Systemic inflammatory response syndrome and sepsis
The American College of Chest Physicians and the Society of Critical Care Medicine published consensus-derived definitions of SIRS, sepsis, and organ failure in 1992 (Table 232-1). In 2001, a list of signs and laboratory findings that should prompt a clinician to consider sepsis in the differential diagnosis was proposed. In addition to tachypnea, tachycardia, and alterations in temperature and white blood cell count, these findings include chills, poor capillary refill, decreased skin perfusion, thrombocytopenia, hypoglycemia, oliguria, alteration in mental status, and skin mottling.
Table 232-1
Consensus Definitions for Sepsis and Organ Failure
| Term | Definition |
| SIRS |
Two or more of the following |
| Sepsis |
The presence of SIRS and either of the following |
| Severe sepsis* | Sepsis associated with organ dysfunction, hypoperfusion, or hypotension. Hypoperfusion and perfusion abnormalities include, but are not limited to, lactic acidosis, oliguria, or mental status changes |
| Sepsis-induced hypotension* | Systolic blood pressure <90 mm Hg, or reduction of >40 mm Hg, in the absence of other causes for hypotension |
| Septic shock* | Sepsis-induced hypotension despite adequate fluid resuscitation plus perfusion abnormalities including, but not limited to, lactic acidosis, oliguria, and mental status changes; patients who receive vasopressor or inotropic agents to maintain blood pressure are considered to be in shock even if they are not hypotensive |
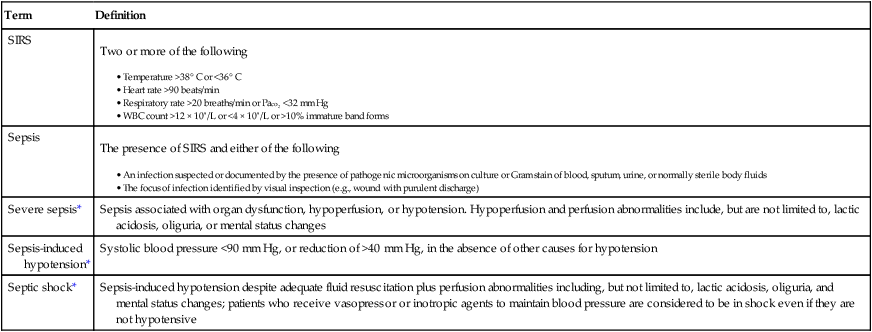
*Or systemic inflammatory response syndrome (SIRS), depending on whether or not infection is present.
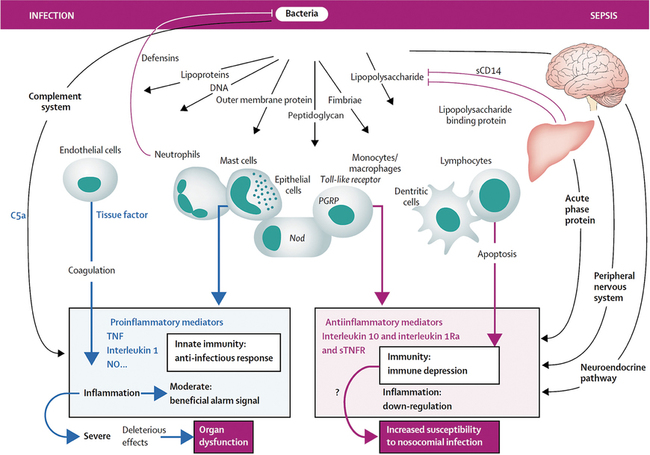
SIRS occurs in the absence of infection and may be secondary to surgical insult, trauma, or inflammatory conditions, such as pancreatitis. Sepsis is the result of a complex interaction among the patient’s immune, inflammatory, and coagulation systems and an infecting organism. In both SIRS and sepsis, at the site of injury or infection, a local inflammatory response is antagonized by a local antiinflammatory response (Figure 232-1). Such proinflammatory and antiinflammatory responses often become systemic. Both excessive and inadequate host-immune responses can lead to progression of disease and organ dysfunction. Further, a large inciting stimulus or a virulent infectious agent may cause organ dysfunction even in the presence of a competent immune system. Neutrophils play a key role in the development of sepsis. An initial toxic stimulus (e.g., bacterial endotoxin) leads to production of proinflammatory cytokines, such as interleukin 1 and tumor necrosis factor. Migration of neutrophils to vascular endothelium subsequently occurs, with concomitant activation of clotting and generation of secondary inflammatory mediators.
