Chapter 90 The Fetus
The major emphasis in fetal medicine involves (1) assessment of fetal growth and maturity, (2) evaluation of fetal well-being or distress, (3) assessment of the effects of maternal disease on the fetus, (4) evaluation of the effects of drugs administered to the mother on the fetus, and (5) identification and treatment of fetal disease or anomalies. Increasing knowledge of fetal physiology has paved the way for effective fetal therapy, intervention during fetal distress, and improved adaptation of a newborn infant to extrauterine life, particularly a premature infant. Some aspects of human fetal growth and development are summarized in Chapter 6.
90.1 Fetal Growth and Maturity
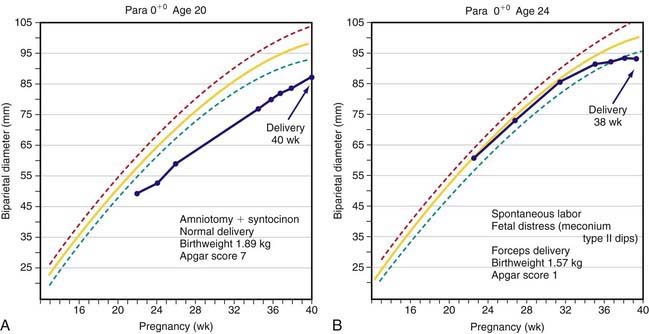
Fetal growth can be assessed by ultrasonography as early as 6-8 wk. The most accurate assessment of gestational age is by 1st-trimester ultrasound measurement of crown-rump length. The biparietal diameter is used to assess gestational age beginning in the 2nd trimester. Through 30 weeks the biparietal diameter accurately estimates gestation to within ± 10 days. Later in gestation, accuracy falls to ± 3 wk. Methods used to assess gestational age at term include measurement of abdominal circumference and femoral length. If a single ultrasound examination is performed, the most information can be obtained with a scan at 18-20 wk, when both gestational age and fetal anatomy can be evaluated. Serial scans may be useful in assessing fetal growth. Two patterns of fetal growth restriction have been identified: continuous fetal growth 2 standard deviations (SD) below the mean for gestational age or a normal fetal growth curve that abruptly slows or flattens later in gestation (Fig. 90-1).
Fetal maturity and dating are usually assessed by history (last menstrual period), physical examination, auscultation of fetal heart sounds at 16-18 wk, maternal perception of fetal movements at 18-20 wk, fundal height, and ultrasound (growth). Lung maturation may be estimated by determining the surfactant content of amniotic fluid (Chapter 95.3).
90.2 Fetal Distress
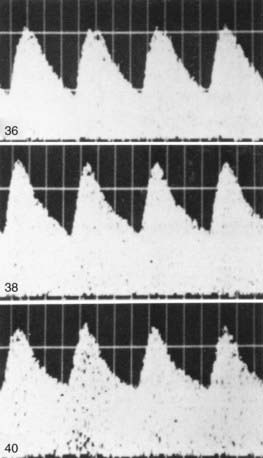
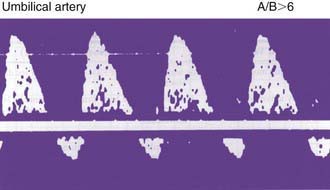
Fetal compromise may occur during the antepartum or intrapartum period; it may be asymptomatic in the antenatal period. Antepartum fetal surveillance is warranted for women at increased risk for fetal death, including those with a history of stillbirth, intrauterine growth restriction (IUGR), oligohydramnios or polyhydramnios, multiple gestation, rhesus sensitization, hypertensive disorders, diabetes mellitus or other chronic maternal disease, decreased fetal movement, and post-term pregnancy. The predominant cause of antepartum fetal distress is uteroplacental insufficiency, which may manifest clinically as IUGR, fetal hypoxia, increased vascular resistance in fetal blood vessels (Figs. 90-2 and 90-3), and, when severe, mixed respiratory and metabolic (lactic) acidosis. The goals of antepartum fetal surveillance are to prevent intrauterine fetal demise, to prevent hypoxic brain injury, and to either prolong gestation in women at risk for preterm delivery when such prolongation is safe or deliver a fetus when it is in jeopardy. Methods for assessing fetal well-being are listed in Table 90-1.
| METHOD | COMMENT(S) AND INDICATION(S) |
|---|---|
| Imaging: | |
| Ultrasound (real-time) | Biometry (growth), anomaly (morphology) detection Biophysical profile Amniotic fluid volume, hydrops |
| Ultrasound (Doppler) | Velocimetry (blood flow velocity) Detection of increased vascular resistance secondary to fetal hypoxia |
| Embryoscopy | Early diagnosis of limb anomaly |
| Fetoscopy | Detection of facial, limb, cutaneous anomalies |
| MRI | Defining of lesions before fetal surgery |
| Fluid analysis: | |
| Amniocentesis | Fetal maturity (L : S ratio), karyotype (cytogenetics), biochemical enzyme analysis, molecular genetic DNA diagnosis, bilirubin, or α-fetoprotein determination Bacterial culture, pathogen antigen, or genome detection |
| Fetal urine | Prognosis of obstructive uropathy |
| Cordocentesis (percutaneous umbilical blood sampling) | Detection of blood type, anemia, hemoglobinopathies, thrombocytopenia, acidosis, hypoxia, polycythemia, immunoglobulin M antibody response to infection Rapid karyotyping and molecular DNA genetic diagnosis Fetal therapy (see Table 90-5) |
| Fetal tissue analysis: | |
| Chorionic villus biopsy | Karyotype, molecular DNA genetic analysis, enzyme assays |
| Skin biopsy | Hereditary skin disease* |
| Liver biopsy | Enzyme assay* |
| Circulating fetal cells or DNA in maternal blood or plasma | Molecular DNA genetic analysis |
| Maternal serum α-fetoprotein concentration: | |
| Elevated | Twins, neural tube defects (anencephaly, spina bifida), intestinal atresia, hepatitis, nephrosis, fetal demise, incorrect gestational age |
| Reduced | Trisomies, aneuploidy |
| Maternal cervix: | |
| Fetal fibronectin | Indicates risk of preterm birth |
| Bacterial culture | Identifies risk of fetal infection (group B streptococcus, Neisseria gonorrhoeae) |
| Fluid | Determination of premature rupture of membranes |
| Antepartum biophysical monitoring: | |
| Nonstress test | Fetal distress; hypoxia |
| Contraction stress test | Fetal distress; hypoxia |
| Biophysical profile and modified biophysical profile | Fetal distress; hypoxia |
| Intrapartum fetal heart rate monitoring | See Fig. 90-4 |
* DNA genetic analysis on chorionic villus samples, amniocytes from amniocentesis, or fetal cells recovered from the maternal circulation may obviate the need for direct fetal tissue biopsy if the gene or genetic marker is available (e.g., the gene for Duchenne muscular dystrophy).
