Chapter 109 Temporal Lobe Operations in Intractable Epilepsy
Epilepsy afflicts 0.5% to 1% of the world’s population and incurs an enormous burden of disease-related disability.1 Approximately one third of patients with epilepsy either fail to achieve adequate seizure control with antiepileptic drugs or are unable to tolerate the side effects induced at the doses necessary to do so.2–4 A substantial literature indicates that poorly controlled epilepsy impairs cognition, reduces employment, diminishes psychosocial function and overall quality of life, and increases both risk of sudden death and overall mortality relative to age-matched controls.5–10
Mesial temporal lobe epilepsy (MTLE) is the most common and well-defined focal epilepsy syndrome, and it is also the most likely to be pharmacoresistant.11,12 For a subset of patients with intractable MTLE, anterior temporal lobectomy (ATL) offers the possibility of a seizure-free life with reduced reliance on antiepileptic drugs. ATL has evolved over the past century into a safe and effective procedure that has been validated with class I evidence.13 It is now in widespread use at epilepsy centers throughout the United States and across the developed world, and contemporary surgical series consistently report seizure-free rates of about 70% in MTLE patients.14
History
The history of surgery for MTLE spans more than a century. Writing in 1898, Jackson and Colman ascribed seizures associated with “tasting movements” and a “dreamy state” to a lesion in the mesial temporal lobe.15 The earliest epilepsy resections excised cortical regions implicated in patients’ seizure semiologies with varying degrees of success, but neocortical removal alone in cases of temporal lobe epilepsy yielded only modest seizure control. In the early 1950s, Penfield et al. used refinements in electroencephalograms (EEGs) and electrocorticography (ECoG) to demonstrate that the mesial limbic structures were central to the pathophysiology of “psychomotor epilepsy.”16 As surgeons identified an increasing number of patients with seizures of mesial temporal origin, interest developed in designing standardized surgical procedures that incorporated resection of the amygdala and hippocampus. Falconer and colleagues17,18 and Morris19 described early versions of procedures that would form the basis for the modern ATL. Niemeyer soon proposed a mesial resection technique that spared the lateral cortex20; this was later adapted by Wieser, Yasargil, and colleagues21–23 and by Olivier,27 and it was the forerunner of the contemporary selective amygdalohippocampectomy (SAH).
Indications and Patient Selection
The selection of appropriate surgical candidates is paramount to the success of ATL and is discussed in detail in the previous chapter. Briefly, patient selection hinges on the concordance of data from several modalities, including neuroimaging, electrophysiologic recordings, and neuropsychological evaluation that (1) identifies the patient as one with the “surgically remediable syndrome” of MTLE,24 (2) localizes a seizure focus suitable for extirpation, and (3) determines that the benefit gleaned from resecting the implicated epileptogenic zone would outweigh any language or memory deficit likely to be experienced.
Surgical Procedure
Anterior Temporal Lobectomy
The standard en bloc ATL, in which the lateral neocortex and mesial hippocampal structures are removed in a single specimen, was developed in the early 1950s17,18 and employed over the ensuing few decades.25 More recently, two-part resection, in which the lateral and mesial portions are resected separately, has been favored by most surgeons.26–28 What follows is one method of performing the two-part ATL, along with references to other variations.
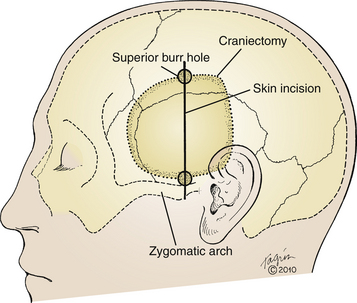
The procedure is usually performed under general anesthesia, unless ECoG or language mapping are planned. In the former case, nitrous oxide should be discontinued during ECoG but the patient should remain paralyzed with depolarizing muscle relaxants; in the latter, the procedure may be performed while the patient is awake.29,30 When ECoG is planned, perioperative use of anxiolytic medications that might suppress EEG activity (diazepam, midazolam, etc.) should be avoided. The patient is positioned supine on the operating room table with the head turned 90 degrees laterally and is stabilized with three-point fixation. A shoulder roll can be employed to assist with lateral head rotation if necessary. The vertex is lowered approximately 10 degrees below horizontal to minimize frontal lobe retraction later in the procedure. The operative area is shaved with clippers, and the skin is prepared with degreasing solution. We employ a linear incision, starting at the root of the zygoma, approximate 1.5 cm anterior to the tragus (Fig. 109-1). The incision is carried superiorly to the superior temporal line. A gentle anterior curve at the superior aspect of the incision may be employed to increase exposure if necessary. Others use a question mark–shaped incision starting at the same location, curving posteriorly over the auricle, and then curving superiorly and anteriorly toward the hairline.26,29,31,32 The incision is marked, and nonsterile clear plastic adhesive drapes are applied to square off the operative field. The region is then sterilely prepped and draped.
Two bur holes are placed at either end of the incision, above the zygomatic process, and at the superior temporal line. Using a footplated bit, the craniotomy is carried out, with particular attention paid to squaring off the posteroinferior corner by cutting directly posteriorly above the ear before turning superiorly (Fig. 109-1). Compared to a gentle curve at that corner, a right angle turn facilitates posterior exposure of the hippocampus later in the procedure. The greater sphenoid wing is further trimmed with rongeurs to increase exposure of the temporal pole, and the inferior margin of the craniotomy is similarly trimmed to access the floor of the middle cranial fossa, taking care not to enter the mastoid air cells. Bleeding from the middle meningeal artery is controlled with bipolar cautery.
Prior to durotomy, we square off the exposure with four fresh towels. The dura is sharply incised in a C-shaped fashion based on the sphenoid wing and reflected over the retracted temporalis muscle. This technique exposes the sylvian fissure, inferior frontal gyrus (IFG), superior temporal gyrus (STG), and middle temporal gyrus (MTG) (Fig. 109-2). The cortical surface is inspected for any abnormalities, as well as for the presence of large draining veins such as the Labbé vein or the middle cerebral vein, which must be preserved. The two-step resection then commences, starting with the neocortical block, followed by the mesial block.
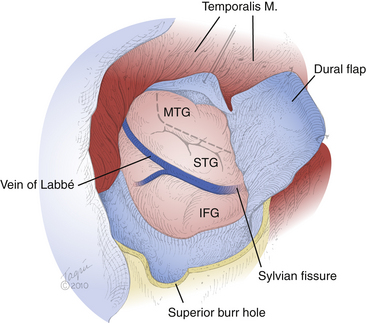
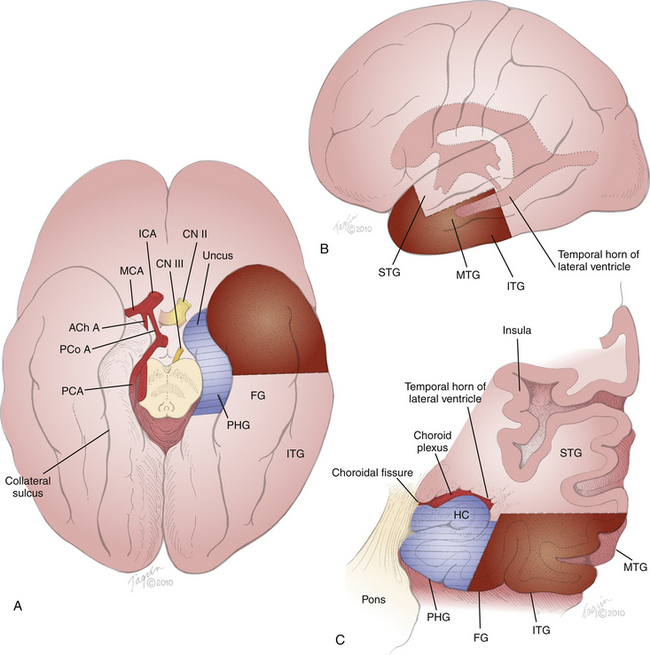
FIGURE 109-2 Cortical exposure. The surgeon’s view of the cortical exposure for a left-sided ATL is depicted, performed through the craniotomy shown in Fig. 109-1. A C-shaped durotomy based on the sphenoid wing exposes the IFG, sylvian fissure, STG, and MTG. The planned corticectomy (dashed line) begins in the MTG and extends anteriorly toward the temporal tip and inferiorly toward the floor of the middle fossa. Superior is the bottom aspect of the figure; anterior is the right aspect of the figure.
