Chapter 42 The Difficult Airway in Neurosurgery
II The Neurosurgical Patient
The American Academy of Neurological Surgeons (AANS) estimates that almost 1 million neurosurgical procedures are performed annually in the United States. Spine procedures are performed at three times the rate of cranial surgeries.1 When considering the range of potential neurosurgical procedures, the variety of patient pathophysiology is substantial. A patient presenting for neurosurgery may appear to be completely normal or can present with clinical symptoms of intracranial hypertension. The airway might be assessed as “normal” but the patient’s head is fixed in a frame. Also, the patient may present with acromegaly for pituitary surgery or may have a previous history of difficult intubation. Additionally, the unanticipated difficult airway becomes an even greater challenge in patients at risk for cerebral aneurysm rupture. Other challenges include the spine surgery patient in the prone position and considerations for extubation after prolonged surgery. Patients with central nervous system (CNS) disease can be sensitive to the effects of hypnotic agents, rendering them susceptible to apnea when premedication is given.
Neurosurgical procedures comprise only 7% of cases in the American Society of Anesthesiologists (ASA) closed-claims database but are associated with settlements that are 1.6 to 4 times more than general surgical procedures.2 Understanding the patient’s physiologic requirements, in addition to the surgeon’s plan, is extremely important in these patients. It is wise to have a number of techniques for achieving, maintaining, and rescuing the neurosurgical airway.
A Intracranial Dynamics and the Airway
Intracranial pressure (ICP) is the pressure within the rigid skull. Airway management in the face of intracranial hypertension is a frequent challenge for the neuroanesthesiologist, as well as the emergency physician. The patient who does not require immediate airway control may benefit from the simple maneuver of elevating the head. The head-up position may have beneficial effects on ICP through changes in mean arterial pressure (MAP), airway pressure, central venous pressure, and cerebrospinal fluid displacement.3 Cerebral perfusion pressure (CPP) is the effective perfusion pressure driving blood through the brain, defined as the difference between mean arterial and intracranial pressures (CPP = MAP − ICP). A frequent consideration in the neurosurgical patient is the need to balance and maintain intracranial dynamics, avoiding increases in ICP, yet maintaining cerebral perfusion. Although head elevation may reduce ICP, raising the head above 30 degrees may place the patient at risk for venous air entrainment and should be done with caution.
Ventilation is intimately related to cerebral blood flow (CBF) and is an integral part of neuroanesthesia management. The avoidance of hypercarbia is essential in management of patients with intracranial hypertension. Carbon dioxide dilates the cerebral blood vessels, increasing the volume of blood in the intracranial vault and therefore increasing ICP. Periarteriolar hydrogen ion concentration, [H+], powerfully influences cerebral arteriolar tone, and as the arterial carbon dioxide tension (PaCO2) rises, [H+] increases, causing arteriolar dilatation. This leads to a concomitant decrease in cerebrovascular resistance, causing an increase in CBF, and an increase in cerebral blood volume (CBV).4 Difficult mask ventilation may quickly lead to hypercarbia, hypoxemia, and increased CBF. Hypoxia also remains one of the more potent arteriolar cerebrovascular dilators. Changes in the arterial oxygen tension (PaO2) are associated with late increases in CBF. Hypoxia or ischemia leads to marked vasodilation, increased arterial vascular volume, and intracranial hypertension.5
Laryngoscopy and intubation, if performed with difficulty or improperly, can severely compromise intracranial dynamics and increase morbidity. Both the sympathetic and the parasympathetic nervous system mediate cardiovascular responses to endotracheal intubation.6 Acute increases in ICP and MAP during laryngoscopy and endotracheal intubation have been well documented.7 In 1975, Burney and Winn8 measured ICP in 12 patients undergoing craniotomy and two patients for carotid arteriography. ICP did not change in response to the injection of contrast medium but increased significantly and dramatically in response to laryngoscopy and intubation. The increase appeared related to the initial ICP of these patients, possibly representing exhaustion of compensatory mechanisms. Special attention must be given to this factor during manipulation of the larynx in neurosurgical patients with initially increased ICP or space-occupying intracranial lesions.
Techniques to blunt this sympathetic response have included (1) an additional dose of thiopental or propofol, (2) use of beta-blockers or other antihypertensive agents, and (3) use of intravenous (IV) lidocaine. Esmolol or lidocaine as an IV bolus of 1.5 mg/kg before laryngoscopy and intubation did not completely prevent the increase in MAP and ICP.9,10 Etomidate has been shown to cause an early “burst” suppression pattern on the electroencephalogram (EEG), minimal changes in CPP, and a marked reduction in ICP. This decrease in ICP was maintained during the first 30 seconds and the following 60 seconds after intubation, as MAP and heart rate remained unchanged.11 Although not practical, this approach demonstrates the extent of efforts often made to obtund this response. Numerous methods have been advocated to prevent undesirable cardiovascular disturbances at intubation.12 Whereas the cardiovascular response can be dramatic and substantial, the ICP response may lag behind and persist longer. Once the patient is intubated, ventilation parameters may be adjusted to the clinical situation.
III Clinical Strategies for the Neurosurgical Patient
A Patient for Craniotomy
Patients who are neurologically intact may demonstrate no evidence of intracranial pathology or alteration. In addition to the history and physical examination, preoperative computed tomography (CT) or magnetic resonance imaging (MRI) scans of the head may give valuable information, because lesions associated with greater than 10 mm in midline shift or cerebral edema usually indicate intracranial hypertension.13 These patients should be appropriately managed to avoid undue increases in ICP and CBF. Such measures include proper head positioning, preoxygenation, and appropriate dosing of induction agents and relaxants to achieve a smooth intubation. The primary challenge in anesthetizing a patient with a supratentorial mass lesion is to avoid further increases in ICP when one has limited intracranial compliance. There is no “ideal anesthetic” for this group of patients, and the perioperative management should be individualized. However, the practitioner should be aware of the effects of anesthetic agents on intracranial dynamics.
The preoperative use of midazolam for anxiety in these patients should not cause harm if they are carefully observed. A 1-mg to 2-mg dose of IV midazolam in adult patients may facilitate the induction of anesthesia without altering intracranial dynamics.14 Opioids, on the other hand, should be restricted to very small amounts and given preoperatively under constant supervision because of possible hypercarbia and resultant effects. The efficacy of depth of anesthesia was recognized early on as a technique for avoiding intracranial hypertension.15 Deep inhalation anesthesia was replaced by a combination of intravenous induction agents, notably thiopental, in combination with fentanyl. Thiopental produces a dose-dependent reduction in CBF and cerebral metabolic rate of oxygen consumption (CMRO2). Other barbiturates, such as pentobarbital and methohexital, essentially have similar effects. ICP is reduced by barbiturates, likely because of the reduction in CBF and CBV. Propofol has largely replaced thiopental as the induction agent of choice for neuroanesthesia. Despite initial concerns about decreasing MAP and CPP, propofol provides a smooth transition to unconsciousness without an increase in heart rate, as observed with thiopental. This often produces less hypertension with laryngoscopy and intubation.16
Clinical doses of most opioids have minimal to modest depressive effects on CBF and CMRO2. Early studies demonstrate that ICP is either not elevated or slightly decreased with fentanyl alone or in combination with droperidol. Reported ICP increases in patients with space-occupying lesions have been attributed to hypercapnia. The variability in response to opioids appears to be caused by the background anesthetic. When vasodilating drugs are used as part of the anesthetic management, the effect of the opioid is consistently that of a vasoconstrictor. Sufentanil was thought to produce an increase in ICP in patients with intracranial mass effect, but this was later attributed to a decrease in MAP.17 Alfentanil produces little changes or slight decreases in CBF.18 The beneficial effect of synthetic opioids is their ability to blunt the hemodynamic response to laryngoscopy and intubation without affecting intracranial dynamics. Remifentanil produces the most profound and consistent response, with lack of hypertension, tachycardia, or increase in ICP.19 A continuous infusion throughout induction may provide the most effective hemodynamic control, while adequate ventilation is maintained.
The volatile agents, including nitrous oxide, can be considered dose-dependent cerebral vasodilators.20 As a component of neuroanesthesia, volatile agents are typically used in moderate doses, in combination with opioids and hypnotic agents. The effects on cerebral circulation and metabolism of sevoflurane and desflurane are largely comparable to isoflurane. Both induce a direct vasodilation of the cerebral vessels, resulting in a less pronounced increase in CBF, compared to the decrease in cerebral metabolism.
Induction may be followed by hyperventilation with a volatile agent to deepen the anesthetic, decrease CMRO2 (and CBF), and provide bronchodilation in patients with asthma or chronic obstructive pulmonary disease. Sevoflurane is useful in both pediatric and adult patients by allowing inhalation induction without the adverse effects of coughing or breath-holding.21 A frequently employed technique in the cooperative patient is the use of active hyperventilation before induction, to initiate hypocapnia and decrease CBF as the patient loses consciousness. The use of topical anesthesia applied to the larynx and trachea can also prevent further response to laryngoscopy and intubation.22 The large number of techniques recommended to suppress cardiovascular responses indicates that no single method has gained widespread acceptance (Table 42-1).
Table 42-1 Anesthetic Techniques to Avoid Increased Intracranial Pressure
| Technique | Precaution(s) |
|---|---|
| Avoid hypercapnia. |
PEEP, Positive end-expiratory pressure.
