The basics
Diagnostic terms, skin anatomy, and stains
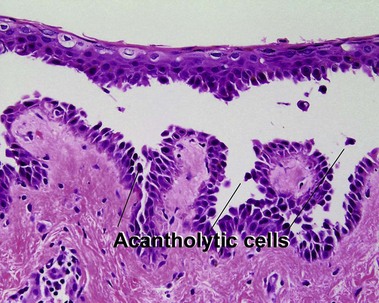
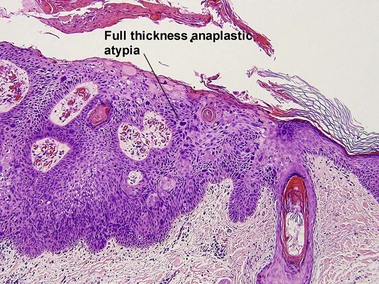
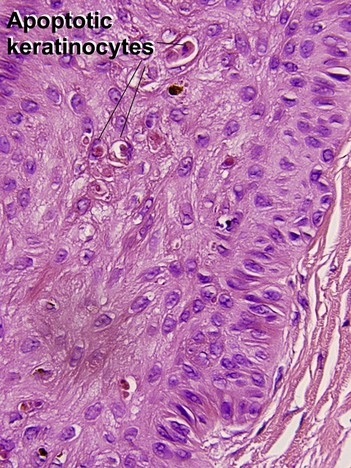
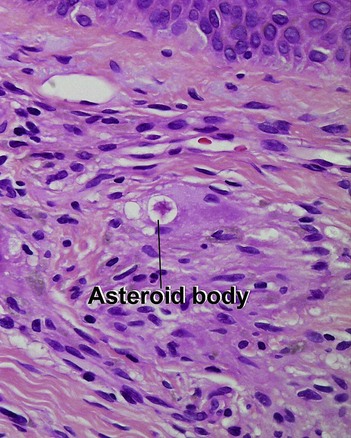
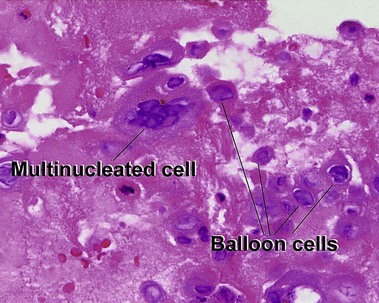
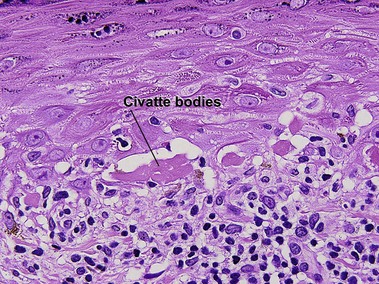
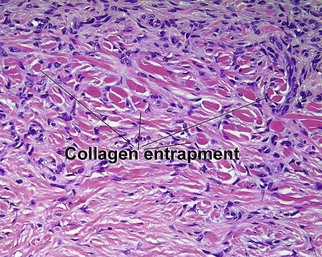
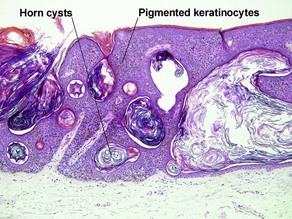
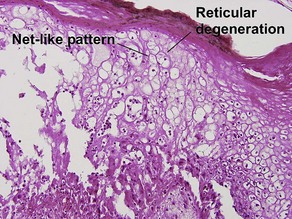
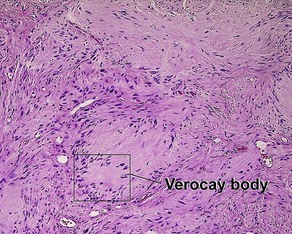
Glossary of terms
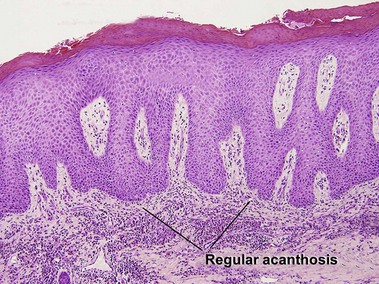
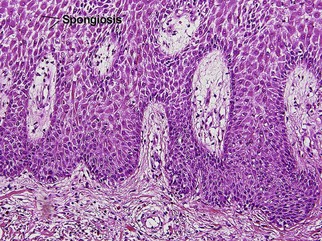
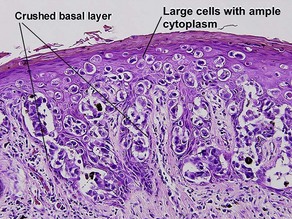
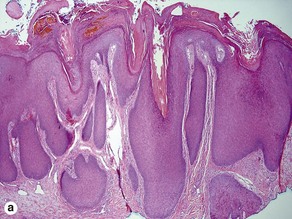
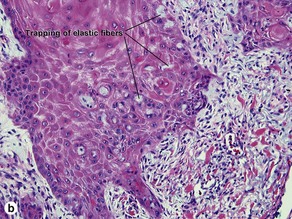
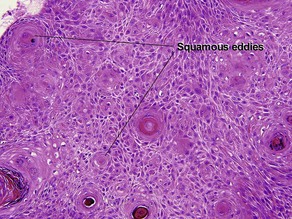
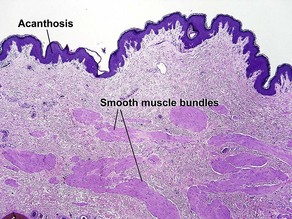
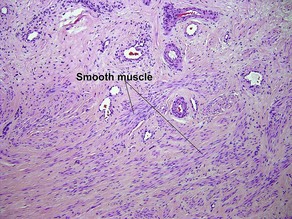
Acanthosis
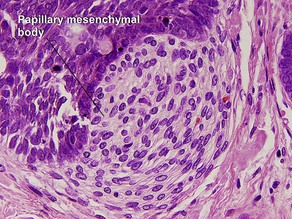
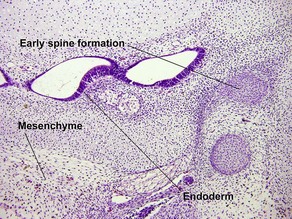
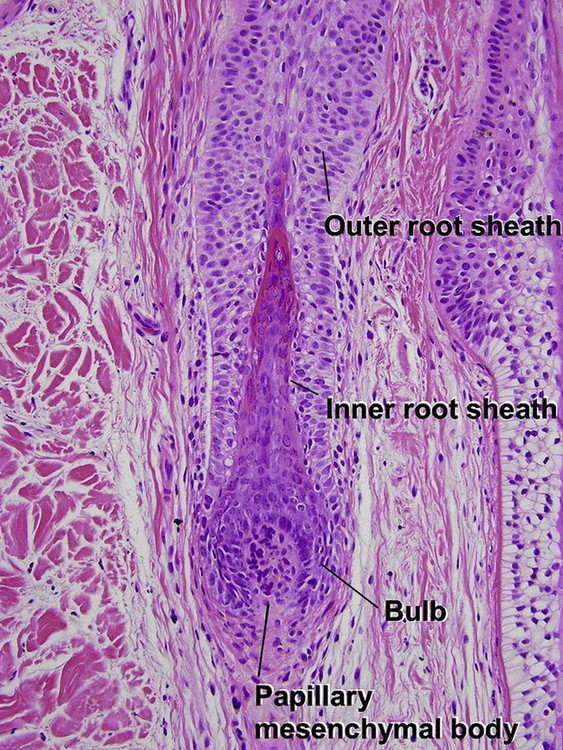
Papillary mesenchymal body
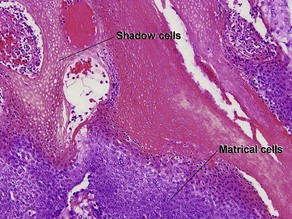
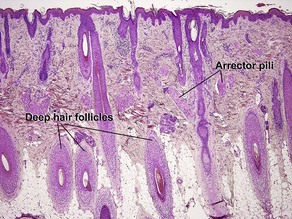
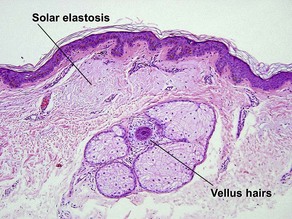
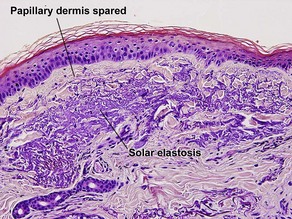
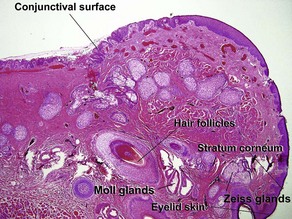
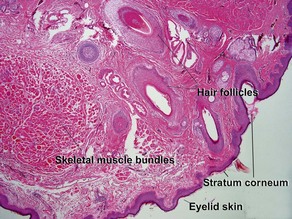
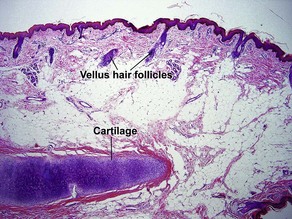
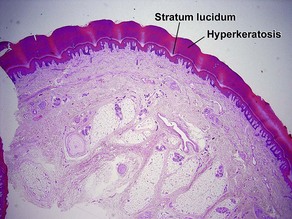
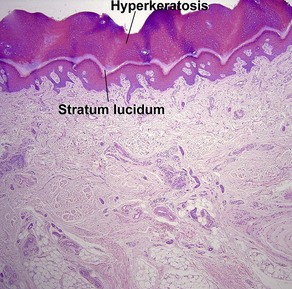
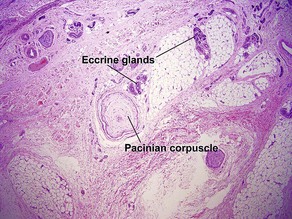
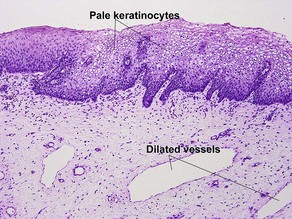
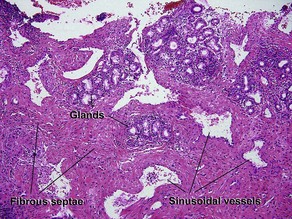
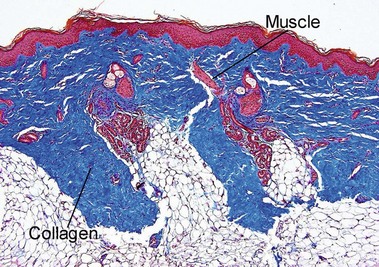
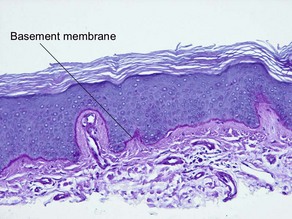
Normal skin anatomy
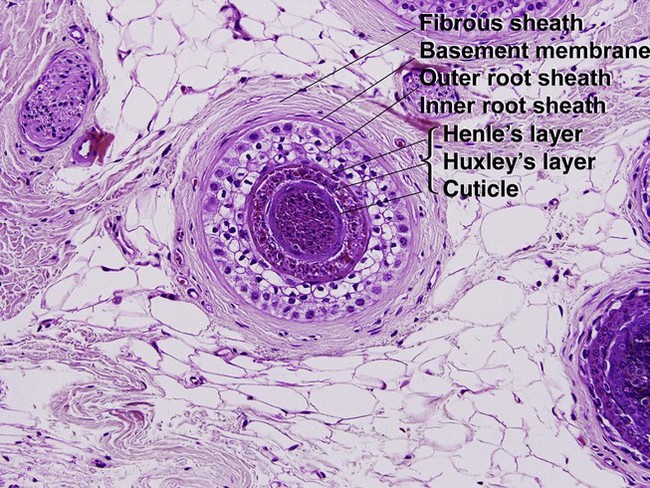
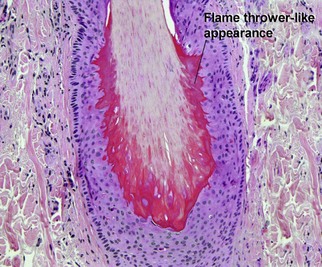
Hair anatomy
Stem
Nail anatomy
Cuticle
• “Visible” cuticle (aka eponychium) is the thick keratinous material that borders the proximal nail fold and adheres to the nail plate
• “True” cuticle is located beneath the “visible” portion and is derived from the ventral part of the proximal nail fold
• The true cuticle is generally not seen, but it is sometimes visible as “flakes” of keratinous material parallel to the proximal nail fold
