Chapter 50 Telescreening for Diabetic Retinopathy
Introduction
The criteria for human diseases amenable to screening approaches were defined by the World Health Organization in 19681 and diabetic retinopathy fulfills all of these. Visual impairment due to diabetic retinopathy is a significant health problem; however, it has a recognizable presymptomatic stage.2 The DCCT and the UKPDS established that intensive diabetes management to obtain near-euglycemic control can prevent and delay the progression of diabetic retinopathy in patients with diabetes.3,4 Timely laser photocoagulation therapy can also prevent loss of vision in a large proportion of patients with sight-threatening diabetic retinopathy.5 Screening for diabetic retinopathy saves vision at a relatively low cost, which has been demonstrated in various studies.6,7 The American Academy of Ophthalmology recommends annual dilated eye examinations beginning at the time of diagnosis for patients with type II diabetes.2 For those with type I diabetes, the recommendation is retinal examination 3–5 years after diagnosis, with annual exams thereafter.2 The barriers for successful screening are numerous and include the high cost of care, poor awareness levels, lack of symptoms in the early stages of disease, socioeconomic factors and poor geographical access to care.8 Current screening programs for diabetic retinopathy are either ophthalmologist-based (with actual presence of the ophthalmologist at the site of screening) or ophthalmologist-led (no ophthalmologist at the site of screening). Table 50.1 summarizes the key differences between the two models. Telemedicine for retinopathy screening is an ophthalmologist-led screening model, which may be a logical potential alternative for patients who have been noncompliant with the traditional face-to-face examination by an ophthalmologist. Telemedicine is the exchange of medical data by electronic telecommunications technology allowing a patient’s medical problems to be evaluated, monitored, and possibly treated while the patient and physician are located at sites physically remote from each other.9 Ophthalmology is uniquely suited for telemedicine as it is a highly visual and image intensive specialty and digital imagery is easily transmitted by electronic means. Remote assessment for diabetic retinopathy provides an ideal model for telehealth screening initiatives and, in fact, has become one of the most common uses for telemedicine in ophthalmology.
Table 50.1 Differences between the ophthalmologist-led and ophthalmologist-based models for screening for diabetic retinopathy
| Ophthalmologist-led model (Telescreening) | Ophthalmologist-based model | |
|---|---|---|
| Brief description | Paramedical staff acquire data/images, which are then transferred for interpretation by ophthalmologist | Screening is performed by ophthalmologist |
| Feasibility | Yes, with less human resources | Needs trained expert |
| Maintenance | Required | Not required |
| Capital expenditure | More | Less |
| Revenue expenditure | Less | More |
| Interobserver bias | Less | More |
| Digital photo archiving | Yes | No |
| Acceptance by community | Yes | Yes |
Guidelines for telescreening program
American Telemedicine Association telehealth practice recommendations for diabetic retinopathy
American Telemedicine Association (ATA), Ocular Telehealth Special Interest Group, and the National Institutes of Standards and Technology Working Group, established the telescreening guidelines for diabetic retinopathy.10 The ATA recommends that telehealth programs for diabetic retinopathy should demonstrate an ability to compare favorably with ETDRS film or digital photography. For screening programs with low thresholds for referral, the International Clinical Diabetic Retinopathy Disease Severity Scale may be used in place of ETDRS scales, however, protocols should state the reference standard used for validation and relevant datasets used for comparison.
Category 1: The program allows identification of patients who have no or minimal diabetic retinopathy and distinguishes them from those who have more than minimal diabetic retinopathy.
Category 2: The program allows identification of patients who do not have sight-threatening diabetic retinopathy and distinguishes them from those who have potentially sight-threatening diabetic retinopathy.
