Chapter 37 The Difficult Airway in Obstetric Anesthesia
III. Anesthesia-Related Maternal Mortality
IV. Obstetric Anesthesia Closed Claims Studies
V. Why Is Advanced Airway Management Important?
VII. Incidence of Difficult or Failed Intubation in Obstetrics
VIII. Prediction of Difficult Airway Management
IX. Recommendations for Management of the Difficult Airway
X. Equipment (Difficult Airway Cart)
XI. Anticipated Difficult Intubation
XII. Airway Management Strategies
XIII. Unanticipated Difficult Tracheal Intubation
I Introduction
Difficult laryngoscopy, failed intubation, and inability to ventilate or oxygenate after induction of general anesthesia (GA) for cesarean delivery (C/D) are major contributory factors leading to maternal morbidity and mortality. In Western countries, the recognition of adverse maternal and neonatal outcomes associated with difficult airway (DA) management has led to a dramatic decline in the use of GA for both elective and emergency C/D.1–3
The overall general anesthesiology practice in industrialized countries in the last 2 decades has undergone a dramatic change. Management of the DA has emerged as one of the most important patient safety issues. Guidelines and strategies for management of the DA have been published by the American Society of Anesthesiologists (ASA),4 the Difficult Airway Society (DAS) in the United Kingdom,5 and the Canadian,6 French7 and Italian8 national societies’ work groups on difficult airway management. These guidelines are applicable to the general surgical population. However, none of the guidelines address management of the DA in an obstetric situation, especially in the context of urgency in delivering the baby.
There are differences among these standards, guidelines, algorithms, recommendations, and protocols, but in practice and in medicolegal cases, the distinction between terminologies can be blurred (Table 37-1).9 Published literature shows that the guidelines from the ASA Task Force on Management of the Difficult Airway have been discussed in 18% of nonobstetric medicolegal cases and were useful both in defending care (defense, 8%) and in criticizing care (plaintiff, 3%).10 Expert witnesses also have used these guidelines in litigated obstetric DA management cases.
| Terms | Definitions | Degree of Obligation |
|---|---|---|
| Standards | Generally accepted principles for patient management; exceptions are rare, and failure to follow is often difficult to justify | Mandatory |
| Strategy | A well-planned series of steps for achieving a goal | Voluntary |
| Guidelines | Systematically developed statements to assist practitioners for specific clinical circumstances; incorporates the best scientific evidence with expert opinion | Voluntary |
| Practice policies | Describe present recommendations issued to influence practitioners in reaching decisions about interventions | Voluntary |
| Recommendations | Suitable and useful strategy; not as strict as standards or guidelines | Voluntary |
| Options | Different possibilities are available; neutral assessment | Voluntary |
| Protocols and algorithms | Stepwise procedures or decision trees to guide practitioners through diagnosis and treatment of various clinical problems | Voluntary |
From Henderson JJ, Popat MT, Latto IP, Pearce AC: Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia 59:675–694, 2004.
II Definitions
There is no consensus on a standard definition of the DA in the literature. The ASA Task Force defined DA as a clinical situation in which a conventionally trained anesthesiologist experiences difficulty with face mask ventilation, difficulty with intubation, or both. The original ASA description of difficult intubation (DI) included a limit of 10 minutes or multiple attempts. The wisdom of this definition must be questioned in obstetrics especially, given the fact that GA is generally reserved for emergency C/D in which delivery of the baby is of the utmost urgency. The common practice in obstetric anesthesia is to use a single dose of succinylcholine when anesthetizing for C/D under GA. In the obstetric situation, difficult intubation is the inability of an experienced anesthesiologist to intubate within one dose of succinylcholine.11 A more apt definition of failed intubation in obstetric patients is inability to secure the airway with two attempts using a conventional laryngoscope or an alternative airway device to assist with tracheal intubation.
III Anesthesia-Related Maternal Mortality
Although the total number of maternal deaths has been decreasing steadily in the last few decades both in the United States and United Kingdom (Table 37-2),12,13 anesthesia-related complications rank seventh among the leading causes of maternal death, in both countries (Fig. 37-1).14,15 Even in the developing countries, anesthesia is emerging as an additional risk for maternal mortality and remains largely under-reported.16,17 Failures in airway management are a primary cause of anesthesia-related maternal deaths in the underdeveloped countries.18
TABLE 37-2 Anesthesia-Related Maternal Deaths in the United States and United Kingdom, 1979-2001
| Year of Death | United States* | United Kingdom† |
|---|---|---|
| 1979-1981 | 4.3 | 8.7 |
| 1982-1984 | 3.3 | 7.2 |
| 1985-1987 | 2.3 | 1.9 |
| 1988-1990 | 1.7 | 1.7 |
| 1991-1993 | 1.4 | 3.5 |
| 1994-1996 | 1.1 | 0.5 |
| 1997-1999 | 1.2 | 1.4 |
| 2000-2002 | 1.0 | 3.0 |
* Maternal deaths per million live births.
† Maternal deaths per million maternities (live births, stillbirths, pregnancy terminations, ectopic pregnancies, and abortions).
(From Hawkins JL, Chang J, Palmer SK, et al: Anesthesia-related maternal mortality in the United States: 1979-2002. Obstet Gynecol 117:71, 2011.)
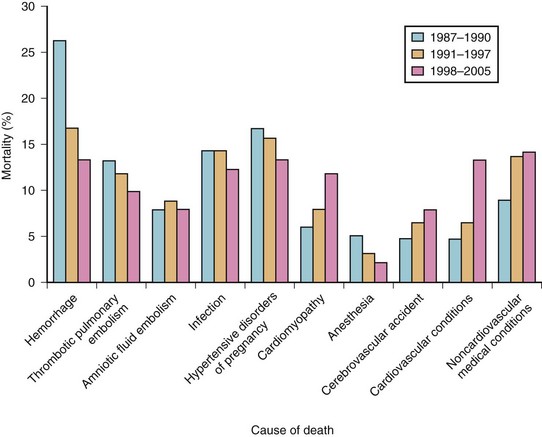
Figure 37-1 Maternal mortality in the United States.
(From Berg CJ, Callaghan WM, Syverson C, Henderson Z: Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol 116:1302–1309, 2010.)
The first national study of anesthesia-related maternal mortality in the United States between 1979 and 1990 was published in 1997.19 Most of these deaths (82%) took place during C/D. Death rates for GA during C/D increased from 20 per million in 1979-1984 to 32.3 per million in 1985-1990. Conversely, the death rate for regional anesthesia (RA) during the same periods declined from 8.6 to 1.9 per million, respectively. The relative risk for GA increased to 16.7 from 1985 to 1990. The case fatality risk ratio for GA was. 2.3 times that of RA from 1979 to 1984 and increased to 16.7 times from 1985 to 1990.19 The majority of maternal deaths were related to difficult or failed intubation, pulmonary aspiration, or respiratory complications.
A follow-up study examined 12 years of anesthesia-related maternal deaths between 1991 and 2002 and compared them with data from 1979 to 1990 to estimate trends over time and to compare the risks of GA and RA during cesarean delivery.13 Results showed that 86 pregnancy-related deaths were associated with complications of anesthesia, accounting for 1.6% of the total. Case-fatality rates for GA declined from 16.8 per million in 1991-1996 to 6.5 per million in 1997-2002 (Table 37-3).
TABLE 37-3 Case-Fatality Rates and Rate Ratios of Anesthesia-Related Deaths during Cesarean Delivery by Type of Anesthesia, United States, 1979-2002
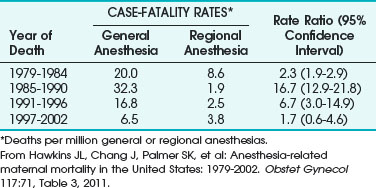
Complications related to anesthesia still occur, despite the decrease of almost 60% in anesthesia-related maternal mortality between 1979-1990 and 1991-2002. Although deaths from GA during C/D declined, about two thirds of the anesthesia-related deaths were caused by intubation failure or induction problems.13 The decline in GA-related case fatalities was explained by improvements in management of the DA and failed intubation and by increased expertise of anesthesiologists with the laryngeal mask airway (LMA) and other airway devices.13
A review of maternal mortality in Michigan from1985 to 2003 identified eight anesthesia-related deaths.20 Seven of these deaths were due to anesthesia-related factors. Interestingly, there were no deaths during induction of GA. The deaths were caused by airway obstruction or hypoventilation during emergence, lapses in monitoring, or lack of supervision in the postoperative period. Other risk factors included African-American race and obesity. This study highlighted the importance of airway-related problems during emergence, particularly in obese patients and in the African-American population, as well as the importance of vigilance in monitoring and management in the postoperative period for prevention of airway-related complications.
In the United Kingdom, despite the decline in the total number of maternal deaths, anesthesia-related causes consistently accounted for approximately 10% of the total direct deaths. During the period 1982-1984, anesthesia was the third leading cause of maternal death, resulting in 19 of 243 deaths, of which 15 were due to airway-related difficulties.21 The confidential enquiry spanning 1994-1996 showed that anesthesia was responsible for only 1 of 268 maternal deaths. In the Confidential Enquiry into Maternal and Child Health (CEMACH) 2000-2002 study, there were six direct deaths, all related to GA.15 Maternal deaths from complications of GA included a risk of 1 maternal death in 20,000. These cardiopulmonary arrests and deaths were related to difficult or failed intubation, difficult pulmonary ventilation resulting in failure to oxygenate, pulmonary aspiration, or acute respiratory distress syndrome (ARDS). In all of these cases, the anesthesia care was considered substandard.15
IV Obstetric Anesthesia Closed Claims Studies
The 2009 closed claims study published in the United States revealed that obstetric anesthesia claims for injuries from 1990 to 2003 had declined compared with claims for injuries before 1990.1 In the period 1990-2003, the proportion of maternal death or brain damage and that of newborn death or brain damage decreased. Respiratory causes of injuries also decreased, from 24% to 4% in claims from 1990 or later. Claims related to inadequate oxygenation or ventilation and those related to aspiration also decreased.
The number of claims related to difficult tracheal intubation after 1990, compared with pre-1990 claims, was unchanged.1 However, the overall improvement in closed claims statistics and the decline in anesthesia-related maternal mortality in more recent years were probably attributable to implementation of a minimum standard of care requiring the use of respiratory system monitors (pulse oximetry and capnography) during anesthesia in modern practice, enhanced awareness of the risk of pulmonary aspiration of gastric contents in the obstetric patient, decreased use of GA in obstetric practice, and use of advanced airway management techniques.
V Why Is Advanced Airway Management Important?
There have been tremendous advances in airway management in recent years, including an explosion in the use of airway devices as adjuncts to airway management. There has been a vast increase in the body of knowledge and in publications worldwide in this field. Improvements in advanced airway management have led to a documented decline in the incidence of airway-related perioperative morbidity in the surgical population.22 Similarly, in the last 2 decades, anesthesiologists’ focus on improving management of the DA and of failed intubation, experience with the LMA, application of the ASA difficult airway algorithm (DAA), and advanced airway management strategies have helped decrease GA-related case fatalities in obstetric cases.13
Because the medical-legal liability associated with airway-related adverse maternal outcomes is high,1 it is essential that all those practicing obstetric anesthesia as part of airway management should do the following:
1. Assess risk factors that predispose to airway-related complications
2. Be cognizant of predictors of the DA
3. Have appropriate algorithms and airway devices and equipment immediately available in the labor and delivery suite and in the operating room to manage the DA
4. Have an airway rescue plan, within the framework of the ASA DAA, for managing the DA
5. Be cognizant of the twin goals of urgently delivering the baby and preventing pulmonary aspiration, which can be hard to balance
VI Risk Assessment
A Airway
Airway changes occur during pregnancy, labor, and delivery.23 Further, the incidence of Mallampati (MP) class III and class IV scores increases during labor compared with the prelabor period, and these changes are not reversed by 48 hours after delivery. Therefore, it is absolutely necessary to examine the airway of a parturient in labor before administering anesthesia for a cesarean delivery.24
An increase in the ground substance of the airway connective tissue, caused by elevated levels of estrogen during pregnancy, an increase in total body water, and an increase in interstitial fluid and blood volume, results in hypervascularity and edema of oropharynx, nasopharynx, and respiratory tract. Excessive weight gain during pregnancy, preeclampsia, iatrogenic fluid overload, excessive bearing-down efforts during labor, and increase in venous pressure all lead to increased upper airway mucosal edema. Additional upper airway changes include tongue engorgement during pregnancy, which leads to decreased mobility of the floor of the mouth and changes in the MP score.25 Several published reports have described development of airway edema during labor and delivery, in preeclampsia, and after massive fluid and blood transfusion resuscitation following postpartum hemorrhage.26–30 In some of these reports, the associated difficulties in tracheal intubation were secondary to changes in the MP score.
Because of the increased vascularity and engorgement of the mucosa, the parturient is at increased risk for epistaxis after manipulation of the nasopharynx with nasotracheal intubation and for swelling of the airway and is vulnerable to increased trauma with repeated attempts at intubation.31 Avoidance of manipulation of the nasopharynx, use of smaller-sized tracheal tubes, and strict adherence to no more than two attempts at tracheal intubation32 are vital measures to avoid airway-related trauma, complications, and catastrophes.