The most commonly used noninvasive tests are the nonstress test (NST), the full and modified biophysical profile (BPP), and less commonly, the contraction stress test (CST). The NST monitors the presence of fetal heart rate accelerations that follow fetal movement. A reactive (normal) NST result demonstrates two fetal heart rate accelerations of at least 15 beats/min lasting 15 sec. A nonreactive NST result suggests fetal compromise and requires further assessment with a CST or the BPP. A CST observes the fetal heart rate response to spontaneous, nipple-stimulated, or oxytocin-stimulated uterine contractions. Fetal compromise is suggested when the majority of contractions in 10 min are followed by late decelerations. A CST is relatively contraindicated in women with preterm premature rupture of membranes, a previous uterine scar from a classic cesarean section, multiple gestations, incompetent cervix, and placenta previa. The goals of fetal monitoring are to prevent intrauterine fetal demise and hypoxic brain injury. Although the CST and NST have low false-negative rates, both have high false-positive rates. The full BPP assesses fetal breathing, body movement, tone, heart rate, and amniotic fluid volume, and it is used to improve the accurate and safe identification of fetal compromise (Table 90-2). A score of 2 is given for each observation present. A total score of 8-10 is reassuring; a score of 6 is equivocal, and retesting should be done in 12-24 hr; and a score of 4 or less warrants immediate evaluation and possible delivery. The BPP has good negative predictive value. The modified BPP consists of the combination of an ultrasound estimate of amniotic fluid volume (the amniotic fluid index) and the NST. When results of both are normal, fetal compromise is very unlikely. Signs of progressive compromise seen on Doppler ultrasonography include reduced, absent, or reversed diastolic waveform velocity in the fetal aorta or umbilical artery (see Fig. 90-3 and Table 90-1). High-risk fetuses often have combinations of abnormalities, such as oligohydramnios, reversed diastolic Doppler umbilical artery blood flow velocity, and a low BPP.
Table 90-2 BIOPHYSICAL PROFILE SCORING: TECHNIQUE AND INTERPRETATION
| BIOPHYSICAL VARIABLE | NORMAL SCORE (2) | ABNORMAL SCORE (0) |
|---|---|---|
| Fetal breathing movements (FBMs) | At least 1 episode of FBM of at least 30 sec duration in 30 min observation | Absence of FBM or no episode ≥30 sec in 30 min |
| Gross body movement | At least 3 discrete body/limb movements in 30 min (episodes of active continuous movement considered a single movement) | 2 or fewer episodes of body/limb movements in 30 min |
| Fetal tone | At least 1 episode of active extension with return to flexion of fetal limb(s) or trunk Opening and closing of hand considered evidence of normal tone |
Either slow extension with return to partial flexion or movement of limb in full extension or absence of fetal movement with the hand held in complete or partial deflection |
| Reactive fetal heart rate (FHR) | At least 2 episodes of FHR acceleration of ≥15 beats/min and at least 15 sec in duration associated with fetal movement in 30 min | Less than 2 episodes of acceleration of FHR or acceleration of <15 beats/min in 30 min |
| Qualitative amniotic fluid (AF) volume * | At least 1 pocket of AF that measures at least 2 cm in 2 perpendicular planes | Either no AF pockets or a pocket <2 cm in 2 perpendicular planes |
* Modification of the criteria for reduced amniotic fluid from less than 1 cm to less than 2 cm would seem reasonable. Ultrasound is used for biophysical assessment of the fetus.
From Creasy RK, Resnik R, Iams JD, editors: Maternal-fetal medicine: principles and practice, ed 5, Philadelphia, 2004, Saunders.
Fetal compromise during labor may be detected by monitoring the fetal heart rate, uterine pressure, and fetal scalp blood pH (Fig. 90-4). Continuous fetal heart rate monitoring detects abnormal cardiac patterns by instruments that compute the beat-to-beat fetal heart rate from a fetal electrocardiographic signal. Signals are derived from an electrode attached to the fetal presenting part, from an ultrasonic transducer placed on the maternal abdominal wall to detect continuous ultrasonic waves reflected from the contractions of the fetal heart, or from a phonotransducer placed on the mother’s abdomen. Uterine contractions are simultaneously recorded from an amniotic fluid catheter and pressure transducer or from a tocotransducer applied to the maternal abdominal wall overlying the uterus. Fetal heart rate patterns show various characteristics, some of which suggest fetal compromise. The baseline fetal heart rate is the average rate between uterine contractions, which gradually decreases from about 155 beats/min in early pregnancy to about 135 beats/min at term; the normal range at term is 110-160 beats/min. Tachycardia (>160 beats/min) is associated with early fetal hypoxia, maternal fever, maternal hyperthyroidism, maternal β-sympathomimetic drug or atropine therapy, fetal anemia, infection, and some fetal arrhythmias. The last do not generally occur with congenital heart disease and may resolve spontaneously at birth. Fetal bradycardia (<110 beats/min) may be normal (e.g., 105-110 beats/min) but may occur with fetal hypoxia, placental transfer of local anesthetic agents and β-adrenergic blocking agents, and, occasionally, heart block with or without congenital heart disease.