Steps of telescreening
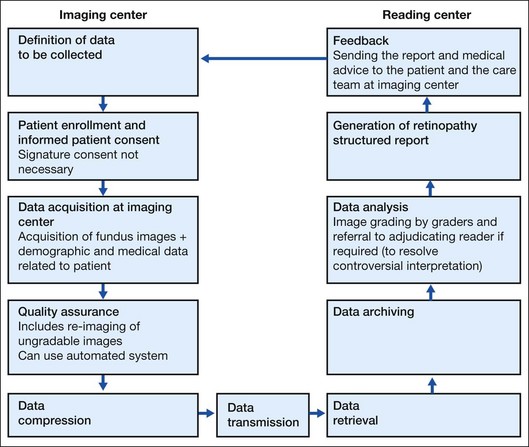
The flow of steps of the telescreening process is diagrammed in Fig. 50.1. In brief, patient enrollment is performed after defining the data to be collected. Since ocular telescreening services for diabetic retinopathy satisfy the criteria of low risk telehealth procedures and are within commonly accepted standards of practice, signature consent may not be required. However, practitioners should provide patients with information about the telescreening program they would reasonably want to know, including differences between care delivered using ocular telehealth approaches versus traditional face-to-face encounters, and a description of what is to be done at the patient’s site and the remote site. The data collected includes fundus images, along with patient examination findings (identification, demographic, and medical information) and some morphological information that is used to make a clinical decision. Fundus images of both eyes of the patient are acquired under a fixed, predetermined imaging protocol. These images are taken by a trained technician using a fundus camera. Due to various factors, the quality of the acquired images may be below the grading standard, thus not providing any meaningful information for examination by the reader. This can be addressed by employing an automatic image quality assessment module. An automatic image quality assessment module will ensure that the images transmitted for diagnosis conform to prescribed gradability standards. During the quality assurance process, the gradable images are selected for compression, whereas the identification of poor quality images can trigger reimaging by the technician. The patient data comprising the clinical data and the fundus images are compressed to make them suitable for low-bandwidth network connectivity. The patient data are transmitted to the servers via the Internet or satellite. At the reading center, the images are graded for presence of retinal lesions and the determination of a diabetic retinopathy level; referred to “next level” graders if necessary; and a retinopathy structured report is generated. Only qualified readers should perform retinal image grading and interpretation. If a reader is not a licensed eye care provider, specific training is required. A licensed, qualified eye care provider with expertise in diabetic retinopathy and familiarity with telescreening program technology should supervise the readers. An adjudicating reader (an ophthalmologist with special qualifications in diabetic retinopathy by training or experience) may resolve discrepant interpretations. Image processing algorithms should undergo rigorous clinical validation. A report comprising the findings, the results and the medical advice given by the expert is made available to the patient and the care team at the remote site through an accessible interface.
Technical considerations
Image acquisition
The gold standard for telescreening is the ETDRS 7 mydriatic standard field 35 mm stereoscopic color fundus photographs.11 However, more practical alternatives, such as digital fundus photography12,13 and nonmydriatic fundus photography,14,15 have been evaluated. Digital imaging has the advantage of faster and easier acquisition, transmission, and storage. Several investigators have reported a high level of correlation between stereoscopic digital imaging and slide film for the identification of most features of diabetic retinopathy.12,13
Regarding nonmydriatic fundus photography, a higher rate of unreadable photographs has been reported through undilated versus dilated pupils.14,15 Diabetic persons often have smaller pupils and a greater incidence of cataracts, which may limit image quality if the procedure is performed through an undilated pupil. Pupil dilation using 0.5% tropicamide is associated with a minimal risk of angle closure glaucoma. Programs using pupil dilation should have a defined protocol to recognize and address this potential complication.
The unsatisfactory performance of nonmydriatic photography has led to the concept of “targeted mydriasis,” offering mydriasis only to a preselected group of patients, in whom undilated photography is known to produce dismal results.15 However, the exact “target” remains to be defined. Based on ROC curve analysis, Raman et al.16 predetermined the cutoff values for “target mydriasis” groups as vision <6/12 (20/40 Snellen equivalent) and age >59 years. Staged mydriasis is another option.17 In this model, a nonmydriatic single digital photograph for screening is taken. If an unsatisfactory nonmydriatic photograph is obtained, the patient undergoes immediate pupillary dilation with 1% tropicamide and the photograph is then repeated. Using this protocol, 75–80% of patients do not require mydriasis.
