Chapter 147 Surgical Resection of Choroidal Melanoma
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
Introduction
Successful exoresection of choroidal melanoma was reported as early as 19251; however, this type of operation is still performed only rarely, not least because of fears about its safety. Stallard reported two cases in 1966 and advocated partial choroidectomy only as a last resort for patients whose tumors did not regress after radiotherapy or who had poor vision in the fellow eye.2 In 1973, Foulds challenged the prevailing dogmas about radical surgery and started performing primary exoresection irrespective of the status of the fellow eye.3 Since then, we have between us performed more than 630 exoresections and 120 endoresections for posterior uveal melanoma.4–9 Others have also adopted these procedures.10–17 This chapter describes our surgical techniques, summarizes the results, identifies the main complications and their management, and discusses the indications in relation to other forms of conservative therapy. This overview reflects our own perspective, with reference to other literature on the subject.
Exoresection
Indications and contraindications
Tumors less than 6 mm in thickness respond satisfactorily to plaque or proton beam radiotherapy unless they have invaded retina or if they extend close to the optic disc. Radiotherapy of more bulky tumors is associated with a significant complication rate, which increases with time, regardless of the type of radiation used, especially when the tumor extends far anteriorly or posteriorly, or if there is an extensive retinal detachment.18–21 Conversely, large tumor size, anterior location, and the presence of exudative retinal detachment tend to make exoresection less difficult. Two matched group studies have reported that with large tumors, the results are better after exoresection than after iodine plaque radiotherapy.22,23
Relative contraindications to local resection include: (1) a tumor diameter >18 mm; (2) tumor extension to within a disc diameter of the optic disc margin; (3) extensive retinal invasion or any retinal perforation; (4) extraocular extension; (5) involvement of more than 2 clock-hours of ciliary body or angle; and (6) general health precluding hypotensive anesthesia. If, however, the patient has poor vision in the fellow eye or if enucleation is refused, then local resection can be performed, with special measures being taken to deal with the increased difficulty. Absolute contraindications include diffuse melanoma and optic nerve invasion. Old age is not in itself a contraindication and even facilitates the anesthesia, because hemorrhage is arrested at a higher blood pressure, which is also easier to control. It is possible to perform exoresection in children.24
Surgical technique
Exposure
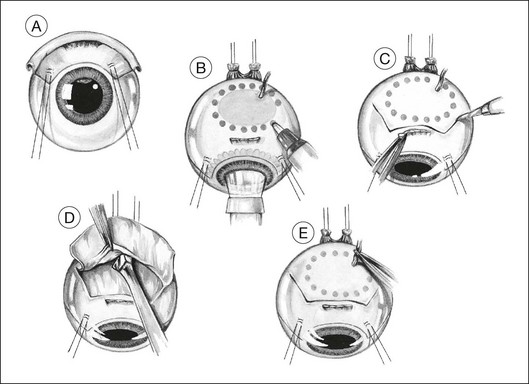
The lashes are trimmed so that they do not get in the way during a delicate part of the operation. The eyelids are retracted using both a wire speculum and traction sutures. The ocular surface is kept moist with 1.5% methylcellulose solution. A 180° limbal conjunctival incision is made. The episclera is scraped away with a No. 15 Bard Parker scalpel. Intervening extraocular muscles are disinserted. Two bridle sutures (i.e., 5–0 braided polyester) for rotating the eye are inserted in the sclera 4 mm from the limbus and clipped with artery forceps (Fig. 147.1A). The tumor margins are identified by transillumination and marked on the sclera with a felt-tipped pen, with care being taken not to be mis-led by any penumbra or subretinal hematoma (Fig. 147.1B).