D Gastrointestinal Changes
Other risk factors include pregnancy-related hormonal, anatomic, and physiologic gastrointestinal changes that place the parturient at risk for gastric regurgitation and pulmonary aspiration during GA. The gravid uterus shifts the stomach cephalad and changes the angle of the gastroesophageal junction, resulting in incompetence of the gastroesophageal pinchcock mechanism. The lower esophageal tone is decreased, leading to increased gastric reflux. There is a progesterone-mediated smooth muscle relaxant effect on the gastrointestinal mucosa. Furthermore, gastric emptying is prolonged during labor. Therefore, the parturient is at risk for gastric regurgitation, active vomiting, and pulmonary aspiration during GA, in case of a DA, or during failed intubation. Aspiration-related deaths during pregnancy occur from complications associated with induction problems such as DI, esophageal intubation, and inadequate attempts at ventilation.12
E Obesity
Weight gain during pregnancy results from the increasing size of the uterus and fetus, increased blood and interstitial fluid volumes, and deposition of new fat. There is a correlation between weight gain and an increase in the MP score.30 Pregnancy also results in a significant increase in breast size and engorgement. In the supine position, the enlarged breasts can encroach into the neck area, impeding effective application of cricoid pressure and leading to difficulty with laryngoscope blade insertion.
Obesity has reached epidemic proportions in the United States, and the incidence of obesity in pregnancy has doubled in the last 10 years. A body mass index (BMI) greater than 25 kg/m2 is considered overweight, and a BMI greater than 30 kg/m2 is considered obese.33 In the nonobstetric population, a BMI greater than 26 kg/m2 results in a threefold increased incidence of DMV and a 10-fold increased incidence of difficult tracheal intubation.34,35 Both prepregnancy obesity and excessive weight gain during pregnancy are associated with comorbidities such as hypertension or preeclampsia with intrauterine growth retardation, diabetes and macrosomia, and dysfunctional labor, thus increasing the incidence of operative cesarean delivery. The incidence of postpartum hemorrhage is higher in these patients, leading to increased likelihood of a GA intervention.
Because weight gain and obesity are associated with an increase in the MP score, the incidence of a partially obliterated oropharyngeal space in an obese parturient is doubled compared with that in nonpregnant patients. The aforementioned changes, the breast engorgement, and anthropometric differences between patients create a risk for difficult laryngoscopy, DI, and DMV.30,36,37 DI is encountered more frequently in morbidly obese parturients weighing more than 130 kg.38 Mask ventilation tends to be difficult because of low chest wall compliance and increased intra-abdominal pressure. In obesity, the respiratory-related changes of pregnancy are even more significant, with a marked decrease in FRC such that the closing capacity exceeds the FRC during tidal breathing, leading to a decrease in arterial oxygen tension and predisposing the parturient to a much higher risk of hypoxemia during a DI or DMV encounter. Obesity compounds all the risk factors associated with normal pregnancy, including DA, difficult laryngoscopy, DI, DMV, and aspiration-related complications.
VII Incidence of Difficult OR Failed Intubation IN Obstetrics
In 1998, the incidence of difficult laryngoscopy or tracheal intubation in the nonobstetric population was 0.1% to 13%.39 In the obstetric population, the incidence of difficult tracheal intubation was found to be 1/25040 to 1/280,41 1/300,42 and 1/750.36
A review of GA for cesarean deliveries from 1990 to 1995 showed the incidence of difficult tracheal intubation to range from a high of 16.3% to a low of 1.3%.2 There was a sentinel CICV incidence of 1 in 536 cases; in this patient, multiple attempts at intubation, unsuccessful mask ventilation, failed Combitube placement, and unsuccessful cricothyroidotomy resulted in cardiopulmonary arrest followed by surgical tracheostomy. Resuscitation was accomplished, but the mother remained in a coma until death, and the baby suffered significant neurologic injury.
In a review of GA for cesarean deliveries from 2000 to 2005, the incidence of CICV was 1 in 98. The single case of CICV occurred after failed tracheal intubation, unsuccessful LMA placement, and hypoxemia; successful cricothyroidotomy resulted in a good outcome for both mother and baby.43
VIII Prediction of Difficult Airway Management
The ASA DAA recommended an airway-related history to detect medical, surgical, and anesthetic factors that might indicate the presence of a DA. The guidelines also recommended an airway physical examination using assessment of multiple airway features before initiation of anesthetic care and airway management in all patients.4 The ASA closed claims analysis (2005) showed that 8% of patients did not have a preoperative history or airway physical examination.10
A retrospective audit was performed of all obstetric GAs, a total of 3430 cases over an 8-year period.44 None of the patients had a failed or esophageal intubation. There were 23 DIs, an incidence of 1:156. Anticipated difficult tracheal intubation occurred in 9 patients, 3 of whom underwent awake fiberoptic intubation; in the remaining 6 patients, who were morbidly obese, the DI was managed by senior trainees or consultants. Unanticipated difficulties occurred in 14 patients (61%). The preoperative assessment was found to be inadequate in these cases, being either not recorded (6 cases) or poorly documented (8 cases).
A Preoperative Assessment
1 History and Evaluation
According to the ASA Task Force, an airway history and a focused review of medical records must be conducted when feasible.4 Obviously, airway evaluation and prediction of DA in an obstetric patient starts with a thorough, focused airway-related history and evaluation of specific airway-related examination findings.45 A thorough history addresses any difficulty with previous GAs, obstructive sleep apnea (OSA) or snoring, head and neck abnormalities, and diseases that might impair the airway and result in difficult tracheal intubation. A history of DA management should be considered a strong predictor of problems unless the history was related to a specific reversible disease process such as a dental abscess. The history may be available from verbal recollections by the patient, previous anesthetic records, hospital notes, a letter describing DA management, or a Medic-Alert bracelet. The introduction of anesthesia information management systems and mandatory electronic medical records should be extremely useful in the future as “airway alerts” are built in to notify future practitioners of the specific details encountered with DA management in a particular patient.
2 Predictors of Difficult Airway
Yentis described the problems with many studies examining the prediction of DA.46 It is appropriate here to delineate the terms used to describe the accuracy and predictive power of these tests. A test to predict DI should have high sensitivity, so that it will identify most of the patients in whom intubation will be truly difficult. It should also have a high positive predictive value (PPV), so that only a few patients with airways actually easy to intubate will be subjected to the protocol for DA management. The test should also have a high specificity, so that it will identify most patients in whom tracheal intubation will be truly easy (Table 37-4).46 Excellent interobserver reliability is essential for any test to have high specificity, sensitivity, and PPV in predicting a DA.
| Term | Calculation |
|---|---|
| True positive (TP) | Difficult intubation that had been predicted to be difficult |
| False positive (FP) | Easy intubation that had been predicted to be difficult |
| True negative (TN) | Easy intubation that had been predicted to be easy |
| False negative (FN) | Difficult intubation that had been predicted to be easy |
| Sensitivity | Percentage of correctly predicted difficult intubations as a proportion of all intubations that were truly difficult—that is, TP/(TP+FN) |
| Specificity | Percentage of correctly predicted easy intubations as a proportion of all intubations that were truly easy—that is, TN/(TN+FP) |
| Positive predictive value | Percentage of correctly predicted difficult intubations as a proportion of all predicted difficult intubations—that is, TP/(TP+FP) |
| Negative predicted value | Percentage of correctly predicted easy intubations as a proportion of all predicted easy intubations—that is, TN/(TN+FN) |
* The sensitivity, specificity, and positive predictive value of each test are calculated using the Cormack-Lehane score as the constant variable.
Adapted from Yentis SM: Predicting difficult intubation: Worthwhile exercise or pointless ritual? Anaesthesia 57:105-109, 2002.
B Quantitative Evaluation of Difficult Intubation
The LEMON mnemonic represents one such assessment that is simple and quick, can be performed on any emergency patient, and has proved to have high PPV.47 The LEMON mnemonic represents the following five elements for preanesthetic assessment (Box 37-1):
L:Look externally—The initial impression of potential airway difficulty is based on assessment for any obvious anatomic distortions or external features that may make intubation difficult, such as facial and periorbital edema in a preeclamptic patient.
E:Evaluate the 3-3-2 Rule—Measuring the geometry of the airway can predict the anesthesia practitioner’s ability to align the oral, pharyngeal, and tracheal axes.
M:Mallampati score—The degree to which the tongue obstructs the visualization of the posterior pharynx has some correlation with the ability to visualize the glottis. The MP score can be estimated by having the patient, in a sitting position, open the mouth fully and protrude the tongue as far as possible without phonation. The relationship of the base of the tongue to the oropharyngeal structures—uvula and tonsillar pillars and fauces—is assessed as follows:
O:Obstruction—Pathologic conditions such as edema, glottic tumor, lingular tonsil, hyperplasia, and trauma can cause obstruction and can make laryngoscopy and ventilation difficult.
N:Neck mobility—This is a vital requirement for successful intubation. The sniffing position is the optimal, classic position of the head and neck for facilitating intubation. The extension of the atlanto-occipital (A-O) joint on the cervical spine so as to be able to align the three axes (oral, pharyngeal, and laryngeal) during laryngoscopy enhances the ease of laryngoscopy and tracheal intubation. Normal A-O joint extension of the head over the neck is 35 degrees. It can be assessed easily by getting the patient to place the chin down on the chest and tilt the head backward as far as possible. A reduction in A-O joint extension of 12 degrees (33%) or more correlates with intubation difficulty; complete joint immobility significantly compromises the laryngeal view. Limited A-O joint extension is present in certain pathologic states such as spondylosis, rheumatoid arthritis, and cervical spine stenosis, resulting in symptoms of nerve compression with cervical extension. Complete A-O joint immobility (e.g., hard-collar neck immobilization) can compromise the view of the glottis during laryngoscopy.
Box 37-1
LEMON Airway Assessment Method
L—Look externally for anatomic features that may make intubation difficult
O—Obstruction: Examine for partial or complete upper airway obstruction
From Reed MJ, Dunn MJ, McKeown DW: Can an airway assessment score predict difficulty at intubation in the emergency department? Emerg Med J 22:99–102, 2005.
C Additional Tests
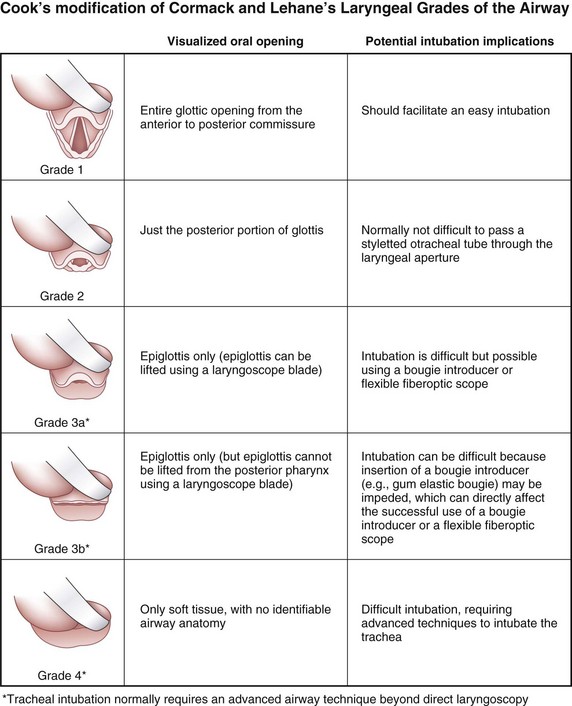
Difficult laryngoscopy was defined by the ASA Task Force as the inability to visualize any part of the vocal cords despite multiple attempts at conventional laryngoscopy.4 The original Cormack and Lehane classification of laryngoscopic views was used to describe the visibility of the glottis during laryngoscopy with a conventional laryngoscope and to predict the ease of intubation.48 The view at laryngoscopy was divided into four grades on the basis of a study in obstetric patients.49 The entire glottis is visible in a grade 1 view, whereas in a grade 2 view only the posterior portion of the glottis is visible; in a grade 3 view, only the epiglottis is seen, and in grade 4 view not even the epiglottis is seen. Grades 3 and 4 are considered to indicate DI.
1 Thyromental Distance
The thyromental distance (TMD) is defined as the distance from the chin (mentum) to the top of the notch of the thyroid cartilage with the head fully extended; it must be measured with a ruler for accuracy. The TMD gives an estimate of the mandibular space and helps in determining how readily the laryngeal axis will fall in line with the pharyngeal axis when the A-O joint is extended50:
• A TMD measurement of 6.5 cm or greater with no other abnormalities indicates the likelihood of easy intubation.
• A TMD measurement of 6.0 to 6.5 cm indicates that alignment of the pharyngeal and laryngeal axes will be challenging and that difficulty with laryngoscopy may result. However, intubation is possible with the use of adjuncts such as an Eschmann introducer or an optical stylet.
• A TMD measurement of less than 6 cm indicates difficult laryngoscopy; specifically, intubation may be impossible.
2 Sternomental Distance
The sternomental distance (SMD) is measured from the sternum to the tip of the mandible with the head fully extended and the mouth closed. The normal measurement is 13.5 cm. The SMD and the corresponding laryngoscopic view were documented in 523 parturients undergoing elective or emergency C/D under GA.51 An SMD of 13.5 cm or less had a sensitivity of 66.7%, a specificity of 71%, and a PPV of only 7.6%. Eighteen patients (3.5%) had a Cormack-Lehane grade 3 or 4 laryngoscopic view and were classified as having potentially difficult tracheal intubations. The SMD on its own as a sole indicator of DI was not useful, and the suggestion was to incorporate it with other tests in the preoperative airway examination.