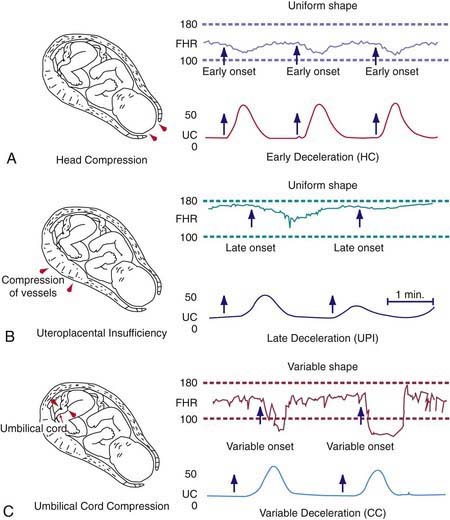
Periodic accelerations or decelerations of the fetal heart rate in response to uterine contractions may also be monitored (see Fig. 90-4). An acceleration is an abrupt increase in fetal heart rate of ≥15 beats/min in ≥15 sec. The presence of accelerations or moderate variability reliably predicts the absence of fetal metabolic acidemia. However, their absence does not reliably predict fetal acidemia or hypoxemia. Early deceleration associated with head compression is a repetitive pattern of gradual decrease and return of the fetal heart rate that is coincidental with the uterine contraction (Table 90-3). Variable deceleration (associated with cord compression) is characterized by variable shape, abrupt onset and occurrence with consecutive contractions, and return to baseline at or after the conclusion of the contraction. Late deceleration, associated with fetal hypoxemia, occurs repetitively after a uterine contraction is well established and persists into the interval following contractions. The late deceleration pattern is usually associated with maternal hypotension or excessive uterine activity, but it may be a response to any maternal, placental, umbilical cord, or fetal factor that limits effective oxygenation of the fetus. Reflex late decelerations with normal beat-to-beat variability are associated with chronic compensated fetal hypoxia, and they occur during uterine contractions that temporarily impede oxygen transport to the heart. Nonreflex late decelerations are more ominous and indicate severe hypoxic depression of myocardial function.
Table 90-3 CHARACTERISTICS OF DECELERATIONS OF THE FETAL HEART RATE
LATE DECELERATION
EARLY DECELERATION
VARIABLE DECELERATION
From Macones GA, Hankins GDV, Spong CY, et al: The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines, Obstet Gynecol 112:661–666, 2008.
If late decelerations are unresponsive to oxygen supplementation, hydration, discontinuation of labor stimulation, and position changes, prompt delivery is indicated. A three-tier system has been developed by a panel of experts for interpretation of fetal heart rate tracings (Table 90-4). Category I tracings are normal and are strongly predictive of normal fetal acid-base status at the time of the observation. Category II tracings are not predictive of abnormal fetal status, but there is insufficient evidence to categorize them as category I or III; further evaluation, surveillance, and reevaluation are indicated. Category III tracings are abnormal and predictive of abnormal fetal acid-base status at the time of observation. Category III tracings require prompt evaluation and efforts to expeditiously resolve the abnormal fetal heart rate as previously discussed for late decelerations.
Table 90-4 THREE-TIER FETAL HEART RATE INTERPRETATION SYSTEM
CATEGORY I
CATEGORY II
Baseline Rate
Baseline FHR Variability
Accelerations
Periodic or Episodic Decelerations
CATEGORY III
From Macones GA, Hankins GDV, Spong CY, et al: The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines, Obstet Gynecol 112:661–666, 2008.
Alfirevic Z, Devane D, Gyte GM: Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour, Cochrane Database Syst Rev (3):CD006066, 2006.
American College of Obstetricians and Gynecologists. Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet Gynecol. 2009;114:192-202.
Bloom SL, Spong CY, Thorn E, et al. Fetal pulse oximetry and cesarean delivery. N Engl J Med. 2006;355:2195-2202.
East CE, Chan FY, Colditz PB: Fetal pulse oximetry for fetal assessment in labour, Cochrane Database Syst Rev (4):CD004075, 2004.
Macones GA, Hankins GDV, Spong CY, et al. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112:661-666.
Pathak S, Lees C. Ultrasound structural fetal anomaly screening: an update. Arch Dis Child Fetal Neonatal Ed. 2009;94:F384-F390.
90.3 Maternal Disease and the Fetus
Noninfectious Diseases (See Table 89-2)
Maternal diabetes increases the risk for neonatal hypoglycemia, hypocalcemia, respiratory distress syndrome and other respiratory problems, polycythemia, macrosomia, myocardial dysfunction, jaundice, and congenital malformations (Chapter 101.1). There is increased risk for incidence of uteroplacental insufficiency, polyhydramnios, and intrauterine death in poorly controlled diabetic mothers. Eclampsia-preeclampsia of pregnancy, chronic hypertension, and chronic renal disease can result in IUGR, prematurity, and intrauterine death, all probably caused by diminished uteroplacental perfusion. Uncontrolled maternal hypothyroidism or hyperthyroidism is responsible for relative infertility, spontaneous abortion, premature labor, and fetal death. Hypothyroidism in pregnant women (even if mild or asymptomatic) can adversely affect neurodevelopment of the child. Maternal immunologic diseases such as idiopathic thrombocytopenic purpura, systemic lupus erythematosus, myasthenia gravis, and Graves disease, all of which are mediated by immunoglobulin (Ig) G autoantibodies that can cross the placenta, frequently cause transient illness in the newborn. Maternal autoantibodies to the folate receptor are associated with NTDs, whereas maternal immunologic sensitization to paternal antigens may be associated with neonatal hemochromatosis. Untreated maternal phenylketonuria results in miscarriage, congenital cardiac malformations, and injury to the brain of a nonphenylketonuric heterozygotic fetus.