Compression
Compression may be used if algorithms have undergone clinical validation. Image data can be compressed using a variety of standards, including JPEG, JPEG Lossless, JPEG 2000, and Run-length encoding (RLE). The International Standards Organization (ISO/IEC JTC1/SC2/WG10) has prepared an International Standard, ISO/IS-15444–1 (JPEG 2000 Part 1), for the digital compression and coding of continuous-tone still images. This standard is known as the JPEG 2000 Standard. Digital Imaging and Communication in Medicine (DICOM) recognizes JPEG and JPEG 2000 for lossy compression of medical images.18 ATA recommends that the compression types and ratios should be periodically reviewed to ensure appropriate clinical image quality and diagnostic accuracy. Some studies have attempted to look at the effect of various levels of compression on the quality of the image with both subjective and objective parameters.19,20 The level of acceptable compression ranges from 1 : 28 to 1 : 52.19,20
Data transfer, archiving, and retrieval
The described telemedicine models reported earlier used the Internet to transmit images.21,22 In rural areas and mobile clinics, satellite transmission is a more preferred option because of poor infrastructure. A variety of technologies are available for data communication and transfer. Telescreening programs should determine specifications for transmission technologies best suited to their needs.
Operational considerations
Detection of diabetic retinopathy and macular edema
As per ATA recommendation, both the ETDRS and International Clinical Diabetic Retinopathy Disease Severity Scales may be used for classifying diabetic retinopathy and macular edema. Nonstereo imaging methods can detect diabetic retinopathy quite well but diabetic macular thickening cannot be detected as reliably. Hence, all nonstereo imaging methods need additional information about retinal thickness for an accurate assessment of macular edema. The retinal thickness analyzer (RTA), which combines two imaging modalities – a wide-angle, red-free black-on-white fundus photograph and a detailed map of retinal thickness at the posterior pole – had mean 93% sensitivity for diagnosis of proliferative diabetic retinopathy and 100% for macular edema when compared with clinical examination.23
Role of the reading center to grade retinal images
Pathways of grading
Pathway 1: Disease/no disease grading
This involves three stages of grading prior to any referral to an arbitration-level grader:
Stage 1: The grader assesses patient image sets to classify into disease and no disease without grading the level of disease. Urgent referrals should be passed for immediate assessment by an ophthalmologist.
Stage 2: A random 10% of the patient’s no disease image sets, together with all the disease image sets are reviewed for an initial full disease grade by a different grader accredited to carry out that level of grading. That second grader should not see the result of the first grader prior to grading.
Stage 3: This is carried out in all cases where an initial full disease grade indicates evidence of diabetic retinopathy in the eye. All referable image sets are reassessed by a different grader who carries out a second full disease grade on these images masked to the initial grading.
Pathway 2: Full disease grading
This involves two stages of grading prior to any referral to an arbitration grader:
Stage 1: A grader carries out a full disease grade on all image sets. Urgent referrals should be passed to the grading center for immediate assessment by an ophthalmologist.
Stage 2: A different masked grader will assess a random 10% of the images where no diabetic retinopathy is evident and carry out a second full grade on all the disease image sets from the stage 1 grade.
Arbitration grade
To make best use of limited resources, it has been proposed that the assessment of image quality and the presence or absence of any diabetic retinopathy could be performed by relatively inexperienced “disease/no disease” graders after a short period of training. Experienced “full disease” graders would then identify patients, deemed to have retinopathy, for referral to an ophthalmologist.24
Reading personnel
The gold standard of image reading by ophthalmologists is impractical in rural areas. Ruamviboonsuk et al.25 evaluated the interobserver differences among the nonphysician personnel (local ophthalmic photographers and certified ophthalmic nurses, who attended an intensive instruction course for this screening program) and ophthalmologists (retina specialists and general ophthalmologists without additional training), in the interpretation of single-field digital fundus images for diabetic retinopathy screening. Retinal specialists had the best agreement among the groups. Photographers were more reliable than the nurses. The authors concluded that the retina specialists should be effective interpreters without additional training, general ophthalmologists may need more training, but nonphysician personnel must have comprehensive training.
Handling of ungradable images
ATA guidelines recommend that the inability to obtain or read images should be considered a positive finding and patients with unobtainable or unreadable images should be promptly re-imaged or referred for evaluation by an eye care specialist. Authors from the Joslin Vision Network (JVN) reported that of those images that were judged ungradable, a large proportion had pathology that required referral for comprehensive examination.26 In the Gloucestershire Study, 3.7% of patients had unassessable images (including those with cataract), of whom 10.3% had referable retinopathy.14
While nonmydriatic photography screening is being successfully used, several factors have been reported to result in ungradable images in nonmydriatic retinal photography. Increasing age is an important factor.27 Media opacity such as cataract and small pupil are other major factors. Scanlon et al.27 suggested that a 20% failure rate for nonmydriatic photography might be acceptable. They also supported the use of nonmydriatic photography for the group of individuals <50 years of age who are at the lowest risk of ungradable images, if the screening programs are directed towards the detection of sight-threatening diabetic retinopathy.