Lamellar scleral dissection
A lamellar scleral flap is fashioned and is hinged posteriorly. This is polyhedral, rather than circular, to facilitate good apposition of the wound edges during closure (Fig. 147.1C). The scleral flap should clear the apparent tumor margins by about 5 mm. By making the flap wider posteriorly, it is possible to reduce the length of the lateral incisions, making closure easier. The flap should be about 80% of the scleral thickness. Any inadvertent buttonholes in the superficial flap are immediately closed with a purse-string suture. Any buttonholes in the deep sclera are sutured or temporarily covered with a plastic patch to prevent prolapse of choroid or tumor when dissecting posterior sclera.
We prepare the flap using a feather blade for the initial scleral incisions and a Desmarres scarifier for lamellar scleral dissection (Fig. 147.1D).
To avoid troublesome hemorrhage, any vortex veins overlying the scleral flap are cauterized before being divided, applying bipolar diathermy both extraocularly (Fig. 147.1E) and to the intrascleral portion of the vein after cautiously exposing as much of the vessel as possible (Fig. 147.1F). Long ciliary vessels overlying the scleral flap are treated similarly. Gentle bipolar cautery of some of the short ciliary vessels adjacent to the optic nerve further reduces hemorrhage.
The lamellar scleral dissection should extend beyond the posterior tumor margin, which is located using the preoperative ultrasound measurements or by transillumination (Fig. 147.1G).
Ocular decompression
If the vitrectomy is delayed until the scleral flap is prepared, then three-port vitrectomy with infusion is unnecessary, the vitrectomy being performed through a single sclerotomy using illumination from the operating microscope (Fig. 147.1H).
Deep scleral incision
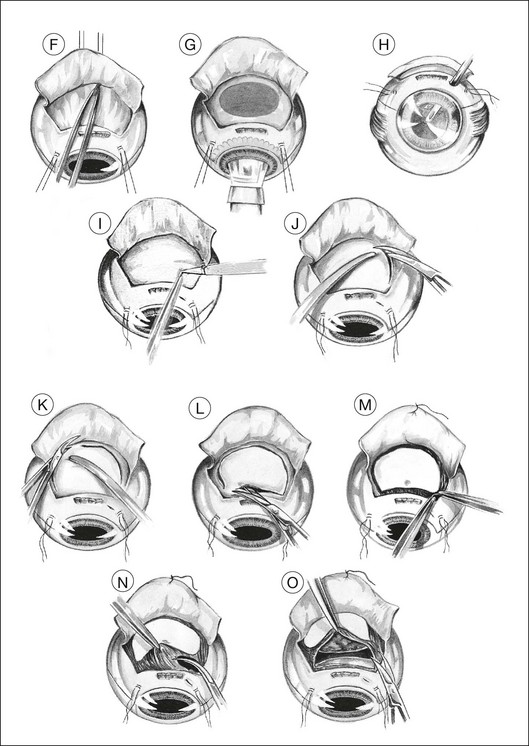
Two small buttonhole incisions are made in the deep sclera about 2 mm inside the superficial scleral incision anterolateral to the tumor (Fig. 147.1I). To avoid damaging choroid, the sclera is pinched with fine-toothed microforceps to create a fold, which is then shaved with a knife until perforation occurs. This scleral incision is extended around the tumor with blunt-tipped corneoscleral scissors (Fig. 147.1J,K). The deep scleral incision is kept 2 mm inside the superficial scleral incisions, so as to create a stepped wound edge, which facilitates subsequent closure (Fig. 147.1L). To prevent excessive bulging of the intraocular contents, the lateral and posterior scleral incisions are completed before the anterior incision is made.
Tumor excision
The subretinal space is entered, preferably at a site where the retina is known to be detached (Fig. 147.1M). This is done by holding the choroid with two pairs of ribbed (not toothed) microforceps and moving them apart to tear the uveal tissue.
