Chapter 87 Systemic chemotherapy for hepatic colorectal cancer
Impact on surgical management
Overview
The liver is the most common site of metastases from colorectal cancer (CRC). Approximately half of all patients with CRC will develop liver metastases at some point during their disease (Ekberg et al, 1987; Steele & Ravikumar, 1989), and half of these will be seen with synchronous liver metastases at the time of diagnosis (Bengmark & Hafstrom, 1969). Resection remains the best treatment for patients with resectable colorectal liver metastases (CLM; see Chapter 81A). Early studies reported 5-year survival rates of 25% to 48% following complete resection of the liver metastases (Adson et al, 1984; Fong et al, 1997; Sugihara et al, 1993) in an era when the only active chemotherapeutic agent used in the United States was the antimetabolite 5-fluorouracil (5-FU). With the single-agent regimen of 5-FU, or 5-FU plus leucovorin (LV), tumor response was seen in only 10% to 20% of those treated, which provided an overall survival of approximately a year in patients who were not candidates for an operation (Meta-Analysis Group in Cancer [MAGIC], 1998; Thirion et al, 2004). Substantial improvements in both response and survival have been reported with the use of the two “modern” cytotoxic agents, irinotecan and oxaliplatin.
Newer chemotherapeutic regimens that combine continuously infused 5-FU and LV with either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) yield overall response rates over 50% and provide a doubling of survival time in patients with unresectable disease (de Gramont et al, 2000; Tournigand et al, 2004). The integration of molecularly targeted agents, such as bevacizumab (monoclonal antibody to vascular endothelial growth factor) and cetuximab (antiepidermal growth factor receptor), into treatment strategies has further increased response rates to an impressive 70% (Folprecht et al, 2010). By combining surgery with these newer chemotherapeutic agents and regimens, 5-year survival rates approaching 60% have been reported following hepatic resection of CLM (Abdalla et al, 2004; Choti et al, 2002; Fernandez et al, 2004).
Chemotherapeutic Agents
Fluoropyrimidines
The fluoropyrimidine 5-FU has remained the backbone of systemic chemotherapy for metastatic CRC for decades. This drug inhibits thymidylate synthase, thereby lowering production of the pyrimidine thymidine for DNA synthesis. It has been used synergistically with leucovorin (folate) either in a daily bolus administration in repeated cycles of 5 days (Mayo Clinic regimen) or on a biweekly infusional schedule (de Gramont regimen). By meta-analysis, continuous infusional administration of 5-FU/LV provides an improved response rate (24% vs. 14% with bolus; P = .002) and decreased grade 3 to 4 toxicity (4% vs. 31% with bolus; P < .001) compared with bolus therapy (MAGIC, 1998; de Gramont et al, 1997). Capecitabine is the orally administered prodrug of 5-FU, which has lower toxicity and provides similar survival rates compared with an intravenous 5-FU/LV regimen (Hoff et al, 2001).
Irinotecan
Irinotecan (CPT-11) is a topoisomerase I inhibitor, which prevents DNA unwinding and results in failure of DNA replication, DNA strand breaks, and cell death. In a randomized trial that evaluated irinotecan in patients with unresectable metastatic CRC, survival after irinotecan alone was equivalent to that of patients treated with standard bolus 5-FU/LV, but the combination of irinotecan and 5-FU/LV improved overall survival by 2.2 months over 5-FU/LV alone (14.8 vs. 12.6 months; P = .04) (Saltz et al, 2000). Subsequently, the combination of irinotecan with 5-FU/LV administered in continuous infusion (FOLFIRI) was associated with further improvement in survival (17.4 vs. 14.1 months; P = .03) (Douillard et al, 2000).
Oxaliplatin
Oxaliplatin is a platinum compound that alkylates DNA strands, thereby inhibiting DNA transcription and replication, resulting in cell death. Although already in use in Europe, oxaliplatin received U.S. Food and Drug Administration (FDA) approval in 2002. Oxaliplatin is commonly used in combination with continuous infusional 5-FU/LV (FOLFOX). Different drug doses and timed regimens—FOLFOX4, FOLFOX6, and FOLFOX7—have been evaluated, but no data support the superiority of any one over another in terms of patient survival. In a national effort, the intergroup N9741 trial (Goldberg et al, 2004) showed that FOLFOX treatment was associated with a 4.5-month improvement in median overall survival compared with continuous infusion of 5-FU/LV alone (19.5 vs. 15 months; P = .001). As no trial has found a difference in efficacy between FOLFOX and FOLFIRI regimens, both remain first-line treatment options for systemic chemotherapy treatment of unresectable metastatic CRC. FOLFOX is used primarily over FOLFIRI because of potentially debilitating diarrhea associated with the latter.
Molecular Targeted Agents
The monoclonal antibodies bevacizumab and cetuximab have emerged as useful adjuncts to systemic chemotherapy for metastatic CRC. Bevacizumab targets and effectively neutralizes circulating vascular endothelial growth factor (VEGF), and several mechanisms of action have been proposed. VEGF is one of the key growth factors required for development and stabilization of new blood vessels that supply tumor growth. Bevacizumab decreases microvascular density and integrity and reduces interstitial tumor pressure in vivo, potentially increasing local delivery of other concurrently administered cytotoxic drugs (Jain, 2001; Willett et al, 2004).
In 2004 Hurwitz and colleagues demonstrated a benefit of adding bevacizumab to systemic chemotherapy for metastatic CRC. In a randomized controlled double-blinded trial, they compared bolus 5-FU/LV plus irinotecan in conjunction with either bevacizumab (n = 402) or placebo (n = 411). Patients in the bevacizumab group had a near 5-month improvement in median survival compared with the placebo group (20.3 vs. 15.6 months; P < .001), and they had an increase in median progression-free survival (10.6 vs. 6.5 months; P = < .001). Although generally well tolerated, the toxicity seen in the bevacizumab group was that of increased gastrointestinal perforation (1.5% of patients) and major thrombotic events (2.5%). In addition, treatment with bevacizumab prior to surgery may be associated with increased wound infections and dehiscences, attributable to inhibition of neovascularization of the healing wound (Scappaticci et al, 2005); however, following hepatic resection for CLM, this phenomenon was not observed in a prospective Phase II trial, in which bevacizumab had been discontinued for 5 weeks (Gruenberger et al, 2008). The current recommendation is still to wait at least 28 days from cessation of bevacizumab to proceed with surgery, but many surgeons prefer to wait 6 weeks.