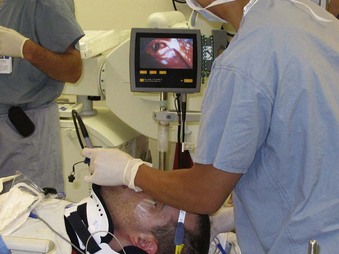
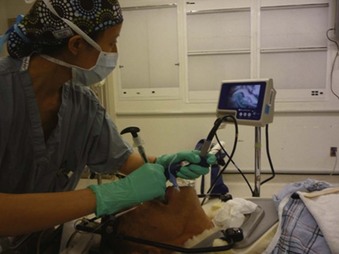
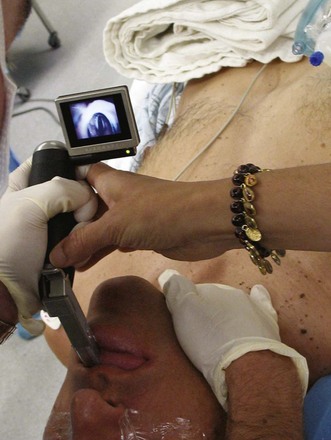
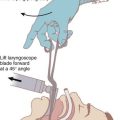
Alternative devices, such as the lightwand, can be useful in failed intubation, particularly in patients with a small chin or limited mouth opening. Because a lightwand is inserted without use of a laryngoscope, there is potential for less hypertension and tachycardia. This finding was demonstrated by Nishikawa and associates23 in 60 patients undergoing awake intubation for emergency surgery. Its successful use in the difficult airway requires experience and practice. Brimacombe and Kihara24 compared the hemodynamic responses of the lightwand and intubating laryngeal mask airway (ILMA) to direct laryngoscopy in hypertensive and normotensive patients. In their series, both the ILMA and the lightwand attenuated the hemodynamic stress response to tracheal intubation compared with direct laryngoscopy in hypertensive, but not in normotensive, anesthetized paralyzed patients. Optical stylets, such as the Shikani Optical Stylet scope or the Clarus Video System, have similar characteristics and insertion technique with the added benefit of laryngoscopic viewing (Fig. 42-1) The ILMA is particularly useful in the failed intubation sequence, and the ability to ventilate is extremely important in neurosurgical patients. The success of the ILMA as a ventilatory device has been impressive, as demonstrated in several of the early evaluation studies.25,26 It is also extremely useful in the setting of a failed fiberoptic intubation.27
The patient for aneurysm surgery who presents with a difficult airway is particularly problematic. If the airway is anticipated or known to be difficult, fiberoptic intubation is often the method of choice. This is assuming that one is skillful using the fiberoptic scope and is prepared to perform this technique in the awake, cooperative patient (see Chapter 11). IV fentanyl and midazolam may be carefully administered if the patient does not exhibit signs of intracranial hypertension. An arterial line is generally placed before induction. Additional techniques include remifentanil infusion (0.05 µg/kg/min) and dexmedetomidine infusion.28,29 Both techniques require careful patient monitoring and may be useful. Once the glottis is viewed, a dose of lidocaine may be given via the fiberoptic scope to prevent coughing and “bucking” with intubation.
B Head-Injured Patient
Traumatic brain injury (TBI) remains a prevalent disease in the United States and the world. The incidence of TBI is 175 to 300 per 100,000 population and accounts for 56,000 deaths per year in the United States.30 With the increased use of seatbelts, motor vehicle crashes are now secondary to gunshot wounds as the leading causes of TBI. Early intubation of the head-injured patient is critical and is often established in the field if providers are so trained. It is essential for optimal management of the patient, providing for efficient ventilation and oxygenation, helping to prevent aspiration of gastric contents, and allowing for suction of the lungs and pulmonary toilet. However, patients who are unconscious and breathing adequately may be transported with oxygen by mask throughout their initial assessment. This is intuitive in the apneic and unresponsive patient with a Glasgow Coma Scale (GCS) score of 8 or less.
The anesthesiologist caring for the patient with TBI must understand that although primary mechanisms of injury (primary insults) are a large determinant of patient outcome, attention to secondary insults, such as hypoxia, hypotension, intracranial hypertension, and decreased CPP, can impact dramatically on morbidity, mortality, and quality of life of the TBI patient.31 Evidence supports this, with mortality from TBI nationally decreasing over the decades.32 Hypoxia in TBI patients is a frequent occurrence, particularly in the prehospital setting. Interestingly, hypoxia was identified in 44% of patients with TBI on arrival in the emergency department.33 Similarly, Jeremitsky and colleagues34 report that hypoxia is one of three predictors of mortality in adult brain-injured patients (with hypothermia and hypoperfusion). Hypoxia dramatically impacts morbidity and mortality in TBI, and hypercapnia further increases mortality.
Hypotension is the secondary insult that has been most frequently cited as contributing to poor outcome after TBI. Hypertension is independently related to mortality in multivariate analysis.34 Information from the Traumatic Coma Data Bank shows that a systolic blood pressure less than 80 mm Hg was one of five factors that worsened patient outcome at 6 months.35 Hypotension during any phase in the brain trauma patient’s hospital course is associated with a greater likelihood of severe disability and vegetative state.36 However, early in the course of brain trauma, especially when combined with hypoxia, hypotension is devastating. When hypotension and hypoxia occur together, mortality is 75%.35
Techniques minimizing head movement should be used in TBI patients and by the most skilled clinicians. However, concern about a cervical fracture should never take precedence over relieving hypoxemia. It is of critical importance to ensure that appropriate monitoring is present throughout airway maneuvers. Nasal intubation should be avoided in head injury, particularly in patients with known or suspected basilar skull fractures and sinus injuries. Alternative airway devices, such as video laryngoscopes, any of the indirect rigid laryngoscopes, ILMA, or fiberoptic stylets, may be useful when the head must remain immobilized.37 Most emergency patients are assumed to have a “full stomach,” so it is important to weigh the risk of aspiration, which is a potential problem during laryngoscopy and intubation. If the situation warrants, surgeons should be prepared to perform a rapid cricothyrotomy if intubation attempts fail and ventilation becomes impossible.
C Patient with Cervical Spine Disease
1 Management of Acute Injury and the Unstable Spine
Spinal injuries occur in approximately 13% to 30% of polytrauma patients, and cervical spine injury (CSI) represents about 0.9% to 3% of all polytrauma patients.38,39 The relative risk of CSI is increased in the presence of severe head injury by a factor greater than 8.40 In the United States, cervical trauma has an incidence of approximately 5 per 10,000 population annually, making up 4% of all blunt trauma. In trauma victims with a GCS score of 13 to 15, the incidence of CSI is 1.4%, but this rises dramatically to 10.2% if the GCS score is less than 8. It is of vital importance to capture all injuries in the unconscious polytrauma patient within an emergent time frame. If a CSI is missed or its detection delayed, the incidence of secondary neurologic deficit increases from 1.4% to 10.5%. For this reason, the Advanced Trauma Life Support (ATLS) protocol was created, constantly updated, and broadly followed in most trauma centers.41 When a diagnosis of CSI is delayed, almost one third of patients may develop permanent neurologic deficit.42 One of the areas of controversy is how best to “clear” the cervical spine in the trauma patient. Detection of CSI requires a variety of modalities that vary in sensitivity, including clinical evaluation, plain radiography, CT, MRI, and dynamic fluoroscopy.
Clinical Evaluation
To clear the cervical spine clinically, the following criteria must be met:
1. GCS score of 15, with the patient alert and oriented
2. Absence of injuries that may draw attention away from a CSI
3. Absence of drugs or intoxicants that may interfere with the patient’s sensorium
4. Absence of signs or symptoms on examining the neck, specifically:
Clearly, there will only be a small number of trauma patients who fulfill these criteria.
Plain Radiography
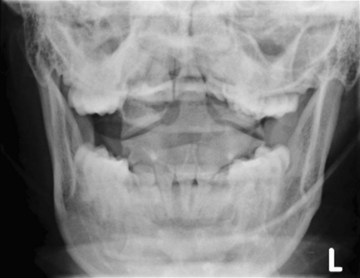
The cross-table lateral view alone, even if technically adequate and interpreted by an expert, will still miss 15% of cervical injuries. Of cross-table lateral films taken in emergency rooms, approximately a quarter of the films are anatomically inadequate, necessitating further imaging modalities for evaluation, usually of the cervicothoracic junction. A three-view cervical series includes the cross-table lateral view, open-mouth odontoid view, and anteroposterior (AP) view (Figs. 42-2 to 42-4). Using these views, the sensitivity increases to detect 90% of those with an actual injury. Again, anywhere from 25% to 50% of these series may be inadequate anatomically. In low-risk patients, plain radiography is an efficient diagnostic examination with specificity of 100%. In high-risk patients, plain radiography is a good adjunctive screening test in conjunction with a CT scan, with sensitivity of 93.3% and specificity of 95%.
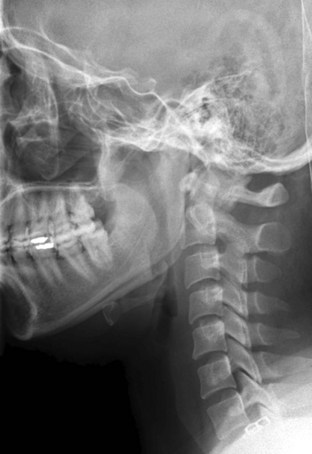
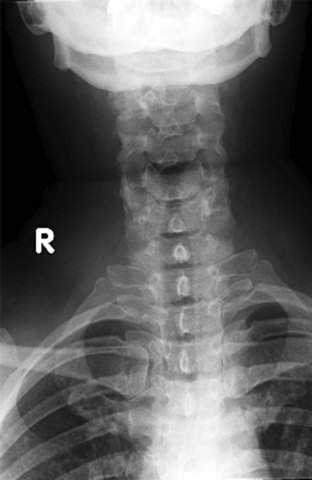
Figure 42-2 Normal lateral cervical x-ray view.
(Courtesy of Prasanna Vibhute, MD, Department of Radiology, Mount Sinai Medical Center.)
Computed Tomography
CT scanning, either of the entire cervical spine or directed at areas missed by plain radiographs, provides a complementary approach when used in addition to the three-view cervical series, reducing the risk of missing a CSI to less than 1%. In the evaluation of the cervical spine, a helical CT scan has higher sensitivity and specificity than plain radiographs in the moderate-risk and high-risk trauma population, but it is more costly. In fact, a helical CT scan is the preferred initial screening test for detection of cervical spine fractures among moderate- to high-risk patients seen in urban trauma centers, reducing the incidence of paralysis resulting from false-negative imaging studies and institutional costs, when settlement costs are taken into account.42
a Intubation
The anesthesiologist or emergency physician may be confronted with a patient with CSI who requires intubation. In one series, 26% of patients admitted to a large trauma center required intubation over the first day of admission. Furthermore, a growing body of literature indicates that any patient with a CSI above C5 should be intubated electively, early in the course of presentation.43 The following survey reviews airway devices and assigns utility based on clinical presentation of cervical injury.
i Direct Laryngoscopy
If performed appropriately, direct laryngoscopy is safe in the patient with CSI.44–46 No neurologic sequelae were noted in a review of 73 patients with known cervical spine fractures intubated after rapid-sequence induction with the application of cricoid pressure and manual in-line stabilization (MILS) of the head and neck and direct laryngoscopy.47 When intubating the patient with direct laryngoscopy, the anterior portion of the hard cervical collar can be removed to facilitate opening of the mouth at intubation.
