The anesthesia provider’s role in the prevention of surgical site infections
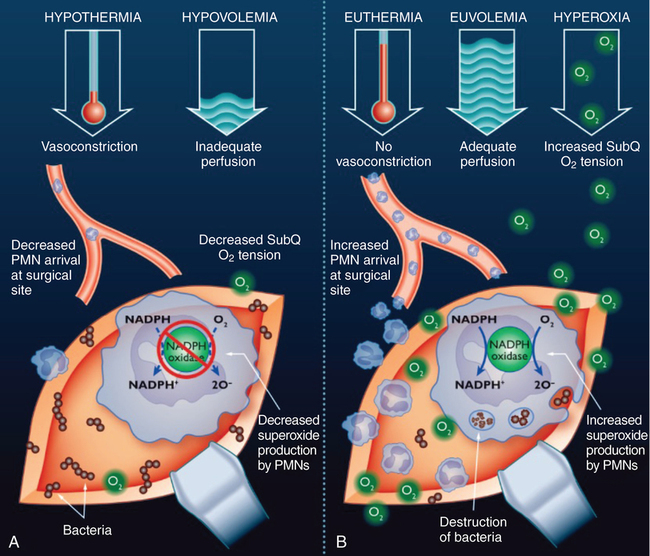
Hyperoxia
In most clinical scenarios, O2 delivery to end organs is vastly more dependent on the amount of O2 bound to hemoglobin than the amount of O2 dissolved in the blood. However, the subcutaneous tissue uses very little O2, compared with the rest of the body. In addition, the mean partial pressure of O2 in the subcutaneous tissue is approximately 60 mm Hg, a level above the range in which O2 readily dissociates from hemoglobin. Lastly, when the microvasculature is traumatized at the site of the wound, the diffusion distance for O2 is increased. These facts likely combine to decrease the importance of hemoglobin-bound O2 on O2 tension at the site of the wound and increase the importance of O2 dissolved in the bloodstream (Figure 162-1).
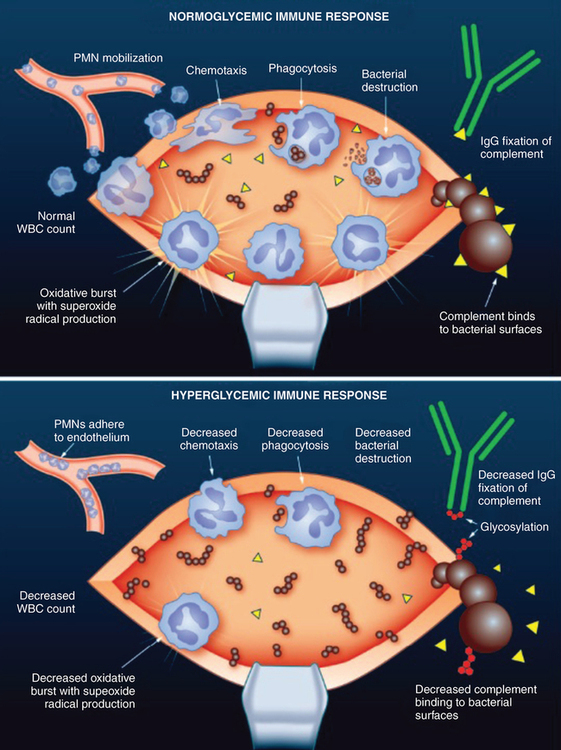
Hyperglycemia
Hyperglycemia has been shown to have numerous deleterious effects on immune function in both in vitro and human models (Figure 162-2). A glucose challenge in healthy subjects induces a transient reduction in leukocyte counts. Hyperglycemia also deactivates immunoglobulins by nonenzymatic glycosylation and glycosylation of the C3 component of complement blocks binding to bacterial surfaces. The importance of neutrophils in preventing SSIs has already been emphasized, and the neutrophils of diabetic patients have numerous functional deficits, including impaired chemotaxis, decreased phagocytic ability, and lower bactericidal capacity. If these dysfunctional neutrophils are placed in a normoglycemic environment, their function can be at least partially restored in a short period of time. Lastly, it has been shown that, in patients undergoing cardiac surgery, glucose control with continuous insulin infusions improves the phagocytic function of neutrophils when compared with the use of intermittent insulin boluses to treat hyperglycemia.