The first stage of the resection is removal of the neocortical block (Fig. 109-3, stippled region). The temporal pole is visualized at the anteriormost recess of the greater wing of the sphenoid bone by gently lifting the dura and peering down the longitudinal axis of the temporal lobe. The distance from the temporal pole is measured along the length of the MTG. We generally resect about 4 cm of neocortex. In nondominant cases, further posterior extension to 5 or 6 cm may possibly be achievable without significant impact on memory, language, or cognitive function. More generous lateral resections are typically reserved for situations in which there is suspicion of epileptogenicity in the neocortex. In these cases, ECoG and language mapping may be useful to tailor the resection to match the pathophysiology and to avoid postoperative language deficit.29,30,33,34 In most cases of standard MTLE, however, ECoG and large lateral resections are not necessary.28,35 A more sparing approach to the lateral resection was developed by Spencer and colleagues, in which only 3 to 3.5 cm of the temporal tip is removed, leaving the STG intact, regardless of laterality.28,31
The MTG pia is cauterized with bipolar forceps at the specified distance from the temporal pole and is sharply cut. This incision is carried directly inferiorly to the floor of the middle fossa (Figs. 109-2 and 109-3B, dashed line), traversing the MTG and inferior temporal gyrus (ITG), and around the basal surface to include the fusiform (lateral occipitotemporal) gyrus, approaching the collateral sulcus (Fig. 109-3). It is deepened into the white matter of the temporal stem approximately 2 to 3 cm toward the temporal horn of the lateral ventricle, using either bipolar coagulation and suction29,32 or ultrasonic aspiration.26,28,36 Some authors plan a coronal trajectory to enter the ventricle at this point,26,29 but we and others31 prefer to approach but not yet enter the ventricle (Fig. 109-3C). The incision is then carried anteriorly, parallel to the superior border of the MTG, to the temporal pole. The STG is initially left intact to protect the sylvian fissure vessels. The incision is carefully deepened parallel to the plane of the sylvian fissure, and the white matter is aspirated progressively inferiorly in an oblique coronal plane until the inferior incision is encountered. The incision plane is continued anteromedially until the temporal pole, freeing the neocortical specimen. The neocortex and underlying white matter can be sent as the first specimen for analysis. The remaining cuff of the MTG and anteriormost 1.5 to 2 cm of the STG are then aspirated with subpial dissection, maintaining the integrity of the arachnoid plane protecting the sylvian vessels. The neocortical resection is thus completed, leaving only the parahippocampal gyrus (PHG), hippocampus, and amygdala.
Attention is then turned to the mesial temporal structures for the second stage of the procedure (Fig. 109-3, hatched region). Prior to commencing, the microscope is brought into position, and self-retaining retractors are placed. We employ two retractors: one on the STG to gently retract the frontal lobe and the other on the cut coronal surface of the temporal lobe at the level of the MTG. Attention should be paid to minimizing frontal lobe retraction, as the structures immediately deep to the frontal retractor include the external capsule and lentiform nucleus of the basal ganglia.
The mesial contents may be resected as a single piece26 or, as described here, in two separate sections,31 one containing the PHG and hippocampus and the other containing the amygdala. The first step of the mesial resection is identification of the ventricle. The temporal horn of the lateral ventricle runs parallel and deep to the MTG (Fig. 109-3A and C). It can usually be encountered approximately 3 to 4 cm posterior to the temporal pole and about 3.5 cm deep to the surface of the MTG. Its location can be confirmed by studying the preoperative films. At this point in the anterior–posterior dimension, the hippocampal formation comprises the inferomedial wall of the ventricle (Figs. 109-3C and 109-4). The choroidal fissure, defined as the space between the two pial leaflets that form the choroid plexus, comprises the superomedial boundary of the ventricle. Further anteriorly, at the level of the uncus, the amygdala forms the superomesial cap of the ventricle.
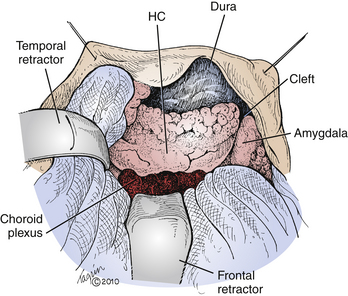
FIGURE 109-4 Mesial resection. The surgeon’s view of the remaining mesial structures is depicted following completion of the lateral resection and opening of the temporal horn of the lateral ventricle in a left ATL. The choroid plexus marks the superior aspect of the ventricle, the amygdala marks the anterior extent, and the smooth ventricular surface of the hippocampal formation (HC) is visualized inferomedially, continuing into the cut edge of the PHG. Immediately anterior to the HC, the ventricle curves sharply medially, forming a cleft separating the HC posteriorly from the amygdala anteriorly (see also Fig. 109-5A). Superior is the bottom aspect of the figure; anterior is the right aspect of the figure.
Entry into the ventricle is confirmed by visualizing the smooth ependymal surface, a gush of cerebrospinal fluid, or the emergence of the choroid plexus. The anterior–posterior extent of the ventricle is opened, revealing the smooth shiny ventricular surface of the hippocampus inferomedially, the choroid plexus superiorly, and the amygdala anteriorly (Fig. 109-4). The boundary between the hippocampal formation posteriorly and the amygdala anteriorly is usually demarcated by a cleft of the ventricle near its anterior tip (Figs. 109-4 and 109-5A). This cleft is deepened until the mesial temporal lobe arachnoid membrane is encountered, thereby cleaving the hippocampus and PHG posteriorly from the amygdala anteriorly. This arachnoid membrane is the medial extent of the resection and should never be violated, as the contents of the ambient cistern, including the circle of Willis vessels and cranial nerves, lie beyond it (Fig. 109-3A).
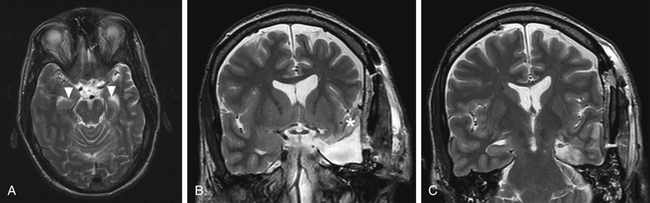
FIGURE 109-5 Preoperative and postoperative MRI. A, Preoperative T2-weighted axial MRI is shown through the level of the midbrain, depicting the cleft (arrowheads) of the temporal horn of the lateral ventricle separating the amygdala anteriorly from the hippocampal formation posteriorly (see also Fig. 109-4). B and C, Postoperative T2-weighted coronal MRI is shown. Anteriorly, at the level of the amygdala and optic chiasm (B), the resection includes all neocortical structures except the STG (asterisk). Posteriorly, at the level of the red nuclei (C), the lateral neocortical structures are spared.
