Chapter 34 Surgical Management of Midline Anterior Skull Base Meningiomas
Surgical Anatomy
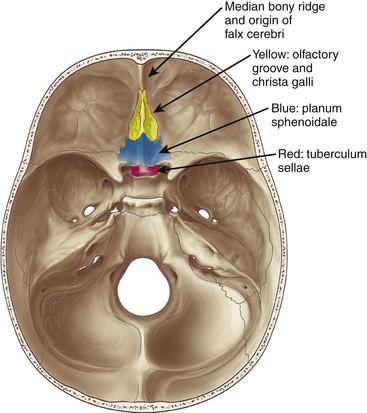
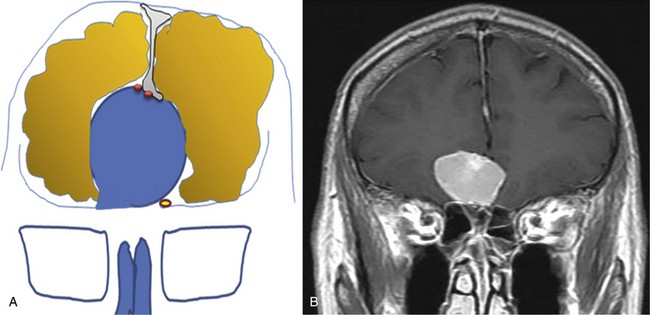
Meningiomas arising in the midline of the anterior fossa are generally separated in the more ventral olfactory groove meningiomas and the more dorsal planum sphenoidale and tuberculum sellae meningiomas. Olfactory groove meningiomas arise over the cribriform plate of the ethmoid bone and the area of the frontosphenoid suture. Those tumors may grow symmetrically around the crista galli and thus may involve any part of the planum of the sphenoid bone or extend predominantly to one side. They occurred with a frequency of less than 6% in our series of 1200 meningiomas. Of all anterior skull base meningiomas, 22% were pure olfactory groove meningiomas. Of these, 7% had at least one additional meningioma at a different location. Planum sphenoidale/tuberculum sellae meningiomas arise from the roof of the sphenoid sinus and the tuberculum sellae, which is an area between the optic nerves and the anterior clinoid processes belonging to the frontal part of the middle cranial fossa. The tuberculum sellae is located between the chiasmatic grooves and on either side at the optic foramen, which transmits the optic nerve and ophthalmic artery to the orbit. Behind the optic foramen, the anterior clinoid process is directed posteriorly and medially and attaches to the tentorium cerebelli. These structures are frequently overgrown by these types of meningiomas, as are the posteriorly located dural folds of the sella turcica and the lateral adjacent cavernous sinus (Fig. 34-1). Planum sphenoidale/tuberculum sellae meningiomas occurred at rates similar to those of olfactory groove meningiomas in our series: less than 6% of all intracranial meningiomas but 21% of anterior skull base meningiomas.
Clinical Presentation
Olfactory groove meningiomas are on average larger than meningiomas at different locations in our series. This is most likely due to the relative lack of focal symptoms at the frontal base with smaller meningiomas. For large tumors, the slow growth rate allows surrounding tissues to adapt. Many symptoms are difficult to localize neurotopically, and the initial consultations of family and physicians often tend toward interpretation of these as functional personality changes rather than focal cerebral symptoms. Personality changes, such as apathy and akinesia, can be common when the tumors grow to larger size; in our series, this was found in up to 13% of patients. Onset of these symptoms is gradual, and they may not be observed early in their course. Other common symptoms include headache and visual deficits, both of which were more frequent in frontal meningiomas than in any other type of meningioma in our series. Because the optic nerves and chiasm are compressed superiorly by the tumor, an inferior visual defect was most common in up to one third of our patients. The Foster-Kennedy syndrome of unilateral optic atrophy and contralateral papilledema, although originally described in olfactory groove meningiomas,1 occurred in only a small number of patients.