Chambers C. Selective serotonin reuptake inhibitors and congenital malformations. BMJ. 2009;339:703-704.
Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549-555.
Harden CL, Pennell PB, Koppel BS, et al. Management issues for women with epilepsy—focus on pregnancy (an evidence-based review) III: vitamin K, folic acid, blood levels, and breast-feeding. Epilepsia. 2009;50:1247-1255.
Lee JY, Huerta PT, Zhang J, et al. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nature Med. 2009;15:91-96.
Leviton A, Paneth N, Reuss ML, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Pediatr Res. 1999;46:566-575.
Li Y, DiNiro E, Vitucci A, et al. Detection of paternally inherited fetal point mutations for β-thalassemia using size-fractionated cell-free DNA in maternal plasma. JAMA. 2005;293:843-849.
Louik C, Lin A, Weler MM, et al. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356:2675-2682.
Waller DR, Shaw GM, Rasmussen SA, et al. Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med. 2007;161:745-750.
Wu YW, Colford JMJr. Chorioamnionitis as a risk factor for cerebral palsy. JAMA. 2000;284:1417-1424.
90.4 Maternal Medication and Toxin Exposure and the Fetus
The use of medications or herbal remedies during pregnancy is potentially harmful to the fetus. Consumption of medications occurs during the majority of pregnancies. The average mother has taken 4 drugs other than vitamins or iron during pregnancy. Almost 40% of pregnant women receive a drug for which human safety during pregnancy has not been established (category C pregnancy risk; see later). Moreover, many women are exposed to potential reproductive toxins, such as occupational, environmental, or household chemicals, including solvents, pesticides, and hair products. The effects of drugs taken by the mother vary considerably, especially in relation to the time in pregnancy when they are taken and the fetal genotype for drug-metabolizing enzymes. Miscarriage or congenital malformations result from the maternal ingestion of teratogenic drugs during the period of organogenesis. Maternal medications taken later, particularly during the last few weeks of gestation or during labor, tend to affect the function of specific organs or enzyme systems, and they adversely affect the neonate rather than the fetus (Tables 90-5 and 90-6).
Table 90-5 AGENTS ACTING ON PREGNANT WOMEN THAT MAY ADVERSELY AFFECT THE STRUCTURE OR FUNCTION OF THE FETUS AND NEWBORN
| DRUG | EFFECT ON FETUS |
|---|---|
| Accutane (isotretinoin) | Facial-ear anomalies, heart disease, CNS anomalies |
| Alcohol | Congenital cardiac, CNS, limb anomalies; IUGR; developmental delay; attention deficits; autism |
| Aminopterin | Abortion, malformations |
| Amphetamines | Congenital heart disease, IUGR, withdrawal |
| Azathioprine | Abortion |
| Busulfan (Myleran) | Stunted growth; corneal opacities; cleft palate; hypoplasia of ovaries, thyroid, and parathyroids |
| Carbamazepine | Spina bifida, possible neurodevelopmental delay |
| Carbimazole | Scalp defects, choanal atresia, esophageal atresia, developmental delay |
| Carbon monoxide | Cerebral atrophy, microcephaly, seizures |
| Chloroquine | Deafness |
| Chorionic villus sampling | Probably no effect, possibly limb reduction |
| Cigarette smoking | Low birthweight for gestational age |
| Cocaine/crack | Microcephaly, LBW, IUGR, behavioral disturbances |
| Cyclophosphamide | Multiple malformations |
| Danazol | Virilization |
| 17α-Ethinyl testosterone (Progestoral) | Masculinization of female fetus |
| Hyperthermia | Spina bifida |
| Lithium | Ebstein anomaly, macrosomia |
| 6-Mercaptopurine | Abortion |
| Methyl mercury | Minamata disease, microcephaly, deafness, blindness, mental retardation |
| Methyltestosterone | Masculinization of female fetus |
| Misoprostol | Arthrogryposis, cranial neuropathies (Möbius syndrome), equinovarus |
| Mycophenolate mofetil | Craniofacial, limb, cardiovascular, CNS anomalies |
| Norethindrone | Masculinization of female fetus |
| Penicillamine | Cutis laxa syndrome |
| Phenytoin | Congenital anomalies, IUGR, neuroblastoma, bleeding (vitamin K deficiency) |
| Polychlorinated biphenyls | Skin discoloration—thickening, desquamation, LBW, acne, developmental delay |
| Prednisone | Oral clefts |
| Progesterone | Masculinization of female fetus |
| Quinine | Abortion, thrombocytopenia, deafness |
| Selective serotonin reuptake inhibitors | Small increased risk of congenital anomalies |
| Statins | IUGR, limb deficiencies, VACTERAL |
| Stilbestrol (diethylstilbestrol [DES]) | Vaginal adenocarcinoma in adolescence |
| Streptomycin | Deafness |
| Tetracycline | Retarded skeletal growth, pigmentation of teeth, hypoplasia of enamel, cataract, limb malformations |
| Thalidomide | Phocomelia, deafness, other malformations |
| Toluene (solvent abuse) | Craniofacial abnormalities, prematurity, withdrawal symptoms, hypertonia |
| Trimethadione and paramethadione | Abortion, multiple malformations, mental retardation |
| Valproate | CNS (spina bifida), facial and cardiac anomalies, limb defects, impaired neurologic function |
| Vitamin D | Supravalvular aortic stenosis, hypercalcemia |
| Warfarin (Coumadin) | Fetal bleeding and death, hypoplastic nasal structures |
CNS, central nervous system; IUGR, intrauterine growth restriction; LBW, low birthweight. VACTERAL, vertebral, anal, cardiac, tracheoesophagcal fistula, renal, arterial, limb.
