CHAPTER 64 Tailored Resections for Epilepsy
Although the first operations performed for the treatment of medically intractable epilepsy were obviously directed by the specific findings in individual patients1,2 and by the large formative experience of Penfield and Jasper in using electrocorticography (ECoG) to guide temporal resection,3 the majority of surgical interventions that have been performed since have been fairly standardized procedures. The recognition of certain epilepsy syndromes and their associated pathologies has enabled widespread application of such procedures as anterior temporal lobe resection, selective amygdalohippocampectomy, and hemispherectomy with remarkable success.4 There are certain settings, however, in which the surgical procedure may be improved in terms of seizure outcome or safety, or both, through tailoring of the surgical procedure to the specific patient. Indeed, in many instances such an approach is the only one possible.
The Basis for Tailored Resection
Temporal Lobe Resection
The role of tailored resection is least agreed on for epilepsy involving the temporal lobe. There are standardized approaches to temporal lobe epilepsy surgery that vary slightly according to side with respect to language dominance and inclusion of mesial structures, and reasonably good seizure outcomes overall have long been reported with these standardized approaches. The 1991 survey of epilepsy surgery centers compiled by Engel for the Second Palm Desert International Conference on the Surgical Treatment of the Epilepsies, held in Indian Wells, California, in February 1992, found seizure-free outcomes in 67.9% of patients undergoing anterior temporal resection.4 Recognition of variability among patients with respect to both the epileptogenic zone and language cortex, however, has led a number of surgical epilepsy teams to advocate tailoring the resection.
Distinguishing between mesial temporal sclerosis and temporal neocortical epilepsy represents one of the first rationales for implementation of this strategy. It was the ability to diagnose seizure onset localized to the hippocampus or amygdala that made selective amygdalohippocampectomy a viable treatment in the first place. Intracranial recording, usually with depth electrodes, made possible the successful selective procedures reported from Zurich5,6; alternatively, neuroimaging showing localized lesions limited to medial structures similarly sufficed. Conversely, neurophysiologic or radiographic data indicating a pathologic process confined to neocortical tissue justified a surgical procedure that spared the amygdala and hippocampus. These strategies are widely implemented today with the use of advanced magnetic resonance imaging (MRI) data or invasive monitoring, or both. Surgical planning and decision making in these instances generally occur outside the operating room, before the actual resective procedure.
Intraoperative “tailoring” of the resection procedure with the assistance of ECoG and functional mapping data obtained at the time of surgery is widely performed today. Intraoperative recordings, with rare exception, necessarily rely on interictal epileptiform discharges, or spikes, rather than ictal onset, which can be diagnosed only with extraoperative studies. The relationship of such interictal spikes to the epileptogenic zone or the area whose resection will result in a seizure-free outcome is not as clear as that for ictal-onset data. Animal models using either penicillin or alumina foci have demonstrated tight correlations between spikes and the epileptogenic zone.7,8 However, human clinical investigations have shown varying degrees of correlation. Using preresection and postresection intraoperative ECoG in a series of 29 patients undergoing standard temporal resection for mesial temporal sclerosis, Schwartz and colleagues investigated the predictive value of corticography for seizure outcome and found no correlation.9 Sugano and associates, in contrast, reported an analysis of 35 temporal lobe epilepsy patients who had undergone either lesionectomy or resection of a lesion plus tissue with residual spike activity (most often the hippocampus) and noted 3-year seizure-free rates of 76.9% and 90.9%, respectively.10 This relationship plus the enhanced ability of ECoG recording to accurately define an epileptogenic zone whose resection will lead to cessation of seizures is one of the two major rationales for tailoring resections.