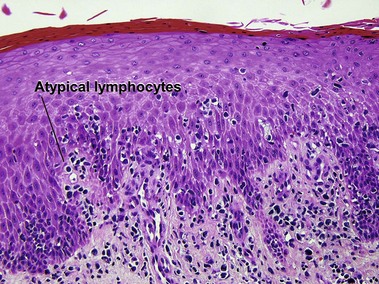
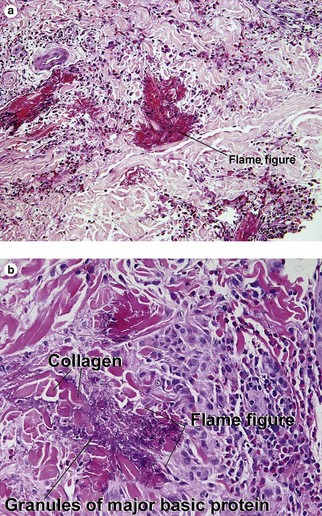
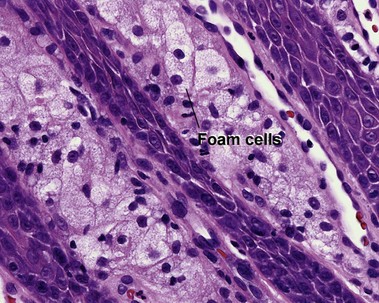
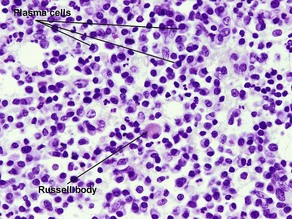
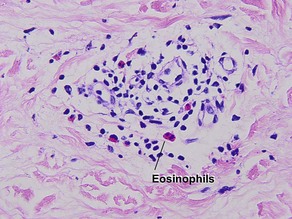
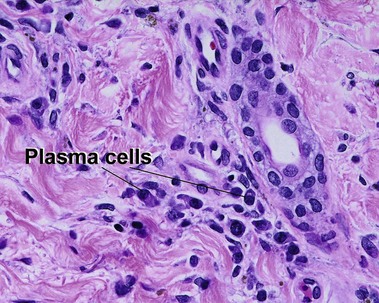
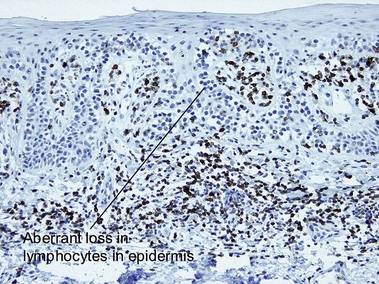
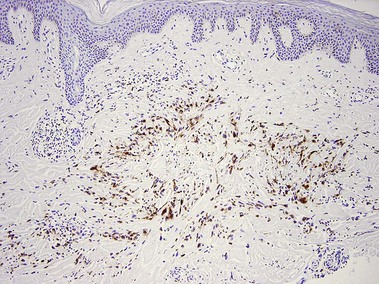
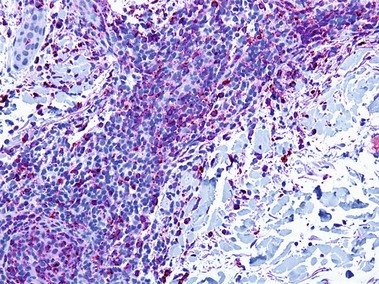
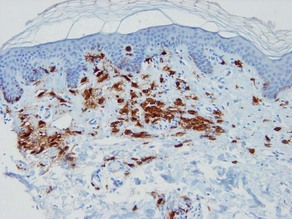
Types of inflammatory cells
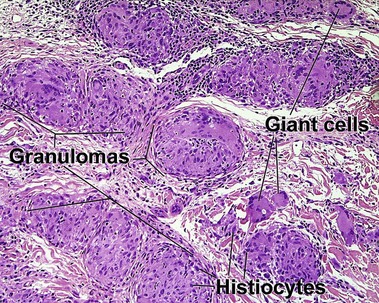
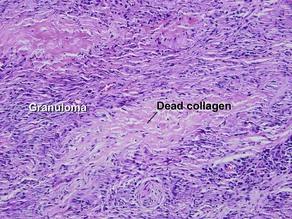
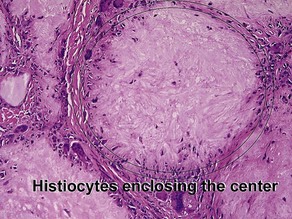
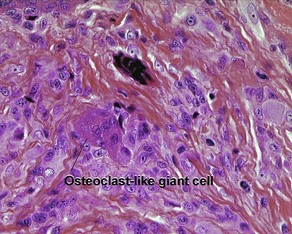
Giant cell
Types
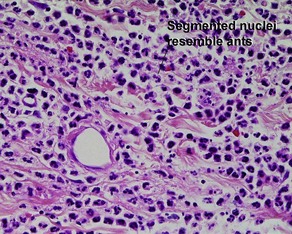
• Foreign-body: nuclei are arranged haphazardly
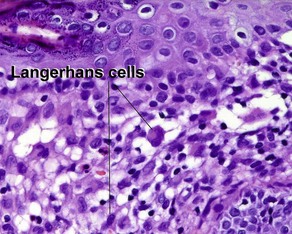
• Langerhans: nuclei are arranged in a wreath shape
• Osteoclast-like: nuclei are arranged haphazardly and eccentrically; cytoplasm is deep pink with a scalloped border that molds to adjacent structures
• Touton: nuclei are arranged in a wreath with foamy cytoplasm peripherally
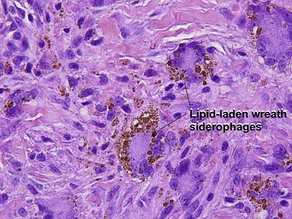
• Ringed siderophage: Touton giant cell with hemosiderin (characteristic of the fibrous histiocytoma type of dermatofibroma)
Histochemical stains
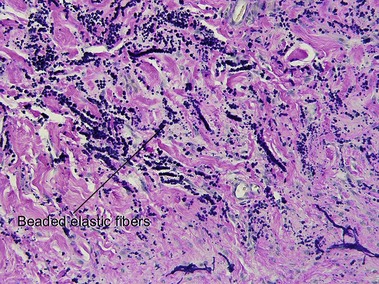
Connective tissue stains
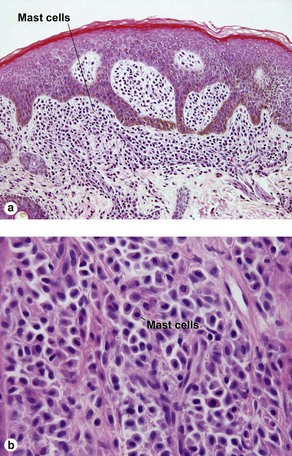
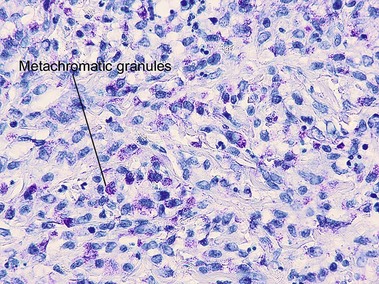
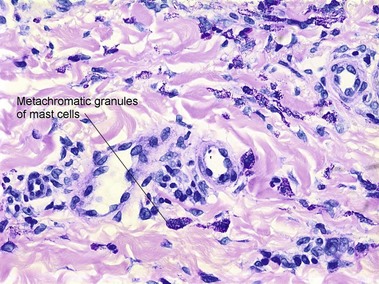
Mast cell stains
Carbohydrate stains
PAS (Periodic acid-Schiff)
• Stains glycogen, neutral mucopolysaccharides (such as basement membrane), and fungi red
• Glycogen is diastase labile, i.e. sections exposed to diastase before staining do not stain red with PAS reaction
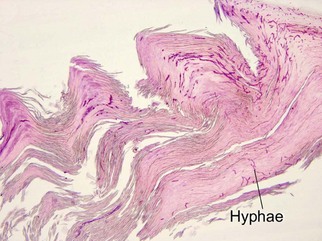
• Useful in clear cell acanthoma, trichilemmoma
• Fungi and neutral mucopolysaccharides (basement membrane) are diastase resistant, i.e. stain red with PAS after diastase exposure
• Useful in tinea corporis, tinea versicolor, candida, basement membrane thickening of lupus erythematosus, thickened vessel walls in porphyria
• Acid mucopolysaccharides, such as hyaluronic acid, do not stain with PAS