3 Jaw Protrusion
Jaw protrusion (also termed prognathism or subluxation) is assessed by the mandibular protrusion test, which demonstrates the extent to which the lower incisors can be slid in front of the upper ones52:
4 Studies Assessing Predictors of Difficult Airway in Obstetrics
a Mallampati Classification
In obstetric patients, the MP score has been used as a single parameter to illustrate the dramatic airway changes that occur in pregnancy and to highlight the importance of preoperative assessment of the airway. Pilkington and coworkers (1995) evaluated the MP class at 12 weeks’ and 38 weeks’ gestation30; photographs taken at the two time periods demonstrated the increase in MP class in the same patient as gestation advanced. The MP score correlated with the increase in body weight, implying that oropharyngeal edema was responsible for the increase in the MP score.
b Multivariate Predictors
Rocke and colleagues (1992) were the first to use multivariate predictors to predict difficult tracheal intubation.36 They evaluated the MP classification as modified by Samsoon and Young, referring to it as the Modified Mallampati Test (MMT), along with other predictors in 3440 patients undergoing elective or emergency C/D under GA. Data were collected on 1606 patients, representing 46.7% of the obstetric surgical patients. Of the patients studied 1500 underwent general anesthesia. Other risk parameters for DA that they assessed included short neck, which equates with decreased A-O joint extension; receding mandible or decreased TMD (<3 finger breadths); and protruding maxillary incisors indicating significant overbite, which would equate to the current class C jaw protrusion or class III upper lip bite test.53
Rocke’s group made subjective assessments of the ease or difficulty of tracheal intubation according to the following scale (Table 37-5)36:
• Grade 1: Easy—intubation at first attempt with no difficulty
• Grade 2: Some difficulty—insertion of tracheal tube not achieved at first attempt; no difficulty but successful intubation after adjustment of laryngoscope blade and/or adjustment of head position, not requiring additional equipment, removal and reinsertion of the laryngoscope, or senior assistance
• Grade 3: Very difficult—requiring removal of the laryngoscope, further oxygenation by mask ventilation, and subsequent intubation with or without the use of airway adjuncts (e.g., Eschmann introducer, alternative laryngoscope blade) or intubation by a senior colleague
• Grade 4: Failed intubation—several attempts at intubation or unrecognized esophageal intubation by resident, followed by subsequent tracheal intubation by senior anesthesiologist
TABLE 37-5 Association between Oropharyngeal Structures Visualized Preoperatively and Subsequent Difficulty at Tracheal Intubation*
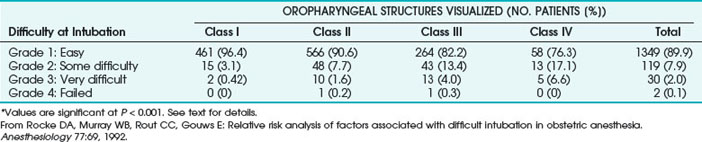
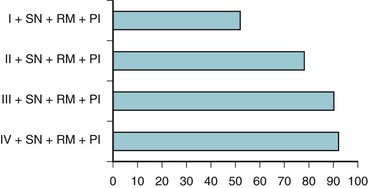
Based on these various parameters, the relative risk of experiencing difficult tracheal intubation (compared with an uncomplicated MMT class I airway) was determined as follows: 3.23 for MMT class II, 7.58 for MMT class III, 11.3 for MMT class IV, 5.01 for short neck, 9.71 for receding mandible, and 8.0 for protruding incisors. The investigators analyzed the univariate individual risk factor (i.e., MMT class) and combinations of the various risk factors and showed that a patient with an MMT III or IV classification plus protruding incisors, a short neck, and a receding mandible would have a probability of difficult laryngoscopy greater than 90% (Fig. 37-2). This study highlighted the importance of preoperative airway assessment to determine the best anesthetic intervention and the importance of prospectively preparing for airway interventions in the true obstetric emergency C/D under GA.
Gupta and colleagues evaluated the obstetric airway,54 using the MMT and the Wilson risk sum, to assess the potential for DA in 372 patients undergoing elective or emergency C/D under GA. The Wilson risk sum score was ascertained by adding scores for five factors (weight, head and neck movement, jaw movement/jaw protrusion, receding mandible, and buck teeth). The investigators also compared the sensitivity, specificity, and PPV of the MMT score (60%, 97.6%, and 65%, respectively) with the data published by Rocke and coworkers36 for laryngoscopy (59.2%, 74.1%, and 4.0%, respectively). The MMT was reproducible and was more sensitive than the Wilson risk sum score. As a screening test for prediction of DI, the Wilson risk sum score was less sensitive (36%) but had almost the same specificity (98.5%) and PPV (64%) as the MMT. When both tests were combined as predictors, the sensitivity was improved to 100%, the specificity was marginally decreased to 96.2%, and the PPV (64.8%) remained almost the same, compared with the MMT score alone. Gupta and colleagues concluded that, in obstetric patients, use of the Wilson risk sum score along with the MMT score resulted in high sensitivity, high specificity, and a high PPV.54 This study highlighted the importance of incorporating multiple predictors rather than using single (univariate) predictors of difficult laryngoscopy and intubation. There was a significant relationship between increased weight and external laryngeal manipulation.
Merah and associates studied the potential of five airway measurements to predict a difficult direct laryngoscopy in 80 West African obstetric patients during C/D under GA.37 The five bedside tests that were evaluated were MMT, TMD, SMD, horizontal length of mandible, and interincisor gap. Eight patients (10%) had difficult laryngoscopy. The MMT as a sole predictor had a sensitivity, specificity, and PPV of 87.1%, 99.6%, and 70%, respectively. In light of the current state of knowledge with respect to airway evaluation, the investigators concluded that this prediction tool would behave similarly in Caucasians as in West Africans. Weight contributed to the prediction of difficult laryngoscopy. The difference between the mean weights of difficult-to-intubate patients (109 ± 12.4 kg) and easy-to-intubate patients (81 ± 12.0 kg) was statistically significant. The combination of MMT and TMD yielded values of 100%, 93.1%, and 61.5% for sensitivity, specificity, and PPV, respectively. The researchers concluded that MMT can be used as the sole predictor of difficult tracheal intubation.
Kodali and colleagues performed a two-part study to evaluate airway changes during labor and delivery.23 In part I, they used the conventional Samsoon modification of the MP airway classification. The airway was photographed at the onset of labor (“prelabor”) and at the end of labor (“postlabor”). Pregnant women with MP class IV airways were excluded from this initial part of the study. In part II, prelabor and postlabor upper airway volumes were measured by acoustic reflectometry. In part I (n = 61), there was a significant increase in MP class between prelabor and postlabor measurements (P < 0.0001). The airway increased by one class in 20 parturients (33%) and by two classes in 3 parturients (5%). At the end of labor, there were 8 parturients with MP class IV (P < 0.01) and 30 with MP class III or IV (P < 0.0001). In Part II (n = 21), there were significant decreases in oral volume (P < 0.05) and in pharyngeal area (P < 0.05) and volume (P < 0.001) after labor and delivery.
Boutonnet and associates methodically evaluated the changes in MP class at four time intervals in 87 pregnant patients: during the eighth month of pregnancy (T1), at placement of the epidural catheter (T2), at 20 minutes after delivery (T3), and at 48 hours after delivery (T4).24 MP class did not change for 37% of the patients. The proportion of patients falling into MP class III or IV at the various times of assessment were as follows: T1, 10.3%; T2, 36.8%; T3, 51%; and T4, 20.7%. The differences in the percentages were all significant (P < 0.01). The incidence of MP classes III or IV increased during labor compared with prelabor period, and these changes were not reversed by 48 hours after delivery.
The studies by Kodali and Boutonnet and their colleagues confirmed the frequent increase in MP score during pregnancy and particularly during the course of labor.23,24 These findings suggest that it is imperative to evaluate the airway in early labor and to reevaluate it before anesthetic management for operative delivery.
c Meta-Analysis of Bedside Screening Test Performance
Shiga and colleagues systematically determined the diagnostic accuracy of bedside tests for predicting difficult tracheal intubation in patients with no airway pathology.55 Thirty-five studies comprising 50,760 patients, including both surgical and obstetric patients, were selected from electronic databases (Table 37-6).
TABLE 37-6 Pooled Estimates of Bayesian Statistics from Six Bedside Tests for Difficult Intubation (DI)*
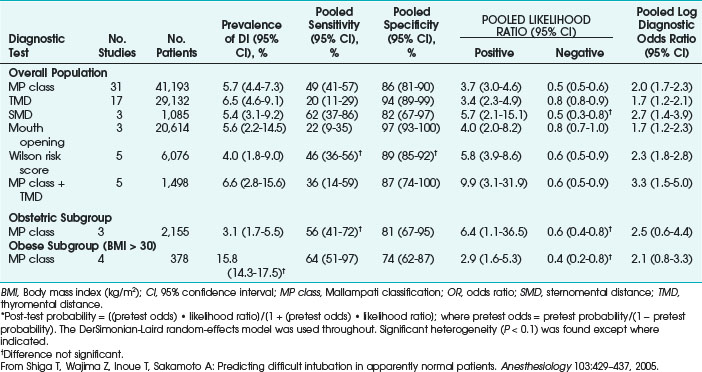
The overall incidence of difficult tracheal intubation was 5.8% (95% confidence interval [CI], 4.5% to 7.5%). Screening tests included the MP oropharyngeal classification, TMD, SMD, mouth opening, and Wilson risk score. Each test yielded poor to moderate sensitivity (20% to 62%) and moderate to fair specificity (2% to 97%). The meta-analysis found that the most useful bedside test for prediction was a combination of the MP classification and TMD (positive likelihood ratio, 9.9; 95% CI, 3.1 to 31.9). The study concluded that in surgical patients, this combination of tests added some incremental diagnostic value compared with the value of each test alone.55
The meta-analysis showed that in the obstetric population (2155 patients), the prevalence of difficult tracheal intubation was 3.1% (95 % CI, 1.7 to 5.5). The diagnostic performance of the MP classification in obstetric and obese populations was similar to that in the overall surgical population. The diagnostic odds ratios in these populations were similar, and the trend toward poor sensitivity and fair specificity remained. In the obstetric patients, the MP classification yielded a sensitivity of 56%, a specificity of 81%, and a likelihood ratio of 0.6%. The meta-analysis data in the obstetric patients remained inconclusive because of the small number of studies and heterogeneity. Whereas in the obese patients (BMI > 30 kg/m2), the incidence of difficult tracheal intubation was 15.8 % or three times higher than in the overall population. Obese patients with a 15% pretest probability of DI had a 34% risk of difficult tracheal intubation after a positive MP class result, which is twice the risk of the overall population with a 5% pretest probability. Similarly, obese pregnant patients also had a higher incidence of difficult tracheal intubation. Because of the higher incidence of difficult tracheal intubation, the MP classification may yield higher post-test probability of difficult tracheal intubation in obese patients than in the overall population.55
5 Trends in Anesthesia in Obstetrics
Cesarean delivery is the commonest major surgical procedure carried out in the United States (U.S. Centers for Disease Control and Prevention National Center for Health Statistics Report 2010) and the incidence is increasing worldwide.56 The increased incidence of maternal morbidity and mortality associated with GA in obstetric patients and the heightened awareness among anesthesia practitioners of the risk for DA and failed intubation in obstetrics has led to an increased use of RA techniques. A 2009 closed claims analysis found that use of RA was 65% before 1990 and increased to more than 80% since then, whereas the use of GA declined from 33% before 1990 to 17% after 1990.1 National Health Service maternity statistics show that the number of obstetric GAs for C/D administered in the United Kingdom fell from over 50% in 1989-90, to as low as 5% in 2004-2005.57 Johnson and colleagues similarly found a marked decline in GA for C/D, from 79% to less than 10% over the same period.58
The current trend in the United States and the United Kingdom is to use GA mainly for the true emergency C/D, if there is insufficient time for a regional technique, or in cases in which there is a contraindication to RA. Despite the decline in the use of GA for C/D, the incidence of difficult tracheal intubation was not found to be significantly different (5% before 1990 versus 3% after 1990).1 Encountering a DA in the obstetric population is not uncommon; therefore, the emerging problem of declining airway skills is important, as evidenced by a rise in the rate of failed tracheal intubation, from 1 : 300 between 1978 and 1983 to 1 : 250 in 1994.40,59,60 It is uncertain whether these concurrent trends are inter-related. The risk of failed tracheal intubation is considerably higher for emergency than for elective C/D, with 80% of airway-related fatalities occurring during emergency C/D (nights and weekends) and usually involving trainees.40 A review of GA for C/D at a tertiary hospital during the period 1990-1995 showed a decline in the use of GA, from 7.2% to 3.6%.2 A further review of GA for C/D from 2000 to 2005 in the same institution showed that GA use had further declined to a low incidence of about 1%.43
Because of the decreased use of GA for C/D, anesthesia trainees’ experience of GA in obstetric patients is also on the decline. The lack of opportunities for training and maintenance of airway skills is an emerging concern.58,61,62
IX Recommendations for Management of the Difficult Airway
Guidelines for the management of anticipated and unanticipated DA have been published by various national societies. The guidelines of the ASA Task Force were originally developed as a consequence of the high number of perioperative respiratory adverse events during airway management.63,64 The updated version of the DAA was published in 2003 (Fig. 37-3).4 The ASA DAA guidelines include evaluation of the airway, physical examination (as described earlier), basic preparation for the DA, and follow-up care. Although these points are applicable to the obstetric patients as well, some elements of the ASA DAA and their adaptability to the obstetric DA cannot be assessed due to the lack of strong evidence. The available evidence on management of the DA in the obstetric patient is mainly in the form of case reports. The ASA DAA provides a framework for management of the DA and should be modified and adapted to the obstetric patient where applicable.