The other rationale for individualization of temporal lobe seizure surgery relates to the known variability in the spatial representation of normal temporal lobe functions, particularly those related to language. Conventional teaching for untailored temporal lobe resection surgery advocates removing no more than the most anterior 4.5 cm of the middle temporal gyrus in the language-dominant hemisphere and preferably sparing the superior temporal gyrus entirely. With extensive experience in mapping language during epilepsy resections, however, Ojemann and coworkers have clearly documented the high variability of cortical language representation, with some critical language sites observed within anterior temporal lobe regions that would be removed during a standardized resection procedure.11–13 On this basis, cortical mapping has been strongly advocated.14 Those who argue against this position would note that others have reported the absence of new language deficits after standard resection.15
Extratemporal Neocortical Resection
Frontal lobe epilepsy resection requires preservation of primary motor cortex and, on the language-dominant side, speech cortex. Although variability in the primary motor areas has been noted, it is considerably less variable than the critical language sites. Under image guidance, planned surgical approaches well anterior to the precentral gyrus may be performed in accordance with the structural anatomy; incorporation of tractography or functional MRI (fMRI) data provides only further reassurance. Resections that are planned closer to eloquent brain regions, however, are facilitated by intraoperative mapping and monitoring. Language preservation with frontal resection requires sparing of Broca’s area in the posterior inferior frontal gyrus, but recent intraoperative studies have documented more extensive and variable frontal lobe language representation as well.16,17
Parietal resections must preserve primary sensory cortex and, on the language-dominant side, perisylvian language areas. Optic radiations, generally deep to the epileptogenic cortex, are at risk with larger resections but are not generally mapped intraoperatively. When operating within the occipital lobe, the primary visual cortex must be preserved. Although the accumulated surgical experience in posterior brain regions is much more limited than that in the temporal and frontal lobes, surgical interventions in these posterior regions are being carried out with increasing frequency and encouraging results.18–23
Multilobar Resection
Multilobar resection for the treatment of medically intractable epilepsy has understandably been used far less frequently than well-recognized interventions such as anterior temporal lobe resection or single-lobe cortical resection. The extent of surgery, its potential morbidity, associated functional consequences, and not least, the lower seizure control efficacy rate underlie the smaller surgical numbers, and the published experience with multilobar surgery is limited. Engel’s 1991 survey described comparative seizure control outcomes, with multilobar resection achieving seizure-free outcomes in 45.2% of patients and improvement in an additional 35.5%; anterior temporal lobe resection and lesionectomy, in contrast, achieved seizure-free outcomes in 67.9% and 66.6% of patients, respectively.4 Patients undergoing these different procedures, however, are distinctly different patient populations, and it would be a misinterpretation of that previous experience to deny multilobar epilepsy patients consideration of possible surgical intervention. Since the time of Engel’s survey, developments in both evaluation—neuroimaging as well as intracranial investigation—and surgical technique have improved the risk-benefit ratio for these patients.24–27
Methodology and Technique
Preoperative Evaluation
Surgical evaluation of an epilepsy patient today requires the multidisciplinary team approach of a dedicated epilepsy service. In addition to a general and epilepsy-focused history and physical examination, routine evaluation will include scalp electroencephalography (EEG) and epilepsy-protocol MRI; in those in whom surgery appears to be a reasonable preliminary consideration, further neuroimaging with positron emission tomography or single-photon emission computed tomography (SPECT), or both, inpatient video-EEG monitoring, ictal SPECT, and neuropsychological testing generally follows. Not all patients require all of these studies. In patients with MRI-negative epilepsy, subtraction SPECT (ictal minus the coregistered baseline) has often proved invaluable in directing subsequent intracranial investigative studies.28,29 Review and consideration of all this information by a multidisciplinary epilepsy team in a regular working conference subsequently ensue. At this point, patients without surgically treatable epilepsy, those who require additional medical management, and those with straightforward conditions such as medial temporal lobe epilepsy or structural lesions confined to a single lobe will have been identified. More challenging patients, such as those with discordant data, apparent multifocal or bilateral disease, MRI-negative epilepsy, or a more extensive pathophysiologic substrate, require further evaluation.
