CHAPTER 47 Surgical Risk of Transmittable Diseases
The first reports of transmission of blood-borne pathogens from patients to surgeons occurred more than 50 years ago.1,2 These early reports of “serum hepatitis” were generally viewed with a detached attitude by surgeons as something that occasionally happened, and these events did not arouse concern about occupational risks. With the recognition of hepatitis A virus (HAV) and hepatitis B virus (HBV) as distinct viral pathogens and the development of specific antibody detection methods, the scope of HBV infection in patients and surgeons was appreciated. Surgeons had a disproportionately higher prevalence of HBV positivity than did the population in general, and it was rapidly appreciated that transmission of the infection from patients to surgeons (and other health care workers [HCWs]) was a far more common event than had been appreciated. Moreover, a nonserotyped hepatitis was identified, and this indicated that yet another form of transmissible hepatitis existed after blood transfusion and other forms of percutaneous blood exposure.3 This nonserotyped hepatitis was labeled non-A, non-B hepatitis (NANBH).4 During the 1970s, evidence was mounting that surgeons and other HCWs worked in an environment with multiple potential hepatitis viruses, but an attitude of indifference persisted with respect to these risks.
In 1981, acquired immunodeficiency syndrome (AIDS) was first identified,5 and subsequent investigations then characterized human immunodeficiency virus (HIV) as the putative agent. HIV infection was associated with blood transfusion and other mechanisms of percutaneous exposure to contaminated blood. During the 1980s, it became apparent that nearly 1 million individuals in the United States had HIV infection and that clinical AIDS was a uniformly fatal disease.6 Furthermore, it became apparent that HIV infection was a latent disease that otherwise healthy-appearing individuals carried for a number of years before AIDS was evident clinically.7 Events surrounding the recognition of AIDS led to great concern and anxiety in the surgical profession about the occupational risks for both AIDS and hepatitis infection.
More than 25 years has passed since the first AIDS cases were reported, and many events have temporized the great fears that surfaced about the occupational risks of this infection in the 1990s. The risk of occupational transmission has been proved to be very uncommon. The development of highly active antiretroviral therapy (HAART) has not eradicated HIV infection but has provided long-term quality life for many of these patients,8 and by virtue of reduced circulating viral loads in these patients receiving treatment, HAART has further reduced the risks of transmission. On the hepatitis front, a highly effective HBV vaccine has been developed from recombinant technology that has dramatically reduced the risk of occupational HBV infection for surgeons. Unfortunately, these developments have created an environment of lassitude and indifference once again about occupational infection in the operating room.
Hepatitis
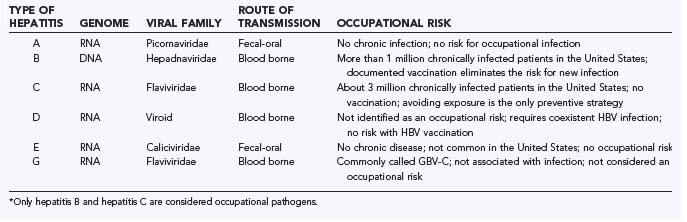
The past 20 years have yielded a dramatic expansion in our understanding of the world of hepatitis infection. Currently, six distinct hepatitis viruses have been identified (Table 47-1). There remains a probability that at least one additional virus remains to be characterized. At present, only HBV and hepatitis C virus (HCV) appear to be of great occupational concern to surgeons. The majority of the following discussion is limited to HBV and HCV infection.
HAV is transmitted by the fecal-oral route and is usually acquired after the ingestion of contaminated water or food products.9 It is an RNA virus that causes an acute and frequently severe hepatitis syndrome. Infected individuals with the hepatitis syndrome (jaundice, malaise, and so on) are acutely ill, but seldom is the outcome of the infection lethal. Importantly, once HAV clinical infection has resolved, there is no state of chronic infection in the aftermath of the acute infection. The absence of a chronic state of infection and the infrequently identified transmission of HAV from blood or blood products do not make this a virus of occupational concern in health care.
Hepatitis E virus is identified primarily in Southeast Asia and is infrequently seen in the United States.10 It too is transmitted by the fecal-oral route, and like HAV, there is no chronic infection after resolution of the acute infection. It is mentioned only for completeness.
Hepatitis D virus, also known as the delta agent, is an incomplete RNA virus that cannot cause infection or replicate without the coexistence of concurrent acute or chronic HBV infection.11 It is seen principally in the intravenous drug abuse population. Infection with hepatitis D virus amplifies the severity of the underlying HBV infection. It is a blood-borne pathogen and theoretically could be an occupational infection for HCWs if preexistent HBV infection were present. Effective vaccination against HBV infection eliminates this risk.
Hepatitis G virus is the most recently identified agent (hepatitis F was putatively identified but has not been validated).12 It is considered the same as the GB virus, where the “GB” initials came from the index infected surgeon who was the source of the virus used in early studies. It is blood borne and found commonly with HBV and HCV infection, and it has genetic homology to HCV. Hepatitis G virus is infrequently found as the sole agent in clinical hepatitis. It is present in as many as 1.4% of blood donors and persists in a chronic state for many years.13 The full scope of its clinical relevance and its risk for occupational transmission continue to be debated.
Hepatitis B
HBV infection is the most thoroughly studied of the blood-borne hepatitis events in humans. HBV is a DNA virus that is very efficiently transmitted by exposure to blood or blood products. Before the era of effective vaccination, HBV infection was the most common and most serious of occupational infections for surgeons. A single hollow-needle percutaneous injury is associated with a 25% to 30% risk of transmission to a naïve host.14 In society, intravenous drug abuse with shared needles has been a major source of transmission of the infection. The virus is a sexually transmitted disease, which has led to a national initiative to vaccinate the pediatric and adolescent populations against HBV.15 Effective screening of the blood supply has virtually eliminated contaminated units of transfused blood as a source of new cases of HBV infection.
