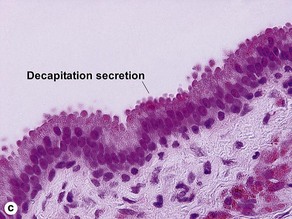
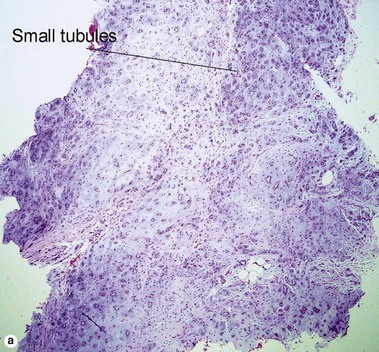
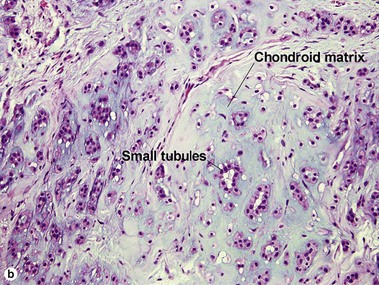
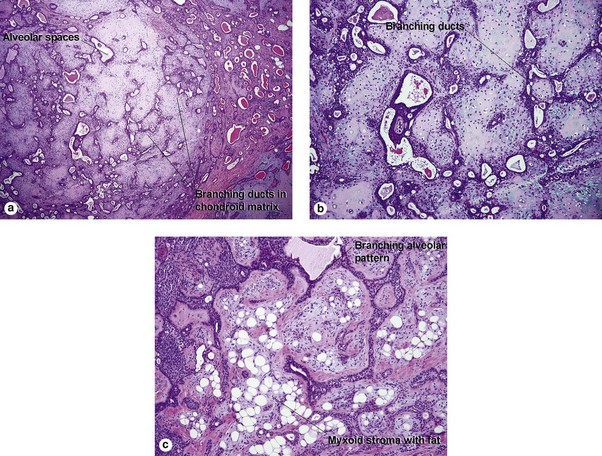
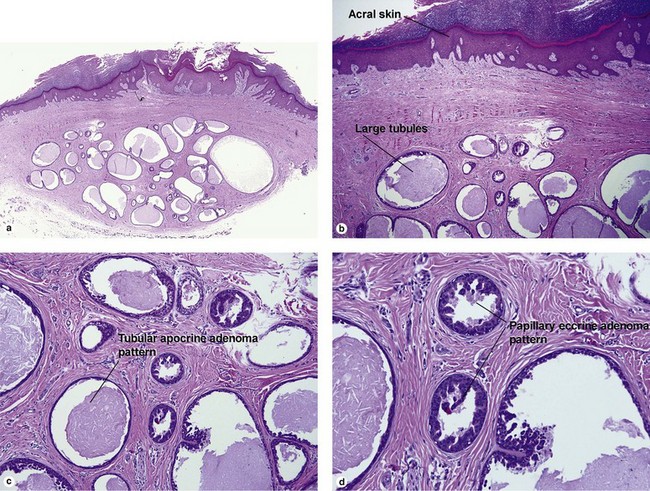
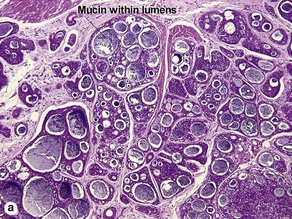
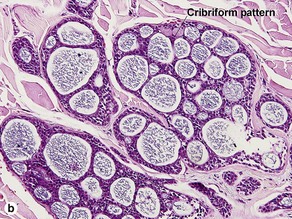
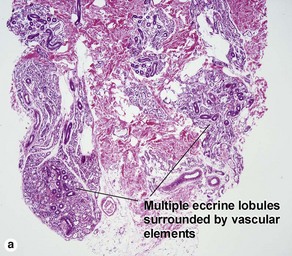
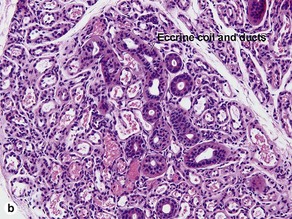
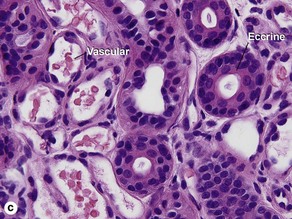
Sweat gland neoplasms
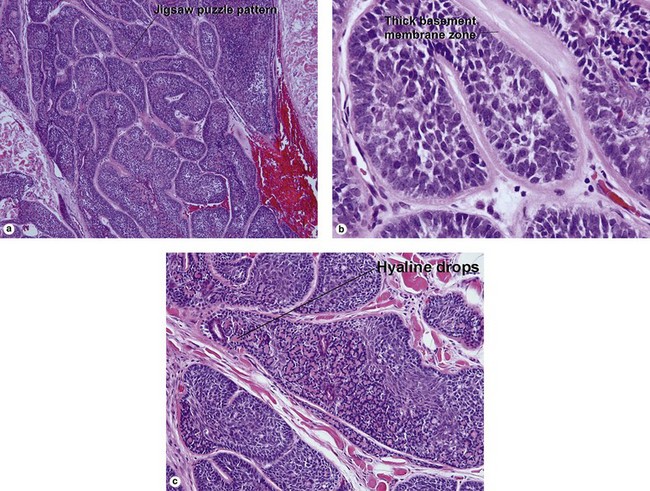
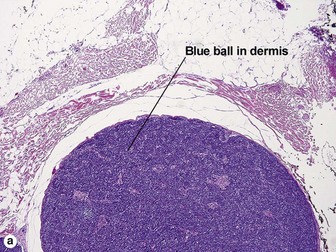
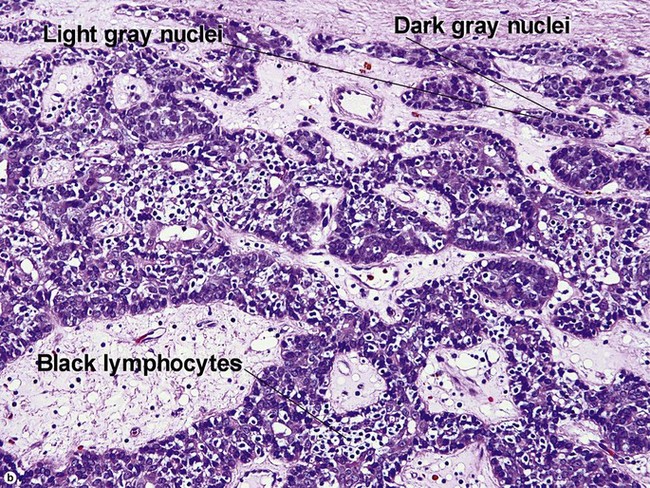
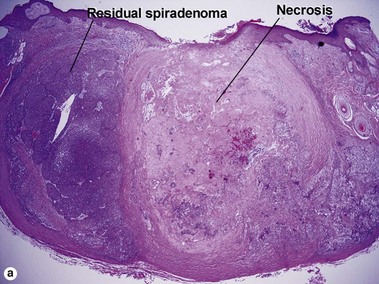
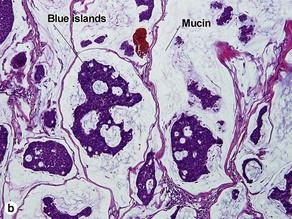
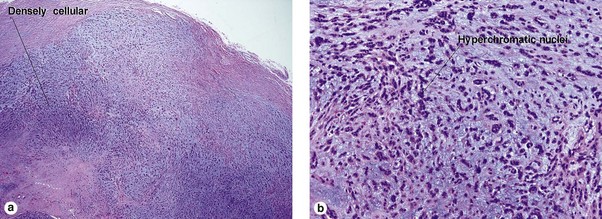
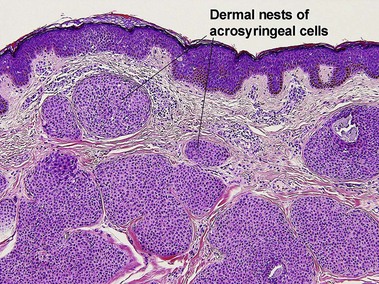
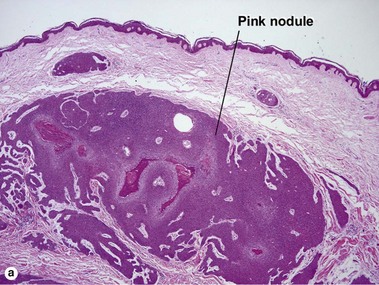
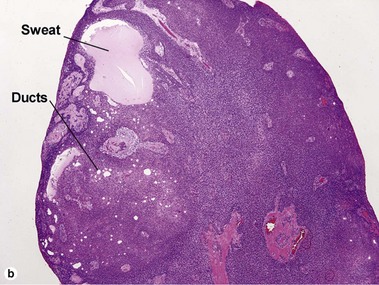
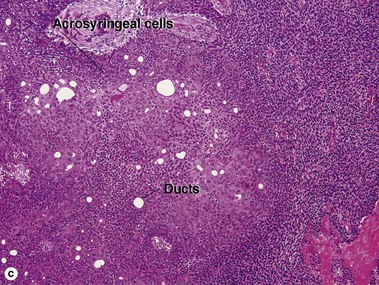