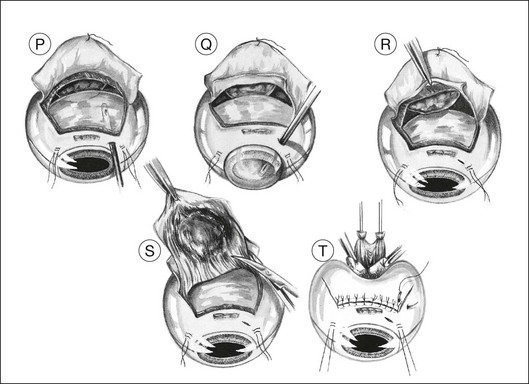
Usually, the choroid is divided in front of the tumor (Fig. 147.1N), then laterally (Fig. 147.1O), and finally posteriorly, using blunt-tipped corneoscleral scissors. If there is excessive retinal bulging, further vitrectomy is performed, with the vitrector being viewed through the retina (Fig. 147.1P) or the pupil (Fig. 147.1Q). If ocular decompression is adequate, the retina should fall away from the tumor so that a space appears between the retina and the normal choroid around the tumor margins (Fig. 147.1R). This allows the uveal tissue posterior to the tumor to be divided with the corneoscleral scissors (Fig. 147.1S). Despite the systemic hypotension, there is usually some oozing of blood, which must be mopped away before clots form, because these are difficult to remove.
As soon as the tumor is excised, the instruments are exchanged for a fresh set to prevent possible seeding of tumor cells. At the earliest opportunity, the intravitreal pressure is increased until the retina bulges slightly in the scleral window so that there is no potential space in which a subretinal hematoma can form. This is achieved by exerting traction on the bridle sutures and by compressing the eye with sponges placed behind the eye posterior to the scleral window (Fig. 147.1T).
Scleral closure
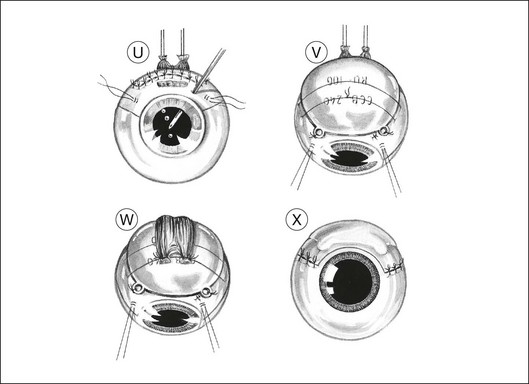
The corners of the flap are sutured first, followed by the anterior margin and finally the lateral margins. Interrupted 8–0 nylon sutures are placed about 2 mm apart. As soon as the suturing of the flap is complete, the globe is reformed by injecting balanced salt solution intravitreally, either using the three-way tap, if an infusion cannula is present, or through a 25-gauge needle attached to a syringe (Fig. 147.1U). Gas tamponade is no longer considered useful, but 2 ml of air is kept in the syringe when injecting fluid, because its compressibility prevents a sudden rise in intraocular pressure, which might rupture the wound.
Adjunctive brachytherapy
Adjunctive plaque radiotherapy is routinely applied, delivering a dose of approximately 100 Gy to a depth of 1–2 mm. We favor the use of a 25-mm ruthenium plaque because of the limited range of beta irradiation, the convenience of a long half-life, and the thin shape of the implant, which facilitates positioning over the site of the previous tumor (Fig. 147.1V). If the superficial flap has inadvertently been buttonholed or if cyclectomy has been performed this brachytherapy is delayed by 1 month.
Eye closure
The muscles are resutured to their original insertions with 5–0 braided polyglycolic acid sutures (Fig. 147.1W). When the muscle insertion is located on the scleral flap, the reinsertion of the muscle is safer if a 1 mm stump of tendon is left in situ at the time of the disinsertion. To compensate for any muscle shortening, the distance from the suture knots to the limbus is measured before the muscle tendon is divided and also at the time of reinsertion so that a sling can be used if necessary.
The conjunctiva is closed with 8–0 braided polyglactin sutures (Fig. 147.1X). Antibiotics, mydriatics and steroids are given in the usual fashion. The entire procedure usually takes between 2 and 3 hours.