The second part of the mesial resection is the amygdalectomy. At this point, the amygdala is conspicuous near the temporal pole as the sole remaining mesial structure. The most important consideration for this stage is the superior limit of resection, an imaginary line between the choroidal point (anteriormost extent of the choroid plexus in the choroidal fissure and point of entry of the anterior choroidal artery into the ventricle) and the limen insulae (threshold of the insula and anterior limit of the insula and transition point between the insular and the frontal cortex). This line is roughly approximated by extending an imaginary line parallel and coaxial with the choroid plexus. Venturing superior to this limit risks injury to the globus pallidus. Adjoining structures to the amygdala, such as the white matter of the neocortex and the hippocampus and PHG, have already been removed. The bulbous structure of the amygdala is removed and sent en bloc for analysis, and the remaining contents of the uncus are peeled off the medial arachnoid membrane. The resection is thus completed (Fig. 109-5B and C).
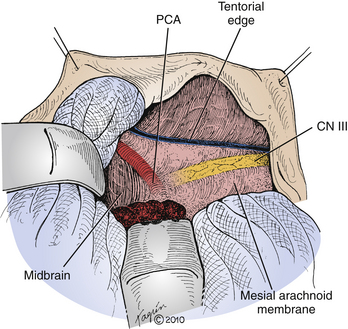
Through the medial arachnoid membrane, the PCA is visible coursing posteriorly around the midbrain, and the oculomotor nerve is seen traveling anteriorly (Fig. 109-6). Bipolar cautery in the vicinity of this arachnoid membrane is therefore not advised. The trochlear nerve may be seen running along and below the edge of the tentorial incisura. After meticulous hemostasis is achieved, the cavity is lined with oxidized cellulose, and the wound is closed in a standard fashion.
Selective Resections
Niemeyer described the first approach for SAH in the late 1950s,20 soon after Falconer et al. developed the standard ATL.18 In this transcortical transventricular approach, an incision 2 to 3 cm long is made in the MTG, approximately 4 cm posterior to the temporal pole. Dissection through this incision leads to the temporal horn of the lateral ventricle, which lies 3 to 3.5 cm deep to the cortical surface. Upon entering the ventricle, the choroid plexus again provides a helpful landmark as the superomedial boundary of the resection. The PHG is subpially aspirated, and the hippocampus is divided at its anterior and posterior extents in a similar fashion to that described earlier. It can then be mobilized laterally, allowing the irrigating vessels in the hippocampal fissure to be bipolar cauterized and sharply sectioned. Aspiration of the fimbria along the medial aspect of the hippocampus frees the specimen. Removal of the amygdala and subpial aspiration of the uncus anteriorly complete the resection. Advantages of this SAH procedure include its straightforward approach, but an important disadvantage is the difficulty of obtaining an en bloc resection for histologic analysis. Variations to the transcortical transventricular SAH have been described by Olivier, with an entry point in the anterior aspect of the STG rather than the MTG.37
In an attempt to leave the lateral temporal neocortex completely intact, Wieser, Yasargil, and colleagues developed the trans-sylvian approach, a technically demanding procedure that accesses the mesial temporal lobe via a sylvian fissure dissection.21,22 The patient’s head is turned laterally 30 degrees, and the vertex is lowered 20 degrees such that the malar eminence is superior. This position allows a vertical angle of dissection through the sylvian fissure. A pterional craniotomy is performed, approximately 2 cm posterior to the typically described location.38 Following craniotomy and durotomy, the sylvian fissure is opened from the carotid bifurcation to about 2 cm beyond the middle cerebral artery bifurcation. A 1- to 2-cm incision is made in the inferior portion of the circular sulcus of the insula. The amygdala lies just a few millimeters deep to the surface. Opening into the ventricle at this point provides a helpful anatomic point of reference. Moving anteroinferiorly, the amygdala is aspirated and the anterior PHG is subpially resected. Attention is then turned posteroinferiorly to the hippocampus and remainder of PHG. The hippocampus is rotated laterally, and the feeding vessels in the hippocampal fissure are divided, freeing the hippocampus medially. It is detached posteriorly by making a transverse section at the posterior aspect of the peduncles. The hippocampus is thus circumferentially freed and removed with the PHG en bloc. This approach is technically challenging, requiring superior microneurosurgical skill and detailed anatomic knowledge of the region. The theoretical advantage of this technique is its sparing of any injury to the temporal cortex, but damage to the sylvian vessels or instigation of vasospasm is a significant source of morbidity.
Finally, a variety of subtemporal approaches have been described.39–41 Motivation behind their development arose from the desire to leave the lateral neocortex and temporal stem intact. Disadvantages are significant, however, including temporal lobe retraction; risk of injury to venous drainage, including the Labbé vein; and difficulties with surgical orientation. As a result, these approaches are usually reserved for mesial temporal lesionectomies requiring neocortical sparing.
Outcomes
Seizure Reduction
Because of a lack of consistency in patient selection, surgical technique, outcome assessment, and timing and duration of follow-up, it can be difficult to compare outcomes across surgical series. The most commonly applied metric of efficacy is reduction in seizure frequency on the Engel classification scheme42 (Table 109-1). Most studies cite rates of seizure freedom (Engel class I), which has been shown to correlate with quality-of-life improvement following epilepsy surgery.43–45 Surgery may not appreciably improve quality of life unless patients are rendered free (or nearly free) of their seizures.46,47
| Class | Description |
|---|---|
| I | Free of disabling seizures |
| II | Rare disabling seizures (e.g., ≤2/yr) |
| III | Worthwhile† reduction in disabling seizures |
| IV | No worthwhile improvement |
† Worthwhile is defined variably, though quality of life and psychosocial measures may not improve significantly for reductions in seizure frequency ≤70%-90%.46,73
Degree of seizure control conferred by ATL has been investigated in several notable studies. In the only randomized, controlled trial of surgical resection versus medical management for MTLE,48 64% of patients who underwent ATL were free of disabling seizures 1 year postoperatively versus only 8% of patients managed medically. A survey by Engel et al. of 100 epilepsy centers revealed that 68% of ATL patients were seizure free following surgery.42 More recent meta-analyses of modern series have also concluded that rates of seizure freedom following resection for MTLE approach 70%.14,49 Most studies report outcomes only to 1 or 2 years postoperatively, and subsequent seizure relapse is certainly possible. There is, however, evidence that 1- or 2-year outcomes predict long-term response50; indeed, longer follow-ups have demonstrated 50% to 70% of patients to be free of disabling seizures in the fifth postoperative year.51–56
Complications
Surgical mortality is rare, and many modern series of several hundred cases or more report no deaths.57–60 Permanent hemiparesis is an uncommon (less than 1%) but disastrous outcome that may stem from manipulation or thrombosis of the middle cerebral or anterior choroidal arteries. Visual field cuts in the contralateral superior quadrant can be detected in a majority of postoperative patients61; these are thought to result from disruption of the optic radiation fibers (Meyer loop) that course over the temporal horn of the lateral ventricle. And while severe deficits (even hemianopia) have been reported, most visual field cuts are subclinical, and they frequently improve with time.61 The incidence of cranial nerve injuries (those to cranial nerve III being more common than those to either cranial nerve IV or cranial nerve VII) resulting in a permanent deficit is less than 1%.
Postoperative psychiatric disturbances are now recognized to be relatively common in the weeks and months following surgery for MTLE, affecting up to 50% of patients.62–66 Risk factors include preexisting psychiatric disorders67 (of which there is a high incidence in candidates for MTLE surgery68,69) and persistent disabling seizures following surgery.63 New-onset depression often occurs in the postoperative period.70,71 By contrast, some patients with preexisting depressive symptoms note improvement following resection72; such gains are most consistently observed in those who are rendered seizure free.73,74 Postoperative psychosis is a recognized phenomenon and has afflicted up to 10% of patients in some series.62,75–77
Neuropsychological Morbidity
Patients manifest a range of neuropsychological changes following ATL. Surgery has been recognized to aggravate preexisting neuropsychological deficits in patients with chronic MTLE,78,79 and a spectrum of new deficits may be introduced as a consequence of resecting eloquent tissue. Of particular concern are memory and language function.
