CHAPTER 370 Surgical Management of Middle Cerebral Artery Aneurysms
The middle cerebral artery (MCA) originates at the bifurcation of the internal carotid artery (ICA). It is the largest branch of the ICA and courses laterally within the sylvian cistern, providing branches along its course, ultimately ending as small cortical or deep lenticulostriate vessels. Approximately 14% to 20% of all intracranial aneurysms originate along the MCA,1–5 most often at the bifurcation of the first segment (M1) with the second segment (M2), and projecting laterally in the plane of the M1 segment.2 MCA aneurysms are somewhat unique for several reasons: they may grow to 20 mm or larger before detection, they are more likely to present with an intraparenchymal clot, and they are more likely to cause symptoms of mass effect than most other intracranial aneurysms.6 They are also, by virtue of their angioarchitecture, more challenging to treat with endovascular techniques than other aneurysms within the intracranial circulation.
Middle Cerebral Artery Anatomy
The sylvian fissure is the gateway to the mesial skull base and its related structures. The fissure is composed of a deep and superficial component. The deep component of the fissure is divided into the anterior (sphenoidal) compartment, which contains the M1 segment of the MCA, and the posterior (operculoinsular) compartment, which contains the M2 and M3 segments. This deep fissure is sometimes known as the sylvian cistern and is contiguous with the basilar cisterns. The superficial part of the sylvian fissure is composed of a stem, which extends from the anterior clinoid process in a medial to lateral direction between the frontal and temporal lobes, and several rami.7
Lying within the sylvian fissure, the MCA is the largest and most variable of the intracranial arteries. It is divided into four segments: M1 (sphenoidal), M2 (insular), M3 (opercular), and M4 (cortical). The M1 segment extends from the bifurcation of the ICA, coursing at the bottom of the sylvian fissure. The M1 segment can be of variable lengths but classically is longer than 15 mm. Short M1 segments have surgical implications because aneurysms on such vessels are, by definition, deeper within the fissure than ordinarily expected. The genu of the MCA is often at or just distal to the limen insulae (a small gyrus at the anteroinferior corner of the insula) and classically corresponds to the bifurcation (78%), trifurcation (12%), or multiple trunks that proceed distally (10%).8 M2 segmental arteries course within the fissure posterosuperiorly at variable depths and branch into M3 vessels at the periphery of the insulae but are variable, and end at the cortical surface where M4 vessels are identified.
The branches of the middle cerebral artery are important for surgical orientation, treatment paradigms and implications, and salvage techniques. The M1 has multiple lenticulostriate arteries that are divided into two groups. The medial lenticulostriate arteries enter the anterior perforating substance superiorly and supply the lentiform nucleus, the caudate, and the internal capsule. The lateral lenticulostriate arteries are more variable in their location, traverse the basal ganglia, and supply the caudate nucleus.9 The anterior temporal artery is a large cortical branch that arises from the M1 segment before the MCA bifurcation and is the first named branch of the M1, supplying the temporal tip. Anomalies of the MCA candelabra are not unusual and include duplications of the MCA, also known as accessory MCAs (1% to 3%),9 arising from the anterior cerebral artery (ACA) and paralleling the MCA (2.7%),10 and other anomalies have been reported and associated with an increased incidence of aneurysm formation.11,12
The venous anatomy of the sylvian fissure is much more variable. The preservation of these veins during opening of the sylvian fissure and aneurysm dissection is critical in preventing venous congestion or even venous infarction.13 We have found that with few exceptions, the sacrifice of any veins is unnecessary, and even frontobasal veins that arise from the temporal side of the fissure can be easily and safely accommodated.
Classification of Middle Cerebral Artery Aneurysms
Classification by Location
Aneurysms of the M1 segment are second in frequency to bifurcation aneurysms and are composed of lenticulostriate or anterior temporal artery saccular aneurysms. Proximal M1 segment lesions represent 2% to 12% of all MCA aneurysms.6,14,15 In patients with multiple intracranial aneurysms, the frequency of proximal MCA aneurysms tends to increase, and nearly three fourths of patients with multiple intracranial aneurysms harbor an MCA aneurysm.6 In fact, Rinne and colleagues examined 561 patients with MCA aneurysms and found that 39% of patients with MCA aneurysms had multiple intracranial aneurysms, significantly higher than the 20% classically quoted for other parts of the intracranial circulation.4,6,16 Additionally, these investigators found that the multiplicity of aneurysms increased the risk for poor outcome.6
Proximal MCA aneurysms can be further divided into superior wall type and inferior wall type, depending on the specific anatomic presentation.17 Superior wall–type M1 segment aneurysms arise at the origins of the lenticulostriate arteries and project into the frontal lobe posterosuperiorly.17 All efforts must be made to preserve such perforating vessels.18–20 Such aneurysms are also often small, making them hazardous for endovascular treatment. Inferior wall M1 aneurysms arise at the origin of the anterior temporal artery or the temporopolar artery and project toward the temporal lobe in an anterolateral projection.