Variations in technique
Retinal adhesion
Problematic adhesion between tumor and retina is more common with relatively thick tumors.8 Adherent retina can often be separated from the tumor by scraping the surface of the tumor with a No. 15 Bard Parker scalpel, about 1–2 mm away from the apparent line of retinal adhesion, so as to divide invisible strands of tissue joining retina to tumor. If this procedure fails, the tumor has probably invaded the retina itself. In this case, our preferred course of action is to top-slice the tumor with the scalpel, leaving the intraretinal portion in situ and treating it with radiotherapy. Another option is to excise the tumor completely, together with the invaded retina, dealing with the retinal defect after closing the sclera. Any retinal defect is managed by total vitrectomy, removal of subretinal hemorrhage, endolaser photocoagulation, and silicone tamponade, with these procedures performed as soon as possible, either immediately after reforming the eye with balanced salt solution or, if this is not possible, a few days later. Our results show that these measures are highly successful at preventing retinal detachment.8
Anesthesia
Intraoperative hemorrhage is reduced by profound hypotensive anesthesia.25 This lowers the systolic blood pressure to approximately 40–50 mmHg for about 60 minutes, from the time when ocular decompression is performed to the moment when the intraocular pressure is increased by intravitreal injection after scleral flap closure.
Outcomes
Visual acuity
In 2011, the authors audited 112 resections performed in the previous 10 years (manuscript in preparation). The tumors had a median diameter of 15.3 mm (range 8.9–22.4) and a median thickness of 8.5 mm, with 41% involving ciliary body and 16% extending to within 3 mm of the optic disc or fovea. The successful resections included several advanced and difficult cases, such as a patient operated on without any hypotensive anesthesia (because she had the thalassemia trait) and one advanced tumor with total funnel retinal detachment touching the lens and an intraocular pressure of 44 mmHg. At the close of the study, 88% of eyes were retained, with 58% having visual acuity of 20/200 or better and 30% having 20/40 or better (Fig. 147.2). In a previous investigation, we showed that the most significant preoperative factors for predicting retention of good vision (20/40 or better) were nasal tumor location (P = 0.002) and distance of more than one disc diameter between the tumor and the optic disc or fovea (P = 0.01).5
Local tumor control
In our 2011 audit, there were no patients with visible residual tumor at the end of the resection procedure. Recurrent tumor developed in 12 patients, after a median of 2.4 years (range 0.6–3.8). Adjunctive brachytherapy seems effective at preventing local tumor recurrences in the irradiated field; however, there have been a few recurrences in nonirradiated areas, that is, in distant parts of the uvea or if adequate coverage of the resection area by the plaque could not be achieved, because the tumor had an irregular shape, or because it extended close to the optic disc, or because the plaque was not large enough to treat a sufficiently wide surround of apparently healthy choroid. We now use a 25 mm plaque instead of a 20 mm plaque. In a previous study of 286 resections performed before the introduction of adjunctive brachytherapy, we showed by Cox multivariate analysis that the predictive factors for recurrent tumor are posterior extension to within a disc diameter of disc or fovea (P = 0.002), presence of epithelioid cells (P = 0.002), and tumor diameter of ≥16 mm or more (P = 0.019). Histological examination of clearance margins was unreliable.7
Tumor recurrence can arise at the margin of the surgical coloboma as an indistinct gray, brown, or white swelling. Rarely, the tumor recurs within the coloboma as a result of intraretinal or intrascleral tumor invasion. Exceptionally, a satellite lesion develops in a distant part of the choroid.26 Noncontiguous recurrences have also been seen after plaque radiotherapy.27 Residual and recurrent tumors need to be distinguished from reactive pigment epithelial hyperplasia and organized subretinal hematomas. Sequential fundus photography is invaluable for distinguishing tumor from other conditions. If there is any doubt about the diagnosis, the suspicious lesion should be ablated by phototherapy as a precautionary measure.
