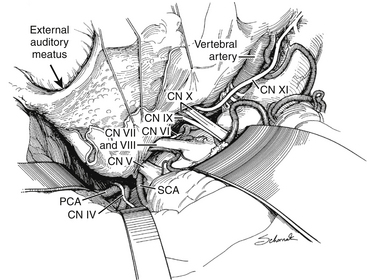
Chapter 77 Surgical Management of Midbasilar and Lower Basilar Aneurysms
Epidemiology
In most neurosurgical series, aneurysms of the midbasilar and lower basilar artery represent less than 1% of cerebral aneurysms. Yamaura1 reported a frequency of 10 midbasilar and lower basilar artery aneurysms in 202 posterior circulation aneurysms (5%), whereas Sano and colleagues2 reported that they accounted for 7 of 1480 (0.5%) total cerebral aneurysms and for 7 of 116 (6%) posterior circulation aneurysms. In their earlier work, Peerless and Drake3 reported that midbasilar and lower basilar artery aneurysms accounted for 193 of all 1266 (15.2%) of their cases of posterior circulation aneurysms. In their more recent publication describing 1767 patients, aneurysms of the basilar trunk and vertebrobasilar junction were seen in 14.7% of patients.4
Recent large multicenter studies may overestimate the frequency of aneurysms in this anatomic location. In the original report from the International Study of Unruptured Intracranial Aneurysms, posterior circulation aneurysms (excluding basilar tip aneurysms) were seen in 6.2% of all patients.5 In a subsequent report from this study, these lesions composed 5.1% of all patients with unruptured aneurysms, 3.9% of which were clipped surgically and 8.9% of which were treated with endovascular coiling.6 Alternatively, the International Subarachnoid Aneurysm Trial probably incorporated a pre-established treatment bias in Europe, since only 2.7% of randomized patients had posterior circulation aneurysms and only one of the 2143 patients (0.05%) analyzed had a basilar trunk aneurysm.7
Principles of Management
Patients presenting with SAH and aneurysms located on the midbasilar and lower basilar artery are managed utilizing the same protocols previously established for other patients with SAH8: cardiorespiratory and basic neurologic supportive care, early ventriculostomy with cerebrospinal fluid (CSF) drainage in patients with Hunt-Hess8 grade IV or V SAH,9 early surgery in suitable cases,9–11 prophylaxis, and, when appropriate, aggressive management of post SAH cerebral vasospasm associated with ischemic deficit10,12,13 and aggressive management of increased intracranial pressure.9,10 Patients with neurologic symptoms related to mass effect of the aneurysm are managed semiurgently. Typically, these patients present with signs of brain stem compression or cranial nerve deficits, and careful consideration of their deficits is required during planning of the treatment approach and of the timing of intervention.
Endovascular management of aneurysms of the middle and lower basilar artery is increasingly reported.14–23 Although evidence of durability of treatment is still lacking, several recent reports have also described enhancements in surgical technique and outcomes associated with these improvements.24–32 Notably, the Pipeline embolization device (Chestnut Medical Technologies, Menlo Park, CA) is a self-expanding, microcatheter-delivered, cylindrical mesh device composed of braided cobalt, chromium, and platinum strands.19 The device, while not yet approved by the U.S. Food and Drug Administration (FDA), has a 30% to 35% metal surface area when fully deployed. Multiple devices can be deployed within each other (telescoped) to create a composite endovascular construct. The degree of metal surface area coverage can be manipulated by varying the technique of device deployment as well as by judiciously choosing the number of devices placed in a particular vascular segment. Evidence of its applicability for large midbasilar and lower basilar artery aneurysms is slowly becoming available—the device can be applied strategically to create an endovascular construct within a parent vessel that is rich with eloquent perforators.19,20 However, the Pipeline device has not yet been approved by the FDA.
The general rules for the anesthetic management of patients undergoing surgical clipping involve the use of preoperative corticosteroids and prophylactic intravenous antibiotics. Intraoperative hypotension is prevented, and intraoperative blood pressure is allowed to run mildly hypertensive, especially during any temporary vessel clipping. During exposure and clipping of the aneurysm, all patients receive intravenous doses of a barbiturate (thiopental) titrated to achieve electroencephalographic (EEG) burst suppression.
Hypothermic Cardiac Standstill
In certain cases, the size of the aneurysm precludes adequate visualization of the parent vessel and perforators. This problem is often most difficult with basilar artery aneurysms. In such situations, additional exposure can be obtained through the use of hypothermic cardiac arrest with barbiturate cerebral protection.33 During cardiac arrest, the aneurysm can be collapsed and the anatomic characteristics defined without the risk of hemorrhage. Since the original report from the Barrow Neurological Institute (BNI) in 1986,33 hypothermic cardiac arrest has been used in many patients with posterior circulation aneurysms.
The successful use of hypothermic cardiac standstill requires an experienced cardiovascular and cerebrovascular team. The success of hypothermic cardiac arrest for clipping complex aneurysms is partially determined by four key variables: depth of hypothermia, duration of circulatory arrest, use of barbiturates, and hemostasis.25 In the original BNI series, the mean brain temperature during standstill was 54°F, and the mean duration of standstill about 22 minutes (range, 3–72 minutes). The absolute maximum safe period of cerebral ischemia is unknown. The duration of cerebral ischemia that can be safely tolerated is significantly increased, however, by the utilization of profound hypothermia and precooling intravenous barbiturates administered to achieve burst suppression of EEG activity.
Anatomic Issues in Surgical Exposure
Previously, aneurysms at this location were treated through a subtemporal-transtentorial approach or a suboccipital approach.2,34–36 Although a transoral transclival or transmaxillary transclival approach has been used to expose the basilar artery by Peerless and Drake3 and others,2,37,38 they are associated with notable technical limitations of exposure and a significant risk of postoperative CSF leakage and meningitis.3 These various techniques can provide access, but they do not meet the requirements of maximal exposure combined with minimal brain retraction. A number of techniques have been devised to maximize lateral bone removal and to provide a relatively short and flat route of access to the front of the brain stem and the basilar artery: the transpetrosal approach,39,40 combined supratentorial-infratentorial approach,41 far-lateral approach,42–44 and far-lateral combined supratentorial-infratentorial approach.39 Each of these techniques, when appropriately matched to the location and size of the aneurysm, can provide excellent access to almost any aneurysm located on the midbasilar and lower basilar artery (Table 77-1).