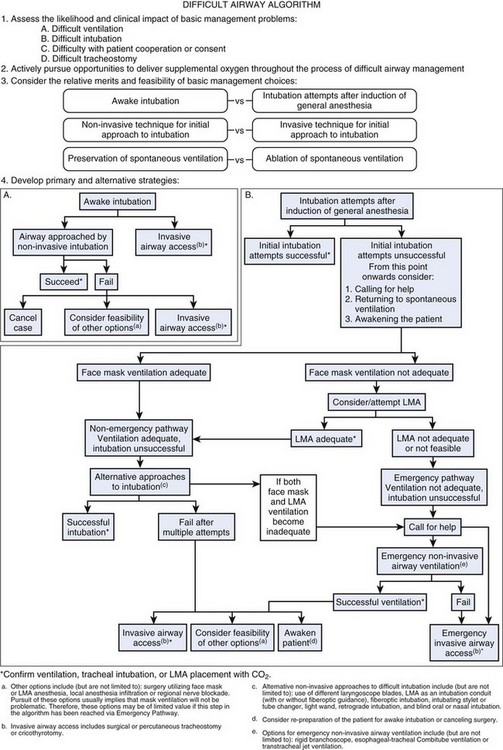
Figure 37-3 Difficult airway algorithm (DAA). LMA, Laryngeal mask airway.
(From American Society of Anesthesiologists Task Force on Management of the Difficult Airway: Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 98:1269–1277, 2003.)
X Equipment (Difficult Airway Cart)
The ASA DAA recommended that anesthesia personnel familiarize themselves with the patients and the airway equipment in advance. Likewise, the ASA Practice Guidelines for Obstetric Anesthesia recommended that airway equipment should be readily available in the labor and delivery area.45 Based on our own experience, we believe that equipment and anesthesia personnel should actually be immediately available in the labor and delivery area, throughout the 24-hour shift, on a daily basis. Our current practice is to have a designated DA cart immediately available inside our obstetric operating rooms for use in airway emergencies, and this policy has proved to be invaluable on many occasions.65–67
• Drawer A: Supraglottic airways of sizes 3 and 4 (LMA Excel, Fastrach LMA, ProSeal LMA) for use in the nonemergency pathway
• Drawer B: Specialized supraglottic airways (Combitube 37 F, King LTS-D sizes 3 and 4) for use in the emergency pathway
• Drawer C: Equipment available for invasive airway access (cricothyroidotomy kit, transtracheal jet cannula with adapter) for use in the emergency pathway, critical airway situation
XI Anticipated Difficult Intubation
Because the incidence of difficult tracheal intubation is significantly higher in the obstetric population, especially during emergency C/D and on nights and weekends,40 it is only prudent to avoid GA for C/D if other safe anesthetic choices are available. In modern obstetric anesthesia practice, neuraxial anesthesia is administered to some patients who would have received GA in the past, such as patients with severe preeclampsia with lower platelet count, placenta previa without active bleeding, or umbilical cord prolapse in a parturient with a functioning epidural catheter.
1. An increased use of epidural anesthesia during labor in high-risk parturients
2. A heightened awareness that an in situ functioning epidural catheter may decrease the necessity for GA in an urgent situation
3. Understanding that the presence of a functioning epidural catheter allows analgesia to be converted to epidural anesthesia, if necessary for a C/D
4. Appreciation of the risks of airway complications associated with GA for C/D
5. Improvement in the quality of intraoperative and postoperative pain management, with the addition of opioids to the local anesthetic, during spinal and epidural blocks
6. Keeping parturients awake and allowing for bonding with the baby during C/D
The current practice of placing epidural catheters prophylactically, early in labor, in high-risk parturients who have clinical conditions such as morbid obesity, DA, or obstetric comorbidities or complications has reduced the risk of an unanticipated GA. This is considered best practice and is in keeping with the most recent ASA guidelines for obstetric anesthesia.45 The practice guidelines advocate this concept of using a prophylactic epidural in high-risk parturients, which basically involves the placement of a catheter and the confirmation of functionality with small amounts of local anesthetic, early in labor, and possibly before a request for analgesia. This provides a readily available neuraxial route of anesthesia, should an emergent operative delivery be necessary. If the epidural catheter is not functioning well, it should be replaced with either a functioning continuous epidural catheter or a continuous spinal catheter.
Current evidence confirms the efficacy of dosing in situ epidural catheters in achieving decision-to-delivery intervals (DDI) for C/D and is comparable to that achieved with GA.68,69 Evidence suggests that it is necessary to allow the shortest DDI for the true fetal distress situation; additionally, the recommended 30-minute DDI for emergency cesarean deliveries is appropriate18 and allows the administration of a spinal anesthetic. Holcroft and colleagues showed the outcomes classified by type of anesthesia.70 DDI for GA was almost 15 minutes shorter than for spinal anesthesia and 13 minutes shorter than for epidural anesthesia (P = 0.0002). The epidural group had functioning labor epidurals that were converted to surgical anesthesia for C/D, whereas the spinal anesthesias were performed after the decision for C/D was made. There were no differences in the incidence of 1-minute Apgar scores lower than 7 based on anesthesia type, but there was a significant increase in 5-minute APGAR scores lower than 7 in the GA group. There was no difference in the umbilical arterial pH between the groups, but the base excess was significantly worse in the GA group. The investigators concluded that although GA is considerably faster than either spinal or epidural anesthesia, it is well established that GA poses greater maternal risk.13,71 Therefore, the benefits of obtaining anesthesia sooner versus the risk of a maternal complication must be weighed and determined by the obstetrician and anesthesiologist working in consultation together in each individual case. The American Congress of Obstetricians and Gynecologists (ACOG) Committee Opinion entitled, “Anesthesia for Emergency Deliveries” endorsed the following stance: “Cesarean deliveries that are performed for a nonreassuring fetal heart rate pattern do not necessarily preclude the use of regional anesthesia.”72 These guidelines require both familiarity and knowledge for application in an urgent C/D situation.
Another option for neuraxial techniques for C/D is deliberate continuous spinal analgesia using the Tuohy needle and an epidural catheter. A recent study by Palanisamy and colleagues confirmed that (1) the rate of use of GA for cesarean delivery between 2000 and 2005 was low, approximately 1%; (2) 85% of GAs were administered for emergent deliveries; (3) most were performed because of a perceived lack of time, particularly for the emergency deliveries; (4) very few GA cases resulted from failure of neuraxial anesthesia techniques; and (5) there was a very low incidence of GA-related morbidity with no cases of mortality.43 This study confirmed first, that the policy of placing epidural catheters prophylactically in high-risk parturients, particularly obese parturients with potential DA, has reduced the risk of unanticipated GA. Second, the adoption of a more aggressive approach toward management of inadequate neuraxial block (by replacing epidural catheters for suboptimal analgesia during labor) may have reduced the incidence of intraoperative conversion to GA. Third, the willingness to perform emergent spinal anesthesia, including intentional continuous spinal techniques, especially in patients with certain comorbid conditions (e.g., severe preeclampsia)73,74 or morbid obesity,75 may have been partly responsible for the reduction in GAs.
The use of a combined spinal/epidural technique for labor analgesia in a high-risk parturient may not be the most prudent choice. Even though several studies have found a lower incidence of failed epidural analgesia after initiation of analgesia with a combined spinal/epidural technique,76,77 there is a disadvantage in that the correct placement of the epidural catheter in the epidural space cannot be verified until the spinal anesthesia wanes. Therefore, if a functioning epidural is important, so as to be able to convert to surgical anesthesia for emergency C/D, especially in a parturient who has a high probability of a DA or a nonreassuring fetal status, then a combined spinal/epidural technique is not the preferred technique for labor analgesia.
XII Airway Management Strategies
A Cesarean Delivery
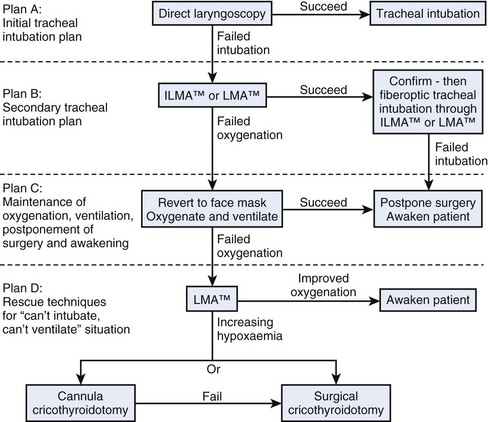
The anesthesia practitioner must have a clear and effective plan for managing a patient with DA, a difficult or failed intubation, or a CICV in a parturient undergoing C/D under GA. The ASA DAA is a beginning for an organized approach to DA problems.4 All anesthesia practitioners must be familiar with the ASA DAA. Although this algorithm is commendable in providing a logical, well-organized overview of the DA, it is not easily memorized and is not easy to implement in emergency situations, especially when two lives (mother and baby) are at risk. The United Kingdom DAS basic guidelines5 are more linear and emphasize a four-point approach with each plan consequent on failure of the previous plan (Fig. 37-4):
• Plan A: Initial intubation attempt
• Plan B: Secondary intubation attempt
• Plan C: Maintain oxygenation and ventilation using rescue techniques with supraglottic devices and bail out
B Awake Fiberoptic Intubation
In a parturient who has a known history of DA, an anticipated DA, morbid obesity, certain anatomic features indicating that tracheal intubation by conventional means is likely to be difficult or impossible, or contraindications to RA and is undergoing C/D, a safe option is to secure the airway with the tracheal tube while the patient remains awake. If RA is not an option and time is not an issue, an awake tracheal intubation can be performed safely with the use of either a flexible fiberscope or a video laryngoscope.78,79 The following paragraphs describe the logical approach to awake tracheal intubation.
• Psychological preparation of the patient.
• Administration of an antisialagogue such as glycopyrrolate, 0.2 to 0.3 mg intravenously, 10 to 15 minutes before the application of topical anesthesia to dry the oropharyngeal secretions. Excessive secretions interfere with the effects of local anesthetics. Glycopyrrolate, being a quaternary ammonium compound, does not cross the placenta.
• Judicious sedation with midazolam (1 mg) and fentanyl (50 to 100 µg) intravenously, so that the patient is calm and cooperative, without risking respiratory depression in the mother or neonate. Opioids produce analgesia and depress the airway reflexes to facilitate smooth oropharyngeal and laryngeal instrumentation.
• Adequate topicalization of the airway with local anesthetic to make it nonreactive to physical stimulation. Topical anesthesia of the oropharynx is achieved with 4 or 5 short sprays of 4% lidocaine to the palate, base of the tongue, and lateral and posterior pharyngeal walls. Two minutes after the spray of local anesthetic, 2% lidocaine gel is spread on the base of the tongue with a tongue blade, to supplement the topical anesthesia and to prevent gagging. Use of a good topical anesthetic before endoscopy prevents coughing, gagging, and laryngeal spasm and contributes to the success of the technique. Depressed gag and airway reflexes can make the parturient more susceptible to pulmonary aspiration of gastric contents. Ovassapian and colleagues80 presented the concept of topicalization of the lower airway and awake fiberoptic tracheal intubation in patients at high risk for pulmonary aspiration and have helped diffuse that controversy.
• The “Spray as you go” technique with local anesthetic spray of 2% lidocaine. This is used for application of the topical anesthetic and for anesthetizing the upper airway from the tip of the tongue to the trachea.
• The advanced airway management skills of the operator. The skill of the anesthetist is equally important for success of the technique.
Research has documented the successful use of awake fiberoptic tracheal intubation in 60 parturients with DAs undergoing C/D.81 These parturients received judicious sedation and underwent full topicalization, including transtracheal injection of lidocaine (except that those parturients with coagulopathy received nebulized lidocaine), and there were no cases of pulmonary aspiration. Lower esophageal tone is maintained in patients who are not oversedated and prevents pulmonary aspiration regardless of the extent of topicalization. In our own institution, we are occasionally faced with similar clinical situations and have successfully used awake, oral fiberoptic intubation to secure the airway before administration of GA for cesarean delivery.82
XIII Unanticipated Difficult Tracheal Intubation
A Step 1: Initial Tracheal Intubation Attempt
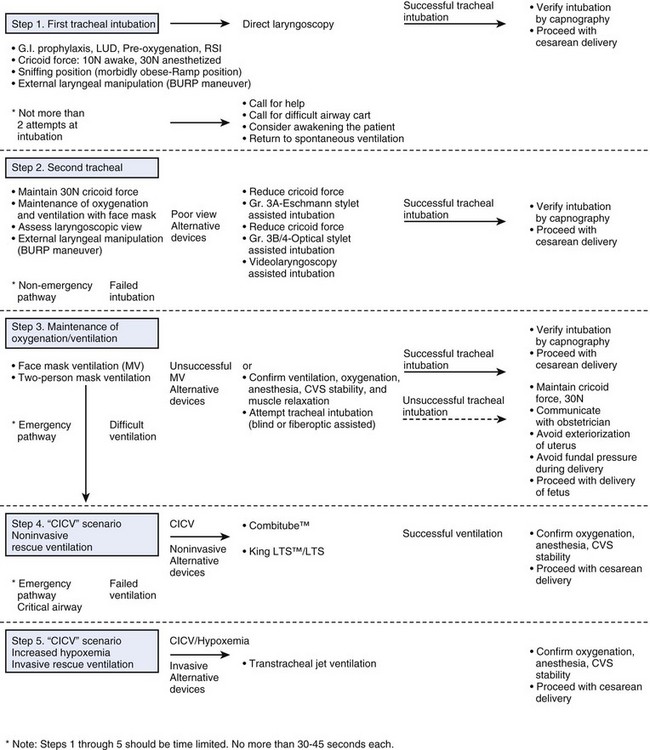
We propose a five-step flow chart for unanticipated difficult tracheal intubation after induction of GA in the obstetric patient (Fig. 37-5). It is based on the ASA DAA and the U.K. DAS algorithm and is modified for the obstetric patient. Each step should not take longer than 30 to 45 seconds, so no more than 5 minutes should elapse before the decision is made to use invasive airway access in the emergency pathway—critical airway scenario.