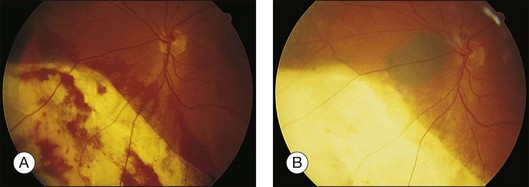
Residual or recurrent tumor is best treated with plaque or proton beam radiotherapy, unless it is very small or situated close to the optic disc or fovea, in which case transpupillary thermotherapy can be attempted (Fig. 147.3). Failure to detect and treat a recurrent tumor effectively can result in tumor extension extraocularly or involvement of the optic disc so that enucleation becomes necessary.
Retinal detachment
Before the introduction of ocular decompression, retinal tears tended to occur because of retinal prolapse when the tumor was being excised. Today, retinal tears occur almost exclusively when attempting to separate tumor from adherent retina. Retinal breaks can usually be identified immediately and rarely result in retinal detachment if adequate vitreoretinal surgery is performed promptly. Our 2011 audit showed the rate of retinal detachment to be 9%. We previously demonstrated retinal detachment to correlate with tumor thickness.8
Metastatic death
Nonrandomized, matched group studies suggest that the incidence of metastatic disease after local excision of uveal melanoma is not significantly different from that after enucleation4 or after plaque radiotherapy.28 As with cutaneous melanoma, breast cancer, and several other malignancies, radical treatment does not seem to give any survival benefit as compared with more conservative and less mutilating therapy.
In a series of 332 patients treated by transscleral local resection before 1996, we showed the probability of metastatic death to correlate with epithelioid cellularity, and large tumor diameter.6 Although highly significant statistically, these correlations had little clinical relevance because they provided only an approximate indication of the prognosis as far as an individual patient was concerned. In 1996, Prescher et al. showed loss of chromosome 3 (i.e., monosomy 3) within uveal melanoma cells to be highly predictive of metastatic disease and death.29 Since 1999, we have therefore determined the chromosome 3 status as a routine service, first using fluorescent in situ hybridization (FISH)30 and, more recently, multiplex ligation-dependent probe amplification.31 We have developed and validated an online tool for predicting survival according to clinical stage, histologic grade and genetic type also taking the patient’s age and sex into account.32 These genetic studies have enabled us to reassure those with an excellent prognosis for survival, while referring high-risk patients to an oncologist for specialized care. Local resection provides large specimens for prognostic studies and in the future, may be useful for selecting systemic treatment or for developing vaccines or other forms of therapy.
Endoresection
The main objective of primary endoresection of choroidal melanoma is to avoid optic neuropathy and maculopathy after radiotherapy,33,34 and local tumor recurrence and extraocular extension after transpupillary thermotherapy.35,36
Surgical technique
Briefly, the technique involves the following steps: (1) pars plana vitrectomy with a 20-gauge vitreous cutter; (2) the creation of a retinotomy over the tumor; (3) piecemeal tumor removal; (4) endodiathermy to the margins and bed of the coloboma; (5) fluid–air exchange to flatten the retina; (6) endolaser retinopexy to attach retina around the coloboma; (7) endolaser photocoagulation to the entire scleral bed, to destroy any residual tumor; (8) air–silicone exchange to maintain retinal flattening and to prevent postoperative hemorrhage; (9) indentation with cryotherapy to any entry site tears; and (10) cryotherapy to the sclerotomies, in case of tumor seeding.30 Recent developments include the use of heavy liquids in some cases, and bimanual surgery. Adjunctive ruthenium plaque radiotherapy is administered at a later date if histologic studies indicate a high grade of malignancy.