Table 90-6 AGENTS ACTING ON PREGNANT WOMEN THAT MAY ADVERSELY AFFECT THE NEWBORN INFANT
CNS, central nervous system; G6PD, glucose-6-phosphate dehydrogenase; IUGR, intrauterine growth restriction; SSRI, selective serotonin reuptake inhibitor.
Ackerman JP, Riggins T, Black MM. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125:554-565.
Alwan S, Reefhuis J, Rasmussen SA, et al. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356:2684-2692.
Andrade SE, Gurwitz JH, Davis RL, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004;191:398-407.
Boyle RJ. Effects of certain prenatal drugs on the fetus and newborn. Pediatr Rev. 2002;23:17-23.
Czeizel AE. The primary prevention of birth defects: multivitamins or folic acid? Int J Med Sci. 2004;1:50-61.
Dubnov-Raz G, Juurlink DN, Fogelman R, et al. Antenatal use of selective serotonin-reuptake inhibitors and QT interval prolongation in newborns. Pediatrics. 2008;122:e710-e715.
Edison RJ, Muenke M. Mechanistic and epidemiologic considerations in the evaluation of adverse birth outcomes following gestational exposure to statins. Am J Med Gen. 2004;131A:287-298.
Foulds N, Walpole I, Elmslie F, et al. Carbimazole embryopathy: an emerging phenotype. Am J Med Gen. 2005;132A:130-135.
Gill SK, O’Brien L, Einarson TR, et al. The safety of proton pump inhibitors (PPIs) in pregnancy: a meta-analysis. Am J Gastroenterol. 2009;104:1541-1545.
Gonzalez C, Marquez-Dias M, Kim C, et al. Congenital abnormalities in Brazilian children associated with misoprostol misuse in the first trimester of pregnancy. Lancet. 1998;351:1624-1627.
Honein MA, Paulozzi LJ, Mathews TJ, et al. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285:2981-2986.
Jones KJ, Lacro RV, Johnson KA, et al. Pattern of malformations in the children of women treated with carbamazepine during pregnancy. N Engl J Med. 1989;320:1661-1666.
Kaufman RH, Adam E, Hatch EE, et al. Continued follow-up of pregnancy outcomes in diethylstilbestrol-exposed offspring. Obstet Gynecol. 2000;96:483-489.
Khattak S, K-Moghtader G, McMartin K, et al. Pregnancy outcome following gestational exposure to organic solvents: a prospective controlled study. JAMA. 1999;281:1106-1109.
Koren G, Pastuszak A, Ito S. Drugs in pregnancy. N Engl J Med. 1998;338:1128-1137.
Lumley J, Watson L, Watson M, et al: Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects, Cochrane Database Syst Rev (3):CD001056, 2001.
Lund N, Pedersen LH, Henriksen TB. Selective serotonin reuptake inhibitor exposure in utero and pregnancy outcomes. Arch Pediatr Adolesc Med. 2009;163:949-954.
Matok I, Gorodischer R, Koren G, et al. The safety of metoclopramide use in the first trimester of pregnancy. N Engl J Med. 2009;360:2528-2534.
McDonald SD, Ferguson S, Tam L, et al. The prevention of congenital anomalies with periconceptional folic acid supplementation. J Obstet Gynaecol Can. 2003;25:115-121.
Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360:1507-1605.
Moran LR, Almeida PG, Worden S, et al. Intrauterine baclofen exposure: a multidisciplinary approach. Pediatrics. 2004;114:e267-e269.
Oei J, Abdel-Latif ME, Clark R, et al. Short-term outcomes of mothers and infants exposed to antenatal amphetamines. Arch Dis Child Fetal Neonatal Ed. 2010;95:F36-F41.
Osrin D, Vaidya A, Shrestha Y, et al. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double-blind, randomized controlled trial. Lancet. 2005;365:955-962.
Pedersen LH, Henriksen TB, Vestergaard M, et al. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ. 2009;339:b3569.
Ramos E,St, André M, Rey E, et al. Duration of antidepressant use during pregnancy and risk of major congenital malformations. Br J Psych. 2008;192:344-350.
Seligman NS, Almario CV, Hayes EJ, et al. Relationship between maternal methadone dose at delivery and neonatal abstinence syndrome. J Pediatr. 2010;157:428-433.
Shaw GM, Carmichael SL, Vollset SE, et al. Mid-pregnancy continine and risks of orofacial clefts and neural tube defects. J Pediatr. 2009;154:17-19.
Tomson T, Battino D. Teratogenicity of antiepileptic drugs: state of the art. Curr Opin Neurol. 2005;18:135-140.
Vento M, Aytes AP, Ledo A, et al. Mycophenolate mofetil during pregnancy: some words of caution. Pediatrics. 2008;122:184-185.