Cetuximab and the newer drug panitumumab are two recombinant chimeric monoclonal antibodies, which bind to the epidermal growth factor receptor (EGFR). Cetuximab is sometimes used as first-line therapy of unresectable metastatic colon cancer. By competitively blocking the transmembrane tyrosine kinase EGFR, these agents inhibit cell growth, induce apoptosis, and decrease matrix metalloproteinase and VEGF production; however, the effectiveness of both of these drugs seems to be significantly dependent on the wild-type phenotype of KRAS, as this protein is part of the downstream signal-transduction pathway of EGFR. Results from the CRYSTAL study showed that for patients with wild-type KRAS, the addition of cetuximab to FOLFIRI as first-line treatment for CLM increased the response rate to 59% compared with 43% using FOLFIRI alone (P = .003). A slight increase in progression-free survival was found (9.9 vs. 8.7 months; P = .017), a benefit not seen among patients with KRAS mutations (Van Cutsem et al, 2008). The OPUS study was constructed similarly and assessed the addition of cetuximab to FOLFOX, and it also showed benefits for the KRAS wild-type subgroup (61% vs. 37%; P = .011) but not for patients with KRAS mutations (Bokemeyer et al, 2009). Based on these results, the American Society of Clinical Oncology published a consensus statement that patients who have tumors with KRAS mutations in codon 12 or 13 were unaffected by EGFR inhibition, and they recommended that anti-EGFR drugs not be used as part of the treatment regimen for this patient population (Allegra et al, 2009).
The colorectal liver metastases (CELIM) study randomized patients with unresectable colorectal liver metastases to receive FOLFOX plus cetuximab or FOLFIRI plus cetuximab in a nonblinded fashion. Response rates for the 53 patients in each arm were similar (68% and 57%; P = not significant [NS]). When analyzed for KRAS mutation, an impressive overall response rate of 70% was found for the entire group (Folprecht et al, 2010). Following a median of eight cycles, complete resection was achieved in 41 patients (34%), suggesting that this regimen was useful for converting unresectable cases to resectable ones. In a secondary analysis, 68 patients were included in a retrospective reevaluation by seven experienced hepatobiliary surgeons. Twenty-two patients (32%) were considered to be resectable based on initial staging imaging studies, which increased to 41 patients (60%) based on restaging studies after chemotherapy (P = .001).
Conversion Therapy
The first retrospective reports from France (Adam et al, 2001; Bismuth et al, 1996; Giacchetti et al, 1999) were updated in 2004 with a series of 1439 patients who underwent resection of colorectal liver metastases between 1988 and 1999. Among these, 1104 patients were described who were initially considered to have unresectable disease based on large tumor size, poor tumor location, multinodularity, or presence of extrahepatic disease. After receiving an average of 10 cycles of systemic chemotherapy (5-FU and chronomodulated oxaliplatin), 138 patients (12.5%) demonstrated enough response to become anatomically resectable, but within the median follow-up time of 49 months, 111 (80%) recurred. Recurrence was limited to the liver in 29%. Five-year overall and disease-free survival were 33% and 22%, both of which were lower than for those patients considered resectable prior to chemotherapy (48% and 30%, respectively; P = .01). The authors identified four preoperative factors independently associated with shorter survival: a rectal primary, three or more metastases, maximum tumor size greater than 10 cm, and a carbohydrate antigen (CA) 19-9 greater than 100 U/L. Mean adjusted 5-year survival rates—according to the presence of 0, 1, 2, 3, or 4 of these factors—were 59%, 30%, 7%, 0%, and 0%, respectively (Adam et al, 2004a).
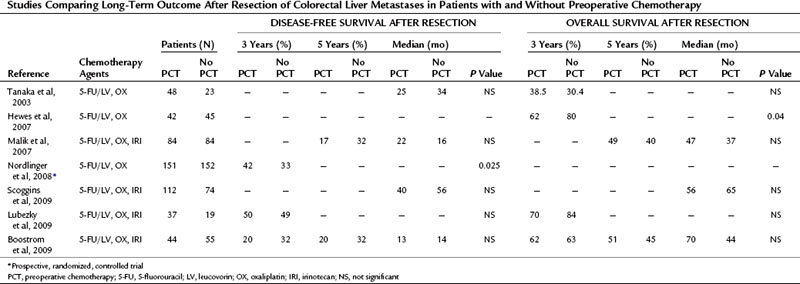
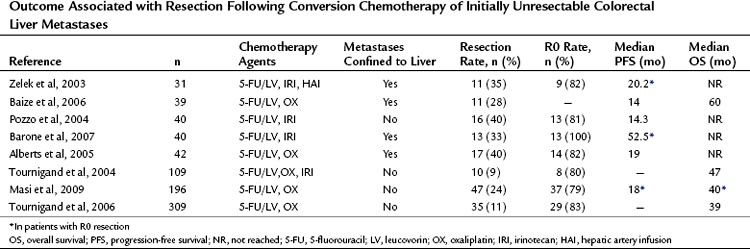
Since those first retrospective reports, prospective trials have provided additional data concerning outcomes of conversion chemotherapy, but few studies report on long-term outcome after resection (Table 87.1). Resectability rates following preoperative chemotherapy for 20 patients presenting with unresectable CLM have been reported as high as 80% (Mentha et al, 2006). Subsequent reports with larger sample sizes provide more modest results with respect to conversion to resectability after chemotherapy (15% to 24%) (Masi et al, 2009; Tournigand et al, 2006). Variability in chemotherapy type, duration, and timing with respect to resection and a lack of standard definitions of resectability make comparison between studies impossible.
Table 87.1 Outcome Associated with Resection Following Conversion Chemotherapy of Initially Unresectable Colorectal Liver Metastases
Two recent prospective studies compared long-term outcomes between primary resection and secondary resection following systemic therapy. Capussotti and colleagues (2006) found no statistical difference in overall survival after resection in 34 patients who were converted to resectable, compared with 116 patients who underwent immediate partial hepatectomy (median survival 41 vs. 50 months, respectively; P = NS). At a median follow-up of 35 months, a 94% recurrence rate was observed in the conversion group compared with 66% in the immediate-resection cohort (P = .001). Disease-free survival was 9 months in the converted group compared with 37 months in the immediate-surgery group (P = .001). These authors also noted that extrahepatic recurrence after resection was greater in patients who were converted from unresectable to resectable (Capussotti et al, 2006).
The second study by Nuzzo and colleagues (2007) compared the outcomes of 60 initially resectable patients to that of a cohort of 15 patients with initially unresectable CLM who underwent complete resection following conversion chemotherapy. This study also found no significant difference in overall survival between initially resectable and unresectable patients with a mean survival of 46 and 47 months, respectively (P = NS). At a median follow-up of 34 months, disease recurrence rates were higher in initially unresectable patients (53%) compared with those of the primarily resectable comparison group (28%), and 3-year disease-free survival rates were 31% and 58%, respectively (P = .04) (Nuzzo et al, 2007).
Combining Systemic Therapy with Surgery
Preoperative Versus Postoperative Chemotherapy
The use of adjuvant chemotherapy in patients who have undergone resection of colorectal liver metastases has the theoretic benefit of decreased recurrence rates from addressing potential micrometastases before they become too large to treat medically. Early evidence of the effect of systemic chemotherapy on resected patients was published by Figueras and colleagues in 2001. They reported on a series of 235 patients who underwent partial hepatectomy with curative intent. Of 180 patients considered for adjuvant treatment with six cycles of 5-FU, only 99 were actually treated, and several patients received oxaliplatin in addition. Patients receiving adjuvant therapy had an improved 5-year survival rate of 53%, compared with 25% for those intended to receive therapy but denied treatment, or for those who declined for other reasons (P < .001). Multivariate analysis revealed adjuvant chemotherapy as an independent predictor of survival (Figueras et al, 2001). Selection bias in this report limits interpretation of the results.