Access of the HBV virus to the host results in binding and internalization of the virus within hepatocytes. Viral replication occurs at varying rates after infection. In only about 25% of acute infections is there a clinically discernible hepatitis syndrome.16 The majority of cases either are characterized by a mild malaise without jaundice or have a completely indolent character. Among all acute infections, about 5% of cases result in chronic sustained infection that persists indefinitely.17 The incidence of chronic infection is not related to whether acute infection was identified, which means that many individuals with chronic disease are unaware of the disease. This chronic state of infection is associated with sustained damage to the liver, although selected cases may have a persistent viremia without evidence of continued liver damage. Hepatocellular carcinoma, portal hypertension, and end-stage liver disease from hepatic cirrhosis are the consequences of the chronic disease for many patients.18 An individual with chronic HBV infection is a reservoir of virus for the infection of others. It is currently estimated that more than 1 million individuals in the United States have chronic HBV infection,19 and the numbers in the international community are many millions more.
HBV infection in surgeons in the era before the availability of a vaccine was quite common. In a 1996 study, about a third of surgeons in practice for more than 10 years had serologic evidence of previous HBV infection.20 About a third had been vaccinated, but a third were serologically devoid of antibody and remained vulnerable to acute infection. It was estimated in the late 1980s by the Centers for Disease Control and Prevention (CDC) that 250 HCWs die annually from the consequences of occupationally acquired chronic HBV infection that had obviously been contracted many years previously.21 A more recent analysis has attributed 75 to 250 deaths to occupationally acquired HBV infection for the year 2002.22
In the 1980s, a highly effective HBV vaccine was developed by using attenuated virus from infected patients.23 Recombinant technology rapidly emerged and resulted in the development of an equally effective vaccine that was not derived from other persons. The vaccine is administered in three doses, with the second and third doses being given 1 and 6 months after the initial administration. About 95% of individuals will have an appropriate antibody response to the surface antigen of HBV. Documentation of antibody response is essential, with revaccination being necessary for those who do not seroconvert from the initial immunization effort. Revaccination after a failed initial attempt has a 30% to 50% probability of being successful.24 Vaccination of all surgeons and HCWs is necessary, and not being vaccinated is unacceptable.
Hepatitis C
HCV was identified in 1989 and has for the most part been the virus responsible for NANBH.25 HCV is an RNA virus with multiple different serotypes. It is a source of occupational infection for surgeons and HCWs, but it is less efficiently transmitted than HBV. A percutaneous needlestick from a hollow needle has about a 2% risk of transmission of the infection.26 HCV shares many of the same epidemiologic characteristics of HBV with respect to high-risk populations of patients and means of infection within society.27 Screening of the blood supply for antibody to the virus has dramatically reduced the risk for transfusion-associated infection.
The clinical sequelae after infection of the hepatocyte follow patterns similar to those of HBV. Like HBV, the majority of acute infections are clinically indolent and not associated with a clinical picture of hepatitis.28 However, unlike HBV infection, rates of chronic infection are 60% to 80%.29 The natural history of chronic disease is highly variable, with some patients progressing to end-stage liver disease or hepatocellular carcinoma and others having chronic antigenemia but not an evolving pattern of liver damage.30 Still others may have spontaneous resolution of the infection at a later time. It has an unpredictable time course. Individuals who are antigen positive are infectious to others. There are 3 to 4 million persons in the United States who have chronic HCV infection,31 and HCV has become the primary cause of disease leading to hepatic transplantation.32
There is no vaccine for HCV, although progress has been made with antiviral treatment of this infection.33 HCV infection results in a circulating antibody that is believed to ineffectively neutralize the virus. There are multiple different serotypes of the virus, and reinfection can occur with the same viral type in patients who actually cleared the initial infection. The prospects for a vaccine are challenging when even acute infection does not confer protective immunity for the host against future infection. The antibody response may be delayed for up to 6 months after acute infection, which makes HCV detection in the blood supply more difficult in donors with recent acute infection.
Human Immunodeficiency Virus
HIV infection is transmitted by sexual contact and by intravenous drug abuse. Vertical transmission from infected mothers to newborns has been dramatically reduced in frequency in the United States by the use of antepartum antiretroviral therapy.34 Transmission secondary to blood transfusion has essentially been eliminated with effective screening procedures in the United States and western Europe. HIV infection remains an international pandemic, especially in the African continent, where preventive strategies have been ineffective and treatment of established infection has been unavailable. At present, about 750,000 people are living with HIV infection in the United States, and nearly 600,000 have died since recognition of the disease.35
Considerable effort has been extended in the evaluation and prevention of occupational HIV infection in HCWs. A serologic survey of more than 3000 orthopedic surgeons at a national meeting identified only 2 cases of HIV infection, both of which occurred in individuals with nonoccupational risks for infection.36 Prospective evaluation of mucous membrane and percutaneous exposure events in HCWs has documented 57 cases of occupational infection (Table 47-2).37 The rate of transmission to HCWs from percutaneous exposure is thought to be 0.3%.38 Epidemiologic evaluation of HCWs in whom HIV infection has developed but who do not have nonoccupational risk factors for the disease has resulted in the identification of 139 cases of probable occupational transmission (Table 47-3).
TABLE 47-2 Number of Patients with Documented Seroconversion to HIV after a Specific Exposure Incident*
| OCCUPATION | NO. DOCUMENTED OCCUPATIONAL HIV INFECTIONS |
|---|---|
| Nurses | 24 |
| Clinical laboratory workers | 16 |
| Physicians, nonsurgical | 6 |
| Nonclinical laboratory workers | 3 |
| Housekeeping/maintenance workers | 2 |
| Surgical technician | 2 |
| Embalmer/morgue technician | 1 |
| Health aide/attendant | 1 |
| Respiratory therapist | 1 |
| Dialysis technician | 1 |
| Total | 57 |
* All patients had negative serology at the time of the exposure event and then seroconverted to a positive HIV status after the event.