90.5 Teratogens
When an infant or child has a congenital malformation or is developmentally delayed, the parents often wrongly blame themselves and attribute the child’s problems to events that occurred during pregnancy. Because benign infections occur and several nonteratogenic drugs are often taken during many pregnancies, the pediatrician must evaluate the presumed viral infections and the drugs ingested to help parents understand their child’s birth defect. The causes of approximately 40% of congenital malformations are unknown. Although only a relatively few agents are recognized to be teratogenic in humans (see Tables 90-5 and 90-6), new agents continue to be identified. Overall, only 10% of anomalies are due to recognizable teratogens (Chapter 102). The time of exposure is usually at less than 60 days of gestation during organogenesis. Specific agents produce predictable lesions. Some agents have a dose or threshold effect; below the threshold, no alterations in growth, function, or structure occur. Genetic variables such as the presence of specific enzymes may metabolize a benign agent into a more toxic-teratogenic form (e.g., phenytoin conversion to its epoxide). In many circumstances, the same agent and dose may not consistently produce the lesion.
Bertrand J, Floyd LL, Weber MK. Guidelines for identifying and referring persons with fetal alcohol syndrome. Fetal Alcohol Syndrome Prevention Team, Division of Birth Defects and Developmental Disabilities, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. MMWR Recomm Rep. 2005;54:1-14.
DeMarini DM, Preston RJ. Smoking while pregnant: transplacental mutagenesis of the fetus by tobacco smoke. JAMA. 2005;293:1264-1265.
Graham JMJr, Edwards MJ, Edwards MJ. Teratogen update: gestational effects of maternal hyperthermia due to febrile illnesses and resultant patterns of defects in humans. Teratology. 1998;58:209-221.
Lammer EJ, Chen CT, Hoar RM, et al. Retinoic acid embryopathy. N Engl J Med. 1985;313:837-841.
Moretti ME, Bar-Oz B, Fried S, et al. Maternal hyperthermia and the risk for neural tube defects in offspring: systematic review and meta-analysis. Epidemiology. 2005;16:216-219.
90.7 Intrauterine Diagnosis of Fetal Disease (See Table 90-1 and Chapter 90.2)
Diagnostic procedures are used to identify fetal diseases when abortion is being considered, when direct fetal treatment is possible, or when a decision is made to deliver a viable but premature infant to avoid intrauterine fetal demise. Fetal assessment is also indicated in a broader context when the family, medical, or reproductive history of the mother suggests the presence of a high-risk pregnancy or a high-risk fetus (Chapters 89 and 90.3).
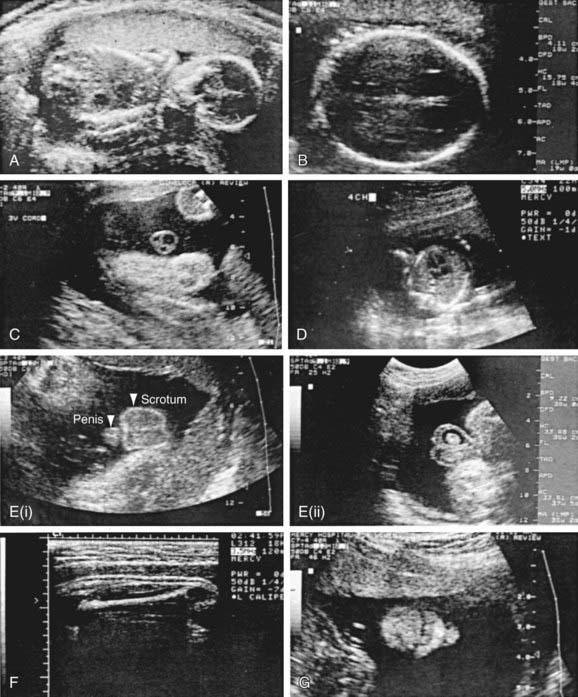
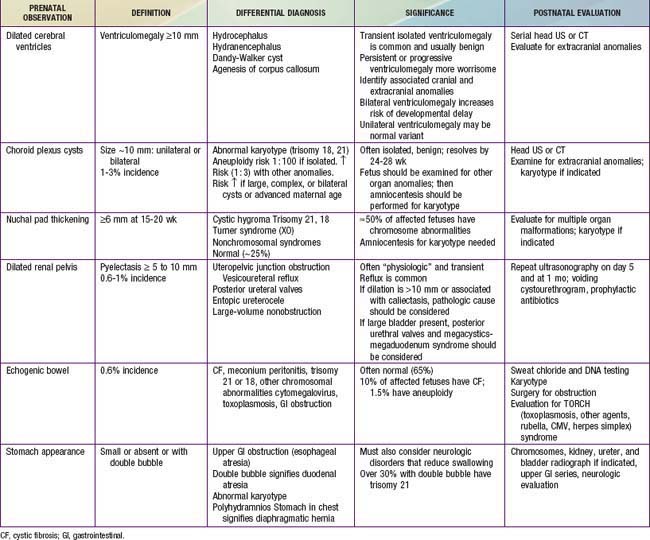
Various methods are used for identifying fetal disease (see Table 90-1). Fetal ultrasonographic imaging may detect fetal growth abnormalities (by biometric measurements of biparietal diameter, femoral length, or head or abdominal circumference) or fetal malformations (Fig. 90-5). Although 89% of fetuses whose biparietal diameter is 9.5 cm or more are at least in the 37th wk of gestation, the lungs of these fetuses may not be mature. Serial determinations of growth velocity and the head-to-abdomen circumference ratio enhance the ability to detect IUGR. Real-time ultrasonography may identify placental abnormalities (abruptio placentae, placenta previa) and fetal anomalies such as hydrocephalus, NTDs, duodenal atresia, diaphragmatic hernia, renal agenesis, bladder outlet obstruction, congenital heart disease, limb abnormalities, sacrococcygeal teratoma, cystic hygroma, omphalocele, gastroschisis, and hydrops (Table 90-7).