Double vision is a rare symptom, occurring in less than 6% of patients. In our series, smelling disorders up to anosmia were apparent in 64.5% of the patients that were diagnosed based purely on the routine preoperative workup. Only 7.1% were completely anosmic preoperatively. Interestingly, anosmia is not an important symptom for most patients, most likely because it develops slowly. In Cushing and Eisenhardt’s series, the sense of smell was the primary symptom in only 3 of the 29 patients.2 Bakay reported that even if anosmia was apparent, it was not the leading symptom.3
Evaluation of Radiologic Studies in Planning the Operation
In recent years, angiography generally has not been indicated unless embolization is planned. The classic angiographic appearance of a meningioma is that of increasing hypervascular tumor blush throughout the arterial phase, persisting well into the late venous phase with slow washout. Hypervascularity may complicate and lengthen the operation. Therefore, embolization may be considered, which involves the devascularization of the tumor’s blood supply through the placement of an embolic agent via a microcatheter into the feeding arteries. However, the surgeon should be aware of a considerable rate of hemorrhagic and ischemic complications when using small particles for embolization.4–7
General Aspects of Surgical Management
As the majority of meningiomas are benign, well-circumscribed extra-axial tumors, complete surgical removal should be the primary goal in most instances. The neurologic integrity has to be preserved as it would be in any other neurosurgical procedure. Due to the relation of anterior midline meningiomas to adjacent neurologic eloquent areas, complete tumor removal even with resection of infiltrated dura or removal of infiltrated bone might be achieved with low morbidity in most cases. However, when tumors are firmly attached to the anterior vessels or the optic chiasm, complete removal might constitute a high risk for damage of those structures. In these cases a small piece of adherent capsule might remain and is controlled by periodic MRI scans. Upon recurrence, reoperation and adjuvant radiation therapy may be considered.
Bifrontal Approach
General Considerations
The bifrontal approach was first described by Horsley8 and Cushing9 and was later proposed by Tönnes,10 who preserved the frontal brain tissue by a subfrontal approach.11 Many others have used the bifrontal approach for large tumors of the frontal base, such as Al-Mefty,12 Nakamura et al.,13 and Ransohoff and Nockels.14 A bifrontal craniotomy might be considered for patients with large tumors because this approach gives direct access to all sides of the tumor. Due to the wide exposure, retraction on the frontal lobes is minimal. It simultaneously allows interruption of the blood supply, preparation of the frontobasal matrix of the tumor, and concomitant decompression. There is usually no problem from the ligation of the anterior sagittal sinus. However, venous drainage should be evaluated by preoperative imaging to avoid venous congestion, and coagulation of draining veins from the anterior frontal lobe should be avoided of possible. A navigation system might be used to avoid opening the frontal sinus; however, if the frontal sinus is entered, meticulous closure of the defect should prevent any complications.