Induction of anesthesia is preceded by preoxygenation to effectively denitrogenate the lungs; effective use of a preoxygenation technique that maximizes oxygen stores (preoxygenation for 3 to 5 minutes or 4 deep breaths over 30 seconds)83; and the first attempt at direct laryngoscopy should always be performed in optimal conditions. After succinylcholine administration and confirmation of adequate muscle relaxation, appropriate head and neck position, and use of effective cricoid pressure, proceed with RSI and tracheal intubation. The application of optimal external laryngeal manipulation to optimize the laryngoscopic view may be critical. All these elements are important in managing the DI scenario (see Fig. 37-5, Step 1) and are discussed in detail in the following sections.
2 Positioning
Proper positioning of the patient is an often missed critical step in facilitating laryngoscopy, tracheal intubation, and mask ventilation, if needed. The optimal sniff position (slight flexion of the neck and extension of the head on the neck), which aligns the oral, pharyngeal, and laryngeal axes into a straight line, is mandatory to facilitate tracheal intubation.52 The neck should be flexed on a pillow and the A-O joint extended to achieve the optimal sniffing position. However, in morbidly obese patients including parturients, one should utilize the head-elevated laryngoscopy (HELP) position and create a ramp, using folded towels or blankets under the shoulders and head to ensure that the head and shoulders are higher than the chest.84 The HELP position is determined by drawing an imaginary horizontal line that connects the patient’s sternal notch with the external auditory meatus, so that the head and neck are at a slightly higher elevation than the chest. The Troop Elevation Pillow (Mercury Medical, Clearwater, FL) is shaped like a ramp and is designed to optimize the HELP and sniffing positions.
3 Cricoid Pressure
Cricoid pressure has played an important role in prevention of pulmonary aspiration since its introduction by Sellick.85 It is an integral part of the flow chart for the patient having RSI for cesarean delivery. Sellick first described a neck maneuver to compress the esophagus between the cricoid cartilages anteriorly and the body of the sixth cervical vertebra posteriorly in order to prevent regurgitated gastric contents from entering the hypopharynx during induction of GA.85 Typically, a cephalad and posteriorly pointing force of 10 newtons (N), applied by the thumb and index finger of the assistant, is required in the awake patient; this force increases up to 30 N in the unconscious patient.86,87 On the other hand, as important as cricoid pressure is to prevent regurgitation of gastric contents into the oropharynx,88 excessive cricoid pressure may obscure the glottic view by displacing the vocal cords anteriorly or laterally.89 The assistant may be asked to transiently reduce or release cricoid pressure, with suction at hand, so that the glottic area and vocal cords can be visualized, despite the possible risk of pulmonary aspiration in the event of gastric regurgitation.90,91 Aspiration is treatable, whereas irreversible hypoxia is not.
4 External Laryngeal Manipulation
Various steps taken to optimize the laryngoscopic view with external laryngeal manipulation, referred to as the BURP maneuver (i.e., backward, upward, rightward pressure on the thyroid cartilage) and the use of an Eschmann introducer can maximize the success of tracheal intubation. The use of optimal external manipulation (i.e., the BURP maneuver) involves pressure on the thyroid cartilage and displacement of the larynx in three specific directions—posteriorly against the cervical vertebrae, as far superiorly as possible, and slightly laterally to the right.92 Because the thyroid cartilage is the surface marking for the laryngeal aperture, proper application of the BURP maneuver improves the laryngoscopic view. In a study comparing glottic views with and without use of the BURP maneuver, it was shown that the BURP maneuver improved the glottic view by at least one whole grade and reduced the incidence of failure to view any portion of the glottis from approximately 9.2% to 1.6%.92
B Step 2: Second Tracheal Intubation Attempt
1 Consider Calling for Help, Returning to Spontaneous Ventilation, and Awakening the Patient
Despite use of optimal positioning and the BURP maneuver, if the first attempt at intubation fails because of a poor view, particularly a grade 3 laryngoscope view, other alternative intubation devices (i.e., Eschmann introducer, optical stylet, video laryngoscope) should be considered to assist in the second attempt at intubation (see Fig. 37-5, Step 2). There should be no more than two attempts at tracheal intubation in an obstetric patient. The second attempt at laryngoscopy should be considered the “best attempt at tracheal intubation.” To increase the success rate, it should be performed by a reasonably experienced anesthesiologist, the optimal sniff or ramped position should be used, and external laryngeal manipulation should be applied. Additionally, the laryngoscope blade type and handle may need to be changed. If necessary, cricoid pressure may be transiently released to optimize the glottic view.
Persistent attempts during emergency tracheal intubation have shown that there was a significant increase in the rate of airway-related complications as the number of laryngoscopic attempts increased (comparing ≤2 with >2 attempts), resulting in hypoxemia (11.8% versus 70%), regurgitation of gastric contents (1.9% versus 22%), aspiration of gastric contents (0.8% versus 13%), bradycardia (1.6% versus 21%), and cardiac arrest (0.7% versus 11%) (P < 0.001).32 We also recommend limiting the intubation attempts to two in an emergent obstetric case.93–95
2 Role of Eschmann Introducer (Gum Elastic Bougie)—Grade 3A Laryngoscopic View
The Eschmann introducer, commonly referred to as the gum elastic bougie, is used universally to facilitate difficult tracheal intubation.96 The original Cormack and Lehane classification of laryngoscopic view50 was recently modified by Cook,97 who proposed subdividing grade 3 into grades 3A and 3B (Fig. 37-6). In grade 3A, the glottic aperture cannot be seen but the epiglottis can be visualized and elevated; hence, a role for indirect methods, such as the Eschmann introducer, is indicated. Success rates have been shown to be similar between the Eschmann introducer and optical stylets in the grade 3A airway (31 versus 29.2 seconds).98
3 Role of Optical Stylets—Grade 3B/4 Laryngoscopic View
In grade 3B, when the glottic aperture cannot be seen and the epiglottis is visualized but cannot be elevated, other alternative methods may be used (see Fig. 37-6).99 Optical stylets have been used successfully to facilitate rapid tracheal intubation in the grade 3B laryngoscopic view and to help confirm tracheal tube placement.98 The success rate has been shown to be higher with optical stylets than with the Eschmann introducer, and the time required is shorter with optical stylets in the grade 3B view (31 versus 45.6 seconds).98
4 Role of Video Laryngoscopy
Video technology has become widespread across medical/surgical disciplines and allows improved visualization of anatomic detail. Besides allowing management of the DA, video laryngoscopy can be a useful teaching tool during both direct and indirect laryngoscopy.100 The portability of video laryngoscopes should facilitate their use in the operating room and also in managing airway calls in emergency departments, intensive care units, endoscopy suites, and radiology suites. It seems only natural that video laryngoscopy will become the norm in managing and teaching the DA in the near future.
Perhaps, in situations in which traditional laryngoscopy fails during the first attempt, the next attempt (best attempt at tracheal intubation) should be the use of a video laryngoscope with which the operator is familiar. Some of the devices available include the Berci Kaplan DCI, Glidescope, McGrath Scope, and CMAC Storz. The Glidescope has been successfully used in large studies and case series.78,101 Potential difficulty can be overcome by using a rigid stylet and shaping the tracheal tube before attempting insertion.
Channeled video laryngoscopes include the AirTraq, Pentax, and KingVision. These scopes are designed to provide an easy glottic view without aligning the oral, pharyngeal, and tracheal axes and have a channel for holding the tracheal tube. There has been one report of two cases of rapid tracheal intubation using Airtraq in morbidly obese parturients undergoing emergency cesarean delivery after failed tracheal intubation.102
C Step 3: Maintenance of Oxygenation and Ventilation
The goals and priorities in airway management strategies after a failed second tracheal intubation should include (1) maintenance of maternal oxygenation, (2) prevention of gastric regurgitation, (3) airway protection, and (4) expeditious delivery of the fetus (see Fig. 37-5, Step 3).
Maintaining oxygenation in the parturient is of paramount importance. Pregnancy-related changes can result in rapid development of hypoxemia and acidosis.103 Because adequate oxygenation is critical, mask ventilation in the presence of cricoid pressure is attempted. If mask ventilation is difficult, an optimal or best attempt at ventilation (i.e., two-person mask ventilation via a conventional face mask) is initiated while cricoid pressure (30 N) is maintained.95
After a failed second attempt at tracheal intubation, if oxygen saturation is maintained, the patient is considered to be on the nonemergency pathway (cannot intubate, but can ventilate), and use of the classic LMA, the intubating laryngeal mask airway (ILMA), or the ProSeal laryngeal mask airway (PLMA) as a rescue device must be considered. The revised ASA DAA, based on evidence in the literature, supports the role of the LMA in airway management.104,105 The LMA not only is useful to provide maternal oxygenation, ventilation, and anesthesia but also serves as a conduit for intubation. Cricoid pressure may have to be reduced or released transiently to allow proper placement of the LMA.91 The LMA or ILMA may be used as a conduit for tracheal intubation, either blind or fiberoptically assisted (see Fig. 37-5, Step 3).
1 The Classic Laryngeal Mask Airway
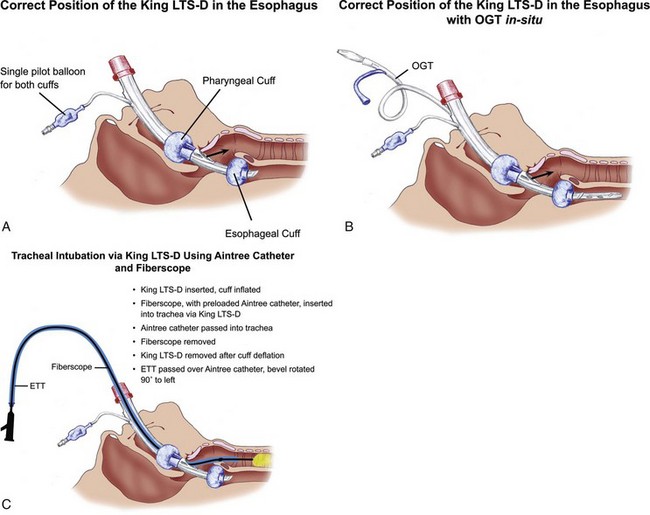
The classic LMA has been widely used for the difficult obstetric airway without any episodes of gastric regurgitation or pulmonary aspiration.40,106–109 Han and colleagues successfully used the classic LMA as a ventilatory device in 1060 of 1067 parturients undergoing elective cesarean delivery.110 No episodes of hypoxia, regurgitation, or aspiration were reported. Multiple case reports in the literature have described successful use of the classic LMA after failed tracheal intubation in obstetric patients.107,111–114 In patients requiring tracheal intubation, fiberoptic-guided tracheal intubation through the classic LMA is reliable.115 However, a longer tracheal tube (e.g., Endotrol, Microlaryngeal, or Nasal Ring-Adair-Elwyn) is needed. The Aintree exchange catheter (Cook Medical, Bloomington, IL) may also be used to facilitate intubation through the classic LMA.
The fiberscope/Aintree catheter exchange technique is extremely useful in switching from an LMA to a tracheal tube.116 The Aintree catheter is 56 cm long and, once preloaded on the fiberscope, allows the distal 3 to 4 cm of the fiberscope to be available for manipulation and passage via the LMA into the trachea. The inner diameter of the Aintree catheter is 4.8 mm, allowing it to be preloaded on no larger than a 4.0-mm pediatric fiberscope. Its removable Rapi-fit connector allows for oxygen insufflation, if necessary, during the airway exchange. Its external diameter is 6.5 mm, allowing it to be used as an airway exchange catheter (AEC) for tracheal tubes that have an internal diameter of 7 mm or more. This airway exchange technique involves the following steps (see Fig. 35-2 in Chapter 35):
1. Insertion of LMA and cuff inflation
2. Confirmation for easy ventilation and oxygenation
3. Passage of fiberscope, preloaded with Aintree catheter, via the LMA into the trachea under vision
4. Removal of fiberscope, leaving the Aintree catheter in the trachea, with the LMA in situ
5. Deflation of LMA cuff, followed by removal of LMA
6. Passage of tracheal tube of at least 7 mm internal diameter over the Aintree catheter into the trachea
2 The Intubating Laryngeal Mask Airway
The LMA Fastrach or ILMA is designed to specifically overcome the problems associated with blind tracheal intubation through the classic LMA.117 The ILMA is particularly useful during failed intubation in an emergency C/D because it provides oxygenation and a conduit for blind tracheal intubation and prevents pulmonary aspiration. Several studies have shown successful use of the ILMA to help visually unassisted tracheal intubation in patients with a DA.115,117,118 The ILMA was used successfully after failed tracheal intubation during an emergency C/D in a patient who was morbidly obese and eclamptic and in a second case in which RA had failed and was followed by GA resulting in failed tracheal intubation.66,67 The ILMA proved to be a life-saving device in both parturients.
3 The ProSeal Laryngeal Mask Airway
The PLMA is a unique device that represents a substantial change in LMA design. The PLMA offers several advantages over the classic LMA for failed tracheal intubation in obstetrics: (1) the seal is 10 cm H20 higher, giving it greater ventilatory capability119; (2) it enables correct positioning in which the glottis is isolated from the esophagus and therefore may provide airway protection and protection against pulmonary aspiration120,121; (3) it facilitates gastric tube insertion to empty the stomach of fluid and air insufflated during difficult face mask ventilation. The PLMA has been used successfully after failed intubation during emergency C/D.120,122–124
After failed tracheal intubation, and once the anesthesia practitioner is able to successfully achieve pulmonary ventilation and oxygenation through the LMA, caution must be used in selecting a nonirritating inhalation anesthetic and in providing an adequate depth of anesthesia. Sevoflurane provides rapid, smooth induction and adequate depth of anesthesia; it is the least irritating volatile agent,125 and it helps facilitate tracheal intubation through the LMA in patients with a DA.126
If tracheal intubation is difficult via the LMA and the fetal or maternal condition warrants immediate surgery, the obstetrician should be asked to proceed with cesarean delivery while the anesthesia care team maintains cricoid pressure and provides oxygenation and anesthesia via the LMA. Communication with the obstetrician should include the need to avoid both exteriorization of the uterus and fundal pressure during cesarean delivery, because of the unprotected airway and the risk of regurgitation and aspiration (see Fig. 37-5, Step 3).