Memory deficits following temporal resection range from disabling global amnesia to subclinical declines in material-specific memory. Global memory deficits in the context of epilepsy surgery were first described by Milner and Penfield80; these are disabling yet rare, with a frequency of 1% or less in contemporary series. Material-specific memory deficits are far more frequent and include decrements in short-term verbal and nonverbal memory. Short-term verbal memory is commonly impaired following dominant resections; one study estimated an incidence of 25% to 50%.81 Another mixed series of ATL and SAH patients identified declines following 40% and 29% of left- and right-sided operations, respectively.82 This has provided an impetus for prospectively identifying patients at risk for verbal memory loss. Two scenarios of concern have been identified among patients undergoing dominant resection83–85: (1) hippocampal atrophy on the side to be resected but impaired memory function on the contralateral side (the “functional reserve” model) and (2) both intact memory and normal-appearing hippocampus on the resected side (the “functional adequacy” model).86 Visuospatial memory tends to be less affected than verbal memory, and it has been noted to improve after dominant resection and decline after nondominant resection.
Language function is also at risk during dominant resections, but few patients develop severe sequelae and some actually improve on certain measures. One study found that patients undergoing left-sided standard resections (without mapping) tended to have relatively poor language function preoperatively, and after resection, most patients were either stable or had improved.87 Another study of language in MTLE patients also suggested that ATL of the dominant hemisphere can be accomplished without measurable compromise of language function.88 And as with cognition and memory, it appears that postoperative seizure freedom may confer gains in language.89 Language deficits do occur, however, and transient postoperative dysnomia has an incidence of up to 25% following dominant resection.90 Patients are vulnerable even when resections are tailored using intra- or extraoperative language mapping.91 These symptoms are typically short lived, and persistent, severe dysphasia is reported in at least 1% of operated patients.
Cognitive changes after temporal resection are mixed, and outcomes appear to depend on both resection side and underlying pathologic substrate.92–94 Overall intellectual function tends to remain stable.53 When surgery effectively reduces seizure frequency, selected cognitive measures may improve.95,96 Gains in general metrics of cognition, such as performance and full-scale IQ, also have been documented.97–99 These improvements following surgery may be attributable to either the tapering of anticonvulsants or the abatement of ictal disturbances. When surgery is unsuccessful in controlling seizures, however, cognitive decline tends to accelerate.8
Extent of Resection
In the early era of epilepsy surgery, removal of the lateral temporal cortex was emphasized, and this approach yielded modest rates of seizure freedom. Neocortical resection alone is now recognized to be ineffective for patients with MTLE, and the demonstrated efficacy of selective mesial techniques (e.g., SAH) suggests that removal of the anterolateral temporal lobe is not always necessary for achieving seizure freedom. With regard to ATL, the question of optimal extent of lateral resection has been addressed by several studies. Cascino et al.100 reported that extent of lateral resection does not correlate with surgical outcome. Later, a prospective, randomized trial found no difference in surgical outcome between patients in whom the STG was resected and those in whom it spared.101 Thus, present data suggest that the extent of lateral resection pursued in ATL, assuming no concern for a neocortical epileptogenic focus, does not affect seizure outcome.
Removal of both the hippocampus and the amygdala is now recognized to be critical to successful MTLE surgery, but the optimal extent of hippocampal resection remains unsettled. Some have argued for limited hippocampectomies, pointing to studies indicating that posterior migration of the hippocampal resection margin does not improve seizure outcome.102–105 Several lines of evidence, however, favor more generous resection. First, some have reported that when an initial MTLE surgery is unsuccessful, reoperation to remove lingering hippocampal tissue frequently leads to seizure freedom.106,107 In addition, magnetic resonance imaging (MRI) volumetry studies have positively correlated mediobasal108 and hippocampal109,110 resection extent with good seizure outcome. Finally, a prospective, randomized trial in which patients were assigned to either limited or radical hippocampectomy (resection to the anterior edge of the cerebral peduncle or to the level of the superior colliculus, respectively) found a superior rate of seizure freedom in the total hippocampectomy group (69% vs. 38%) 1 year after surgery.111 Importantly, this benefit in seizure reduction was not attended by excess neurologic or neuropsychological morbidity.
Tailored Resections
The data linking ECoG-detected epileptiform abnormalities with seizure outcome are inconsistent. Early studies affirmed that interictal discharges, especially when absent before resection or abundant after resection, held predictive value. Reports have suggested that preresection spikes predict outcomes,112 but others found no association.100,113–115 Residual discharges following resection have been reported to portend persistent seizures by some,116–120 but a majority of studies do not support this conclusion.100,114,115,121–129 A recent meta-analysis concluded that though ECoG may have a role in selected infrequent cases, there is no well-defined relationship between ECoG discharges and outcome within the MTLE population.14
Given the potential for language and memory deficits following dominant ATL, some have advocated mapping with cortical stimulation to tailor the lateral resection margins. Ojemann and Dodrill used this method to establish correlations between postoperative verbal memory scores and neocortical resection extent.130 They observed that sparing regions with stimulation-evoked changes in memory or naming reduces postoperative declines in memory and language measures. Other groups, however, have not demonstrated such differences by tailoring resections.87,88,131–133 In addition, it is uncommon for areas with stimulation-elicited language disturbances (which are rarely more anterior than 4.5 cm from the dominant temporal pole along the MTG134) to fall within the resection boundaries of the technique described here.
Selective Mesial Resection
To date, there have been no prospective, randomized trials comparing SAH to ATL with respect to reducing seizure frequency. Indirect comparisons of seizure outcomes from large single-center series suggest that the two techniques yield similar results,82,135–137 though patient selection criteria for SAH are generally more restrictive than those for ATL. Comparisons of two SAH techniques (transcortical and trans-sylvian), including one prospective, randomized trial, also revealed no differences in seizure-free outcome.138
Part of the impetus for developing selective mesial resection techniques for MTLE was the presumption that sparing the nonepileptogenic lateral cortex would minimize neurologic and neuropsychological morbidity. A number of studies have reported modest (though inconsistent) benefits in neuropsychological outcomes for SAH over ATL. With respect to overall neuropsychological performance, Clusmann et al. noted a higher rate of improvement and a lower rate of deterioration.139 Some studies also favored SAH in tests of recall,78 verbal memory,136,137,140 and verbal IQ.103 However, other studies have not found differences in functions including naming, general cognition,132 memory,141 and learning and retention.79 Thus, while there is scattered evidence for better outcomes for SAH versus ATL on some neuropsychological measures, a prospective, randomized trial with standardized and thorough postoperative assessment is needed to adequately address this issue.142
Binder D.K., Schramm J. Resective surgical techniques: mesial temporal lobe epilepsy. In: Luders H., editor. Textbook of Epilepsy Surgery. London: Informa Healthcare; 2008:1081-1092.
Chelune G.J. Hippocampal adequacy versus functional reserve: predicting memory functions following temporal lobectomy. Arch Clin Neuropsychol. 1995;10(5):413-432.
Engel J.Jr. Etiology as a risk factor for medically refractory epilepsy: a case for early surgical intervention. Neurology. 1998;51(5):1243-1244.
Engel J.Jr., Cascino G., Shields W. Surgically remediable syndromes. In: Engel J., Pedley T. Epilepsy: a comprehensive textbook. Philadelphia: Lippincott-Raven; 1998:1687-1696.