Operative Technique
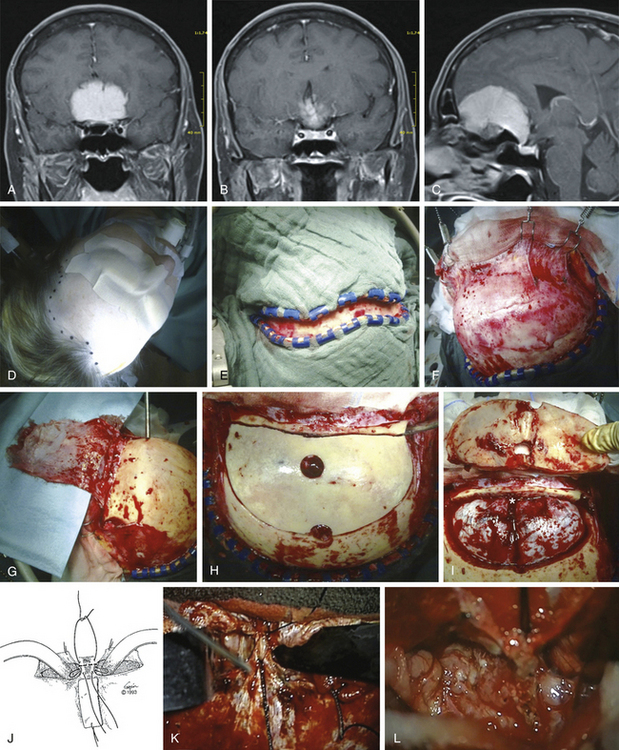
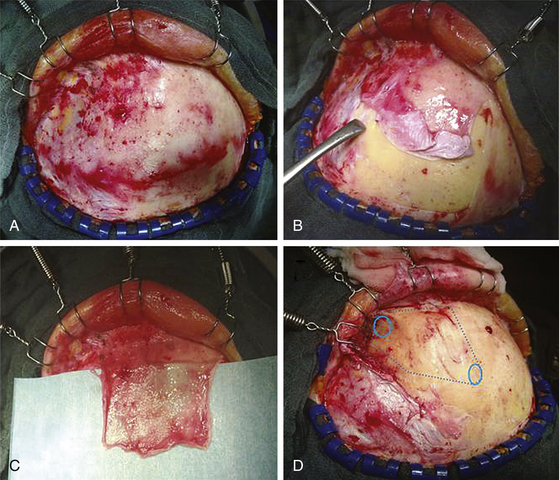
The patient is placed in the supine position with the knees slightly flexed and the head slightly elevated and extended. A three-point skeletal-fixation headrest system is used. Usually, only a small area of hair needs to be shaved to prepare a coronal incision through the skin while preserving the pericranial tissue. The skin of the posterior aspect of the incision is elevated, and the pericranial tissue is incised below the skin. This step gives extra pericranial tissue, which might be used later to cover the floor of the anterior fossa and to patch the convex dura as needed. The skin flap and underlying tissue, including the pericranial tissue, are then turned down together using fishhooks. According to the size and extension of the tumor, bur holes are placed just below the end of the anterior temporal line or keyholes are placed above the pterion and on each side of the sagittal sinus anterior to the skin incision (Figs. 34-2 and 34-3).
The frontal sinuses are entered frequently. Whether the mucosa needs to be completely removed remains controversial. A multilayered closure of the frontal sinus is prepared. First, a layer of bone wax is used to close the sinus during the intracerebral part of the operation. For final closure, a pericranial flap is formed (Fig. 34-3) that can be inserted once the tumor is removed. The pericranial flap should be sutured to the frontal edge of the dura and intradurally as far posterior as possible to cover any defects (Fig. 34-4). In addition, frontobasal dura gaps can be covered that occurred during tumor and dural matrix removal. In case of an opened ethmoid sinus, the pericranial tissue should cover this too. Sutures are placed along the edge of the craniotomy to control epidural bleeding.
A slightly curved dural incision is performed over each medial inferior frontal lobe adjacent to the edge of the craniotomy opening but leaving a sufficient rim for safe and convenient closure of the dura. Depending on the bony removal, the dural opening can be curved basally or upward. This incision can be tailored to the specific line of approach to the meningioma. If a lateral approach is needed, in addition to the midline approach, the dural opening is extended as needed. It is carried medially near the edge of the sagittal sinus, thereby allowing good exposure yet protecting most of both frontal lobes during the operation. Bridging veins from the anterior frontal lobe to the midline area might be coagulated and divided if necessary. To obtain better exposure of the sagittal sinus, the frontal lobes might be carefully retracted. Then the sagittal sinus is divided between two silk sutures, and the falx is cut (Fig. 34-3). The frontal lobes are gently retracted slightly laterally and posteriorly to open the view to the anterior and superior surface of the tumor, lying in the midline with attachments to the falx and crista galli.