D Step 4: CICV—Noninvasive Rescue Ventilation
1 Emergency Pathway
If face mask ventilation was not adequate and the LMA was ineffective in providing oxygenation and ventilation, the patient is considered to be on the emergency pathway (CICV) and will require rescue ventilation with other noninvasive devices4 such as the Combitube. King LTS/LTS-D may be a suitable alternative to the Combitube (see Fig. 37-5, Step 4).
2 Combitube
The Combitube should be considered in emergency airway situations, especially when patients are at risk for pulmonary aspiration, tracheal intubation has failed, and LMA is not effective.127,128 The ASA DAA suggests use of the Combitube after failed pulmonary ventilation with conventional face mask and LMA.4 The successful use of the Combitube has been described after failed tracheal intubation in an emergency C/D.127
When properly positioned, the Combitube allows ventilation with a higher seal pressure than the classic LMA, protects against regurgitation, and allows a subsequent attempt at fiberoptic intubation while the inflated esophageal cuff maintains airway protection.127,129 Although there have been failures,130 the Combitube has been used successfully in cases of difficult tracheal intubation127,131 and CICV,132,133 including failure with the LMA.134 The Combitube may offer significant advantages over the LMA in parturients. These advantages include isolation of the stomach from the glottic area and minimal need for preparation. Oxygenation and pulmonary ventilation can be achieved rapidly, especially because the parturient is prone to rapid arterial oxygen desaturation. The Combitube has been shown to prevent pulmonary aspiration during cardiopulmonary resuscitation and to protect the airway from pulmonary aspiration of gastric contents during obstetric anesthesia.129,135
The Combitube is a disposable double-lumen tube with two cuffs and two pilot balloons that is designed for blind insertion. If blind insertion is difficult or unsuccessful, the Combitube may be placed in the upper esophagus under direct vision with the use of a laryngoscope. Ventilation is initially attempted through lumen 1, which forces air into the trachea (see Fig. 35-3 in Chapter 35). Causes of failed ventilation with the Combitube include inadvertent tracheal placement (5%), deep insertion laryngospasm, and bronchospasm. The steps in troubleshooting Combitube use are important to understand, especially when one is confronted with a critical airway. The Combitube is designed to enter the esophagus after blind insertion. If ventilation is difficult through the blue lumen 1, the Combitube could be in the trachea, and ventilation must be switched to the clear lumen 2, allowing air to enter the trachea directly through the open end. If the Combitube is inserted too deep (see Fig. 35-5A in Chapter 35), ventilation is impossible and the pharyngeal balloon must be deflated, the Combitube pulled back 1 to 2 cm, and ventilation switched back to the blue lumen 2 (see Fig. 35-5B in Chapter 35). The original Combitube is a large, bulky device and can cause esophageal damage; therefore, the small adult (SA) size is recommended.136
3 Other Alternative Airway Devices
The King LT (known as the laryngeal tube in Europe) differs from the Combitube by being smaller, shorter, and softer and having a non-latex oropharyngeal cuff. The design of the King LT allows easy insertion, provides vertical positioning latitude, uses low-pressure cuffs,119,137 and allows passage of an AEC while simultaneously providing an esophageal barrier (Fig. 37-7A).138 It is a versatile airway device that can be used in elective or emergent conditions or DA, and ventilation can be spontaneous or controlled.139
The King LTS/LTS-D is the double-lumen version. It has dedicated ventilation and gastric access lumens and allows passage of an 18-F orogastric tube via the gastric drainage lumen (see Fig. 37-7B). Ventilatory seal with the King LT and airway pressure of 40 cm H2O was found to be possible without gastric inflation in 30 patients.140 The LMA was compared with King LT in 22 patients, and the mean leak pressure was significantly greater for the King LT141; gastric insufflation did not occur with the King LT. The King LTS/LTS-D may be useful in situations in which the patient is at risk for aspiration. In a case report, the King LTS was used successfully to establish ventilation and oxygenation after failed intubation in an emergency C/D.142
Exchange of the King LT for a tracheal tube can be accomplished with a fiberscope and an AEC (see Fig. 37-7C).138 The incidence of complications and adverse airway events is minimal with the use of this device.119
If oxygenation and ventilation are not established with either the LMA, the Combitube, or the King LT and the patient develops increasing hypoxemia (associated with bradycardia), the patient is considered to be in the emergency pathway—critical airway situation, which is a life-threatening emergency requiring immediate invasive intervention and rescue ventilation with either percutaneous cricothyroidotomy, TTJV, or surgical tracheostomy (see Fig. 37-5, Step 5).4,5
E Step 5: CICV with Increasing Hypoxemia—Invasive Rescue Ventilation
1 Emergency Pathway—Critical Airway
All current airway guidelines4–6 recommend management of the CICV situation using either cannula cricothyroidotomy with percutaneous TTJV or surgical cricothyroidotomy.
2 Cricothyroidotomy and Transtracheal Jet Ventilation
The risks of invasive rescue techniques must be constantly weighed against the risks of hypoxic brain damage or maternal death.143 In Palanisamy’s study,3 among the 98 parturients who received GA for C/D, there was a sentinel case of DI, resulting in a critical airway incident (CICV) that necessitated a surgical cricothyroidotomy. The investigators reported that the total time taken from RSI of anesthesia to surgical cricothyroidotomy was less than 5 minutes.3
The anesthesia practitioner must be prepared to use invasive techniques to secure the airway via the cricothyroid membrane. Success depends on understanding the anatomy of the cricothyroid membrane and factors that determine efficacy of ventilation airway devices.144,145
a Cannula Cricothyroidotomy with Percutaneous TTJV
This technique involves the combination of insertion of a cannula or catheter through the cricothyroid membrane and use of a high-pressure source for ventilation. TTJV can provide effective ventilation146,147; however, it is fraught with barotrauma hazards and is associated with a low success rate.148–150 It is important to keep the upper airway patent and open to allow for deflation of the lungs and exhalation through the upper airway. If an LMA has been used, it can be left in place to allow for exhalation.
b Surgical Cricothyroidotomy
A simplified cricothyroidotomy technique can be performed in 30 seconds.151 This technique consists of the following four steps:
Step 1—Identification of the cricothyroid membrane
Step 2—Horizontal stab incision (no. 20 scalpel) through skin and membrane
Step 3—Caudal traction on the cricoid membrane with a tracheal hook
Cricothyroidotomy can be particularly difficult in the obese patient. Insertion of the tube can be facilitated by passage of an introducer (bougie) through the incision or by the use of a tracheal retractor.152
Guidewire techniques of cricothyroidotomy can be successful. The Melker guidewire intubation set is now available with a cuffed tube. It has been shown that the technique can be performed in 40 seconds after practice in a mannequin.153
3 Extubation
The ASA Task Force regarded the concept of an extubation strategy as a logical extension of the extubation process.4 The closed claims analysis for DA management in surgical patients concluded that development of management strategies covering emergence and the recovery phase after extubation may improve patient safety.10
An emerging problem in obstetric patients is respiratory-related complications after emergence and maternal mortality after extubation.20 The data from the Confidential Enquiries into Maternal Death from the United Kingdom and a study from the United States on anesthesia-related deaths in Michigan indicated that airway management of the obstetric patient during induction of GA,20,154 including intubation, was without complications; however, critical airway and ventilation incidents including hypoventilation or airway obstruction occurred during emergence, extubation, or recovery.
Current literature does not provide a sufficient basis for evaluating the merits of an extubation strategy.4 The focus has been mainly on addressing strategies, safety concerns, and techniques for intubating the trachea after induction of anesthesia, with relatively little attention paid to extubation strategies. The U.K. data specifically mentioned lack of training as partly responsible for the undesirable outcomes after extubation. The review from Michigan identified eight anesthesia-related deaths between 1985 and 2003. All of these deaths occurred as a result of airway obstruction or hypoventilation during emergence and recovery. Lapses in standard postoperative monitoring and inadequate supervision by an anesthesiologist were identified. Obesity and African-American race also seemed to be important risk factors for anesthesia-related maternal mortality.
Further attention is required for high-risk extubation patients, such as obese parturients and patients with an edematous airway, obstructive sleep apnea, or known or suspected airway management difficulties. Development of a strategy to maintain continuous access to the airway, so as to facilitate potential rescue of the airway, must be considered. Postextubation hypoventilation, airway compromise, ventilation-perfusion inequalities, and airway obstruction due to respiratory fatigue or apneic episodes may affect the patient in the operating room during emergence or in the immediate postoperative recovery period. Maintenance of continuous access to the airway after extubation with an AEC can be an important component of an extubation strategy in these selected patients.155 The AEC is well tolerated by most patients (90%) and therefore is a valuable option.155 Currently, there are no evidence-based guidelines regarding the optimal period of time for which to maintain airway access after extubation via an indwelling AEC. Experts have suggested at least 30 to 60 minutes or until the likelihood of reintubation is minimized.156–159 Patients with the potential for periglottic edema may benefit from extending the duration of the in-dwelling AEC to 60 to 120 minutes.155
XIV Communication and Documentation
1. Comprehensive documentation in the patient’s chart outlining the problem and measures taken to manage the airway
2. Communication with the patient and the family
3. A detailed letter for the patient to carry describing the events that took place and their subsequent management
4. An airway alert in the electronic medical record
XVI Clinical Pearls
• Cesarean delivery is the most common major surgical operation carried out in the United States (U.S. Centers for Disease Control and Prevention’s National Center for Health Statistics report) and is increasing in incidence throughout the world.
• Anesthesia-related maternal complications rank seventh in the United States and the United Kingdom as a cause of maternal mortality; airway-related causes feature as a predominant category and are preventable.
• Pregnancy-related anatomic and physiologic changes predispose parturients to difficulty with intubation, ventilation, and extubation.
• Early preoperative assessment for patients undergoing labor and delivery must include a thorough airway history and examination and a rescue plan for potential failed tracheal intubation. Appropriate airway equipment and personnel must be immediately available in labor and delivery sites to manage the difficult airway (DA).
• Regional anesthesia (RA) is safe in most parturients undergoing cesarean delivery; in certain exceptional situations, an awake tracheal intubation is considered the safest choice in cases of anticipated or known DA in a parturient undergoing cesarean delivery.
• Adequate preoxygenation, left uterine displacement, head-elevated positioning, and use of supplementary high-flow oxygen via nasal cannula enhances oxygenation.
• Alternative airway devices such as optical stylets or video laryngoscopy should be used after a failed second attempt at tracheal intubation in the unanticipated DA (cannot intubate, can ventilate) scenario.
• First generation supraglottic airways such as the laryngeal mask airway (LMA) and second-generation supraglottic airways such as the King LTS-D should be considered as ventilatory devices in the unanticipated DA (“cannot intubate, cannot ventilate” [CICV]) scenario.
• Invasive airway access with a percutaneous/surgical cricothyrotomy or transtracheal jet ventilation (TTJV) may be necessary in the situation of critical airway with hypoxemia.
• Problems related to emergence and extubation have emerged as the most common cause of airway-related maternal mortality in recent reports from United States and the United Kingdom.
• Documentation should be provided in the patient’s record if difficulty is encountered with laryngoscopy, tracheal intubation, ventilation, or tracheal extubation.
All references can be found online at expertconsult.com.
1 Davies JM, Posner KL, Lee LA, et al. Liability associated with obstetric anesthesia: A closed claims analysis. Anesthesiology. 2009;110:131–139.
3 Palanisamy A, Mitani AA, Tsen LC. General anesthesia for cesarean delivery at a tertiary care hospital from 2000 to 2005: A retrospective analysis and 10-year update. Int J Obstet Anesth. 2010;20:10–16.
4 American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98:1269–1277.
5 Henderson JJ, Popat MT, Latto IP, Pearce AC. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia. 2004;59:675–694.
10 Peterson GN, Domino KB, Caplan RA, et al. Management of the difficult airway: A closed claims analysis. Anesthesiology. 1995;103:33–39.
12 Hawkins JL. Anesthesia-related maternal mortality. Clin Obstet Gynecol. 2003;46:679–687.
20 Mhyre JM, Riesner MN, Polley LS, Naughton NN. A series of anesthesia-related maternal deaths in Michigan, 1985-2003. Anesthesiology. 2007;106:1096–1104.
24 Boutonnet M, Faitot V, Katz A, et al. Mallampati class changes during pregnancy, labour, and after delivery: Can these be predicted? Br J Anaesth. 2010;104:67–70.
93 Wali A, Suresh MS. Maternal morbidity, mortality, and risk assessment. Anesthesiol Clin. 2008;26:197–230. ix
97 Cook TM. Classification of laryngoscopic view. Anaesthesia. 2000;55:1029–1030.
98 Kovacs G, Law JA, McCrossin C, et al. A comparison of a fiberoptic stylet and a bougie as adjuncts to direct laryngoscopy in a manikin-simulated difficult airway. Ann Emerg Med. 2007;50:676–685.
101 Cooper RM, Pacey JA, Bishop MJ, McCluskey SA. Early clinical experience with a new videolaryngoscope (GlideScope) in 728 patients. Can J Anaesth. 2005;52:191–198.
153 Wong DT, Prabhu AJ, Coloma M, et al. What is the minimum training required for successful cricothyroidotomy?: A study in mannequins. Anesthesiology. 2003;98:349–353.
155 Mort TC. Continuous airway access for the difficult extubation: The efficacy of the airway exchange catheter. Anesth Analg. 2007;105:1357–1362.