TABLE 47-3 Number of Health Care Personnel Who Are Thought to Represent Possible Seroconversions for Occupational HIV Infection*
| OCCUPATION | NO. POSSIBLE OCCUPATIONAL HIV INFECTIONS |
|---|---|
| Nurses | 35 |
| Clinical laboratory workers | 17 |
| Health aide/attendants | 15 |
| Housekeeping/maintenance workers | 13 |
| Nonsurgical physicians | 12 |
| Emergency medical technicians | 12 |
| Other technicians/therapists | 9 |
| Surgical physicians | 6 |
| Dental workers/dentists | 6 |
| Dialysis technicians | 3 |
| Surgical technicians | 2 |
| Embalmers/morgue technicians | 2 |
| Respiratory therapists | 2 |
| Others | 5 |
| Total | 139 |
* These cases were identified from the epidemiologic evaluation of health care workers reported to the Centers for Disease Control and Prevention with HIV infection but who were determined after case evaluation not to have nonoccupational risk factors for the infection.
Prevention of Occupational Infection
Personal Protective Barriers
Much has been made of the value of using eye shields and double gloving to prevent contact of blood with the skin or mucous membranes of the surgeon. Every surgeon has had the experience of an arterial or irrigation spray in the face during an operation. Protective eyewear and face shields are available in every operating room and are mandated by the Occupational and Safety Health Administration (OSHA).39 Observations in many operating rooms today will give testimony to the lassitude about occupational infection when one sees inadequate or no eye protection during operations where eye exposure is a real risk.
Double gloving has been shown in many studies to prevent blood contact by the hands of surgeons.40–42 If surgeons wash their hands with isopropyl alcohol after a lengthy craniotomy or a major spine procedure, all of the stinging about the cuticles and elsewhere will be validation that nonintact skin is present. Sustained blood contact by hands with nonintact skin means that occupational infection is a potential risk. Double gloving will prevent blood contact, and the use of indicator systems permits prompt recognition when the glove barrier has been breeched.43
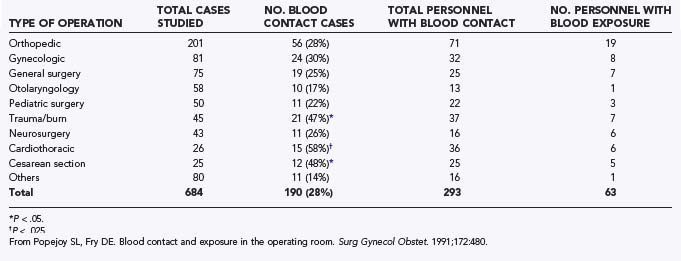
In a previous study, 90% of blood contact with the skin of the operating room team occurred on the hands and forearms (Table 47-4).44 Reinforcement of the forearms with sleeve covers combined with double gloving extending above the level of the seam of the glove cuff of the surgical gown provides a double layer of protection to the area of the body most vulnerable to blood contact during operations. Use of these sleeves is most appropriate for operations involving the chest or abdomen, but they are certainly worth considering during craniotomy for trauma and major spine procedures, where significant blood loss can be anticipated. Wearing a plastic apron underneath the surgical gown and trauma boots that cover the feet up to the level of the knee are also options when intracranial hematomas are being managed surgically.
Technical Considerations
Safe surgery means a zero tolerance for avoidable behavior that leads to percutaneous injury. Sharp instruments must be handled with respect. Blunt-needle technology has been shown to reduce injuries45 and has been endorsed by the American College of Surgeons (ACS)46 and by the National Institute for Occupational Safety and Health (NIOSH).47 Use of the surgical “way” station (e.g., Mayo stand) for the passage of loaded needle holders will prevent injury to the surgeon and scrub personnel from direct hand-to-hand exchanges.48 Use of an electrocautery instead of a scalpel may reduce risks from knife injury. Some have advocated that selected common procedures be done without sharp instruments of any kind in the surgical field.49 Towel barriers to cover bony edges of the cranial vault or spicules of bone in spine procedures can minimize inadvertent abrasions, glove tears, and punctures from these structures. Drills, saws, and rongeurs are all part of selected procedures and require attention to avoid injury. Avoidance of injury in the operating room is more attitude than technique.
Response to Exposure
When exposure from a patient with known or suspected infection has occurred, a specific course of action is necessary. Current serologic testing for the index viruses must be performed for the exposed surgeon to document that preexistent disease is not present.50 For HBV exposure, the current antibody status of the surgeon is important. Previous vaccination with a positive antibody response for the HBV surface antigen means that nothing further needs to be done. If the serology indicates a weak reaction or no antibody response, a dose of HBV immune globulin and a dose of HBV vaccine should be given. If the exposed individual has not had a previous course of full vaccination, a dose of HBV immune globulin and HBV vaccine should be administered. The exposed surgeon should complete the full 6-month course of vaccination. If a surgeon is positive for the core antibody of HBV, that surgeon has previously had HBV infection. If this is a new finding for the surgeon, the presence of the core antigen for HBV needs to be evaluated because the surgeon may be one with unrecognized chronic infection. With the increased emphasis on HBV vaccination, it is hoped that there will be very few chronic HBV infections discovered after an exposure event in the operating room.