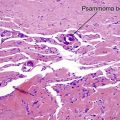
Spiradenoma
Table 5-1
Characteristics of cylindroma versus spiradenoma
| Characteristic | Cylindroma | Spiradenoma |
| Blue cells with little cytoplasm | Yes | Yes |
| Dark and pale blue nuclei | Yes | Yes |
| Peppered with black lymphocytes | No | Yes |
| Hyaline droplets in nests | Yes | Yes |
| Jigsaw-puzzle pattern | Yes | No |
| Deeply eosinophilic basement membrane | Yes | No |
| Inheritance | Autosomal dominant | Sporadic |
| Tender | No | Yes |
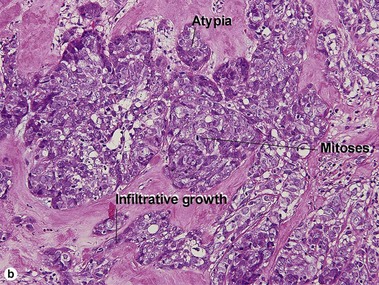
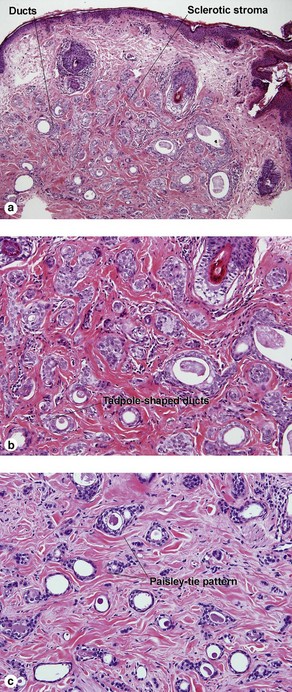
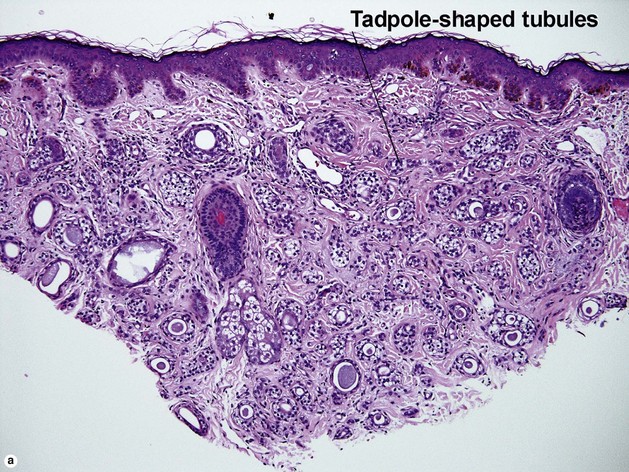
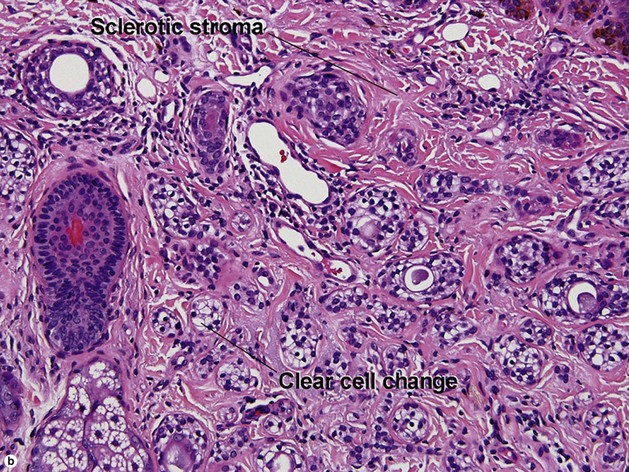
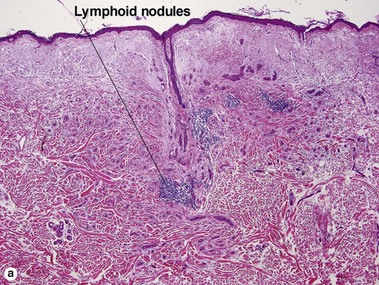
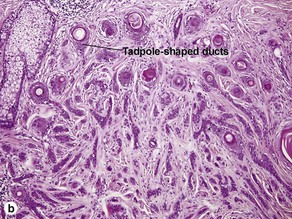
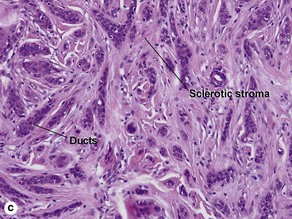
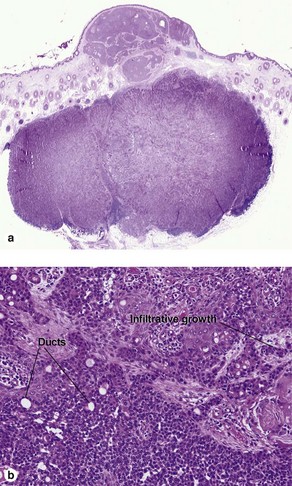
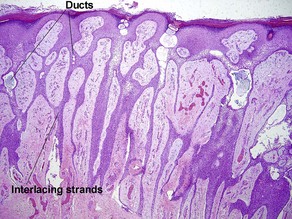