The major concern during the initial management of patients with potential CSI is the further deterioration of the neurologic function caused by pathologic motion of the injured vertebrae. Therefore, to protect the spinal cord, it is crucial to maintain spinal alignment and preserve spinal stability by establishing early immobilization of the spine. Techniques to provide cervical spine immobilization include sandbag-tape immobilization and cervical collars of various consistency.48 The same rationale is applied when management of the airway is required in the patient with suspicious CSI. The goal is to achieve endotracheal intubation as quickly as possible with the least amount of cervical motion. Although thorough evaluation for respiratory failure is necessary, current consensus is that early intubation is mandatory in patients with complete CSI, and evidence of respiratory failure should prompt immediate airway intervention.49 As currently recommended by ATLS protocol, direct laryngoscopy with MILS is most often performed and has been extensively investigated.50
The effects of direct laryngoscopy have been studied in a range of patients, including those with normal neck anatomy under anesthesia, as well as in cadavers, including those with cervical lesions caused to simulate fractures at a variety of levels. In the anesthetized patient with normal cervical anatomy, using neuromuscular blockade and a no. 3 Macintosh blade, a variety of movements occur. On elevation of the blade to obtain a view of the larynx, there is superior rotation of the occiput and C1 in the sagittal plane, C2 remains near-neutral, and there is mild inferior rotation of C3 to C5.51 The most significant movement is produced at the atlanto-occipital and atlantoaxial joints.45 In cadaveric models of unstable cervical segments (C1-C2), the movements associated with maneuvers such as chin lift and jaw thrust are greater than those produced by the intubation itself. The application of cricoid pressure produced no significant movement at the site of injury in these patients.6
ii Immobilization
In view of the risk of secondary neurologic injury to the acutely injured, unstable cervical spine, it is widely viewed as standard of care to immobilize the cervical spine when this is suspected. The most common measures include manual in-line immobilization, immobilization of the head between two sandbags, and placement of a rigid cervical collar and a spinal board. This management is itself associated with significant morbidity and mortality. It may increase the difficulty of intubation or increase the likelihood of airway compromise and risk of aspiration. Nonetheless, the use of manual in-line immobilization (not traction) is the best means to minimize movement of the cervical spine during airway manipulations and should always be practiced. It should be recognized, however, that the presence of a cervical collar does not necessarily protect against movement at the occipitocervical and cervicothoracic junctions.52
In all these studies, a certain degree of movement of the cervical spine was detected during direct laryngoscopy with MILS. The magnitude of the reported displacement was within the physiologic ranges. In addition, no difference was detected in the movements recorded by using three different blades (Macintosh, Miller, McCoy). Santoni and colleagues53 recently reported a direct correlation between the worsening of the glottic view caused by MILS and the increase in maximum applied pressure by the laryngoscope blade. They concluded that this increase in the force applied through the laryngoscope could worsen cervical instability. In the presence of cervical instability, impaired glottic visualization and secondary increases in pressure application with MILS have the potential to increase pathologic craniocervical motion.
The laryngoscopic pressure, which reflects a degree of difficulty in glottic visualization, can be significantly diminished by using video laryngoscopes, such as the Airtraq and the Pentax AirwayScope54,55 (Fig. 42-5). These channeled video laryngoscopes allow for indirect laryngoscopy and provide optimal view of the glottis without alignment of the oropharyngeal and orotracheal axes. For these reasons, they have been successful in allowing tracheal intubation in the presence of cervical collars. The GlideScope is a widely used video laryngoscope with a record of success in cervical spine immobilization.56,57 Using cinefluoroscopy, Robaitaille and colleagues58 found that the GlideScope did not produce less cervical spine movement than the Macintosh blade but did provide an improved laryngoscopic view and successful intubation (Fig. 42-6).
iii Awake Intubation
In a cooperative patient, awake fiberoptic intubation can be performed. One of the benefits of this technique is that it allows for the patient to be intubated without movement of the cervical spine. It may be performed with a hard collar in place. The patient’s airway may be topicalized, but this may, in theory, increase the potential for aspiration in patients at risk for regurgitation and aspiration. Ovassapian and others,59 however, found no evidence of aspiration in 105 patients at risk. Awake fiberoptic intubation may prove to be time-consuming and requires expert topicalization and operator skills for success. Because of the lack of assurance of expedient intubation and the risk of aspiration, we advocate that the fiberoptic scope be used in the cooperative patient in the urgent situation and in the nonurgent patient who is not at risk of aspiration. This recommendation is a general guideline, and expertise with any given airway device must be considered when using an airway technique in a specific clinical situation.
iv Laryngeal Mask Airway
Another alternative to direct laryngoscopy is the intubating LMA. Waltl and associates60 reported that the ILMA produced less extension of the upper cervical spine than direct laryngoscopy.61 Ferson and colleagues,62 in 254 difficult-to-manage airways, reported that 70 patients with acutely unstable necks were all successfully intubated with the ILMA, 92.6% on the first attempt and 7.4% on the second attempt.62 There was no report of worsening neurologic outcome or aspiration as a result of this intervention. The authors were skilled users of the device and practicing anesthetists who had vast clinical experience with the ILMA. Other studies were not as successful. Bilgin and Bozkurt63 reported that optimum conditions for ventilation through the ILMA could be achieved at the first attempt only in 59% of the patients wearing a semirigid neck collar, and that two to four attempts were necessary in 42% of the patients. Successful blind intubation could be performed in all patients, but only 53% at the first attempt. On the contrary, first-attempt and overall success rates were reported to be higher than with blind techniques using a flexible fiberoptic scope or lightwand-guided tracheal intubation under vision through the ILMA. The clear disadvantage of this approach was the prolonged intubation time.63
This information must be viewed, however, in light of cadaveric experiments in which the intubating LMA has been demonstrated to create posterior pressure on the midportion of the cervical spine.64 This may be particularly relevant in cervical flexion injuries. If the ILMA is to be used in a patient in a hard cervical collar with cricoid pressure, one should be aware of difficulties described in this scenario. Wong and associates65 presented two cases where the ILMA was used in awake topicalized patients with unstable cervical spine without difficulty. In light of these studies indicating that the ILMA may produce cervical motion and excessive pressure on the cervical spine, and that it is difficult to place with application of cricoid pressure and the presence of a hard cervical collar, the ILMA cannot be recommended as a primary device in the patient with acute cervical injury. It should be viewed as a rescue device if direct or fiberoptic intubation fails.
v Cricothyrotomy
Although suggested as an alternative to direct laryngoscopy in patients with cervical neck trauma,66 cricothyrotomy may produce a small but significant movement of the cervical spine.67 Although often suggested as a primary mode of intubation of the unstable patient with CSI, the procedure is associated with a high complication rate.68 In one study of long-term complications in emergency departments in the United Kingdom, only 41.5% survived to hospital discharge. A mere 25.9% of these patients who survived experienced no long-term complications (10.9% of all patients receiving emergency cricothyrotomy).69 This high incidence of complications may be related to the decreasing number of cricothyrotomies performed and the unfamiliarity of many physicians with the procedure. The incidence of emergency surgical airway in the setting of trauma has decreased over the past several decades. Therefore, emergency cricothyrotomy should be reserved as a rescue procedure in the management of the airway in patients with acute cervical spine injuries.
vi Alternative Strategies
Optimal airway management strategies in patients with an unstable CSI remain controversial. Additional alternative devices to the laryngoscopes are a lightwand or an intubating fiberoptic stylets, such as the Shikani Optical Stylet and the Bonfils Retromolar Intubation Fiberscope. These tools are designed to allow blind tracheal intubation, avoiding hyperextension of the neck. However, few objective data guide the selection of appropriate devices. Different study series report that intubation with the Trachlight was successful at first attempt in 90% of patients. Inoue and colleagues70 conducted a prospective randomized study of 148 patients receiving general anesthesia for procedures related to clinical and radiographic evidence of cervical abnormality. Trachlight or ILMA was used for tracheal intubation, with the head and neck held in a neutral position. In the Trachlight group, intubation was successful at the first attempt in 67 of 74 patients (90.5%) and at the second attempt in five (6.8%). In contrast, in the ILMA group, 54 of 74 patients (73.0%) were intubated within the protocol. The mean time for successful tracheal intubation at the first attempt was significantly shorter in the Trachlight group than in the ILMA group. The Trachlight may be more advantageous for orotracheal intubation in patients with CSI than the Fastrach with respect to reliability, rapidity, and safety. Skill and experience with the device are essential to achieving this success (Box 42-1).
2 Chronic Spine Disease with Myelopathy
In the same manner as patients with ischemic heart disease present for noncardiac surgery, patients with cervical spine disease present for surgery for non-neurosurgical procedures. As such, their airway management is of interest to all, not solely those providing anesthesia for complex spinal surgery. One of the problems in relation to this patient group is predicting difficulty with intubation, both by direct laryngoscopy and fiberoptic intubation.71
Difficult intubation (DI) is well recognized as more common in patients with cervical spine disease. In particular, ankylosing spondylitis, rheumatoid arthritis, and Klippel-Feil abnormality present additional difficulty.72 One of the problems with predicting DI is its incidence and the sensitivity and specificity of the tests used to detect it.73 DI in the undifferentiated anesthesia community has an incidence of approximately 1%. The positive predictive value (PPV; proportion of difficult cases predicted to be difficult) of the common tests such as Mallampati or Wilson Risk Sum is about 8%, and the PPV of the tests used in combination is approximately 30%.74 Tests predicting DI with sensitivity of 95% and specificity of 99% will have a 51% false-positive rate. However, if the prevalence of DI is theoretically 10%, the problem of false-positive cases would decrease to 8.7% (with sensitivity of 95% and specificity of 99%). One might therefore expect prediction of DI to be more rewarding in a patient subgroup with a high incidence of DI, such as those with cervical spine disease.
A number of important correlates have emerged from this examination. As previously discussed, when performing direct laryngoscopy, the most significant movement is produced at the atlanto-occipital and atlantoaxial joints.45 Not surprisingly, therefore, patients with reduced mobility at this level present increased difficulty of intubation.75 However, a highly significant association exists between disease of the occipito-atlanto-axial complex and impaired mandibular protrusion. This is mainly, but not uniquely, caused by rheumatoid disease. Also, extension at the craniocervical junction is needed to open the mouth fully, another limiting factor with direct laryngoscopy.
a Rheumatoid Arthritis
Three especially relevant areas in which rheumatoid arthritis (RA) affects the airway and cervical spine are cricoarytenoid arthritis, temporomandibular arthritis, and atlantoaxial instability.76 Laryngeal involvement in RA has a prevalence of 45% to 88%. Depending on the investigation, 59% of patients with RA show laryngeal involvement on physical examination, 14% show extrathoracic airway obstruction on spirometry, and 69% show one or more signs of laryngeal involvement. Of the latter, 75% have symptoms of breathing difficulty. For these RA patients, the greatest risk is after extubation. Intubation, even if of brief duration, can lead to sufficient mucosal edema to cause postextubation stridor and airway obstruction. Interestingly, the incidence of postextubation stridor is much lower after FOI (1%) than after direct laryngoscopy (14%).77
Up to two thirds of patients with long-standing RA may have limited temporomandibular joint (TMJ) mobility with consequent limited mouth opening. Of those with severe TMJ destruction, up to 70% may undergo episodes of airway obstruction similar to that seen in patients with micrognathia or obstructive sleep apnea syndrome.78
Atlantoaxial instability is present in about 25% of all patients with RA and is more likely in those with severe peripheral rheumatoid involvement. Symptoms correlate poorly with radiologic findings, and a serious concern is that some series have found atlantoaxial instability in approximately 5% of RA patients presenting for elective orthopedic surgery. The direction of the instability is variable, and a significant percentage will exhibit vertical subluxation, or cranial “settling.” This can also result in impingement of the odontoid peg on the brainstem.79
IV Failed Intubation or Anticipated Difficult Airway
A Patient in a Halo Frame or Stereotactic Headframe
Early halo immobilization is a common practice in patients with potentially unstable cervical injuries and may facilitate the diagnostic work-up and treatment of trauma patients with multiple injuries.80 The halo device provides the most rigid form of external cervical immobilization. Although the halo frame is an effective form of cervical immobilization, complications can occur. This cumbersome device prevents easy access to the patient’s airway and also prevents extension of the head.