Quality assurance
In 2000, the UKNSC stressed the integration of quality assurance as a core feature of telescreening programs for diabetic retinopathy and proposed the criteria and minimal/achievable standards for each quality assurance objective.28 Since then, other ongoing quality assurance programs have published their methods and outcomes.29,30 Different screening programs have different criteria for image regrading, resulting in different numbers of retinal photographs that need to be regraded for quality assurance (6% to 46%).31
There are two categories of quality assurance:32
(a) Internal quality assurance that is integrated as part of the day-to-day workflow in a screening program measured against national standards.
(b) External quality assurance which has three main functions: the monitoring of ongoing program performance against the quality standards, the organization of peer-review visits, and the administration of an external proficiency testing system for all graders.
There is international consensus that screening programs for diabetic retinopathy should achieve at least 80% sensitivity, 95% specificity, and <5% technical failure rates.33 The quality assurance group of the National Screening Committee has recommended that quality assurance should involve the second examination of all images initially reported to have any diabetic retinopathy, together with 10% of the negative images, independently as part of the internal quality assurance system.34
The ATA and UKNSC have established the national standards for quality assurance for a diabetic retinopathy screening program. ATA has specified major categories of performance to be evaluated, as applicable to the program, at the level of the originating site and at the reading center. The UKNSC has provided 19 standards or parameters for quality assurance. Several authors have suggested further measures. Leese et al.31 recommended the use of automated grading systems running in parallel with manual grading; and concentrating quality assurance in the smaller number of patients with high-risk nonproliferative disease.
Evaluating telescreening programs
Efficacy
Telescreening has been shown to detect diabetic retinopathy and macular edema with a reasonably high sensitivity and specificity.9,35 Whited et al.35 reviewed the available literature and noted that the sensitivity and specificity values ranged from 50% to 93% for detection of diabetic retinopathy. Similar high efficacy was reported on comparing teleophthalmology for macular edema detection to both gold standards, i.e. slit-lamp biomicroscopy and stereoscopic photography.35
Patient satisfaction
Since telescreening involves remote care without evaluation by the doctor in person, there are concerns regarding lack of satisfaction among patients. Studies have shown, however, that telescreening has equal, if not better, satisfaction than in-person evaluation by a doctor.36,37 Paul et al. assessed patient satisfaction levels and factors influencing satisfaction during teleophthalmology consultation in India using a patient satisfaction questionnaire. He found that 37.34% of the patients felt that telescreening is more satisfying than an in-person evaluation, and 60% felt that both models are equally satisfying. It was also noted that patients who asked questions during the screening were 2.18 times more likely to be satisfied with teleophthalmology than those who did not.
Cost-effectiveness
Bjorvig et al.,38 in an economic analysis, concluded that telemedicine was a less costly option for screening in places with higher patient workloads. Telemedicine was also proven to be cost-effective in the prison populations by Aoki et al.,39 where it may have special utility due to costs and safety issues associated with transporting prisoners.
Gomez-Ulla et al.6 did a comparative cost analysis of diabetic retinopathy telescreening versus standard ophthalmoscopy, from both Public Healthcare System (PHS) and patient perspectives. The authors concluded that from the PHS perspective, direct fundus examination is less costly than telescreening owing to the higher capital costs required for the purchase of digital imaging equipment. From a global perspective, however, the digital imaging alternative is more convenient because the travel cost and loss of income for the patient are lower.
Recently, Jones et al.7 reviewed the evidence available on cost-effectiveness. They concluded that telemedicine is cost-effective for retinopathy screening in remote and rural communities and other groups with travel difficulties and the cost-effectiveness increases with an increase in patient workload.
