Controversies Surrounding Pediatric Psychopharmacology
The past several decades have witnessed an increased awareness of mental health disorders and neurobehavioral problems in children [1]. These behavioral issues now represent a growing portion of pediatric visits. Addressing and appropriately managing early-onset disorders may improve quality of life while providing hope that adult morbidity may be attenuated [2]. Given the shortage of mental health providers and the concept of the pediatric practice as “medical home,” many pediatricians are poised to receive this population. Caring for this population of children is riddled with systemic obstacles. Lack of time, training, support, and compensation, together with poor integration of collaboration with mental health resources, all contribute to this challenge. Children’s mental health has become a priority issue for the American Academy of Pediatrics [3].
A better understanding of the magnitude of mental health issues facing children was highlighted by the recently completed National Comorbidity Study, which examined the prevalence of psychiatric disorders in a sample of more than 10,000 children between the ages of 13 and 18 years. The investigators found that almost 50% of adolescents would have had enough symptoms at some point in their life to meet criteria for a psychiatric disorder by 18 years of age [1]. Twenty-two percent were found to have significant impairment as a result of their symptomatology. These numbers are alarming. The fact that there are fewer than 8000 child psychiatrists and fewer than 600 developmental behavioral pediatricians in the United States to care for these children highlights the tremendous shortage of subspecialty providers, and compounds the problem of providing adequate care.
In response to the imbalance between the number of patients and providers, the American Academy of Pediatrics (AAP) Committee of Psychosocial Aspect of Child and Family Health and Task Force on Mental Health has set a goal for pediatricians to be competent in the use of medications to treat attention-deficit/hyperactivity disorder (ADHD), major depressive disorder, and anxiety [4]. The AAP has created several valuable tools in the area of mental health (Addressing Mental Health Concerns in Primary Care: A Clinician’s Toolkit, Autism Toolkit, ADHD Toolkit) to support pediatricians in assessment, decision-making, management, coding, and so forth, and these are now available for purchase. These tools are helpful, but it will be important for clinicians to familiarize themselves with current research and the medications used to treat these common problems.
At present there seems to be an overdependence on psychotropic medication solutions for the treatment of mental health disorders. This situation is influenced by many issues. Our understanding of the neurobiological basis of psychiatric illness has greatly advanced over the past two decades, with even more emphasis on the role of medication as a viable treatment. Furthermore, there now is an understanding that these psychiatric conditions that were thought to only affect adults often originate in childhood. This convergence of ideas, together with a lack of community resources to support children suffering from psychiatric conditions, has contributed to this tendency to resort to medication. In addition, clinicians may be influenced by nonpediatric psychopharmacology research or their anecdotal prescribing experience as well as the experience of colleagues. The popular media and the pharmaceutical industry also play a role in providing sensationalized appeals or biased opinions that may influence even the most critically minded physician and parent.
The goal of this article is to bring up-to-date information regarding advances in medical psychopharmacology research to general pediatricians. The article identifies the common psychiatric disorders in children, pivotal research shaping treatment recommendations, and some of the controversies surrounding the use of psychotropic medication in children. Care providers can thereby develop an informed approach to management of psychotropic medication.
The National Comorbidity Study found that the top 3 pediatric mental health disorders in decreasing frequency are anxiety (specific phobia at 19.3%, social phobia at 9.1%), depression (11.7%), and ADHD (8.7%). This finding is consistent with previous studies [5,6]. Although autism is not considered among the most common disorders (frequency of 1:110) [7], it is considered to be the fastest-growing developmental disability [8]. Therefore, autism and its pharmacotherapy are also described in this article.
Empirically driven treatment models
Management of many common disorders in medicine often relies on treatment models that are based on a comprehensive base of research and observed clinical outcomes in adult patients. Until recently, most medication interventions in pediatric mental health were extrapolated from adult treatment patterns and used in an off-label fashion [9,10]. However, research over the past two decades has led to the emergence of pediatric-specific practice parameters for several common conditions, including some neurobehavioral disorders. Psychopharmacologic agents are now being used with greater frequency than ever before. The use of second-generation antipsychotics has more than doubled in the past decade [11,13]. Almost one-third of psychotropic medications are prescribed by non–mental health providers [14].
Both anecdotal experience and empirical clinical data suggest that medication can play an important role in managing behavioral disorders in children. Only recently have large and well-designed treatment studies been conducted for childhood mental health disorders. These studies help to inform the medical community on the application of evidence-based interventions. While these studies are far from comprehensive, they are a step toward creating treatment algorithms that incorporate the dual priorities of safety and efficacy in the care of children with psychiatric disorders. Before examining the specific classes of medications, it is important to be aware of these seminal research works guiding treatment for children. Familiarizing oneself with these studies will help the practitioner to understand best-practice medication and therapeutic alternatives.
Anxiety
The Surgeon General estimates that more than 13% of children have an anxiety disorder, which includes specific phobia, social phobia, obsessive-compulsive disorder (OCD), and generalized anxiety disorder [15]. A recent large multisite study, the Child/Adolescent Multimodal Study (CAMS), reported the effects of anxiety treatments on a randomly assigned group of 488 children. Children aged 7 to 17 years were given 1 of 4 treatments for 12 weeks: sertraline alone, cognitive-behavioral therapy (CBT) alone, combination sertraline and CBT, or control. The results showed that 81% in the combination group showed significant improvement, 60% of those in the CBT-only group, 55% in the antidepressant group (sertraline), and 24% in the placebo group. This study suggests that first-line treatment for anxiety disorders should include CBT. In addition, this study found that the sertraline group had no more side effects than the placebo group, suggesting safety and tolerability [16].
Obsessive thoughts and compulsive behaviors are important presentations of childhood anxiety. The large multisite Pediatric Obsessive Compulsive Treatment Study II (POTS) focused specifically on OCD. One hundred and twelve children with OCD, aged 7 to 17 years, entered 1 of 4 study arms: CBT alone, sertraline alone, combination sertraline and CBT, or placebo, over 12 weeks. In this study combination therapy proved to be the superior treatment, followed by CBT or sertraline (neither of which showed superiority to one another). All active treatments were superior to placebo. These results support the use of combination treatment for children with OCD, and identify the lesser impact of CBT or sertraline used alone [17].
It should be noted that these studies looked at CBT and not play therapy, talk therapy, or other therapies. However, many therapists are not trained in this therapeutic method, which highlights 3 common challenges for the pediatrician: identification of patients who might benefit from nonpharmacologic mental health treatment, locating community resources that can apply best-practice mental health treatments (in this case CBT) with children, and circumventing financial barriers that limit access [18].
Depression
Depression is another common pediatric mental health disorder. Childhood depression can have very serious consequences. In 2005, 16.9% of United States high school students reported seriously considering suicide during the 12 months preceding the survey. More than 8% of students reported that they had actually attempted suicide one or more times during the same period [19]. Many adult physicians treat depression with antidepressants, such as selective serotonin reuptake inhibitors (SSRIs). However, until recently limited pediatric data were available to support an evidence-based approach for the management of depression in children. The Treatment for Adolescent Depression Study (TADS) study examined 327 adolescents with depression in 1 of 4 treatment groups: fluoxetine, CBT, combination of fluoxetine and CBT, or placebo. The results of this important study showed that all 3 treatment groups showed improvement over placebo (Fig. 1). In the first few weeks all 4 groups improved. By 6 weeks post intervention the 3 active treatments were superior to placebo, with the combination treatment group and fluoxetine group showing the greatest changes. Surprisingly, this difference dissipates over time and all 3 treatment groups show significant and equivalent benefits over placebo by 3 to 6 months (see Fig. 1). Therefore, if symptoms require rapid amelioration, such as when depression is severe or associated with significant suicidality, medication should be used in conjunction with CBT [20]. However, in patients with mild to moderate symptoms of depression and taking into account the potential side effects of a medication, CBT is an appropriate first option for families who can access this treatment with an appropriate pediatric CBT provider. Follow-up of the cohort revealed that those children who continued to receive intervention, regardless of the treatment, fared better. This finding would support the idea that ongoing support is helpful in maintaining a positive outcome. The length of this ongoing support is not clear. Interpretation of studies examining antidepressant response in children must be interpreted carefully and replicated, as there is a known high placebo response rate.
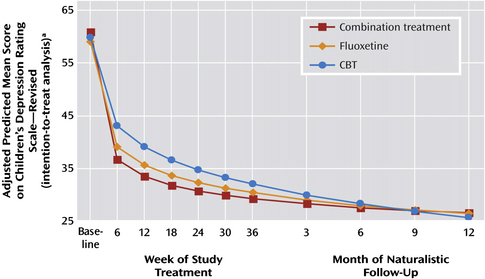
Fig. 1 Depression scores from baseline to end of naturalistic follow-up of 327 adolescents with major depressive disorder treated with fluoxetine, CBT, or combination.
