Chapter 2 Surgical Navigation with Intraoperative Imaging
Special Operating Room Concepts
For high-grade gliomas, in 1994 Albert reported that post-operative imaging showed tumor remnants in 77% of patients who were presumed to have undergone gross total resection.1 In 2006,2 Stummer et al. published a multicenter randomized study, which, as a byproduct, showed residual tumors in 64% of the patients undergoing conventional microsurgical tumor resection (only patients with high-grade gliomas were included, which was deemed—by imaging criteria—to be fully resectable). With the importance of the extent of resection for high-1,2 as well as low-grade gliomas,3,4 these findings emphasize the need for improvement.
In neurovascular surgery the routine use of intraoperative angiography has been advocated to avoid undetected residual disease.5,6 In spinal surgery, the significant percentage of misplaced screws could be reduced from 10%, but still occurs with approximately 5%, even with modern navigation techniques.7 These findings underscored the desire to complement advanced preoperative evaluation with intraoperative quality control. Thus various surgical groups proceeded to integrate imaging into their procedures.
The earliest attempts were made with ultrasound (US) and computed tomography (CT). The immediate impact on surgical procedures was small, due to limited resolution (US and CT) and cumbersome integration into the operating room (CT). Another avenue opened with the introduction of image-guided neuronavigation (IGN) systems.8,9 These systems allowed the transfer of increasingly refined presurgical image information into the operating theater to guide surgical procedures. However, intraoperative changes (“brain shift”) critically limited their application accuracy.10,11 The concept of intraoperative imaging resurfaced. With magnetic resonance imaging (MRI) becoming the method of choice for the imaging of the central nervous system, pioneering efforts to introduce this modality into surgery provided proof of the concept.12–14 These initial experiences with intraoperative MRI (iMRI)15–17 ignited diversification into a variety of approaches.
Computer-Assisted, Image-Guided Neuronavigation
The major link between imaging and integration of this information into surgery is provided by navigation systems. Diagnostic computer-based image-analysis and three-dimensional (3D) modeling facilitated the spatial definition of complex pathologic processes. The desire to use this information directly in the surgical field led to the introduction of IGN systems in the mid-1980s8,9 and their commercial availability in the early 1990s. These systems provided the surgeon with a tool that allowed the transfer of presurgical image information in an intuitive and interactive fashion into the surgical field (see Chapter 3 for more detail on neuronavigation).
Meanwhile, the technology has proceeded from being a novelty to an established asset for neurosurgical procedures. Questions of prior consideration, that is, application accuracy and integration of instruments, were overcome. However, the major shortcoming was the dependence on preoperative image data. Since intraoperative changes (e.g., CSF drainage, tumor resection, sagging of the cortex, swelling of underlying tissue, summarized as “brain shift”), accumulate throughout surgery, preoperative data become invalidated.10,11,18 This has particular influence on glioma surgery. While enabling precise approach planning and localization, resection control is generally beyond the capacity of these systems, since they cannot account for intraoperative changes. Intraoperative imaging resolved this issue directly. It enables continued use of these systems with newly acquired accurate data.
A different avenue investigates mathematical models to compensate for brain shift. Various algorithms can characterize and calculate deformation matrixes.10,11,19 Various brain shift patterns were identified. A multimodal approach appears potentially useful, which uses intraoperative “sparse” US data20–22 to calculate a deformation matrix, which is then used to elastically deform preoperative MRI images. Albeit all these efforts advances were meager and the only option to provide precise updated navigation remains the integration of intraoperative images.
Intraoperative Imaging
Intraoperative Fluoroscopy
Operating theaters for stereotactic neurosurgery had built-in biplane x-ray to eliminate parallax artifacts in imaging of electrode placement. With the limited scope of this application, these ORs remained rare and have largely been replaced by standard fluoroscopy, or more recently intraoperative MRI.23,24
In instrumented spinal surgery, fluoroscopy is used as an online imaging modality for planning and verifying screw positioning. Combinations with navigation systems have been propagated. Intraoperative angiography has been employed by major vascular centers for quality insurance in aneurysm and AVM surgery.5,6
Intraoperative Ultrasound
Intraoperative US (IoUS) was one of the first to be employed as an intraoperative imaging modality in neurosurgery.25 With subsequent new generations, image quality improved and miniaturization of the hand-pieces enhanced applicability. Advantages are the dynamic, surgeon-driven, on-line character of the information.26 Particularly in vascular surgery, the flow-related analysis of duplex sonography provides additional flexibility. Further major developments were the introduction of spatially accurate 3D ultrasound,27 of contrast agents28 and the integration of US into navigation systems.26,29–31 In particular, the last aspect provided the means for easier interpretation of the images, which generally demands experience.
For the last 20 years, IoUS has been regarded as the most promising system for online information acquisition in neurosurgery. Still, these systems remain limited in their distribution. Potential reasons may be the unfamiliarity with the technique of ultrasound and its limitations in tissue differentiation,32 differing from the most widely distributed primary diagnostic modality of MRI.33
Major indications are circumscribed lesions, such as metastasis, cavernomas, vascular pathologies, and for spinal intradural lesions. With its integration into conventional navigation systems and in combination with iMRI34 the unfamiliarity with this modality might potentially be overcome.
Intraoperative Computed Tomography
Shalit and Lunsford first reported the integration of a stationary CT into OR.35,36 The next generation of CTs was mobile, permitting shared application in the OR and the ICU. However, image quality and radiation exposure limited the application and further implementation of this modality. Further advances in CT- and OR-table technology and integration with navigation systems have led to a reappraisal.
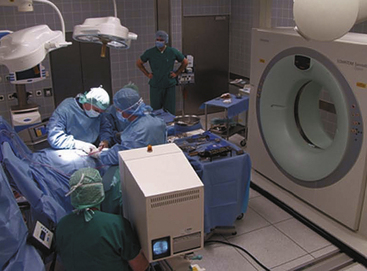
Modern CT-OR (Fig. 2-1) solutions use a rail system to move the CT between a parking position and the patient for scanning,37 which provides full access to the patient. In spine surgery, intraoperatively acquired images can be used to update navigation systems to provide additional image guidance for screw placement, as well as verification of correct positioning. For neurovascular surgery, intraoperative CT-angiography has the potential to provide information on obtained occlusion of vascular pathologies, but also with perfusion CT on potential vascular compromise.