Outcomes
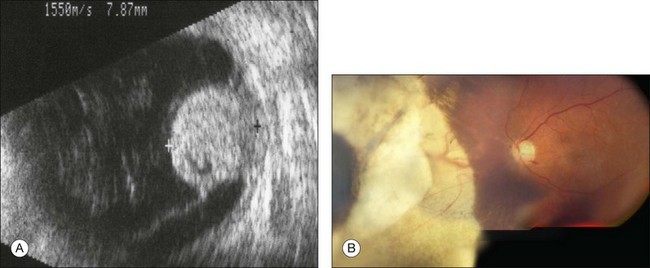
The techniques of endoresection have improved since our initial publication in 1998.9 The outcomes depend largely on the tumor location (Fig. 147.4). In an interim analysis of an ongoing study, 52 out of 53 eyes operated on between 2007 and 2011 were conserved, with 42% having vision of counting fingers or better, 25% having 20/200 or better and 11% retaining 20/40 or better (unpublished data). Four of these patients developed retinal detachment and two had local tumor recurrence, which consisted of subconjunctival seeding in one case. The tumors had a median diameter of 9.8 mm (range 4.8–14.5), with 32% touching the disc and another 17% involving the fovea. Two patients died of metastatic disease.
As with other forms of therapy, local recurrence after endoresection tends to occur from untreated residual tumor in the adjacent choroid and in the sclera, and such recurrent tumor can extend extraocularly if not treated promptly.37 So far, we have not seen widespread intraocular recurrences from intraoperative tumor seeding. In one patient, an untreated marginal recurrence spread through the defect into the retina and around the vitreous cavity.38 This patient, who lived far from our center, had been discharged back to her retinal surgeon, who was unable to monitor the fundus because of cataract. Such spread would not have occurred if the eye had been enucleated as soon as ophthalmoscopy was no longer possible, a policy we have always followed after all types of conservative treatment. Another patient developed subconjunctival seeding, a complication we have also seen with tumor biopsy.39 Several authors precede endoresection with radiotherapy to reduce the risk of iatrogenic tumor seeding.14,15 Our impression is that such seeding is rare, at least in patients with a small melanoma, so that many patients must be developing radiation-induced complications unnecessarily. There would seem to be scope for randomized studies.
Secondary local resection for “toxic tumor” after radiotherapy
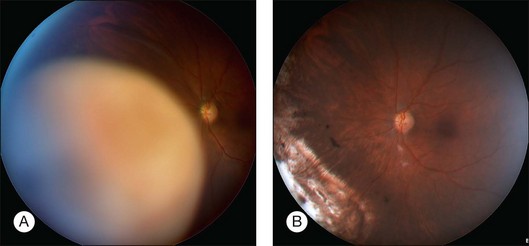
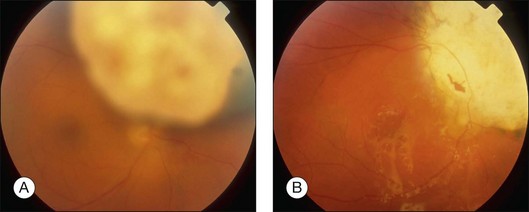
Neovascular glaucoma is a common problem after radiotherapy of uveal melanoma, especially with large tumors. Some have suggested that this complication is due to extensive irradiation of healthy ocular tissues.40 However, we believe that this complication is, at least in part, caused by the presence of a large volume of irradiated tumor, either because it becomes ischemic or because it causes extensive retinal detachment, or both (i.e., “toxic tumor syndrome”). We have successfully treated these complications in several patients by performing exoresection or endoresection of the toxic tumor. Figure 147.5 shows the left eye of a 75-year-old man, who had choroidal melanoma measuring 13.2 × 10.5 mm in diameter with a thickness of 9.3 mm in the left eye. The patient was keen to retain the eye because the right eye was amblyopic with visual acuity of 20/100. He was treated with proton beam radiotherapy because he was being anticoagulated for cardiac arrhythmia. The irradiated eye developed exudative retinal detachment with reduction of vision to 20/100 and neovascular glaucoma with an intraocular pressure of 46 mmHg. Exoresection was performed with minimal hypotensive anaesthesia. The retinal detachment resolved within a day. The iris neovascularization regressed within a month, and 24 months after the resection the intraocular pressure was normal without topical medications. Some 8 years after the surgery, the patient was well and when tested with a LogMAR chart was able to see 66 letters with the affected eye and 60 letters with the fellow eye (Fig. 147.5).