Table 77-1 Surgical Approaches to Midbasilar and Lower Basilar Artery
| Transpetrosal Approaches |
| Retrolabyrinthine |
| Translabyrinthine |
| Transcochlear |
| Combined Supra- and Infra-Tentorial Approaches |
| Retrolabyrinthine |
| Translabyrinthine |
| Transcochlear |
| Far-Lateral Approach |
| Extreme Lateral Craniocervical Approach |
| Far Lateral: Combined Supra- and Infra-Tentorial (“Combined-Combined”) Approaches |
| Retrolabyrinthine |
| Translabyrinthine |
| Transcochlear |
| Anterior Transclival Approaches |
| Transoral (transpalatal) |
| Transmaxillary |
| Transfacial |
| Other Approaches |
| Extended orbitozygomatic |
| Anterior petrosectomy |
| Subtemporal |
| Unilateral suboccipital |
Transpetrosal Approaches
The anterior brain stem and clival regions can be reached through removal of portions of the petrosal bone with almost no brain or brain stem retraction.45 There are three variations of the temporal (petrous) bone dissection. The retrolabyrinthine technique involves petrous bone resection and preserves hearing. The translabyrinthine technique increases the amount of petrous bone resected and sacrifices hearing. Finally, the transcochlear technique involves maximal petrous bone resection, sacrifices hearing, and requires transposition of the facial nerve.41 Moving through these three variations represents a gradual increase in the amount of petrous bone resected and in the exposure of the brain stem and clivus.
Retrolabyrinthine Approach
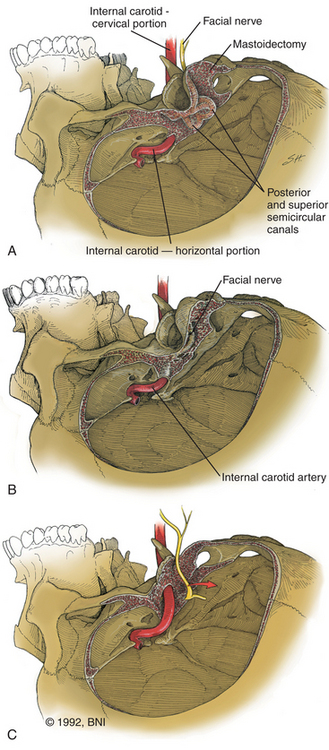
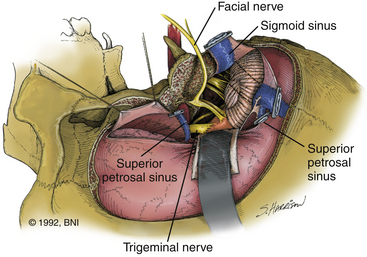
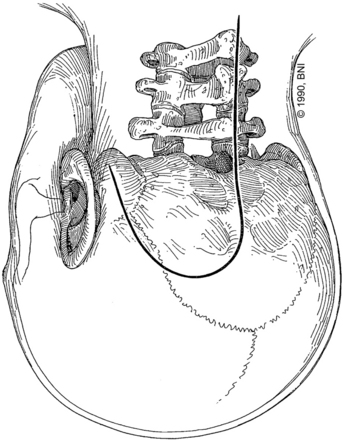
The retrolabyrinthine approach provides excellent access into the cerebellopontine angle but does not allow significant anterior visualization of the brain stem; therefore, it has a limited, isolated role in management of aneurysms of the midbasilar and lower basilar artery. If hearing is to be preserved, a retrolabyrinthine approach is used. The posterior and superior semicircular canals are skeletonized by drilling as far anteriorly as possible, both above and below the otic capsule, to expose as much dura as possible (Fig. 77-1A). The bone overlying the superior petrosal sinus and sigmoid sinus is removed with the drill. The endolymphatic sac and duct are preserved.
Recent studies describe a variation of the retrolabyrinthine approach designed to improve the surgical corridor to the midbasilar region but without necessarily jeopardizing hearing.28,30 Incidental or accidental injuries to the semicircular canals are not universally associated with hearing loss. Consequently, the transmastoid partial labyrinthectomy has been described as a modification of the standard retrolabyrinthine approach. The posterior and superior semicircular canals are occluded and resected, but the vestibule and lateral semicircular canal are preserved. Serviceable hearing can be preserved in 80% of patients using this technique.28
Translabyrinthine Approach
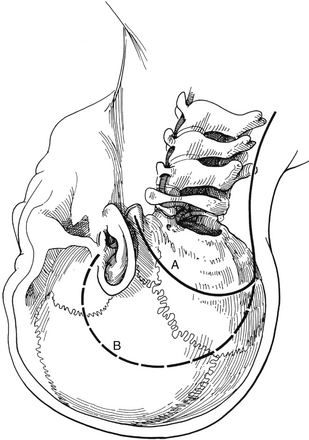
If greater exposure is required, the standard translabyrinthine approach, which sacrifices hearing, can be used. The initial part of this approach is performed as described for the retrolabyrinthine approach but involves the additional complete removal of all three semicircular canals and skeletonization of the posterior half of the internal auditory canal (Fig. 77-1B). More bone is removed from the face of the petrous pyramid than is possible through the retrolabyrinthine technique. Removal of all bone overlying the sigmoid sinus and, if necessary, the jugular bulb provides greater exposure of the inferior aspect of the clivus. The posterior external auditory canal and the bone overlying the mastoid segment of the facial nerve should also be thinned to minimize the obstruction to visualization of the clivus. The distal end of the superior vestibular nerve in the vestibule is used as a reference for identification of the facial nerve as it exits the internal auditory canal. The bone overlying the labyrinthine segment of the facial nerve is also thinned with cautious drilling utilizing a diamond bit and continuous intraoperative monitoring of facial nerve function.