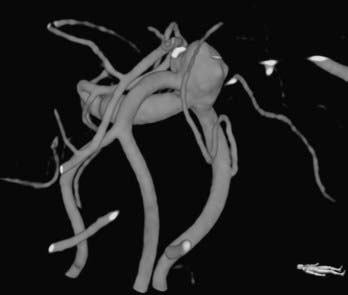
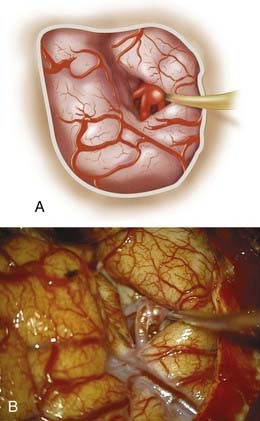
Bifurcation and trifurcation aneurysms represent up to 90% of all MCA aneurysms and are the lesions most uniformly referred for surgical consideration (Fig. 370-1). As noted in the previous brief discussion of MCA anatomy, the bifurcation (or trifurcation) can be highly variable, classically with one division supplying the frontal lobe and another supplying the temporal lobe. In a large Finnish series of MCA aneurysms, the direction of MCA bifurcation aneurysms varied considerably. Thirty-four percent were directed inferiorly in both the lateral and anteroposterior planes.6 With three-dimensional rotational digital subtraction angiography (DSA) and computed tomographic angiography (CTA), the precise orientation of the aneurysm relative to the vascular and cranial anatomy can be imaged preoperatively.
Classification by Etiology
Saccular
With regard to etiology, the precise pathogenesis of the common saccular (or berry) aneurysm is incompletely understood. These aneurysms classically form at sites of arterial curves or branching.21 Hemodynamic forces are likely to be an important contributing factor in the forced segmentation of the arterial elastic membrane, which may be an important factor in the aneurysm formation cascade.22
Fusiform Aneurysms
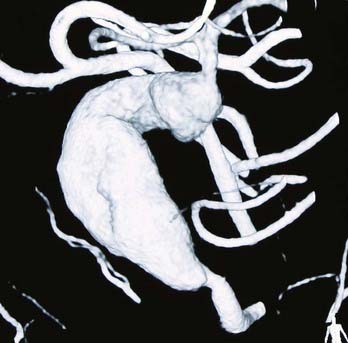
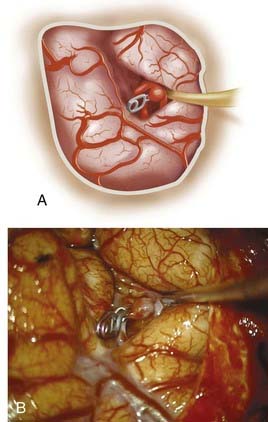
Fusiform lesions are most frequently seen in the posterior circulation and have far different management considerations than saccular aneurysms (Fig. 370-2). Although rare,6 there is no doubt that they represent a different disease process than saccular aneurysms, and in the MCA distribution, they can grow quite large, incorporating multiple branch vessels. MCA fusiform aneurysms can be found in all segments of the artery, although classically they are found at the bifurcation.
Infectious Aneurysms
Infectious or mycotic aneurysms are most commonly found along the distal branches of the cerebral arteries.23–25 They are typically secondary to infectious emboli, with subsequent aneurysm formation. Usually, bacterial endocarditis (65%) is implicated in infectious aneurysms, but other idiopathic bacterial or fungal sources have been implicated.23,26 Other embolic sources include neoplastic disease, such as choriocarcinoma and atrial myxoma.
Dissecting Aneurysms
Dissecting MCA aneurysms are rare and may be associated with infection, connective tissue diseases such as Marfan’s syndrome, cystic medial degeneration, and fibromuscular dypslasia.23,27–30 It is assumed in such scenarios that there exists a congenital weakness of the vessel wall with rupture of the elastic interna.30,31 The dissection is classically located between the internal elastic lamina and medial layers, which is different from dissecting aneurysms of the aorta or peripheral arteries.30 The intima is folded and displaced by the second lumen, causing the true lumen to be narrowed or completely blocked. Angiographically, if flow is present, there is an asymmetric narrowing32 or rippled appearance due to the separated or folded intima.30 The pathologic findings make the treatment of this rare disease process challenging. If infarction has been avoided and patency has been preserved, trapping with bypass or endovascular stenting, or both, may be considered. Although most present with ischemia, 43% of patients with an MCA aneurysmal dissection present with SAH, and 75% of these patients are male.33
Traumatic Aneurysms
Major cranial trauma is often associated with intracranial arterial dissection.25,34–36 Traumatic aneurysms are uncommon and are most often associated with the ACA and its branches because of its proximity to both the skull base and the falx. Traumatic MCA aneurysms are unusual, are most classically associated with a skull fracture, and have a high rupture rate.37 These lesions are most frequently distal on M3 and M4 segments and often present with delayed rupture (average, 4.7 days) after the inciting trauma.37 Classically, these are managed with surgical trapping and excision with or without bypass.
Classification by Size
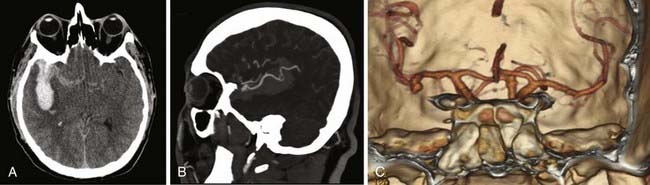
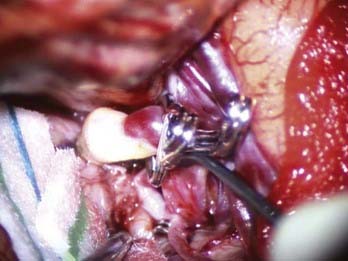
Cerebral aneurysms that reach large (>15 mm) or giant (>25 mm) size are more frequently seen in the middle cerebral artery distribution than in other arterial distributions and can represent up to 9% of MCA aneurysms.6 As with smaller aneurysms, they come in two types, saccular and fusiform, but because of their size, they represent significant treatment dilemmas (Fig. 370-3). Although often asymptomatic, they can also present with symptoms associated with mass effect or with transient ischemic attacks (or stroke) due to thrombus or flow reduction. About half have a neck that is amenable to clipping.2 Advanced surgical management may include clip reconstruction, bypass, wrapping, aneurysmorrhaphy, or combination techniques, including surgical and endovascular management. Some lesions are best managed by expectant management or regional referral, depending on surgeon and patient preferences.