(From Treatment for Adolescents with Depression Study (TADS) team. The Treatment for Adolescents with Depression Study (TADS): Outcomes over 1 year of naturalistic follow-up. Am J Psychiatry 2009;166:1141–9; with permission from American Journal of Psychiatry (Copyright © 2008), American Psychiatric Association.)
Attention-deficit/hyperactivity disorder
Epidemiologic studies revealed prevalence rates for ADHD generally ranging from 4% to 12% in the general population of 6- to 12-year-olds [5,6]. ADHD is characterized by inattention and/or hyperactivity and impulsivity. Many Americans question the very existence of ADHD, and feel that ADHD treatment is unjustified. However, decades of detailed analysis validate the presence, prevalence, morbidity, progression, and scientific validity of this mental health disorder. Until the 1990s there was considerable debate over the management ADHD. In 1995 the Multimodal Treatment Study of children with ADHD (MTA) explored a variety of treatment options. The MTA is the largest study to systematically examine the role of behavioral interventions and/or medication in the treatment of disruptive behavior in children. This study examined 579 children with combined-type ADHD, aged 7.9 to 9.9 years, over a 14-month period. There were 4 treatment arms: treatment with stimulant medication, behavioral intervention, combination of medication and behavioral treatment, and control. The results showed the medicine and medicine plus behavior groups experienced the largest reductions in ADHD symptoms, and the responses were considered superior to those seen by children receiving behavioral treatment alone or in the control group. Furthermore, the behavior plus medication did not show superiority to medication alone in ADHD symptoms [21]. There are limitations to the study, but the overall message is clear. Medication can be an important component of a successful short- to medium-duration intervention for children with ADHD. Behavioral interventions are important tools for the treatment of ADHD symptoms and for children with ADHD and common comorbid conditions. This seminal study led to practice parameters from the American Academy of Child and Adolescent Psychiatry (AACAP) and AAP recommending that medication, specifically stimulants, are an option for the first-line treatment of ADHD in school-aged children [22,23].
Autism
The behaviors associated with autism such as anxiety, hyperkinetic behavior, agitation, and aggression are often severe and debilitating. More than two-thirds of children with autism ultimately try medication to manage these behavior problems [24]. Medication is NOT a treatment for the core symptoms of autism but rather is used to control secondary behaviors that create barriers to progress or danger for patients or others. There are no large or multimodal studies guiding comprehensive medication management for autism. The primary treatment is a multimodal treatment program that incorporates intensive therapy such as applied behavioral analysis, language therapy, occupational therapy, social cognition interventions, and cognitive support [25]. Only when a child fails to respond to interventions or when behaviors are extremely disruptive should medications be considered.
Psychopharmacology
In addition to understanding the major research that influences medication treatment, physicians should understand the nuances of psychopharmacologic agents. The decision to use medication to treat a behavioral disorder is not a decision that parents take lightly. Parents commonly consult friends, family, and the internet before making a decision with their child’s physician. Thus, in this “electronic age,” physicians need to be ultra-prepared to anticipate questions and to provide reassurance about the risks and benefits associated with each of the major classes of medications for pediatric patients. What follows is a discussion addressing commonly expressed concerns regarding the most frequently used psychopharmacologic agents used in pediatrics: antidepressants, stimulants and stimulant alternatives, and antipsychotics. This discourse will arm the pediatrician with information to educate parents and guide medication choice and treatment.
Selective serotonin reuptake inhibitors
SSRIs are a class of compounds typically used in the treatment of depression and anxiety disorders. SSRIs are among the most commonly prescribed antidepressant drugs on the market today. The use of SSRIs in the treatment of behavioral disorders in children is based on the assumption that abnormalities exist in the serotonergic activity of the brain. The primary action of SSRIs is to block the action of the presynaptic serotonin reuptake pump, thereby increasing the level of serotonin in the synaptic gap. Thus the postsynaptic neuron is exposed to more serotonin to treat what is suspected as the cause of many emotional and behavioral disorders [26].
There are 6 SSRIs currently being regularly prescribed in the United States: fluoxetine, sertraline, fluvoxamine, paroxetine, citalopram, and escitalopram. Of the 6, fluoxetine (Prozac) is one of only two SSRIs approved for use by the Food and Drug Administration (FDA) for the treatment of children and adolescents aged 8 to 17 years who suffer from depression. It is also approved for the treatment of OCD in children 7 to 17 years of age. Fluoxetine has the longest half-life (between 4 and 7 days) of all the SSRIs. It also takes the longest time to achieve steady state, between 2 and 4 weeks. Its long half-life decreases the symptoms associated with the abrupt discontinuation of therapy, which is a major advantage in regulating treatment and limiting side effects from accidental noncompliance [27]. Fluoxetine tends to be the most activating of the SSRIs. Sertaline (Zoloft) is licensed by the FDA for treatment of children from 12 to 17 years of age who are diagnosed with OCD. Paroxetine (Paxil) is not licensed by the FDA for treatment of children with depression or anxiety. Fluvoxamine (Luvox) is the least activating of all the SSRIs, but interacts with many common medications. It is approved for use in children older than 8 years who are diagnosed with OCD, but has not been approved for treatment of depression in children in the United States. Fluvoxamine has the shortest half-life of all SSRIs, at 15 hours [27]. Because of its pharmokinetics, citalopram (Celexa) is thought to be the SSRI with the fewest drug-to-drug interactions. Escitalopram (Lexapro) is the S-stereoisomer of citalopram. It is typically twice as potent as the racemic version citalopram. Escitalopram is FDA-approved for the treatment of depression in adolescents aged 12 to 17 years [28].
When measured in adults, all of the SSRIs have similar efficacy and side-effect profiles. Studies of their effectiveness in treating depression in children are limited. The different pharmacodynamic properties of the SSRIs, especially their drug-drug interaction potential, should be considered when selecting a distinct SSRI for treatment. The following sections highlight some of the controversies surrounding SSRI use in children.
Suicidal ideation
There is ongoing debate about the risk of SSRIs and other antidepressants increasing the chance of suicidal behavior in children and adolescents. Evidence for and against the association of suicidal risk with antidepressant therapy emerged from several randomized trials on adolescents and children [20,29]. In addition, observational studies and population-based studies were reviewed, and the rates of suicide during treatment with antidepressants compared. No clear-cut causal relationship was identified between the use of antidepressants and suicide. This uncertainty led to the creation of an FDA advisory panel to study the issue in more depth. The panel’s meta-analysis determined that there was a small but real increased risk of suicidal thought or behaviors in children and adolescents on SSRIs when compared with placebo. There were no completed suicides in the subjects participating in the studies. The review also found that the time for highest risk of suicidal ideation was in the period of initiation of treatment with antidepressants [30,32]. This finding mirrors the adult treatment experience with SSRIs. It is generally accepted that in the adult population the greatest risk for suicide attempt occurs directly after starting antidepressant therapy. One theory is that the first symptom to improve after initiation of an antidepressant is the patient’s low energy level. This activation may enable a still depressed adult to act on their thoughts. Alternatively, the medication may depress mood or raise anxiety or distress in a minority of treated individuals. The findings of the FDA meta-analysis led the FDA in 2004 to ask the manufacturers of all antidepressant medication to change their labeling to inform patients and families about the issue. The “Black Box” warning states that the use of antidepressants may increase the risk of suicidal ideation and behavior in adolescents and children. In addition, all pharmacies were instructed to provide a patient medication guide along with each prescription. This guide highlights important safety issues and warnings, and is required when families receive any antidepressant [33,34].
Subsequent studies seemed to support that the Black Box warning was necessary [20]. On the other hand, other studies have not supported the need for these warnings. In 2004 the American College of Neuropsychopharmacology (ACNP) Task Force on SSRI and Suicidal Behavior in Youth included published and unpublished evidence for 15 trials regarding the safety and effectiveness of 5 different SSRI medications (citlopram, fluoxetine, paroxetine, sertraline, venlafaxine). There were no completed suicides and the rate of suicidal behavior was no different between treatment and placebo groups in the studies examined. In addition, the investigators found that only fluoxetine had strong statistical evidence of efficacy [32].