Engel J.Jr., Van Ness P., Rasmussen T., et al. Outcome with respect to epileptic seizures. In: Engel J., editor. Surgical treatment of the epilepsies. New York: Raven Press; 1993:609-621.
Engel J.Jr., Wiebe S., French J., et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60(4):538-547.
Engel J.Jr. Surgery for seizures. N Engl J Med. 1996;334(10):647-652.
Hermann B.P., Wyler A.R., Ackerman B., et al. Short-term psychological outcome of anterior temporal lobectomy. J Neurosurg. 1989;71(3):327-334.
Kwan P., Brodie M.J. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319.
McIntosh A.M., Wilson S.J., Berkovic S.F. Seizure outcome after temporal lobectomy: current research practice and findings. Epilepsia. 2001;42(10):1288-1307.
Niemeyer P. The transventricular amygdalohippocampectomy in temporal lobe epilepsy. In: Baldwin M., Baily P. Temporal Lobe Epilepsy. Springfield, IL: Charles C Thomas; 1958:461-482.
Ojemann G.A. Intraoperative tailoring of temporal lobe resections. In: Engel J., editor. Surgical Treatment of the Epilepsies. New York, NY: Raven Press; 1993:481-488.
Ojemann G.A. Temporal lobectomy tailored to electrocorticography and functional mapping. In: Spencer S.S., Spencer D.D. Surgery for Epilepsy. Boston: Blackwell Scientific Publications; 1991:137-145.
Penfield W, Henri Jasper H: Epilepsy and the functional anatomy of the human brain? London J. and A. Churchill, 1954;584-586.
Schramm J. Temporal lobe epilepsy surgery and the quest for optimal extent of resection: a review. Epilepsia. 2008;49(8):1296-1307.
Semah F., Picot M.C., Adam C., et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51(5):1256-1262.
Spencer D.D. Anteromedial temporal lobectomy: directing the surgical approach to the pathologic substrate. In: Spencer S.S., Spencer D.D. Surgery for Epilepsy. Boston, MA: Blackwell Scientific Publications; 1991:129-137.
Spencer D.D., Doyle W.K. Temporal lobe operations for epilepsy: radical hippocampectomy. In: Schmidek H.H., Sweet W.H. Operative Neurosurgical Techniques: Indications, Methods, and Results. Philadelphia, PA: WB Saunders Company; 1995:1305-1316.
Spencer D.D., Spencer S.S., Mattson R.H., et al. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery. 1984;15(5):667-671.
Sperling M.R., O’Connor M.J., Saykin A.J., et al. Temporal lobectomy for refractory epilepsy. JAMA. 1996;276(6):470-475.
Wiebe S., Blume W.T., Girvin J.P., et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311-318.
Wieser H.G., Yasargil M.G. Selective amygdalohippocampectomy as a surgical treatment of mesiobasal limbic epilepsy. Surg Neurol. 1982;17(6):445-457.
Wyler A.R., Hermann B.P., Somes G. Extent of medial temporal resection on outcome from anterior temporal lobectomy: a randomized prospective study. Neurosurgery. 1995;37(5):982-990.
Yam D., Nicolle D., Steven D.A., et al. Visual field deficits following anterior temporal lobectomy: long-term follow-up and prognostic implications. Epilepsia. 2010;51(6):1018-1023.
Yasargil M.G., Teddy P.J., Roth P. Selective amygdalo-hippocampectomy. Operative anatomy and surgical technique. Adv Tech Stand Neurosurg. 1985;12:93-123.
1. Engel J.Jr. Surgery for seizures. N Engl J Med. 1996;334(10):647-652.
2. Cockerell O.C., Johnson A.L., Sander J.W., et al. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet. 1995;346(8968):140-144.
3. Kwan P., Brodie M.J. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319.
4. Mohanraj R., Brodie M.J. Diagnosing refractory epilepsy: response to sequential treatment schedules. Eur J Neurol. 2006;13(3):277-282.
5. Elwes R.D., Marshall J., Beattie A., et al. Epilepsy and employment. A community based survey in an area of high unemployment. J Neurol Neurosurg Psychiatr. 1991;54(3):200-203.
6. Walczak T.S., Leppik I.E., D’Amelio M., et al. Incidence and risk factors in sudden unexpected death in epilepsy: a prospective cohort study. Neurology. 2001;56(4):519-525.
7. Helmstaedter C., Kurthen M., Lux S., et al. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003;54(4):425-432.
8. Elger C.E., Helmstaedter C., Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3(11):663-672.
9. Sperling M.R. The consequences of uncontrolled epilepsy. CNS Spectr. 2004;9(2):98-101. 106–109
10. Austin K., deBoer H. Disruptions in social functioning and services facilitating adjustment for the child and adult. In: Engel J., Pedley T. Epilepsy: A Comprehensive Textbook. Philadelphia: Lippincott-Raven; 1997:2237-2246.
11. Engel J.Jr. Etiology as a risk factor for medically refractory epilepsy: a case for early surgical intervention. Neurology. 1998;51(5):1243-1244.
12. Semah F., Picot M.C., Adam C., et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51(5):1256-1262.
13. Engel J.Jr., Wiebe S., French J., et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60(4):538-547.
14. McIntosh A.M., Wilson S.J., Berkovic S.F. Seizure outcome after temporal lobectomy: current research practice and findings. Epilepsia. 2001;42(10):1288-1307.
15. Jackson J., Colman W. Case of epilepsy with tasting movements and “dreamy state”—very small patch of softening in the left uncinate gyrus. Brain. 1898;21:580-590.
16. Penfield W, Henri Jasper H: Epilepsy and the functional anatomy of the human brain? London, J. and A. Churchill, 1954;584-586.
17. Hill D., Falconer M.A., Pampiglione G., et al. Discussion on the surgery of temporal lobe epilepsy. Proc R Soc Med. 1953;46(11):965-976.
18. Falconer M.A., Meyer A., Hill D., et al. Treatment of temporal-lobe epilepsy by temporal lobectomy; a survey of findings and results. Lancet. 1955;268(6869):827-835.
19. Morris A.A. Temporal lobectomy with removal of uncus, hippocampus, and amygdala; results for psychomotor epilepsy three to nine years after operation. AMA Arch Neurol Psychiatry. 1956;76(5):479-496.
20. Niemeyer P. The transventricular amygdalohippocampectomy in temporal lobe epilepsy. In: Baldwin M., Baily P. Temporal Lobe Epilepsy. Springfield, IL: Charles C Thomas; 1958:461-482.
21. Wieser H.G., Yasargil M.G. Selective amygdalohippocampectomy as a surgical treatment of mesiobasal limbic epilepsy. Surg Neurol. 1982;17(6):445-457.
22. Yasargil M.G., Teddy P.J., Roth P. Selective amygdalo-hippocampectomy. Operative anatomy and surgical technique. Adv Tech Stand Neurosurg. 1985;12:93-123.
23. Wieser H.G. Selective amygdalo-hippocampectomy for temporal lobe epilepsy. Epilepsia. 1988;29(Suppl 2):S100-113.
24. Engel J.Jr., Cascino G., Shields W. Surgically remediable syndromes. In: Engel J., Pedley T. Epilepsy: A Comprehensive Textbook. Philadelphia: Lippincott-Raven; 1998:1687-1696.
25. Crandall P.H. Standard en bloc anterior temporal lobectomy. In: Spencer S.S., Spencer D.D. Surgery for Epilepsy. Boston: Blackwell Scientific Publications; 1991:118-129.