Presentation and Evaluation of Middle Cerebral Artery Aneurysms
Subarachnoid or intracranial hemorrhage is the most common presentation of MCA aneurysms. Because of their propensity to become quite large before detection, they may occasionally become symptomatic without SAH. Giant aneurysms are reported to cause seizures more often than smaller ones, and this may be due to mass effect, ischemic changes, or repeated subclinical hemorrhages.38–41 The evaluation and treatment of patients with aneurysms varies depending on whether an aneurysm has ruptured or not. Nonruptured aneurysms are increasingly being discovered incidentally because of the frequency of use of screening studies (computed tomography [CT], CTA, magnetic resonance angiography [MRA]) in the evaluation of any number of unrelated symptoms. Occasionally, patients with a strong family history of aneurysms undergo screening as well. Unless treatment is not likely to be eventually recommended, we generally evaluate these patients with a catheter angiogram to fully assess the aneurysm, its morphology, and the remaining cerebral vasculature.
In symptomatic cases, CT usually reveals the presence of subarachnoid blood; however, a lack of blood necessitates a lumbar puncture (LP) if the history is suggestive of an aneurysm rupture.42 In patients with SAH on CT or LP, we typically perform a cerebral angiogram, with three-dimensional reconstruction of any pathology if possible.
Surgical Treatment of Middle Cerebral Artery Aneurysms
MCA aneurysms tend to be highly variable with regard to their ease or difficulty of surgical clipping. Variables include presence of SAH, size and configuration of the aneurysm, inclusion of branch vessels, and surgeon’s experience. Because of their frequently broad-based configuration, many MCA aneurysms are unsuitable for current endovascular options. In addition, the relatively small caliber of surrounding branches often precludes the use of stents, and their classic bifurcation location makes recurrence more likely. For all these reasons, and the fact that the lesions are usually readily accessible surgically, MCA aneurysms are preferentially clipped at most cerebrovascular centers.6,43,44
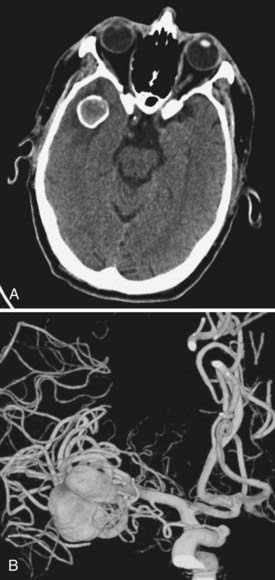
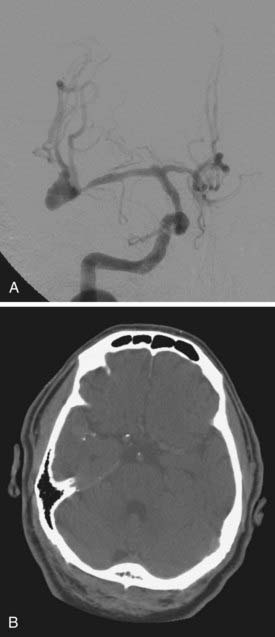
We attempt to treat ruptured MCA aneurysms at the first available opportunity.45,46 Treatment is generally scheduled for the next available operative day, ideally before postbleed day 3. Occasionally, patients are delayed in transfer and arrive with significant vasospasm as documented on DSA or CTA. In these situations, we have found it prudent to wait until after the vasospasm window, classically postbleed days 11 to 14, and manage these patients expectantly in the intensive care unit. Occasionally, but classically, MCA aneurysms can present with a large intracerebral hemorrhage that requires urgent or emergent clot evacuation and exploration and treatment of the aneurysm (Fig. 370-4). Unruptured MCA aneurysms that present incidentally are counseled regarding the various management options. Age of the patient, size and multiplicity of the aneurysm, family history, tobacco use, control of hypertension (if present), overall medical condition, and patient and family wishes are considered and discussed, using our knowledge of the natural history as a guide.47,48
Preparation
Cerebrospinal fluid drainage is often indicated in patients with acute hydrocephalus after aneurysmal SAH. Although there are anecdotal reports of rupture, several small studies have not confirmed an increased risk,49,50 and the relaxed brain makes surgery safer in ruptured aneurysm surgery. However, in patients with good-grade (Hunt-Hess grades I and II) disease, we often defer placement of a ventriculostomy, or may consider placement of a lumbar drain. Patients with unruptured aneurysms generally do not have preoperative placement of a lumbar drain. Most patients receive a bolus of mannitol at the beginning of the operation to assist in brain relaxation.
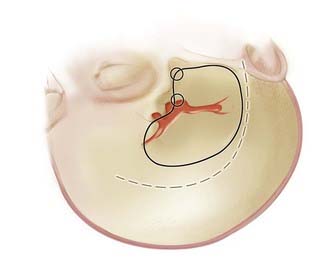
As with all cranial surgery, the positioning of the incision and head are of paramount importance and cannot be overlooked. A radiolucent cranial fixation device is used in all cases to assist with intraoperative angiography. For MCA aneurysms, the head is turned about 30 degrees to the opposite side with the vertex slightly extended (Fig. 370-5).