The largest retrospective multicenter study from Parks and colleagues (2007) included 247 patients treated with adjuvant 5-FU–based chemotherapy following resection of CLM and 518 patients who received no adjuvant treatment. They found an increase in overall survival with the use of adjuvant chemotherapy with a 47-month median survival and a 5-year survival of 37% compared with 36 months and 31%, respectively, for patients who did not receive chemotherapy (P = .007). Stratifying patients according to the clinical risk scores (Fong et al, 1999), they noted that patients who received adjuvant chemotherapy had improved survival in each category (range, 1.3- to 2.0-fold) (Parks et al, 2007).
Subsequently, Portier and colleagues (2006) conducted a multicenter, randomized, controlled trial that compared surgery combined with adjuvant 5-FU/LV to surgery alone. After recruiting 173 patients who had undergone R0 resection, this trial was aborted early because of low accrual rates. Although the study was underpowered, the investigators reported their results. At a median follow-up of 87 months, they demonstrated an improved 5-year disease-free survival rate in the adjuvant chemotherapy group compared with that of the surgery-alone group (33.5% vs. 26.7%; P = .028); they also found a nonsignificant trend toward increased 5-year overall survival (51.1% vs. 41.9%; P = .13). Recently, Mitry and colleagues (2008) pooled the results of this trial with another trial reported in abstract form to overcome the lack of statistical power. By combining patients, they were able to increase the study population to 278, of which 138 (50%) received six cycles of adjuvant 5-FU/LV following complete resection. In this combined dataset, a trend toward improved median progression-free survival was noted (27.9 months vs. 18.8 months; P = .058) and a trend toward increased overall survival (62.2 months vs. 47 months; P = .095) for the adjuvant chemotherapy group. Treatment with adjuvant chemotherapy was independently associated with improved disease-free and overall survival by multivariate analysis.
Although these data suggest a benefit for adjuvant systemic therapy following hepatic resection for patients with CLM, other investigators have raised the possibility of using a preoperative approach, as has been championed in the treatment of rectal cancer (Bosset et al, 2005; Sauer et al, 2004). The rationale and theoretic benefits of preoperative chemotherapy include the ability to 1) treat micrometastatic disease prior to exposing patients to the regional growth factors that attend surgery following partial hepatectomy; 2) downsize tumors; 3) ensure that patients will get some systemic therapy, as complications after partial hepatectomy may preclude this; and 4) identify those patients who have aggressive tumor biology with disease progression while on systemic therapy, who would not likely have benefited from surgical resection. On the other hand, the risks of preoperative chemotherapy include liver toxicity, increased risk of disease progression or development of new metastatic sites, and selection of more resistant cell clones (Kemeny, 2007).
The best data have been published by the European Organization for Research and Treatment of Cancer (EORTC). Their prospective randomized controlled trail enrolled 364 patients and compared a group who received perioperative FOLFOX and surgery to a group who received surgery alone. A median of six cycles were given prior to and following resection, and all patients had four or fewer lesions and were deemed resectable prior to enrollment. This trial demonstrated a 7.3% progression-free survival benefit, from 28.1% (95.66% confidence interval [CI], 21.3 to 35.5) to 35.4% (CI, 28.1 to 42.7; hazard ratio [HR], 0.79 [0.62 to 1.02]; P = .06) for patients receiving chemotherapy along with surgery (Nordlinger et al, 2008). Only 63% of patients (115 of 151) in the chemotherapy group actually received chemotherapy following surgery, of which only 70% (n = 80) received all six cycles. Thirty-six patients did not receive postoperative chemotherapy, mainly because of patient refusal, perioperative complications, toxic effects of preoperative chemotherapy, or disease progression. Overall, the results of this study support the hypothesis that chemotherapy is beneficial for some patients undergoing partial hepatectomy for CLM, but it does not address the question of whether treating patient with preoperative chemotherapy impacts prognosis.
The existing retrospective studies that compare survival after resection of CLM with and without preoperative chemotherapy typically include patients who also received adjuvant chemotherapy. To date none of these studies demonstrates a significant benefit from preoperative chemotherapy (Table 87.2).
In Vivo Response to Systemic Treatment
It seems clear that chemotherapy improves the results of resection, but it remains unclear whether such therapy is optimally given before or after surgery. One of the potential benefits of administering chemotherapy prior to hepatic resection for CLM is to assess in vivo response of disease. Adam and colleagues (2004b) investigated the response to chemotherapy as a predictor of long-term outcome in 131 patients after potentially curative resection of multiple liver metastases (≥4). After a median of nine courses of 5-FU/LV with either irinotecan or oxaliplatin, they observed objective tumor response in 58 patients (44%), stable disease in 39 (30%), and tumor progression in 34 (26%). Response to chemotherapy correlated with overall survival following hepatectomy at 37%, 30%, and 8%, respectively, at 5 years (P < .001). In another recent retrospective analysis of 88 patients treated with preoperative oxaliplatin or irinotecan-based chemotherapy regimens followed by partial hepatectomy for resectable CLM, Chiappa and colleagues (2009) also noted that response to chemotherapy on restaging was associated with a significantly improved 5-year survival compared with that of nonresponders (71% vs. 15 %; P = .026).
Two studies from Memorial Sloan-Kettering Cancer Center (MSKCC) addressed the question of chemoresponsiveness of CLM and survival in patients who had staged resections. Allen and colleagues (2003) found that the 46 patients who did not progress on chemotherapy administered prior to hepatic resection saw a 5-year survival benefit over that of the 54 patients who did not receive chemotherapy (85% vs. 35%; P = .03). The results of this analysis suggest that a clinical response to chemotherapy can identify a subset of patients with biologically favorable tumors, and treating clinicians can select them for more aggressive therapy. In a separate study by Gallagher and colleagues (2009) of 111 patients from MSKCC who underwent hepatic resection after preoperative chemotherapy, no differences were seen in median overall survival among patients who responded, had stable disease, or showed progression of disease (58 months vs. 65 months vs. 61 months; P = .98); however, it should be noted that the protocol for this evaluation was to proceed with hepatectomy at the first clinical sign of progression, as opposed to proceeding with resection at the first sign of response.
It remains unclear what the optimal duration of preoperative chemotherapy should be and when to proceed with hepatic resection; however, more surgeons are advocating proceeding with hepatic resection at the first signs of tumor response to avoid extended exposure of the liver to cytotoxic chemotherapy (see Chapter 65). To address this question, White and colleagues (2008) reviewed data for 35 patients with CLM who received FOLFOX with or without bevacizumab and evaluated radiologic tumor changes at 2-month intervals after the start of treatment; they found that the mean cumulative tumor reduction among responders (n = 30) was 39% at 2 months, and it increased to 56% at 4 months (P < .01) after commencing therapy. Importantly, no significant incremental tumor size reduction occurred between 4 and 6 months. Although this study was constructed to assess the time of maximal response in initially unresectable patients, these data suggest that 4 months of preoperative therapy may be sufficient to achieve the maximal tumor response in potentially resectable patients.