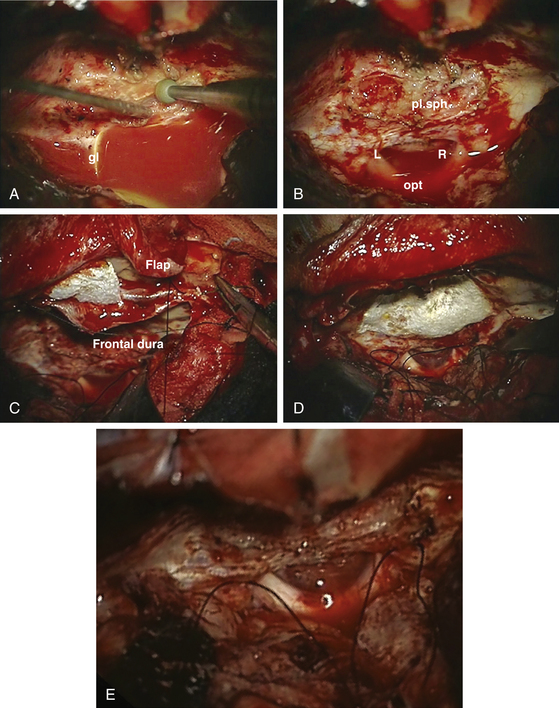
The alternative procedure is to remove the core of the tumor first. This leads to higher blood loss and poorer control of the blood supply during debulking. However, preparation of the skull base after debulking of the tumor is easier compared to the initial devascularization procedure. The initial step in that procedure is to open the anterior capsule of the tumor. If histology is in doubt, a biopsy might be taken now for frozen-section histology. Depending on the amount of bleeding, large parts of the anterior and midline portion of the tumor can be removed before the part of the tumor capsule that is attached at the anterior midline is divided. Numerous small blood vessels that penetrate the bone in the frontal fossa usually constitute the main blood supply for those tumors. Therefore, in cases of heavy bleeding after starting debulking the tumor, it might be necessary to switch strategies and do the basal devascularization first. Those vessels are occluded by bipolar coagulation and, occasionally, bone wax or soft drilling with a diamond drill.
Devascularization of the tumor as early as possible is especially important in large olfactory groove meningiomas. As the debulking progresses, the remaining tumor is brought down away from the brain tissue toward the cribriform plate, where most of the work takes place. Thus, it is an alternating procedure of dissection along the base and internal decompression of the tumor, resulting in the exposure of the base and removal of the bulk of the tumor. For decompression, an ultrasound surgical aspirator can be used that is effective even for tumors of firm consistency. By preventing the frontal lobes from falling into the decompression area by self-retaining retractors, there is enough space to dissect the tumor capsule from the adjacent frontal brain parenchyma. Care should be taken to avoid pressure on the frontal lobes and consecutive postoperative edema or even intraparenchymal hemorrhage. The preparation of the posterior tumor capsule is the most challenging step, because branches of the anterior artery complex might be adjacent to or even involved in the tumor capsule. In most cases, at least the larger vessels can be separated by a small rim of cerebral cortex and arachnoid between the adventitia and the outer tumor capsule. However, when those vessels are firmly embedded within the tumor capsule, a tumor remnant should be left in place. Smaller branches or the frontopolar artery being spread in the capsule might be considered to be occluded and divided if there is no alternative that may not cause a problem.15
After identification of the involved adjacent neurologic structures and removal of the bulk of the tumor, the area of dural attachment should be meticulously coagulated or preferentially excised. Infiltrated hyperostotic bone should be removed by a high-speed diamond drill. In our view, hyperostosis may contain remaining tumor cells, and the osseous and tumorous vascular supply usually originates from this area. Similarly, any tumor entering the ethmoid sinus should be resected. The region of the excised dura should be covered with a sheet of Tacho-seal,TachoSil, TM or something similar; with a vascularized galeal–periosteal patch or fascia lata over the defect, the patch should be sutured to the remaining dura margin with single stitches and sealed along the border with fibrin glue (Fig. 34-4). Another layer of gelatin sponge is placed over the fascia. The layers might be augmented with fibrin glue.