Intraoperative Magnetic Resonance Imaging
Postoperative MRI remains the gold-standard for defining the extent of resection in neurooncology1,2 and pituitary lesions.38
The desire to employ the potential of MRI to monitor open neurosurgical procedures, as means to quality insurance, resection control, and complication detection led to the combination of MRI and surgery.13 Presently intraoperative MRI is used primarily for gliomas and pituitary lesions,38–40 but also for vascular41 and epilepsy surgery.42
The “twin operating theater”14,16 combined surgery and imaging (low-field, open 0.2 T MR system with a horizontal opening) by using two adjacent rooms. The patient was transferred between surgical and imaging site. Thus conventional OR equipment could be used without MR-safety or compatibility issues. To minimize the time for the transfer, this approach was modified by operating in the vicinity of the MRI, the “fringe field.”43,44
The open magnet design (“double doughnut”)12,13,17 aimed at a full integration of surgery and MRI. The vertical opening provided the surgeon with access to the patient. Surgical and imaging site were merged, a transfer was unnecessary. For practical reasons, surgery was discontinued during scanning. However, this design held the potential to provide real-time imaging, such as in biopsies, or through “continuous imaging” protocols.45 Furthermore, a navigation system was an integral part of the MRI. With a localizer, the surgeon controlled the scanning plane of the MRI interactively.46,47 Specially developed software for intraoperative navigation extended the functionality.48,49 This solution is closest to the symbiosis of surgery and imaging. However, by operating in a magnetic field, constraints in regards to technical equipment, in particular the microscope, the 56-cm gap for the surgeon, and the need for nonferromagnetic instruments, microneurosurgical standards were difficult to uphold.
These pioneering clinical experiences proved that the vision50 to bring MRI into the surgical surrounding could be realized. Biopsies as well as interstitial therapies could be blended with MRI into a novel procedure. However, it became evident, that the synthesis of open surgery and MRI into a comprehensive new method proved too complex. Either imaging potential, in comparison to preoperative high-field diagnostic scans, patient access or both, were restricted.
With emphasis on accessibility, a minimized, compact open MRI (0.12 T, 0.15 T) was introduced, which fit beneath the surgical table.51,52 To integrate high-field (1.5 T and higher) imaging, while providing ample patient access and only minimal influence on microneurosurgical instruments and techniques, surgical and imaging sites were separated. This can be achieved within an integrated OR-MR design (“dedicated”),15,40,53 or by arranging MR and OR into separate adjacent modules/rooms41,54–56 (“shared resources”).
A comprehensive classification, which encompasses present arrangements and accommodates potential future developments and expansions, cannot be based on variable characteristics such as field-strength and MR-design. Since the original concept was to merge surgery and imaging, it is reasonable to use work flow to distinguish among different installations. Specific issues for the integration of MRI into the surgical surrounding such as MR safety and compatibility of equipment, field strength, shielding, MR design,47,57,58 and imaging characteristics will be outlined before discussing various MR-OR integrations.
MR Safety, Compatibility, and Shielding
The introduction of a magnet into a surgical surrounding raises safety issues pertaining to interaction of the magnetic field and OR equipment.47,58–61 The magnet can exert a pull on ferromagnetic instruments. Generally the strength of the pull is related to field strength (and MR shielding) and distance to the MRI. The so-called 5-gauss line demarcates the inner area, in which the pull increases and the outer zone, in which ferromagnetic instruments can be safely used without being drawn into the MRI. In most MR-ORs, this demarcation is indicated on the floor. The immediate area around the 5-gauss line, which is within the magnetic field but still has no significant pull, is called the “fringe” field.
Shielding is necessary to prevent the interaction of the magnet with radio-frequency (RF) technology. Normally the entire room is shielded to prevent the magnet’s influence on electrical devices and vice versa. Alternatively, a specific shielding can be laced around the patient for scanning. While all nonessential electrical equipment can be turned off during scanning, or is primarily based outside the shielded room (e.g., the computer for image guided navigation), special anesthesia equipment is used to prevent RF noise (artifacts) in the images.
MR Design (“Open-Bore” and “Closed-Bore” Systems) and Field Strength
The static magnetic field of the MRI is generated within its bore. In open-bore (i.e., open-magnet) systems, the magnet is divided into two poles. The gap can be horizontal or vertical (“double doughnut”),59 resulting in different access to the patient.
Generally the open-magnet design has lower field strength than the “short-bore” closed systems. Higher field strength generally promotes acquisition speed (temporal resolution) as well as quality of the subsequently acquired images (spatial resolution). A wider range of image sequences is available (e.g., spectroscopy, DTI, fMRI, dynamic scanning).15,62,63 Furthermore, the homogeneity of the magnetic field increases, reducing geometric distortions. This issue is of major importance in low- and mid-field scanners. Phantom studies performed on the compact 0.12 T system provided acceptable application accuracy.52 However, studies in a stronger magnetic field (mid-field 0.5 T, open MR system) have shown that significant geometric distortions are present,64 which are machine- and patient-induced. These findings have to be considered when using non–high-field MR units (below 1 T) for resection control and updated neuronavigation.
Imaging
Which imaging to choose depends on the lesion’s imaging characteristics in diagnostic studies. Enhanced and nonenhanced T1WI, T2, and occasionally FLAIR answer most questions.39,40,53,54,62,65 For low-grade lesions, T2 and FLAIR images are the most appropriate.40,53,54 For enhancement, pre- and post-contrast T1 images are acquired.