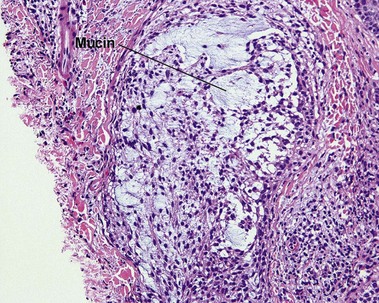
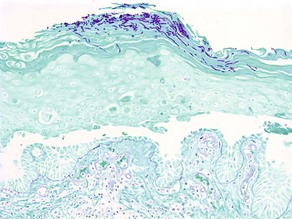
Alcian blue
• Demonstrates acid mucopolysaccharides by staining them blue
• In normal skin, most mucin is sulfated acid mucopolysaccharide (heparin, chondroitin, and dermatan sulfates). In most pathologic states with increased dermal mucin, the mucin is predominantly non-sulfated hyaluronic acid
• Non-sulfated acid mucopolysaccharides (hyaluronic acid) stain with Alcian blue at pH 2.5 but not at pH 0.5
• Examples: follicular mucinosis, granuloma annulare, myxoid cyst, dermal mucin in lupus erythematosus
• Sulfated acid mucopolysaccharides stain with Alcian blue at both pH 2.5 and pH 0.5
• Alcian blue can be used with and without hyaluronidase to differentiate hyaluronic acid from other mucopolysaccharides
Colloidal iron
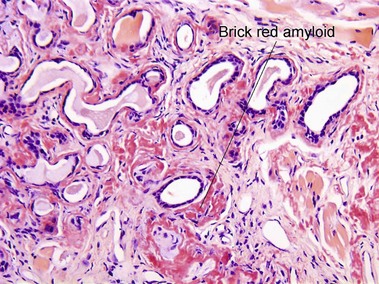
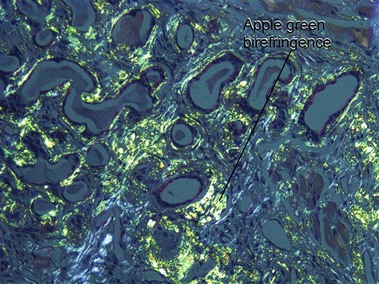
Amyloid
Iron
Calcium
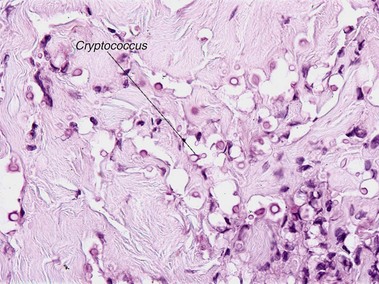
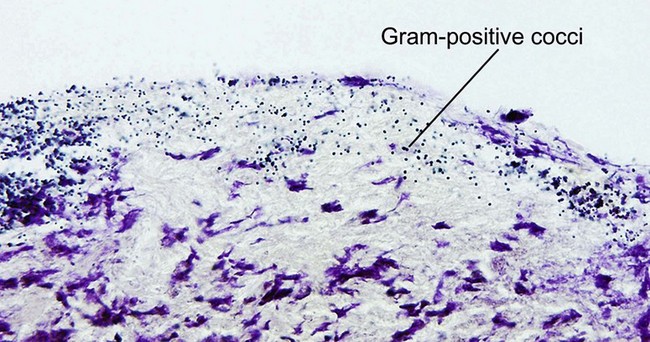
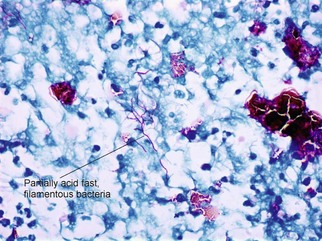
Mycobacteria
Ziehl–Neelsen acid-fast stain; Fite acid-fast stain; Kinyoun’s acid-fast stain
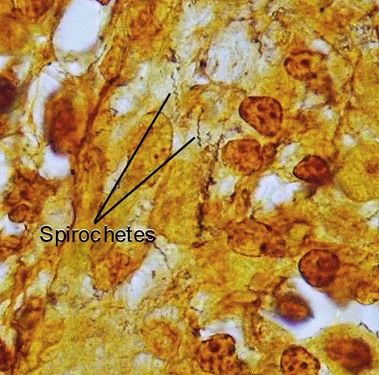
Other “special” stains
Giemsa
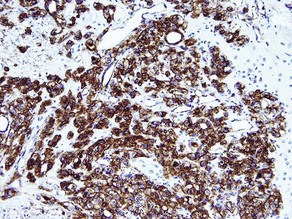
Epithelial markers
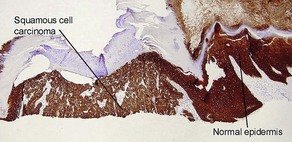
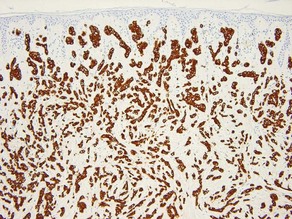
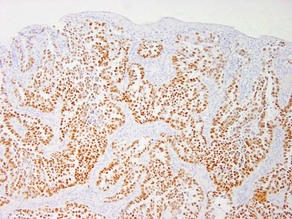
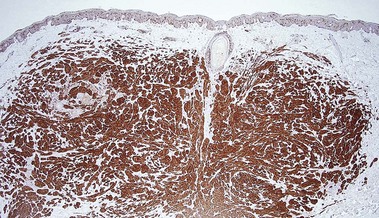
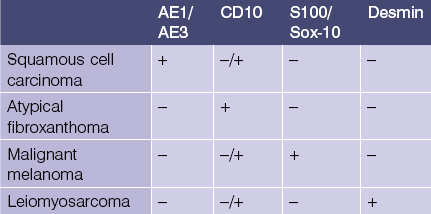
AE1/AE3
• Cocktail of high- and low-molecular-weight monoclonal cytokeratin antibodies
• Expressed in the epidermis and adnexal epithelium
• Stains all epithelial tumors (squamous cell carcinoma and adnexal tumors) and generally excludes mesenchymal, melanocytic, and hematopoietic tumors
• Also stains epithelioid sarcoma, synovial sarcoma and mesothelioma
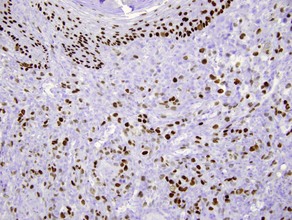
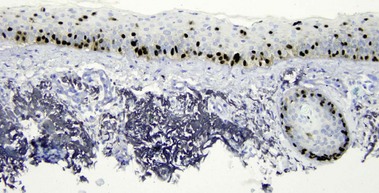
p63
• Expressed in basal and spinous cells of the epidermis, germinative cells of sebaceous glands, and myoepithelial cells of the sweat glands