There is no vaccine for HIV, but some epidemiologic evidence suggests that prophylactic antiretroviral therapy should be administered for known or suspected exposure.51 The treatment should be continued for a full course with three drugs and serologic evaluation for seroconversion then completed.52
The Infected Surgeon
A great source of debate during the 1990s was whether surgeons who had contracted one of the blood-borne viral infections, by whatever means, should continue to engage in invasive procedures. Some have argued for routine testing of physicians and surgeons and recommend suspension of privileges for those who are positive. Others have pointed to an absence of evidence demonstrating that infected surgeons are a risk to their patients. The identification of a Florida dentist who apparently transmitted HIV infection to several of his patients, presumably by intention, really ignited the controversy.53,54 Many states passed laws requiring various levels of action if physicians were known to harbor HBV or HIV infection. Although the intensity of the debate has receded, many punitive laws about infected surgeons remain.
There have been many clusters of HBV infection that have been transmitted from infected surgeons and dentists to patients.55–57 The common feature for transmission is that the surgeon has had a high concentration of viral units per milliliter of blood. The “e” antigen of HBV has been a marker to identify such surgeons. The e antigen of HBV is a degradation product of the viral nucleocapsid and is seen in patients with active viral replication in the liver.58 Because an epitope of the HBV virus has been identified that is not associated with the e antigen,59 actual viral counts in blood are considered a better predictor of the risk for transmission to patients. A nonsurgical group has recommended that surgeons who are e antigen positive should not continue in the surgical care of patients.60 An e antigen–positive surgeon known to have chronic HBV infection should adhere to the recommendations of the ACS and have an expert local panel convened to make recommendations about future surgical practice (Table 47-5).61
TABLE 47-5 Key Points for Emphasis from the Statement on the Surgeon and Hepatitis by the American College of Surgeons*
| POINT OF EMPHASIS ON HEPATITIS INFECTION | COMMENT |
|---|---|
| Surgeons have an ethical obligation to care for patients with HBV or HCV infection. | Hepatitis infection is not covered by the Americans with Disabilities Act. The moral imperative remains while exercising appropriate standards of infection control in the health care setting. |
| Surgeons should know their HBV and HCV infection status. | Significantly improved antiviral chemotherapy for these infections is currently available. Future additional therapies are being pursued. |
| HBV “e” antigen–positive surgeons are at potential risk for transmission to their patients. | These surgeons should be evaluated by an expert panel to make recommendations about the prevention of infection in patients. |
| All surgeons should know their antibody status for HBV infection and should be immunized against HBV infection. | Surgeons must all be vaccinated against HBV infection. Documentation of an antibody response to vaccination is important. |
| HCV-infected surgeons can safely continue to practice surgery. | A surgeon with HCV infection should adhere to all standards of infection control in the care of patients. It is advisable to seek expert advise about currently available treatment of the infection. |
| Surgeons should seek expert consultation when documented or suspected exposure to a chronic HBV- or HCV-infected patient has occurred. | The surgeon should confer with local experts.† |
* http://www.facs.org/fellows_info/statements/st-22.html.
† National Clinicians’ Postexposure Prophylaxis Hotline at 1-888-448-4911 or visit the website at http://www.nccc.ucsf.edu/Hotlines/PEPline.
Although many cases of HBV transmission to patients from infected surgeons have been identified, only four surgeons have been associated with transmission of HCV infection to patients. The most notable case was from Spain.62 A cardiac surgeon with high viral blood counts (107 viruses/mL) was identified as transmitting infection to at least five patients. Single surgeon-to-patient transmissions of HCV infection have been reported from a cardiac surgeon from the United Kingdom,63 and single transmissions involving a gynecologist64 and an orthopedic surgeon have been reported from Germany.65 No occupational infection has been identified at this time from a neurosurgeon to a patient.
Surgeons and other physicians have been vectors in the transmission of infection even though they may not be infected with HCV themselves. Several clusters of nosocomial HCV infection in patients have resulted from contaminated multidose vials,66,67 contaminated radiopharmaceuticals,68 unsafe injection practices,69 reused needles and syringes,70 and poor hand hygiene.71
To date, no occupational transmission of HIV infection has occurred in the United States except for the dental cases noted earlier. A single case report from France identified a potential transmission from an HIV-infected orthopedic surgeon.72 Studies of patients from the practices of surgeons with HIV infection have not identified evidence of transmission.73 The position of the ACS with respect to HIV-infected surgeons continues to be a valid one (Table 47-6).74 It is important for HIV-positive surgeons to know that they are infected so that effective treatment can be given.
TABLE 47-6 Key Points for Emphasis in the Statement on the Surgeon and HIV Infection from the American College of Surgeons*
| POINT OF EMPHASIS ON HIV INFECTION | COMMENT |
|---|---|
| Surgeons have an ethical obligation to give care to HIV patients. | It is the law that a health care provider must provide care for HIV-infected patients based on the Americans with Disabilities Act. |
| Contemporary standards of infection control practice should be used in all venues of patient care. | In the era of other nosocomial pathogens that can be transmitted in the course of patient care, infection control practice is a must in all patient contacts. |
| HIV-infected surgeons may continue to practice with invasive procedures under the provision that standards of infection control are used and the surgeon is physically fit to practice. | HIV-infected surgeons, especially when given highly effective antiretroviral chemotherapy, should have privileges to practice in the same context as those with diabetes or hypertension. Functional considerations of health should dictate. When there are questions, the surgeon’s personal physician or a locally convened group of experts in HIV should provide recommendations about the continuation of surgical practice. |
| Postexposure prophylaxis with antiretroviral chemotherapy is recommended. | Although the data are not completely convincing, exposure to an HIV-infected patient or an unusually severe event involving exposure to a patient of unknown serology should initiate the triple-drug prophylaxis regimen. |
| All surgeons should know their HIV status. | Because effective treatment is available, surgeons should be tested. The attitude of the early 1990s, when treatment was less effective and restrictions of practice loomed in the background, has passed. |
| All surgeons and the leadership in U.S. surgery must remain sensitive to the issues of patient safety and workplace risks for HIV infection. | Individual surgeons and the national organizations that represent them should maintain an interest in all developments surrounding HIV, its treatment, and transmission. All should maintain interest for patient safety. |
Legal Issues
It is understandable that issues of transmissible infection passing from surgeons or other HCWs to patients would generate some legal issues. The legal and political issues were triggered by recommendations from the CDC for the prevention of HIV and HBV infection during “exposure-prone invasive procedures.”75 Among these recommendations, it was advised that “exposure-prone procedures should be identified by medical/surgical/dental organizations and institutions at which the procedures are performed.” Moreover, “HCWs who are infected with HIV or HBV (and are e-antigen positive) should not perform exposure-prone procedures unless they have sought counsel from an expert review panel and been advised under what circumstances, if any, they may continue to perform these procedures. Such circumstances would include notifying prospective patients of the HCWs’ seropositivity before they undergo exposure-prone invasive procedures.” This led to passage of Public Law 102-141 by Congress in October 1991, which required states to implement the CDC’s recommendations, or their equivalent, as a condition for the receipt of Public Health Service funds. All states complied, although laws were very different from state to state. Although the furor and passion about this subject have waned, fines and imprisonment remain in the law of many states for surgeons who do not follow the requirements established by the CDC in 1991.