Further sequences may potentially yield additional information,53 such as location of functional centers or fiber tracts. Both features can be extracted from intraoperative MRI, especially the latter.66
The intraoperative MRI is essentially a surgical tool. It is implemented to support surgical decision making. Thus the surgeon has to define his or her intention and the subsequent question, which primarily relates to the achieved extent of resection (residual tumor and its localization) and complication avoidance (distance to critical structures). It is essential that the surgeon acquires a good working knowledge of MRI to compile the individual imaging protocol and analyze the images according to surgical objectives.40,41,53,62
Practical challenges in interpreting intraoperative images largely pertain to nonspecific contrast enhancement (“spread enhancement”). The surgical result is described by “removed percent of contrast-enhancing lesion.” Since contrast enhancement merely reflects the local breakdown of the blood–brain barrier, it is unsurprising that contrast spreads into surrounding regions over time. While almost inconsequential in diagnostic imaging, acknowledging this phenomenon is of major importance for intraoperative MRI (iMRI) to avoid over-resection. Thus scans for the initial neuronavigation-assisted resection should be acquired prior to surgery. When imaging is for resection control, pre- and post-contrast T1 images and subtraction are compared to identify residual contrast enhancement. New sequences capturing the dynamic nature of neovascularized areas, in particular dynamic susceptibility contrast-weighted perfusion MRI (DSC-MRI), provide more accurate intraoperative information than conventional contrast-enhanced T1WI.67 Future development of specific contrast media may lead to a resolution of this problem.68,69
Integration of Intraoperative Navigation and MRI
The shortcomings of image-guided navigation in detecting intraoperative changes were a major motivation to implement intraoperative imaging. Since surgery and imaging take place in different coordinate systems, the transfer of the images between these venues represents the crucial integrating step. IGN provides this essential link.40,70,71
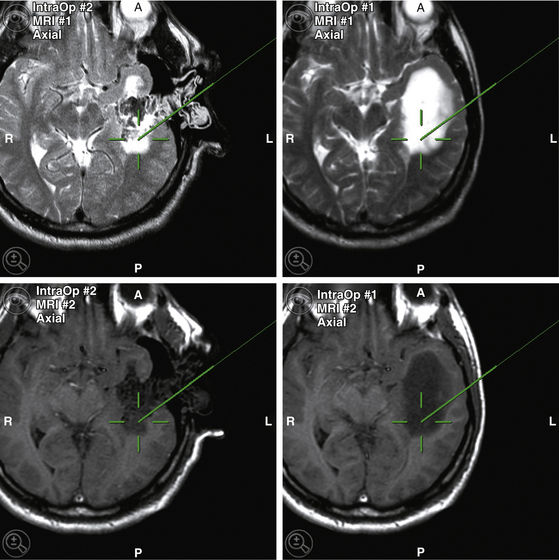
In most MRI-installations, navigation systems are ceiling mounted. Initial navigation is performed with preoperative images until the surgeon deems an update necessary to regain accurate navigation. The intraoperative images are sent directly from the scanner console to the navigation system. The images are fused (automated image fusion algorithm) to the already registered preoperative images and shown on the display (Fig. 2-2). With the DRF reattached in its original position, the images can be used for updated navigation without additional re-referencing. Thus intraoperative updates for neuronavigation can be acquired at the surgeon’s discretion, and used for updated navigation.
OR-MR Integrations
The horizontal systems were mostly adjacent to a conventional OR.14,16 The patient was moved from the surgical site to the imaging site. An improved workflow left the patient within the fringe field to shorten the transfer.43,44
The vertical units (i.e., “double doughnut”) had the advantage, that patient transport was not necessary because imaging and surgical sites were the same. The vertical orientation of the gap between the poles gave, however confined, acceptable access to the positioned patient. This facilitated the workflow but posed high demands on the equipment and surgical workflow.17,47,50
Shared Resources and Multimodal Imaging OR Concepts
The separated room concept for surgical and imaging sites was developed to allow the unimpeded usage of surgical tools as well as perfect imaging. An additional economic aspect was that while surgery was progressing, the idle MRI could be used for routine imaging—hence, the notion of shared resources. However, this demanded special arrangements for connecting surgical and imaging sites. Potentially, the patient can be brought to the MRI or the MRI to the patient.
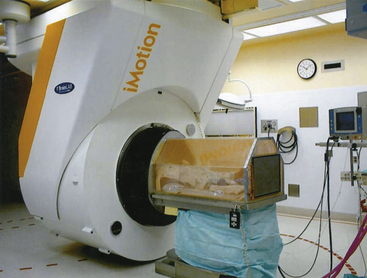
The first mobile MRI (Fig. 2-3) was developed and installed in Calgary.41 The 1.5-T unit is mounted on a ceiling rail system, which permits transporting the MRI into the surgical area (overhead crane technology). The specially designed operating table is MR-compatible, as patient positioning can be adjusted hydraulically. Furthermore, the RF coils are integrated into the surgical table. The upper detachable portion can be repositioned for imaging. The MRI usually resides in a separate room. On its way in and during scanning, ferromagnetic instruments have to be removed from its path and beyond the 5-gauss line. If not needed during the procedure, the magnet can be potentially used as a shared resource for conventional scanning, or serve adjacent ORs connected by a common rail system.
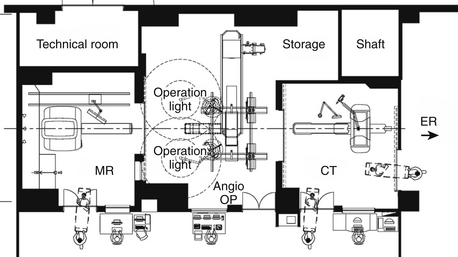
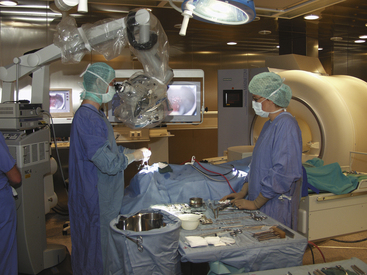
Stationary MRIs in separated rooms are presently 3-T MRI units, where the higher field necessitates more elaborate shielding (Fig. 2-4). The 5-gauss line extends farther away from the MRI, raising demands on MR-safe and compatible equipment and instruments. This and the fact that 3-T systems are not yet widely used led to implementation as separated rooms, permitting shared imaging resources between surgery and radiology.54,56
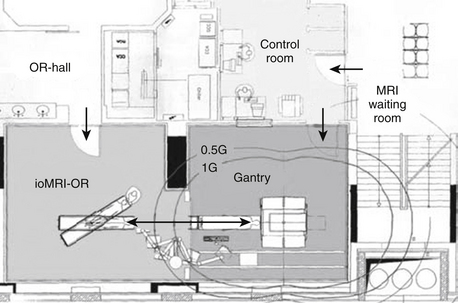
FIGURE 2-4 Example of shared-resources layout.