1 Davies JM, Posner KL, Lee LA, et al. Liability associated with obstetric anesthesia: A closed claims analysis. Anesthesiology. 2009;110:131–139.
2 Tsen LC, Pitner R, Camann WR. General anesthesia for cesarean section at a tertiary care hospital 1990-1995: Indications and implications. Int J Obstet Anesth. 1998;7:147–152.
3 Palanisamy A, Mitani AA, Tsen LC. General anesthesia for cesarean delivery at a tertiary care hospital from 2000 to 2005: A retrospective analysis and 10-year update. Int J Obstet Anesth. 2010;20:10–16.
4 American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98:1269–1277.
5 Henderson JJ, Popat MT, Latto IP, Pearce AC. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia. 2004;59:675–694.
6 Crosby ET, Cooper RM, Douglas MJ, et al. The unanticipated difficult airway with recommendations for management. Can J Anaesth. 1998;45:757–776.
7 Boisson-Bertrand D, Bourgain JL, Camboulives J, et al. [Difficult intubation. French Society of Anesthesia and Intensive Care. A collective expertise]. Ann Fr Anesth Reanim. 1996;15:207–214.
8 Frova G. [The difficult intubation and the problem of monitoring the adult airway. Italian Society of Anesthesia, Resuscitation, and Intensive Therapy (SIAARTI)]. Minerva Anestesiol. 1998;64:361–371.
9 Heidegger T, Gerig HJ, Henderson JJ. Strategies and algorithms for management of the difficult airway. Best Pract Res Clin Anaesthesiol. 2005;19:661–674.
10 Peterson GN, Domino KB, Caplan RA, et al. Management of the difficult airway: A closed claims analysis. Anesthesiology. 2005;103:33–39.
11 Pearce A. Evaluation of the airway and preparation for difficulty. Best Pract Res Clin Anaesthesiol. 2005;19:559–579.
12 Hawkins JL. Anesthesia-related maternal mortality. Clin Obstet Gynecol. 2003;46:679–687.
13 Hawkins JL, Chang J, Palmer SK, et al. Anesthesia-related maternal mortality in the United States: 1979-2002. Obstet Gynecol. 2011;117:69–74.
14 Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116:1302–1309.
15 Cooper GM, McClure JH. Maternal deaths from anaesthesia: An extract from Why Mothers Die 2000-2002, the confidential enquiries into maternal deaths in the United Kingdom. Chapter 9: Anaesthesia. Br J Anaesth. 2005;94:417–423.
16 Onwuhafua PI, Onwuhafua A, Adze J. The challenge of reducing maternal mortality in Nigeria. Int J Gynaecol Obstet. 2000;71:211–213.
17 Kampikaho A, Irwig LM. Incidence and causes of maternal mortality in five Kampala hospitals, 1980-1986. East Afr Med J. 1991;68:624–631.
18 McKenzie AG. Operative obstetric mortality at Harare Central Hospital 1992-1994: An anaesthetic view. Int J Obstet Anesth. 1998;7:237–241.
19 Hawkins JL, Koonin LM, Palmer SK, Gibbs CP. Anesthesia-related deaths during obstetric delivery in the United States, 1979-1990. Anesthesiology. 1997;86:277–284.
20 Mhyre JM, Riesner MN, Polley LS, Naughton NN. A series of anesthesia-related maternal deaths in Michigan, 1985-2003. Anesthesiology. 2007;106:1096–1104.
21 Turnbull A, Tindall VR, Beard RW, et al. Report on confidential enquiries into maternal deaths in England and Wales 1982-1984. Rep Health Soc Subj (Lond). 1989;34:1–166.
22 Cheney FW. The American Society of Anesthesiologists Closed Claims Project: What have we learned, how has it affected practice, and how will it affect practice in the future? Anesthesiology. 1999;91:552–556.
23 Kodali BS, Chandrasekhar S, Bulich LN, et al. Airway changes during labor and delivery. Anesthesiology. 2008;108:357–362.
24 Boutonnet M, Faitot V, Katz A, et al. Mallampati class changes during pregnancy, labour, and after delivery: Can these be predicted? Br J Anaesth. 2010;104:67–70.
25 Mallampati SR, Gatt SP, Gugino LD, et al. Can Anaesth Soc J. 1985;32:429–434.
26 Jouppila R, Jouppila P, Hollmen A. Laryngeal oedema as an obstetric anaesthesia complication: Case reports. Acta Anaesthesiol Scand. 1980;24:97–98.
27 Farcon EL, Kim MH, Marx GF. Changing Mallampati score during labour. Can J Anaesth. 1994;41:50–51.
28 Heller PJ, Scheider EP, Marx GF. Pharyngolaryngeal edema as a presenting symptom in preeclampsia. Obstet Gynecol. 1983;62:523–525.
29 Bhavani-Shankar K, Lynch EP, Datta S. Airway changes during cesarean hysterectomy. Can J Anaesth. 2000;47:338–341.
30 Pilkington S, Carli F, Dakin MJ, et al. Increase in Mallampati score during pregnancy. Br J Anaesth. 1995;74:638–642.
31 Dildy GA. Critical care in obstetrics. Dildy GA, III., Belfort MA, eds. ed 4. Blackwell Malden, MA: Science; Malden, MA:19–42.
32 Mort TC. Emergency tracheal intubation: Complications associated with repeated laryngoscopic attempts. Anesth Analg. 2004;99:607. 613
33 Bray GA. Overweight is risking fate. Definition, classification, prevalence, and risks. Ann N Y Acad Sci. 1987;499:14–28.
34 Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92:1229–1236.
35 el-Ganzouri AR, McCarthy RJ, Tuman KJ, et al. Preoperative airway assessment: Predictive value of a multivariate risk index. Anesth Analg. 1996;82:1197–1204.
36 Rocke DA, Murray WB, Rout CC, Gouws E. Relative risk analysis of factors associated with difficult intubation in obstetric anesthesia. Anesthesiology. 1992;77:67–73.
37 Merah NA, Foulkes-Crabbe DJ, Kushimo OT, Ajayi PA. Prediction of difficult laryngoscopy in a population of Nigerian obstetric patients. West Afr J Med. 2004;23:38–41.
38 Hood DD, Dewan DM. Anesthetic and obstetric outcome in morbidly obese parturients. Anesthesiology. 1993;79:1210–1218.
39 Arne J, Descoins P, Fusciardi J, et al. Preoperative assessment for difficult intubation in general and ENT surgery: Predictive value of a clinical multivariate risk index. Br J Anaesth. 1998;80:140–146.
40 Hawthorne L, Wilson R, Lyons G, Dresner M. Failed intubation revisited: 17-Yr experience in a teaching maternity unit. Br J Anaesth. 1996;76:680–684.
41 Samsoon GL, Young JR. Difficult tracheal intubation: A retrospective study. Anaesthesia. 1987;42:487–490.
42 Lyons G. Failed intubation: Six years’ experience in a teaching maternity unit. Anaesthesia. 1985;40:759–762.
43 Palanisamy A, Mitani AA, Tsen LC. General anesthesia for cesarean delivery at a tertiary care hospital from 2000 to 2005: A retrospective analysis and 10-year update. Int J Obstet Anesth. 2001;20:10–16.
44 Djabatey EA, Barclay PM. Difficult and failed intubation in 3430 obstetric general anaesthetics. Anaesthesia. 2009;64:1168–1171.
45 American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Practice guidelines for obstetric anesthesia: An updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Anesthesiology. 2007;106:843–863.
46 Yentis SM. Predicting difficult intubation: Worthwhile exercise or pointless ritual? Anaesthesia. 2002;57:105–109.
47 Reed MJ, Dunn MJ, McKeown DW. Can an airway assessment score predict difficulty at intubation in the emergency department? Emerg Med J. 2005;22:99–102.
48 Cormack RS, Lehane JR, Adams AP, Carli F. Laryngoscopy grades and percentage glottic opening. Anaesthesia. 2000;55:184.
49 Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39:1105–1111.
50 Patil VU, Stehling LC, Zauder HL. Fiberoptic endoscopy in anesthesia. Chicago: Year Book Medical Publishers; 1983.
51 Al Ramadhani S, Mohamed LA, Rocke DA, Gouws E. Sternomental distance as the sole predictor of difficult laryngoscopy in obstetric anaesthesia. Br J Anaesth. 1996;77:312–316.
52 Calder I, Calder J, Crockard HA. Difficult direct laryngoscopy in patients with cervical spine disease. Anaesthesia. 1995;50:756–763.
53 Khan ZH, Kashfi A, Ebrahimkhani E. A comparison of the upper lip bite test (a simple new technique) with modified Mallampati classification in predicting difficulty in endotracheal intubation: a prospective blinded study. Anesth Analg. 2003;96:595–599.
54 Gupta S, Pareek S, Dulara SC. Comparison of two methods for predicting difficult intubation in obstetric patients. Middle East J Anesthesiol. 2003;17:275–285.
55 Shiga T, Wajima Z, Inoue T, Sakamoto A. Predicting difficult intubation in apparently normal patients: A meta-analysis of bedside screening test performance. Anesthesiology. 2005;103:429–437.
56 Menacker F, Hamilton B. Recent trends in cesarean delivery in the United States. NCHS Data Brief 35. CDC; March 2010.
57 NHS maternity statistics, England 2004-2005. Leeds, West Yorkshire, UK: NHS Information Centre, 2006.
58 Johnson RV, Lyons GR, Wilson RC, Robinson AP. Training in obstetric general anaesthesia: A vanishing art? Anaesthesia. 2000;55:179–183.
59 Barnardo PD, Jenkins JG. Failed tracheal intubation in obstetrics: A 6-year review in a UK region. Anaesthesia. 2000;55:690–694.
60 Cormack RS. Failed intubation in obstetric anaesthesia. Anaesthesia. 2006;61:505–506. author reply 506
61 Rahman K, Jenkins JG. Failed tracheal intubation in obstetrics: No more frequent but still managed badly. Anaesthesia. 2005;60:168–171.
62 Tsen LC, Camann W. Training in obstetric general anaesthesia: A vanishing art? Anaesthesia. 2000;55:712–713.
63 Practice guidelines for management of the difficult airway: A report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 1993;78:597–602.
64 Caplan RA, Posner KL, Ward RJ, Cheney FW. Adverse respiratory events in anesthesia: A closed claims analysis. Anesthesiology. 1990;72:828–833.
65 Suresh MS, Gardner M, Key E. Intubating laryngeal mask airway (ILMA): A life saving rescue device following failed tracheal intubation during cesarean section. Presented at the 36th annual meeting of the Society for Obstetric Anesthesia and Perinatology, Ft. Myers, FL, 2004. Anesthesiology. 2004;100(Suppl 1):A135.
66 Suresh MS, Chandrasekhar S, Munnur U. Management of unanticipated difficult intubation with intubating LMA (Fastrach LMA) and review of literature with comparison to classic LMA. Presented at the 42nd annual meeting of the Society for Obstetric Anesthesia and Perinatology, San Antonio, TX. 2010.
67 Suresh MS, Mason CL. Airway management using the Levitan optical stylet in an emergency cesarean section. Presented at the 41st annual meeting of the Society for Obstetric Anesthesia and Perinatology, Washington, DC. 2009.
68 Lam DT, Ngan Kee WD, Khaw KS. Extension of epidural blockade in labour for emergency caesarean section using 2% lidocaine with epinephrine and fentanyl, with or without alkalinisation. Anaesthesia. 2001;56:790–794.
69 Popham P, Buettner A, Mendola M. Anaesthesia for emergency caesarean section, 2000-2004, at the Royal Women’s Hospital, Melbourne. Anaesth Intensive Care. 2007;35:74–79.
70 Holcroft CJ, Graham EM, Aina-Mumuney A, et al. Cord gas analysis, decision-to-delivery interval, and the 30-minute rule for emergency cesareans. J Perinatol. 2005;25:229–235.
71 Harvey K, Davies R, Evans A, et al. A comparison of the use of Trachlight and Eschmann multiple-use introducer in simulated difficult intubation. Eur J Anaesthesiol. 2007;24:76–81.
72 Committee on Obstetrics. Maternal and Fetal Medicine. Anesthesia for emergency deliveries. ACOG committee opinion no. 104, March 1992. Int J Gynaecol Obstet. 1992;39:148.
73 Aya AG, Mangin R, Vialles N, et al. Patients with severe preeclampsia experience less hypotension during spinal anesthesia for elective cesarean delivery than healthy parturients: A prospective cohort comparison. Anesth Analg. 2003;97:867–872.
74 Visalyaputra S, Rodanant O, Somboonviboon W, et al. Spinal versus epidural anesthesia for cesarean delivery in severe preeclampsia: A prospective randomized, multicenter study. Anesth Analg. 2005;101:862–868.
75 Soens MA, Birnbach DJ, Ranasinghe JS, van Zundert A. Obstetric anesthesia for the obese and morbidly obese patient: An ounce of prevention is worth more than a pound of treatment. Acta Anaesthesiol Scand. 2008;52:6–19.
76 Norris MC. Are combined spinal-epidural catheters reliable? Int J Obstet Anesth. 2000;9:3–6.
77 Pan PH, Bogard TD, Owen MD. Incidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: A retrospective analysis of 19,259 deliveries. Int J Obstet Anesth. 2004;13:227–233.
78 Doyle DJ. Awake intubation using the GlideScope video laryngoscope: Initial experience in four cases. Can J Anaesth. 2004;51:520–521.
79 Sinofsky AH, Milo SP, Scher C. The awake Glidescope intubation: An additional alternative to the difficult intubation. Middle East J Anesthesiol. 2010;20:743–746.