Complete Response
As a result of increased effectiveness of systemic chemotherapy, it is now more common for tumors to demonstrate a complete radiographic response. This creates a new set of issues for managing patients with CLM following conversion or preoperative chemotherapy. The question remains whether these lesions are undetectable but still present and therefore likely to grow back. Elias and colleagues (2004) reported on 11 patients in whom at least one liver metastasis disappeared on imaging and could not be localized at laparotomy. Local recurrence was seen in three of these patients (27%), with a median follow-up time of 31.1 months. Few conclusions can be drawn from this initial report, given the small sample size, but the result from this analysis suggests that a durable response is possible in a majority of patients following complete radiographic resolution.
Subsequently, Benoist and colleagues (2006) reported on 66 liver metastases that disappeared radiographically following chemotherapy, which represented 20% of all lesions in the cohort. At the time of exploration, 31 lesions (47%) could not be identified, even with intraoperative ultrasonography, and they were consequentially not removed. By 1 year of follow-up, 23 (74%) of these lesions recurred. Of the other 35 liver lesions (53%) identified and removed at surgery, 32 (91%) contained viable disease, suggesting that tumors are still present, even when they disappear on imaging. Thus 55 of the original 66 lesions (83%) either harbored residual viable disease or returned following complete radiographic response.
Even more compelling is the question of a complete histologic response and how it impacts patient survival. In a study from the M.D. Anderson Cancer Center (MDACC), 305 patients received a median of five cycles of oxaliplatin- and irinotecan-based chemotherapy, with or without bevacizumab, prior to hepatic resection, and they were then stratified according to histopathologic response (Blazer et al, 2008). Complete response with no residual histologic evidence of tumor was observed in 9%, a major response (1% to 49% residual cells) was seen in 36%, and a minor response (≥50% residual cancer cells) was seen in 55% of tumors in the resection specimen. The 5-year survival rates for these three groups were 75%, 56%, and 33%, respectively (P < .05), and pathologic response to preoperative chemotherapy was independently associated with improved survival on multivariate analysis.
A report by Chun and colleagues (2009) assessed the association of morphologic radiologic response with pathologic response. A total of 234 lesions were examined from 50 patients who underwent hepatic resection after treatment with bevacizumab. In the cohort that had an “optimal” morphologic radiologic response, the median number of residual tumor cells was 20%, and the median survival was not yet reached by the time of the study’s conclusion. In contrast, patients who had an “incomplete or absent” response had 50% to 70% residual tumor cells on pathologic assessment and had a shorter median survival (31 months; P = .03). This report represents an early assessment of the correlation between radiographic and histologic findings and how they correlate with patient outcome.
These data suggest that all original sites of metastases should be addressed surgically or with ablative therapy if possible, even if they are no longer present on restaging imaging, as they are likely to harbor viable malignant cells that may progress at some point. Identifying hepatic metastases after a complete radiologic response remains a technical challenge that can be overcome by use of a simple coil-marking technique described by Zalinski and colleagues (2009). This is especially valuable in steatotic, chemotherapy-treated livers, given the muted contrast between the lesions and the liver background as a result of impaired perfusion (Peppercorn et al, 1998). Medical oncologists are urged to refer potential resection candidates to surgeons prior to starting chemotherapy, and surgeons are urged to review available imaging obtained prior to preoperative chemotherapy.
Chemotherapy-Induced Liver Damage (See Chapter 65)
With increased use of preoperative chemotherapy, treating physicians are becoming more aware of the toxic effects of chemotherapy on liver parenchyma and how these changes affect postoperative outcome (Fong & Bentrem, 2006; Zorzi et al, 2007). Investigators have shown a wide spectrum of histopathologic changes to the underlying liver parenchyma in resection specimens (see Chapter 65 for a detailed description). Prolonged use of 5-FU and irinotecan-based chemotherapy has been associated with fatty liver changes ranging from mild forms of steatosis to more severe forms of steatohepatitis with additional necroinflammatory components (Peppercorn et al, 1998; Vauthey et al, 2006). Oxaliplatin-based regimens have been associated with venoocclusive injuries, termed sinusoidal obstruction syndrome (SOS) (Rubbia-Brandt et al, 2004). Although the association between chemotherapy and histopathologic changes has been well documented, the impact of chemotherapy-associated hepatotoxicity on surgical management of colorectal liver metastases remains somewhat ill defined.
From the transplant literature, we know that hepatic steatosis can impair liver function and postoperative regeneration. In a study of 485 patients undergoing liver resections for neoplasms (325 steatotic livers, 160 matched controls), steatosis was found to be an independent predictor of postoperative complications on multivariate analysis (P < .01) (Kooby et al, 2003). Overall and infective complication rates of 62% and 43% for the marked steatosis group (n = 102) were significantly higher than in the control group, at 35% and 14%, respectively (P < .01). The association between steatosis and previous exposure to chemotherapy was noted. Vauthey and colleagues (2006) systematically analyzed hepatic injury in 158 patients treated with varying preoperative chemotherapy regimens for metastatic CRC and correlated them to postoperative outcomes after hepatic resection. This study distinguished steatosis and steatohepatitis as separate pathologic findings and demonstrated that steatohepatitis induced by irinotecan-based chemotherapy was associated with an increase in 90-day mortality following major hepatectomy for CLM (14.7% vs. 1.4%, no steatohepatitis; P = .001). Furthermore, patients with steatohepatitis have a higher risk of death from postoperative liver failure compared with all other patients (6% vs. 1%; P = .01).
Karoui and colleagues (2006) reported that chemotherapy was significantly associated with sinusoidal dilation (P = .005), present in 49% of patients in the chemotherapy group (two thirds receiving oxaliplatin) compared with 14% in controls. Furthermore, significantly greater postoperative morbidity after major hepatectomy was found in the chemotherapy group compared with the control group (38% vs. 14%; P = .03), which was mostly attributed to the high incidence of transient liver failure in the chemotherapy group (11% vs. 0% in controls; P = NS). The addition of bevacizumab to oxaliplatin-based chemotherapy may have a protective effect on sinusoidal injury. Ribero and colleagues (2007) found that the addition of bevacizumab to oxaliplatin-based chemotherapy reduced the incidence of sinusoidal dilation of any grade by half (27% vs. 54%) and lowered severe, grade 2 to 3 sinusoidal dilation (8% vs. 28%) to about a third of reviewed pathologic specimens.