Real-time ultrasonography also facilitates performance of cordocentesis and the BPP by imaging fetal breathing, body movements, tone, and amniotic fluid volume (see Table 90-2). Doppler velocimetry assesses fetal arterial blood flow (vascular resistance) (see Figs. 90-2 and 90-3). Roentgenographic examination of the fetus has been replaced by real-time ultrasonography, MRI, and fetoscopy.
Amniocentesis, the transabdominal withdrawal of amniotic fluid during pregnancy for diagnostic purposes (see Table 90-1), is frequently performed to determine the timing of delivery of fetuses with erythroblastosis fetalis or the need for fetal transfusion. It is also done for genetic indications, usually between the 15th and 16th wk of gestation, with results available within 1-2 wk. The most common indication for genetic amniocentesis is advanced maternal age (the risk for chromosome abnormality at age 21 years is 1 : 526, vs 1 : 8 at age 49). The amniotic fluid may be directly analyzed for amino acids, enzymes, hormones, and abnormal metabolic products, and amniotic fluid cells may be cultivated to permit detailed cytologic analysis for prenatal detection of chromosomal abnormalities and DNA-gene or enzymatic analysis for the detection of inborn metabolic errors. Analysis of amniotic fluid may also help in identifying NTDs (elevation of α-fetoprotein), adrenogenital syndrome (elevation of 17-ketosteroids and pregnanetriol), and thyroid dysfunction. Chorionic villus biopsy (transvaginal or transabdominal) performed in the 1st trimester also provides fetal cells but may pose a slightly increased risk for fetal loss and limb reduction defects. Fetal cells circulating in maternal blood and fetal DNA in maternal plasma are potential noninvasive sources of material for prenatal diagnosis. This technology may eliminate the need for amniocentesis or chorionic villus sampling.
Saturated phosphatidylcholine or phosphatidylglycerol concentrations in amniotic fluid may be more specific and sensitive predictors of pulmonary maturity, especially in high-risk pregnancies such as those occurring in women with diabetes (Chapters 89 and 101.1).
Cordocentesis, or percutaneous umbilical blood sampling, is used to diagnose fetal hematologic abnormalities, genetic disorders, infections, and fetal acidosis (see Table 90-1). Under direct ultrasonographic visualization, a long needle is passed into the umbilical vein at its entrance to the placenta or fetal abdominal wall. Umbilical blood may be withdrawn to determine fetal hemoglobin, platelet concentration, lymphocyte DNA, the presence of infection, or PaO2, pH, PCO2, and lactate levels.
Transfusion or administration of drugs can be performed through the umbilical vein (Table 90-8). Serum screening is offered to pregnant women at midgestation to evaluate the risk for Down syndrome (trisomy 21) and congenital malformations known to cause elevations of various markers, including abdominal wall and NTDs. A combination of these biochemical markers (including α-fetoprotein, inhibin A, estriol, pregnancy-associated plasma protein A, and β-HCG [human chorionic gonadotropin]) and ultrasound increases the positive predictive value of these screening tests. Additionally, families with a known genetic syndrome may be offered prenatal genetic testing from amniotic fluid or amniocytes obtained via amniocentesis or chorionic villus sampling.
| DISORDER | POSSIBLE TREATMENT |
|---|---|
| HEMATOLOGIC | |
| Anemia with hydrops (erythroblastosis fetalis) | Umbilical vein packed red blood cell transfusion |
| Thalassemia | Fetal stem cell transplantation |
| Isoimmune thrombocytopenia | Umbilical vein platelet transfusion, maternal IVIG |
| Autoimmune thrombocytopenia (ITP) | Maternal steroids and IVIG |
| Chronic granulomatous disease | Fetal stem cell transplantation |
| METABOLIC-ENDOCRINE | |
| Maternal phenylketonuria (PKU) | Phenylalanine restriction |
| Fetal galactosemia | Galactose-free diet (?) |
| Multiple carboxylase deficiency | Biotin if responsive |
| Methylmalonic acidemia | Vitamin B12 if responsive |
| 21-Hydroxylase deficiency | Dexamethasone |
| Maternal diabetes mellitus | Tight insulin control during pregnancy, labor, and delivery |
| Fetal goiter | Maternal hyperthyroidism—maternal propylthiouracil |
| Fetal hypothyroidism—intra-amniotic thyroxine | |
| Bartter syndrome | Maternal indomethacin may prevent nephrocalcinosis and postnatal sodium losses |
| FETAL DISTRESS | |
| Hypoxia | Maternal oxygen, position |
| Intrauterine growth restriction | Maternal oxygen, position, improve macronutrients and micronutrients if deficient |
| Oligohydramnios, premature rupture of membranes with variable deceleration | Amnioinfusion (antepartum and intrapartum) |
| Polyhydramnios | Amnioreduction (serial), indomethacin (if due to increased urine output) if indicated |
| Supraventricular tachycardia | Maternal digoxin,* flecainide, procainamide, amiodarone, quinidine |
| Lupus anticoagulant | Maternal aspirin, prednisone |
| Meconium-stained fluid | Amnioinfusion |
| Congenital heart block | Dexamethasone, pacemaker (with hydrops) |
| Premature labor | Magnesium sulfate, antibiotics sympathomimetics, indomethacin |
| RESPIRATORY | |
| Pulmonary immaturity | Betamethasone |
| Bilateral chylothorax—pleural effusions | Thoracentesis, pleuroamniotic shunt |
| CONGENITAL ABNORMALITIES† | |
| Neural tube defects | Folate, vitamins (prevention); fetal surgery‡ |
| Obstructive uropathy (with oligohydramnios but without renal dysplasia) | >24 wk <32 wk of gestation, vesicoamniotic shunt plus amnioinfusion |
| Cystic adenomatoid malformation (with hydrops) | Pleuroamniotic shunt or resection‡ |
| Fetal neck masses | Secure an airway with EXIT procedure‡ |
| INFECTIOUS DISEASE | |
| Group B streptococcus colonization | Ampicillin, penicillin |
| Chorioamnionitis | Antibiotics |
| Toxoplasmosis | Spiramycin, pyrimethamine, sulfadiazine, and folic acid |
| Syphilis | Penicillin |
| Tuberculosis | Antituberculosis drugs |
| Lyme disease | Penicillin, ceftriaxone |
| Parvovirus | Intrauterine red blood cell transfusion for hydrops, severe anemia |
| Chlamydia trachomatis | Erythromycin |
| HIV-AIDS | Zidovudine (AZT) plus protease inhibitors |
| Cytomegalovirus | Ganciclovir by umbilical vein |
| OTHER | |
| Nonimmune hydrops (anemia) | Umbilical vein packed red blood cell transfusion |
| Narcotic abstinence (withdrawal) | Maternal low-dose methadone |
| Severe combined immunodeficiency disease | Fetal stem cell transplantation |
| Sacrococcygeal teratoma (with hydrops) | In utero resection or catheter directed vessel obliteration |
| Twin-twin transfusion syndrome | Repeated amniocentesis, yttrium-aluminum-garnet (YAG) laser photocoagulation of shared vessels |
| Twin reversed arterial perfusion (TRAP) syndrome | Digoxin, indomethacin, cord occlusion |
| Multifetal gestation | Selective reduction |
| Neonatal hemochromatosis | Maternal IVIG |
EXIT, Ex utero intrapartum treatment; IVIG, intravenous immunoglobulin; (?), possible but not proved efficacy.