By 2003 there was genuine concern that the Black Box warning may have scared providers, and prescribing rates of these very important medications declined. Olfson and colleagues [34] found that in the decade before the Black Box warning, adolescent suicide rates were declining at rates comparable to the increase in prescribing rates of antidepressant medications. This trend stopped after the Black Box warning was introduced. An increase in suicides and behavior was noted (Fig. 2). New evidence for studies that assessed the effect of the resulting Black Box warning began to emerge. Gibbons and colleagues [35] found that the suicide rates were lower in counties with higher SSRI prescribing rates. These studies have resulted in a shift in opinion about the use of SSRI to treat major depression in adolescents and children. Many clinicians now have accepted a view that the potential benefits of antidepressants outweigh the suicide risk.
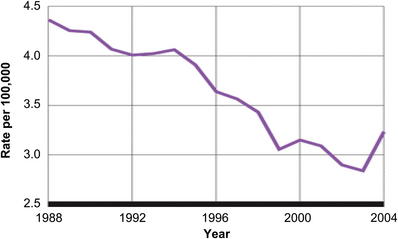
Fig. 2 Suicide rate among youth leaps from 2003 to 2004. After a steady decline since 1988 in suicide among youth aged 5 to 19 years, the number jumped suddenly in 2004, to a rate of 3.23 per 100,000, back to where it was in the mid-1990s. The shift in suicide rate in youth corresponds to a shift in opinion of SSRI use.
(From Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry 2007;164:1356–63; with permission from American Journal of Psychiatry (Copyright © 2007), American Psychiatric Association.)
Many of the pivotal pediatric studies (TADS, CAMS, POTS) support a consideration of medication in the treatment algorithms for childhood mental health disorders [16,17,20]. These studies all support the combined use of medications along with other modalities. The risk of suicide most often seems to emerge primarily from the depression itself. A decision to not treat a major depressive episode with medication may at times be a bigger risk than the risk of using SSRIs. Clinicians must consider the severity of illness, the availability of nonmedication therapies, and the literature on antidepressants and suicide risk when choosing a path for treatment of their individual patients.
Monitoring
The FDA Black Box warning led to an additional recommendation for a follow-up schedule to monitor children started on SSRI medications. This procedure was extremely restrictive, suggesting weekly visits for 4 weeks, then every other week for 4 weeks, and so forth. These recommendations were found to be unrealistic and were not followed by practicing providers for obvious reasons. The new recommendations for monitoring children and adolescents started on SSRI state that they “…should be monitored and observed carefully by mental health providers and family members” [36,37]. Monitoring should include the watching for a worsening of depression or the onset of suicidal ideation. Also important in the initiation phase of intervention is the need to pay attention to potential side effects of SSRIs. This change in monitoring recommendation allows practitioners to develop an individualized approach that includes in-person visits combined with telephone follow-up monitor a patient’s response to medication. There is no recommendation to check laboratory tests or electrocardiogram (ECG) when using SSRIs.
Other adverse effects
SSRIs are generally well tolerated. The recognized side effects of SSRI include headache, abdominal pain, nausea, diarrhea, sleep changes, jitteriness or agitation, diaphoresis, and change in sexual function. As with all antidepressants there is the possibility of an induced manic or hypomanic episode, particularly in individuals with a predisposition to bipolar disorder. Anecdotally, some children and adolescents are very sensitive to even low doses of SSRIs. Common behavioral side effects of even low-dose SSRIs include agitation, an escalation in anxiety, and an increase in impulsiveness or disinhibition [38]. The possibility of these negative side effects makes it imperative to monitor carefully, especially in vulnerable populations such as those with autism, traumatic brain injury, or cognitive deficiency.
Serotonin syndrome
The use of SSRIs is also associated with a small risk of serotonin syndrome. Sometimes the cause is idiopathic or metabolic in nature, but also may be the result of drug-to-drug interaction or overdose [39]. Serotonin syndrome is the consequence of flooding the central nervous system with serotonin. The symptoms emerge suddenly and may include a change in mental status: anxiety, agitated delirium, restlessness, or disorientation. Physical manifestations can include autonomic effects such as diaphoresis, tachycardia, hyperthermia, hypertension, vomiting, and diarrhea, and neuromuscular hyperactivity such as tremor, muscle rigidity, myoclonus, and hyperreflexia [40]. Serotonin syndrome can be life threatening.
Dosage
The recommended dosage ranges that are currently published in the literature are controversial, and may reflect more the historical ranges that are recommended for adults. Primary care pediatric providers are best served by a much more conservative approach. Adjusting the adult dosage in the table downward to between one-half to one-third of the adult dosage is a common approach [38]. For example, fluoxetine might start at 4 mg in a preadolescent. Although it may sound like an oversimplification, many practitioners ascribe to the “common lore” of “start low and go slow.”
“SSRI addiction”
The abrupt termination of SSRI usage by patients has been recognized as a major issue. This withdrawal syndrome has lent credence to the idea that SSRIs are addictive. There is no evidence to support this idea, but abrupt cessation of these medications can trigger a cascade of uncomfortable symptoms, which can be avoided if the medications are tapered over a period of weeks. The symptoms of serotonin withdrawal syndrome are disequilibrium, nausea and vomiting, fatigue and lethargy, myalgia, tremor, insomnia, migraine-like auras, agitation, anxiety, crying spells, irritability, low mood, and vivid dreams. Some medications have a much higher likelihood of causing this problem. The medications with shorter half-lives have a significantly higher chance of causing withdrawal symptoms. Fluoxetine is the least likely to cause severe symptoms of withdrawal, and paroxetine or fluvoxamine the most likely to cause discontinuation symptoms [41]. Some consideration could be given to switching from a short-acting SSRI to fluoxetine as a tool for tapering, with a reduced chance of causing distress.
Fetal exposure
The use of SSRIs in treating moderate to severe depression during pregnancy has escalated since their introduction in 1988. There is a growing awareness of the need to determine the effects, if any, of this use on the developing fetus. At present there are no reliable data on the short-term or long-term effects on the developing child’s neurodevelopment. Neonates born with antenatal exposure in the third trimester can experience symptoms similar to adults with SSRI discontinuation syndrome [42]. Neonates can experience either a single symptom or a constellation of symptoms including jitteriness, tremors, diarrhea, emesis, irritability, agitation, hypoglycemia, hyperreflexia, hypotonia or hypertonia, respiratory distress, excessive crying, difficulty with feeding, sleep disturbances, and seizures. It is important to bear in mind that the actual incidence of neonatal behavioral syndrome in the exposure of SSRI use has not been established [43,44]. Long-term antenatal exposure to SSRI has also been implicated in disorders such as persistent pulmonary hypertension, low birth weight, and being small for gestational age [45].
Autism
The use of SSRI medication for the treatment of comorbidity associated with autism has become commonplace in both pediatric and psychiatric practice. It is well known that children on the autism spectrum can suffer with anxiety and depression as well as obsessive-compulsive behaviors. In 2010 a Cochrane review by Williams and colleagues [46] evaluated 7 different studies with 271 subjects to determine whether SSRI medications (1) improve the core features of autism; (2) improve other noncore behaviors such as self-abusive behaviors; (3) improve the quality of life of the children and their caregivers; (4) has short-term and long-term effects on outcome; and (5) causes harm. Four SSRIs were evaluated: fluoxetine, fluvoxamine, fenfluramine, and citalopram. The subjects of these studies were both children and adults with autism [46]. One of the larger studies in children found no benefit in the treatment of stereotypies in autism [47]. Two small studies in adults showed positive outcomes for the Clinical Global Impression (CGI) and obsessive-compulsive behavior (OCB). One study showed improvement in aggression and another improvement in anxiety. The investigators concluded that there is only limited evidence on the effectiveness of SSRIs in adults and children with autism. There was no evidence of harm found in any of the studies.
On the surface, the results of this review would suggest that the use of SSRI medications in the treatment of autistic individuals is without merit. However, it would be a mistake to base clinical practice on this limited number of studies with such a small number of subjects. Common sense suggests that the use of SSRIs, because of their low incidence of side effects, is a preferable choice to the use of antipsychotic medications with their multitude of negative side effects. Antidotal evidence in clinical practice suggests that the use of subclinical doses of SSRI can sometimes improve anxiety levels that might not be well captured on the existing clinical assessment scales. Managing the often difficult behaviors of autistic people makes it essential that more comprehensive studies be done in an effort to determine what, if any, benefit SSRIs can provide for this population.
Stimulants/stimulant alternatives
Psychostimulants are very well studied, with initial reports of significant efficacy by Bradley dating back to 1937 in the American Journal of Psychiatry [48]. These medications are used to treat hyperactivity, inattention, and impulsivity as seen in ADHD, or are used off-label for disturbances of attention in other disorders such as autism, traumatic brain injury, or depression. Stimulants act by blocking dopamine and norepinephrine reuptake transporters, stimulating release of dopamine from the presynaptic storage vesicles, and weakly stimulating the release of norepinephrine.