Transcochlear Technique
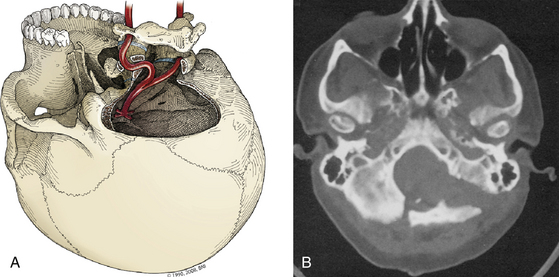
For the greatest exposure of the clivus, the transcochlear technique is used (Fig. 77-1C). The external auditory canal is transected and oversewn in two layers. After the translabyrinthine exposure, the facial nerve is removed from its temporal bone canal,46 the greater superficial petrosal nerve is sectioned, and the facial nerve is transposed posteriorly, utilizing the dura of the internal auditory canal to protect part of the nerve. The entire tympanic portion of the temporal bone is removed with exposure of the periosteum of the temporomandibular joint. The internal auditory canal and cochlea are then removed. The jugular bulb is exposed by removal of the bone that separates it from the internal carotid artery at the skull base. The bone surrounding the carotid artery is removed to the siphon.
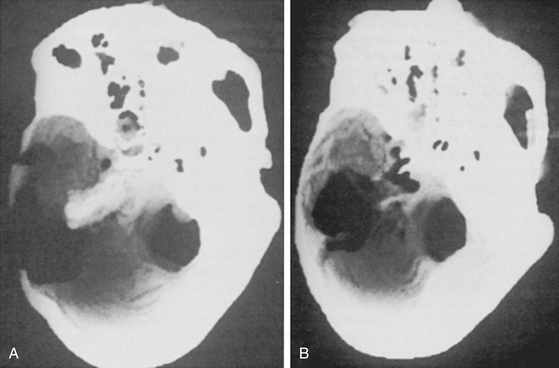
If direct exposure of the internal carotid artery is unnecessary, a thin rim of bone can be left encasing the vessel. The option of extensive internal carotid artery exposure allows creation of a direct saphenous vein bypass graft from the petrous portion of the internal carotid artery to the subarachnoid internal carotid artery if necessary.39,47 Bone is also removed from the floor of the plate of the middle fossa to the horizontal segment of the internal carotid artery. The difference in the amount of resection of the petrous ridge between the retrolabyrinthine and the transcochlear approaches can be appreciated on postoperative computed tomographic (CT) reconstruction scans (Fig. 77-2).
Intradural Exposure and Closure
Regardless of which of the previous extradural bony exposures is used, the dura mater is incised just inferior and parallel to the superior petrosal sinus and just superior to the jugular bulb. These two dural incisions meet at the sinodural angle and the porus acusticus. The dura mater of the internal auditory canal is opened, and the cerebellopontine angle is entered. The surgical procedure then proceeds according to the specific principles of aneurysm surgery as discussed earlier. After the aneurysm has been obliterated, the surgical field is closed in anatomic layers when possible. The temporal and occipital dura is reapproximated with 4-0 braided nylon suture. Abdominal adipose tissue, temporalis muscle, and fibrin glue are used to obliterate the eustachian tube in the translabyrinthine transcochlear approaches and to fill the void created by the temporal bone resection. Temporary lumbar spinal drainage of CSF is used for 3 to 5 days to prevent CSF leakage through the wound.
Selection of Surgical Approach
The salient features of the various approaches can be summarized as follows:
• The retrolabyrinthine approach provides excellent exposure of the cerebellopontine angle but not of the anterior brain stem and preserves function of both hearing and the facial nerves.
• The translabyrinthine approach offers greater exposure of the cerebellopontine angle and significantly improves exposure of the anterolateral and anterior brain stem, but at the expense of hearing and with an increased risk of CSF leakage.
• The transcochlear technique achieves the maximal exposure possible but accomplishes it with not only the disadvantages associated with the translabyrinthine technique but also an increased risk of facial nerve paralysis.
Apart from the morbidity associated with bone removal, a problematic aspect of all the transpetrosal approaches is that the surgical corridor to the aneurysm is necessarily lateral to the lesion by the nature of the bony exposure. This geometry can increase the difficulty of clipping the aneurysm because the surgeon may need to explore around the dome of the aneurysm during dissection in preparation for clipping and also may need to manipulate the aneurysm to visualize the entire neck during clipping. The senior author (RFS) has explored the use of alternate approaches, specifically the extended orbitozygomatic approach for lesions involving the distal two-fifths of the basilar artery or the far-lateral approach for lesions of the proximal two fifths of the basilar artery and vertebrobasilar junction.26 These approaches leave only the truly midbasilar (i.e., middle one fifth of the basilar artery) for transpetrosal approaches. The advantage of these alternate strategies, with or without hypothermic cardiac arrest, is that they allow visualization along the axis of the basilar artery, with good exposure of the neck and perforating vessels, facilitating aneurysm obliteration with a straight clip.
Using this more selected approach led to good scores on the Glasgow Outcome Scale (GOS)48 in 75% of patients (GOS 4 and 5).26 Only 11% had permanent treatment-related neurologic deficits. There were four deaths but only one occurred in the perioperative period.
Combined Supra- and Infra-Tentorial Approaches
Variations of the combined approach have been described since the first report in 1905 by Borchardt. However, it was mostly abandoned, partially as a result of a high mortality rate and improvements in the suboccipital approach offered by Cushing and Dandy. In 1966 Hitselberger and House40 described a combined suboccipital-petrosal approach for the removal of large cerebellopontine angle tumors and discussed how the wide exposure obtained with this technique overcame the individual limitations of the suboccipital and translabyrinthine approaches for dealing with extensive lesions. Their technique extended the translabyrinthine approach beyond the sigmoid sinus into the suboccipital areas, to achieve a wide view of the cerebellopontine angle with minimal brain retraction, and allowed the sigmoid sinus to be mobilized or divided to improve the surgical exposure.
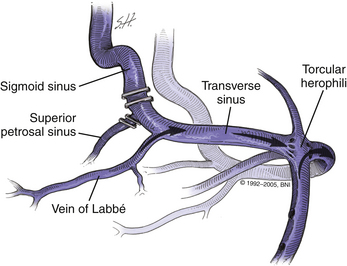
Malis49 popularized the idea of combining the subtemporal and posterior fossa (suboccipital) approaches to improve the surgical exposure when treating extensive lesions of the clivus or medial petrous region. In Malis’s version, the petrosal bone is not extensively drilled, and the superior anastomotic vein (vein of Labbé) is preserved by ligation of the transverse sinus between the entrance of the superior anastomotic vein posteriorly and the sigmoid sinus and superior petrosal sinus anteriorly. After the transverse sinus is divided, the tentorium is split along the petrosal apex with sparing of the superior petrosal sinus. Elevation of the tentorium, transverse sinus, temporal lobe, and superior anastomotic vein allows exposure of the clivus down to the foramen magnum.