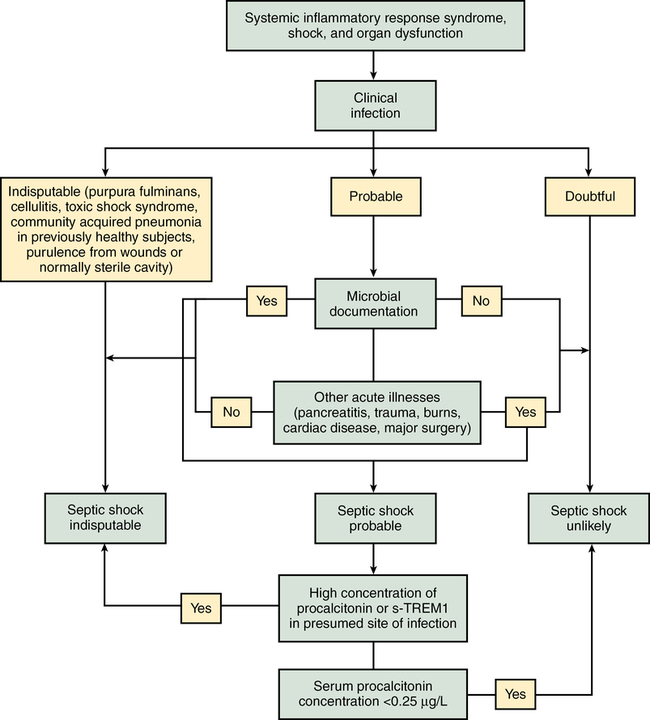
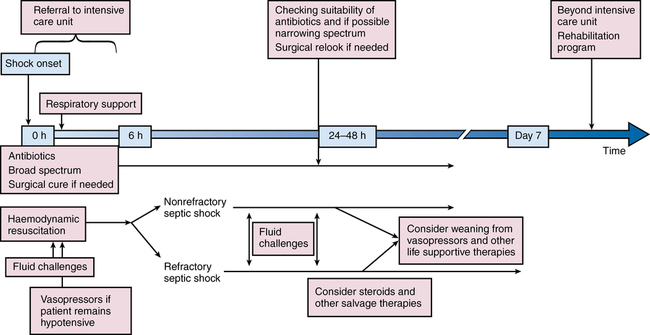
Severe sepsis and septic shock are medical emergencies requiring prompt intervention. Guidelines for management have been developed by a multinational multidisciplinary collaboration of experts as part of an education initiative known as the Surviving Sepsis Campaign, the latest iteration being the 2012 version. Many institutions have incorporated these therapies into “sepsis bundles” to promote best practice. Suggested algorithms for investigating potential sepsis and managing patients with sepsis are provided in Figures 232-2 and 232-3. Elements of sepsis management include initial resuscitation, diagnosis, antibiotic therapy, source control, and supportive therapy.
Antibiotic therapy and source control
Antibiotic therapy, directed against likely pathogens, should be intravenously administered as soon as possible and within the first hour after severe sepsis or septic shock is recognized. Antimicrobial agents that effectively penetrate into the presumed site of infection should be chosen. The initial therapy should cover a wide spectrum of pathogens, with subsequent daily reassessment based on culture data and clinical response. Antimicrobial options are presented in Table 232-2. Identification of an anatomic site of infection should prompt consideration of intervention to control the source (e.g., drainage of empyema or intraabdominal abscess, débridement of infected necrotic tissue, removal of an infected device.)