Advances in telescreening
Automated retinal image analysis
Over the past decade several attempts have been made to either semi-automate or fully-automate retinal image analysis. Tools have been developed for analyzing and enhancing the image quality (correction of illumination, increasing image contrast, histogram equalization, vessel segmentation, edge sharpening, and image deconvolution), and for providing automated identification of pathologic retinal lesions (neural networks, region growing, morphological analysis, and classification algorithms).40 Automated identification of retinal lesions can identify the absence or presence of diabetic retinopathy based on the detection of microaneurysms and dot hemorrhages (dark lesions),41 or can detect referable retinopathy based on the detection of exudates (bright lesions) and blot hemorrhages (dark lesions).42
Winder et al.40 conducted a structured survey of algorithms for the automatic detection of retinopathy in digital color retinal images. The authors pointed out the need for clear guidelines and goals in image processing research in order to avoid producing results that are difficult to compare in terms of the success of the algorithms or techniques.
1 Wilson JMG, Jungner G. Principles and practice of screening for disease. WHO Chronicle. 1968;22:473.
2 American Diabetes Association. Standards of medical care in diabetes – 2008. Diabetes Care. 2008;31(Suppl 1):S12–S54.
3 The DCCT Research Group. The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. Arch Ophthalmol. 1995;113:36–51.
4 UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
5 Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation treatment for diabetic retinopathy, ETDRS report number 9. Ophthalmology. 1991;98:766–785.
6 Gomez-Ulla F, Alonso F, Aibar B, et al. A comparative cost analysis of digital fundus imaging and direct fundus examination for assessment of diabetic retinopathy. Telemed J E Health. 2008;14:912–918.
7 Jones S, Edwards RT. Diabetic retinopathy screening: a systematic review of the economic evidence. Diabet Med. 2010;27:249–256.
8 Hazin R, Barazi MK, Summerfield M. Challenges to establishing nationwide diabetic retinopathy screening programs. Curr Opin Ophthalmol. 2011;22:174–179.
9 Zimmer-Galler IE, Zeimer R. Telemedicine in diabetic retinopathy screening. Ophthalmol Clin. 2009(Spring);49:75–86.
10 Cavallerano J, Lawrence MG, Zimmer-Galler I, et al. Telehealth practice recommendations for diabetic retinopathy. American Telemedicine Association, Ocular Telehealth Special Interest Group; National Institute of Standards and Technology Working Group. Telemed J E Health. 2004;10:469–482.
11 Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786–806.
12 Tennant MT, Greve MD, Rudnisky CJ, et al. Identification of diabetic retinopathy by stereoscopic digital imaging via teleophthalmology: a comparison to slide film. Can J Ophthalmology. 2001;36:187–196.
13 Liesenfeld B, Kohner E, Piehlmeier W, et al. A telemedical approach to the screening of diabetic retinopathy: digital fundus photography. Diabetes Care. 2000;23:345–348.
14 Scanlon PH, Malhotra R, Thomas G, et al. The effectiveness of screening for diabetic retinopathy by digital imaging photography and technician ophthalmoscopy. Diabet Med. 2003;20:467–474.
15 Murgatroyd H, Ellingford A, Cox A, et al. Effect of mydriasis and different field strategies on digital image screening of diabetic eye disease. Br J Ophthalmol. 2004;88:920–924.
16 Raman R, Rani PK, Mahajan S, et al. The tele-screening model for diabetic retinopathy: evaluating the influence of mydriasis on the gradability of a single-field 45 degrees digital fundus image. Telemed J E Health. 2007;13:597–602.
17 Murgatroyd H, Cox A, Ellingford A, et al. Can we predict which patients are at risk of having an ungradeable digital image for screening for diabetic retinopathy? Eye (Lond). 2008;22:344–348.
18 Digital Imaging and Communications in Medicine (DICOM) Supplement 61:JPEG 2000 Transfer Syntaxes. Available at ftp://medical.nema.org/medical/dicom/final/sup61_ft.pdf
19 Eikelboom RH, Yogesan K, Barry CJ, et al. Methods and limits of digital image compression of retinal images for telemedicine. Invest Ophthalmol Vis Sci. 2000;41:1916–1924.
20 Newsom RSB, Clover A, Costen MTJ, et al. Effect of digital image compression on screening for diabetic retinopathy. Br J Ophthalmol. 2001;85:799–802.