(From Pamir MN, Ozduman K, Dincer A, Yildiz E, Peker S, Ozek MM. First intraoperative, shared-resource, ultrahigh-field 3-Tesla magnetic resonance imaging system and its application in low-grade glioma resection. J Neurosurg. 2010;112:57-69, Fig. 1.)
The surgical site is a conventional operating theater. The patient is positioned on a surgical OR table with a floating top, which can be connected to the MR system. Either a rail system56 or a wheeled transfer table54 is used. The head-holder can be either separated from flexible surface coils,56 or integrated into the rigid imaging coils.54 In the latter case, a removable sterile top portion is disconnected for surgery and replaced for scanning.
The rooms have additional entrances to provide access to the MR while surgery progresses in the adjacent room. Thus during the surgical time, routine diagnostics can be performed. Costs and function can be shared between neurosurgery and radiology. While the economic aspect is appealing, the concept of obtaining image-information on demand for surgical decision making is impeded. If the MR is occupied, the surgical patient has to wait. Presently the transfer distance, as well as the preparations to provide safety, represents an additional delay.56 It becomes cumbersome, and thus less likely, that repeated intraoperative scans are obtained.
In the MRXO55 concept (Fig. 2-5), the central OR-angiography room is connected to a 1.5-T MRI and a CT suite. Both suites have separate entrances to admit patients from radiology (MRI) and the emergency room (CT), adherent to the shared-resources concept. This setup is primarily designed for neurosurgical applications.
In the planned AMIGO (advanced multimodality image guided operative)72 design, the fully equipped surgical room (fluoroscopy, US, navigation system) will be flanked by a 3-T MRI unit and a PET-CT. With this design, the applicability of the suite is not only as a shared resource in regards to simultaneous imaging of other patients during surgery, but also expandable to an interdisciplinary suite serving different specialties.
Dedicated OR-MR Environment
Dedicated systems realize the close integration of MRI and surgery within one OR-MR environment.
Dedicated Low-Field System
The 0.15-T MRI (previous generation 0.12 T) is an open-bore system with two poles.51,52,73 The MRI is positioned beneath the patient’s head (Fig. 2-6). On demand the magnet is raised to place the surgical field in the imager. Images are acquired and transmitted to the connected navigation unit for updated navigation. The OR has to be shielded to avoid RF interference, and all other nonessential equipment has to be turned off. Alternatively, the patient and the scanner can be shielded separately.74 This compact MRI provides the closest approximation to the original concept of merging imaging and surgery in space. The application can be integrated into a conventional OR, provided shielding is implemented and used on demand by raising the poles to imaging level. Despite the application comfort, the low field holds challenges in regards to homogeneity (spatial resolution and geometric distortions) and field of view (120–160 mm vs. 220 mm in high-field systems). These systems are used for intraoperative imaging in glioma51,52 and pituitary surgery.75,76
Dedicated High-Field System
These installations combine a fully equipped neurosurgical OR with a high-field scanner, primarily 1.5-T MR units, into a comprehensive unit.15,40,53 Two main setups provide this dedicated environment.15,40,53 The 5-gauss border represents a demarcation that permits the spatial division of the OR-MR suite.40,53,60 Surgical and imaging site are connected by a surgical table, which attaches directly to the scanner. The surgical area is reached by disconnecting the table and rotating it either by 30 degrees15,40 (Fig. 2-7) or 160 degrees53 (Fig. 2-8) away from the MR axis, to place the operating field outside the 5-gauss line.
The primary fully equipped surgical site for microneurosurgery is outside the 5 gauss line, where ferromagnetic tools and equipment (e.g., microscope, ultrasonic aspirator, bipolar coagulation, and cortical stimulation) can be used unimpeded. The rigid head fixation has to be fully MR compatible. The material of the pins has no influence on the overall imaging (local artifacts with metal pins). The head fixation can be integrated into the rigid RF coil with restricted degrees of freedom.53 More flexibility for positioning is achieved by a modified carbon-fiber, MR-compatible Mayfield clamp attached to the table top used with surface coils.40 One coil is positioned below the patient’s head, within the Mayfield clamp, while the top coil is removed during surgery and replaced for scanning.
For surgical navigation, the dynamic reference frame (DRF) is attached to the head-holder. The navigation system is registered, the craniotomy planning finalized. In integrated MR-OR solutions the navigation system is ceiling mounted, with the computer placed outside the shielded room. After craniotomy, the operation is performed, using state-of-the art microneurosurgical techniques. For lesions in eloquent areas the authors’ group utilizes the technique of awake craniotomy with cortical stimulation.77
In both arrangements,40,53 the interval from stopping the surgery to initial scanning commonly takes about 3 to 5 min.
The surgeon determines the imaging protocols based on presurgical imaging characteristics. The images are transferred to the navigation system as soon as they are acquired for updated accurate navigation.40,71
The surgical field is redraped on top of the previous draping. If residual tumor is identified, updated neuronavigation allows the precise localization for resection. If no further resection is necessary or deemed feasible, surgery is concluded.
Biopsies and burr-hole procedures in a dedicated, high-field MRI can be performed in the primary surgical site outside the 5-gauss line with standard equipment (conventional stereotactic frames; computer-guided, navigated free-hand biopsies; navigated endoscopy).40 More sophisticated in-bore procedures use the capacity of real-time imaging. Burr-hole and dural opening are performed outside the 5-gauss line with standard equipment. After a MR-compatible burr hole mounted device is attached, the patient is transferred into the MR for scanning. The mounted guide is fixed to preserve the planed trajectory. During the probe’s advance in-plane imaging (1–3 images/second) provides real-time control and final image confirmation of the target point.78 With short-bore MRs, the needle can be advanced by the surgeon reaching into the bore. However, remote control or robotic devices provide more comfortable reach and can be potentially employed within MRIs with less access.78,79 Current studies discuss in-bore procedures for deep brain stimulation.24
Within the integrated system originally described by Hall et al.15 a secondary surgical site can be used for specific tasks.40,65 When the MRI table is extended beyond the back of the MRI, the patient’s head can be accessed freely for surgery. This area is within the 5-gauss line, thus necessitating the use of MR-safe equipment. While ultrasonic aspirator and bipolar coagulation can be used in this site, microscopes which provide familiar illumination and magnification qualities are not available. The transfer to the MRI is much shorter, repeated imaging becomes easier. This secondary surgical site returns to the idea of a close interlacing of imaging and surgery. However, as long as microneurosurgical techniques are hampered, the utility of this area is limited to smaller interventions.