Although stereotactic localization may be useful for very distal M3 and M4 aneurysms,51,52 a standard pterional incision, as popularized by Yasargil,53 is adequate for most MCA aneurysms. This incision is made from the midline, behind the hairline, to the zygoma in a curvilinear fashion, 1 cm in front of the ear to avoid the frontotemporal branches of the facial nerve (see Fig. 370-5).54 The skin flap is then separated from the periosteum and temporalis fascia and is turned anteroinferiorly down to the level of the fat pad just over the zygoma.
Many methods are available to mobilize the temporalis, and indeed one of the cosmetic concerns of patients after surgery is temporalis atrophy. There are several factors causing atrophy of the temporalis, including denervation, loss of blood supply, and injury to the muscle.55 We generally prefer a slightly modified opening as described by Heros and coworkers, using a muscle-splitting incision just posterior to the fat pad of the temporalis, with a second perpendicular muscle-splitting incision just inferior to the superior temporal line, leaving a generous cuff for reapproximation and closure.54 The bulk of the temporalis is held in place posteroinferiorly, which avoids having the muscle bulk displaced anteriorly, obscuring the surgeon’s line of site. A subperiosteal dissection of both remaining segments of the temporalis may lessen atrophy, allowing the bone flap to extend as much as needed to the floor of the middle fossa anteriorly. A common variant is to elevate the temporalis in a muscle-splitting incision with the skin incision. Although this may slightly lessen visualization into the temporal fossa, for most MCA aneurysms, this exposure is perfectly adequate.
Craniotomy
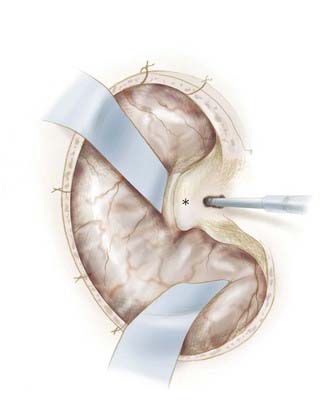
Although the pterional craniotomy has been well described by many,20,53,54 a brief review and several nuances deserve attention. We generally place two bur holes, one at the keyhole and one just superior to the zygoma (see Fig. 370-5). In older patients, we may place a third bur hole at the posterior aspect of the superior temporal line. The dura is stripped from the cranium with a Penfield No. 3, and the pterional craniotomy flap is created with a high-speed drill with footplate. The lesser wing of the sphenoid is flattened to the level of the superior orbital fissure using a combination of round cutting bur and rongeurs before opening the dura (Fig. 370-6). The squamous temporal bone is removed to allow an unencumbered view of the sylvian fissure. By definition, this also allows the thinning of the superior and lateral orbital walls, and care should be taken to minimize transgression of the wall and orbital fat. Bleeding from the pterion may be problematic and can usually be addressed by careful application of bone wax, followed by application of small sheets of Avitene into the epidural space of the temporal fossa. Wire-pass drill holes are placed around the periphery of the craniotomy for dural tack-up sutures, and the dura is opened in a curvilinear fashion based on the pterion and tacked in place.
Fissure Dissection
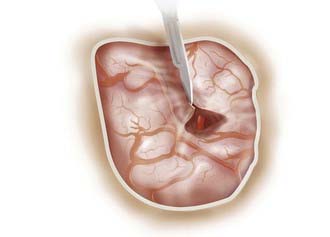
The microscope is brought into the field, and the arachnoid over the sylvian fissure is opened sharply with an arachnoid knife or a No. 11 knife blade (Fig. 370-7). This should always be done on the frontal side of all identifiable veins or simply adjacent to the frontal lobe. Applying counter-tension with a suction tip will allow for a more straightforward entry. After a 3- to 4-mm opening has been made with a knife, microscissors are used to continue to open the superficial arachnoid over the fissure. Lining up the surgeon’s hand and arm with the sylvian fissure may assist an otherwise awkward angle that sometimes is encountered as the surgeon begins dissection.
There are several approaches to general dissection of the fissure that deserve discussion. In general, we use a combination of methods. Yasargil described the inside-out method of entering the sylvian fissure distally, working deeply, and splitting the fissure as the surgeon moves proximally along the MCA until the fissure is dissected.20 The obvious advantage of this is being able to use the cerebrospinal fluid that has completely filled the fissure to aid in the dissection. The disadvantage of this technique is that proximal control for MCA aneurysms is not necessarily established before the aneurysm is seen. The alternative is to identify and open the opticocarotid cistern, establish proximal control, and then work along the ICA from proximal to distal, splitting the fissure and working along the M1 segment until the pathology is encountered. We often use a combined approach of sylvian fissure dissection, providing some brain relaxation and early proximal control of the aneurysm by opening the opticocarotid cistern first. The distal fissure is then dissected in the plane that is most easily and readily identifiable. The goal is identification of the M2 or M3 vessels distal to the aneurysm. These can then be used as landmarks aiding in more proximal dissection and ultimately aneurysm identification.
Several pitfalls of sylvian fissure dissection are worth mentioning. Dissection may occasionally result in an inadvertent subpial dissection plane, particularly in patients with SAH. Aside from the obvious epileptogenic and neurological consequences, it can sometimes be difficult and tedious to rectify. Avoidance of injury to any arterial structure is obviously of paramount importance. The surgeon should also be careful not to occlude any lenticulostriate vessels with temporary clips because such trauma may result in permanent occlusion. Sylvian veins likewise should be protected at all costs because their injury or sacrifice can have unpredictable and occasionally catastrophic consequences. Injury to the sylvian veins increases the postoperative risk for hemorrhage and edema,56 and certainly all experienced cerebrovascular surgeons can recount patients in whom an otherwise technically sound case had a poor outcome that was likely attributable to a venous injury.