26. Binder D.K., Schramm J. Resective surgical techniques: mesial temporal lobe epilepsy. In: Luders H., editor. Textbook of Epilepsy Surgery. London: Informa Healthcare; 2008:1081-1092.
27. Olivier A. Surgery of epilepsy: overall procedure. In: Apuzzo M.L.J., editor. Neurosurgical Aspects of Epilepsy. Park Ridge, IL: American Association of Neurological Surgeons; 1990:117-148.
28. Spencer D.D. Anteromedial temporal lobectomy: directing the surgical approach to the pathologic substrate. In: Spencer S.S., Spencer D.D. Surgery for Epilepsy. Boston, MA: Blackwell Scientific Publications; 1991:129-137.
29. Grossman R.G., Hamilton W.J. Temporal lobe operations for drug-resistant epilepsy. In: Schmidek H.H., Sweet W.H. Operative Neurosurgical Techniques: Indications, Methods, and Results. Philadelphia, PA: WB Saunders Company; 1995:1383-1393.
30. Ojemann G.A. Temporal lobectomy tailored to electrocorticography and functional mapping. In: Spencer S.S., Spencer D.D. Surgery for Epilepsy. Boston: Blackwell Scientific Publications; 1991:137-145.
31. Spencer D.D., Spencer S.S., Mattson R.H., et al. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery. 1984;15(5):667-671.
32. Maxwell R.E., Tummala R. Standard temporal lobectomy and transsylvian amygdalohippocampectomy. In: Winn H.R., editor. Youmans Neurological Surgery. Philadelphia, PA: WB Saunders; 2004:2605-2614.
33. Miles A.N., Ojemann G.A. Tailored resections for epilepsy. In: Winn H.R., editor. Youmans Neurological Surgery. Philadelphia, PA: WB Saunders; 2004:2615-2628.
34. Ojemann G.A. Intraoperative tailoring of temporal lobe resections. In: Engel J., editor. Surgical Treatment of the Epilepsies. New York, NY: Raven Press; 1993:481-488.
35. Spencer D.D., Doyle W.K. Temporal lobe operations for epilepsy: radical hippocampectomy. In: Schmidek H.H., Sweet W.H. Operative Neurosurgical Techniques: Indications, Methods, and Results. Philadelphia, PA: WB Saunders Company; 1995:1305-1316.
36. Immonen A., Jutila L., Kalviainen R., et al. Preoperative clinical evaluation, outline of surgical technique and outcome in temporal lobe epilepsy. Adv Tech Stand Neurosurg. 2004;29:87-132.
37. Olivier A. Transcortical selective amygdalohippocampectomy in temporal lobe epilepsy. Can J Neurol Sci. 2000;27(Suppl 1):S68-76.
38. Yasargil M.G., Microneurosurgery, Vol I;Stuttgart, Thieme, 1984.
39. Hori T., Tabuchi S., Kurosaki M., et al. Subtemporal amygdalohippocampectomy for treating medically intractable temporal lobe epilepsy. Neurosurgery. 1993;33(1):50-56.
40. Shimizu H., Suzuki I., Ishijima B. Zygomatic approach for resection of mesial temporal epileptic focus. Neurosurgery. 1989;25(5):798-801.
41. Park T.S., Bourgeois B.F., Silbergeld D.L., et al. Subtemporal transparahippocampal amygdalohippocampectomy for surgical treatment of mesial temporal lobe epilepsy. Technical note. J Neurosurg. 1996;85(6):1172-1176.
42. Engel J.Jr., Van Ness P., Rasmussen T., et al. Outcome with respect to epileptic seizures. In: Engel J., editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1993:609-621.
43. Ferguson S.M., Rayport M., Schell C.A. Life after surgery for temporolimbic seizures. Int Rev Neurobiol. 2006;76:87-116.
44. Spencer S.S., Berg A.T., Vickrey B.G., et al. Health-related quality of life over time since resective epilepsy surgery. Ann Neurol. 2007;62(4):327-334.
45. Tanriverdi T., Poulin N., Olivier A. Life 12 years after temporal lobe epilepsy surgery: a long-term, prospective clinical study. Seizure. 2008;17(4):339-349.
46. McLachlan R.S., Rose K.J., Derry P.A., et al. Health-related quality of life and seizure control in temporal lobe epilepsy. Ann Neurol. 1997;41(4):482-489.
47. Birbeck G.L., Hays R.D., Cui X., et al. Seizure reduction and quality of life improvements in people with epilepsy. Epilepsia. 2002;43(5):535-538.
48. Wiebe S., Blume W.T., Girvin J.P., et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311-318.
49. Kim R., Spencer D. Surgery for mesial temporal sclerosis. In: Schmidek H., Sweet W. Operative Neurosurgical Techniques. Philadelphia: WB Saunders; 2000:1436-1444.
50. Foldvary N., Nashold B., Mascha E., et al. Seizure outcome after temporal lobectomy for temporal lobe epilepsy: a Kaplan-Meier survival analysis. Neurology. 2000;54(3):630-634.
51. Elwes R.D., Dunn G., Binnie C.D., et al. Outcome following resective surgery for temporal lobe epilepsy: a prospective follow up study of 102 consecutive cases. J Neurol Neurosurg Psychiatr. 1991;54(11):949-952.
52. Berkovic S.F., McIntosh A.M., Kalnins R.M., et al. Preoperative MRI predicts outcome of temporal lobectomy: an actuarial analysis. Neurology. 1995;45(7):1358-1363.
53. Sperling M.R., O’Connor M.J., Saykin A.J., et al. Temporal lobectomy for refractory epilepsy. JAMA. 1996;276(6):470-475.
54. So E.L., Radhakrishnan K., Silbert P.L., et al. Assessing changes over time in temporal lobectomy: outcome by scoring seizure frequency. Epilepsy Res. 1997;27(2):119-125.
55. Bien C.G., Kurthen M., Baron K., et al. Long-term seizure outcome and antiepileptic drug treatment in surgically treated temporal lobe epilepsy patients: a controlled study. Epilepsia. 2001;42(11):1416-1421.
56. McIntosh A.M., Kalnins R.M., Mitchell L.A., et al. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004;127(Pt 9):2018-2030.
57. Behrens E., Schramm J., Zentner J., et al. Surgical and neurological complications in a series of 708 epilepsy surgery procedures. Neurosurgery. 1997;41(1):1-9.
58. Rydenhag B., Silander H.C. Complications of epilepsy surgery after 654 procedures in Sweden, September 1990-1995: a multicenter study based on the Swedish National Epilepsy Surgery Register. Neurosurgery. 2001;49(1):51-56.
59. Salanova V., Markand O., Worth R. Temporal lobe epilepsy surgery: outcome, complications, and late mortality rate in 215 patients. Epilepsia. 2002;43(2):170-174.
60. Tanriverdi T., Ajlan A., Poulin N., et al. Morbidity in epilepsy surgery: an experience based on 2449 epilepsy surgery procedures from a single institution. J Neurosurg. 2009;110(6):1111-1123.
61. Yam D., Nicolle D., Steven D.A., et al. Visual field deficits following anterior temporal lobectomy: long-term follow-up and prognostic implications. Epilepsia. 2010;51(6):1018-1023.
62. Fenwich P.B., Fenwick P.B. Psychiatric assessment and temporal lobectomy. Acta Neurol Scand Suppl. 1988;117:96-102.
63. Blumer D., Wakhlu S., Davies K., et al. Psychiatric outcome of temporal lobectomy for epilepsy: incidence and treatment of psychiatric complications. Epilepsia. 1998;39(5):478-486.
64. Ring H.A., Moriarty J., Trimble M.R. A prospective study of the early postsurgical psychiatric associations of epilepsy surgery. J Neurol Neurosurg Psychiatr. 1998;64(5):601-604.