* Drug of choice (may require percutaneous umbilical cord sampling and umbilical vein administration if hydrops is present). Most drug therapy is given to the mother, with subsequent placental passage to the fetus.
† Detailed fetal ultrasonography is needed to detect other anomalies; karyotype is also indicated.
‡ EXIT permits surgery and other procedures.
Bianchi DW, Lo YM. Fetomaternal cellular and plasma DNA trafficking: the yin and the yang. Ann N Y Acad Sci. 2001;945:119-131.
Ismaili K, Avni FE, Wissing M, et al. Long-term clinical outcome of infants with mild and moderate fetal pyelectasis: validation of neonatal ultrasound as a screening tool to detect significant nephrouropathies. J Pediatr. 2004;144:759-765.
Stenhouse EJ, Crossley JA, Aitken DA, et al. First trimester combined ultrasound and biochemical screening for Down syndrome in routine clinical practice. Prenat Diagn. 2004;24:774-780.
Wright CF, Chitty LS. Cell-free fetal DNA and RNA in maternal blood: implications for safer antenatal testing. BMJ. 2009;339:161-164.
90.8 Treatment and Prevention of Fetal Disease
Management of a fetal disease depends on coordinated advances in diagnostic accuracy and knowledge of the disease’s natural history; an understanding of fetal nutrition, pharmacology, immunology, and pathophysiology; the availability of specific active drugs that cross the placenta; and therapeutic procedures. Progress in providing specific treatments for accurately diagnosed diseases has improved with the advent of real-time ultrasonography and cordocentesis (see Tables 90-1 and 90-8).
The incidence of sensitization of Rh-negative women by Rh-positive fetuses has been reduced by prophylactic administration of Rh(D) immunoglobulin to mothers early in pregnancy and after each delivery or abortion, thus reducing the frequency of hemolytic disease in their subsequent offspring. Fetal erythroblastosis (Chapter 97.2) may be accurately diagnosed by amniotic fluid analysis and treated with intrauterine intraperitoneal or, more often, intraumbilical vein transfusions of packed Rh-negative blood cells to maintain the fetus until it is mature enough to have a reasonable chance of survival.
Pharmacologic approaches to fetal immaturity (e.g., administration of steroids to the mother to accelerate fetal lung maturation and decrease the incidence of respiratory distress syndrome [Chapter 95.3] in prematurely delivered infants) are successful. Inhibiting labor with tocolytic agents is unfortunately not successful in most patients with premature labor. Management of definitively diagnosed fetal genetic disease or congenital anomalies consists of parental counseling or abortion; rarely, high-dose vitamin therapy for a responsive inborn error of metabolism (biotin-dependent disorders) or fetal transfusion (with red blood cells or platelets) may be indicated. Fetal surgery (see Table 90-8) remains an experimental approach to therapy and is available only in a few highly specialized perinatal centers. The nature of the defect and its consequences, as well as ethical implications for the fetus and the parents, must be considered.
Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993-1004.
Flake AW, Zanjani ED. In utero hematopoietic stem cell transplantation. JAMA. 1997;278:932-937.
Holmes N, Harrison MR, Baskin LS. Fetal surgery for posterior urethral valves: long-term postnatal outcomes. Pediatrics. 2001;108:E7.
Johnson MP, Sutton LN, Rintoul N, et al. Fetal myelomeningocele repair: short-term clinical outcomes. Am J Obstet Gynecol. 2003;189:582-587.
Morris LM, Lim FY, Crombleholme TM. Ex utero intrapartum treatment procedure: a peripartum management strategy in particularly challenging cases. J Pediatr. 2009;154:126-131.
Prontera W, Jaeggi ET, Pfizenmaier M, et al. Ex utero intrapartum treatment (EXIT) of severe fetal hydrothorax. Arch Dis Child. 2002;86:F58-F60.
Senat MV, Deprest J, Boulvain M, et al. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl J Med. 2004;351:136-144.
Sutton L, Sun P, Adzick N. Fetal neurosurgery. Neurosurgery. 2001;48:124-142.