Aneurysm Dissection and Clipping
In patients without SAH, early identification of an M2 or M3 branch is usually fairly straightforward and can provide a useful guide into the fissure and the bifurcation. In patients with an SAH, identification methods are similar but often more challenging (Fig. 370-8). The brain is more friable, the blood obscures the fissure and its contents, and small veins that are normally easily avoided can be injured even during skilled dissections. Plenty of irrigation by the assistant will help speed the clearance of the clot. We find that the morbidity of making a larger fissure opening is less than the potential morbidity of additional retraction or incomplete opening and exposure. When an M2 branch has been identified in the fissure, we work proximally, using the Yasargil inside-out method of fissure dissection described previously.20 In the case of an unruptured aneurysm or a small, laterally pointing ruptured aneurysm of the bifurcation, we often work first distally and then ultimately on the medial surface (undersurface) of the bifurcation to access the M1 segment, gaining proximal control without opening the proximal fissure first. If there is a larger or ruptured aneurysm or uncertain anatomy, fissure dissection should be wide and include proximal control, initially at the opticocarotid cistern, and then working distally until the proximal M1 is identified.
The proximal MCA is prepared for possible temporary occlusion before the aneurysm is dissected. Intraoperative rupture of an aneurysm can dramatically change the course of the surgery and its outcome. In the seminal article regarding this intraoperative misadventure, rupture was more frequent, and outcome was substantially worse with blunt dissection than with unintended ruptures seen with sharp dissection.57 Sharp dissection is performed along the artery, carefully exposing the neck of the aneurysm (Fig. 370-9). A self-retaining retractor may facilitate final dissection and visualization at this point. If used, self-retaining retractors should be used to maintain exposure and protect the brain. Actual retraction should be avoided or minimized. We generally identify a “provisional” proximal and distal neck at this early juncture to allow a clip to be placed if an unintended rupture occurs. Sharp dissection is then carried out, working carefully around the neck of the aneurysm until the anatomy is understood, and the clip may be applied atraumatically. The body of the aneurysm may be retracted forward for final dissection if absolutely necessary, although we find that adjusting the position of the microscope and table is usually adequate. Identification of small vessels that are often hidden from initial dissection is essential before clip placement. There are often small branches adherent to the dome of the aneurysm that impede straightforward clip placement and require additional dissection to prevent their occlusion or avulsion. Clipping of the aneurysm is generally done only when all the branches have been visualized.
Although there is no one particular method to clip all MCA aneurysms, several general tenets apply. When possible, clips should be applied parallel to the branch vessels to avoid possible small remnants and to reestablish normal anatomic flow; in general, the simpler the clip construct, the better (Fig. 370-10). Care should be taken when applying a clip immediately adjacent to a wall or branch point because this may result in narrowing or kinking that may compromise flow. This may cause an aneurysm to tear at the base if calcification or atherosclerosis is present.
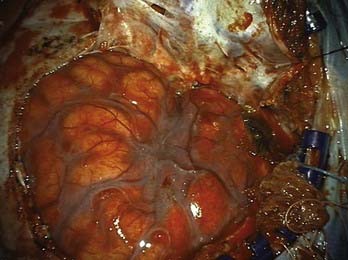
Multilobed aneurysms often require more than one clip and often imaginative clip constructs. Proximal temporary clip occlusion may help in decreasing the turgor of the aneurysm and thus allow successful clip placement with less danger of intraoperative rupture. Large, multilobulated aneurysms often also have calcified or atheromatous walls (Fig. 370-11), making the closure of clips tricky. In these situations, the tandem-clipping technique may be helpful by applying a fenestrated clip through the distal neck and then completing the proximal neck occlusion with a nonfenestrated clip.43,58 Wide-necked or large and giant aneurysms often require that the clip be placed several millimeters above the neck so that when the clip is closed, the arterial wall is reconstructed without limiting flow through the vessel. Occasionally, in large ruptured MCA aneurysms, the dome may be clipped to provide some element of security for final dissection, allowing the surgeon to perform final dissection with some of the mass of the aneurysm obliterated by the temporary clip.
Clipping Adjuncts
Aneurysmorrhaphy
Giant MCA aneurysms present additional difficulties regarding surgical management. The visualization of branches or small lenticulostriate arteries is often poor; elements of the proximal or distal vessels are often incorporated into the base of the aneurysm; and there is usually calcification or atheroma, or both, present. Typically, these large sacs contain intraluminal thrombus that must be removed before clip application. This requires temporary trapping and opening of the sac to evacuate the thrombus. Brain ischemic protection, with modest hypothermia, induced hypertension, and barbiturates is used. Additional protection can be provided by a bypass to the distal MCA. An ultrasonic aspirator is often used to evacuate the intraluminal thrombus more rapidly to reduce temporary occlusion time. Occasionally, atheroma and calcification can limit surgical and endovascular treatment in patients who might be otherwise considered for treatment (Fig. 370-12).