21 Luzio S, Hatcher S, Zahlmann G, et al. Feasibility of using the TOSCA telescreening procedures for diabetic retinopathy. Diabet Med. 2004;21:1121–1128.
22 Aiello LM, Bursell SE, Cavallerano J, et al. Joslin Vision Network Validation Study: pilot image stabilization phase. J Am Optom Assoc. 1998;69:699–710.
23 Neubauer AS, Welge-Lüssen UC, Thiel MJ, et al. Tele-screening for diabetic retinopathy with the retinal thickness analyzer. Diabetes Care. 2003;26:2890–2897.
24 Harding S, Greenwood R, Aldington S, et al. Grading and disease management in national screening for diabetic retinopathy in England and Wales. Diabet Med. 2003;20:965–971.
25 Ruamviboonsuk P, Teerasuwanajak K, Tiensuwan M, et al. Interobserver agreement in the interpretation of single-field digital fundus images for diabetic retinopathy screening. Thai Screening for Diabetic Retinopathy Study Group. Ophthalmology. 2006;113:826–832.
26 Cavallerano AA, Cavallerano JD, Katalinic P, et al. Use of Joslin Vision Network digital-video nonmydriatic retinal imaging to assess diabetic retinopathy in a clinical program. Retina. 2003;23:215–223.
27 Scanlon PH, Foy C, Malhotra R, et al. The influence of age, duration of diabetes, cataract, and pupil size on image quality in digital photographic retinal screening. Diabetes Care. 2005;28:2448–2453.
28 Garvican L, Clowes J, Gillow T. Preservation of sight in diabetes: developing a national risk reduction programme. Diabet Med. 2000;17:627–634.
29 Arun CS, Young D, Batey D, et al. Establishing ongoing quality assurance in a retinal screening programme. Diabet Med. 2006;23:629–634.
30 Schneider S, Aldington SJ, Kohner EM, et al. Quality assurance for diabetic retinopathy telescreening. Diabet Med. 2005;22:794–802.
31 Leese GP, Ellis JD. Quality assurance for diabetic retinal screening. Diabet Med. 2007;24:579–581.
32 UK National Screening Committee [Internet]. Gloucester, UK: NHS Diabetic Eye Screening Programme; January 2012. [cited 2012 4 Feb]. Workbook 4.4. Essential elements in developing a diabetic retinopathy screening programme. Available at http://diabeticeye.screening.nhs.uk/workbook
33 Taylor R, Broadbent DM, Greenwood R, et al. Mobile retinal screening in Britain. Diabet Med. 1998;15:344–347.
34 Garvican L, Scanlon PH. A pilot quality assurance scheme for diabetic retinopathy risk reduction programmes. Diabet Med. 2004;21:1066–1074.
35 Whited JD. Accuracy and reliability of teleophthalmology for diagnosing diabetic retinopathy and macular edema: a review of the literature. Diabetes Technol Ther. 2006;8:102–111.
36 Luzio S, Hatcher S, Zahlmann G, et al. Feasibility of using the TOSCA telescreening procedures for diabetic retinopathy. Diabetic Med. 2004;21:1121–1128.
37 Paul PG, Raman R, Rani PK, et al. Patient satisfaction levels during teleophthalmology consultation in rural South India. Telemed J E Health. 2006;12:571–578.
38 Bjorvig S, Johansen MA, Fossen K. An economic analysis of screening for diabetic retinopathy. J Telemed Telecare. 2002;8:32–35.
39 Aoki N, Dunn K, Fukui T, et al. Costeffectiveness analysis of telemedicine to evaluate diabetic retinopathy in a prison population. Diabetes Care. 2004;27:1095–1101.
40 Winder RJ, Morrow PJ, McRitchie IN, et al. Algorithms for digital image processing in diabetic retinopathy. Comput Med Imaging Graph. 2009;33:608–622.
41 Philip S, Fleming AD, Goatman KA, et al. The efficacy of automated “disease/no disease” grading for diabetic retinopathy in a systematic screening programme. Br J Ophthalmol. 2007;91:1512–1517.
42 Fleming AD, Goatman KA, Philip S, et al. The role of haemorrhage and exudate detection in automated grading of diabetic retinopathy. Br J Ophthalmol. 2010;94:706–711.