Interestingly despite this restrain, this secondary surgical site allowed a major development: the inception of an integrated, dedicated 3-T system within an operating theater.80,81 Contrary to shared-resources solutions, this setup is the only one to attempt the combination of intraoperative 3-T system and surgery. While there are still significant drawbacks, this installation provides the proof of concept, that dedicated high-field OR-MR rooms for biopsies and open craniotomies can be realized.80–82
Summary
In iMRI suites, intraoperative imaging has taken its most elaborate form. Despite its cost, special demands, and labor intensiveness, this field keeps expanding, due to the unparalleled imaging capabilities for the analysis of structural pathology as well as physiologic investigations of the central nervous system (e.g., neurooncology, epilepsy surgery). Various groups reported more complete resections for high-grade gliomas and pituitary lesions employing intraoperative low-,52,83 mid-,84,85 and high-field systems.39,86 Increased resection percentages were also shown for low-grade lesions.53,87
The attempt to decrease MRI size to facilitate integration, resulted in a compact low-field system, albeit with limited imaging potential.51 Higher-field MRIs providing diagnostic imaging capability are integrated primarily through their suite design, which separates surgical and imaging site within the same (dedicated systems) or adjacent rooms (twin operating theater, shared resource). In dedicated systems, the transfer between surgical and imaging site can be achieved swiftly.40,53 In shared resource concepts, transfer is longer. Furthermore potential conflicts in using the imaging for surgery and routine diagnostics may lead to prolonged waiting periods before intraoperative imaging can be commenced.54,56
Long envisioned intraoperative MRI has been successfully combined with standard microneurosurgical and navigation techniques into comprehensive units for neurosurgical procedures.50,88 High-field MRI and its intraoperative application represent a major interdisciplinary challenge and opportunity. Further refinements may lead back to the original concept of merging therapy and MRI, with robotic devices for surgery, focused US for noninvasive ablative procedures88 or open high-field magnet designs.
The essential link between imaging and surgery is the computer-assisted IGN system. It represents the platform on which the pre- and intra-operative multimodal imaging information coalesces to enable surgical decision making.71,88–90
In current systems, imaging interrupts surgery for various periods of time. While microneurosurgical techniques remain unrestricted, the surgical workflow is disrupted and the procedure prolonged. Thus intraoperative imaging represents a compromise balancing the additional value of the imaging information versus timely conclusion of the surgery.
Especially for MRI, the overabundance of high-quality image information becomes a challenge in its own right. Fiber tracking has been employed in sophisticated ways, delineating the major fiber connections.91,92 Spectroscopy has been used to guide stereotactic biopsies,93 and with further refinement may yield information on resection borders in open surgery.54
Conclusion
Intraoperative imaging and navigation have developed from a vision50 to a neurosurgical reality. The development of various OR designs to accommodate intraoperative imaging and surgery has accelerated. Solutions apparently prohibitive in scope and cost 10 years ago have been implemented and surpassed. The higher the expectation of image information, the more complex the resulting design. The most intricate, but at present also the most flexible and informative modality for cranial neurosurgery, is intraoperative MRI. The multitude of designs, implementations, and field strengths make this a most multifaceted area of expertise. Incorporation of magnets with increasing field strengths and multimodal imaging concepts (MRXO) including metabolic information (AMIGO) represent the next challenges.
Black P.M., Moriarty T., Alexander E.3rd, et al. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery. 1997;41:831-842.
Claus E.B., Horlacher A., Hsu L., et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103:1227-1233.
Hadani M., Spiegelman R., Feldman Z., et al. Novel, compact, intraoperative magnetic resonance imaging-guided system for conventional neurosurgical operating rooms. Neurosurgery. 2001;48:799-807.
Hall W.A., Galicich W., Bergman T., Truwit C.L. 3-Tesla intraoperative MR imaging for neurosurgery. J Neurooncol. 2006;77:297-303.
Hall W.A., Martin A.J., Liu H., et al. High-field strength interventional magnetic resonance imaging for pediatric neurosurgery. Pediatr Neurosurg. 1998;29:253-259.
Jankovski A., Francotte F., Vaz G., et al. Intraoperative magnetic resonance imaging at 3-T using a dual independent operating room-magnetic resonance imaging suite: development, feasibility, safety, and preliminary experience. Neurosurgery. 2008;63:412-424.
Jolesz F.A., Nabavi A., Kikinis R. Integration of interventional MRI with computer-assisted surgery. J Magn Reson Imaging. 2001;13:69-77.
Matsumae M., Koizumi J., Fukuyama H., et al. World’s first magnetic resonance imaging/x-ray/operating room suite: a significant milestone in the improvement of neurosurgical diagnosis and treatment. J Neurosurg. 2007;107:266-273.
Nabavi A., Black P.M., Gering D.T., et al. Serial intraoperative magnetic resonance imaging of brain shift. Neurosurgery. 2001;48:787-797.
Nabavi A., Dorner L., Stark A.M., Mehdorn H.M. Intraoperative MRI with 1.5 Tesla in neurosurgery. Neurosurg Clin North Am. 2009;20:163-171.
Nabavi A., Goebel S., Doerner L., et al. Awake craniotomy and intraoperative magnetic resonance imaging: patient selection, preparation, and technique. Top Magn Reson Imaging. 2009;19:191-196.
Nimsky C., Fujita A., Ganslandt O., et al. Volumetric assessment of glioma removal by intraoperative high-field magnetic resonance imaging. Neurosurgery. 2004;55:358-370.
Nimsky C., Ganslandt O., Cerny S., et al. Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery. 2000;47:1070-1079.
Nimsky C., Ganslandt O., Kober H., et al. Intraoperative magnetic resonance imaging combined with neuronavigation: a new concept. Neurosurgery. 2001;48:1082-1089.
Nimsky C., Ganslandt O., Von Keller B., et al. Intraoperative high-field-strength MR imaging: implementation and experience in 200 patients. Radiology. 2004;233:67-78.
Pamir M.N., Ozduman K., Dincer A., et al. First intraoperative, shared-resource, ultrahigh-field 3-Tesla magnetic resonance imaging system and its application in low-grade glioma resection. J Neurosurg. 2010;112:47-69.