In evaluating treatment options for children, clinicians must consider both functional impairments and risks/benefits. Several controversies exist regarding the use of these treatments, including growth effects, tics, sudden cardiac death, substance abuse potential, and the potential for early intervention in preschoolers. Each of these areas is addressed here.
Growth effects
The most common short-term effects of stimulant use on children are appetite suppression and weight loss, which leads to parental concern regarding stunted growth. Spencer and colleagues [49] have reported the absence of stimulant-related growth suppression. Findings from the MTA study indicate that long-term side effects of mild growth suppression are likely to occur and accumulate over the initial 3 years of treatment, with little or no rebound before puberty [50,51]. However, Biederman and colleagues [52] recently completed a 10-year case-controlled, longitudinal study following 124 children without ADHD compared with 137 children with ADHD on and off medication. The investigators found no relationship between height, weight, ADHD, or ADHD medication status in the long term. Many of the studies, including the Biederman study, showed growth inhibition in the first year of medication use, which appears to dissipate with time [52,53]. The current message to clinicians is that while these medications do not appear to have a long-term effect on growth, there is enough data on either side of the issue to continue the debate. Larger studies will need to be conducted to further assess this issue. It is important to monitor height, weight, and body mass index (BMI) biannually as a means of surveillance. If a child shows a significant change in height, drug holidays or altered dosages should be considered [23].
Tics
There is long-standing concern regarding the possibility that stimulants may cause tics. ADHD is common in children with tics [54]. Often the onset of ADHD symptoms predates the onset of tics, whether or not they have been exposed to medication. Therefore, is it sometimes difficult to ascribe cause. The literature suggests that 15% to 30% of children develop tics while on stimulants, most of which are transient [22]. The withdrawal of methylphenidate can result in a decrease in severity and frequency of tics, with an increase when reinstated [55]. The literature suggests that a similar number of children develop tics whether they are on medication or not [54,56].
Children with preexisting tics or tic disorders commonly have ADHD and disruptive behavior disorders. The use of stimulants in this population is another area for debate. The Physicians’ Desk Reference guided by the FDA lists tics or a family history of tics as a contraindication for the use of stimulants. There are now good alternatives to stimulants, but there are times when these medications fail or are not tolerated and where a stimulant may still be beneficial. A preponderance of the evidence suggests that the use of stimulants in the treatment of ADHD and co-occurring tics is both safe and effective [57,59]. While many children with a tic disorder show a lessening of tics over time on medication, there is a minority of children with a tic disorder who do appear to show at least a temporary worsening of symptoms [58,59]. One interesting study conducted by the Tourette Syndrome Study group, which examined 136 children with ADHD and a tic disorder, showed that stimulants at a low dose can be used to treat ADHD effectively without exacerbating the tic disorder in 75% of the children studied, with only a slight increase in the remaining 25% [60]. Although there are nonstimulant alternatives available that are not associated with increased tic generation, stimulants should still play a role as a treatment consideration in a child with a tic disorder.
Hematologic findings
It is not necessary to order routine laboratory tests as part of safety monitoring unless there are other medical comorbidities that suggest a medical necessity. The cost and discomfort of blood sampling during short-term or long-term stimulant use outweighs the potential benefit, as no clinically significant effects on blood biochemistry in general, and on hematologic findings in particular, are found [61,62].
Sudden cardiac death
In 2005 Health Canada suspended sales of mixed amphetamine salts (Adderall) based on postmarketing reports of 12 children and adolescent deaths in the United States over a 4-year period (1999–2003), but without full investigation. Half of the deaths had underlying serious heart conditions that were not detected prior to death. Those that died had aberrant origins of coronary arteries, bicuspid aortic valves, cardiomyopathy, toxic levels from overuse, family history of ventricular arrhythmias, extreme exercise, and dehydration, with numbers slightly higher in the amphetamine group than in the methylphenidate group [63]. Later that year, Health Canada determined that the incidence of sudden unexplained death was similar to, and even lower than, that which occurred in the general population. The risk for such an event was calculated by the FDA to be between 1.0 and 8.5 per 100,000 [64]. Regardless, such medications were labeled by the FDA to warn against the use of stimulants in children with existing cardiac symptoms, and to use caution regarding use in children with prior cardiac disease or significant family history.
Previous studies looking at the effects of stimulants have shown increases of 3 to 6 beats/min in heart rate and 2 to 4 mm Hg in systolic/diastolic blood pressure [65,67]. Although statistically significant, these numbers are not clinically relevant. There has been debate regarding the need for prior ECGs and/or echocardiograms before initiating medications. At this time, the AAP and AACAP are not recommending an ECG or echocardiogram before starting stimulants or a serotonin norepinephrine reuptake inhibitor (SNRI) [22,23]. Instead, a thorough cardiac family history (arrhythmia, sudden unexpected death, hypertrophic cardiomyopathy, or Marfan syndrome) and personal cardiac history (palpitations, syncope, chest pain, prior surgeries, congenital heart conditions, murmur, or arrhythmia) should be obtained. Examination would also include blood pressure and auscultation. These components are an important part of the ADHD evaluation [68,70]. A positive cardiac history or concerning findings on physical examination necessitates further evaluation with ECG, echocardiogram, and/or cardiology referral [71]. Prior cardiac history does not preclude stimulant use, but necessitates appropriate assessment of potential benefit and acknowledgment of the FDA warning.
Substance abuse potential
ADHD in childhood is a risk factor for later substance abuse. This risk is present regardless of exposure to stimulant medication. Stimulant treatment does not appear to increase the risk of substance abuse [72,74]. The association between ADHD and substance abuse is more closely linked to whether a young adult develops conduct disorder than to whether they have ADHD [75].
Claims that childhood treatment with stimulants decreases the danger of future substance abuse [72] were not supported as “protective” either by the recent publications of long-term follow-up of these cohorts or by the MTA study [74,76]. The hypothesis that taking medications during adolescence is protective against substance abuse should be addressed by a prospective, longitudinal study of children with ADHD on and off medication and matched controls, from treatment onset into adulthood.
Preschoolers
ADHD historically has been viewed as a disorder of elementary school-aged children. Increasingly, ADHD has become one of the most commonly recognized behavioral disorders in preschoolers. The startling increase in stimulant prescriptions written for this age group along with the lack of diagnostic and treatment consensus for these children, led to the Preschool ADHD Treatment Study (PATS), a 6-site study funded by the National Institute of Mental Health (NIMH). Because the lowest age approved for use of methylphenidate by FDA guidelines is 6 years, one of the primary aims of the study was to investigate its safety and efficacy in 3- to 5.5-year-olds (at screening).
Multiple publications have emerged from the PATS. A pharmacokinetic substudy is highlighted here. The pharmacokinetic substudy demonstrated slower clearance in preschoolers than in school-aged children with ADHD. Thus, preschoolers respond differently to stimulants than do school-aged children, even when weight is controlled, accounting for a different volume of distribution from older children [77].
Overall, the PATS study has helped to validate the diagnosis of ADHD in preschoolers and to contribute a guide to assessment in this age group [78]. The initial findings of this study suggest that stimulants are safe and effective for preschoolers. It was also noted that preschoolers are more sensitive to side effects and have a small dosage window of therapeutic effect, which make the planning of treatment more challenging. Taken together, the main message to clinicians is to proceed cautiously with the use of stimulants in very young children. Nonmedication interventions were used prior to medication treatment in the PATS. Clinicians should seek behavioral treatment for their patients before turning to stimulant medications in this age group.
Monitoring stimulants
Children started on stimulants should be seen after the first month to follow progress and review for side effects. Children can then be followed at regular intervals. Seeing children every 3 months on a stable dose helps to ensure safe management and monitoring of emergent side effects of treatment. Rating scales should be used to ensure optimal treatment. One should watch for mood alterations, explore social and family relations, and encourage a structured, positive behavior system. Laboratory surveillance is not indicated [23].
Stimulant alternatives
This discussion is not inclusive of all alternative medication options. The goal is to focus on the most commonly used medications.