Patients treated with halo fixation present unique challenges in terms of airway control. The halo frame prevents proper positioning for laryngoscopy by restricting atlanto-occipital extension. Oral intubation is often possible, but it is a function of other variables, such as mouth opening, tongue size, upper dentition, and ability to prognath the lower jaw forward. In the nonemergent setting, fiberoptic bronchoscopy can overcome the difficulties in intubating these patients,81 but in an emergency setting, these intubations can be extremely difficult. Sims and Berger82 reported a retrospective survey of 105 patients managed with halo fixation at a level 1 trauma center. In this series, 14 of the patients (13%) required emergent or semiemergent airway control with almost half the patients dying in the attempts or shortly after. Based on their findings, the authors suggest that early tracheostomy be considered in hospitalized trauma patients requiring halo fixation who present with a high Injury Severity Score (ISS), history of cardiac disease, or a condition requiring intubation on arrival. Patients who are intubated on arrival may be more likely to require emergent reintubation during their hospital stay. Older patients and those with a history of cardiac disease are more at risk for arrest-related death (Box 42-2).
Respiratory failure or airway obstruction in the patient wearing a halo frame becomes a serious emergency. If the airway needs to be secured and tracheal intubation has failed, use of adjuncts may be life-saving. The halo frame immobilizes the head and neck and prevents use of the “sniffing position” for laryngoscopy or assisted ventilation. Case reports have described a variety of techniques for airway rescue in the patient wearing halo fixation. The Bullard laryngoscope has been used after failed laryngoscopy in a patient who additionally had a difficult airway.83 The ILMA was successfully used in an awake patient when a fiberoptic scope was unavailable.84 This device was also used by one of the authors after a failed intubation attempt following a respiratory arrest. A Combitube was used in a 78-year-old patient with respiratory deterioration after extubation when LMA insertion proved impossible.85 In recent reports, 15 patients in halo-vest fixation were electively intubated for surgery under general anesthesia using the GlideScope, and a 14-year-old patient unable to tolerate awake FOI was successfully intubated with the Pentax AWS86,87 (Fig. 42-7).
Stereotactic Headframes
Stereotactic localization is widely used in neurosurgery and has revolutionized practice over the past 30 years. The term stereotactic originated from the Greek words stereo meaning “three-dimensional” and tactos meaning “touched.” Lars Leksell is best known as the neurosurgeon who brought stereotaxis into clinical use, although it was originally described by Horsley and Clarke in 1908.88 In 1949, Leksell designed the first instrument to be based on the arc-center principle, a system that provided precise mechanical three-dimensional control in intracranial space. It served to identify the target and to calculate the angles and distances to be used with the frame. The stereotactic system has undergone many refinements over the years. The early stereotactic frames produced by Leksell provided head fixation but significantly interfered with airway access. The later frames have a cross-bar that may be directed cephalad for easier access to the nose and mouth. The cross-bar can be removed by unscrewing two screws with an Allen wrench (which should always be available). Newer designs feature a movable front piece (Fig. 42-8). Despite moderate access to the airway, head positioning and fixation to the table can make proper positioning for airway management extremely challenging.
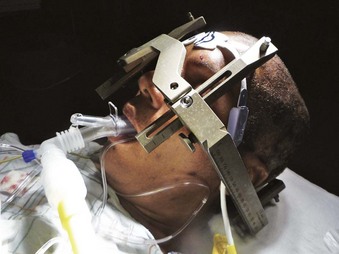
Figure 42-8 Leksell frame allows placement of a laryngeal mask airway (LMA Supreme; LMA North America, San Diego).
Applications of stereotaxis are increasing and are presently used for biopsy, craniotomy, and procedures for movement disorders. Neuronavigational techniques require the acquisition of radiologic studies, such as CT or MRI, while the patient is wearing the stereotactic frame. Stereotactic neurosurgery may require general anesthesia or conscious sedation. Cooperative patients who are neurologically intact may easily tolerate frame placement under sedation with local anesthetic applied at the pin sites. Conscious sedation is desirable when patients must be transported for diagnostic radiologic procedures in the headframe (Fig. 42-9). This is the anesthetic technique appropriate for intracranial biopsies and the surgical treatment of movement disorders and Parkinson’s disease. The use of intravenous sedation must be carefully monitored, and the agents chosen should provide analgesia, sedation, and cardiovascular stability.89 Oxygen should be administered by nasal cannula, and monitoring of capnography is extremely useful. It is essential to monitor head positioning during frame fixation to the operating table. Excessive head flexion may lead to airway obstruction when sedative agents are given.90
Potential complications of the surgical procedure include bleeding and the potential for air entrainment. Air entrainment may occur from the surgical site if near the venous sinuses or from the pins sites if placed near diploic veins.91 This is usually noted by the development of coughing, dyspnea, and decreased oxygen saturation. It is important to make the diagnosis and inform the surgeon, who should immediately flood the operative field to prevent further air entry. Another and more serious risk is internal bleeding. Postoperatively, patients usually undergo a CT scan to check for signs of hemorrhage or hematoma formation.
Patient cooperation is an important factor in these procedures; pediatric patients, obtunded patients, and those at risk for seizures present increased management challenges. The obese patient or the patient prone to airway obstruction requires careful consideration for the stereotactic headframe technique, perhaps performed under general anesthesia. When this decision is made, the patient is anesthetized and intubated before frame placement and must be ventilated, sedated, and monitored for transport to and from a diagnostic radiologic area. Alternatively, a cooperative patient may tolerate placement of the headframe and diagnostic radiology, although the lesion is in the occipital region, requiring prone positioning. This problem may be solved by awake intubation in the headframe, followed by positioning after anesthesia is induced. If awake intubation fails, an alternative technique may be used. The LMA is extremely useful in this scenario and may be used as the sole airway in smaller patients having the procedure in the supine position. It is important to be familiar with a number of airway techniques and to have a plan for alternative methods of airway management should these challenges occur92 (Box 42-3).
Box 42-3 Airway Management Techniques for Stereotactic Surgery
B Patient for Awake Craniotomy or Embolization Procedures
Craniotomy in the awake state has been performed since ancient times. Current indications include resection of a lesion in the eloquent or speech center of the brain. Surgical procedures for the treatment of epilepsy, tumors, or arteriovenous malformation are sometimes performed in the awake patient. With refinement of neurophysiologic monitoring techniques, awake craniotomies are necessary in only a small percentage of patients. However, surgery for movement disorders has again increased the use of this technique. Intraoperative complications of awake craniotomy include restlessness and agitation.93 This may occur when the patient is oversedated but experiences discomfort. More serious complications are hypoventilation, nausea, and seizures.94 Changing the level of sedation will often resolve these problems. It is important to maintain the good rapport with the patient established preoperatively. Comfortable positioning of the patient to avoid discomfort, allowing surgical access and avoiding a claustrophobic atmosphere, is essential and requires cooperation of the entire operating room (OR) staff.
The evolution of anesthetic technique has progressed from fentanyl/droperidol to the current use of propofol infusion with alfentanil or remifentanil or dexmedetomidine.95,96 Intraoperative nausea is rare with the use of propofol infusions. A newer agent is dexmedetomidine (Precedex), a selective α2-adrenergic agonist used for continuous IV sedation. Dexmedetomidine has been shown to produce sedation and analgesia without respiratory depression. The onset is slower than with propofol, and dexmedetomidine must be administered by infusion. This may be beneficial for the older patient, pediatric patient, or potentially debilitated patient and has been used throughout intraoperative testing. Seizure control is sometimes necessary; methohexital (1 mg/kg) or a benzodiazepine (midazolam) is effective. Terminating the seizure requires careful titration of sedatives to avoid apnea.
If necessary, general anesthesia may be required for the uncooperative or the very young patient.97 The “asleep-awake-asleep” technique has been used by some centers in an effort to minimize patient discomfort and provide better operating conditions for the surgeon. The patient undergoes a “light general anesthetic” with additional local anesthesia and is awakened intraoperatively for testing at the appropriate time.
Airway management can be challenging, and several maneuvers have been reported. Huncke and colleagues98 used awake fiberoptic intubation, which was accomplished in 10 patients. This effective but arduous technique required significant skill and a special catheter to deliver local airway anesthetic. Some clinicians have also used nasal airways and blind nasal intubation, assuming that bleeding or significant discomfort is avoided. The most useful technique in recent years has been the laryngeal mask airway for control of the airway.99 The LMA has been described in several reports and can be achieved (with skill) without having to remove drapes or change patient position.100 In our experience, using the LMA Classic and the LMA-ProSeal, patients could be induced and re-anesthetized for the resection after intraoperative testing. This allows the surgeon a “quiet field,” because many patients become hypercarbic while awake and sedated. The LMA-ProSeal is particularly advantageous for the ability to provide positive-pressure ventilation and deliberate entry into the gastric tract.101
Embolization Procedures
The endovascular treatment of intracranial aneurysm and arteriovenous malformation (AVM) is now an option for many patients. This new therapy offers significantly reduced morbidity, mortality, and hospital stay compared with craniotomy.1 In patients with acute subarachnoid hemorrhage (SAH), considerations must be made for the likelihood of increased ICP, changes in transmural pressure, and cerebral ischemia. During endovascular treatment, the two most serious potential complications are cerebral infarction and hemorrhage. Endovascular coiling may be safely applied within hours of the aneurysm rupture with low probability of aneurysm perforation. General anesthesia is preferred for patients with acute SAH. Despite concern for neurologic evaluation, most neuroradiologists now prefer general anesthesia for optimal imaging of studies and techniques. Airway control through an ETT or LMA allows for improved oxygenation, anesthetic administration, and a motionless patient. Radiologic imaging methods include high-resolution fluoroscopy and high-speed digital subtraction angiography (DSA) with a “roadmapping” function.2 The computer superimposes images onto live fluoroscopy so that the progress of the radiopaque catheter tip can be seen. Any motion during this stage of the procedure profoundly degrades the image. The anesthesiologist is typically off to the side of the patient and must negotiate around the myriad of monitors and equipment, which are part of this terrain. One benefit of this environment is the ability to obtain fluoroscopic confirmation of ETT positioning, confirm proper central line location, if placed, or make the diagnosis of atelectasis.