Intraoperative Mapping and Electrocorticography
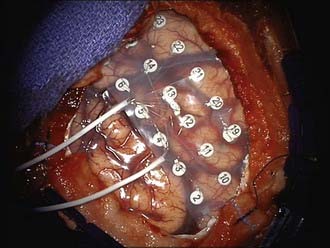
When used, intraoperative ECoG may be performed with either awake or general anesthesia; in either instance, attention to anesthetic considerations is critically important. Wide exposure of the suspected cortical region is essential. After visual inspection of the cortical surface, electrodes of a variety of types may be used. A combination of grids and strips can be used to obtain the desired number of contacts, regularity of spacing, and access to less exposed surfaces such as the inferior temporal or orbitofrontal cortex. Large 4 × 8 or 8 × 8 subdural grids with 1-cm spacing of contacts may be placed easily over the convexity (Fig. 64-1); smaller subdural grids and strips (most typically 1 × 8) may supplement the larger grids. In lesional cases, image guidance may be helpful in directing manual placement of these electrode arrays. Intraoperatively placed depth electrodes may be used, particularly electrodes placed into the medial temporal lobe structures, but this has become less common. Once placed, ample recording is performed, and in collaboration with electrocorticographers in the operating room, the epileptogenic zone that would ideally be resected is defined. After the initial recording, mapping (see the next paragraph), and subsequent resection, further ECoG is often performed, although the significance of postresection spikes and the appropriate surgical response are debated. Advocates of this technique cite reports of better outcomes associated with absence of residual epileptiform activity,30–32 although resection in pursuit of residual spike activity is controversial and not universally advised.9
Intraoperative mapping, most commonly for language, motor, or sensory function (or any combination of these functions), typically follows the initial ECoG. For the primary sensorimotor cortex, mapping has a long and successful history. Integration of preoperative MRI or ideally fMRI data through the use of image guidance methods greatly facilitates this activity. Placement of a subdural strip perpendicular to the presumed central sulcus is a method that can be used to obtain somatosensory evoked potentials and assist in localization of the precentral and postcentral gyri. Direct motor mapping may be performed with either a handheld stimulator or a 1 × 4 subdural strip, with appropriate electromyographic recording increasing the sensitivity. Once the motor cortex has been identified, an extremely useful practice involves placing a subdural strip electrode over that area, delivering repeated electrical stimuli, and monitoring motor evoked responses to examine the integrity of the corticospinal tract during the resection.33–35
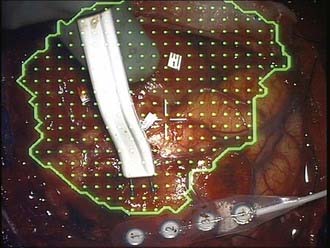
Language mapping requires an awake craniotomy, and with an experienced team, this approach can be used routinely in an efficient and safe manner. Numerous descriptions of awake craniotomy techniques have been published.13,16 A handheld bipolar stimulator consisting of 1-mm electrodes with a separation of 5 mm is used to apply a 5-second, 60-Hz, biphasic square-wave constant-current or constant-voltage stimulus at sequential sites across the exposed cortex of interest, beginning with low-stimulation parameters and gradually increasing the stimulation intensity until either language is disrupted or the maximal subthreshold for afterdischarges is reached (Fig. 64-2). Adjacent electrodes monitor the cortex for afterdischarges, and cold irrigation or intravenous propofol is available as needed to manage stimulation-induced seizures. Broca’s area is most easily identified, with arrest of speech during counting being readily elicited; speech interference by a direct motor response must be excluded. Beyond Broca’s area, naming is assessed by using pictures of objects presented on an appropriately positioned computer screen during application of the stimulus. The cortex is investigated in this manner at approximately 1-cm intervals, with each site typically tested three times. Numbered labels are used to identify sites that have been tested, and intraoperative photographs can be used to document the results of the functional mapping procedure. Subsequent resections are generally kept a minimum of 1 cm from positive language sites, although this conventional dictum does not take into account sulcal patterns that may warrant its modification.
Extraoperative Intracranial Investigation