Atomoxetine
Atomoxetine belongs to the class of drugs known as the SNRIs. It is the first nonstimulant approved by the FDA for the treatment of ADHD in children. It has a very low abuse potential. While it is considered a first-line treatment for ADHD, it has a lower effect size of about 0.6 [79], suggesting moderate effectiveness; this is less than the reported effect sizes for traditional stimulants (0.8) [79], suggesting their stronger effectiveness. As an alternative to the stimulants, atomoxetine may offer the benefit of longer activity and lower abuse potential. Originally explored as an antidepressant, it seems to have some additional benefit in the area of anxiety, but this has not been clearly corroborated by empiric data. Atomoxetine has a long onset to therapeutic benefit (2–6 weeks). Its side-effect profile is similar to the stimulants with the exception of tics (atomoxetine is not associated with exacerbation of tics), but it carries additional side effects such as cough, dizziness, and an idiosyncratic hepatotoxicity risk. In 2005, the FDA added a Black Box warning suggesting a small but real risk of increased suicidal behavior (the same as for SSRIs). There are “long-term” studies at 4.8 years that show no change in growth and ECG, and no unexpected new adverse events [80]. This medication should be considered for a child with ADHD and anxiety or a tic disorder, or for a child who needs medication with a longer duration of action. Although not studied, atomoxetine is often used adjunctively with stimulants.
α2-Adrenergic agonists
Despite having been used for years, neither immediate-release clonidine nor guanfacine are approved by the FDA for the treatment of ADHD. These agents seem to have their best effect on the hyperactive/impulsive part of this disorder by dampening the norepinephrine-mediated arousal system. These drugs have also been shown to reduce tic production and improve working memory, planning, and behavioral inhibition. Guanfacine XR has been FDA-approved for the treatment of ADHD and clonidine LA is the first drug to be approved for use adjunctively to stimulants. Some of the noted side effects include sedation, which appears to lessen over the first 2 to 4 weeks, hypotension, bradycardia, constipation, dry mouth, and increased appetite. α2-Adrenergic agonists are likely to play a role as second-line treatment agents for ADHD.
Neurofeedback
While obviously not a medication, biofeedback has emerged as an important and controversial treatment for ADHD. Limited randomized controlled trials (RCTs) are available examining its effectiveness. In 2005 Loo and Barkley [81] published an analysis of available neurofeedback studies, and concluded that electroencephalographic feedback is not well established as a treatment or adjunct to medication in the treatment of ADHD. However, they did not address some promising studies. A recent small, but controlled study shows a modest effect on ADHD symptoms, with an effect size of 0.71 [82,83]. Although this effect size is still lower than that often reported for stimulant medications used to treat ADHD, there appears to be a significant positive effect on the disorder in this study. Neurofeedback has not yet been accepted by most physicians as a component of treatment for ADHD, but this topic deserves more investigation. Biofeedback should be factored into the comprehensive understanding of treatment alternatives.
Antipsychotics
Antipsychotics are the subset of psychopharmacologic agents that historically have been used to treat schizophrenia and psychotic disorders. Over the years their applications have been broadened to treat bipolar disorder, agitation in autism, tics, anxiety, impulsivity, sleep, and aggression. These medications are not part of the typical repertoire generally prescribed by pediatricians. However, they are discussed here because they are increasingly part of the psychopharmacologic landscape. Their very use in children creates great controversy secondary to their significant side-effect profile. These medications are often necessary to treat severe behavior disturbance in children. After stimulants and SSRIs, they are now among the psychotropic medications most frequently used to treat childhood psychiatric disorders. Great caution must be used when prescribing antipsychotic medications to children and adolescents.
The traditional antipsychotics such as haloperidol, pimozide, and thorazine have been replaced by what was perceived to be the safer atypical or second-generation antipsychotics (SGAs). These alternatives were supposed to have higher efficacy and fewer side effects. However, they may have merely switched one set of side effects, such as extrapyrimidal side effects (EPS), for metabolic syndrome including weight gain, elevated lipid profile, and insulin resistance.
Thus far, only a few of the SGAs have received approval from the FDA for pediatric indications. Risperidone and aripiprazole are approved for the treatment of schizophrenia in children older than 13 years, bipolar disorder, mania, or mixed episodes in children 10 years and older. SGAs are also approved for the treatment of irritability associated with autism for children between 5 and 17 years of age. It should be noted that the FDA originally refused the application for risperidone because of its significant side effects. However, given additional studies and the severity of the untreated conditions, the benefits of treatment with SGAs were determined to ultimately outweigh the risks in enough cases to warrant FDA approval for children, with warnings.
Despite these relatively few recommended indications, antipsychotic use in children is on the increase. The number of prescriptions for the SGAs in the pediatric population rose 160% in the United States between 1990 and 2000 [84]. This trend continues, with an additional 22% increase in the prescriptions of atypical antipsychotics between 2004 and 2008 for children younger than 17 years [12,13,85]. The majority of the prescriptions were written by psychiatrists for mood disorders. The most popular medication was risperidone [86]. A study in the United States found that antipsychotics are often used to treat ADHD and hyperactivity despite no empirical evidence supporting their use for this indication [12,87].
Mechanism
The mechanism of action among the older and newer antipsychotics differ somewhat. The traditional antipsychotics such as haloperidol strongly block dopamine (DA2) receptors while having more minor effects on other neuroreceptors to differing degrees. The atypical or newer antipsychotics are known as serotonin dopamine antagonists (SDAs), again with slightly differing activity profiles based on effects at other neuroreceptor sites. The associated potencies and side effects are determined by the neuroreceptors affected by a particular antipsychotic. For example, if drug X has the strongest affinity for the dopamine receptor it will have both the strongest antidopamine potency and side effects. This profile determines a medication’s action and its side effects (Table 1).
Table 1 Depression – response to treatment
| Neuroreceptor | Effect | Example (Strongest Effect) |
|---|---|---|
| Dopamine blockade | Extrapyramidal signs (EPS), tardive dyskinesia (TD), prolactin stimulation (improves positive symptoms) | Haloperidol |
| Serotonin stimulation | Anti-EPS, sexual dysfunction, antidepressant activity, gastric upset | Aripiprazole |
| α-Adrenergic blockade | Postural hypotension, dizziness, syncope | Thioridazine |
| Muscarinic blockade | Memory, cognition, urinary retention, constipation | Olanzepine, chlorpromazine |
| Histamine blockade | Sedation, weight gain, protective against EPS (improve negative symptoms) | Olanzepine, seroquel |
Side effects
It is not the intention to cover all side effects associated with antipsychotics. What follows is a discussion of some of the more concerning side effects that influence the decision to use these medications, which should be included in any discussion of informed consent.
Extrapyramidal side effects
It was originally thought that EPS were not ascribed to the atypical antipsychotics, but were commonly associated primarily with the traditional antipsychotics. EPS comprise a set of involuntary movement disorders such as dystonia, tardive dyskinesia, akathesia, or parkinsonian-like movements. This response is attributable to D2 blockade in the basal ganglia, which is strongest with the typical antipsychotics. The newer medications still have an effect at the D2 receptor and thus can also cause EPS, albeit to a lesser extent [88–91]. Some of these movement disorders, such as tardive dyskinesia, are irreversible.
Blood dyscrasias
A variety of blood dyscrasias have been reported in children on atypical antipsychotics. These effects have been mostly in anecdotal and case reports, but have occurred with enough frequency to warrant obtaining a complete blood count (CBC) at regular intervals, perhaps after 3 months then annually.
Prolactin
Prolactin levels are also affected by both the older and newer antipsychotics. The effect seems to be greater with the older antipsychotics but also occurs with the newer ones. Several studies show a transient increase in prolactin, and a few others show a more sustained increase in almost a quarter of children on antipsychotics [91,93]. The effect of the increase is unclear. However, altered menstrual cycles are a common phenomenon, and suggest the need to lower the dose or consider a change of medication. This type of endocrine abnormality brings into play fertility, breast tissue development in males, and nipple discharge.
Metabolic syndrome
Metabolic syndrome is probably the most significant issue facing this newer class of medications. By 2004 these medications were clearly linked to weight gain and altered glucose levels [92]. Out of concern for cardiometabolic effects, the FDA issued a warning. The evidence continued to mount as the use of these medications continued to escalate. In the Second-Generation Antipsychotic Treatment Indications, Effectiveness and Tolerability in Youth (SATIETY) study, Correll and colleagues [94,95] corroborated the move by the FDA. This study found that 10% to 36% of 272 patients between 4 and 19 years of age who were antipsychotic medication–naïve became overweight or obese during the 11-week study. There was minimal weight change in the control group who did not take the medications. The study monitored treatment with aripiprazole, olanzapine, quetiapine, or risperidone to treat psychosis, mood disorder, aggression, or spectrum disorders in children. The greatest weight gain was seen with olanzepine (8.5 kg) and the least with aripiprazole (4.4 kg). It must be borne in mind that this is 8.5 kg in only 11 weeks. Adverse changes in levels of total cholesterol, triglycerides, and non–high-density lipoprotein (HDL), and in the ratio of triglycerides to HDL cholesterol were also noted.