Schulder M. Intracranial surgery with a compact, low-field-strength magnetic resonance imager. Top Magn Reson Imaging. 2009;19:179-189.
Steinmeier R., Fahlbusch R., Ganslandt O., et al. Intraoperative magnetic resonance imaging with the magnetom open scanner: concepts, neurosurgical indications, and procedures: A preliminary report. Neurosurgery. 1998;43:739-747.
Sutherland G.R., Kaibara T., Louw D., et al. A mobile high-field magnetic resonance system for neurosurgery. J Neurosurg. 1999;91:804-813.
Sutherland G.R., Latour I., Greer A.D. Integrating an image-guided robot with intraoperative MRI: a review of the design and construction of neuroarm. IEEE Eng Med Biol Mag. 2008;27:59-65.
Tronnier V.M., Wirtz C.R., Knauth M., et al. Intraoperative diagnostic and interventional magnetic resonance imaging in neurosurgery. Neurosurgery. 1997;40:891-900.
Uhl E., Zausinger S., Morhard D., et al. Intraoperative computed tomography with integrated navigation system in a multidisciplinary operating suite. Neurosurgery. 2009;64:231-239.
1. Albert F.K., Forsting M., Sartor K., et al. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery. 1994;34:45-60.
2. Stummer W., Pichlmeier U., Meinel T., et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392-401.
3. Claus E.B., Black P.M. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973-2001. Cancer. 2006;106:1358-1363.
4. Smith J.S., Chang E.F., Lamborn K.R., et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338-1345.
5. Lopez K.A., Waziri A.E., Granville R., et al. Clinical usefulness and safety of routine intraoperative angiography for patients and personnel. Neurosurgery. 2007;61:724-729.
6. Klopfenstein J.D., Spetzler R.F., Kim L.J., et al. Comparison of routine and selective use of intraoperative angiography during aneurysm surgery: a prospective assessment. J Neurosurg. 2004;100:230-235.
7. Kosmopoulos V., Schizas C. Pedicle screw placement accuracy: a meta-analysis. Spine (Phila Pa 1976). 2007;32:E111-120.
8. Watanabe E., Watanabe T., Manaka S., et al. Three-dimensional digitizer (neuronavigator): new equipment for computed tomography-guided stereotaxic surgery. Surg Neurol. 1987;27:543-547.
9. Roberts D.W., Strohbehn J.W., Hatch J.F., et al. A frameless stereotaxic integration of computerized tomographic imaging and the operating microscope. J Neurosurg. 1986;65:545-549.
10. Nimsky C., Ganslandt O., Cerny S., et al. Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery. 2000;47:1070-1079.
11. Nabavi A., Black P.M., Gering D.T., et al. Serial intraoperative magnetic resonance imaging of brain shift. Neurosurgery. 2001;48:787-797.
12. Black P.M., Moriarty T., Alexander E.3rd, et al. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery. 1997;41:831-842.
13. Jolesz F.A. 1996 RSNA Eugene P. Pendergrass New Horizons Lecture. Image-guided procedures and the operating room of the future. Radiology. 1997;204:601-612.
14. Tronnier V.M., Wirtz C.R., Knauth M., et al. Intraoperative diagnostic and interventional magnetic resonance imaging in neurosurgery. Neurosurgery. 1997;40:891-900.
15. Hall W.A., Martin A.J., Liu H., et al. High-field strength interventional magnetic resonance imaging for pediatric neurosurgery. Pediatr Neurosurg. 1998;29:253-259.
16. Steinmeier R., Fahlbusch R., Ganslandt O., et al. Intraoperative magnetic resonance imaging with the magnetom open scanner: concepts, neurosurgical indications, and procedures: A preliminary report. Neurosurgery. 1998;43:739-747.
17. Black P.M., Alexander E.3rd, Martin C., et al. Craniotomy for tumor treatment in an intraoperative magnetic resonance imaging unit. Neurosurgery. 1999;45:423-431.
18. Hill D.L., Maurer C.R.Jr., Maciunas R.J., et al. Measurement of intraoperative brain surface deformation under a craniotomy. Neurosurgery. 1998;43:514-526.
19. Ferrant M., Nabavi A., Macq B. Serial registration of intraoperative MR images of the brain. Med Image Anal. 2002;6:337-359.
20. Miga M.I., Roberts D.W., Hartov A., et al. Updated neuroimaging using intraoperative brain modeling and sparse data. Stereotact Funct Neurosurg. 1999;72:103-106.
21. Sun H., Lunn K.E., Farid H., et al. Stereopsis-guided brain shift compensation. IEEE Trans Med Imaging. 2005;24:1039-1052.
22. Miga M.I., Paulsen K.D., Hoopes P.J., et al. In vivo quantification of a homogeneous brain deformation model for updating preoperative images during surgery. IEEE Trans Biomed Eng. 2000;47:266-273.
23. Starr P.A., Martin A.J., Ostrem J.L., et al. Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy. J Neurosurg. 2010;112(3):479-490.
24. Starr P.A., Martin A.J., Larson P.S. Implantation of deep brain stimulator electrodes using interventional MRI. Neurosurg Clin North Am. 2009;20:193-203.
25. Dohrmann G.J., Rubin J.M. History of intraoperative ultrasound in neurosurgery. Neurosurg Clin North Am. 2001;12:155-166. ix
26. Koivukangas J., Louhisalmi Y., Alakuijala J., Oikarinen J. Ultrasound-controlled neuronavigator-guided brain surgery. J Neurosurg. 1993;79:36-42.
27. Unsgaard G., Rygh O.M., Selbekk T., et al. Intra-operative 3D ultrasound in neurosurgery. Acta Neurochir (Wien). 2006;148:235-253. discussion 253
28. Ellegala D.B., Leong-Poi H., Carpenter J.E., et al. Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta3. Circulation. 2003;108:336-341.
29. Ji S., Wu Z., Hartov A., et al. Mutual-information-based image to patient re-registration using intraoperative ultrasound in image-guided neurosurgery. Med Phys. 2008;35:4612-4624.
30. Sergeeva O., Uhlemann F., Schackert G., et al. Integration of intraoperative 3D-ultrasound in a commercial navigation system. Zentralbl Neurochir. 2006;67:197-203.