Temporary Occlusion
The literature is varied regarding the utility, technique, and success of temporary occlusion. Obviously, tolerance varies with different sites of temporary occlusion. Samson and Batjer demonstrated that patients tolerated 14 minutes of temporary clip occlusion time with burst suppression, a finding similar to other studies, with a poorer tolerance for longer occlusion times,59,60 although others have found that occlusion times of less than 10 minutes are preferable.61,62 We generally work expeditiously and, if possible, opt to reperfuse after 3 to 5 minutes, even with a controlled but bleeding aneurysm. There is no consensus in the literature regarding specific cerebroprotective agents, and many have been examined. Barbiturates have been associated with myocardial contractile depression and lengthy postoperative neurological recovery periods secondary to the long half-life of barbiturates and therefore are not without risk.59 If needed, we generally use thiopental or propofol at our institution for brain protection during temporary occlusion.
Bypass
When performing the craniotomy for all MCA aneurysms, we preserve at least one branch of the STA in the event an unanticipated STA-MCA bypass is needed. If a low-flow bypass is anticipated preoperatively, the vessel pedicle is dissected out with the opening. High-flow extracranial-intracranial bypasses using a saphenous vein or radial artery graft may need to be considered for fusiform aneurysms involving more than one branch when successful clip ligation is considered unlikely without it. Although the detailed technical nuances are beyond the scope of this chapter, several points deserve mentioning. If a bypass is considered likely, it is important to take the time to prepare the graft before it is necessary because usually by the time it is necessary, it is too late. We occasionally perform a preemptive bypass in anticipation of the need for flow augmentation, whether or not it is ultimately needed. In salvage situations, the anterior temporal artery may be dissected and used as donor vessel if no bypass has been planned and time is of essence.63 Additionally, we have used M2 side-to-side anastomosis when one branch is unexpectedly occluded and cannot be properly revascularized.
Baskaya MK, Coscarella E, Tummala RP, et al. Surgical management of middle cerebral artery aneurysms: surgical anatomy, approaches, and pitfalls. Neurosurg Q. 2005;15:201.
Batjer H, Samson D. Intraoperative aneurysmal rupture: incidence, outcome, and suggestions for surgical management. Neurosurgery. 1986;18:701.
Charbel FT, Ausman JI, Diaz FG, et al. Temporary clipping in aneurysm surgery: technique and results. Surg Neurol. 1991;36:83-90.
Figueiredo EG, Deshmukh P, Nakaji P, et al. The minipterional craniotomy: technical description and anatomic assessment. Neurosurgery. 2007;61:256.
Gibo H, Carver CC, Rhoton ALJr, et al. Microsurgical anatomy of the middle cerebral artery. J Neurosurg. 1981;54:151-169.
Haley EC, Kassell NF, Torner JC. The International Cooperative Study on the Timing of Aneurysm Surgery. The North American experience. Stroke. 1992;23:205-214.
Hellingman CA, van den Bergh WM, Beijer IS, et al. Risk of rebleeding after treatment of acute hydrocephalus in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:96.
Heros RC, Fritsch MJ. Surgical management of middle cerebral artery aneurysms. Neurosurgery. 2001;48:780.
Heros RC, Lee SH. The combined pterional/anterior temporal approach for aneurysms of the upper basilar complex: technical report. Neurosurgery. 1993;33:244.
Horiuchi T, Nakagawa F, Miyatake M, et al. Traumatic middle cerebral artery aneurysm: case report and review of the literature. Neurosurg Rev. 2007;30:263-267.
International Study Group of Unruptured Intracranial Aneurysm Investigators (ISUIA). Unruptured intracranial aneurysms: risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725-1733.
Kashiwagi S, Kato S, Moroi J, et al. Characteristics of the M1 segment aneurysms and consideration for surgical treatment. SCS Surg Cerebral Stroke. 2001;29:192-195.
Kassell NF, Torner JC, Haley ECJr, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg. 1990;73:18-36.
Lupret V, Sajko T, Beroš V, et al. Advantages and disadvantages of the supraorbital keyhole approach to intracranial aneurysms. Acta Clin Croat. 2006;45:91-94.
Miyaoka M, Sato K, Ishii S. A clinical study of the relationship of timing to outcome of surgery for ruptured cerebral aneurysms. A retrospective analysis of 1622 cases. J Neurosurg. 1993;79:373-378.
Molyneux A. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
Osborn AG. Diagnostic Cerebral Angiography. Philadelphia: Lippincott Williams & Wilkins; 1999.
Oyesiku NM, Barrow DL. Infectious, traumatic, and other unusual aneurysms. In: Awad IA, editor. Current Management of Cerebral Aneurysms. Park Ridge, Ill: American Association of Neurological Surgeons; 1993:23-246.
Rinkel GJE, Djibuti M, Algra A, et al. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998;29:251-256.
Rinne J, Hernesniemi J, Niskanen M, et al. Analysis of 561 patients with 690 middle cerebral artery aneurysms: anatomic and clinical features as correlated to management outcome. Neurosurgery. 1996;38:2.
Sundt TMJr. Part I. Basic considerations: basic principles and techniques. In: Surgical Techniques for Saccular and Giant Intracranial Aneurysms. Baltimore: Williams & Wilkins; 1990:39-58.
Tanriover N, Rhoton ALJr, Kawashima M, et al. Microsurgical anatomy of the insula and the sylvian fissure. J Neurosurg. 2004;100:891-922.
Yasargil MG. General operative techniques. In: Microneurosurgery, vol 1. Microsurgical Anatomy of the Basal Cisterns and Vessels of the Brain. Stuttgart: Georg Thieme; 1984. 208-271
1 Atkinson JL, Sundt TMJr, Houser OW, et al. Angiographic frequency of anterior circulation intracranial aneurysms. J Neurosurg. 1989;70:551-555.