Weiss and colleagues [87] further corroborate these findings in a retrospective study of emergency room admissions in Canada. This study found that children treated with atypical antipsychotics were much more likely (27%) than unexposed controls (2.9%) to exhibit metabolic syndrome or associated features. Furthermore, children were much more likely than adults to exhibit metabolic syndrome. These studies suggest that the benefits of atypicals must be balanced with the cardio-metabolic risks suggested by the research. These findings portray significant risks.
Traditional versus newer antipsychotics
As discussed, there are several side effects associated with these medications. Atypical antipsychotics were billed as safer, better alternatives to the traditional antipsychotics. The atypical antipsychotics are similar to the classic antipsychotics in terms of reducing psychotic symptoms, but there does not appear to be superiority, with the exception of clozapine. Clozapine (the first atypical) has shown efficacy in patients for whom other medications have failed [96,97].
There are some benefits to these newer medications, which are used at a frequency of 9:1 over the traditional medications. The atypical antipsychotics seem to offer some treatment benefits over the traditional medications with improved depression, less energy suppression, and a less flattened affect. An adult study was initiated to investigate this question. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) by the NIMH set out to compare atypical to traditional antipsychotics. In this multicenter study, adults with schizophrenia were randomized to receive olanzapine, perphenazine, quetiapine, risperidone, and ziprasidone [98]. The outcome showed that olanzapine appeared to be more effective than the other drugs studied, but it also showed the highest weight gain and changes in hemoglobin A1c, cholesterol, and triglycerides. There did not appear to be any difference in efficacy between the remaining traditional or atypical medications. Although the study does not examine the pediatric population, it is important to reconsider the notion that the atypical antipsychotics are safer than the traditional antipsychotics. Instead, this study emphasizes judicious use of all antipsychotic medication.
A study in 2008 had similar findings in children. Sikich and colleagues [99], in the Treatment of the Early Onset Schizophrenia Spectrum Study (TEOSS study), compared a typical antipsychotic to 2 SGAs in the treatment of schizophrenia in 119 children. This study did not show any superiority of the SGAs. Furthermore, each medication had its own profile of side effects. Risperidone had the greatest increase in prolactin; olanzepine led to greatest weight gain, and increase in insulin and cholesterol. This result would suggest that the SGAs may not be superior in benefit and seem to have substantial, albeit different, side effects as compared with the typical antipsychotics.
Antipsychotics in the treatment of autism
One of the more pivotal studies related to autism examined the use of risperidone in the treatment of agitation or other serious behaviors in children with autism by the Research Units on Pediatric Psychopharmacology [100]. In this multicenter study, 101 children aged 7 to 17 years received either risperidone or placebo. Sixty-nine percent of those receiving risperidone were rated by clinicians as improved or very much improved compared with 19% in the placebo group. This study had the most robust outcome for any study in autism to date. However, this study also showed that risperidone is not benign, with side effects including significant weight gain.
Brief summary of antipsychotics
Clozapine has shown superiority over other antipsychotics, but has a significant risk of weight gain and metabolic syndrome. It is the only antipsychotic that requires regular CBC monitoring, due to the small but life-threatening risk of agranulocytosis. Olanzepine has shown genuine benefit over some of the other medications in adults but has also showed the greatest weight gain and sedation. Risperidone is the most commonly used medication in children. It has FDA approval for the treatment of pediatric schizophrenia, bipolar disorder, mania, and autism. Of the newer medications it has a higher tendency toward prolactin abnormalities, EPS, and weight gain. Ziprasidone is the only medication associated with QTc prolongation and therefore necessitates an ECG. Quetiapine may cause significant sedation and weight gain. Aripiprazole tends to be the most weight neutral, among the least EPS generating, and may show the most qualitative improvement in depressive symptoms. Quetiapine has FDA approval for childhood schizophrenia, mania, bipolar disorder, and autism.
Monitoring
Despite the recognition of significant and potentially harmful side effects, most patients placed on antipsychotics are not appropriately screened and monitored. A study in the American Journal of Psychiatry showed that the younger the patient was, the less likely they were to be screened [101]. While a psychiatrist may prescribe these medications, it may be necessary for the primary care physician to help monitor for side effects. Traditional medical visits can be used to monitor personal and medical history, pulse, blood pressure, height, weight, and BMI. Each visit should include a review of systems addressing potential side effects such as akathisia, dystonias, sedation, and reproductive dysfunction. After 3 months laboratory studies should be obtained, including a CBC, fasting blood glucose, lipids, and liver function tests. Blood glucose, lipids, and perhaps hemoglobin A1c should be reviewed every 6 months thereafter. Comprehensive chemistry panels should be repeated annually for children who continue to receive these medications. Prolactin levels should be checked if the child is symptomatic, and an ECG should be done if a child is taking ziprasidone [102] (Table 2).
Table 2 Monitoring strategies in children and adolescents treated with antipsychotic agents: antipsychotic medication and receptor impact
| Assessment | Routine Follow-Up |
|---|---|
| Personal and family medical history | Annually |
| Lifestyle behaviors | Each visit |
| Sedation/somnolence | Each visit |
| Sexual/reproductive dysfunction | During titration, then every 3 months |
| Parkinsonism, akathisia | During titration, at 3 months, then annually |
| Height, weight, body mass index | Each visit |
| Blood pressure and pulse | At 3 months and annually |
| Electrolytes, blood count, renal and liver function | Annually |
| Fasting blood glucose and lipids | At 3 months, then every 6 months |
| Liver function tests | At 3 months, then annually |
| Prolactin | Only if symptomatic |
| Electrocardiography | Only if taking ziprasidone |
From Correll CU, Kratochvil CJ, Antipsychotic use in children and adolescents: minimizing adverse affects to maximize outcomes. J Am Acad Child Adolesc Psychiatry 2008;47(1):9–20; with permission.
Summary
Mental health conditions are some of the most significant and chronic concerns for children and adolescents. Parents and some physicians are often reluctant to consider a potentially long-term psychotropic medication treatment for these children. Pediatricians can serve a vital role by helping families sift through the vast amount of information on psychotropic medication to help them make informed decisions regarding their child. Despite all of the aforementioned controversy regarding the use of psychotropic medications, they can be invaluable for children whose well-diagnosed conditions are not responsive to other interventions. Although the risks are undeniable, there are valid and important reasons to use these medications. Depression, impulsivity, inattention, psychosis, agitation, and mood lability can all profoundly affect daily living, independence, and the well-being of children. Untreated, these disorders negatively affect relationships with parents, family members, teachers, and peers. Physicians who can help children and families weigh risks and benefits of treatment, inform, and monitor appropriately, can provide important care for their patients with neurobehavioral disorders.
There is a growing understanding that the subspecialty health care system is not able to accommodate the vast numbers of children with the most common mental health issues: anxiety, depression, and ADHD. The AAP is emphasizing the need for pediatricians to play a major role in the identification, assessment, and management of this population. There is clearly a role for both behavioral treatments and medication in that care. The ability to integrate the research presented will be important in creating comprehensive treatment pathways.
This movement toward a broader scope of practice is admittedly daunting for many who were not trained to provide this care. It necessitates pediatricians to deepen their knowledge in the area of behavioral health. It also emphasizes the need for system-wide change incorporating more collaboration and shared care between psychiatrists, pediatricians, psychologists, and other behavioral support services [103]. Understanding the roles of behavior and medicine in the care of children with common mental health conditions is an important step toward this goal.
References
[1] K.R. Merikangas, Jp He, M. Burstein, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
[2] C. Arango, M. Parellada, D.M. Moreno. Clinical effectiveness of new generation antipsychotics in adolescent patients. Eur Neuropsychopharmacol. 2004;14:471-479.
[3] J.M. Foy, P. Duncan, B. Frankowski, et alAmerican Academy of Pediatrics Task Force on Mental Health. Enhancing pediatric mental health care: report from the American Academy of Pediatrics Task Force on Mental Health. Introduction. Pediatrics. 2010;125(Suppl 3):S69-S74.
[4] W.L. Coleman, M.I. Dobbins, A.S. Garner, et alCommittee on Psychosocial Aspects of Child and Family Health and Task Force on Mental Health. The future of pediatrics: mental health competencies for pediatric primary care. Pediatrics. 2009;124:410-421.
[5] R.T. Brown, W.S. Freeman, J.M. Perrin, et al. Prevalence and assessment of attention-deficit/hyperactivity disorder in primary care settings. Pediatrics. 2000;107(3):e43.
[6] E.J. Costello, A. Angold, J. Burns, et al. The great smoky mountains study of youth. goals, design, methods, and the prevalence of DSM-III-R disorders. Arch Gen Psychiatry. 1996;53(12):1129-1136.