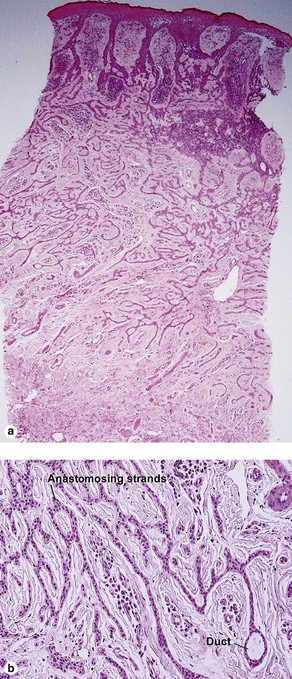
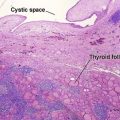
Microcystic adnexal carcinoma
Table 5-2
Characteristics of microcystic adnexal carcinoma versus syringoma
| Characteristic | Microcystic adnexal carcinoma | Syringoma |
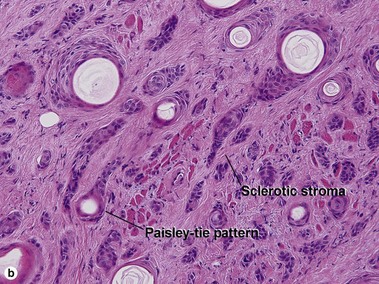
| Paisley-tie pattern | Yes | Yes |
| Stroma | Red, sclerotic | Red, sclerotic |
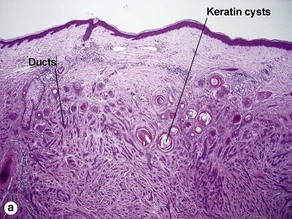
| Horn cysts | Common | May occur |
| Size | Large | Small |
| Shape | Infiltrative | Round |
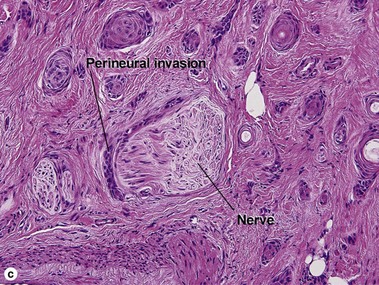
| Perineural extension | Yes | No |
| Basaloid islands | +/− | No |
| Clinical appearance | Plaque on upper lip, cheek, chin | Small papules on eyelids or eruptive papules |
Table 5-3
Characteristics of microcystic adnexal carcinoma versus morpheaform basal cell carcinoma