Localization of the area of seizure onset, as well as cortical function, can also be achieved extraoperatively with implanted electrode arrays. Although this strategy necessitates an additional operative procedure, there are also advantages related to intraoperative recording. ECoG data recorded outside the operating room are obtained in a setting that more closely resembles the patient’s normal environment, and these recordings can be obtained for periods of days or even weeks. Actual seizure onset may be recorded rather than relying on interictal spike activity as its surrogate. Repeated seizures can be captured, and when a consistent pattern is observed across all events, it is likely that these data accurately reflect the patient’s usual pattern of epilepsy. With respect to mapping normal function, the extraoperative setting enables more extensive and repeated testing without the constraints of anesthetic requirements or operative time. Extraoperative methods are most valuable in a patient in whom the actual region of seizure onset has not been confidently defined. Bilateral, multilobar subdural strip and depth electrode placement, for example, may identify seizure onset in patients who would otherwise be deemed unsuitable for surgical resection. Particularly in patients with MRI-negative epilepsy, placing multiple electrode types and large grid arrays over the general regions of interest may be the only effective strategy for identifying a resectable seizure focus. The utility and low morbidity associated with subdural grid electrodes have been well documented.36
Intracranial Electrode Implantation Procedure
The craniotomy is performed with standard neurosurgical technique. There are two schools of thought regarding management of the bone flap. Many centers in the past have replaced the bone flap at the close of the implantation, in the same manner as for any craniotomy. Alternatively, in an attempt to reduce the risk for subsequent infection by bacterial colonization along the electrode leads and at the same time to simplify the procedure, the bone flap may be left out during the interval while the patient is monitored, with replacement at the time of the subsequent procedure for electrode removal and resection. We keep the bone flap sterile in a freezer and have not experienced significant morbidity or other difficulties related to using this approach. Particular attention to hemostasis during the opening as well as throughout the procedure reduces the risk for development of a symptomatic epidural or subdural hematoma; the postoperative appearance of some blood in the region of the electrodes has been specifically reported but is without clinical significance.37
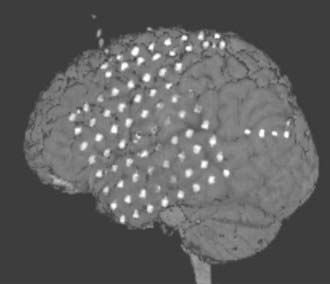
Postoperatively, a fine-resolution CT scan of the head is obtained before the patient goes to the epilepsy monitoring unit, and this study is then fused with the patient’s preoperative MRI for optimal understanding of the final electrode positions (Fig. 64-3). The patient is now monitored until (ideally) at least three typical seizures have been recorded, during which time prophylactic antibiotics are administered, although this is a recognized controversial precaution. Use of anticonvulsants is weaned if necessary. Recording is usually performed for 2 days up to 4 weeks, with an average time of 7 days. Functional mapping, unless not required, is performed during this interval. The data are reviewed by the multidisciplinary team, and surgical recommendations are developed and discussed with the patient and family.
Seizure Outcomes
For anterior temporal lobe resection, with or without tailoring, Engel’s survey had found seizure-free outcomes in 67.9% of patients.4 A recent series of 434 consecutive temporal resections selectively using ECoG for tailoring in patients without medial temporal sclerosis has been reported by Elsharkawy and colleagues, who achieved Engel class I seizure outcomes at 2 years in 72.3% of patients.38
The report of Sugano and colleagues already alluded to involved 35 patients with temporal lesions who underwent lesionectomy alone or lesionectomy plus resection of additional tissue shown to generate spike activity on postresection ECoG; seizure-free outcomes were achieved in 90.9% of the latter group (versus 76.9% with lesionectomy alone).10
Kanner and colleagues analyzed their results of tailored anterior temporal resection and assessed outcome with respect to the extent of resection based on postoperative MRI. Through the use of intraoperative recording, they were frequently able to spare the amygdala or hippocampus, or both, and yet achieve seizure-free outcomes in 21 of 24 patients, with rare seizures in the remaining 3.32
It should also be noted, however, that untailored temporal resections continue to produce good results. Cukiert and coauthors reported a series of untailored corticoamygdalohippocampectomy procedures in 100 patients with mesial temporal sclerosis, with 89 patients becoming seizure free or experiencing simple partial seizures only; the other 11 patients all experienced greater than a 90% reduction in seizure frequency.39 Also to be noted is the report of Schwartz and associates, who in a series of 29 patients undergoing untailored temporal resection with ECoG performed before and after resection, found no correlation between seizure outcomes and the presence or absence of residual epileptiform activity on the postresection recording.9