• Lack of reactivity in metastatic carcinoma assists in differentiation from primary cutaneous adnexal neoplasms
• Useful in identification of cutaneous spindle cell squamous cell carcinoma from other spindle cell neoplasms
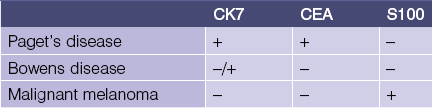
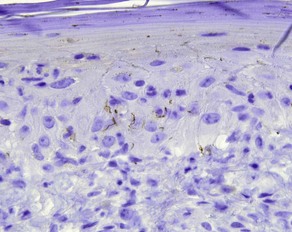
CK7
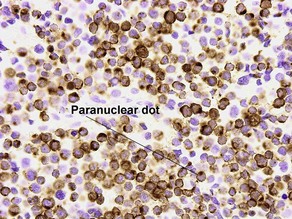
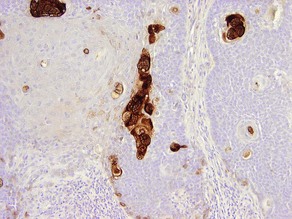
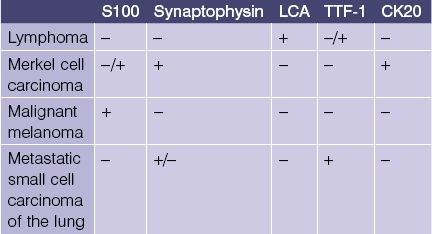
CK20
• Marks Merkel cell carcinoma predominantly in a paranuclear pattern and distinguishes from metastatic oat cell carcinoma of the lung that is typically negative
• Used in determining the origin of metastatic carcinoma (see Table 1.1)
• Marker of gastrointestinal adenocarcinoma
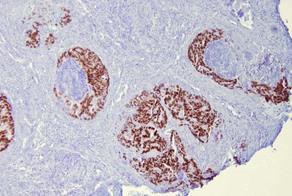
• Highlights sparse Merkel cells within the basaloid islands of desmoplastic trichoepithelioma but not basal cell carcinoma
Table 1-1
Metastatic carcinoma of unknown origin
| CK20− | CK20+ | |
| CK7+ | Breast, lung, mesothelioma | Bladder, pancreatic |
| CK7− | Hepatocellular, prostate, renal, neuroendocrine and squamous carcinoma of lung | Colon |
CDX2
Thyroid transcription factor (TTF-1)
Epithelial membrane antigen (EMA)
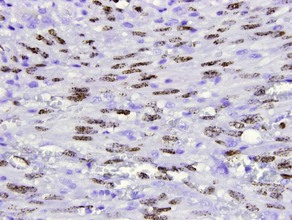
Carcinoembryonic antigen (CEA)
Mesenchymal markers
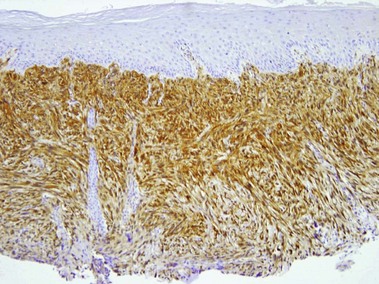
Desmin
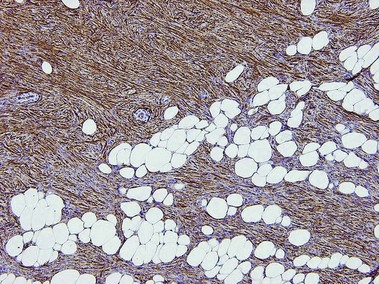
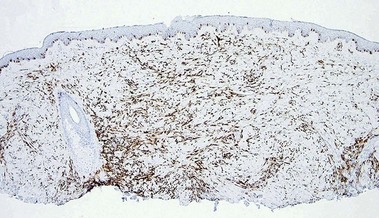
CD34
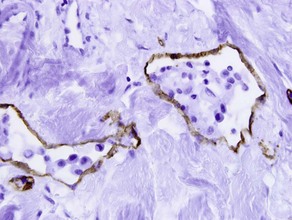
• Marker of vascular endothelium and hematopoietic progenitor cells
• Positive in dermatofibrosarcoma protuberans and negative in dermatofibroma
• Positive in spindle cell lipoma, sclerotic fibroma, solitary fibrous tumor, superficial acral fibromyxoma, pleomorphic fibroma, and pleomorphic hyalinizing angiectatic tumor
• Decreased staining in morphea
• Increased staining in nephrogenic systemic fibrosis
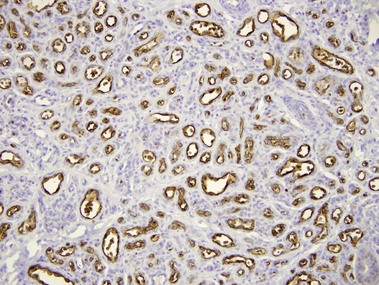
• Stains connective tissue around normal hair follicles
• Typically highlights the stroma of trichoepitheliomas but not basal cell carcinomas
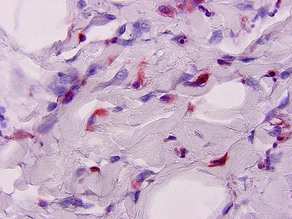
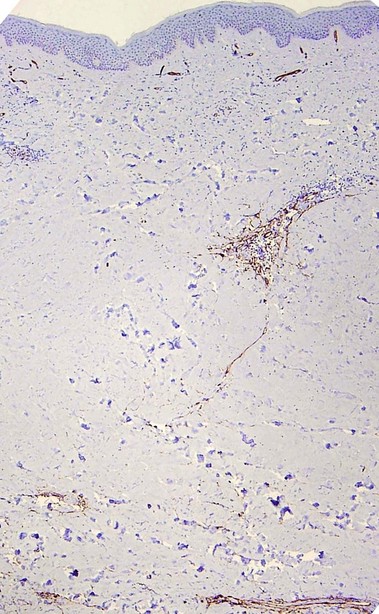