Unilateral Subfrontal Approach
General Considerations
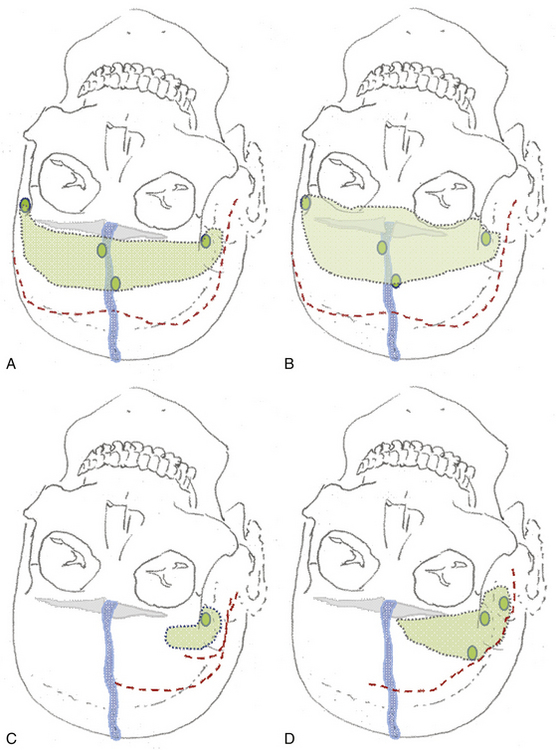
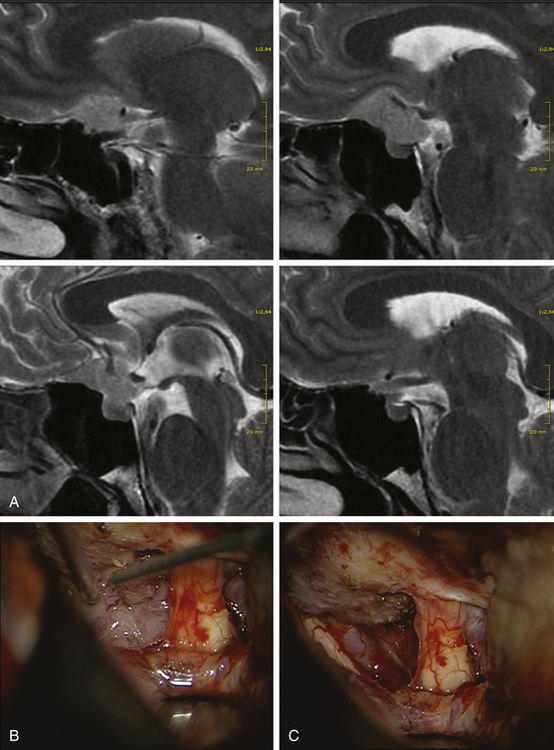
The unilateral approach is preferred if the tumor grows prominently on one side and does not have an extensive basal dural attachment onto the contralateral side and if the contralateral olfactory nerve is expected to have a clear plane of dissection (Fig. 34-5A).
Operative Technique
The patient’s position depends on the involvement of the midline structures, the displacement and orientation of the A2 segments, and the optic nerve structures. In general, the head is slightly extended posteriorly to allow the frontal lobes to follow gravity. A regular frontal skin incision behind the hair line is used that might be extended over the midline, if anterior and medial visualization are considered. A pericranial flap is always prepared to cover potential dural tears or removed tumor matrix in case of anterior middle fossa involvement (Fig. 34-6). This is not necessary for isolated tuberculum sellae tumors. The craniotomy should aim to visualize the sylvian fissure if posterior retraction along the sphenoid wing is required, remain very low above the plane of the frontal skull base, and extend close to the midline as far as necessary. The craniotomy should be shaped according to the expected irregularities of the frontal skull base, which might otherwise hinder clear visualization along a one-sided trajectory.
Tumor Removal
The alternative technique to initial devascularization is debulking, which was introduced as early as 1938 in the classic monograph by Cushing and Eisenhardt.1 However, even central decompression rarely leads to shrinking of the tumor because these are usually rather stiff and attached to the surrounding brain. The preparation should aim to preserve the olfactory nerves and A2 segments. Therefore, these structures should be visualized as early as possible. Before approaching the dural matrix of the tumor, the olfactory nerve should be freed of the surrounding arachnoid scarring, which is easier from a lateral approach. Piecemeal preparation might be necessary to maintain olfactory nerve integrity because this nerve is particularly vulnerable to bipolar cauterization and inadvertent suctioning. We prefer to protect the nerve with a nonadherent covering such as Bicol collagen sponges rather than using a Cottonoid. If the optic nerves are reached by the tumor, dissection should proceed in an arachnoid plane, which can be of varying thickness and clarity. If a thin layer of tumor is approaching the optic nerves and seems to enter the middle cranial fossa, careful inspection of this side using a bayonet mirror or an endoscope should facilitate visualization of all tumor compartments and their removal using curettes and coagulation of the remaining matrix using an angled bipolar cautery.