C Patient with Acromegaly
Acromegaly is a rare condition afflicting 3 to 4 per 1 million people.102 After Marie’s 1882 description of the disease,103 Chappel104 reported the death of an acromegalic patient secondary to airway obstruction in 1886. Airway obstruction is one of several mechanisms associated with DI in these patients. The association between difficult airway management and acromegaly has long been recognized in the anesthesiology community. Compared with the nonacromegalic population, acromegalic patients have a higher incidence of DI, unpredictable difficult airway, and problematic mask ventilation.
The occurrence of the difficult airway in acromegaly is well described, and the incidence of difficult laryngoscopy in these patients ranges from 9% to 33%.105 The hypersecretion of growth hormone characterizing acromegaly results in a number of alterations in airway anatomy. Patients develop hypertrophy of the facial bones with coarsening of the features. The hypertrophy of the mandibular bone leads to prognathism. In addition to significant macroglossia, hypertrophy of laryngeal and pharyngeal soft tissues and structures (e.g., vocal cords, arytenoepiglottic and ventricular folds) is well documented. Schmitt and associates106 found a 26% incidence of Cormack and Lehane grade III views on direct laryngoscopy in acromegalic patients.
Past case reports described the inability to ventilate and intubate the acromegalic patient, but heightened awareness and preparation have led to increased success with these patients.107 Preinduction airway assessment may not correlate with difficulty of intubation. Although Mallampati classification may be helpful, the thyromental distance is an insensitive indicator of DI. The lack of large prospective studies on acromegaly precludes an absolute statement regarding predictive value of preoperative assessment to difficulty of intubation at this time. Sharma and colleagues108 recently compared the modified Mallampati classification with the upper lip bite test as predictor for difficult laryngoscopy in acromegalic patients, concluding that “sensitivity and accuracy of both tests were less in the acromegalic population than the non-acromegalic controls.”
Airway management in the agromegalic patient remains problematic. Some advocate the use of awake fiberoptic intubation in patients with acromegaly to avoid the creation of a surgical airway.109,110 This approach is gradually changing. While awake FOI remains the present standard of care for difficult airways, acromegalic patients have considerable redundant tissue and large tongues. Hakala and colleagues111 reported that FOI may prove difficult or fail in patients with acromegaly. When considering awake FOI, the increased incidence of coronary artery disease in patients with acromegaly should also be considered.112 An anesthetic plan must be formulated to balance the risks of losing the airway and precipitating myocardial ischemia. The plan should include (1) a second anesthesiologist if a difficult airway is anticipated, (2) a difficult airway cart, and (3) a surgeon skilled in performing a surgical airway.
In addition to difficulty with intubation of acromegalic patients, ventilation may also be challenging. Various explanations have been described as the reason for difficulty in ventilating these patients. The prognathic jaw may impede proper mask placement; the large tongue or redundancy of soft tissue may lead to airway obstruction with recumbency and use of muscle relaxants; and the decreased range of neck motion secondary to cervical osteophyte formation may impede the attainment of proper sniffing position. There is a 16% to 30% incidence of upper airway obstruction diagnosed by spirometry in patients with acromegaly.113 Additionally, the incidence of sleep apnea is increased in these patients.114 A history of obstructive sleep apnea, hoarseness, or stridor should alert the anesthesiologist to possible glottic and infraglottic involvement and the potential for difficulty with intubation and ventilation. This propensity toward airway obstruction must also be considered in the immediate postoperative period, especially in the patient with bilateral nasal packing.115,116 Postoperative negative-pressure pulmonary edema has been described in acromegalic patients from partial obstruction after extubation (Box 42-4).
Box 42-4 Airway Considerations in Patients with Acromegaly
The ILMA has been advocated as an adjunct for intubating patients with acromegaly, but the failure rate may be too high for its use as a primary tool. Law-Koune and others117 reported a 47.4% first-attempt failure rate with the ILMA in acromegalic patients induced with propofol, concluding that the “rate of failed blind intubation through the ILMA precludes its use as a first choice for elective airway management.” Further research is required to address if the use of the ILMA as a conduit for lightwand- or fiberoptic-assisted intubation will improve the success rate. As a rescue strategy in failed laryngoscopic or fiberoptic intubation, the ILMA may be an option, if awakening the patient is not a realistic alternative. Also, we used FOI with the LMA Classic (sizes 5 and 6) in two patients with acromegaly when the ILMA did not allow successful intubation.
Use of video laryngoscopy is likely to be beneficial in acromegalic patients. Prospective studies are lacking, but we continue to have excellent results at our institutions using the GlideScope and the McGrath video laryngoscopes as primary or secondary instruments for intubating patients with acromegaly (Fig. 42-10). The construction of most blade-type video laryngoscopes allows easy navigation around the large tongue and usually provides excellent visualization of the glottic opening.118 Experience with video laryngoscopy is recommended in normal airways before attempting use in a potentially difficult airway.
D Role of Laryngeal Mask Airway in Neurosurgery
Although use of both the LMA and the intubating LMA for neurosurgical procedures has been previously described, this section addresses additional issues and further details their use in the neurosurgical patient population. Although the LMA cannot substitute for the ETT, it can be used in a number of situations where an ETT would be difficult or impossible to insert. In addition, the beneficial effects on cardiovascular and intracranial reflexes make the LMA a wise choice in certain neurosurgical procedures. This assumes that the clinician is skilled in LMA placement and manages the anesthetic appropriately. Several case reports describe LMA use in craniotomy, but these were scenarios of failed intubation and the necessity for an airway in fasted patients.119,120 Although the intubating LMA has been discussed extensively as a device for airway management in patients with limited neck movement, Combes and colleagues121 demonstrated its role in the failed-intubation scenario. In their prospective study of unanticipated difficult intubation, they concluded that the ILMA and the gum elastic bougie effectively solve most problems occurring during unexpected difficult airway management.121 This is particularly important for the neurosurgical patient, who may not easily tolerate repeated laryngoscopy attempts, inadequate ventilation, and excessive hypertension and tachycardia.
The LMA can also be used as a conduit for FOI and as a rescue airway technique that is preferred by some clinicians rather than the ILMA.122 The LMA, as well as the ILMA, can be inserted in a variety of patient positions. This becomes useful in the dreaded situation of extubation in the prone position, as well as loss of the airway in a sedated patient fixed in a headframe.120Assuming the mouth opening is adequate, the device can be easily placed by facing the patient and using the thumb to insert along the hard palate. A case report describes anesthetic induction and management in the prone position for a penetrating spine injury at C1-C2 using a LMA.123 Elective use in the prone position, although considered controversial by some, can be safely performed in appropriate patients with proper positioning. There is a growing body of experience and literature on the utility of this technique with the LMA Supreme. Studies have shown the ease of insertion with the patient positioned prone for surgery. This obviates the need for turning an anesthetized patient and allows for efficient use of OR time.124,125
Hypertension, coughing, and bucking preferably are avoided in the neurosurgical patient. When these are a particular consideration or the patient has severe asthma or chronic obstructive pulmonary disease (COPD), extubation may be facilitated by exchanging the ETT for the LMA (using LMA as “bridge to extubation”).126 This is performed while the patient is still deeply anesthetized, without airway reflexes. The exchange technique is also useful when an elaborate head draping is required and excessive neck movement will likely provoke coughing and bucking. The technique is also known as the “Bailey maneuver,” used at the Royal Throat, Nose and Ear Hospital in London.127 The laryngeal mask provides a number of airway options for the neurosurgical patient and should always be readily available.
V Postoperative Considerations
A Airway Injury and Function after Neurosurgery
1 After Supratentorial Craniotomy
In addition to general issues regarding impaired level of consciousness, as previously mentioned, patients may present with airway problems who have previously undergone temporoparietal or pterional craniotomy. When these patients subsequently present for surgery, either further neurosurgery or other non-neurosurgical procedures, they may now have a difficult airway because of limited mouth opening not evident at the original craniotomy. This is as a consequence of scar formation in the region of the temporalis muscle.128 This is much more likely to occur in those who have had a period of sedation and ventilation in the intensive care unit following their original craniotomy, because they do not resume normal eating and talking activities. Even patients extubated immediately after craniotomy and who do resume normal eating and talking are at risk of developing restricted mouth opening if, for reasons of excessive pain, they themselves limit jaw movement and subsequently develop restrictive scarring. Kawaguchi and associates129 were able to characterize postcraniotomy changes in mouth opening that occurred in 92 patients after surgery. The postoperative reduction in maximal mouth opening was greater in the group who underwent frontotemporal craniotomy (vs. parietal or occipital regions). Limited mouth opening resolved after 3 months in most patients. Supratentorial craniotomies separated by short intervals can increase the risk of limited mouth opening, which may result in a difficult intubation.
2 After Cervical Spine Surgery
Anterior cervical spinal surgery may result in recurrent laryngeal nerve injury or hematoma, causing airway obstruction after extubation. The most common cause of vocal cord paralysis is compression of the recurrent laryngeal nerve within the endolarynx. Monitoring of ETT cuff pressure and release after retractor placement may prevent injury to the recurrent laryngeal nerve.130 Edema may also develop in the tissues of the neck because the esophagus and trachea are retracted during these procedures to obtain access to the cervical spine. In contrast to problems associated with recurrent laryngeal nerve injury, such as angioedema or hematoma, which tend to occur early, this edema may not develop for 2 to 3 days postoperatively. Common features in these patients require reintubation with an increasing number of levels manipulated; the higher the operated levels, the longer the surgery, and the greater the intraoperative blood loss, the greater is the risk for reintubation. If these patients require reintubation, it is often difficult to perform, with significant rates of mortality and hypoxic sequelae. Consequently, attempts have been made to identify the risks previously described and the strategies to predict at-risk groups and plan alternative management, as discussed next.
Sagi and colleagues131 demonstrated that 19 patients (6.1%) had an airway complication, six patients (1.9%) required reintubation, and one patient died. Symptoms developed on average 36 hours postoperatively. All complications except for two were attributable to pharyngeal edema. Variables found to be statistically associated with an airway complication were exposing more than three vertebral bodies; intraoperative blood loss greater than 300 mL; exposures involving C2, C3, or C4; and surgery longer than 5 hours. A history of myelopathy, spinal cord injury, pulmonary problems, smoking, anesthetic risk factors, and the absence of a drain did not correlate with an airway complication. Thus, patients with prolonged procedures (5 hours), exposing more than three vertebral levels that include C2, C3, or C4 and with more than 300-mL blood loss, should remain intubated or should be extubated over an airway exchange catheter and watched carefully for respiratory insufficiency.