Morrison and King50 also described a translabyrinthine transtemporal approach to provide extensive exposure by drilling the petrous bone from the cerebellopontine angle upward into the middle fossa. Exposure is increased by division of the superior petrosal sinus and tentorium. In 1977, Fisch51 described the infratemporal fossa approach for extensive tumors of the temporal bone and base of the skull. Fisch combined a partial posterior and inferior petrosectomy with a cervicofacial approach. This approach provided good exposure of the jugular foramen but frequently required permanent anterior displacement of the facial nerve, resection of the zygomatic arch and mandibular condyle, and complete obliteration of the pneumatic spaces of the temporal bone. House and Hitselberger52 modified the translabyrinthine approach by adding the transcochlear technique. Pellet and colleagues53 combined the infratemporal approach of Fisch51 and the transcochlear approach of House and Hitselberger52 to create the widened transcochlear approach. This approach utilizes a petrosectomy to connect the posterior fossa to the superior carotid region, allowing for the single-stage removal of lesions that extend from the infratemporal region to the posterior fossa. The basic principles for the combined approach had thus been defined: variable amounts of petrous bone dissection with a supratentorial-infratentorial craniotomy and division of the tentorium. An important variable in the combined approach concerns the significance and fate of both the dural sinuses (superior petrosal, sigmoid, or transverse sinus) and the superior anastomotic vein.
A number of authors have also presented their experiences using variations of the combined infratemporal and posterior fossa approach for removal of large lesions involving the clivus and medial petrous region.41,54–57 Some advocate preservation of the major dural sinus,56,57 and others, with appropriate consideration for patency of the opposite transverse sinus, commonly sacrifice the sigmoid41,54 or transverse sinus.49
Surgical Technique
The patient is positioned supine on the operating table with the head positioned parallel to the floor, inclined slightly downward, and fixed to the operating table in a Mayfield headrest. A soft roll is placed under the ipsilateral shoulder to give appropriate support. The skin incision begins at the level of the zygoma, 1 cm anterior to the ear, and continues in a gentle curving fashion around the ear to just below the mastoid tip. To obtain greater exposure, the posterior part of the incision can be extended farther posteriorly. To obtain an exposure that includes the foramen magnum, this combined approach can be combined with the far-lateral suboccipital approach (discussed later).39,42–44 The scalp flap is retracted inferiorly with fishhooks attached to a Leyla bar. This maneuver exposes the lateral aspect of the skull: the zygoma, lateral temporal bone, external auditory meatus, and mastoid region. The neuro-otologist performs the approach through the temporal (petrous) bone, after which the neurosurgeon performs a craniotomy with exposure of the remaining sigmoid sinus and transverse sinus, followed by the intradural part of the procedure.
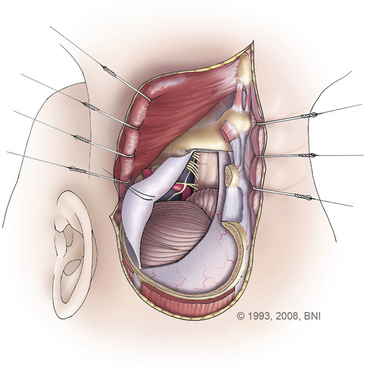
After the neuro-otologist has completed the petrous bone resection, the neurosurgeon proceeds with a subtemporal suboccipital craniotomy that crosses the transverse sinus and exposes the remainder of the sigmoid sinus. This craniotomy exposes a large dural surface. Brain relaxation, if required, is achieved initially with hyperventilation, administration of mannitol or barbiturates, or spinal drainage of CSF. Comprehensive electrophysiologic monitoring is routinely used: compressed spectral EEG, somatosensory-evoked potentials, brain stem auditory responses in both ears, or just the contralateral ear if ipsilateral hearing is to be sacrificed, and functional evaluation of facial and other appropriate cranial nerves. The anterior part of the dural incision (Fig. 77-3) is made over the temporal lobe and extends posteriorly to at least 1 cm below the site where the superior petrosal sinus enters the sigmoid sinus. Uncommonly, a low-lying superior anastomotic vein is found attached to the temporal dura or tentorium, and care must be exercised to prevent damaging this important vascular structure. If the sigmoid sinus is to be preserved, the dural incision crosses the superior petrosal sinus to join with a dural incision in front of the sigmoid sinus. The superior petrosal sinus can usually be cauterized or clipped and subsequently divided. Another incision can be made behind the sigmoid sinus (Fig. 77-3, inset)56 to allow access, if necessary, in front of and behind the sinus.
Sacrifice of Sigmoid Sinus
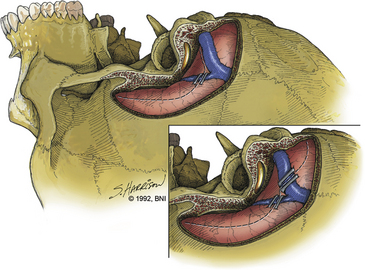
Sacrifice of the sigmoid sinus can be considered if the contralateral transverse sinus and sigmoid sinus are patent (Fig. 77-4) and if these sinuses communicate with the sagittal sinus and ipsilateral transverse sinus through a patent confluence of the sinuses (confluens sinuum). To obtain further assurance that the sigmoid sinus can be sacrificed, we assess intravascular pressure within the sigmoid sinus before and after temporary occlusion of the sinus. Once the superficial petrosal sinus has been divided, a no. 25 needle is inserted into the sigmoid sinus just proximal to the temporary clip location and pressure is recorded. In our experience when the sinuses are patent, intravascular pressure has not increased by more than 7 mm Hg after sigmoid sinus occlusion. Should pressure in the sigmoid sinus increase more than 10 mm Hg with temporary occlusion, the sinus should be kept intact. If the sigmoid sinus is sacrificed, the ipsilateral superior anastomotic vein drains contralaterally, because it consistently and reliably enters the transverse sinus above the junction of the superior petrosal sinus and sigmoid sinus (Fig. 77-4). We now keep the sigmoid sinus intact in most patients.