For lesional epilepsy surgery, Engel’s survey reported that 66.6% of patients became seizure free.4 There are two clinical series of lesionectomy alone without resection of surrounding cortex. In the extended (4-year) follow-up series of Cascino and coworkers, 13 of 23 (56.5%) lesional patients achieved seizure-free outcomes, and 17 patients (73.9%) experienced greater than a 90% reduction in seizure frequency.40 In Giulioni and colleagues’ series of 15 children, 13 (86.6%) became seizure free.41 Comparing the results of lesionectomy alone with resection tailored by ECoG, Jooma and coauthors reported a study of 16 lesional epilepsy patients treated by lesionectomy and 14 patients whose resection was guided by intraoperative ECoG. Seizure-free outcomes were achieved in just 3 patients (18.8%) in the lesionectomy-alone group but in 62.5% of the tailored resection patients.42 In a similar comparison, Sugano and associates’ previously cited study showed improvement in seizure-free outcomes from 76.9% for lesionectomy alone to 90.9% for tailored resection.10
Extratemporal resections in Engel’s series led to seizure-free outcomes in 45.1% of patients.4 In more recent surgical series, Engel class I outcomes have been reported in 49.5% to 60% of patients operated on for frontal lobe epilepsy43–45 and in 39.5% to 90% of patients with parietal or occipital lobe epilepsy.18,46–48 With respect to larger, multilobar resections, Engel’s survey found seizure-free outcomes in 45.2% of patients.4 More recent series have reported seizure-free outcomes in 30% to 47% of patients.24,25,49
For the present purposes it is useful to distinguish whether patients included in the series had lesional or nonlesional epilepsy. In nearly every series in which it has been examined, the presence of a discrete MRI-detectable lesion has been associated with a better outcome.43,45,50 In patients with no structural abnormality found on neuroimaging, reliance on intracranial recording and mapping, with subsequent formulation of a tailored resection plan, is essential. In addition to determining an epileptogenic region for resection, individualized intracranial monitoring can serve to enable sparing of suspected but then exonerated uninvolved structures. With such a strategy, seizure-free outcome rates have been reported to be in the range of 29% to 41%, with a considerable additional percentage of patients becoming almost seizure free.45,50 Although generally not as favorable as results seen with lesional epilepsy, these surgical outcomes are best compared with the results of nonoperative treatment, the only actual alternative.
Binder DK, Podlogar M, Clusmann H, et al. Surgical treatment of parietal lobe epilepsy. J Neurosurg. 2009;110:1170.
Cascino GD, Hulihan JF, Sharbrough FW, et al. Parietal lobe lesional epilepsy: electroclinical correlation and operative outcome. Epilepsia. 1993;34:522.
Cascino GD, Kelly PJ, Sharbrough FW, et al. Long-term follow-up of stereotactic lesionectomy in partial epilepsy: predictive factors and electroencephalographic results. Epilepsia. 1992;33:639.
Elsharkawy AE, Alabbasi AH, Pannek H, et al. Long-term outcome after temporal lobe epilepsy surgery in 434 consecutive adult patients. J Neurosurg. 2009;110:1135.
Hata D, Isu T, Nakanishi M, et al. Intraoperative electrocorticography and successful focus resection in a case of Sturge-Weber syndrome. Seizure. 1998;7:505.
Hermann BP, Wyler AR, Somes G. Language function following anterior temporal lobectomy. J Neurosurg. 1991;74:560.
Jeha LE, Najm I, Bingaman W, et al. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007;130:574.
Jooma R, Yeh HS, Privitera MD, et al. Lesionectomy versus electrophysiologically guided resection for temporal lobe tumors manifesting with complex partial seizures. J Neurosurg. 1995;83:231.
Kanner AM, Kaydanova Y, deToledo-Morrell L, et al. Tailored anterior temporal lobectomy. Relation between extent of resection of mesial structures and postsurgical seizure outcome. Arch Neurol. 1995;52:173.
Kasowski HJ, Stoffman MR, Spencer SS, et al. Surgical management of parietal lobe epilepsy. Adv Neurol. 2003;93:347.
Leiphart JW, Peacock WJ, Mathern GW. Lobar and multilobar resections for medically intractable pediatric epilepsy. Pediatr Neurosurg. 2001;34:311.
Mosewich RK, So EL, O’Brien TJ, et al. Factors predictive of the outcome of frontal lobe epilepsy surgery. Epilepsia. 2000;41:843.
Ojemann G, Ojemann J, Lettich E, et al. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316.
Olivier A, Boling WJr. Surgery of parietal and occipital lobe epilepsy. Adv Neurol. 2000;84:533.
Salanova V, Andermann F, Rasmussen T, et al. Parietal lobe epilepsy. Clinical manifestations and outcome in 82 patients treated surgically between 1929 and 1988. Brain. 1995;118:607.
Schwartz TH, Bazil CW, Walczak TS, et al. The predictive value of intraoperative electrocorticography in resections for limbic epilepsy associated with mesial temporal sclerosis. Neurosurgery. 1997;40:302.
Sugano H, Shimizu H, Sunaga S. Efficacy of intraoperative electrocorticography for assessing seizure outcomes in intractable epilepsy patients with temporal-lobe-mass lesions. Seizure. 2007;16:120.
Van Gompel JJ, Worrell GA, Bell ML, et al. Intracranial electroencephalography with subdural grid electrodes: techniques, complications, and outcomes. Neurosurgery. 2008;63:498.