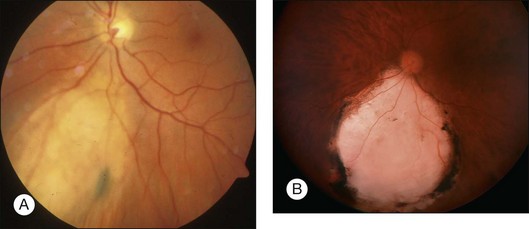
Local resection is also effective for recurrent tumor after other forms of therapy, selecting exoresection for large anterior tumors and endoresection for smaller, posterior tumors (Fig. 147.6).
1 Schubert F. Operation eines leucosarkomas der choroidea mit erhaltung des auges dauer heilung. Wien Klin Wochenschr. 1925;24:677–678.
2 Stallard HB. Partial choroidectomy. Br J Ophthalmol. 1966;50:660–662.
3 Foulds WS. The local excision of choroidal melanomata. Trans Ophthalmol Soc UK. 1973;93:343–346.
4 Foulds WS, Damato BE, Burton RL. Local resection versus enucleation in the management of choroidal melanoma. Eye. 1987;1:676–679.
5 Damato BE, Paul J, Foulds WS. Predictive factors of visual outcome after local resection of choroidal melanoma. Br J Ophthalmol. 1993;77:616–623.
6 Damato BE, Paul J, Foulds WS. Risk factors for metastatic uveal melanoma after trans-scleral local resection. Br J Ophthalmol. 1996;80:109–116.
7 Damato BE, Paul J, Foulds WS. Risk factors for residual and recurrent uveal melanoma after trans-scleral local resection. Br J Ophthalmol. 1996;80:102–108.
8 Damato B, Groenewald CP, McGalliard JN, et al. Rhegmatogenous retinal detachment after transscleral local resection of choroidal melanoma. Ophthalmology. 2002;109:2137–2143.
9 Damato BE, Groenewald C, McGalliard J, et al. Endoresection of choroidal melanoma. Br J Ophthalmol. 1998;82:213–218.
10 Peyman GA, Cohen SB. Ab interno resection of uveal melanoma. Int Ophthalmol. 1986;9:29–36.
11 Peyman GA, Juarez CP, Diamond JG, et al. Ten years experience with eye wall resection for uveal malignant melanomas. Ophthalmology. 1984;91:1720–1725.
12 Shields JA, Shields CL, Shah P, et al. Partial lamellar sclerouvectomy for ciliary body and choroidal tumors. Ophthalmology. 1991;98:971–983.
13 Bechrakis NE, Petousis VE, Willerding G, et al. Ten year results of transscleral resection of large uveal melanomas: Local tumour control and metastatic rate. Br J Ophthalmol. 2010;94:460–466.
14 Bechrakis NE, Foerster MH. Neoadjuvant proton beam radiotherapy combined with subsequent endoresection of choroidal melanomas. Int Ophthalmol Clin. 2006;46:95–107.
15 Schilling H, Bornfeld N, Talies S, et al. Endoresection of large uveal melanomas after pretreatment by single-dose stereotactic convergence irradiation with the Leksell gamma knife – first experience on 46 cases. Klin Monatsbl Augenheilkd. 2006;223:513–520.
16 Garcia-Arumi J, Zapata MA, Balaguer O, et al. Endoresection in high posterior choroidal melanomas: long-term outcome. Br J Ophthalmol. 2008;92:1040–1045.
17 Char DH, Miller T, Crawford JB. Uveal tumour resection. Br J Ophthalmol. 2001;85:1213–1219.
18 Foss AJ, Whelehan I, Hungerford JL, et al. Predictive factors for the development of rubeosis following proton beam radiotherapy for uveal melanoma. Br J Ophthalmol. 1997;81:748–754.
19 Shields CL, Naseripour M, Cater J, et al. Plaque radiotherapy for large posterior uveal melanomas (> or =8-mm thick) in 354 consecutive patients. Ophthalmology. 2002;109:1838–1849.