Intradural Exposure
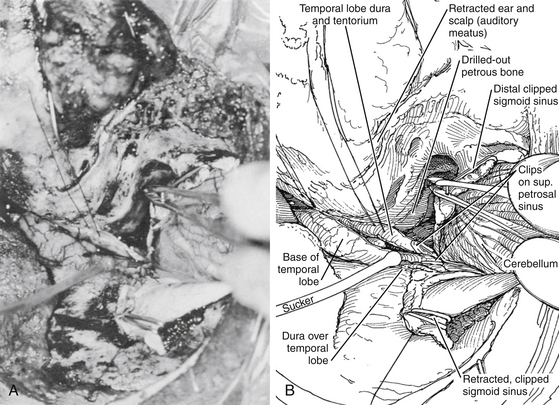
After the dural incisions have been completed, the superior petrosal sinus has been divided, and the fate of the sigmoid sinus has been determined, the dural incision is extended down through the tentorium (posterior to the fourth cranial nerve) to the tentorial hiatus, thus connecting the supra- and infra-tentorial compartments (Fig. 77-5). If the sigmoid sinus has been preserved, the posterior temporal lobe must be elevated, with care taken to protect the superior anastomotic vein, which is indirectly tethered to the skull base by the sigmoid sinus. The superior anastomotic vein can be mobilized by dissection from the cortical surface to minimize tension on the vessel.57 These final maneuvers expose the ipsilateral petrous region, the entire clivus and brain stem, and the cranial nerves and major arterial vessels of the brain stem. With microscopic technique, tumors and vascular lesions can be resected or clipped between any pair of adjacent cranial nerves. These surgical approaches provide maximal angle of exposure along the base of the skull with minimal or no brain retraction (Figs. 77-5 and 77-6).
Closure
When possible, the surgical field is closed in anatomic layers. The temporal and occipital dura is reapproximated with 4-0 braided nylon suture. Abdominal adipose tissue, temporalis muscle, and fibrin glue are used to obliterate the eustachian tube (translabyrinthine) and the void created by temporal bone resection. Temporary lumbar drainage of CSF is used for 3 to 5 days to prevent CSF leakage through the wound. A standard closure of the fascial and skin layers is then undertaken.
Treatment Complications
A detailed analysis of 46 patients reported in 199241 summarizes the operative risks associated with the combined approach. There was no operative mortality in this series, and the postoperative morbidity and mortality data compare favorably with those of previously published reports for this approach. The incidence of facial nerve paralysis in our patient series has remained about 30%. The incidence of CSF leakage is about 13% and that of abducens paralysis is about 7%. The incidence of other operative complications (numbness, aphasia, sepsis, hemiparesis, pneumonia, and hematoma) ranges between 2% and 4% for each.
Far-Lateral Approach
The far-lateral approach to the inferior clivus and upper cervical region is a technical modification that achieves lateral extension to the unilateral suboccipital approach and has been a neurosurgical “standard” for more than a century.42–4458 This modification greatly enhances anterior exposure of this region (the inferior brain stem and clivus, upper cervical spine, and anterior spinal canal) and provides excellent access to the lower basilar artery and vertebrobasilar junction.
Patient Positioning
The patient is placed in a modified park-bench position that is different from the standard park-bench position in two features (Figs. 77-7 and 77-8): the position of the body and dependent arm, and the position of the head. Each of these features is critical to obtaining maximal exposure of the lesion with greatest ease for the surgeon and optimal safety for the patient.
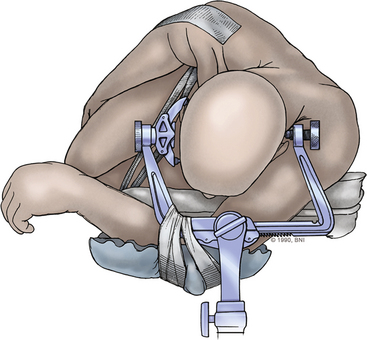
The patient is placed in a Mayfield three-pin headrest (Fig. 77-7) and positioned laterally on the operating table with the side to be treated facing upward. The operating table is extended by placing a 34-inch plastic board under the mattress and pulling the mattress and board 15 to 20 cm beyond the end of the table. The patient’s dependent arm is allowed to drop off the extended end of the operating table and is carefully cradled in foam underneath the edge of the table, within the gap between the Mayfield headrest and the table attachment (Figs. 77-7 and 77-8). A foam roll is placed in the axilla. This placement of the arm improves venous return and decreases the risk of brachial plexus compression. Furthermore, the amount of cranial rotation and flexion that can be achieved is maximized by allowing the dependent arm to drop.
The head is not positioned in the standard straight lateral position. The position of the head is achieved with a sequence of three maneuvers that places the inferior clivus perpendicular to the floor and that maximally opens the posterior cervical-suboccipital angle, thereby allowing greater movement of the operating microscope around the operative field. The head is positioned with the midline parallel to the floor. Then the head and neck are (1) flexed in the anteroposterior plane until the chin is one fingerbreadth from the sternum, (2) rotated 45 degrees downward (to the contralateral side, away from the lesion), and (3) laterally flexed 30 degrees downward toward the opposite shoulder. With the patient in this position, the ipsilateral mastoid process is at the highest point in the operative field. The upper shoulder is pulled down toward the feet and taped, thereby further enlarging the working space for the surgeon (Fig. 77-8). The knees and upper arm are well padded, and the entire body is secured with tape to allow for full rotation of the operating table.