Factor XIIIa
D2-40 (podoplanin)
Neuroectodermal markers
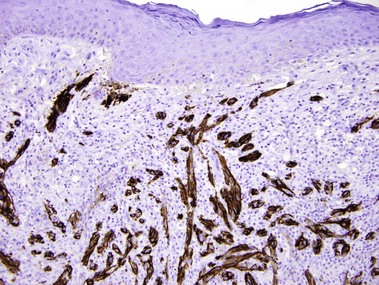
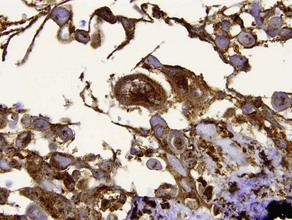
S100
• Reactivity is observed for neural crest-derived cells and some mesenchymal lines
• Stains melanocytes, Langerhans cells, sweat glands, nerves, schwann cells, myoepithelial cells, fat, muscle, and chondrocytes
• Useful in differential diagnosis of spindle cell neoplasms
• Examples: desmoplastic melanoma, Langerhans cell histiocytosis, granular cell tumor, Rosai–Dorfman disease
S100A6 (calcyclin)
• Member of the S100 protein superfamily
• Stains melanocytes, Schwann cells, Langerhans cells, and dermal dendrocytes thus expressed in nevi, particularly those with type C Schwann cell-like features, and some neural and fibrohistiocytic tumors
• Positive in cellular neurothekeoma while S100 is negative
• Reactive in most atypical fibroxanthomas but is not specific and also stains other entities in the cutaneous spindle cell tumor differential diagnosis
• Has been reported to stain Spitz nevi strongly and diffusely but spitzoid melanomas have weak or patchy staining in the dermis only
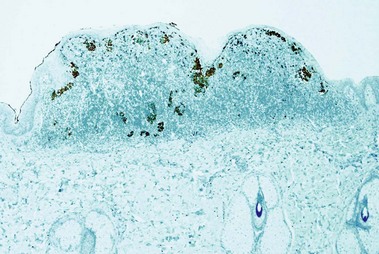
HMB-45
p75 (nerve growth factor receptor)
Neuroendocrine markers
Hematopoietic markers
CD20
CD10 (CALLA)
• Common acute lymphoblastic leukemia antigen (CALLA) is an early marker of B-cell differentiation
• Useful in differential diagnosis of B-cell lymphoproliferative disorders (see Table 1.2)
• Positive in periadnexal mesenchymal cells, staining only the stroma of trichoblastomas but the epithelial cells of basal cell carcinoma
• Expressed in most atypical fibroxanthomas but not uncommonly seen in the other tumors in the differential diagnosis of cutaneous spindle cell tumors
• Marker of renal cell carcinoma but also expressed in other cutaneous clear cell lesions including: balloon cell nevi, clear cell hidradenoma, and sebaceous tumors
CD30 (Ki-1, BERH2)
ALK-1
• Anaplastic lymphoma kinase expressing chromosomal translocation t (2,5)