20 Egger E, Zografos L, Schalenbourg A, et al. Eye retention after proton beam radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys. 2003;55:867–880.
21 Puusaari I, Heikkonen J, Summanen P, et al. Iodine brachytherapy as an alternative to enucleation for large uveal melanomas. Ophthalmology. 2003;110:2223–2234.
22 Kivelä T, Puusaari I, Damato B. Transscleral resection versus iodine brachytherapy for choroidal malignant melanomas 6 millimeters or more in thickness: a matched case-control study. Ophthalmology. 2003;110:2235–2244.
23 Bechrakis NE, Bornfeld N, Zoller I, et al. Iodine 125 plaque brachytherapy versus transscleral tumor resection in the treatment of large uveal melanomas. Ophthalmology. 2002;109:1855–1861.
24 Russo A, Coupland SE, O’Keefe M, et al. Choroidal melanoma in a 7-year-old child treated by trans-scleral local resection. Graefes Arch Clin Exp Ophthalmol. 2010;248:747–749.
25 Jones AG. Profound hypotension: ethical considerations. Hosp Med. 2002;63:92–94.
26 Kim JW, Damato BE, Hiscott P. Noncontiguous tumor recurrence of posterior uveal melanoma after transscleral local resection. Arch Ophthalmol. 2002;120:1659–1664.
27 Duker JS, Augsburger JJ, Shields JA. Noncontiguous local recurrence of posterior uveal melanoma after cobalt 60 episcleral plaque therapy. Arch Ophthalmol. 1989;107:1019–1022.
28 Augsburger JJ, Lauritzen K, Gamel JW, et al. Matched group study of surgical resection versus cobalt-60 plaque radiotherapy for primary choroidal or ciliary body melanoma. Ophthalmic Surg. 1990;21:682–688.
29 Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347:1222–1225.
30 Damato B, Duke C, Coupland SE, et al. Cytogenetics of uveal melanoma: a 7-year clinical experience. Ophthalmology. 2007;114:1925–1931.
31 Damato B, Dopierala JA, Coupland SE. Genotypic profiling of 452 choroidal melanomas with multiplex ligation-dependent probe amplification. Clin Cancer Res. 2010;16:6083–6092.
32 Damato B, Eleuteri A, Taktak AF, et al. Estimating prognosis for survival after treatment of choroidal melanoma. Prog Retin Eye Res. 2011;30:285–295.
33 Gragoudas ES, Li W, Lane AM, et al. Risk factors for radiation maculopathy and papillopathy after intraocular irradiation. Ophthalmology. 1999;106:1571–1577.
34 Meyer A, Lévy C, Blondel J, et al. Optic neuropathy after proton-beam therapy for malignant choroidal melanoma. J Fr Ophtalmol. 2000;23:543–553.
35 Shields CL, Shields JA, Perez N, et al. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: outcomes and limitations. Ophthalmology. 2002;109:225–234.
36 Singh AD, Eagle RC, Jr., Shields CL, et al. Clinicopathologic reports, case reports, and small case series: enucleation following transpupillary thermotherapy of choroidal melanoma: clinicopathologic correlations. Arch Ophthalmol. 2003;121:397–400.
37 Damato B, Wong D, Green FD, et al. Intrascleral recurrence of uveal melanoma after transretinal “endoresection”. Br J Ophthalmol. 2001;85:114–115.
38 Hadden PW, Hiscott PS, Damato BE. Histopathology of eyes enucleated after endoresection of choroidal melanoma. Ophthalmology. 2004;111:154–160.
39 Raja V, Russo A, Coupland S, et al. Extraocular seeding of choroidal melanoma after a transretinal biopsy with a 25-gauge vitrector. Retin Cases Brief Rep. 2010;5:194–196.
40 Daftari IK, Char DH, Verhey LJ, et al. Anterior segment sparing to reduce charged particle radiotherapy complications in uveal melanoma. Int J Radiat Oncol Biol Phys. 1997;39:997–1010.