Surgical Technique
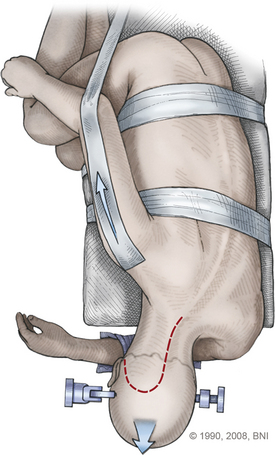
An inverted hockey-stick incision starts at the mastoid prominence and proceeds under the superior nuchal line to the midline. The incision follows the midline to the C3 or C4 spinous process (Fig. 77-9). A 1-cm edge of nuchal fascia and muscle is left at the upper incision to allow anatomic closure of the wound. This nuchal cuff is obtained by dissecting under the muscle with a narrow periosteal elevator, starting from the midline and ending at the mastoid prominence. The fascia and muscle are divided at the inferior edge of the instrument. At closure, the Mayfield headrest is temporarily loosened from the table attachment, and the neck is extended to help reapproximate the cervical musculature to the nuchal fascia. The paraspinal muscles are split along the midline ligament until the spinous processes of C1 and C2 are identified. Subperiosteal dissection is used to expose the occipital bone, spinous processes, and ipsilateral laminae of C1 and C2. The myocutaneous flap is retracted inferiorly and laterally with fishhooks attached to a Leyla bar. The midline part of the incision can be retracted contralaterally with fishhooks from a second Leyla bar.
A limited suboccipital, retrosigmoid craniotomy is performed with the same drill, extending from the contralateral margin of the foramen magnum (just past the midline) to as far lateral as possible and back down again to the foramen magnum, just medial to the level at which the vertebral artery enters the dura. The other dimension of the craniotomy—the height (the distance between the foramen magnum and the superior margin of the bone opening)—can extend to the level of the transverse sinus and is determined primarily by the location and size of the lesion (Fig. 77-10). Lesions confined to the foramen magnum do not require exposure to the transverse sinus. For access to clival lesions that extend beyond the reach of the far-lateral approach, the craniotomy can be expanded with a transpetrosal procedure or beyond the level of the transverse sinus and tentorium when it is combined with the combined approach (see section titled Far-Lateral Combined Supra- and Infra-Tentorial Approach).
The next stage involves removal of bone from the remaining ipsilateral lateral rim of the foramen magnum and occipital condyle. The rim of the foramen magnum is removed with rongeurs, and the posterior occipital condyle and the superior lateral mass and facet of C1 are removed with a high-speed drill. The inner portion of the condyle is drilled until only a thin shell of cortical bone remains. This “inside-out” approach, leaving a protective layer of cortical bone over the surrounding structures, facilitates safe removal in a restricted space. This shell is carefully removed with microcurettes. The extradural vertebral artery is protected with a small dissector while the condyle is drilled away.
Because the hypoglossal canal is situated in the anterior medial third of the occipital condyle, it is not threatened by removal of the posterior lateral third of the condyle.59 This extreme removal of lateral bone from the condyle and lateral mass of C1 is the crucial final step for approaching the anterior brain stem from an inferolateral angle with minimal brain retraction (Fig. 77-10). Even more extensive exposure can be obtained by drilling away the mastoid process and the occipitoatlantal articular facet; however, bony fusion of the craniocervical junction is then needed to maintain postoperative stability.60
The dura is opened in a curvilinear fashion with its base hinged laterally and pulled tight against the lateral aspect of the craniotomy with 4-0 braided nylon sutures. These tenting sutures are placed so that after passage of the needle through the dura, both limbs of the suture are on the inside surface of the dura, thereby aiding in retracting the dura tightly against the underlying bone. The extensive extradural removal of the lateral bone, without retraction, eliminates the last obstruction to direct vision of the inferior clivus, anteroinferior brain stem, and upper anterior cervical spinal cord (Fig. 77-11). With arachnoidal microdissection and minimal elevation of the cerebellar tonsil, visualization is improved as far rostrally as the pontomedullary junction. This approach also allows excellent proximal control of the vertebral artery and its branches.
Salas and colleagues have recently described a variation of the far-lateral approach designed to improve exposure and visualization of aneurysms at the vertebrobasilar junction.21 The transtubercular approach initially involves extradural resection of the posteromedial one third of the occipital condyle and C1 lateral mass. Partial resection of jugular tubercle is also performed extradurally. The remainder of the tubercle is craniectomized intradurally using a drill and working around the lower cranial nerves, with or without prior division of the sigmoid sinus (using similar principles for sinus division as described earlier).
Far-Lateral Combined Supra- and Infra-Tentorial Approach
Occasionally, a more extensive lateral exposure of the clivus is required to deal with giant aneurysms involving the midbasilar and lower basilar artery. Such exposure can be accomplished by combining the far-lateral approach with either a transpetrosal approach or the combined supra- and infra-tentorial approach.39 This “combined-combined” approach offers a wide, flat route to the entire length of the clivus.
The combined-combined approach can be accomplished using the principles previously outlined for the individual procedures. The skin incision for the far-lateral approach is expanded to encompass the exposure needed for the transpetrosal or combined approach, thereby drawing the superior limb of the incision up over the ear, down to the level of the zygoma (Fig. 77-12). The transpetrosal drilling is completed by the neuro-otologist. The neurosurgeon follows with the far-lateral exposure. After the C1 hemilaminectomy has been completed, a craniotomy that starts at the foramen magnum and encompasses the suboccipital and temporal regions can be performed in two sections (Fig. 77-13) or as a single unit. The dural opening for the far-lateral and transpetrosal or combined techniques is completed, and the two are joined if the sigmoid sinus is ligated and divided (Fig. 77-14). The exposure of the anterior brain stem and basilar artery that can be obtained is exquisite. The dural opening and wound are closed as previously described.
Anterior Transclival Approaches
The various transclival approaches are seldom used to treat aneurysms of the midbasilar and lower basilar artery. They are usually the subject of case reports or small institutional series. In general, the risks of CSF leakage and meningitis are higher than with the previously described approaches. There is also risk of palate dysfunction when the transpalatal route is used and when a transmaxillary approach Le Fort osteotomy is used. Ogilvy and colleagues developed the transfacial transclival approach through a lateral rhinotomy incision, an alternative to the other transclival approaches to optimize exposure without maxillotomy and to minimize complications.25 Using this approach, they treated five patients with excellent surgical exposure, good cosmetic results, and no palatal dysfunction. Although three developed CSF leaks, none had any long-term sequelae of meningitis.
Baldwin H.Z., Miller C.G., van Loveren H.R., et al. The far lateral/combined supra- and infratentorial approach. A human cadaveric prosection model for routes of access to the petroclival region and ventral brain stem. J Neurosurg. 1994;81(1):60-68.