[7] C. Rice, et al. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, United States, 2006. Mobid Mortal Week Rep. 2009;58(Suppl 10):1-20.
[8] A.T. Cavagnaro. Autistic spectrum disorders: changes in the California caseload, an update June 1987-June 2007: California Health and Human Services Agency. State of California 2003 survey of developmental disabilities. Available at: http://www.dds.ca.gov/autism/docs/AutismReport_2007.pdf Accessed December 5, 2011
[9] D.C. Radley, S.N. Finkelstein, R.S. Stafford. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166:1021-1026. [psychiatric drugs]
[10] J.M. Zito, A.T. Derivan, C.J. Kratochvil, et al. Off-label psychopharmacologic prescribing for children: history supports close clinical monitoring. Child Adolesc Psychiatry Ment Health. 2008;2:24.
[11] W.O. Cooper, G.B. Hickson, D.C. Fuchs, et al. New users of antipsychotic medication among children enrolled in TennCare. Arch Pediatr Adolesc Med. 2004;158(8):753-759.
[12] E. Cascade, A. Kalali, R. Findling. Use of antipsychotics in children. Psychiatry (Edgmont). 2009;6(6):21-23.
[13] N.C. Patel, R.J. Sanchez, M.T. Johnsrud, et al. Trends in antipsychotic use in a Texas Medicaid population of children and adolescents: 1996 to 2000. J Child Adolesc Psychopharmacol. 2002;12(3):221-229.
[14] W.O. Cooper, P.G. Arbogast, H. Ding, et al. Trends in prescribing of antipsychotic medications for US children. Ambul Pediatr. 2006;6(2):79-83.
[15] Available at: http://www.surgeongeneral.gov/library/mentalhealth/chapter3/sec6.html Accessed December 5, 2011
[16] J.T. Walkup, A.M. Albano, J. Piacentini, et al. Cognitive-behavioral therapy, sertraline and their combination for children and adolescents with anxiety disorders: acute phase efficacy and safety. N Engl J Med. 2008;359(17):2753-2766.
[17] M. Franklin, E. Foa, J. March. Pediatric obsessive-compulsive disorder treatment Study. J Am Acad Child Adolesc Psychiatry. 2004;292:1969-1976.
[18] J.M. Foy, J. Perrin, American Academy of Pediatrics Task Force on Mental Health. Enhancing pediatric mental health care: strategies for preparing a community. Pediatrics. 2010;125:S75-S86.
[19] D.K. Eaton, L. Kann, S.A. Kinchen, et al. Youth risk behavior surveillance—United States, 2005. MMWR Surveill Summ. 2006;55(Suppl 5):1-108.
[20] J. March, S. Silva, J. Curry, et al. Treatment for adolescents with Depression Study (TADS): long-term effectiveness and safety. Arch Gen Psychiatry. 2007;64(10):1132-1143.
[21] The MTA Cooperative Group: a 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56:1073-1086.
[22] American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder and Committee on Quality Improvement. Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108(4):1033-1044.
[23] S. Pliszka, AACAP Work Group. Practice parameters for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
[24] D.P. Oswald, N.A. Sonenklar. Medication use among children with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2007;l17(3):348-355.
[25] Available at: www.Nationalautismcenter.org Study Accessed December 5, 2011
[26] S.H. Preskorn. Clinically relevant pharmacology of selective serotonin reuptake inhibitors: an overview with emphasis on drug metabolism. Clinical Pharmacokinet. 1997;32:1-21.
[27] C.L. DeVane. Pharmacokinetics of the selective serotonin reuptake inhibitors. J Clin Psychiatry. 1992;53:13-20.
[28] L.K. Leslie, T.B. Newman, P.J. Chesney, et al. The Food and Drug Administration’s deliberations on antidepressants use in pediatric patients. Pediatrics. 2005;116:195-201.
[29] A. Khan, S. Khan, R. Kolts, et al. Suicide rates in clinical trials of SSRIs other antidepressants and placebo: analysis of FDA reports. Am J Psychiatry. 2003;160:790-792.
[30] T.A. Hammad, T. Laughren, J. Racoosin. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63:332-339.
[31] H. Jick, J. Kaye, S. Jick. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292:338-343.
[32] American College of Neuropsychopharmacology, preliminary report of the task force on SSRI’s and suicidal behavior in youth. Available at: www.ancp.org, 2004. Accessed December 5, 2011
[33] U.S. FDA Public Health Advisory: suicidality in children and adolescents being treated with antidepressant medications; 2004.
[34] M. Olfson, D. Shaffer, S. Marcus, et al. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry. 2003;60:978-982.
[35] R.D. Gibbons, H. Brown, K. Hur, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164:1356-1363.
[36] M.M. Mamdani. Health advisories: when good intentions go bad. CMAJ. 2008;178:1025-1026.
[37] Ly Katz, A.L. Kozyrskyj, H.J. Prior, et al. Effects of regulatory warnings on antidepressant prescribing rates, use of health services and outcomes among children, adolescents and young adults. CMAJ. 2008;178:1005-1011.
[38] B. Birmaher, D. Brent, W. Bernet, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:1503-1536.
[39] E.W. Boyer, M. Shannon. The serotonin syndrome. N Engl J Med. 2005;352:1112-1120.
[40] P. Birmes, D. Coppin, L. Schmitt, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168:1439-1442.
[41] N.J. Coupland, C.J. Bell, J.P. Potokar. Serotonin reuptake inhibitor withdrawal. J Clin Psychopharmacol. 1996;16:356-362.
[42] E.L. Moses-Kolko, D. Bogen, J. Perel, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. 2005;293:2372-2383.
[43] k Laine, T. Heikkinen, U. Ekblad, et al. Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch Gen Psychiatry. 2003;60:720-726.
[44] H. Nordeng, R. Lindemann, A. Perminov. Neonatal withdrawal syndrome after in utero exposure to serotonin reuptake inhibitors. Acta Paediatr. 2001;90:288-291.
[45] C.D. Chambers, S. Hernandez-Diaz, L.J. Van Marter, et al. Selective serotonin reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354:579-587.
[46] K. Williams, D.M. Wheeler, N. Silove, et al. Selective serotonin reuptake inhibitors (SSRI) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 8, 2010. CD004677
[47] B.H. King, E. Hollander, L. Sikich, et al. (STAART Psychopharmacology Network): lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66(6):583-590.
[48] C. Bradley. The behavior of children receiving benzedrine. Am J Psychiatry. 1937;94:577-585.
[49] T. Spencer, J. Biederman, M. Harding, et al. Growth deficits in ADHD children revisited: evidence for disorder-associated growth delays? J Am Acad Child Adolesc Psychiatry. 1996;35:1460-1469.
[50] J.M. Swanson, G.R. Elliott, L.L. Greenhill, et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007;46:1015-1027.
[51] J.M. Swanson, S.P. Hinshaw, L.E. Arnold, et al. Secondary evaluations of MTA 36-month outcomes: propensity score and growth mixture model analyses. J Am Acad Child Adolesc Psychiatry. 2007;46:1002-1013.
[52] J. Biederman, T.J. Spencer, M.C. Monuteaux, et al. A naturalistic 10-year prospective study of height and weight in children with attention-deficit hyperactivity disorder grown up: sex and treatment effects. J Pediatr. 2010;157(4):635-640. e1
[53] H. Zhang, H. Du, H. Zhuang. Impact of long-term treatment of methylphenidate on height and weight of school age children with ADHD. Neuropediatrics. 2010;41:55-59.
[54] M.H. Bloch, K.E. Panza, A. Landeros-Weisenberger, et al. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(9):884-893.
[55] M.A. Riddle, K.A. Lynch, L. Scahill, et al. Effects of methylphenidate discontinuation and reinitiation in children with Tourette’s syndrome and ADHD. J Child Adolesc Psychopharmacol. 1995;5:205.
[56] S.F. Law, R.J. Schachar. Do typical clinical doses of methylphenidate cause tics in children treated for attention-deficit hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 1999;38(8):944-951.
[57] K.D. Gadow, J. Sverd, J. Sprafkin, et al. Efficacy of methylphenidate for attention-deficit hyperactivity disorder in children with tic disorder. Arch Gen Psychiatry. 1995;52(6):444-455.
[58] K.D. Gadow, J. Sverd, J. Sprafkin, et al. Long term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch Gen Psychiatry. 1999;56:330-336.
[59] Y. Poncin, D.G. Sukhodolsky, J. McGuire, et al. Drug and non-drug treatments of children with ADHD and tic disorders. Eur Child Adolesc Psychiatry. 2007;16(Suppl 1):78-88.
[60] The Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58:527-536.