• Positive in most systemic anaplastic large cell lymphoma and negative in primary cutaneous anaplastic large cell lymphoma
• Those few patients with ALK-1-negative systemic anaplastic large cell lymphoma have a poor prognosis
CD117 (c-Kit)
BCL2
• An oncogene that inhibits apoptosis
• Useful in differential diagnosis of B-cell lymphoproliferative disorders (see Table 1.2)
• Most basal cell carcinomas reveal diffuse BCL2 staining, whereas trichoepitheliomas only show staining of the outermost epithelial layers of the tumor islands
Table 1-2
Cutaneous B-cell lymphoproliferative disorders
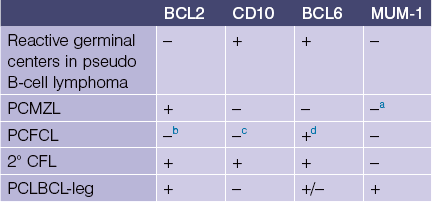
PCMZL, primary cutaneous marginal zone lymphoma
PCFCL, primary cutaneous follicle center lymphoma
2° CFL, secondary cutaneous follicular lymphoma
PCLBCL-leg, primary cutaneous large B-cell lymphoma, leg type
aPlasma cells are positive with MUM-1 but the neoplastic B cells are negative in PCMZL.
bOnly 10–20% of PCFCL are BCL-2 positive.
cMost PCFCL have a diffuse histologic pattern and are CD10 negative but cases with a follicular pattern are CD10 positive.
dThe BCL6-positive malignant cells are outside the follicle.
Multiple myeloma oncogene-1 (MUM-1)
• Expressed in plasma cells, activated T cells, and subset of germinal center cells
• Distinguishes primary cutaneous diffuse large B-cell lymphoma, leg type from diffuse follicle center lymphoma
• Reactivity reported in anaplastic large cell lymphoma, lymphomatoid papulosis, and blastic plasmacytoid dendritic cell neoplasm
Proliferation markers
Alcaraz, I, Cerroni, L, Rütten, A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012; 34(4):347–393.
Anstey, A, Cerio, R, Ramnarain, N, et al. Desmoplastic malignant melanoma. An immunocytochemical study of 25 cases. Am J Dermatopathol. 1994; 16(1):14–22.
Bahrami, S, Malone, JC, Lear, S, et al. CD10 expression in cutaneous adnexal neoplasms and a potential role for differentiating cutaneous metastatic renal cell carcinoma. Arch Pathol Lab Med. 2006; 130(9):1315–1319.
Benner, MF, Jansen, PM, Meijer, CJ, et al. Diagnostic and prognostic evaluation of phenotypic markers TRAF1, MUM1, BCL2 and CD15 in cutaneous CD30-positive lymphoproliferative disorders. Br J Dermatol. 2009; 161(1):121–127.