31. Unsgaard G., Gronningsaeter A., Ommedal S., Nagelhus Hernes T.A. Brain operations guided by real-time two-dimensional ultrasound: new possibilities as a result of improved image quality. Neurosurgery. 2002;51:402-411.
32. Tronnier V.M., Bonsanto M.M., Staubert A., et al. Comparison of intraoperative MR imaging and 3D-navigated ultrasonography in the detection and resection control of lesions. Neurosurg Focus. 2001;10:E3.
33. Hartov A., Roberts D.W., Paulsen K.D. A comparative analysis of coregistered ultrasound and magnetic resonance imaging in neurosurgery. Neurosurgery. 2008;62:91-99.
34. Katisko J.P., Koivukangas J.P. Optically neuronavigated ultrasonography in an intraoperative magnetic resonance imaging environment. Neurosurgery. 2007;60:373-380.
35. Shalit M.N., Israeli Y., Matz S., Cohen M.L. Experience with intraoperative CT scanning in brain tumors. Surg Neurol. 1982;17:376-382.
36. Lunsford L.D. A dedicated CT system for the stereotactic operating room. Appl Neurophysiol. 1982;45:374-378.
37. Uhl E., Zausinger S., Morhard D., et al. Intraoperative computed tomography with integrated navigation system in a multidisciplinary operating suite. Neurosurgery. 2009;64:231-239.
38. Fahlbusch R., Ganslandt O., Buchfelder M., et al. Intraoperative magnetic resonance imaging during transsphenoidal surgery. J Neurosurg. 2001;95:381-390.
39. Nimsky C., Fujita A., Ganslandt O., et al. Volumetric assessment of glioma removal by intraoperative high-field magnetic resonance imaging. Neurosurgery. 2004;55:358-370.
40. Nabavi A., Dorner L., Stark A.M., Mehdorn H.M. Intraoperative MRI with 1.5 Tesla in neurosurgery. Neurosurg Clin North Am. 2009;20:163-171.
41. Sutherland G.R., Kaibara T., Louw D., et al. A mobile high-field magnetic resonance system for neurosurgery. J Neurosurg. 1999;91:804-813.
42. Buchfelder M., Fahlbusch R., Ganslandt O., et al. Use of intraoperative magnetic resonance imaging in tailored temporal lobe surgeries for epilepsy. Epilepsia. 2002;43:864-873.
43. Rubino G.J., Farahani K., McGill D., et al. Magnetic resonance imaging-guided neurosurgery in the magnetic fringe fields: the next step in neuronavigation. Neurosurgery. 2000;46:643-653.
44. Lewin J.S., Nour S.G., Meyers M.L., et al. Intraoperative MRI with a rotating, tiltable surgical table: a time use study and clinical results in 122 patients. AJR Am J Roentgenol. 2007;189:1096-1103.
45. Kacher D.F., Maier S.E., Mamata H., et al. Motion robust imaging for continuous intraoperative MRI. J Magn Reson Imaging. 2001;13:158-161.
46. Moriarty T.M., Quinones-Hinojosa A., Larson P.S., et al. Frameless stereotactic neurosurgery using intraoperative magnetic resonance imaging: Stereotactic brain biopsy. Neurosurgery. 2000;47:1138-1145. discussion 1145-1136
47. Nabavi A., Kacher D.F., Gering D.T., et al. Neurosurgical procedures in a 0.5 Tesla, open-configuration intraoperative MRI: planning, visualization and navigation. Automedica. 2001;20:163-197.
48. Gering D.T., Nabavi A., Kikinis R., et al. An integrated visualization system for surgical planning and guidance using image fusion and an open MR. J Magn Reson Imaging. 2001;13:967-975.
49. Nabavi A., Gering D.T., Kacher D.F., et al. Surgical navigation in the open MRI. Acta Neurochir Suppl. 2003;85:121-125.
50. Jolesz F.A., Kikinis R., Shtern F. The Vision of Image-Guided Surgery: the High-Tech Operating Room. In: Taylor R.H., Lavalle S., Burdea G.C., Moesges R. Computer-Integrated Surgery. Cambridge: MIT Press, 1996.
51. Hadani M., Spiegelman R., Feldman Z., et al. Novel, compact, intraoperative magnetic resonance imaging-guided system for conventional neurosurgical operating rooms. Neurosurgery. 2001;48:799-807.
52. Schulder M. Intracranial surgery with a compact, low-field-strength magnetic resonance imager. Top Magn Reson Imaging. 2009;19:179-189.
53. Nimsky C., Ganslandt O., Von Keller B., et al. Intraoperative high-field-strength MR imaging: implementation and experience in 200 patients. Radiology. 2004;233:67-78.
54. Pamir M.N., Ozduman K., Dincer A., et al. First intraoperative, shared-resource, ultrahigh-field 3-Tesla magnetic resonance imaging system and its application in low-grade glioma resection. J Neurosurg. 2010;112:57-69.
55. Matsumae M., Koizumi J., Fukuyama H., et al. World’s first magnetic resonance imaging/x-ray/operating room suite: a significant milestone in the improvement of neurosurgical diagnosis and treatment. J Neurosurg. 2007;107:266-273.
56. Jankovski A., Francotte F., Vaz G., et al. Intraoperative magnetic resonance imaging at 3-T using a dual independent operating room-magnetic resonance imaging suite: development, feasibility, safety, and preliminary experience. Neurosurgery. 2008;63:412-424.
57. Kacher D.F., Nabavi A., Kanan A.R., et al. Design and implementation of surgical instruments, devices and receiver coils for intraoperative MRI-guided neurosurgical and neuroablative procedures. Automedica. 2001;20:89-134.
58. Hushek S.G., Russell L., Moser R.F., et al. Safety protocols for interventional MRI. Acad Radiol. 2005;12:1143-1148.
59. Schenck J.F., Jolesz F.A., Roemer P.B., et al. Superconducting open-configuration MR imaging system for image-guided therapy. Radiology. 1995;195:805-814.
60. Hall W.A. The safety and efficacy of stereotactic biopsy for intracranial lesions. Cancer. 1998;82:1749-1755.
61. Johnston T., Moser R., Moeller K., Moriarty T.M., Intraoperative M.R.I. safety. Neurosurg Clin North Am. 2009;20:147-153.