Swallowing difficulties and dysphonia may occur in patients undergoing anterior cervical discectomy and fusion. The etiology and incidence of these abnormalities are not well defined. Once again, there is a tendency for patients undergoing multilevel surgery to demonstrate increased incidence of swallowing abnormalities on postoperative radiographic studies.132 Patients undergoing multilevel procedures are at an increased risk for these complications, in part because of soft tissue swelling in the neck. Although more rare, the risk of migration of the bone, synthetic graft, or plate into the airway or compressing the airway with resultant obstruction may also occur. In addition to necessitating reintubation, this has the added hazards of intubation being required in a patient with a potentially unstable cervical spine and the need for further surgery.133,134
3 After Posterior Fossa Surgery
Because of the position of the lower cranial nerves in relation to the posterior fossa, the patient’s ability to maintain the airway may be compromised postoperatively. Performance of a careful history preoperatively may unmask subtle impairment of gag reflex with increased episodes of choking on food, and family members may have noticed changes in character of speech. Duration of surgery, proximity of the surgical site to the lower cranial nerves, and the presence of either edema or hematoma in relation to these nerves may result in both loss of gag reflex and loss of the ability to maintain and protect the airway after posterior fossa surgery. Because of the proximity of the brainstem, further hazards are presented postoperatively because central control of respiration may be jeopardized, and these factors will dictate the safety of timing of extubation.135,136
Potential postoperative airway problems divide essentially into those of the prone and sitting position, and those related to surgery on the structures in the posterior fossa. Basically, patients are at risk even before the end of these surgeries because securing the ETT in this setting is problematic because secretions and skin preparation solutions are ongoing threats to its security. Even if securely fastened to the skin, facial edema may result in the ETT migrating out of the trachea, especially in children in whom the distance between endobronchial intubation and extubation is small. Facial edema itself is not necessarily hazardous to the airway, but macroglossia and oropharyngeal edema clearly are problematic.134
A variety of mechanisms have been proposed to account for the macroglossia seen after posterior cervical spinal and posterior fossa surgery.137 Clearly, if the tongue becomes inadvertently trapped between the teeth, lingual edema will result. The venous drainage of the tongue may be obstructed by the presence of an oropharyngeal airway, and if an esophageal stethoscope is there along with an oral ETT, these further risk impairing the venous and lymphatic drainage of the tongue in the prone position. Other factors that may contribute to the formation of edema are lateral rotation of the head and neck and flexion of the neck, because these two maneuvers may impair venous drainage of the head and neck.138 The duration of surgery, as well as blood loss and fluid replacement, should also be taken into account as to the likelihood of developing macroglossia.139
In all these settings, although macroglossia may be immediately apparent and may preclude extubation, the oropharyngeal airway and the ETT may be the only elements maintaining the airway, and only on their removal does the edema become apparent, risking airway compromise. A rarer neurologic complication impacting on airway function after surgery in this position is quadriplegia. This may be caused by a combination of prolonged hyperflexion, overstretching of the cord, and compromised blood supply to the cord.140 The devastating complication of quadriplegia has been reported as another risk of surgery in the seated position. Clearly, extubation of the neurosurgical patient after prolonged surgery requires careful consideration, a review of the patient’s intraoperative course, and assessment of airway and neurologic responses. Patients who appear to have obvious facial or airway swelling with minimal response to the ETT are best left intubated until they are fully recovered and meet all criteria for extubation.
VII Clinical Pearls
• In addition to airway assessment, a neurologic examination or communication with the surgeon is invaluable before induction of anesthesia for neurosurgical procedures.
• Patients with an unstable cervical spine may be unable to cooperate with an awake fiberoptic intubation because of intoxication, hypoxia, or head injury. The need for a CSI patient’s airway to be secured is often urgent because of the cervical spine injury or associated head or facial injury.
• A rigid cervical collar may make airway management difficult, impeding mouth opening and application of cricoid pressure. Therefore, with manual in-line immobilization in place, the front part of the collar should be removed or opened before attempted intubation.
• Patients with acromegaly frequently have obstructive sleep apnea and should be induced and ventilated with caution. Direct laryngoscopy and video laryngoscopy are the most effective intubation techniques.
• Elective patients who demonstrate neurologic symptoms of the extremities with neck flexion or extension should have awake, topicalized endotracheal intubation.
• Become familiar with alternative airway devices and practice in normal airways before treating patients with difficult airways.
• Be attentive and inspect the degree of neck flexion in patients positioned prone, lateral, or in any head fixation device.
• A cuff leak test may be helpful before extubation after prolonged surgery in the prone position; always have reintubation strategies and plans.
All references can be found online at www.expertconsult.com.
30 Bedell E, Pough DS. Anesthetic management of traumatic brain injury. Anesthesiol Clin North Am. 2002;20:417–439.
56 Bathory I, Frascarlo P, Kern C, et al. Evaluation of the GlideScope for tracheal intubation in patients with cervical spine immobilization by a semi-rigid collar. Anaesthesia. 2009;64:1337–1341.
60 Waltl B, Melischek M, Schuschnig C. Tracheal intubation and cervical spine excursion: direct laryngoscopy vs. intubating laryngeal mask. Anaesthesia. 2001;56:221–226.
79 Paus AC, Steen H, Røislien J. High mortality rate in rheumatoid arthritis with subluxation of the cervical spine: a cohort study of operated and nonoperated patients. Spine (Phila Pa 1976). 2008;33:2278.
82 Sims CA, Berger DL. Airway risk in hospitalized trauma patients with cervical injuries requiring halo fixation. Ann Surg. 2002;225:280–284.
105 Nemergut EC, Zuo Z. Airway management in patients with pituitary disease: a review of 746 patients. J Neurosurg Anesthesiol. 2006;18:73–77.
115 Spiekermann BF, Stone DJ, Bogdonoff DL. Airway management in neuroanaesthesia. Can J Anaesth. 1996;43:820–834.
131 Sagi HC, Beutler W, Carroll E, Connolly PJ. Airway complications associated with surgery on the anterior spine. Spine. 2002;27:949–953.
134 Bruder N, Ravussin P. Recovery from anesthesia and postoperative extubation of neurosurgical patients: a review. J Neurosurg Anesth. 1999;11:282–293.
1 American Academy of Neurological Surgeons: National neurosurgical statistics report, 1999.
2 Lee LA, Posner KL, Cheney FW, et al. ASA Closed Claims Project: an analysis of claims associated with neurosurgical anesthesia. Anesthesiology. 2003;99:A362.
3 Ng I, Lim J, Wong HB. Effects of head posture on cerebral hemodynamics: its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery. 2004;54:593–597.
4 Ito H, Kanno I, Ibaraki M, et al. Changes in cerebral blood flow and cerebral blood volume during hypercapnia and hypocapnia measured by positron emission tomography. J Cereb Blood Flow Metab. 2003;23:665–670.
5 Shapiro HM, Wyte SR, Harris AB, Galindo A. Acute intraoperative intracranial hypertension in neurosurgical patients: mechanical and pharmacologic factors. Anesthesiology. 1972;37:399–405.
6 Traystman RJ, et al. Cerebral circulatory responses to arterial hypoxia in normal and chemodenervated dogs. Circ Res. 1978;42:469.
7 Bekker AY, Mistry A, Ritter AA, et al. Computer simulation of intracranial pressure changes during induction of anesthesia: comparison of thiopental, propofol, and etomidate. J Neurosurg Anesthesiol. 1999;11:69–80.
8 Burney RG, Winn R. Increased cerebrospinal fluid pressure during laryngoscopy and intubation for induction of anesthesia. Anesth Analg. 1975;54:687–690.
9 Unni VK, Johnston RA, Young HS, McBride RJ. Prevention of intracranial hypertension during laryngoscopy and endotracheal intubation: use of a second dose of thiopentone. Br J Anaesth. 1984;56:1219–1223.
10 Samaha T, Ravussin P, Claquin C, Ecoffey C. Prevention of increase of blood pressure and intracranial pressure during endotracheal intubation in neurosurgery: esmolol versus lidocaine. Ann Fr Anesth Reanim. 1996;15:36–40.
11 Modica PA, Tempelhoff R. Intracranial pressure during induction of anaesthesia and tracheal intubation with etomidate-induced EEG burst suppression. Can J Anaesth. 1992;39:236–241.
12 Ugur B, Ogurlu M, Gezer E, et al. Effects of esmolol, lidocaine and fentanyl on haemodynamic responses to endotracheal intubation: a comparative study. Clin Drug Invest. 2007;27:269–277.
13 Bedford RF, Morris L, Jane JA. Intracranial hypertension during surgery for supratentorial tumor: correlation with preoperative tomography scans. Anesth Analg. 1982;61:430–433.
14 Forster A, Juge O, Morel D. Effects of midazolam on cerebral blood flow in human volunteers. Anesthesiology. 1982;56:453–455.
15 Moss E. Volatile anesthetic agents in neurosurgery. Br J Anaesth. 1989;63:4–6.
16 Tseitlin AM, Lubnin AIu, Baranov OA, Luk’ianov VI. The use of propofol (Diprivan) for inducing anesthesia in neurosurgical patients. II. Its effect on intracranial pressure and on cerebral perfusion pressure. Anesteziol Reanimatol. 1998;4:39–43.
17 Werner C, Kochs E, Bause H, et al. Effects of sufentanil on cerebral hemodynamics and intracranial pressure in patients with brain injury. Anesthesiology. 1995;83:721–726.
18 Souter MJ, Andrew PJD, Piper IR, et al. Effects of alfentanil on cerebral hemodynamics in an experimental model of traumatic brain injury. Br J Anaesth. 1997;79:97–102.
19 Coles JP, Leary TS, Monteiro JN, et al. Propofol anesthesia for craniotomy: a double-blind comparison of remifentanil, alfentanil, and fentanyl. J Neurosurg Anesthesiol. 2000;12:15–20.
20 De Deyne C, Joly LM, Ravussin P. Newer inhalation anaesthetics and neuro-anaesthesia: what is the place for sevoflurane or desflurane? Ann Fr Anesth Reanim. 2004;23:367–374.
21 Fairgrieve R, Rowney DA, Karsli C, Bissonnette B. The effect of sevoflurane on cerebral blood flow velocity in children. Acta Anaesthesiol Scand. 2003;47:1226–1230.
22 Takita K, Morimoto Y, Kemmotsu O. Tracheal lidocaine attenuates the cardiovascular response to endotracheal intubation. Can J Anesth. 2001;48:732–736.