A more serious issue for neurosurgeons and for surgeons in general is the Americans with Disabilities Act (ADA).76 The ADA was passed in 1990 and prohibits discrimination on the basis of a person’s disability, which specifically includes HIV/AIDS patients. It explicitly prohibits private providers of public accommodations (dental/medical services) from discrimination based on the defined disability. The Supreme Court of the United States ruled in favor of a lawsuit filed by an HIV-positive patient in 1998 in Bragdon v Abbott.77 In this case a dentist refused to fill a cavity for this patient in his office, but rather chose to do it in the hospital because of concerns of safety from HIV infection. The Supreme Court ruled that the patient posed no direct threat to the dentist and that damages were incurred in the discriminatory expense of having the dental work performed at a hospital.
In yet another case, a private neurosurgery group was required to pay $40,000 in monetary compensation and a $10,000 civil penalty to the United States.78 A neurosurgeon in the group allegedly refused to provide care for a patient with a back condition who was HIV positive. An Assistant Attorney General stated, “The Department of Justice will not stand idly by when doctors refuse medical services, including surgery to people with HIV disease. … A discriminatory refusal of medical care is especially egregious where, as here, the refusal affects a population so dependent on the availability of medical services.”
Future Considerations
It is unlikely that all risks from blood-borne pathogens have been fully described.79 Additional potential hepatitis viral threats remain.80 Among NANBH patients, HCV has accounted for only about 80% of these infections.81 Another hepatitis virus probably exists. The TT virus has been described in Japan,82 and although its significance as a pathogen in humans is not clear, its presence does give testimony that other undefined viruses are potentially present in the blood of our patients. The SEN virus is yet another novel virus that is potentially a hepatic pathogen83 and is transmissible from patient to patient.84
New acute viral disease entities have stormed the clinical scene all over the world and must be considered a threat. West Nile virus infection has been transmitted by transfusion and must be considered a potential occupational pathogen during the viremic phase of the disease.85 Severe acute respiratory syndrome (SARS)86 and the Asian avian influenza87 epidemic are examples of acute infections that are not viewed as being caused by blood-borne pathogens but must have an asymptomatic viremic phase that may make them potentially transmissible. The efficiency of international travel makes these scenarios plausible.
Perhaps the most intriguing of future issues in blood-borne transmissible disease is prion disease, or “infectious proteins.” Transmission from a diseased host to a naïve recipient of the abnormal neuroprotein results in the development of new variant Creutzfeldt-Jakob disease (CJD) (Table 47-7).88 The abnormal prion particle is only protein with no DNA or RNA. It is thought to transmit disease by the abnormally folded pathogen protein serving as a template that results in the normally occurring prion protein assuming the abnormal configuration. The disease has not yet been fully defined, but transmission results in some hosts having progressive degenerative CJD and others potentially being chronic asymptomatic carriers of the protein while being “infectious” to others.89 Iatrogenic disease has been transmitted from neural transplants and the administration of human-derived neural proteins.90 Four cases of transmission from contaminated neurosurgical instruments have occurred. The full scope of transmissible CJD remains unclear. Clinical91 and experimental92 evidence implicates transfusion as a means of transmission. Occupational transmission is a potential consideration.
TABLE 47-7 Sources of Iatrogenically Transmitted Creutzfeldt-Jakob Disease (CJD) in 405 Patients
| SOURCE OF IATROGENIC CJD | NO. CASES | COMMENT |
|---|---|---|
| Surgical procedures | ||
| Dura mater grafts | 196 | 123 cases from Japan |
| Surgical instruments | 4 | 3 cases from the United Kingdom, 1 from France; all underwent standard sterilization after the index procedure |
| Electroencephalographic needles | 2 | Both cases from Switzerland |
| Corneal transplants | 2 | 1 case from Germany and 1 from the United States |
| Hormone therapy | ||
| Growth hormone | 194 | 107 cases from France, 51 from the United Kingdom, 26 from the United States |
| Gonadotropin | 4 | All cases from Australia |
| Blood transfusion | 3 | All cases from the United Kingdom |
Adapted from Brown P, Brandel J-P, Preece M, et al. Iatrogenic Creutzfeldt-Jakob disease: the waning of an era. Neurology. 2006;67:389-393.
Alter HJ. Emerging, re-emerging and submerging infectious threats to the blood supply. Vox Sang. 2004;87(Suppl. 2):S56-S61.
Berguer R, Heller PJ. Preventing sharps injuries in the operating room. J Am Coll Surg. 2004;199:462-467.