Table 232-2
Antimicrobial Choices in Sepsis
| Patient Population/Site of Infection | Likely Pathogen | Recommended Antimicrobial Agent or Agents |
| Immunocompetent | Gram-positive Gram-negative |
Give ureidopenicillins + one of the following β-Lactamase inhibitors Carbapenems Third- and fourth-generation cephalosporins Add antipseudomonal fluoroquinolone if Pseudomonas aeruginosa is a likely pathogen. Add vancomycin or linezolid if there is concern for MRSA. Add linezolid if there is concern for VRE. |
| Immunocompromised | Gram-positive Gram-negative Fungal |
Treat as for an immunocompetent patient, with inclusion of vancomycin or linezolid and antipseudomonal agent. Add antifungal (amphotericin B, caspofungin, or voriconazole) if patient is at high risk for fungal infection. |
| Intravascular catheter–related infections | Gram-positive Gram-negative Fungal |
Provide broad-spectrum antimicrobial coverage In settings with a significant MRSA prevalence, vancomycin should be administered. Add antipseudomonal agent in immunocompromised patients. Add intravenously administered amphotericin B or fluconazole if fungemia is suspected. |
| VAP, HCAP, HAP* | Streptococcus pneumoniae, Haemophilus influenzae, MSSA, enteric gram-negative bacilli | In the absence of risk factors that necessitate use of broad-spectrum antibiotics, fluoroquinolone, ampicillin/sulbactam, or ceftriaxone can be given. With recognized risk factors, use antipseudomonal cephalosporin (cefipime, ceftazidime), antipseudomonal carbapenem (imipenem, meropenem), or piperacillin/tazobactam AND antipseudomonal fluoroquinolone (ciprofloxacin, levofloxacin) or aminoglycoside. Add vancomycin or linezolid if there is concern for MRSA. Add macrolide or fluoroquinolone if there is concern for Legionella pneumophila. |
| Severe community-acquired pneumonia | Typical organisms (S. pneumoniae, H. influenzae, S. aureus) and atypical organisms (Mycoplasma pneumoniae, Chlamydia pneumoniae, L. pneumophila) | Give third-generation cephalosporin and intravenously administered macrolide or nonpseudomonal fluoroquinolone. Give antipseudomonal fluoroquinolone if Pseudomonas aeruginosa is a likely pathogen. |
| Fungal infections | Candida spp., Aspergillus | Caspofungin, amphotericin B, voriconazole, itraconazole, or fluconazole may be chosen depending on individual patient and organism factors. |
*Certain patients (e.g., those with recent antibiotic therapy, prolonged hospitalization, or immunosuppression or on dialysis) require broad-spectrum antibiotics targeting gram-positive, gram-negative, and atypical organisms, such as Legionella pneumophila and MRSA. (methicillin-resistant Staphylococcus aureus)
Vasopressors and inotropes
Mean arterial pressure should be maintained at a minimum of 65 mm Hg, though preexisting comorbid conditions (e.g., longstanding hypertension) may alter this pressure goal. Surviving Sepsis Campaign recommendations for hemodynamic support are provided in Box 232-1. Norepinephrine is recommended as the first-choice vasopressure in septic shock, though epinephrine may be used as an alternative when the patient is poorly responsive to the initial choice. Patients with sepsis may have a relative vasopressin deficiency that contributes to vasodilatation, and intravenously administered vasopressin is increasingly used by many as a vasoconstrictor. Phenylephrine is devoid of β-adrenergic effects and is not recommended as a first-line agent because it is likely to decrease stroke volume. Vasopressor agents should be administered through a central venous catheter as soon as a catheter is available. When myocardial dysfunction is suggested by elevated cardiac filling pressures and low cardiac output, dobutamine should be administered to attain a normal (though not supranormal) cardiac index.
Supportive therapy
Multiple supportive therapies are often required for patients with sepsis. Noninvasive or invasive mechanical ventilation may be needed for patients with acute respiratory distress syndrome. Mechanical ventilatory support should be based on the principles discussed in Chapter 227. When required, sedation should be guided by protocols that target predetermined end points (e.g., sedation scales), with daily interruption or lightening of sedation with awakening and retitration of sedative agents. Neuromuscular blocking agents should be avoided, if possible, to decrease the likelihood of the development of critical illness polyneuromyopathy, although a short (≤ 48 h) course of neuromuscular blocking agents is recommended for patients with acute respiratory distress syndrome and sepsis. Glycemic control in critically ill patients has been the subject of considerable debate, with evolution of target values over the past decade. The NICE-SUGAR (Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation) study has greatly influenced the Surviving Sepsis Guidelines in this regard. A use of a protocol-based approach is recommended, commencing with intravenously administered insulin when two consecutive blood glucose levels are greater than 180 mg/dL, and targeting an upper blood glucose level of 180 mg/dL or less.