23 Nishikawa K, Kawana S, Namiki A. Comparison of the lightwand technique with direct laryngoscopy for awake endotracheal intubation in emergency cases. J Clin Anesth. 2001;13:259–263.
24 Kihara S, Brimacombe J, Yaguchi Y, et al. Hemodynamic responses among three intubation devices in normotensive and hypertensive patients: a comparison among the Macintosh laryngoscope, Trachlight lightwand and the intubating laryngeal mask airway Fastrach. Anesth Analg. 2003;96:890–895.
25 Baskett PJ, Parr MJ, Nolan JP. The intubating laryngeal mask. Results of a multicentre trial with experience of 500 cases. Anaesthesia. 1998;53:1174–1179.
26 Cros AM, Maigrot F, Esteben D. Fastrach laryngeal mask and difficult intubation. Ann Fr Anesth Reanim. 1999;18:1041–1046.
27 Watson NC, Hokanson M, Maltby JR, Todesco JM. The intubating laryngeal mask airway in failed fibreoptic intubation. Can J Anaesth. 1999;46:376–378.
28 Reusche MD, Egan TD. Remifentanil for conscious sedation and analgesia during awake fiberoptic tracheal intubation: a case report with pharmacokinetic simulations. J Clin Anesth. 1999;11:64–68.
29 Grant SA, Breslin DS, MacLeod DB, et al. Dexmedetomidine infusion for sedation during fiberoptic intubation: a report of three cases. J Clin Anesth. 2004;16:124–126.
30 Bedell E, Pough DS. Anesthetic management of traumatic brain injury. Anesthesiol Clin North Am. 2002;20:417–439.
31 Schreiber M, Aoki N, Scott B, et al. Determinants of mortality in patients with severe blunt head injury. Arch Surg. 2002;137:285–290.
32 Reilly PL. Brain injury: the pathophysiology of the first hours (review). “Talk and die revisited.”. J Clin Neurosci. 2001;8:398–403.
33 Silverston P. Pulse oximetry at the roadside: a study of pulse oximetry in immediate care. BMJ. 1989;298:711–713.
34 Jeremitsky E, Omert L, Dunham CM, et al. Harbingers of poor outcome the day after severe brain injury: hypothermia, hypoxia, and hypoperfusion. J Trauma. 2003;54:312–319.
35 Chestnut RM, Marshall SB, Piek J, et al. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir. 1993;59(S):121–125.
36 Chestnut RM. Avoidance of hypotension: condition sine qua non of successful severe head-injury management. J Trauma. 1997;42:S4–S9.
37 Mosier JM, Stolz U, Chiu S, Sakles JC. Difficult airway management in the emergency department: GlideScope videolaryngoscopy compared to direct videolaryngoscopy. J Emerg Med. 2011. [Epub ahead of print]
38 Schmidt OI, Gahr RH, Gosse A, et al. ATLS and damage control in spine trauma. World J Emerg Surg. 2009;4:9. http://www.wjes.org/content/4/1/9.
39 Goldberg W, Mueller C, Panacek E, et al. Group distribution and patterns of blunt traumatic cervical spine injury. Ann Emerg Med. 2001;38:17–21.
40 Nguyen GK, Clark R. Adequacy of plain radiography in the diagnosis of cervical spine injuries. Emerg Radiol. 2005;11:158–161.
41 American College of Surgeons. Advanced trauma life support. Chicago: ACS; 2008. pp 157-169
42 Grogan EL, Morris JA, Jr., Dittus RS, et al. Cervical spine evaluation in urban trauma centers: lowering institutional costs and complications through helical CT scan. J Am Coll Surg. 2005;200:160–165.
43 Velmahos GC, Toutouzas K, Chan L, et al. Intubation after cervical spinal cord injury: to be done selectively or routinely? Am Surg. 2003;69:891–894.
44 Patterson H. Emergency department intubation of trauma patients with undiagnosed cervical spine injury. Emerg Med J. 2004;21:302–305.
45 Sawin PD, Todd MM, Traynelis VC, et al. Cervical spine motion with direct laryngoscopy and orotracheal intubation: An in vivo cinefluoroscopic study of subjects without cervical abnormality. Anesthesiology. 1996;85:26–36.
46 Smith CE. Cervical spine injury and tracheal intubation: a never-ending conflict. Trauma Care. 2000;10:20–26.
47 Criswell JC, Parr MJ, Nolan JP. Emergency airway management in patients with cervical spine injuries. Anaesthesia. 1994;49:900–903.
48 Schneider AM, Hipp JA, Nguyen L, et al. Reduction in head and intervetebral motion provided by 7 contemporary cervical orthroses in 45 individuals. Spine (Phila Pa 1976). 2007;32:E1–E7.
49 Hassid VJ, Schinco MA, Tepas JJ, et al. Definitive establishment of airway control is critical for optimal outcome in lower cervical spinal cord injury. J Trauma. 2008;65:1328–1332.
50 Lennarson PJ, Smith D, Todd MM, et al. Segmental spine motion during orotracheal intubation of the intact and injured spine with and without external stabilization. J Neurosurg (Spine). 2000;92:201–206.
51 Hastings RH, Wood PR. Head extension and laryngeal view during laryngoscopy with cervical spine stabilization maneuvers. Anesthesiology. 1994;80:825–831.
52 Manoach S, Paladino L. Manual in-line stabilization for acute airway management of suspected cervical spine injury: historical review and current questions. Ann Emerg Med. 2007;50:236–245.
53 Santoni BG, Hindman BJ, Puttlitz CM, et al. Manual in-line stabilization increases pressures applied by the laryngoscope blade during direct laryngoscopy and orotracheal intubation. Anesthesiology. 2009;110:24–31.
54 Maharaj CH, Buckley E, Harte BH, et al. Endotracheal intubation in patients with cervical spine immobilization: a comparison of Macintosh and Airtraq laryngoscopes. Anesthesiology. 2007;107:53–59.
55 Aoi Y, Inagawa G, Nakamura K, et al. Airway scope versus Macintosh laryngoscope in patients with simulated limitation of neck movements. J Trauma. 2010;69:838–842.
56 Bathory I, Frascarlo P, Kern C, et al. Evaluation of the GlideScope for tracheal intubation in patients with cervical spine immobilization by a semi-rigid collar. Anaesthesia. 2009;64:1337–1341.
57 Agro F, Barzoi G, Montecchia F. Tracheal intubation using a Macintosh laryngoscope or a GlideScope in 15 patients with cervical spine immobilization. Br J Anaesth. 2003;90:705–706.
58 Robitaille A, Williams SR, Tremblay MH, et al. Cervical spine motion during tracheal intubation with manual in-line stabilization: direct laryngoscopy versus GlideScope videolaryngoscopy. Anesth Analg. 2008;106:935–941.
59 Ovassapian A, Krejceie TC, Yelich SJ. Awake fiberoptic intubation in the patient at high risk of aspiration. Br J Anaesth. 1989;62:13–16.
60 Waltl B, Melischek M, Schuschnig C. Tracheal intubation and cervical spine excursion: direct laryngoscopy vs. intubating laryngeal mask. Anaesthesia. 2001;56:221–226.
61 Wahlen BM, Gercek E. Three-dimensional cervical spine movement during intubation using the Macintosh and Bullard laryngoscopes, the Bonfils fiberscope and the intubating laryngeal mask airway. Eur J Anaesthesiol. 2004;21:907–913.
62 Ferson DZ, Rosenblatt WH, Johansen MJ, et al. Use of the intubating LMA-Fastrach in 254 patients with difficult-to-manage airways. Anesthesiology. 2001;95:1175–1181.
63 Bilgin H, Bozkurt M. Tracheal intubation using the ILMA, C-Trach or McCoy laryngoscope in patients with simulated cervical spine injury. Anaesthesia. 2006;61:685–691.
64 Brimacombe J, Keller C, Kunzel KH, et al. Cervical spine motion during airway management: a cinefluoroscopic study of the posteriorly destabilized third cervical vertebrae in fresh cadavers. Anesth Analg. 1999;89:1296–1300.
65 Wong JK, Tongier WK, Armbruster SC, White PF. Use of the intubating laryngeal mask airway to facilitate awake orotracheal intubation in patients with cervical spine disorders. J Clin Anesth. 1999;11:346–348.
66 Domino KB. Care of the acutely unstable patient. In: Cottrell JE, Smith DS. Anesthesia and neurosurgery. St Louis: Mosby; 2001:251–274.
67 Gerling MC, Davis DP, Hamilton RS. Effect of surgical cricothyrotomy on the unstable cervical spine in a cadaver model of intubation. J Emerg Med. 2001;20:1–5.
68 Ratnayake B, Langford RM. A survey of emergency airway management in the United Kingdom. Anaesthesia. 1996;51:908–911.
69 Isaacs JH. Emergency cricothyrotomy: long-term results. Am Surg. 2001;67:346–349.
70 Inoue Y, Koga K, Shigematsu A. A comparison of two tracheal intubation techniques with Trachlight and Fastrach in patients with cervical spine disorders. Anesth Analg. 2002;94:667–671.
71 Maktabi MA, Titler SS, Kadia S, et al. When fiberoptic intubation fails in patients with unstable craniovertebral junctions. Anesth Analg. 2009;108:1937–1940.
72 Stallmer ML, Vanaharam V, Mashour GA. Congenital cervical spine fusion and airway management: a case series of Klippel-Feil syndrome. J Clin Anesth. 2008;20:447–451.
73 Iohom G, Ronayne M, Cunningham AJ. Prediction of difficult tracheal intubation. Eur J Anaesthesiol. 2003;20:31–36.
74 Oates JD, Macleod AD, Oates PD, et al. Comparison of two methods for predicting difficult intubation. Br J Anaesth. 1991;66:305–309.
75 Calder I, Calder J, Crockard HA. Difficult direct laryngoscopy in patients with cervical spine disease. Anaesthesia. 1995;50:756–763.
76 Bandi V, Munnur U, Braman SS. Airway problems in patients with rheumatologic disorders. Crit Care Clin. 2002;18:749–765.
77 Kolman J, Morris I. Cricoarytenoid arthritis: a cause of acute airway obstruction in rheumatoid arthritis. Can J Anesth. 2002;49:729–732.
78 Horton WA, Fahy L, Charters P. Disposition of the cervical vertebrae, atlanto-axial joint, hyoid and mandible during x-ray laryngoscopy. Br J Anaesth. 1989;63:435–438.
79 Paus AC, Steen H, Røislien J. High mortality rate in rheumatoid arthritis with subluxation of the cervical spine: a cohort study of operated and nonoperated patients. Spine (Phila Pa 1976). 2008;33:2278.