Brown P, Brandel J-P, Preece M, et al. Iatrogenic Creutzfeldt-Jakob disease: the waning of an era. Neurology. 2006;67:389-393.
Centers for Disease Control and Prevention (CDC). Evaluation of blunt suture needles in preventing percutaneous injuries among health-care workers during gynecologic surgical procedures—New York City, March 1993–June 1994. MMWR Morb Mortal Wkly Rep. 1997;46(2):25-29.
Department of Justice. Neurosurgery group pays $50,000 to settle HIV discrimination claim with Justice Department. Available at http://www.usdoj.gov/opa/pr/2000/December/709cr.htm
Esteban JI, Gomez J, Martell M, et al. Transmission of hepatitis C virus by a cardiac surgeon. N Engl J Med. 1996;334:555-560.
Fry DE. The ABCs of hepatitis. Adv Surg. 1999;33:413-437.
Harpaz R, von Seidlein L, Averhoff FM, et al. Transmission of hepatitis B virus to multiple patients from a surgeon without evidence of inadequate infection control. N Engl J Med. 1996;334:549-554.
Howard RJ, Fry DE, Davis JM, et al. Hepatitis C virus infection in healthcare workers. J Am Coll Surg. 1997;184:540-552.
Makary MA, Pronovost PJ, Weiss ES, et al. Sharpless surgery: a prospective study of the feasibility of performing operations using non-sharp techniques in an urban, university-based surgical practice. World J Surg. 2006;30:1224-1229.
Shapiro CN. Occupational risk of infection with hepatitis B and hepatitis C virus. Surg Clin North Am. 1995;75:1047-1056.
Shapiro CN, Tokars JI, Chamberland ME, American Academy of Orthopaedic Surgeons Serosurvey Study Committee. Use of hepatitis-B vaccine and infection with hepatitis B and C among orthopaedic surgeons. J Bone Joint Surg Am. 1996;78:1791-1800.
U.S. Public Health Service. Updated U.S. Public Health Service guidelines for the management of occupational exposure to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2001;50(RR-11):1-52.
1 Trumbell ML, Greiner DJ. Homologous serum jaundice: an occupational hazard to medical personnel. JAMA. 1951;145:965-969.
2 Kuh C, Ward WE. Occupational virus hepatitis: an apparent hazard for medical personnel. JAMA. 1951;143:631-634.
3 Wick MR, Moore S, Taswell HF. Non-A, non-B hepatitis associated with blood transfusion. Transfusion. 1985;25:93-101.
4 Alter MJ, Hadler SC, Francis DP, et al. The epidemiology of non-A, non-B hepatitis in the United States. Prog Clin Biol Res. 1985;182:71-79.
5 Gottlieb MS, Schroff R, Schanker HM, et al. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981;305:1425-1431.
6 HIV prevalence estimates and AIDS case projections for the United States: report based on a workshop. MMWR Recomm Rep. 1990;39(RR-16):1-31.
7 Lifson AR, Rutherford GW, Jaffe HW. The natural history of human immunodeficiency virus infection. J Infect Dis. 1988;158:1360-1367.
8 Bartlett JA, Fath MJ, Demasi R, et al. An updated systematic overview of triple combination therapy in antiretroviral-naïve HIV-infected adults. AIDS. 2006;20:2051-2064.
9 Cuthbert JA. Hepatitis A: old and new. Clin Microbiol Rev. 2001;14:38-58.
10 Reyes GR, Purdy MA, Kim JP, et al. Isolation of a cDNA from the virus responsible for enteric transmitted non-A, non-B hepatitis. Science. 1990;247:1335-1339.
11 Wang K-S, Choo Q-L, Weiner AJ, et al. Structure, sequence and expression of the hepatitis delta viral genome. Nature. 1986;323:508-514.
12 Linnen J, Wages JJr, Zhang-Keck Z-Y, et al. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science. 1996;271:505-508.
13 Alter HJ, Nakatsuji Y, Melpolder J, et al. The incidence of transfusion-associated hepatitis G virus infection and its relation to liver disease. N Engl J Med. 1997;336:747-754.
14 Shapiro CN. Occupational risk of infection with hepatitis B and hepatitis C virus. Surg Clin North Am. 1995;75:1047-1056.
15 Mast EE, Margolis HS, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendation of the Advisory Committee of Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54(RR-16):1-31.
16 Fry DE. The ABCs of hepatitis. Adv Surg. 1999;33:413-437.
17 Seeff LB, Koff RS. Evolving concepts of the clinical and serologic consequences of hepatitis B virus infection. Semin Liver Dis. 1986;6:11-22.
18 Befeler AS, DiBisceglie AM. Hepatitis B. Infect Dis Clin North Am. 2000;14:617-632.
19 Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745.
20 Shapiro CN, Tokars JI, Chamberland ME, American Academy of Orthopaedic Surgeons Serosurvey Study Committee. Use of hepatitis-B vaccine and infection with hepatitis B and C among orthopaedic surgeons. J Bone Joint Surg Am. 1996;78:1791-1800.
21 Centers for Disease Control and Prevention (CDC). Guidelines for prevention of transmission of human immunodeficiency virus and hepatitis B virus to health-care and public-safety workers—a response to P.I. 100-607 The Health Omnibus Program Extension Act of 1988. MMWR Morb Mortal Wkly Rep. 1989;38(S-6):3-37.
22 Sepkowitz KA, Eisenberg L. Occupational deaths among healthcare workers. Emerg Infect Dis. 2005;11:1003-1008.
23 Zajac BA, West DJ, McAleer WJ, et al. Overview of clinical studies with hepatitis B vaccine made with recombinant DNA. J Infect Dis. 1986;13(Suppl):39-45.
24 Hadler SC, Francis DP, Maynard JE, et al. Long term immunogenicity and efficacy of hepatitis B vaccine in homosexual men. N Engl J Med. 1986;315:209-214.