80 Ovassapian A, Krejcie TC, Yelich SJ, Dykes MH. Awake fibreoptic intubation in the patient at high risk of aspiration. Br J Anaesth. 1989;62:13–16.
81 Trevisan P. Fibre-optic awake intubation for caesarean section in a parturient with predicted difficult airway. Minerva Anestesiol. 2002;68:775–781.
82 Suresh MS, Krasuski P, Shukla N, et al. Fiberoptic intubation in patients undergoing cesarean section. Presented at the 34th annual meeting of the Society for Obstetric Anesthesia and Perinatology, San Diego. CA. 2002.
83 Norris MC, Dewan DM. Preoxygenation for cesarean section: A comparison of two techniques. Anesthesiology. 1985;62:827–829.
84 Levitan RM. Patient safety in emergency airway management and rapid sequence intubation: Metaphorical lessons from skydiving. Ann Emerg Med. 2003;42:81–87.
85 Sellick BA. Cricoid pressure to control regurgitation of stomach contents during induction of anaesthesia. Lancet. 1961;2(7199):404–406.
86 Herman NL, Carter B, Van Decar TK. Cricoid pressure: Teaching the recommended level. Anesth Analg. 1996;83:859–863.
87 Wraight WJ, Chamney AR, Howells TH. The determination of an effective cricoid pressure. Anaesthesia. 1983;38:461–466.
88 Fanning GL. The efficacy of cricoid pressure in preventing regurgitation of gastric contents. Anesthesiology. 1970;32:553–555.
89 Allman KG. The effect of cricoid pressure application on airway patency. J Clin Anesth. 1995;7:197–199.
90 Aoyama K, Takenaka I, Sata T, Shigematsu A. Cricoid pressure impedes positioning and ventilation through the laryngeal mask airway. Can J Anaesth. 1996;43:1035–1040.
91 Brimacombe J, White A, Berry A. Effect of cricoid pressure on ease of insertion of the laryngeal mask airway. Br J Anaesth. 1993;71:800–802.
92 Knill RL. Difficult laryngoscopy made easy with a “BURP.”. Can J Anaesth. 1993;40:279–282.
93 Wali A, Suresh MS. Maternal morbidity, mortality, and risk assessment. Anesthesiol Clin. 2008;26:197. 230, ix
94 Suresh MS. Difficult airway in the parturient. Probl Anesth. 2001;13:88–99.
95 Suresh MS, Wali A. Failed intubation in obstetrics: Airway management strategies. Anesthesiol Clin North Am. 1998;16:477–498.
96 Arya VK, Makker S, Bedi SP. Impacted gum-elastic bougie in an endotracheal tube after successful intubation of a difficult airway. J Clin Anesth. 2007;19:566.
97 Cook TM. Classification of laryngoscopic view. Anaesthesia. 2000;55:1029–1030.
98 Kovacs G, Law JA, McCrossin C, et al. A comparison of a fiberoptic stylet and a bougie as adjuncts to direct laryngoscopy in a manikin-simulated difficult airway. Ann Emerg Med. 2007;50:676–685.
99 Cook TM, Godfrey I, Rockett M, Vanner RG. Cricoid pressure: Which hand? Anaesthesia. 2000;55:648–653.
100 Kaplan MB, Ward D, Hagberg CA, et al. Seeing is believing: The importance of video laryngoscopy in teaching and in managing the difficult airway. Surg Endosc. 2006;20(Suppl 2):S479–S483.
101 Cooper RM, Pacey JA, Bishop MJ, McCluskey SA. Early clinical experience with a new videolaryngoscope (GlideScope) in 728 patients. Can J Anaesth. 2005;52:191–198.
102 Dhonneur G, Ndoko S, Amathieu R, et al. Tracheal intubation using the Airtraq in morbid obese patients undergoing emergency cesarean delivery. Anesthesiology. 2007;106:629–630.
103 Archer GW, Jr., Marx GF. Arterial oxygen tension during apnoea in parturient women. Br J Anaesth. 1974;46:358–360.
104 Parmet JL, Colonna-Romano P, Horrow JC, et al. The laryngeal mask airway reliably provides rescue ventilation in cases of unanticipated difficult tracheal intubation along with difficult mask ventilation. Anesth Analg. 1998;87:661–665.
105 Campo SL, Denman WT. The laryngeal mask airway: Its role in the difficult airway. Int Anesthesiol Clin. 2000;38:29–45.
106 Godley M, Reddy AR. Use of LMA for awake intubation for caesarean section. Can J Anaesth. 1996;43:299–302.
107 Chadwick IS, Vohra A. Anaesthesia for emergency caesarean section using the brain laryngeal airway. Anaesthesia. 1989;44:261–262.
108 Vanner RG. Laryngeal mask airway in the failed intubation drill. Int J Obstet Anesth. 1995;4:191. 192; author reply 192–194
109 Gataure PS, Hughes JA. The laryngeal mask airway in obstetrical anaesthesia. Can J Anaesth. 1995;42:130–133.
110 Han TH, Brimacombe J, Lee EJ, Yang HS. The laryngeal mask airway is effective (and probably safe) in selected healthy parturients for elective cesarean section: A prospective study of 1067 cases. Can J Anaesth. 2001;48:1117–1121.
111 McClune S, Regan M, Moore J. Laryngeal mask airway for caesarean section. Anaesthesia. 1990;45:227–228.
112 Priscu V, Priscu L, Soroker D. Laryngeal mask for failed intubation in emergency caesarean section. Can J Anaesth. 1992;39:893.
113 Storey J. The laryngeal mask for failed intubation at caesarean section. Anaesth Intensive Care. 1992;20:118–119.
114 Brimacombe JR, Berry A. The incidence of aspiration associated with the laryngeal mask airway: A meta-analysis of published literature. J Clin Anesth. 1995;7:297–305.
115 Parr MJ, Gregory M, Baskett PJ. The intubating laryngeal mask: Use in failed and difficult intubation. Anaesthesia. 1998;53:343–348.
116 Atherton DP, O’Sullivan E, Lowe D, Charters P. A ventilation-exchange bougie for fibreoptic intubations with the laryngeal mask airway. Anaesthesia. 1996;51:1123–1126.
117 Lim CL, Hawthorne L, Ip-Yam PC. The intubating laryngeal mask airway (ILMA) in failed and difficult intubation. Anaesthesia. 1998;53:929–930.
118 Brain AI, Verghese C, Addy EV, et al. The intubating laryngeal mask. II: A preliminary clinical report of a new means of intubating the trachea. Br J Anaesth. 1997;79:704–709.
119 Brimacombe J, Keller C, Fullekrug B, et al. A multicenter study comparing the ProSeal and classic laryngeal mask airway in anesthetized, nonparalyzed patients. Anesthesiology. 2002;96:289–295.
120 Keller C, Brimacombe J, Lirk P, Puhringer F. Failed obstetric tracheal intubation and postoperative respiratory support with the ProSeal laryngeal mask airway. Anesth Analg. 2004;98:1467. 1470
121 Miller DM, Light D. Laboratory and clinical comparisons of the Streamlined Liner of the Pharynx Airway (SLIPA) with the laryngeal mask airway. Anaesthesia. 2003;58:136–142.
122 Awan R, Nolan JP, Cook TM. Use of a ProSeal laryngeal mask airway for airway maintenance during emergency caesarean section after failed tracheal intubation. Br J Anaesth. 2004;92(1):144–146.
123 Sharma B, Sahai C, Sood J, Kumra VP. The ProSeal laryngeal mask airway in two failed obstetric tracheal intubation scenarios. Int J Obstet Anesth. 2006;15:338–339.
124 Vaida SJ, Gaitini LA. Another case of use of the ProSeal laryngeal mask airway in a difficult obstetric airway. Br J Anaesth. 2004;92:905. author reply 905
125 Doi M, Ikeda K. Airway irritation produced by volatile anaesthetics during brief inhalation: Comparison of halothane, enflurane, isoflurane and sevoflurane. Can J Anaesth. 1993;40:122–126.
126 MacIntyre PA, Ansari KA. Sevoflurane for predicted difficult tracheal intubation. Eur J Anaesthesiol. 1998;15:462–466.
127 Baraka A, Salem R. The Combitube oesophageal-tracheal double lumen airway for difficult intubation. Can J Anaesth. 1993;40:1222–1223.
128 Eichinger S, Schreiber W, Heinz T, et al. Airway management in a case of neck impalement: Use of the oesophageal tracheal combitube airway. Br J Anaesth. 1992;68:534–535.
129 Urtubia RM, Aguila CM, Cumsille MA. Combitube: A study for proper use. Anesth Analg. 2000;90:958–962.
130 Jaehnichen G, Golecki N, Lipp MD. A case report of difficult ventilation with the Combitube: Valve-like upper airway obstruction confirmed by fibreoptic visualisation. Resuscitation. 2000;44:71–74.
131 Banyai M, Falger S, Roggla M, et al. Emergency intubation with the Combitube in a grossly obese patient with bull neck. Resuscitation. 1993;26:271–276.
132 Tunstall ME, Geddes C. “Failed intubation” in obstetric anaesthesia: An indication for use of the “esophageal gastric tube airway. Br J Anaesth. 1984;56:659–661.
133 Bigenzahn W, Pesau B, Frass M. Emergency ventilation using the Combitube in cases of difficult intubation. Eur Arch Otorhinolaryngol. 1991;248:129–131.
134 Mercer M. Respiratory failure after tracheal extubation in a patient with halo frame cervical spine immobilization: Rescue therapy using the Combitube airway. Br J Anaesth. 2001;86:886–891.
135 Frass M, Frenzer R, Rauscha F, et al. Evaluation of esophageal tracheal Combitube in cardiopulmonary resuscitation. Crit Care Med. 1987;15:609–611.
136 Hartman RA, Castro T, Jr., Matson M, Fox DJ. Rapid orotracheal intubation in the clenched-jaw patient: A modification of the lightwand technique. J Clin Anesth. 1992;4:245–246.
137 Hagberg C, Bogomolny Y, Gilmore C, et al. An evaluation of the insertion and function of a new supraglottic airway device, the King LT, during spontaneous ventilation. Anesth Analg. 2006;102:621–625.
138 Genzwuerker HV, Vollmer T, Ellinger K. Fibreoptic tracheal intubation after placement of the laryngeal tube. Br J Anaesth. 2002;89:733–738.
139 Gaitini LA, Vaida SJ, Somri M, et al. An evaluation of the laryngeal tube during general anesthesia using mechanical ventilation. Anesth Analg. 2003;96:1750. 1755
140 Dorges V, Ocker H, Wenzel V, Schmucker P. The laryngeal tube: A new simple airway device. Anesth Analg. 2000;90:1220–1222.
141 Asai T, Kawashima A, Hidaka I, Kawachi S. The laryngeal tube compared with the laryngeal mask: Insertion, gas leak pressure and gastric insufflation. Br J Anaesth. 2002;89:729–732.
142 Zand F, Amini A. Use of the laryngeal tube-S for airway management and prevention of aspiration after a failed tracheal intubation in a parturient. Anesthesiology. 2005;102:481–483.
143 Tighe SQ. Failed tracheal intubation. Anaesthesia. 1992;47:356.
144 Burkey B, Esclamado R, Morganroth M. The role of cricothyroidotomy in airway management. Clin Chest Med. 1991;12:561–571.
145 Bennett JD, Guha SC, Sankar AB. Cricothyrotomy: The anatomical basis. J R Coll Surg Edinb. 1996;41:57–60.
146 Zornow MH, Thomas TC, Scheller MS. The efficacy of three different methods of transtracheal ventilation. Can J Anaesth. 1989;36:624–628.
147 Patel RG. Percutaneous transtracheal jet ventilation: A safe, quick, and temporary way to provide oxygenation and ventilation when conventional methods are unsuccessful. Chest. 1999;116:1689–1694.
148 Metz S, Parmet JL, Levitt JD. Failed emergency transtracheal ventilation through a 14-gauge intravenous catheter. J Clin Anesth. 1996;8:58–62.
149 Egol A, Culpepper JA, Snyder JV. Barotrauma and hypotension resulting from jet ventilation in critically ill patients. Chest. 1985;88:98–102.
150 Benumof JL, Gaughan SD. Concerns regarding barotrauma during jet ventilation. Anesthesiology. 1992;76:1072–1073.
151 Brofeldt BT, Panacek EA, Richards JR. An easy cricothyrotomy approach: The rapid four-step technique. Acad Emerg Med. 1996;3:1060–1063.
152 Morris A, Lockey D, Coats T. Fat necks: Modification of a standard surgical airway protocol in the pre-hospital environmental. Resuscitation. 1997;35:253–254.
153 Wong DT, Prabhu AJ, Coloma M, et al. What is the minimum training required for successful cricothyroidotomy?: A study in mannequins. Anesthesiology. 2003;98:349–353.
154 Cooper GM, McClure JH. Anaesthesia chapter from saving mothers’ lives: Reviewing maternal deaths to make pregnancy safer. Br J Anaesth. 2008;100:17–22.
155 Mort TC. Continuous airway access for the difficult extubation: The efficacy of the airway exchange catheter. Anesth Analg. 2007;105:1357–1362.
156 Cooper RM. The use of an endotracheal ventilation catheter in the management of difficult extubations. Can J Anaesth. 1996;43:90–93.
157 Dosemeci L, Yilmaz M, Yegin A, et al. The routine use of pediatric airway exchange catheter after extubation of adult patients who have undergone maxillofacial or major neck surgery: A clinical observational study. Crit Care. 2004;8:R385–R390.
158 Miller KA, Harkin CP, Bailey PL. Postoperative tracheal extubation. Anesth Analg. 1995;80:149–172.
159 Topf AI, Eclavea A. Extubation of the difficult airway. Anesthesiology. 1996;85:1213–1214.