Wennberg R, Quesney F, Olivier A, et al. Electrocorticography and outcome in frontal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1998;106:357.
Wieser HG, Yasargil MG. Selective amygdalohippocampectomy as a surgical treatment of mesiobasal limbic epilepsy. Surg Neurol. 1982;17:445.
1 Horsley V. Brain surgery. BMJ. 1886;2:670.
2 Wolf P. The history of surgical treatment of epilepsy in Europe. In: Lüders H, editor. Epilepsy Surgery. New York: Raven Press; 1991:9-17.
3 Penfield W, Jasper H. Epilepsy and the Functional Anatomy of the Human Brain. Boston: Little Brown; 1954.
4 Engel JJr, Van Ness PC, Rasmussen TB, et al. Outcome with respect to epileptic seizures. In: Engel J Jr, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:609-621.
5 Wieser HG, Yasargil MG. Selective amygdalohippocampectomy as a surgical treatment of mesiobasal limbic epilepsy. Surg Neurol. 1982;17:445.
6 Wieser HG, Siegel AM, Yasargil GM. The Zurich amygdalo-hippocampectomy series: a short up-date. Acta Neurochir Suppl. 1990;50:122.
7 Lüders H, Bustamante L, Zablow L, et al. Quantitative studies of spike foci induced by minimal concentrations of penicillin. Electroencephalogr Clin Neurophysiol. 1980;48:80.
8 Harris AB, Lockard JS. Absence of seizures or mirror foci in experimental epilepsy after excision of alumina and astrogliotic scar. Epilepsia. 1981;22:107.
9 Schwartz TH, Bazil CW, Walczak TS, et al. The predictive value of intraoperative electrocorticography in resections for limbic epilepsy associated with mesial temporal sclerosis. Neurosurgery. 1997;40:302.
10 Sugano H, Shimizu H, Sunaga S. Efficacy of intraoperative electrocorticography for assessing seizure outcomes in intractable epilepsy patients with temporal-lobe-mass lesions. Seizure. 2007;16:120.
11 Ojemann GA, Whitaker HA. Language localization and variability. Brain Lang. 1978;6:239.
12 Ojemann GA. Individual variability in cortical localization of language. J Neurosurg. 1979;50:164.
13 Ojemann G, Ojemann J, Lettich E, et al. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316.
14 Ojemann GA. Intraoperative tailoring of temporal lobe resections. In: Engel J Jr, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:481-488.
15 Hermann BP, Wyler AR, Somes G. Language function following anterior temporal lobectomy. J Neurosurg. 1991;74:560.
16 Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18.
17 Ojemann G. Localization of language in frontal cortex. Adv Neurol. 1992;57:361.
18 Binder DK, Podlogar M, Clusmann H, et al. Surgical treatment of parietal lobe epilepsy. J Neurosurg. 2009;110:1170.
19 Kim DW, Lee SK, Yun CH, et al. Parietal lobe epilepsy: the semiology, yield of diagnostic workup, and surgical outcome. Epilepsia. 2004;45:641.
20 Kasowski HJ, Stoffman MR, Spencer SS, et al. Surgical management of parietal lobe epilepsy. Adv Neurol. 2003;93:347.
21 Olivier A, Boling WJr. Surgery of parietal and occipital lobe epilepsy. Adv Neurol. 2000;84:533.
22 Rasmussen T. Surgery for central, parietal and occipital epilepsy. Can J Neurol Sci. 1991;18:611.
23 Williamson PD, Boon PA, Thadani VM, et al. Parietal lobe epilepsy: diagnostic considerations and results of surgery. Ann Neurol. 1992;31:193.
24 Patil AA, Andrews RV, Torkelson R. Surgical treatment of intractable seizures with multilobar or bihemispheric seizure foci (MLBHSF). Surg Neurol. 1997;47:72.
25 Koszewski W, Czarkwiani L, Bidzinski J. Multilobar resections in surgical treatment of medically intractable epilepsy. Neurol Neurochir Pol. 1998;32(suppl 2):81.
26 Leiphart JW, Peacock WJ, Mathern GW. Lobar and multilobar resections for medically intractable pediatric epilepsy. Pediatr Neurosurg. 2001;34:311.
27 Daniel RT, Meagher-Villemure K, Roulet E, et al. Surgical treatment of temporoparietooccipital cortical dysplasia in infants: report of two cases. Epilepsia. 2004;45:872.