25 Choo Q-L, Kuo G, Weiner AJ, et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359-362.
26 Recommendations for prevention and control of Hepatitis C virus infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1-39.
27 Howard RJ, Fry DE, Davis JM, et al. Hepatitis C virus infection in healthcare workers. J Am Coll Surg. 1997;184:540-552.
28 Koretz RL, Brezina M, Polito AJ, et al. Non-A, non-B posttransfusion hepatitis: comparing C and non-C hepatitis. Hepatology. 1993;17:361-365.
29 Alter MJ. Community-acquired viral hepatitis B and C in the United States. Gut. 1993;34(Suppl 2):517-519.
30 Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26:62S-65S.
31 Sheppard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558-567.
32 American Liver Foundation. Liver Transplant. Available at http://www.liverfoundation.org/education/info/transplant
33 Degertekin B, Lok AS. Update on viral hepatitis: 2007. Curr Opin Gastroenterol. 2008;24:306-311.
34 Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173-1180.
35 Centers for Disease Control and Prevention (CDC). HIV/AIDS Surveill Rep, 17. 2005:1-49. http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2005report/pdf/2005SurveillanceReport.pdf.
36 Tokars JI, Chamberland ME, Schable CA, et al. A survey of occupational blood contact and human immunodeficiency virus infection among orthopedic surgeons. The American Academy of Orthopaedic Surgeons Serosurvey Study Committee. JAMA. 1992;268:489-494.
37 Centers for Disease Control and Prevention (CDC). Surveillance of healthcare personnel with HIV/AIDS, as of December 2002. Available at http://www.cdc.gov/ncid0d/dhqp/bp_hiv_hp_with.html
38 Centers for Disease Control and Prevention (CDC). Exposure to blood—what health-care workers need to know, 2003. Available at http://www.cdc.gov/ncidod/dhqp/pdf/bbp/Exp_to_Blood.pdf
39 Occupational Safety and Health Administration. Occupational exposure to bloodborne pathogens: final rule. Fed Reg. 1991;56:64004.
40 Quebbeman EJ, Telford GL, Wadsworth K, et al. Double gloving: protecting surgeons from blood contacts in the operating room. Arch Surg. 1992;127:213-216.
41 Jensen SL, Kristensen B, Fabrin K. Double gloving as self protection in abdominal surgery. Eur J Surg. 1997;163:163-167.
42 Chapman S, Duff P. Frequency of glove perforations and subsequent blood contact in association with selected obstetric surgical procedures. Am J Obstet Gynecol. 1993;168:1354-1357.
43 Florman S, Burgdorf M, Finigan K, et al. Efficacy of double gloving with an intrinsic indicator system. Surg Infect. 2005;6:385-395.
44 Popejoy SL, Fry DE. Blood contact and exposure in the operating room. Surg Gynecol Obstet. 1991;172:480-483.
45 Centers for Disease Control and Prevention (CDC). Evaluation of blunt suture needles in preventing percutaneous injuries among health-care workers during gynecologic surgical procedures—New York City, March 1993–June 1994. MMWR Morb Mortal Wkly Rep. 1997;46(2):25-29.
46 American College of Surgeons. [ST-52] Statement on blunt suture needles. Available at http://www.facs.org/fellows_info/statements/st-52.html
47 National Institute for Occupational Safety and Health. Use of blunt-tip needles to decrease percutaneous injuries to surgical personnel. Safety and Health Information Bulletin. DHHS (NIOSH) Publication No. 2008-101. Available at http://www.cdc.gov/niosh/docs/2008-101/pdfs/2008-101.pdf
48 Berguer R, Heller PJ. Preventing sharps injuries in the operating room. J Am Coll Surg. 2004;199:462-467.
49 Makary MA, Pronovost PJ, Weiss ES, et al. Sharpless surgery: a prospective study of the feasibility of performing operations using non-sharp techniques in an urban, university-based surgical practice. World J Surg. 2006;30:1224-1229.
50 U.S. Public Health Service. Updated U.S. public health service guidelines for the management of occupational exposure to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2001;50(RR-11):1-52.
51 Centers for Disease Control and Prevention (CDC). Case controlled study of HIV seroconversion in health-care workers after percutaneous exposure to HIV-infected blood—France, United Kingdom, and United States. January 1988–August 1994. MMWR Morb Mortal Wkly Rep. 1995;44(50):929-933.
52 Panillo AL, Cardo DM, Grohskopf LA, et al. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54(RR-9):1-17.
53 Centers for Disease Control and Prevention (CDC). Update—transmission of HIV infection during an invasive dental procedure—Florida. MMWR Morb Mortal Wkly Rep. 1991;40:21-27. 33
54 Ciesielski C, Marianos D, Qu CY, et al. Transmission of human immunodeficiency virus in a dental practice. Ann Intern Med. 1992;116:798-805.
55 Heptonstall J. Outbreaks of hepatitis B virus infection associated with infected surgical staff. CDR (Lond Engl Rev). 1991;1(8):R81-R85.
56 Harpaz R, von Seidlein L, Averhoff FM, et al. Transmission of hepatitis B virus to multiple patients from a surgeon without evidence of inadequate infection control. N Engl J Med. 1996;334:549-554.
57 Spijkerman IJB, van Doorn L-J, Jansssen MHW, et al. Transmission of hepatitis B virus from a surgeon to his patients during high-risk and low-risk surgical procedures during 4 years. Infect Control Hosp Epidemiol. 2002;23:306-312.
58 Slusarczyk J, Hess G, Meyer zum Buschenfelde K-H. Association of hepatitis B e antigen (HBeAg) with the core of the hepatitis B virus. Liver. 1985;5:48-53.
59 Heptonstall J, Incident Investigation Team. Transmission of hepatitis B to patients from four infected surgeons without hepatitis B e antigen. N Engl J Med. 1997;336:178-184.