[61] L.L. Greenhill, R.L. Findling, J.M. Swanson, ADHD Study Group. A double-blind, placebo-controlled study of modified-release methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2002;109:e39.
[62] S.B. Wigal, T.E. Wilens, M. Wolraich, et al. Hematologic and blood biochemistry monitoring during methylphenidate treatment in children with attention-deficit/hyperactivity disorder: 2-year, open-label study results. Pediatrics. 2007;120:e120-e128.
[63] U.S. Food and Drug Administration (2005) public health advisory for Adderall and Adderall XR. Available at: http://www.fda.gov/cder/drug/advisory/adderall.htm Accessed January 24, 2006
[64] J. Rosack. US regulators puzzled by Canada’s ruling on safety of ADHD drug. Psychiatr News. 2005;40:5-9.
[65] Adderall XR [package insert]. Wayne, PA: Shire US Inc; 2010.
[66] Concerta [package insert]. Titusville, NJ: McNeil Pediatrics; 2008.
[67] Focalin XR [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2008.
[68] V.L. Vetter, J. Elia, C. Erickson, et al. A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407-2423.
[69] A.E. Warren, R.M. Hamilton, S.A. Belanger, et al. Cardiac risk assessment before the use of stimulant medications in children and youth: a joint position statement by the Canadian Paediatric Society, the Canadian Cardiovascular Society, and the Canadian Academy of Child and Adolescent Psychiatry. Can J Cardiol. 2009;25(11):625-630.
[70] S.B. Wigal, A. Jun, A. Wong. Does prior exposure to stimulants in children with ADHD impact cardiovascular parameters from lisdexamfetamine dimesylate? Postgrad Med. 2010;122(5):27-34.
[71] J.M. Perrin, R.A. Friedman, T.K. Knilans. Cardiovascular monitoring and stimulant drugs for attention deficit hyperactivity disorder. Pediatrics. 2008;122:451-453.
[72] T.E. Wilens, S.V. Faraone, J. Biederman, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? a meta-analytic review of the literature. Pediatrics. 2003;111:179-185.
[73] S. Mannuzza, R.G. Klein, N.L. Truong, et al. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. Am J Psychiatry. 2008;165(5):604-609.
[74] J. Biederman, M.C. Monuteaux, T. Spencer, et al. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008;165(5):597-603.
[75] E.R. Disney, I.J. Elkins, M. McGue, et al. Effects of ADHD, conduct disorder, and gender on substance use and abuse in adolescence. Am J Psychiatry. 1999;156:1515-1521.
[76] B.S.G. Molina, K. Flory, S.P. Hinshaw, et al. Delinquent behavior and emerging substance use in the MTA at 36 months: prevalence, course, and treatment effects. J Am Acad Child Adolesc Psychiatry. 2007;46:1028-1040.
[77] S.B. Wigal, S. Gupta, L. Greenhill, et al. Pharmacokinetics of methylphenidate in preschoolers with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17(2):153-164.
[78] T. Wigal, S. Wigal, A. Shanklin, et al. ADHD in preschoolers: a diagnosis—or just a “PhAse”? Consultant for Pediatricians. 2008;8(Suppl):S2-S8.
[79] S.V. Faraone, J. Beiderman, T.J. Spencer. Comparing the efficacy of medications for ADHD using meta-analysis. Medscape General Medicine. 2006;8(4):4.
[80] C. Donnelly, M. Bangs, P. Trzepacz, et al. Safety and tolerability of atomoxetine over 3 to 4 years in children and adolescents with ADHD. J Am Acad Child Adolesc Psychiatry. 2009;48(2):176-185.
[81] S. Loo, R. Barkley. Clinical utility of EEG in attention deficit hyperactivity disorder. Appl Neuropsychol. 2005;12(2):64-76.
[82] H. Gevensleben, B. Holl, B. Albrecht, et al. Neurofeedback training in children with ADHD: 6-month follow-up of a randomized controlled trial. Eur Child Adolesc Psychiatry. 2010;19(9):715-724.
[83] H. Gevensleben, B. Holl, B. Albrecht, et al. Is neurofeedback an efficacious treatment for ADHD? A randomized controlled clinical trial. J Child Psychol Psychiatry. 2009;50(7):780-789.
[84] N.C. Patel, M.L. Crismon, K. Hoagwood, et al. Trends in the use of typical and atypical antipsychotics in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2005;44:548-556.
[85] M. Olfson, C. Blanco, L. Liu, et al. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry. 2006;63(6):679-685.
[86] B. Vitiello, C. Correll, B. van Zwieten-Boot, et al. Antipsychotics in children and adolescents: increasing use, evidence for efficacy and safety concerns. Eur Neuropsychopharmacol. 2009;19(9):629-635.
[87] M. Weiss, C. Panagiotopoulos, L. Giles, et al. A naturalistic study of predictors and risks of atypical antipsychotic use in an attention-deficit/hyperactivity disorder clinic. J Child Adolesc Psychopharmacol. 2009;19(5):575-582.
[88] R. Kumar, S. Perminder. Akathesia and second generation antipsychotic drugs. Curr Opin Psychiatry. 2009;22(3):293-299.
[89] B.M. Cooper, P.E. Keck, A. Satlin, et al. Prevalence and severity of akathisia in patients on clozapine. Biol Psychiatry. 1991;29:1215-1219.
[90] A.A. Shirazadi, S.N. Ghaemi. Side effects of atypical antipsychotics: extrapyramidal symptoms and the metabolic syndrome. Harv Rev Psychiatry. 2006;14:152-164.
[91] R.L. Findling. Paediatric psychopharmacology: closing the gap between science and practice. Expert Opin Pharmacother. 2001;2:523-525.
[92] P. Laita, A. Cifuentes, A. Doll, et al. Antipsychotic-related abnormal involuntary movements and metabolic and endocrine side effects in children and adolescents. J Child Adolesc Psychopharmacol. 2007;17:487-502.
[93] C.U. Correll, H.E. Carlson. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:771-791.
[94] C.U. Correll. Assessing and maximizing the safety and tolerability of antipsychotics used in the treatment of children and adolescents. J Clin Psychiatry. 2008;69(Suppl 4):26-30.
[95] C.U. Correll, P. Manu, V. Olshanskiy, et al. Cardiometabolic risk of second generation antipsychotic medications during first time use in child and adolescents. JAMA. 2009;302(16):1765-1773.
[96] S. Kumra, J.V. Oberstar, L. Sikich, et al. Efficacy and tolerabilityof second-generation antipsychotics in children and adolescent with schizophrenia. Schizophr Bull. 2008;34:60-71.
[97] P. Shaw, A. Sporn, N. Gogtay, et al. Childhood-onset schizophrenia: a double-blind, randomized clozapine-olanzapine comparison. Arch Gen Psychiatry. 2006;63:721-730.
[98] J.A. Lieberman, T.S. Stroup, J.P. McEvoy, et al. (Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators): effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223.
[99] L. Sikich, J.A. Frazier, J. McClellan, et al. Double blind comparison of first- and second-generation antipsychotics in early onset schizophrenia: findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420-1431.
[100] J.T. McCracken, J. Mcgough, B. Shah. Risperidone in children with autism and serious behavior problems. N Engl J Med. 2002;347:314-321.
[101] D.W. Haupt, L.C. Rosenblatt, E. Kim, et al. Prevalence and predictors of lipid and glucose monitoring in commercially insured patients treated with second-generation antipsychotic agents. Am J Psychiatry. 2009;166:345.
[102] C.U. Correll. Antipsychotic use in children and adolescents: minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47(1):9-20.
[103] J.M. Foy, K.J. Kelleher, D. Laraque, et alAmerican Academy of Pediatrics Task Force on Mental Health. Enhancing pediatric mental health care: strategies for preparing a primary care practice. Pediatrics. 2010;125(Suppl 3):S87-S108.
Disclosures: Dr Robin Steinberg-Epstein is a consultant for McNeil; has received grant/research support from Addrenex, Eli Lilly & Co., McNeil, Next Wave Pharmaceuticals, Otsuka, Psychogenics, Quintiles, Shionogi Pharm., Shire, and the NIMH; is on the speaker or advisory boards for McNeil. Ms. Book has nothing to disclose. Dr Sharon B. Wigal is a consultant for Abbott, McNeil, NuTec, Shire, Taisho, and the NIMH; has received grant/research support from Addrenex, Eli Lilly & Co., McNeil, Next Wave Pharmaceuticals, Otsuka, Psychogenics, Quintiles, Shionogi Pharm., Shire, NICHD, and the NIMH; is on the speaker or advisory boards for Eli Lilly & Co., McNeil, Shionogi Pharm., and Shire.