Drake C.G., Peerless S.J., Hernesniemi J.A., et al. Surgery of Vertebrobasilar Aneurysms: London, Ontario Experience on 1767 Patients. Vienna: Springer-Verlag; 1996.
Fiorella D., Kelly M.E., Albuquerque F.C., Nelson P.K. Curative reconstruction of a giant midbasilar trunk aneurysm with the pipeline embolization device. Neurosurgery. 2009;64(2):212-217.
Heros R.C. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg. 1986;64(4):559-562.
Higa T., Ujiie H., Kato K., et al. Basilar artery trunk saccular aneurysms: morphological characteristics and management. Neurosurg Rev. 2009;32(2):181-191.
Hitselberger W.E., House W.F. A combined approach to the cerebellopontine angle: a suboccipital-petrosal approach. Arch Otolaryngol. 1966;84:49-67.
House W.F. Translabyrinthine approach. In: House W.F., Luetje C.M. Acoustic Tumors II: Management. Baltimore: University Park Press; 1979:43-87.
House W.F., Hitselberger W.E. The transcochlear approach to the skull base. Arch Otolaryngol. 1976;102(6):334-342.
Hunt W.E., Hess R.M. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28(1):14-20.
Lanzino G., Wakhloo A.K., Fessler R.D., et al. Efficacy and current limitations of intravascular stents for intracranial internal carotid, vertebral, and basilar artery aneurysms. J Neurosurg. 1999;91(4):538-546.
Molyneux A., Kerr R., Stratton I., et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267-1274.
de Oliveira E., Rhoton A.L.Jr., Peace D., et al. Microsurgical anatomy of the region of the foramen magnum. Surg Neurol. 1985;24(3):293-352.
Peerless S.J., Drake C.G. Surgical techniques of posterior circulation aneurysms. In: Schmidek H.H.S., Sweet W.H. Operative Neurosurgical Techniques. 2nd ed. Philadelphia: WB Saunders; 1988:973-989.
Sanai N., Tarapore P., Lee A.C., et al. The current role of microsurgery for posterior circulation aneurysms: a selective approach in the endovascular era. Neurosurgery. 2008;62(6):1236-1249.
Seifert V., Raabe A., Zimmermann M. Conservative (labyrinth-preserving) transpetrosal approach to the clivus and petroclival region—indications, complications, results and lessons learned. Acta Neurochir (Wien). 2003;145(8):631-642.
Seifert V., Stolke D. Posterior transpetrosal approach to aneurysms of the basilar trunk and vertebrobasilar junction. J Neurosurg. 1996;85(3):373-379.
Sen C.N., Sekhar L.N. An extreme lateral approach to intradural lesions of the cervical spine and foramen magnum. Neurosurgery. 1990;27(2):197-204.
Sen C.N., Sekhar L.N. Surgical management of anteriorly placed lesions at the craniocervical junction—an alternative approach. Acta Neurochir (Wien). 1991;108(1-2):70-77.
Spetzler R.F., Daspit C.P., Pappas C.T. The combined supra- and infratentorial approach for lesions of the petrous and clival regions: experience with 46 cases. J Neurosurg. 1992;76(4):588-599.
Spetzler R.F., Graham T.F. The far lateral approach to the inferior clivus and the upper cervical region: technical note. BNI Q. 1990;6:35-38.
Uda K., Murayama Y., Gobin Y.P., et al. Endovascular treatment of basilar artery trunk aneurysms with Guglielmi detachable coils: clinical experience with 41 aneurysms in 39 patients. J Neurosurg. 2001;95(4):624-632.
Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med. 1998;339(24):1725-1733. 10
Van Rooij W.J., Sluzewski M., Menovsky T., Wijnalda D. Coiling of saccular basilar trunk aneurysms. Neuroradiology. 2003;45(1):19-21.
Wiebers D.O., Whisnant J.P., Huston J.III, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103-110.
Yamaura A. Surgical management of posterior circulation aneurysms: Part I. Contemp Neurosurg. 1985;7:1-6.
1. Yamaura A. Surgical management of posterior circulation aneurysms: Part I. Contemp Neurosurg. 1985;7:1-6.
2. Sano K., Asano T., Tamura A. Surgical technique. In: Sano K., Asano T., Tamura A. Acute Aneurysm Surgery: Pathophysiology and Management. New York: Springer-Verlag; 1987:194-246.
3. Peerless S.J., Drake C.G. Surgical techniques of posterior circulation aneurysms. In: Schmidek H.H.S., Sweet W.H. Operative Neurosurgical Techniques. 2nd ed. Philadelphia: W.B. Saunders; 1988:973-989.
4. Drake C.G., Peerless S.J., Hernesniemi J.A. Surgery of Vertebrobasilar Aneurysms: London, Ontario Experience on 1767 Patients. Vienna: Springer-Verlag; 1996.
5. Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med. 1998;339(24):1725-1733. 10
6. Wiebers D.O., Whisnant J.P., Huston J.III, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103-110. 12
7. Molyneux A., Kerr R., Stratton I., et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267-1274. 26
8. Hunt W.E., Hess R.M. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28(1):14-20.
9. Bailes J.E., Spetzler R.F., Hadley M.N., Baldwin H.Z. Management morbidity and mortality of poor-grade aneurysm patients. J Neurosurg. 1990;72(4):559-566.
10. Awad I.A., Carter L.P., Spetzler R.F., et al. Clinical vasospasm after subarachnoid hemorrhage: response to hypervolemic hemodilution and arterial hypertension. Stroke. 1987;18(2):365-372.
11. Higa T., Ujiie H., Kato K., et al. Basilar artery trunk saccular aneurysms: morphological characteristics and management. Neurosurg Rev. 2009;32(2):181-191.
12. Kassell N.F., Peerless S.J., Durward Q.J., et al. Treatment of ischemic deficits from vasospasm with intravascular volume expansion and induced arterial hypertension. Neurosurgery. 1982;11(3):337-343.
13. Findlay J.M., Macdonald R.L., Weir B.K. Current concepts of pathophysiology and management of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Cerebrovasc Brain Metab Rev. 1991;3(4):336-361.
14. Lanzino G., Wakhloo A.K., Fessler R.D., et al. Efficacy and current limitations of intravascular stents for intracranial internal carotid, vertebral, and basilar artery aneurysms. J Neurosurg. 1999;91(4):538-546.