65. Inoue Y., Mihara T. Psychiatric disorders before and after surgery for epilepsy. Epilepsia. 2001;42(Suppl 6):13-18.
66. Fenwick P., Blumer D., Caplan R., et al. Presurgical psychiatric assessment. In: Engel J., editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1993:649-661.
67. Crandall P. Postoperative management and criteria for evaluation. In: Purpura D., Penry J., Walter R. Advances in Neurology. New York: Raven Press; 1975:265-279.
68. Glosser G., Zwil A.S., Glosser D.S., et al. Psychiatric aspects of temporal lobe epilepsy before and after anterior temporal lobectomy. J Neurol Neurosurg Psychiatr. 2000;68(1):53-58.
69. Matsuura M. Indication for anterior temporal lobectomy in patients with temporal lobe epilepsy and psychopathology. Epilepsia. 2000;41(Suppl 9):39-42.
70. Naylor A.S., Bá R.-H., Kessing L., et al. Psychiatric morbidity after surgery for epilepsy: short-term follow up of patients undergoing amygdalohippocampectomy. J Neurol Neurosurg Psychiatr. 1994;57(11):1375-1381.
71. Quigg M., Broshek D.K., Heidal-Schiltz S., et al. Depression in intractable partial epilepsy varies by laterality of focus and surgery. Epilepsia. 2003;44(3):419-424.
72. Altshuler L., Rausch R., Delrahim S., et al. Temporal lobe epilepsy, temporal lobectomy, and major depression. J Neuropsychiatry Clin Neurosci. 1999;11(4):436-443.
73. Hermann B.P., Wyler A.R., Ackerman B., et al. Short-term psychological outcome of anterior temporal lobectomy. J Neurosurg. 1989;71(3):327-334.
74. Derry P.A., Rose K.J., McLachlan R.S. Moderators of the effect of preoperative emotional adjustment on postoperative depression after surgery for temporal lobe epilepsy. Epilepsia. 2000;41(2):177-185.
75. Jensen I., Larsen J.K. Mental aspects of temporal lobe epilepsy. Follow-up of 74 patients after resection of a temporal lobe. J Neurol Neurosurg Psychiatr. 1979;42(3):256-265.
76. Mace C.J., Trimble M.R. Psychosis following temporal lobe surgery: a report of six cases. J Neurol Neurosurg Psychiatr. 1991;54(7):639-644.
77. Christodoulou C., Koutroumanidis M., Hennessy M.J., et al. Postictal psychosis after temporal lobectomy. Neurology. 2002;59(9):1432-1435.
78. Helmstaedter C., Elger C.E. Cognitive consequences of two-thirds anterior temporal lobectomy on verbal memory in 144 patients: a three-month follow-up study. Epilepsia. 1996;37(2):171-180.
79. Jones-Gotman M., Zatorre R.J., Olivier A., et al. Learning and retention of words and designs following excision from medial or lateral temporal-lobe structures. Neuropsychologia. 1997;35(7):963-973.
80. Milner B., Penfield W. The effect of hippocampal lesions on recent memory. Trans Am Neurol Assoc (80th Meeting). 1955:42-48.
81. Martin R.C., Sawrie S.M., Roth D.L., et al. Individual memory change after anterior temporal lobectomy: a base rate analysis using regression-based outcome methodology. Epilepsia. 1998;39(10):1075-1082.
82. Clusmann H., Schramm J., Kral T., et al. Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. J Neurosurg. 2002;97(5):1131-1141.
83. Trenerry M.R., Jack C.R., Ivnik R.J., et al. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993;43(9):1800-1805.
84. Hermann B.P., Seidenberg M., Dohan F.C., et al. Reports by patients and their families of memory change after left anterior temporal lobectomy: relationship to degree of hippocampal sclerosis. Neurosurgery. 1995;36(1):39-44.
85. Davies K.G., Bell B.D., Bush A.J., et al. Naming decline after left anterior temporal lobectomy correlates with pathological status of resected hippocampus. Epilepsia. 1998;39(4):407-419.
86. Chelune G.J. Hippocampal adequacy versus functional reserve: predicting memory functions following temporal lobectomy. Arch Clin Neuropsychol. 1995;10(5):413-432.
87. Davies K.G., Maxwell R.E., Beniak T.E., et al. Language function after temporal lobectomy without stimulation mapping of cortical function. Epilepsia. 1995;36(2):130-136.
88. Hermann B.P., Wyler A.R., Somes G. Language function following anterior temporal lobectomy. J Neurosurg. 1991;74(4):560-566.
89. Hermann B., Wyler A. Comparative results of dominant temporal lobectomy under general or local anesthesia: language outcome. Journal of Epilepsy. 1988;1(3):127-134.
90. Langfitt J.T., Rausch R. Word-finding deficits persist after left anterotemporal lobectomy. Arch Neurol. 1996;53(1):72-76.
91. Stafiniak P., Saykin A.J., Sperling M.R., et al. Acute naming deficits following dominant temporal lobectomy: prediction by age at 1st risk for seizures. Neurology. 1990;40(10):1509-1512.
92. McMillan T.M., Powell G.E., Janota I., et al. Relationships between neuropathology and cognitive functioning in temporal lobectomy patients. J Neurol Neurosurg Psychiatr. 1987;50(2):167-176.
93. York M.K., Rettig G.M., Grossman R.G., et al. Seizure control and cognitive outcome after temporal lobectomy: a comparison of classic Ammon’s horn sclerosis, atypical mesial temporal sclerosis, and tumoral pathologies. Epilepsia. 2003;44(3):387-398.
94. Phillips N.A., McGlone J. Grouped data do not tell the whole story: individual analysis of cognitive change after temporal lobectomy. J Clin Exp Neuropsychol. 1995;17(5):713-724.
95. Novelly R.A., Augustine E.A., Mattson R.H., et al. Selective memory improvement and impairment in temporal lobectomy for epilepsy. Ann Neurol. 1984;15(1):64-67.
96. Hermann B.P., Wyler A.R., Richey E.T. Wisconsin Card Sorting Test performance in patients with complex partial seizures of temporal-lobe origin. J Clin Exp Neuropsychol. 1988;10(4):467-476.
97. Rausch R., Crandall P.H. Psychological status related to surgical control of temporal lobe seizures. Epilepsia. 1982;23(2):191-202.
98. Olivier A. Risk and benefit in the surgery of epilepsy: complications and positive results on seizures tendency and intellectual function. Acta Neurol Scand. 1988;117(Suppl):114-121.
99. Wachi M., Tomikawa M., Fukuda M., et al. Neuropsychological changes after surgical treatment for temporal lobe epilepsy. Epilepsia. 2001;42(Suppl 6):4-8.
100. Cascino G.D., Trenerry M.R., Jack C.R., et al. Electrocorticography and temporal lobe epilepsy: relationship to quantitative MRI and operative outcome. Epilepsia. 1995;36(7):692-696.
101. Hermann B., Davies K., Foley K., et al. Visual confrontation naming outcome after standard left anterior temporal lobectomy with sparing versus resection of the superior temporal gyrus: a randomized prospective clinical trial. Epilepsia. 1999;40(8):1070-1076.
102. Kanner A.M., Kaydanova Y., deToledo-Morrell L., et al. Tailored anterior temporal lobectomy. Relation between extent of resection of mesial structures and postsurgical seizure outcome. Arch Neurol. 1995;52(2):173-178.
103. Renowden S.A., Matkovic Z., Adams C.B., et al. Selective amygdalohippocampectomy for hippocampal sclerosis: postoperative MR appearance. AJNR Am J Neuroradiol. 1995;16(9):1855-1861.