| Characteristic | Microcystic adnexal carcinoma | Morpheaform basal cell carcinoma |
| Paisley-tie pattern | Throughout tumor | Sometimes superficially |
| Stroma | Red, sclerotic | Red, sclerotic |
| Horn cysts | Common | Occasional |
| Deeply invasive | Yes | Yes |
| Perineural invasion | Yes | Yes |
| Clinical appearance | Plaque on upper lip, cheek, chin | Scar-like |
Table 5-4
Characteristics of microcystic adnexal carcinoma versus desmoplastic trichoepithelioma
| Characteristic | Microcystic adnexal carcinoma | Desmoplastic trichoepithelioma |
| Paisley-tie pattern | Yes | Yes |
| Stroma | Red, sclerotic | Red, sclerotic |
| Horn cysts | Common | Common |
| Calcification | Rare | Common |
| Lymphoid aggregates | Typical | Rare |
| Perineural extension | Yes | No |
| Central dell | No | Typical |
| Clinical appearance | Plaque on upper lip, cheek, chin | Firm doughnut |
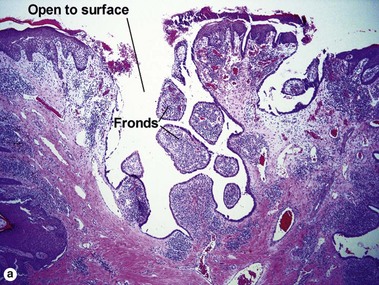
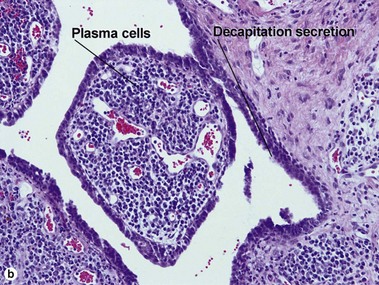
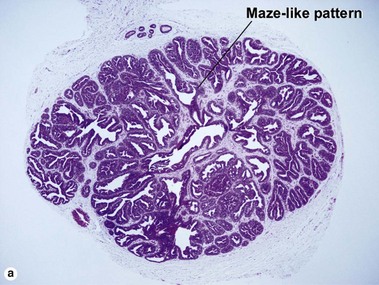
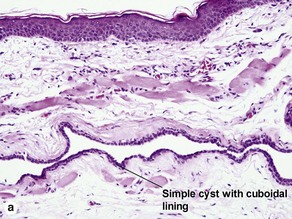
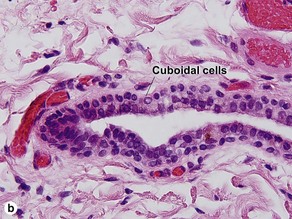
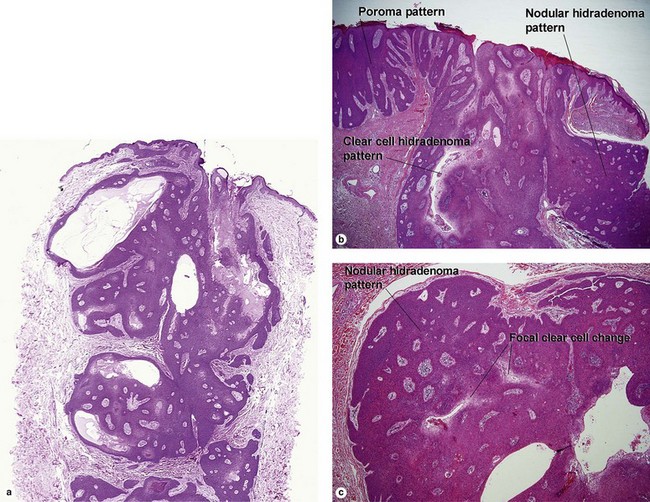
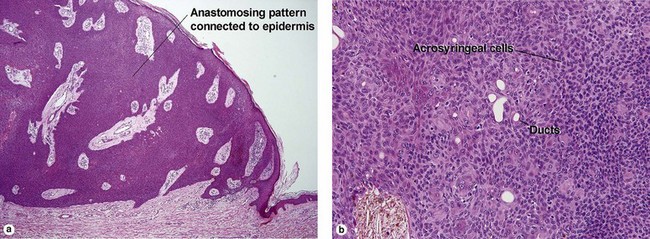
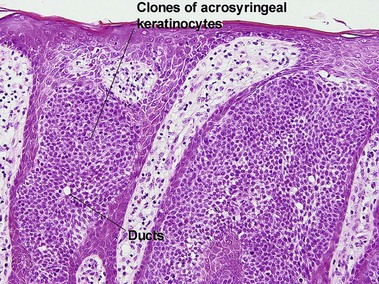
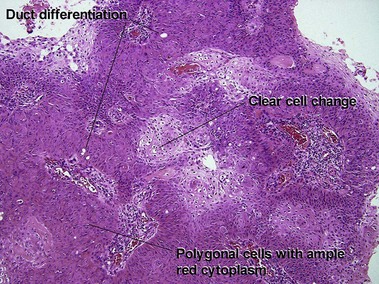
Acrospiromas
Acrospiromas comprise a large family of sweat gland tumors that includes poromas, hidradenomas, dermal duct tumors, and hidroacanthoma simplex. They are all composed of cells that resemble those of the acrosyringium. They all demonstrate duct differentiation within tumor islands. Hybrid forms are common.
Duke, WH, Sherrod, TT, Lupton, GP. Aggressive digital papillary adenocarcinoma (aggressive digital papillary adenoma and adenocarcinoma revisited). Am J Surg Pathol. 2000; 24(6):775–784.
Fischer, S, Breuninger, H, Metzler, G, et al. Microcystic adnexal carcinoma: an often misdiagnosed, locally aggressive growing skin tumor. J Craniofac Surg. 2005; 16(1):53–58.
Halachmi, S, Lapidoth, M. Approach to the rare eccrine tumors. Dermatol Surg. 2011; 37(8):1194–1195.
Ishiko, A, Shimizu, H, Inamoto, N, et al. Is tubular apocrine adenoma a distinct clinical entity? Am J Dermatopathol. 1993; 15(5):482–487.
Komine, M, Hattori, N, Tamaki, K. Eccrine syringofibroadenoma (Mascaro): an immunohistochemical study. Am J Dermatopathol. 2000; 22(2):171–175.
Lago, EH, Piñeiro-Maceira, J, Ramos-e-Silva, M, et al. Primary adenoid cystic carcinoma of the skin. Cutis. 2011; 87(5):237–239.
Matin, RN, Gibbon, K, Rizvi, H, et al. Cutaneous mucinous carcinoma arising in extramammary Paget disease of the perineum. Am J Dermatopathol. 2011; 33(7):705–709.