60 AIDS/TB Committee of the Society for Healthcare Epidemiology of America. Management of healthcare workers with hepatitis B virus, hepatitis C virus, human immunodeficiency virus, or other bloodborne pathogens. Infect Control Hosp Epidemiol. 1997;18:349-363.
61 American College of Surgeons. [ST-22] Statement on the surgeon and hepatitis. Available at http://www.facs.org/fellows_info/statements/st-22.html
62 Esteban JI, Gomez J, Martell M, et al. Transmission of hepatitis C virus by a cardiac surgeon. N Engl J Med. 1996;334:555-560.
63 Duckworth GJ, Heptonstall J, Aitken C. Transmission of hepatitis C virus from a surgeon to a patient. The incident control team. Commun Dis Public Health. 1999;2:188-192.
64 Ross RS, Viazov S, Thromahlen M, et al. Risk of hepatitis C virus transmission from an infected gynecologist to patients: results of a 7-year retrospective investigation. Arch Intern Med. 2002;162:805-810.
65 Ross RS, Viazov S, Roggendorf M. Phylogenetic analysis indicates transmission of hepatitis C virus from an infected orthopaedic surgeon to a patient. J Med Virol. 2002;66:461-467.
66 Germain JM, Carbonne A, Thiers V, et al. Patient-to-patient transmission of hepatitis C virus through the use of multidose vials during general anesthesia. Infect Control Hosp Epidemiol. 2005;26:789-792.
67 Krause G, Trepka MJ, Whisenhunt RS, et al. Nosocomial transmission of hepatitis C virus associated with the use of multidose saline vials. Infect Control Hosp Epidemiol. 2003;24:122-127.
68 Patel PR, Larson AK, Castel AD, et al. Hepatitis C virus infections from a contaminated radiopharmaceutical used in myocardial perfusion studies. JAMA. 2006;296:2005-2011.
69 Centers for Disease Control and Prevention (CDC). Acute hepatitis C virus infections attributed to unsafe injection practices at an endoscopy clinic—Nevada 2007. MMWR Morb Mortal Wkly Rep. 2007;57(19):513-517.
70 Comstock RD, Mallonee S, Fox JL, et al. A large nosocomial outbreak of hepatitis C and hepatitis B among patients receiving pain remediation treatments. Infect Control Hosp Epidemiol. 2004;25:576-583.
71 Savey A, Simon F, Izopet J, et al. A large nosocomial outbreak of hepatitis C virus infections at a hemodialysis center. Infect Control Hosp Epidemiol. 2005;26:752-760.
72 Lot F, Seguier J-C, Fegueux S, et al. Probable transmission of HIV from an orthopedic surgeon to a patient in France. Ann Intern Med. 1999;130:1-6.
73 Robert LM, Chamberland ME, Cleveland JL, et al. Investigation of patients of health care workers infected with HIV. The Centers for Disease Control and Prevention database. Ann Intern Med. 1995;122:653-657.
74 American College of Surgeons. [ST-13] Statement on the surgeon and HIV infection. Available at http://www.facs.org/fellows_info/statements/st-13.html
75 Recommendations for preventing transmission of human immunodeficiency virus and hepatitis B virus to patients during exposure-prone invasive procedures. MMWR Recomm Rep. 1991;40(RR-8):1-9.
76 . Title 42 of the Unites States Code: Americans with Disabilities Act of 1990. Available at http://www.ada.gov/pubs/ada.htm
77 Centers for Disease Control and Prevention (CDC). Business responds to AIDS/Labor responds to AIDS; HIV and the law. Available at http://www.hivatwork.org/law/americans-disbilities-act.htm
78 Department of Justice. Neurosurgery group pays $50,000 to settle HIV discrimination claim with justice department. Available at http://www.usdoj.gov/opa/pr/2000/December/709cr.htm
79 Alter HJ. Emerging, re-emerging and submerging infectious threats to the blood supply. Vox Sang. 2004;87(Suppl. 2):S56-S61.
80 Narayanan Menon KV. Non-A to E hepatitis. Curr Opin Infect Dis. 2002;15:529-534.
81 Seeff LB, Hollinger FB, Alter HJ, et al. Long-term mortality and morbidity of transfusion-associated non-A, non-B, and type C hepatitis: a National Heart, Lung, and Blood Institute collaborative study. Hepatology. 2001;33:455-463.
82 Nishizawa T, Okamoto H, Konishi K, et al. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun. 1997;241:92-97.
83 Umemura T, Yeo AE, Sottini A, et al. SEN virus infection and its relationship to transfusion-associated hepatitis. Hepatology. 2001;33:1303-1311.
84 Pirovano S, Bellinzoni M, Ballerini C, et al. Transmission of SEN virus from mothers to babies. J Med Virol. 2002;66:421-427.
85 Hayes EB, Gubler DJ. West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med. 2006;57:181-194.
86 Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170:1136-1147.
87 Thomas JK, Noppenberger J. Avian influenza: a review. Am J Health Syst Pharm. 2007;64:149-165.
88 Aguzzi A, Glatzel M. Prion infections, blood and transfusions. Nat Clin Pract Neurol. 2006;2:321-329.
89 Ironside JW. Variant Creutzfeldt-Jakob disease: risk of transmission by blood transfusion and blood therapies. Haemophilia. 2006;12(Suppl. 1):8-15.
90 Brown P, Brandel J-P, Preece M, Sato T. Iatrogenic Creutzfeldt-Jakob disease: the waning of an era. Neurology. 2006;67:389-393.
91 Llewelyn CA, Hewitt PE, Knight RS, et al. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417-421.
92 Houston F, Foster JD, Chong A, et al. Transmission of BSE by blood transfusion in sheep. Lancet. 2000;356:999-1000.