62. Hall W.A., Truwit C.L. Intraoperative MR-guided neurosurgery. J Magn Reson Imaging. 2008;27:368-375.
63. Nimsky C., Ganslandt O., Fahlbusch R. Comparing 0.2 tesla with 1.5 tesla intraoperative magnetic resonance imaging analysis of setup, workflow, and efficiency. Acad Radiol. 2005;12:1065-1079.
64. Archip N., Clatz O., Whalen S., et al. Compensation of geometric distortion effects on intraoperative magnetic resonance imaging for enhanced visualization in image-guided neurosurgery. Neurosurgery. 2008;62:209-215.
65. Hall W.A., Martin A.J., Liu H., et al. Brain biopsy using high-field strength interventional magnetic resonance imaging. Neurosurgery. 1999;44:807-813.
66. Nimsky C., Ganslandt O., Hastreiter P., et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2007;61:178-185.
67. Ulmer S., Hartwigsen G., Riedel C., et al. Intraoperative dynamic susceptibility contrast MRI (iDSC-MRI) is as reliable as preoperatively acquired perfusion mapping. Neuroimage. 2010;49(3):2158-2162.
68. Neuwelt E.A., Varallyay C.G., Manninger S., et al. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: a pilot study. Neurosurgery. 2007;60:601-611.
69. Knauth M., Egelhof T., Roth S.U., et al. Monocrystalline iron oxide nanoparticles: possible solution to the problem of surgically induced intracranial contrast enhancement in intraoperative MR imaging. AJNR Am J Neuroradiol. 2001;22:99-102.
70. Wirtz C.R., Bonsanto M.M., Knauth M., et al. Intraoperative magnetic resonance imaging to update interactive navigation in neurosurgery: method and preliminary experience. Comput Aided Surg. 1997;2:172-179.
71. Nimsky C., Ganslandt O., Kober H., et al. Intraoperative magnetic resonance imaging combined with neuronavigation: a new concept. Neurosurgery. 2001;48:1082-1089.
72. . Advanced Multimodality Image Guided Operating (AMIGO) Suite, 2008 Available at http://www.ncigt.orgt/pages/AMIGO
73. Schulder M., Azmi H., Biswal B. Functional magnetic resonance imaging in a low-field intraoperative scanner. Stereotact Funct Neurosurg. 2003;80:125-131.
74. Levivier M., Wikler D., De Witte O., et al. PoleStar N-10 low-field compact intraoperative magnetic resonance imaging system with mobile radiofrequency shielding. Neurosurgery. 2003;53:1001-1006. discussion 1007
75. Baumann F., Schmid C., Bernays R.L. Intraoperative magnetic resonance imaging-guided transsphenoidal surgery for giant pituitary adenomas. Neurosurg Rev. 2010;33(1):83-90.
76. Gerlach R., du Mesnil de Rochemont R., Gasser T., et al. Feasibility of Polestar N20, an ultra-low-field intraoperative magnetic resonance imaging system in resection control of pituitary macroadenomas: lessons learned from the first 40 cases. Neurosurgery. 2008;63:272-284.
77. Nabavi A., Goebel S., Doerner L., et al. Awake craniotomy and intraoperative magnetic resonance imaging: patient selection, preparation, and technique. Top Magn Reson Imaging. 2009;19:191-196.
78. Hall W.A., Liu H., Martin A.J., et al. Brain biopsy sampling by using prospective stereotaxis and a trajectory guide. J Neurosurg. 2001;94:67-71.
79. Sutherland G.R., Latour I., Greer A.D. Integrating an image-guided robot with intraoperative MRI: a review of the design and construction of neuroArm. IEEE Eng Med Biol Mag. 2008;27:59-65.
80. Hall W.A., Galicich W., Bergman T., Truwit C.L. 3-Tesla intraoperative MR imaging for neurosurgery. J Neurooncol. 2006;77:297-303.
81. Truwit C.L., Hall W.A. Intraoperative magnetic resonance imaging-guided neurosurgery at 3-T. Neurosurgery. 2006;58:ONS-338-345.
82. Kim P.D., Truwit C.L., Hall W.A. Three-Tesla high-field applications. Neurosurg Clin North Am. 2009;20:173-178.
83. Knauth M., Wirtz C.R., Tronnier V.M., et al. Intraoperative MR imaging increases the extent of tumor resection in patients with high-grade gliomas. AJNR Am J Neuroradiol. 1999;20:1642-1646.
84. Trantakis C., Tittgemeyer M., Schneider J.P., et al. Investigation of time-dependency of intracranial brain shift and its relation to the extent of tumor removal using intra-operative MRI. Neurol Res. 2003;25:9-12.
85. Schneider J.P., Schulz T., Schmidt F., et al. Gross-total surgery of supratentorial low-grade gliomas under intraoperative MR guidance. AJNR Am J Neuroradiol. 2001;22:89-98.
86. Hatiboglu M.A., Weinberg J.S., Suki D., et al. Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: a prospective volumetric analysis. Neurosurgery. 2009;64:1073-1081.
87. Claus E.B., Horlacher A., Hsu L., et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103:1227-1233.
88. Jolesz F.A., Nabavi A., Kikinis R. Integration of interventional MRI with computer-assisted surgery. J Magn Reson Imaging. 2001;13:69-77.
89. Nimsky C., von Keller B., Schlaffer S., et al. Updating navigation with intraoperative image data. Top Magn Reson Imaging. 2009;19:197-204.
90. Nimsky C., Ganslandt O., Fahlbusch R. Functional neuronavigation and intraoperative MRI. Adv Tech Stand Neurosurg. 2004;29:229-263.
91. Nimsky C., Grummich P., Sorensen A.G., et al. Visualization of the pyramidal tract in glioma surgery by integrating diffusion tensor imaging in functional neuronavigation. Zentralbl Neurochir. 2005;66:133-141.
92. Nimsky C., Ganslandt O., Fahlbusch R. Implementation of fiber tract navigation. Neurosurgery. 2007;61:306-317.
93. Martin A.J., Hall W.A., Roark C., et al. Minimally invasive precision brain access using prospective stereotaxy and a trajectory guide. J Magn Reson Imaging. 2008;27:737-743.