pediatr Orthop. 1992;12:143-150.
Liu YF, Chen WM, Lin YF, et al. Type II collagen gene variants and inherited osteonecrosis of the femoral head. N Engl J Med. 2005;352:2294-2301.
Martinez AG, Weinstein SL, Dietz FR. The weight-bearing abduction brace for the treatment of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 1992;74:12-21.
Noonan KJ, Price CT, Kupiszewski SJ, et al. Results of femoral varus osteotomy in children older than 9 years of age with Perthes disease. J Pediatr Orthop. 2001;21:198-204.
Thompson GH, Price CT, Roy D, et al. Legg-Calvé-Perthes disease: current concepts. Instr Course Lect. 2002;51:367-384.
670.4 Slipped Capital Femoral Epiphysis
Classification
An acute SCFE has been characterized as one occurring in a patient who has prodromal symptoms for ≤3 weeks and should be distinguished from a purely traumatic separation of the epiphysis in a previously normal hip (a true Salter-Harris type I fracture; Chapter 675). The patient with an acute slip usually has some prodromal pain in the groin, thigh, or knee and usually reports a relatively minor injury (a twist or fall) that is not sufficiently violent to produce an acute fracture of this severity. Osteonecrosis is a significant and common complication of acute SCFE, with a reported incidence of 17-47%.
Clinical Manifestations
The classic patient presenting with a SCFE is an obese, African-American boy between the ages of 11 and 16 yr. Girls present earlier, usually between 10 and 14 yr of age. Patients with chronic and stable SCFEs tend to present after weeks to months of symptoms. Patients usually limp to some degree and have an externally rotated lower extremity. Physical examination of the affected hip reveals a restriction of internal rotation, abduction, and flexion. Commonly, the examiner notes that as the affected hip is flexed, the thigh tends to rotate into progressively more external rotation (Fig. 670-14). Most patients complain of groin symptoms, but isolated thigh pain or knee pain is a common presentation from referred pain along the course of the obturator nerve. In fact, missed or delayed diagnosis often occurs in children who present with knee pain and do not receive appropriate imaging of the hip. Patients with unstable SCFEs usually present in an urgent fashion. Children typically refuse to allow any range of motion of the hip; much like a hip fracture, the extremity is shortened, abducted, and externally rotated.
Diagnostic Studies
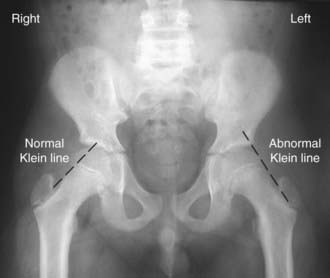
Plain radiography including AP and frog-leg lateral views of both hips is usually the only imaging study needed to make the diagnosis. Because ~25% of patients have a contralateral slip on initial presentation, it is vital that both hips be carefully evaluated by the treating physician. Radiographic findings include widening and irregularity of the physis, a decrease in epiphyseal height in the center of the acetabulum, a crescent-shaped area of increased density in the proximal portion of the femoral neck, and the “blanch sign of Steel” corresponding to the double density created from the anteriorly displaced femoral neck overlying the femoral head. In an unaffected patient, the Klein line, a straight line drawn along the superior cortex of the femoral neck on the AP radiograph, should intersect some portion of the lateral capital femoral epiphysis. With progressive displacement of the epiphysis, the Klein line no longer intersects the epiphysis (Fig. 670-15). Although some of these radiographic findings can be subtle, most diagnoses can be readily made on the frog-leg lateral view, which reveals the characteristic posterior and inferior displacement of the epiphysis in relation to the femoral neck.
Treatment
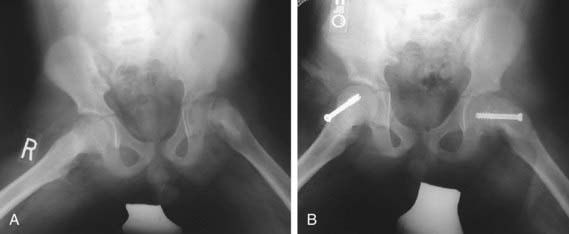
The goal of treatment is to prevent further progression of the slip and to stabilize (i.e., close) the physis. Although various forms of treatment have been used in the past, including spica casting, the current gold standard for the treatment of SCFE is in situ pinning with a single, large screw (Fig. 670-16). The term “in situ” implies that no attempt is made to reduce the displacement between the epiphysis and femoral neck because doing so increases the risk of osteonecrosis. Screws are typically placed percutaneously under fluoroscopic guidance. Postoperatively, most patients are allowed partial weight-bearing with crutches for 4-6 wk, followed by a gradual return to normal activities. Patients should be monitored with serial radiographs to be sure that the physis is closing and that the slip is stable. After healing from the initial stabilization, patients with severe residual deformity may be candidates for proximal femoral osteotomy to correct the deformity, reduce impingement, and improve range of motion.
Clarke NMP, Kendrick T. Slipped capital femoral epiphysis. BMJ. 2009;339:1198-1199.
Dobbs MT, Weinstein SL. Natural history and long-term outcomes of slipped capital femoral epiphysis. Instr Course Lect. 2001;50:571-575.
Kocher MS, Bishop JA, Hresko MT, et al. Prophylactic pinning of the contralateral hip after slipped capital femoral epiphysis. J Bone Joint Surg Am. 2004;86:2658-2665.
Loder RT, Aronsson DD, Dobbs MT, et al. Slipped capital femoral epiphysis. Instr Course Lect. 2001;50:555-570.
Lubicky JP. Chondrolysis and avascular necrosis: complications of slipped capital femoral epiphysis. J Pediatr Orthop. 1996;5:162-167.
Reynolds RA. Diagnosis and treatment of slipped capital femoral epiphysis. Curr Opin Pediatr. 1999;11:80-83.
Riad J, Bajelidze G, Gabos PG. Bilateral slipped capital femoral epiphysis. J Pediatr Orthop. 2007;27:411-414.
Tosounidis T, Stengel D, Kontakis G, et al. Prognostic significance of stability in slipped upper femoral epiphysis: a systematic review and meta-analysis. J Pediatr. 2010;157:674-680.
Warner WCJr, Beaty JH, Canale ST. Chondrolysis after slipped capital femoral epiphysis. J Pediatr Orthop. 1996;5:168-172.
Erol B, Dormans JP. Hip disorders. In: Dormans JP, editor. Pediatric orthopedics: core knowledge in orthopedics. Philadelphia: Mosby; 2005:224-264.
Tamai J, Erol B, Dormans JP. Hip disorders. In: Dormans JP, Bell LM, editors. Pediatric orthopedics and sports medicine: the requisites in pediatrics. St Louis: Mosby; 2004:175-212.