28 Lewis PJ, Siegel A, Siegel AM, et al. Does performing image registration and subtraction in ictal brain SPECT help localize neocortical seizures? J Nucl Med. 2000;41:1619.
29 Thadani VM, Siegel AH, Lewis P, et al. SPECT in neocortical epilepsies. Adv Neurol. 2000;84:425.
30 Wennberg R, Quesney F, Olivier A, et al. Electrocorticography and outcome in frontal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1998;106:357.
31 Hata D, Isu T, Nakanishi M, et al. Intraoperative electrocorticography and successful focus resection in a case of Sturge-Weber syndrome. Seizure. 1998;7:505.
32 Kanner AM, Kaydanova Y, deToledo-Morrell L, et al. Tailored anterior temporal lobectomy. Relation between extent of resection of mesial structures and postsurgical seizure outcome. Arch Neurol. 1995;52:173.
33 Macdonald DB. Intraoperative motor evoked potential monitoring: overview and update. J Clin Monit Comput. 2006;20:347.
34 Szelenyi A, Kothbauer K, de Camargo AB, et al. Motor evoked potential monitoring during cerebral aneurysm surgery: technical aspects and comparison of transcranial and direct cortical stimulation. Neurosurgery. 2005;57:331.
35 Cedzich C, Taniguchi M, Schafer S, et al. Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery. 1996;38:962.
36 Van Gompel JJ, Worrell GA, Bell ML, et al. Intracranial electroencephalography with subdural grid electrodes: techniques, complications, and outcomes. Neurosurgery. 2008;63:498.
37 Mocco J, Komotar RJ, Ladouceur AK, et al. Radiographic characteristics fail to predict clinical course after subdural electrode placement. Neurosurgery. 2006;58:120.
38 Elsharkawy AE, Alabbasi AH, Pannek H, et al. Long-term outcome after temporal lobe epilepsy surgery in 434 consecutive adult patients. J Neurosurg. 2009;110:1135.
39 Cukiert A, Buratini JA, Machado E, et al. Seizure-related outcome after corticoamygdalohippocampectomy in patients with refractory temporal lobe epilepsy and mesial temporal sclerosis evaluated by magnetic resonance imaging alone. Neurosurg Focus. 2002;13(4):ecp2.
40 Cascino GD, Kelly PJ, Sharbrough FW, et al. Long-term follow-up of stereotactic lesionectomy in partial epilepsy: predictive factors and electroencephalographic results. Epilepsia. 1992;33:639.
41 Giulioni M, Galassi E, Zucchelli M, et al. Seizure outcome of lesionectomy in glioneuronal tumors associated with epilepsy in children. J Neurosurg. 2005;102:288.
42 Jooma R, Yeh HS, Privitera MD, et al. Lesionectomy versus electrophysiologically guided resection for temporal lobe tumors manifesting with complex partial seizures. J Neurosurg. 1995;83:231.
43 Elsharkawy AE, Alabbasi AH, Pannek H, et al. Outcome of frontal lobe epilepsy surgery in adults. Epilepsy Res. 2008;81:97.
44 Jeha LE, Najm I, Bingaman W, et al. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007;130:574.
45 Mosewich RK, So EL, O’Brien TJ, et al. Factors predictive of the outcome of frontal lobe epilepsy surgery. Epilepsia. 2000;41:843.
46 Kim CH, Chung CK, Lee SK, et al. Parietal lobe epilepsy: surgical treatment and outcome. Stereotact Funct Neurosurg. 2004;82:175.
47 Salanova V, Andermann F, Rasmussen T, et al. Parietal lobe epilepsy. Clinical manifestations and outcome in 82 patients treated surgically between 1929 and 1988. Brain. 1995;118:607.
48 Cascino GD, Hulihan JF, Sharbrough FW, et al. Parietal lobe lesional epilepsy: electroclinical correlation and operative outcome. Epilepsia. 1993;34:522.
49 D’Agostino MD, Bastos A, Piras C, et al. Posterior quadrantic dysplasia or hemi-hemimegalencephaly: a characteristic brain malformation. Neurology. 2004;62:2214.
50 Smith JR, Lee MR, King DW, et al. Results of lesional vs. nonlesional frontal lobe epilepsy surgery. Stereotact Funct Neurosurg. 1997;69:202.