104. Son E.I., Howard M.A., Ojemann G.A., et al. Comparing the extent of hippocampal removal to the outcome in terms of seizure control. Stereotact Funct Neurosurg. 1994;62(1-4):232-237.
105. McKhann G.M., Schoenfeld-McNeill J., Born D.E., et al. Intraoperative hippocampal electrocorticography to predict the extent of hippocampal resection in temporal lobe epilepsy surgery. J Neurosurg. 2000;93(1):44-52.
106. Hennessy M.J., Elwes R.D., Binnie C.D., et al. Failed surgery for epilepsy. A study of persistence and recurrence of seizures following temporal resection. Brain. 2000;123(Pt 12):2445-2466.
107. Salanova V., Markand O., Worth R. Temporal lobe epilepsy: analysis of failures and the role of reoperation. Acta Neurol Scand. 2005;111(2):126-133.
108. Nayel M.H., Awad I.A., Luders H. Extent of mesiobasal resection determines outcome after temporal lobectomy for intractable complex partial seizures. Neurosurgery. 1991;29(1):55-60.
109. Awad I.A., Katz A., Hahn J.F., et al. Extent of resection in temporal lobectomy for epilepsy. I. Interobserver analysis and correlation with seizure outcome. Epilepsia. 1989;30(6):756-762.
110. Bonilha L., Yasuda C.L., Rorden C., et al. Does resection of the medial temporal lobe improve the outcome of temporal lobe epilepsy surgery? Epilepsia. 2007;48(3):571-578.
111. Wyler A.R., Hermann B.P., Somes G. Extent of medial temporal resection on outcome from anterior temporal lobectomy: a randomized prospective study. Neurosurgery. 1995;37(5):982-990.
112. Binnie C.D., Alarcon G., Elwes R.D., et al. Role of ECoG in “en bloc” temporal lobe resection: the Maudsley experience. Electroencephalogr Clin Neurophysiol Suppl. 1998;48:17-23.
113. McBride M.C., Binnie C.D., Janota I., et al. Predictive value of intraoperative electrocorticograms in resective epilepsy surgery. Ann Neurol. 1991;30(4):526-532.
114. Tran T.A., Spencer S.S., Marks D., et al. Significance of spikes recorded on electrocorticography in nonlesional medial temporal lobe epilepsy. Ann Neurol. 1995;38(5):763-770.
115. Schwartz T.H., Bazil C.W., Walczak T.S., et al. The predictive value of intraoperative electrocorticography in resections for limbic epilepsy associated with mesial temporal sclerosis. Neurosurgery. 1997;40(2):302-309.
116. Bengzon A.R., Rasmussen T., Gloor P., et al. Prognostic factors in the surgical treatment of temporal lobe epileptics. Neurology. 1968;18(8):717-731.
117. So N., Olivier A., Andermann F., et al. Results of surgical treatment in patients with bitemporal epileptiform abnormalities. Ann Neurol. 1989;25(5):432-439.
118. Fiol M.E., Gates J.R., Torres F., et al. The prognostic value of residual spikes in the postexcision electrocorticogram after temporal lobectomy. Neurology. 1991;41(4):512-516.
119. Stefan H., Quesney L.F., Abou-Khalil B., et al. Electrocorticography in temporal lobe epilepsy surgery. Acta Neurol Scand. 1991;83(2):65-72.
120. Salanova V., Andermann F., Rasmussen T., et al. The running down phenomenon in temporal lobe epilepsy. Brain. 1996;119(Pt 3):989-996.
121. Tuunainen A., Nousiainen U., Mervaala E., et al. Postoperative EEG and electrocorticography: relation to clinical outcome in patients with temporal lobe surgery. Epilepsia. 1994;35(6):1165-1173.
122. Kanazawa O., Blume W.T., Girvin J.P. Significance of spikes at temporal lobe electrocorticography. Epilepsia. 1996;37(1):50-55.
123. Radhakrishnan K., So E.L., Silbert P.L., et al. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: a multivariate study. Neurology. 1998;51(2):465-471.
124. Rasmussen T.B. Surgical treatment of complex partial seizures: results, lessons, and problems. Epilepsia. 1983;24(Suppl 1):S65-S76.
125. Wyllie E., Lüders H., Morris H.H., et al. Clinical outcome after complete or partial cortical resection for intractable epilepsy. Neurology. 1987;37(10):1634-1641.
126. Fenyes I., Zoltan L., Fenyes G. Temporal epilepsies with deep-seated epileptogenic foci. Postoperative course. Arch Neurol. 1961;4:559-571.
127. Devinsky O., Canevini M., Sato S., et al. Quantitative electrocorticography in patients undergoing temporal lobectomy. Journal of Epilepsy. 1992;5(3):178-185.
128. Graf M., Niedermeyer E., Schiemann J., et al. Electrocorticography: information derived from intraoperative recordings during seizure surgery. Clin Electroencephalogr. 1984;15(2):83-91.
129. Chatrian G.E., Tsai M.L., Temkin N.R., et al. Role of the ECoG in tailored temporal lobe resection: the University of Washington experience. Electroencephalogr Clin Neurophysiol. 1998;48(Suppl):24-43.
130. Ojemann G.A., Dodrill C.B. Verbal memory deficits after left temporal lobectomy for epilepsy. Mechanism and intraoperative prediction. J Neurosurg. 1985;62(1):101-107.
131. Katz A., Awad I.A., Kong A.K., et al. Extent of resection in temporal lobectomy for epilepsy. II. Memory changes and neurologic complications. Epilepsia. 1989;30(6):763-771.
132. Wolf R.L., Ivnik R.J., Hirschorn K.A., et al. Neurocognitive efficiency following left temporal lobectomy: standard versus limited resection. J Neurosurg. 1993;79(1):76-83.
133. Hermann B.P., Perrine K., Chelune G.J., et al. Visual confrontation naming following left anterior temporal lobectomy: a comparison of surgical approaches. Neuropsychology. 1999;13(1):3-9.
134. Ojemann G., Ojemann J., Lettich E., et al. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71(3):316-326.
135. Arruda F., Cendes F., Andermann F., et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol. 1996;40(3):446-450.
136. Pauli E., Pickel S., Schulemann H., et al. Neuropsychologic findings depending on the type of the resection in temporal lobe epilepsy. Adv Neurol. 1999;81:371-377.
137. Paglioli E., Palmini A., Portuguez M., et al. Seizure and memory outcome following temporal lobe surgery: selective compared with nonselective approaches for hippocampal sclerosis. J Neurosurg. 2006;104(1):70-78.
138. Lutz M.T., Clusmann H., Elger C.E., et al. Neuropsychological outcome after selective amygdalohippocampectomy with transsylvian versus transcortical approach: a randomized prospective clinical trial of surgery for temporal lobe epilepsy. Epilepsia. 2004;45(7):809-816.
139. Clusmann H., Kral T., Gleissner U., et al. Analysis of different types of resection for pediatric patients with temporal lobe epilepsy. Neurosurgery. 2004;54(4):847-859.
140. Tanriverdi T., Olivier A. Cognitive changes after unilateral cortico-amygdalohippocampectomy unilateral selective–amygdalohippocampectomy mesial temporal lobe epilepsy. Turk Neurosurg. 2007;17(2):91-99.
141. Gleissner U., Helmstaedter C., Schramm J., et al. Memory outcome after selective amygdalohippocampectomy: a study in 140 patients with temporal lobe epilepsy. Epilepsia. 2002;43(1):87-95.
142. Schramm J. Temporal lobe epilepsy surgery and the quest for optimal extent of resection: a review. Epilepsia. 2008;49(8):1296-1307.