2 Heros RC, Fritsch MJ. Surgical management of middle cerebral artery aneurysms. Neurosurgery. 2001;48:780.
3 Kassell NF, Torner JC, Haley ECJr, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg. 1990;73:18-36.
4 Molyneux A. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
5 Raps EC, Rogers JD, Galetta SL, et al. The clinical spectrum of unruptured intracranial aneurysms. Arch Neurol. 1993;50:265-268.
6 Rinne J, Hernesniemi J, Niskanen M, et al. Analysis of 561 patients with 690 middle cerebral artery aneurysms: anatomic and clinical features as correlated to management outcome. Neurosurgery. 1996;38:2.
7 Tanriover N, Rhoton ALJr, Kawashima M, et al. Microsurgical anatomy of the insula and the sylvian fissure. J Neurosurg. 2004;100:891-922.
8 Gibo H, Carver CC, Rhoton ALJr, et al. Microsurgical anatomy of the middle cerebral artery. J Neurosurg. 1981;54:151-169.
9 Osborn AG. Diagnostic Cerebral Angiography. Philadelphia: Lippincott Williams & Wilkins; 1999.
10 Tacconi L, Johnston FG, Symon L. Accessory middle cerebral artery: case report. J Neurosurg. 1995;83:916-918.
11 Kai Y, Hamada J, Morioka M, et al. Treatment of unruptured duplicated middle cerebral artery aneurysm: case report. Surg Neurol. 2006;65:190-193.
12 Takahashi T, Suzuki S, Ohkuma H, et al. Aneurysm at a duplication of the middle cerebral artery. AJNR Am J Neuroradiol. 1994;15:1166-1168.
13 Kazumata K, Kamiyama H, Ishikawa T, et al. Operative anatomy and classification of the sylvian veins for the distal transsylvian approach. Neurol Med Chir. 2003;43:427-434.
14 Flamm ES, Fein JM. Middle cerebral artery aneurysms. Cerebrovasc Surg. 1985;3:861-877.
15 Ojemann RG, Heros RC, Crowell RM. Middle cerebral artery aneurysms. In: Surgical Management of Cerebrovascular Disease. Baltimore: Williams & Wilkins; 1988. 241-252
16 Wirth FP, Laws ERJr, Piepgras D, et al. Surgical treatment of incidental intracranial aneurysms. Neurosurgery. 1983;12:507.
17 Hosoda K, Fujita S, Kawaguchi T, et al. Saccular aneurysms of the proximal (M1) segment of the middle cerebral artery. Neurosurgery. 1995;36:441.
18 Iwama T, Yoshimura S, Kaku Y, et al. Considerations in the surgical treatment of superior-wall type aneurysm at the proximal (M1) segment of the middle cerebral artery. Acta Neurochir. 2004;146:967-972.
19 Kashiwagi S, Kato S, Moroi J, et al. Characteristics of the M1 segment aneurysms and consideration for surgical treatment. SCS Surg Cerebral Stroke. 2001;29:192-195.
20 Yasargil MG. Middle cerebral artery aneurysms. Microneurosurgery. 1984;2:124-164.
21 Ferguson GG. Physical factors in the initiation, growth, and rupture of human intracranial saccular aneurysms. J Neurosurg. 1972;37:666-677.
22 Foutrakis GN, Yonas H, Sclabassi RJ. Saccular aneurysm formation in curved and bifurcating arteries. Am J Neuroradiol. 1999;20:1309-1317.
23 Chun JY, Smith W, Halbach VV, et al. Current multimodality management of infectious intracranial aneurysms. Neurosurgery. 2001;48:1203.
24 Johnson HR, South JR. Traumatic dissecting aneurysm of the middle cerebral artery. Surg Neurol. 1980;14:224-226.
25 Piepgras DG, McGrail KM, Tazelaar HD. Intracranial dissection of the distal middle cerebral artery as an uncommon cause of distal cerebral artery aneurysm: case report. J Neurosurg. 1994;80:909-913.
26 Horten BC, Abbott GF, Porro RS. Fungal aneurysms of intracranial vessels. Arch Neurol. 1976;33:577-579.
27 Schievink WI, Parisi JE, Piepgras DG, et al. Intracranial aneurysms in Marfan’s syndrome: an autopsy study. Neurosurgery. 1997;41:866.
28 Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med. 2004;350:1862-1871.
29 Slovut DP, Olin JW. Fibromuscular dysplasia. Curr Treat Options Cardiovasc Med. 2005;7:159-169.
30 Steiner H, Lammer J, Kleinert R, et al. Dissecting aneurysm of cerebral arteries in congenital vascular deficiency. Neuroradiology. 1986;28:331-334.
31 Mizutani T, Goldberg HI, Parr J, et al. Cerebral dissecting aneurysm and intimal fibroelastic thickening of cerebral arteries: case report. J Neurosurg. 1982;56:571-576.
32 Ojemann RG, Fisher CM, Rich JC. Spontaneous dissecting aneurysm of the internal carotid artery. Stroke. 1972;3:434-440.
33 Kurino M, Yoshioka S, Ushio Y. Spontaneous dissecting aneurysms of anterior and middle cerebral artery associated with brain infarction: a case report and review of the literature. Surg Neurol. 2002;57:428-436.
34 Damasio H, Seabra-Gomes R, da Silva JP, et al. Multiple cerebral aneurysms and cardiac myxoma. Arch Neurol. 1975;32:269-270.