Although multiple studies support that patients with damaged livers have higher perioperative morbidity and mortality rates compared to those with healthy livers, others that directly compare perioperative outcome with and without preoperative chemotherapy for CLM have not been able to demonstrate this phenomenon (Pawlik et al, 2007; Scoggins et al, 2009). The reason for this discrepancy likely lies in factors of timing and dose of preoperative chemotherapy treatments and extent of hepatic resection. Both prolonged chemotherapy treatments and short intervals between the cessation of chemotherapy have been found to be associated with significantly increased morbidity (Aloia et al, 2006; Welsh et al, 2007). Although the optimal regimen and duration of systemic chemotherapy are still being assessed, dialogue between treating medical and surgical oncologists regarding the timing of surgery is critical. Theoretically, a longer interval may provide the liver time to recover from any reversible hepatotoxic effect of chemotherapy. On the other hand, a longer interval prior to hepatic resection may result in progression of disease. With this in mind, most surgeons will proceed with resection between 3 and 6 weeks after the last dose of chemotherapy.
Management of Synchronous Liver Metastases
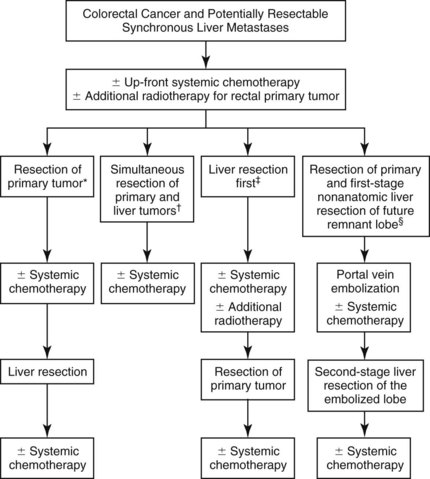
Synchronously diagnosed CRC liver metastases pose additional challenges regarding the timing, sequence, and method of treatment. The traditional approach would be resection of the colorectal primary followed by a course of adjuvant chemotherapy and hepatic resection in the absence of interval disease progression; however, in the era of more active chemotherapy, this approach remains most applicable for patients with unresectable liver metastases, with the hope of conversion to secondarily resectable status. Alternative treatment pathways have also been proposed for patients with initially resectable synchronous liver disease (Fig. 87.1). The first issue specific to this patient subset is the question of whether the bowel or liver should be addressed first, or if they should both be removed at the same time.
In recent years, two large studies have confirmed previous smaller reports that simultaneous colon and hepatic resections are safe and may demonstrate benefits of reduced morbidity and mortality compared with staged procedures (Martin et al, 2003; Reddy et al, 2007). In the earlier report, by Martin and colleagues (2003), simultaneous resections were more likely performed for right colon primaries and for smaller and fewer liver metastases, and they resulted in similar rates of major complications (27% vs. 25%; P = NS) and mortality (4% vs. 4%; P = NS) for patients undergoing major hepatic resections between the groups. Reddy and colleagues (2007) found similar morbidity between simultaneous and staged minor hepatectomy (overall 36% and 38%; P = NS), but a significantly higher rate of severe morbidity (36.1 vs. 17.6%; P = .05) and mortality (8.3 vs. 0%; P = .007) was reported after simultaneous major hepatic resection. Long-term outcomes are similar among simultaneous and staged hepatic resections for synchronous CLM, and more than one study has shown that timing of hepatic resection does not affect long-term outcome (Minagawa et al, 2006; Tanaka et al, 2004).
Another more recent report by de Haas and colleagues (2010) confirms the safety of simultaneous resection, but it suggests that this strategy is an independent predictor of earlier tumor recurrence compared with the delayed hepatectomy approach. The authors compared outcomes from 55 patients who had simultaneous hepatic and colorectal resections to 173 patients who had the staged approach. The morbidity rate was actually lower in the simultaneous group (11% vs. 25%; P = .015), but more recurrences were observed in the simultaneous group at 3 years (85% vs. 64%; P = .002).
The “liver first” approach has been introduced as alternative treatment strategy for patients with advanced CRC with synchronous liver metastases. Patients receive chemotherapy before the liver resection and then undergo resection of the colorectal primary at a later date. This approach resulted from the improved efficacy of chemotherapy and focuses on delivering chemotherapy to the liver without potential delays as a result of prolonged recoveries from colon surgery. This idea is based on the belief that the liver is the component that will ultimately limit survival. Secondly, authors have raised concerns of enhanced metastatic tumor growth after removal of the primary tumor (Demicheli et al, 2008). Published reports of this approach are limited to small case series. Mentha and colleagues (2008) have recently updated their initial series and reported 35 patients, of which 30 completed the pathway. With a clinical risk score of 3 or higher, the reported 5-year survival of 31%, with a median survival of 44 months, is encouraging. Limitations of this approach include risks of secondary obstruction, disease progression, and chemotherapy-induced liver damage.
The role of preoperative chemotherapy in patients with resectable synchronous liver metastases remains a subject of debate. Despite the theoretic benefits of preoperative chemotherapy, data on the actual survival benefits are equivocal. In a study of 151 patients initially evaluated at MSKCC, Fahy and colleagues (2009) reported an overall disease-specific survival of 26.7 months after diagnosis of synchronous colon cancer. Both liver resection and preoperative chemotherapy were treatment modalities significantly associated with survival, but most patients received some sort of postresection therapy. Reddy and colleagues (2009) specifically addressed timing of chemotherapy in their large multiinstitutional study of 499 patients undergoing resection for synchronous CLM. Postoperative chemotherapy alone was independently associated with recurrence-free survival, if given for 6 months or longer (HR, 0.75; P = .04), and with overall survival, if administered for 2 months or longer (HR, 0.59; P = .001). Preoperative chemotherapy alone was not associated with improved survival, but patients with perioperative chemotherapy had similar outcomes to patients who received postoperative chemotherapy alone, thus given the lack of level 1 evidence, the available data do not support use of prehepatectomy chemotherapy uniformly in all resectable synchronous CLM.
Among selected patients prehepatectomy therapy may be useful. Tanaka and colleagues (2003) found that prehepatectomy chemotherapy was associated with improved survival (5-year survival of 39% vs. 52%; P = .04) in a group of 71 patients with bilateral multiple, mostly synchronous CLM. Interestingly, the authors also noted a lower rate of major hepatectomy in their patients who received prehepatectomy chemotherapy (81% vs. 100%; P = .027). Given the fact that patients who received preoperative chemotherapy had unresectable extensive bilobar disease, this is one of the few studies that indicate the possibility for smaller resection after effective treatment; however, no studies directly compare extent of resection to the originally planned procedure before chemotherapy.
In rectal cancer patients, the potential need for locoregional radiotherapy further complicates the matter. Neoadjuvant chemosensitizing radiation has proven to lower local relapse rates to less than 10% (range, 6% to 8%) in locally advanced rectal cancer (T3 to T4) in several prospective randomized trials (Roh et al, 2009; Sauer et al, 2004). This outcome is extremely valuable, because surgical salvage options are slim and are generally associated with poor quality of life and poor survival (Salo et al, 1999); however, this treatment modality has not proven to be beneficial in terms of overall survival, and its role in patients with stage IV disease is unclear. Several potential approaches to integrating neoadjuvant chemoradiation exist in this stage of disease, and the question is in what sequence neoadjuvant chemotherapy, radiation, or chemoradiation should be given. This decision naturally is dictated by the toxicity of such extensive therapy and based on the needs of the individual patient. Although simultaneous resection can theoretically be achieved through one long midline incision, most surgeons recommend a staged procedure with initial resection of the rectal primary (Reddy et al, 2007). Early reports of the liver-first approach have also been published from a European Center (Verhoef et al, 2009). Of 22 patients with advanced local rectal primaries and synchronous liver metastases in this series, 16 patients (73%) completed the full treatment protocol, and all were alive after a median of 19 months. Morbidity of this approach was equivalent to that seen with the rectum-first approach and simultaneous resections in a second small case series (van der Pool et al, 2010).