80 Heary HF, Hunt CD, Kreiger AJ, et al. Acute stabilization of the cervical spine by halo/vest application facilitates evaluation and treatment of multiple trauma patients. J Trauma. 1992;33:445–451.
81 Kang M, Vives MJ, Vaccaro AR. The halo vest: principles of application and management of complications. J Spinal Cord Med. 2003;26:186–192.
82 Sims CA, Berger DL. Airway risk in hospitalized trauma patients with cervical injuries requiring halo fixation. Ann Surg. 2002;225:280–284.
83 Cohn AI, Lau M, Leonard J. Emergent airway management at a remote hospital location in a patient wearing a halo traction device [correspondence]. Anesthesiology. 1998;89:845.
84 Sener EB, Sarihasan B, Ustun E, et al. Awake tracheal intubation through the intubating laryngeal mask airway in a patient with halo traction. Can J Anaesth. 2002;49:610–613.
85 Mercer M. Respiratory failure after tracheal extubation in a patient with halo frame cervical spine immobilization–rescue therapy using the Combitube airway. Br J Anaesth. 2001;86:886–891.
86 Huang SJ, Lee CL, Wang PK, et al. The use of the GlideScope for tracheal intubation in patients with halo vest. Acta Anaesthesiol Taiwan. 2011;49:88–90.
87 Maruyama K, Yamada T, Hara K, et al. Tracheal intubation using an AirWay Scope in a patient with halo-vest fixation for upper cervical spine surgery. Br J Anaesth. 2002;102:565–566.
88 Perkins WJ, Kelly PJ, Faust RJ. Stereotactic surgery. In: Cucchiara RF, Michenfelder JD. Clinical neuroanesthesia. New York: Churchill Livingstone; 1990:379–420.
89 Poon CC, Irwin MG. Anaesthesia for deep brain stimulation and in patients with implanted neurostimulator devices. Br J Anaesth. 2009;103:152–165.
90 Stokes MA, Soriano SG, Tarbell NJ, et al. Anesthesia for stereotactic radiosurgery in children. J Neurosurg Anesthesiol. 1995;7:100–108.
91 Moitra V, Permut TA, Penn RM, et al. Venous air embolism in an awake patient undergoing placement of deep brain stimulators. J Neurosurg Anesthesiol. 2004;16:321–322.
92 Chung YT, Wu HS, Lin YH, et al. Frequent use of alternative airway techniques makes difficult intubations less and easier. Acta Anaesthesiol Taiwan. 2004;42:141–145.
93 Archer DP, Joycelyne MA. Conscious-sedation during craniotomy for intractable epilepsy: a review of 354 consecutive cases. Can J Anaesth. 1998;35:338–344.
94 Danks RA, Rogers M, Agolio LS, et al. Patient tolerance of craniotomy performed with the patient under local anaesthesia with conscious sedation. Neurosurgery. 1998;42:28–34.
95 Manninen PH, Balki M, Lukitto K, et al. Patient satisfaction with awake craniotomy for tumor surgery: a comparison of remifentanil and fentanyl in conjunction with propofol. Anesth Analg. 2006;102:237–242.
96 Ard JL, Jr., Bekker AY, Doyle WK. Dexmedetomidine in awake craniotomy: a technical note. Surg Neurol. 2005;63:114–116.
97 Hagberg C, Berry J, Hacque S. The laryngeal mask for awake craniotomy in pediatric patients. Anesthesiology. 1995;83:A184.
98 Huncke K, Van de Weile B, Fried I, et al. The asleep-awake-asleep anesthetic technique for intraoperative language mapping. Neurosurgery. 1998;42:1312.
99 Sarang A, Dinsmore J. Anaesthesia for awake craniotomy-evolution of a technique that facilitates awake neurological testing. Br J Anaesth. 2003;90:161–165.
100 Shinokuma T, Shono S, Iwakiri S, et al. Awake craniotomy with propofol sedation and a laryngeal mask airway: a case report. Masui. 2002;51:529–531.
101 Brimacombe J, Keller C, Boehler M, et al. Positive pressure ventilation with the ProSeal versus classic laryngeal mask airway: a randomized, crossover study of healthy female patients. Anesth Analg. 2001;93:1351–1353.
102 Melemed S. Acromegaly. N Engl J Med. 1990;322:966–975.
103 Marie P. Sur duex d’acromégalie: hypothroie singulière non congénitale des extrémititiés supérieures, inférieures et céphalique. Rev Med. 1986;6:297–333.
104 Chappel WF. A case of acromegaly with laryngeal and pharyngeal symptoms. J Laryngol Otol. 1896;10:142.
105 Nemergut EC, Zuo Z. Airway management in patients with pituitary disease: a review of 746 patients. J Neurosurg Anesthesiol. 2006;18:73–77.
106 Schmitt H, Buchfelder M, Radespiel-Tröger M. Difficult intubation in acromegalic patients: incidence and predictability. Anesthesiology. 2000;93:110–114.
107 Singelyn FJ, Scholtes JL. Airway obstruction in acromegaly: a method of prevention. Anaesth Intensive Care. 1998;16:491–492.
108 Sharma D, Prabhakar H, Bital P, et al. Predicting difficult laryngoscopy in acromegaly: a comparison of upper lip bite test with modified Mallampati classification. J Neurosurg Anesthesiol. 2010;22:138–143.
109 Ovassapian A, Doka JC, Romsa DE. Acromegaly: use of fiberoptic laryngoscopy to avoid tracheostomy. Anesthesiology. 1981;54:429–430.
110 Young ML, Hanson CW. An alternative to tracheostomy following transsphenoidal hypophysectomy in a patient with acromegaly and sleep apnea. Anesth Analg. 1993;76:446–449.
111 Hakala P, Randell T, Valli H. Laryngoscopy and fibreoptic intubation in patients with acromegaly. Br J Anaesth. 1998;80:345–347.
112 Matta MP, Caron P. Acromegalic cardiomyopathy: a review of the literature. Pituitary. 2003;6:203–207.
113 Evans CC, Hipkin LJ, Murray GM. Pulmonary function in acromegaly. Thorax. 1997;32:322–327.
114 Piper J, Dirks BA, Traynelis VC, et al. Perioperative management and surgical outcome of the acromegalic patient with sleep apnea. Neurosurgery. 1995;36:70–75.
115 Spiekermann BF, Stone DJ, Bogdonoff DL. Airway management in neuroanaesthesia. Can J Anaesth. 1996;43:820–834.
116 Scholtes FJ, Scholtes JL. Airway obstruction in acromegaly: A method of prevention. Anaesthesia and Intensive Care. 1988;16:491–492.
117 Law-Koune JD, Liu N, Szekely B, et al. Using the intubating laryngeal mask airway for ventilation and endotracheal intubation in anesthetized and unparalyzed acromegalic patients. J Neurosurg Anesthesiol. 2004;16:11–13.
118 Sun DA, Warriner CB, Parsons DG. The GlideScope video laryngoscope: randomized clinical trial in 200 patients. Br J Anaesth. 2005;94:381–384.
119 Agarwald A, Shobhana N. LMA in neurosurgery. Can J Anaesth. 1995;42:750.
120 Audu P, Cooper H. Craniotomy performed with LMA: a case report. J Neurosurg Anesthesiol. 2000;12:112–113.
121 Combes X, Le Roux B, Suen P, et al. Unanticipated difficult airway in anesthetized patients. Anesthesiology. 2004;100:1146–1150.
122 Benumof JB. Laryngeal mask airway and the ASA difficult airway algorithm. Anesthesiology. 1996;84:686–699.
123 Valero R, Serrano S, Adalia R, et al. Anesthetic management of a patient in prone position with a drill bit penetrating the spinal canal at C1-C2, using a laryngeal mask. Anesth Analg. 2004;98:1447–1450.
124 Ng A, Raitt DG, Smith G. Induction of anesthesia and insertion of a laryngeal mask airway in the prone position for minor surgery. Anesth Analg. 2002;94:1194–1198.
125 Lopez AM, Valero R, Brimacombe J. Insertion and use of the LMA Supreme in the prone position. Anaesthesia. 2010;65:154–157.
126 Stix MS, Borromeo CJ, Sciortino GJ, Teague PD. Learning to exchange an endotracheal tube for a laryngeal mask prior to emergence. Can J Anaesth. 2001;48:795–799.
127 Nair I, Bailey PM. Review of uses of the laryngeal mask in ENT anaesthesia. Anaesthesia. 1995;50:898–900.
128 Nitzan DW, Azaz B, Constantini S. Severe limitation in mouth opening following transtemporal neurosurgical procedures. J Neurosurg. 1992;76:623–625.
129 Kawaguchi M, Sakamoto T, Furuya H, et al. Pseudoankylosis of the mandible after supratentorial craniotomy. Anesth Analg. 1996;83:731–734.
130 Apfelbaum RI, Kriskovich MD, Haller JR. On the incidence, cause and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine. 2000;25:2906–2912.
131 Sagi HC, Beutler W, Carroll E, Connolly PJ. Airway complications associated with surgery on the anterior spine. Spine. 2002;27:949–953.
132 Frempong-Boadu A, Houten JK, et al. Swallowing and speech dysfunction in patients following anterior cervical discectomy and fusion: a prospective objective preoperative and postoperative assessment. J Spine Discord Tech. 2002;15:362–368.
133 Terao Y, Matsumoto S, Yamashita K, et al. Increased incidence of emergency airway management after combined anterior-posterior cervical spine surgery. J Neurosurg Anesthesiol. 2004;16:282–286.
134 Bruder N, Ravussin P. Recovery from anesthesia and postoperative extubation of neurosurgical patients: a review. J Neurosurg Anesth. 1999;11:282–293.
135 Narayan VB, Umamaheswara GS. Unilateral facial and neck swelling after infratentorial surgery in the lateral position. Anesth Analg. 1999;89:1290–1291.
136 Kimovec MA, Ambrose J. Predictors of airway and respiratory complications after posterior fossa craniotomies. J Neurosurg Anesthesiol. 1992;4:315.
137 Harrop JS, Vaccaro A, Przybylski GJ. Acute respiratory compromise associated with flexed cervical traction after C2 fractures. Spine. 2001;26:E50–E54.
138 Howard R, Mahoney A, Thurlow AC. Respiratory obstruction after posterior fossa surgery. Anaesthesia. 1990;45:222–224.
139 Sinha A, Agarwal A, Gaur A, Pandey CK. Oropharyngeal swelling and macroglossia after cervical spine surgery in the prone position. J Neurosurg Anesthesiol. 2001;13:237–239.
140 Morandi X, Riffaud L, Amlashi SF, Brassier G. Extensive spinal cord infarction in the sitting position: case report. Neurosurgery. 2004;54:1512–1515.