35 Oyesiku NM, Barrow DL. Infectious, traumatic, and other unusual aneurysms. In: Awad IA, editor. Current Management of Cerebral Aneurysms. Park Ridge, Ill: American Association of Neurological Surgeons; 1993:23-246.
36 Sato O, Bascom JF, Logothetis J. Intracranial dissecting aneurysm: case report. J Neurosurg. 1971;35:483-487.
37 Horiuchi T, Nakagawa F, Miyatake M, et al. Traumatic middle cerebral artery aneurysm: case report and review of the literature. Neurosurg Rev. 2007;30:263-267.
38 Ellamushi H, Thorne L, Kitchen N. Unruptured cerebral aneurysms causing seizure disorder (report of two cases). Seizure. 1999;8:310-312.
39 Miele VJ, Bendok BR, Batjer HH. Unruptured aneurysm of the middle cerebral artery presenting with psychomotor seizures: case study and review of the literature. Epilepsy Behav. 2004;5:420-428.
40 Mizobuchi M, Ito N, Tanaka C, et al. Unidirectional olfactory hallucination associated with ipsilateral unruptured intracranial aneurysm. Epilepsia. 1999;40:516-519.
41 Zambrelli E, Cavallini A, Tosi P, et al. A possible case of unruptured middle cerebral artery aneurysm presenting as epileptic seizures. Neurol Sci. 2003;24:141-144.
42 Byyny RL, Mower WR, Shum N, et al. Sensitivity of noncontrast cranial computed tomography for the emergency department diagnosis of subarachnoid hemorrhage. Ann Emerg Med. 2008;51:697-703.
43 Chyatte D, Porterfield R. Nuances of middle cerebral artery aneurysm microsurgery. Neurosurgery. 2001;48:339.
44 Zipfel GJ, Dacey RG. Update on the management of unruptured intracranial aneurysms. Neurosurg Focus. 2004;17:1-10.
45 Haley EC, Kassell NF, Torner JC. The International Cooperative Study on the Timing of Aneurysm Surgery. The North American experience. Stroke. 1992;23:205-214.
46 Miyaoka M, Sato K, Ishii S. A clinical study of the relationship of timing to outcome of surgery for ruptured cerebral aneurysms. A retrospective analysis of 1622 cases. J Neurosurg. 1993;79:373-378.
47 The International Study Group of Unruptured Intracranial Aneurysm Investigators (ISUIA). Unruptured intracranial aneurysms. Risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725-1733.
48 Rinkel GJE, Djibuti M, Algra A, et al. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998;29:251-256.
49 Hellingman CA, van den Bergh WM, Beijer IS, et al. Risk of rebleeding after treatment of acute hydrocephalus in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:96.
50 Ruijs ACJ, Dirven CMF, Algra A, et al. The risk of rebleeding after external lumbar drainage in patients with untreated ruptured cerebral aneurysms. Acta Neurochir. 2005;147:1157-1162.
51 Figueiredo EG, Deshmukh P, Nakaji P, et al. The minipterional craniotomy: technical description and anatomic assessment. Neurosurgery. 2007;61:256.
52 Lupret V, Sajko T, Beroš V, et al. Advantages and disadvantages of the supraorbital keyhole approach to intracranial aneurysms. Acta Clin Croat. 2006:45.
53 Yasargil MG. General operative techniques. In: Microneurosurgery, vol 1. Microsurgical Anatomy of the Basal Cisterns and Vessels of the Brain. Stuttgart: Georg Thieme; 1984. 208-271
54 Heros RC, Lee SH. The combined pterional/anterior temporal approach for aneurysms of the upper basilar complex: technical Report. Neurosurgery. 1993;33:244.
55 Oikawa S, Mizuno M, Muraoka S, et al. Retrograde dissection of the temporalis muscle preventing muscle atrophy for pterional craniotomy. J Neurosurg. 1996;84:297-300.
56 Dean BL, Wallace RC, Zabramski JM, et al. Incidence of superficial sylvian vein compromise and postoperative effects on CT imaging after surgical clipping of middle cerebral artery aneurysms. AJNR Am J Neuroradiol. 2005;26:2019-2026.
57 Batjer H, Samson D. Intraoperative aneurysmal rupture: incidence, outcome, and suggestions for surgical management. Neurosurgery. 1986;18:701.
58 Sundt TMJr. Part I. Basic considerations: basic principles and techniques. In: Surgical Techniques for Saccular and Giant Intracranial Aneurysms. Baltimore: Williams & Wilkins; 1990:39-58.
59 Lavine SD, Masri LS, Levy ML, et al. Temporary occlusion of the middle cerebral artery in intracranial aneurysm surgery: time limitation and advantage of brain protection. Neurosurg Focus. 1997;2:6.
60 Samson D, Batjer HH, Bowman G, et al. A clinical study of the parameters and effects of temporary arterial occlusion in the management of intracranial aneurysms. Neurosurgery. 1994;34:22.
61 Charbel FT, Ausman JI, Diaz FG, et al. Temporary clipping in aneurysm surgery: technique and results. Surg Neurol. 1991;36:83-90.
62 Suzuki J, Yoshimoto T, Kayama T. Surgical treatment of middle cerebral artery aneurysms. No Shinkei Geka. 1984;12:289-296.
63 Baskaya MK, Coscarella E, Tummala RP, et al. Surgical management of middle cerebral artery aneurysms: surgical anatomy, approaches, and pitfalls. Neurosurg Q. 2005;15:201.