Extrahepatic Disease
The presence of extrahepatic disease in the setting of CLM has traditionally been considered a contraindication for hepatic resection. With better systemic chemotherapy, survival has improved in all stages of disease, and surgeons have become more aggressive in considering resection in patients who were not candidates in the past. Several centers have published their experience with liver resection in patients with concomitant extrahepatic disease. Carpizo and colleagues (2009) reported the outcome of 127 patients with extrahepatic disease, which comprised 9% of all patients undergoing liver resection at MSKCC between 1997 and 2007. Overall survival of 47% and 26% at 3 and 5 years was significantly inferior to the 67% and 49% (P < .001) observed in patients without extrahepatic disease. Importantly, the survival observed in patients with extrahepatic disease in this study is similar to that observed for patients with only liver disease resected during the 1980s and 1990s.
It is noteworthy that recurrence was observed in 95% of patients in this group with median follow-up of 24 months. This included both local extrahepatic invasion and distant metastatic sites including lung, ovaries, peritoneum, and regional hepatic lymph nodes. Although this study found that patients with perihepatic lymph node metastases had worse survival than those with lung and ovarian metastases, with a median survival of 26, 45, and 82 months, respectively (P < .05). The incidence of occult microscopic lymph node involvement ranged from 2% to as high as 28% (Beckurts et al, 1997), likely because of the fact that hepatic lymph node dissection is not commonly performed. Adam and colleagues (2008a) found regional lymph node metastases in 6% of 763 patients undergoing hepatic resection for CLM and reported a predicted 5-year survival that was significantly inferior compared with that of patients without nodal involvement (18% vs. 53%; P = .001). The authors pointed out that all patients who survived 5 years had lymph node metastases in the hepatoduodenal ligament, and no long-term survivors were among those with celiac or retroperitoneal lymph node metastases.
Jaeck and colleagues (2002) prospectively performed systematic portal lymphadenectomy on 160 patients and examined differences in survival of different subsites; overall, metastatic lymph nodes were found in 17 patients (11%), with a 3-year survival of 19%. Similarly, this study distinguished between hepatoduodenal ligament nodes (zone 1) and celiac/common hepatic artery nodes (zone 2) and found a substantially worse survival for patients with zone 2 compared with zone 1 lymph node involvement (0% 1-year vs. 38% 3-year survival). The same group recently updated their results of 45 patients with regional lymph node involvement (Oussoultzoglou et al, 2009). After a change in multimodal treatment strategies, the vast majority received modern oxaliplatin- or irinotecan-based chemotherapy, with or without bevacizumab or cetuximab, and the surgical approach shifted toward a more aggressive lymphadenectomy. With a median follow up of 26 months, 3-year overall survival was increased to 29.7%, and 5-year survival of 17.3% was reported. Interestingly, no significant survival difference was found among patients with zone 1 and 2 nodal involvement, with a median survival of 19 and 20 months (P = .44), respectively. Elevated carcinoembryonic antigen (CEA) greater than 200 µg/L was found in 82% of patients and was found to be the only preoperative factor independently associated with overall survival (odds ratio [OR], 7.1; P < .001).
Portal Vein Embolization and Chemotherapy
Preoperative portal vein embolization (PVE) is used to promote compensatory hypertrophy in patients whose future liver remnant (FLR) may be insufficient to support them during the recovery phase following major hepatic resection. Techniques, indications, and results of PVE are outlined in Chapter 93A, Chapter 93B ; they will only be discussed briefly here, as they may be affected by concurrent systemic chemotherapy or underlying chemotherapy-induced liver damage.
PVE increases the safety of hepatectomy for patients with marginal hepatic reserve. The average increase of the estimated FLR is between 8% and 16% at 2 to 4 weeks after PVE, depending on the measurement technique and underlying liver disease (Covey et al, 2005; Madoff et al, 2003; Vauthey et al, 2000). Long-term survival has been found to be comparable for patients with and without PVE before major hepatic resection for CLM, at 40% and 38% at 5 years (Azoulay et al, 2000).
Given the cytotoxic nature of chemotherapeutic agents and their ability to hinder cell proliferation, the question arises whether liver regeneration and hypertrophy are altered by concurrent chemotherapy. In particular, the inhibition of vascular endothelial growth factor (VEGF), as targeted by bevacizumab, has been shown to impair liver regeneration in animal models after partial hepatectomy (Bockhorn et al, 2007). One of the first reports to address this issue was from Goéré and colleagues (2006) in France. Data from 10 patients who had no chemotherapy between static portal embolization and surgery were compared with data from another 10 patients who had continuous chemotherapy. No significant difference was observed in FLR growth between the groups, but the sample size was small. Covey and colleagues (2008) further addressed this question in a study of 100 patients undergoing PVE before resection of CLM, 43 of whom received concurrent chemotherapy of various regimens. This study found no statistically significant difference in liver growth between the patients who had concurrent chemotherapy treatment and those who did not (22% vs. 26%; P = NS). Confirming results were presented by Zorzi and colleagues (2008), who showed no difference in FLR growth after PVE in patients treated without chemotherapy, those treated with systemic chemotherapy, and those treated with chemotherapy that included bevacizumab (10.1% vs. 8.8% vs. 6.8%; P = NS). More recently, one paper suggested that a bevacizumab-containing chemotherapy regimen impairs FLR hypertrophy after PVE (Aussilhou et al, 2009). The authors found a significantly smaller increase in FLR in the bevacizumab-treated group (n = 13) compared with the group that did not receive bevacizumab (n = 27) in their regimens (561 + 171 cm3 vs. 667 + 213 cm3; P = .031). Based on existing data, administration of systemic therapy appears to be acceptable around the time of PVE, but it may be prudent to avoid bevacizumab in these patients.
Data regarding the impact of chemotherapy-induced liver injuries on liver regeneration after PVE are scant. A recent study showed that steatosis was associated with significantly lower hypertrophy volume and hypertrophy ratios after right PVE in 35 patients after hemihepatectomy (Tanaka et al, 2010). Based on existing data, it appears safe to consider PVE in patients who receive chemotherapy prior to hepatic resection; however, the actual benefit of PVE for this set of patients still remains unclear. Our recommendation is to limit the use of PVE to patients who will undergo major hepatic resection with an FLR less than 25%. Further data on PVE in patients with CLM are needed.