Buonaccorsi, JN, Plaza, JA. Role of CD10, wide-spectrum keratin, p63, and podoplanin in the distinction of epithelioid and spindle cell tumors of the skin: an immunohistochemical study of 81 cases. Am J Dermatopathol. 2012; 34(4):404–411.
Córdoba, A, Guerrero, D, Larrinaga, B, et al. Bcl-2 and CD10 expression in the differential diagnosis of trichoblastoma, basal cell carcinoma, and basal cell carcinoma with follicular differentiation. Int J Dermatol. 2009; 48(7):713–717.
Cota, C, Vale, E, Viana, I, et al. Cutaneous manifestations of blastic plasmacytoid dendritic cell neoplasm-morphologic and phenotypic variability in a series of 33 patients. Am J Surg Pathol. 2010; 34(1):75–87.
Elston, DM, Gibson, LE, Kutzner, H. Infectious diseases. In: Lin F, Prichard J, eds. Handbook of Practical Immunohistochemistry. New York: Springer, 2011.
Eyzaguirre, E, Haque, AK. Application of immunohistochemistry to infections. Arch Pathol Lab Med. 2008; 132(3):424–431.
Fox, MD, Billings, SD, Gleason, BC, et al. Expression of MiTF may be helpful in differentiating cellular neurothekeoma from plexiform fibrohistiocytic tumor (histiocytoid predominant) in a partial biopsy specimen. Am J Dermatopathol. 2012; 34(2):157–160.
Fullen, DR, Lowe, L, Su, LD. Antibody to S100a6 protein is a sensitive immunohistochemical marker for neurothekeoma. J Cutan Pathol. 2003; 30(2):118–122.
Fullen, DR, Reed, JA, Finnerty, B, et al. S100A6 expression in fibrohistiocytic lesions. J Cutan Pathol. 2001; 28(5):229–234.
Helm, KF. Immunohistochemistry of skin tumors. In: Dabbs DJ, ed. Diagnostic Immunohistochemistry. Philadelphia: Churchill Livingstone; 2002:313–327.
Hoefnagel, JJ, Vermeer, MH, Jansen, PM, et al. Bcl-2, Bcl-6 and CD10 expression in cutaneous B-cell lymphoma: further support for a follicle centre cell origin and differential diagnostic significance. Br J Dermatol. 2003; 149(6):1183–1191.
Hudson, AR, Smoller, BR. Immunohistochemistry in diagnostic dermatopathology. Dermatol Clin. 1999; 17(3):667–680.