Arteries are frequently embedded within thickened arachnoid layers of the tumor capsule. It is necessary to continuously reevaluate the correct plane of dissection because tumor feeding arteries are difficult to differentiate from frontal lobe branches. When the surgeon is in doubt, every vessel is prepared with its arachnoid covering.
Tuberculum sellae meningiomas that push the optic structures upward and do not extend anteriorly can be approached from a pterional craniotomy. Tuberculum sellae meningiomas extending anteriorly and pushing the optic nerves laterally should be considered for a modified frontal approach (Fig. 34-7). If the carotid artery is engulfed and the medial sphenoid wing or the cavernous sinus is involved, any combined approach that includes a pterional opening is recommended.
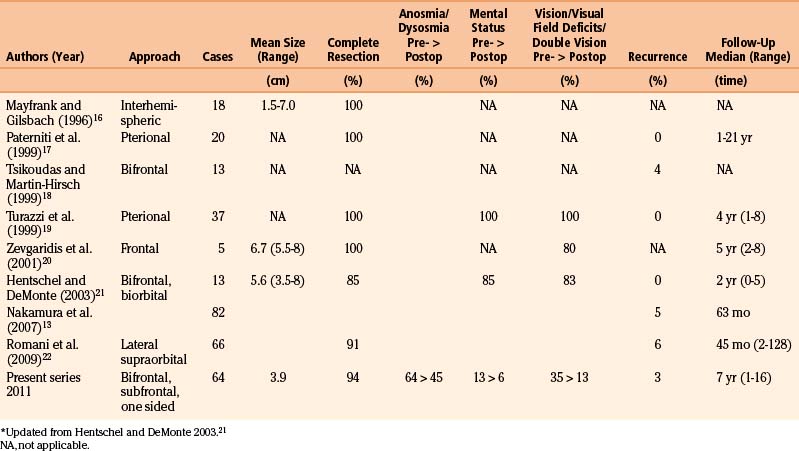
Olfactory groove meningiomas most frequently underwent radical removal (Simpson grade I and II in more than 94%), which was reflected in only two recurrences (3%) compared to 14% of recurrences/residual tumors for all intracranial meningiomas, in our series (Table 34-1).
Al-Mefty O. Surgical technique for the juxtasellar area. In: Al-Mefty O., editor. Surgery of the Cranial Base (Foundations of Neurological Surgery, 2 ed.). Boston: Kluwer Academic Publishers; 2010:73-89.
Bakay L. Olfactory meningiomas. The missed diagnosis. JAMA. 1984;251:53-55.
Bendszus M., Monoranu C.M., Schutz A., et al. Neurologic complications after particle embolization of intracranial meningiomas. AJNR Am J Neuroradiol. 2005;26:1413-1419.
Carli D.F., Sluzewski M., Beute G.N., et al. Complications of particle embolization of meningiomas: frequency, risk factors, and outcome. AJNR Am J Neuroradiol. 2010;31:152-154.
Cushing H., Eisenhardt L. Meningiomas, Their Classification, Regional Behaviour, Life History and Surgical End Results. Springfield, Illinois: Charles C. Thomas; 1938.
Hassler W., Zentner J. Pterional approach for surgical treatment of olfactory groove meningiomas. Neurosurgery. 1989;25:942-945.
Kallmes D.F., Evans A.J., Kaptain G.J., et al. Hemorrhagic complications in embolization of a meningioma: case report and review of the literature. Neuroradiology. 1997;39:877-880.
Nakamura M., Struck M., Roser F., et al. Olfactory groove meningiomas: clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery. 2007;60:844-852.
Ransohoff J., Nockels R.P. Olfactory groove and planum meningiomas. In: Apuzzo M.L.J., editor. Brain Surgery Complication Avoidance and Management. New York: Churchill Livingstone; 1993:203-219.
Wakhloo A.K., Juengling F.D., Van Velthoven V., et al. Extended preoperative polyvinyl alcohol microembolization of intracranial meningiomas: assessment of two embolization techniques. AJNR Am J Neuroradiol. 1993;14:571-582.