Two-Stage Hepatectomy
Two-stage hepatectomy is a relatively new strategy to expand the criteria of resectability. Using this approach, complete resection is feasible in selected patients with bilateral CLM. PVE or portal vein ligation is often required after the first resection to promote hypertrophy of the FLR and to ensure sufficient liver remnant. In the first description by Adam and colleagues (2000), the authors proposed to remove as many tumor sites as possible during the first operation, followed by a course of chemotherapy, with or without PVE, and second-stage resection to clear the remaining liver. Among 13 patients who had both resections, reported 3-year survival was 35%. This strategy was then modified by Jaeck and colleagues (2004) because of concerns of accelerated tumor growth during the regeneration period after major hepatectomy and PVE. The new strategy is to initially remove tumor in the FLR, clear one side of the liver in a usually smaller operation prior to PVE, and resect the remaining tumor-bearing liver in a second operation.
Success rates and long-term outcome of patients who have both hepatic resections largely depend on patient selection and utilization of modern chemotherapy. The literature reports success rates from 69% to 92% (Adam et al, 2000; Jaeck et al, 2004; Tanaka et al, 2007; Wicherts et al, 2008), but perioperative morbidity and mortality rates are uniformly higher after the second hepatectomy. In the largest series of 41 patients who underwent two-stage hepatectomy, the cumulative morbidity was 79%, with an overall morbidity of 20% and 59% and mortality of 0% and 7% after the first- and second-stage hepatectomy, respectively (Wicherts et al, 2008).
The best long-term results were presented in a study by Chun and colleagues (2007) from the MDACC. In this study, all patients received a course of modern oxaliplatin- or irinotecan-based chemotherapy preoperatively, and 81% received additional chemotherapy postoperatively. With a median follow-up of 25 months, they reported an astonishing 3-year overall and disease-free survival of 86% and 51%, respectively, which was favorable when compared with patients who had a one-stage hepatectomy. Although this series presents a well-selected patient population, it demonstrates that in combination with modern chemotherapy, two-stage hepatectomy offers a chance of long-term survival for patients who would otherwise not be candidates for resection.
Abdalla EK, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818-825. discussion 825-817
Adam R, et al. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777-785.
Adam R, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347-353.
Adam R, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644-657. discussion 657-648
Adam R, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052-1061. discussion 1061-1054
Adam R, et al. Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement? J Clin Oncol. 2008;26:3672-3680.
Adam R, et al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol. 2008;26:1635-1641.
Adson MA, et al. Resection of hepatic metastases from colorectal cancer. Arch Surg. 1984;119:647-651.
Alberts SR, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243-9249.
Allegra CJ, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091-2096.
Allen PJ, et al. Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg. 2003;7:109-115. discussion 116-107
Aloia T, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983-4990.
Aussilhou B, et al. Preoperative liver hypertrophy induced by portal flow occlusion before major hepatic resection for colorectal metastases can be impaired by bevacizumab. Ann Surg Oncol. 2009;16:1553-1559.
Azoulay D, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480-486.
Baize N, et al. Long-term survival of patients downstaged by oxaliplatin and 5-fluorouracil combination followed by rescue surgery for unresectable colorectal liver metastases. Gastroenterol Clin Biol. 2006;30:1349-1353.
Barone C, et al. Final analysis of colorectal cancer patients treated with irinotecan and 5-fluorouracil plus folinic acid neoadjuvant chemotherapy for unresectable liver metastases. Br J Cancer. 2007;97:1035-1039.
Beckurts KT, et al. Significance of lymph node involvement at the hepatic hilum in the resection of colorectal liver metastases. Br J Surg. 1997;84:1081-1084.
Bengmark S, Hafstrom L. The natural history of primary and secondary malignant tumors of the liver. I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer. 1969;23:198-202.
Benoist S, et al. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol. 2006;24:3939-3945.
Bismuth H, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509-520. discussion 520-502
Blazer DG3rd, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344-5351.
Bockhorn M, et al. VEGF is important for early liver regeneration after partial hepatectomy. J Surg Res. 2007;138:291-299.
Bokemeyer C, et al. Overall survival of patients with KRAS wild-type tumours treated with FOLFOX4 +/− cetuximab as 1st-line treatment for metastatic colorectal cancer: the OPUS study. EJC Supplements. 7, 2009. 346–346
Boostrom SY, et al. Impact of neoadjuvant chemotherapy with FOLFOX/FOLFIRI on disease-free and overall survival of patients with colorectal metastases. J Gastrointest Surg. 2009;13:2003-2009. discussion 2009-2010
Bosset JF, et al. Enhanced tumoricidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results—EORTC 22921. J Clin Oncol. 2005;23:5620-5627.
Capussotti L, et al. Neoadjuvant chemotherapy and resection for initially irresectable colorectal liver metastases. Br J Surg. 2006;93:1001-1006.
Carpizo DR, et al. Liver resection for metastatic colorectal cancer in patients with concurrent extrahepatic disease: results in 127 patients treated at a single center. Ann Surg Oncol. 2009;16:2138-2146.
Chiappa A, et al. Neoadjuvant chemotherapy followed by hepatectomy for primarily resectable colorectal cancer liver metastases. Hepatogastroenterology. 2009;56:829-834.
Choti MA, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759-766.
Chun YS, et al. Systemic chemotherapy and two-stage hepatectomy for extensive bilateral colorectal liver metastases: perioperative safely and survival. J Gastrointest Surg. 2007;11(11):1498-1504.
Chun YS, et al. Comparison of two methods of future liver remnant volume measurement. J Gastrointest Surg. 2008;12:123-128.
Chun YS, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338-2344.
Covey AM, et al. Safety and efficacy of preoperative portal vein embolization with polyvinyl alcohol in 58 patients with liver metastases. AJR Am J Roentgenol. 2005;185:1620-1626.
Covey AM, et al. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg. 2008;247:451-455.
de Gramont A, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol. 1997;15:808-815.
de Gramont A, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947.
de Haas RJ, et al. Comparison of simultaneous or delayed liver surgery for limited synchronous colorectal metastases. Br J Surg. 2010;97:1279-1289.
Demicheli R, et al. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. 2008;19:1821-1828.
Douillard JY, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047.
Ekberg H, et al. Pattern of recurrence in liver resection for colorectal secondaries. World J Surg. 1987;11:541-547.
Elias D, et al. Evolution of missing colorectal liver metastases following inductive chemotherapy and hepatectomy. J Surg Oncol. 2004;86:4-9.
Fahy BN, et al. Synchronous hepatic metastases from colon cancer: changing treatment strategies and results of surgical intervention. Ann Surg Oncol. 2009;16:361-370.
Fernandez FG, et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg. 2004;240:438-447. discussion 447-450
Figueras J, et al. Resection rate and effect of postoperative chemotherapy on survival after surgery for colorectal liver metastases. Br J Surg. 2001;88:980-985.
Folprecht G, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38-47.
Fong Y, Bentrem DJ. CASH (chemotherapy-associated steatohepatitis) costs. Ann Surg. 2006;243:8-9.
Fong Y, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938-946.