Kucher, C, Xu, X, Pasha, T, et al. Histopathologic comparison of nephrogenic fibrosing dermopathy and scleromyxedema. J Cutan Pathol. 2005; 32(7):484–490.
Lau, SK, Chu, PG, Weiss, LM. Immunohistochemical expression of Langerin in Langerhans cell histiocytosis and non-Langerhans cell histiocytic disorders. Am J Surg Pathol. 2008; 32(4):615–619.
Lazova, R, Tantcheva-Poor, I, Sigal, AC. P75 nerve growth factor receptor staining is superior to S100 in identifying spindle cell and desmoplastic melanoma. J Am Acad Dermatol. 2010; 63(5):852–858.
Lora, V, Kanitakis, J. CDX2 expression in cutaneous metastatic carcinomas and extramammary Paget’s Disease. Anticancer Res. 2009; 29(12):5033–5037.
Macarenco, RS, Ellinger, F, Oliveira, AM. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med. 2007; 131(4):625–636.
Mohamed, A, Gonzalez, RS, Lawson, D, et al. SOX10 expression in malignant melanoma, carcinoma, and normal tissues. Appl Immunohistochem Mol Morphol. 2012.
Murphy, M, Fullen, D, Carlson, JA. Low CD7 expression in benign and malignant cutaneous lymphocytic infiltrates: experience with an antibody reactive with paraffin-embedded tissue. Am J Dermatopathol. 2002; 24(1):6–16.
North, PE, Waner, M, Mizeracki, A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangioma. Hum Pathol. 2000; 31(1):11–22.
Ostler, DA, Prieto, VG, Reed, JA, et al. Adipophilin expression in sebaceous tumors and other cutaneous lesions with clear cell histology: an immunohistochemical study of 117 cases. Mod Pathol. 2010; 23(4):567–573.
Perna, AG, Smith, MJ, Krishnan, B, et al. CD10 is expressed in cutaneous clear cell lesions of different histogenesis. J Cutan Pathol. 2005; 32(5):348–351.
Pilloni, L, Bianco, P, Difelice, E, et al. The usefulness of c-Kit in the immunohistochemical assessment of melanocytic lesions. Eur J Histochem. 2011; 55(2):e20.
Plaza, JA, Ortega, PF, Stockman, DL, et al. Value of p63 and podoplanin (D2-40) immunoreactivity in the distinction between primary cutaneous tumors and adenocarcinomas metastatic to the skin: a clinicopathologic and immunohistochemical study of 79 cases. J Cutan Pathol. 2010; 37(4):403–410.
Poniecka, AW, Alexis, JB. An immunohistochemical study of basal cell carcinoma and trichoepithelioma. Am J Dermatopathol. 1999; 21(4):332–336.
Pouryazdanparast, P, Yu, L, Cutlan, JE, et al. Diagnostic value of CD163 in cutaneous spindle cell lesions. J Cutan Pathol. 2009; 36(8):859–864.
Ribé, A, McNutt, NS. S100A6 protein expression is different in Spitz nevi and melanomas. Mod Pathol. 2003; 16(5):505–511.
Rose, AE, Christos, PJ, Lackaye, D, et al. Clinical relevance of detection of lymphovascular invasion in primary melanoma using endothelial markers D2-40 and CD34. Am J Surg Pathol. 2011; 35(10):1441–1449.
Roullet, M, Gheith, SM, Mauger, J, et al. Percentage of γδ T cells in panniculitis by paraffin immunohistochemical analysis. Am J Clin Pathol. 2009; 131(6):820–826.
Sachdev, R, Robbins, J, Kohler, S, et al. CD163 expression is present in cutaneous histiocytomas but not in atypical fibroxanthomas. Am J Clin Pathol. 2010; 133(6):915–921.
Saglam, A, Uner, AH. Immunohistochemical expression of Mum-1, Oct-2 and Bcl-6 in systemic anaplastic large cell lymphomas. Tumori. 2011; 97(5):634–638.
Schach, CP, Smoller, BR, Hudson, AR, et al. Immunohistochemical stains in dermatopathology. J Am Acad Dermatol. 2000; 43:1094–1100.
Schimming, TT, Grabellus, F, Roner, M, et al. pHH3 immunostaining improves interobserver agreement of mitotic index in thin melanomas. Am J Dermatopathol. 2012; 34(3):266–269.
Shin, J, Vincent, JG, Cuda, JD, et al. Sox10 is expressed in primary melanocytic neoplasms of various histologies but not in fibrohistiocytic proliferations and histiocytoses. J Am Acad Dermatol. 2012; 67(4):717–726.
Skobieranda, K, Helm, KF. Decreased expression of the human progenitor cell antigen (CD34) in morphea. Am J Dermatopathol. 1995; 17(5):471–475.
Smith, KJ, Tuur, S, Corvette, D, et al. Cytokeratin 7 staining in mammary and extramammary Paget’s disease. Mod Pathol. 1997; 10(11):1069–1074.
Su, LD, Schnitzer, B, Ross, CW, et al. The t(2; 5)-associated p80 NPM/ALK fusion protein in nodal and cutaneous CD30+ lymphoproliferative disorders. J Cutan Pathol. 1997; 24(10):597–603.
Tellechea, O, Reis, JP, Domingues, JC, et al. Monoclonal antibody Ber EP4 distinguishes basal-cell carcinoma from squamous-cell carcinoma of the skin. Am J Dermatopathol. 1993; 15(5):452–455.
Vitte, F, Fabiani, B, Bénet, C, et al. Specific skin lesions in chronic myelomonocytic leukemia: a spectrum of myelomonocytic and dendritic cell proliferations: a study of 42 cases. Am J Surg Pathol. 2012; 36(9):1302–1316.
White, WL. Immunomicroscopy in diagnostic dermatopathology: an update on cutaneous neoplasms. Adv Dermatol. 1999; 14:359–397.
Wood, WS, Tron, VA. Analysis of HMB-45 immunoreactivity in common and cellular blue nevi. J Cutan Pathol. 1991; 18(4):261–263.