1. Al-Mefty O. Surgical technique for the juxtasellar area. In: Al-Mefty O., editor. Surgery of the Cranial Base (Foundations of Neurological Surgery, 2 ed.). Boston: Kluwer Academic Publishers; 2010:73-89.
2. Cushing H., Eisenhardt L. Meningiomas: Their Classification, Regional Behaviour, Life History, and Surgical End Results. Springfield, Illinois: Charles C. Thomas; 1938.
3. Bakay L. Olfactory meningiomas. The missed diagnosis. JAMA. 1984;251:53-55.
4. Bendszus M., Monoranu C.M., Schutz A., et al. Neurologic complications after particle embolization of intracranial meningiomas. AJNR Am J Neuroradiol. 2005;26:1413-1419.
5. Carli D.F., Sluzewski M., Beute G.N., et al. Complications of particle embolization of meningiomas: frequency, risk factors, and outcome. AJNR Am J Neuroradiol. 2010;31:152-154.
6. Kallmes D.F., Evans A.J., Kaptain G.J., et al. Hemorrhagic complications in embolization of a meningioma: case report and review of the literature. Neuroradiology. 1997;39:877-880.
7. Wakhloo A.K., Juengling F.D., Van Velthoven V., et al. Extended preoperative polyvinyl alcohol microembolization of intracranial meningiomas: assessment of two embolization techniques. AJNR Am J Neuroradiol. 1993;14:571-582.
8. Horsley V. On the technique of operations on the central nervous system. Br. Med. J. 1906;2:411-423.
9. Cushing H. The meningiomas arising from the olfactory groove and their removal by the aid of electrosurgery. Lancet. 1927;1:1329-1339.
10. Tönnies W. Zur Operation der Meningeome der Siebbeinplatte. Zentralblatt für Neurochirurgie. 1938;1:1-7.
11. Hassler W., Zentner J. Pterional approach for surgical treatment of olfactory groove meningiomas. Neurosurgery. 1989;25:942-945.
12. Al-Mefty O. Surgical technique for the juxtasellar area. In: Al-Mefty O., editor. Surgery of the Cranial Base (Foundations of Neurological Surgery, 2 ed.). Boston: Kluwer Academic Publishers; 2010:73-89.
13. Nakamura M., Struck M., Roser F., et al. Olfactory groove meningiomas: clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery. 2007;60:844-852.
14. Ransohoff J., Nockels R.P. Olfactory groove and planum meningiomas. In: Apuzzo M.L.J., editor. Brain Surgery Complication Avoidance and Management. New York: Churchill Livingstone; 1993:203-219.
15. Ojemann R.G., Martuza R.L. Surgical management of olfactory groove meningiomas. In: Schmidek H.H., Roberts D.W. Operative neurosurgical techniques: indications, methods, and results. Philadelphia: WB Saunders Company; 2006:207.
16. Mayfrank L., Gilsbach J.M. Interhemispheric approach for microsurgical removal of olfactory groove meningiomas. Br J Neurosurg. 1996;10:541-545.
17. Paterniti S., Fiore P., Levita A., et al. Basal meningiomas. A retrospective study of 139 surgical cases. J Neurosurg Sci. 1999;43:107-113.
18. Tsikoudas A., Martin-Hirsch D.P. Olfactory groove meningiomas. Clin Otolaryngol Allied Sci. 1999;24:507-509.
19. Turazzi S., Cristofori L., Gambin R., et al. The pterional approach for the microsurgical removal of olfactory groove meningiomas. Neurosurgery. 1999;45:821-825.
20. Zevgaridis D., Medele R.J., Muller A., et al. Meningiomas of the sellar region presenting with visual impairment: impact of various prognostic factors on surgical outcome in 62 patients. Acta Neurochir (Wien). 2001;143:471-476.
21. Hentschel S.J., DeMonte F. Olfactory groove meningiomas. Neurosurg Focus. 2003;14:e4.
22. Romani R., Lehecka M., Gaal E., et al. Lateral supraorbital approach applied to olfactory groove meningiomas: experience with 66 consecutive patients. Neurosurgery. 2009;65:39-52.