Fong Y, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-318. discussion 318-321
Gallagher DJ, et al. Response to neoadjuvant chemotherapy does not predict overall survival for patients with synchronous colorectal hepatic metastases. Ann Surg Oncol. 2009;16:1844-1851.
Giacchetti S, et al. Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann Oncol. 1999;10:663-669.
Goéré D, et al. Chemotherapy does not impair hypertrophy of the left liver after right portal vein obstruction. J Gastrointest Surg. 2006;10:365-370.
Goldberg RM, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23-30.
Gruenberger B, et al. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol. 2008;26:1830-1835.
Hewes JC, et al. Preoperative chemotherapy and the outcome of liver resection for colorectal metastases. World J Surg. 2007;31:353-364. discussion 365-356
Hoff PM, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19:2282-2292.
Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342.
Jaeck D, et al. Significance of hepatic pedicle lymph node involvement in patients with colorectal liver metastases: a prospective study. Ann Surg Oncol. 2002;9:430-438.
Jaeck D, et al. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037-1049. discussion 1049-1051
Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987-989.
Karoui M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1-7.
Kemeny N. Presurgical chemotherapy in patients being considered for liver resection. Oncologist. 2007;12:825-839.
Kooby DA, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034-1044.
Lubezky N, et al. Is there a survival benefit to neoadjuvant versus adjuvant chemotherapy, combined with surgery for resectable colorectal liver metastases? World J Surg. 2009;33:1028-1034.
Mackay HJ, et al. A multicenter phase II study of “adjuvant” irinotecan following resection of colorectal hepatic metastases. Am J Clin Oncol. 2005;28(6):547-554.
Madoff DC, et al. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness—study in 26 patients. Radiology. 2003;227:251-260.
Malik HZ, et al. A critical appraisal of the role of neoadjuvant chemotherapy for colorectal liver metastases: a case-controlled study. Ann Surg Oncol. 2007;14:3519-3526.
Martin R, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg. 2003;197:233-241. discussion 241-232
Masi G, et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg. 2009;249:420-425.
Mentha G, et al. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg. 2006;93:872-878.
Mentha G, et al. Liver first” approach in the treatment of colorectal cancer with synchronous liver metastases. Dig Surg. 2008;25:430-435.
Meta-Analysis Group In Cancer. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. Meta-Analysis Group In Cancer. J Clin Oncol. 1998;16:301-308.
Minagawa M, et al. Selection criteria for simultaneous resection in patients with synchronous liver metastasis. Arch Surg. 2006;141:1006-1012. discussion 1013
Mitry E, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26:4906-4911.
Nordlinger B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016.
Nuzzo G, et al. Liver resection for primarily unresectable colorectal metastases downsized by chemotherapy. J Gastrointest Surg. 2007;11:318-324.
Oussoultzoglou E, et al. Long-term survival after liver resection for colorectal liver metastases in patients with hepatic pedicle lymph nodes involvement in the era of new chemotherapy regimens. Ann Surg. 2009;249:879-886.
Parks R, et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg. 2007;204:753-761. discussion 761-753
Pawlik TM, et al. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg. 2007;11:860-868.
Peppercorn PD, et al. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil-based therapy for advanced colorectal cancer. Br J Cancer. 1998;77:2008-2011.
Portier G, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976-4982.
Pozzo C, et al. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004;15:933-939.
Reddy SK, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14:3481-3491.
Reddy SK, et al. Timing of multimodality therapy for resectable synchronous colorectal liver metastases: a retrospective multi-institutional analysis. Ann Surg Oncol. 2009;16:1809-1819.
Ribero D, et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761-2767.
Roh MS, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124-5130.
Rubbia-Brandt L, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460-466.
Salo JC, et al. Surgical salvage of recurrent rectal carcinoma after curative resection: a 10-year experience. Ann Surg Oncol. 1999;6:171-177.
Saltz LB, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-914.
Sauer R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740.
Scappaticci FA, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol. 2005;91:173-180.
Scoggins CR, et al. Preoperative chemotherapy does not increase morbidity or mortality of hepatic resection for colorectal cancer metastases. Ann Surg Oncol. 2009;16:35-41.
Steele GJr, Ravikumar TS. Resection of hepatic metastases from colorectal cancer: biologic perspective. Ann Surg. 1989;210:127-138.
Sugihara K, et al. Pattern of recurrence after hepatic resection for colorectal metastases. Br J Surg. 1993;80:1032-1035.
Tanaka K, et al. Role of neoadjuvant chemotherapy in the treatment of multiple colorectal metastases to the liver. Br J Surg. 2003;90:963-969.
Tanaka K, et al. Outcome after simultaneous colorectal and hepatic resection for colorectal cancer with synchronous metastases. Surgery. 2004;136:650-659.
Tanaka K, et al. Remnant liver regeneration after two-stage hepatectomy for multiple bilobar colorectal metastases. Eur J Surg Oncol. 2007;33:329-335.
Tanaka K, et al. Influence of chemotherapy on liver regeneration induced by portal vein embolization or first hepatectomy of a staged procedure for colorectal liver metastases. J Gastrointest Surg. 2010;14:359-368.
Thirion P, et al. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol. 2004;22:3766-3775.
Tournigand C, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237.
Tournigand C, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer—a GERCOR study. J Clin Oncol. 2006;24:394-400.
Van Cutsem E, et al. The CRYSTAL study: assessment of the predictive value of KRAS status on clinical outcome in patients with MCRC receiving first-line treatment with cetuximab or cetuximab plus FOLFIRI. Ann Oncol. 2008;19:17-18.
van der Pool AE, et al. Optimizing the outcome of surgery in patients with rectal cancer and synchronous liver metastases. Br J Surg. 2010;97:383-390.
Vauthey JN, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512-519.
Vauthey JN, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065-2072.
Verhoef C, et al. The “liver-first approach” for patients with locally advanced rectal cancer and synchronous liver metastases. Dis Colon Rectum. 2009;52:23-30.
Welsh FK, et al. Safe liver resection following chemotherapy for colorectal metastases is a matter of timing. Br J Cancer. 2007;96:1037-1042.
White RR, et al. Assessing the optimal duration of chemotherapy in patients with colorectal liver metastases. J Surg Oncol. 2008;97:601-604.
Wicherts DA, et al. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008;248:994-1005.
Willett CG, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145-147.
Zalinski S, et al. A marking technique for intraoperative localization of small liver metastases before systemic chemotherapy. Ann Surg Oncol. 2009;16:1208-1211.
Zelek L, et al. Multimodal therapy with intravenous biweekly leucovorin, 5-fluorouracil and irinotecan combined with hepatic arterial infusion pirarubicin in non-resectable hepatic metastases from colorectal cancer (a European Association for Research in Oncology trial). Ann Oncol. 2003;14:1537-1542.
Zorzi D, et al. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274-286.
Zorzi D, et al. Chemotherapy with bevacizumab does not affect liver regeneration after portal vein embolization in the treatment of colorectal liver metastases. Ann Surg Oncol. 2008;15:2765-2772.