15. Uda K., Murayama Y., Gobin Y.P., et al. Endovascular treatment of basilar artery trunk aneurysms with Guglielmi detachable coils: clinical experience with 41 aneurysms in 39 patients. J Neurosurg. 2001;95(4):624-632.
16. Van Rooij W.J., Sluzewski M., Menovsky T., Wijnalda D. Coiling of saccular basilar trunk aneurysms. Neuroradiology. 2003;45(1):19-21.
17. Islak C., Kocer N., Kantarci F., et al. Endovascular management of basilar artery aneurysms associated with fenestrations. AJNR Am J Neuroradiol. 2002;23(6):958-964.
18. Yoon S.M., Chun Y.I., Kwon Y., Kwun B.D. Vertebrobasilar junction aneurysms associated with fenestration: experience of five cases treated with Guglielmi detachable coils. Surg Neurol. 2004;61(3):248-254.
19. Fiorella D., Kelly M.E., Albuquerque F.C., Nelson P.K. Curative reconstruction of a giant midbasilar trunk aneurysm with the pipeline embolization device. Neurosurgery. 2009;64(2):212-217.
20. Juszkat R., Nowak S., Wieloch M., Zarzecka A. Complete obliteration of a basilar artery aneurysm after insertion of a self-expandable Leo stent into the basilar artery without coil embolization. Korean J Radiol. 2008;9(4):371-374.
21. Hassan T., Ezura M., Takahashi A. Treatment of giant fusiform aneurysms of the basilar trunk with intra-aneurysmal and basilar artery coil embolization. Surg Neurol. 2004;62(5):455-462.
22. Kang H.S., Oh C.W., Han M.H., et al. Treatment of a sequential giant fusiform aneurysm of the basilar trunk. Korean J Radiol. 2005;6(2):125-129.
23. Kis B., Weber W., Berlit P., Kuhne D. Elective treatment of saccular and broad-necked intracranial aneurysms using a closed-cell nitinol stent (Leo). Neurosurgery. 2006;58(3):443-450.
24. Seifert V., Stolke D. Posterior transpetrosal approach to aneurysms of the basilar trunk and vertebrobasilar junction. J Neurosurg. 1996;85(3):373-379.
25. Ogilvy C.S., Barker F.G., Joseph M.P., et al. Transfacial transclival approach for midline posterior circulation aneurysms. Neurosurgery. 1996;39(4):736-741.
26. Lawton M.T., Daspit C.P., Spetzler R.F. Technical aspects and recent trends in the management of large and giant midbasilar artery aneurysms. Neurosurgery. 1997;41(3):513-520.
27. Salas E., Sekhar L.N., Ziyal I.M., et al. Variations of the extreme-lateral craniocervical approach: anatomical study and clinical analysis of 69 patients. J Neurosurg. 1999;90(suppl 2):206-219.
28. Sekhar L.N., Schessel D.A., Bucur S.D., et al. Partial labyrinthectomy petrous apicectomy approach to neoplastic and vascular lesions of the petroclival area. Neurosurgery. 1999;44(3):537-550.
29. Seifert V., Raabe A., Stolke D. Management-related morbidity and mortality in unselected aneurysms of the basilar trunk and vertebrobasilar junction. Acta Neurochir (Wien). 2001;143(4):343-348.
30. Shehab Z.P., Walsh R.M., Thorp M.A., et al. Partial labyrinthectomy approach for brainstem vascular lesions. J Otolaryngol. 2001;30(4):224-230.
31. Horie N., Kitagawa N., Morikawa M., et al. Giant thrombosed fusiform aneurysm at the basilar trunk successfully treated with endovascular coil occlusion following bypass surgery: a case report and review of the literature. Neurol Res. 2007;29(8):842-846.
32. Sanai N., Tarapore P., Lee A.C., Lawton M.T. The current role of microsurgery for posterior circulation aneurysms: a selective approach in the endovascular era. Neurosurgery. 2008;62(6):1236-1249.
33. Spetzler R.F., Hadley M.N., Rigamonti D., et al. Aneurysms of the basilar artery treated with circulatory arrest, hypothermia, and barbiturate cerebral protection. J Neurosurg. 1988;68(6):868-879.
34. Sugita K., Kobayashi S., Takemae T., et al. Aneurysms of the basilar artery trunk. J Neurosurg. 1987;66(4):500-505.
35. Sugita K., Kobayashi S., Shintani A., Mutsuga N. Microneurosurgery for aneurysms of the basilar artery. J Neurosurg. 1979;51(5):615-620.
36. Wascher T.M., Spetzler R.F. Surgical approaches to lesions involving the brain stem. BNI Q. 1992;8(4):18-28.
37. Archer D.J., Young S., Uttley D. Basilar aneurysms: a new transclival approach via maxillotomy. J Neurosurg. 1987;67(1):54-58.
38. de los Reyes R.A., Kantrowitz A.B., Detwiler P.W., et al. Transoral-transclival clipping of a giant lower basilar artery aneurysm. Surg Neurol. 1992;38(5):379-382.
39. Baldwin H.Z., Miller C.G., van Loveren H.R., et al. The far lateral/combined supra- and infratentorial approach. A human cadaveric prosection model for routes of access to the petroclival region and ventral brain stem. J Neurosurg. 1994;81(1):60-68.
40. Hitselberger W.E., House W.F. A combined approach to the cerebellopontine angle: a suboccipital-petrosal approach. Arch Otolaryngol. 1966;84:49-67.
41. Spetzler R.F., Daspit C.P., Pappas C.T. The combined supra- and infratentorial approach for lesions of the petrous and clival regions: experience with 46 cases. J Neurosurg. 1992;76(4):588-599.
42. Spetzler R.F., Graham T.F. The far lateral approach to the inferior clivus and the upper cervical region: technical note. BNI Q. 1990;6:35-38.
43. Heros R.C. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg. 1986;64(4):559-562.
44. Sen C.N., Sekhar L.N. An extreme lateral approach to intradural lesions of the cervical spine and foramen magnum. Neurosurgery. 1990;27(2):197-204.
45. Seifert V., Raabe A., Zimmermann M. Conservative (labyrinth-preserving) transpetrosal approach to the clivus and petroclival region—indications, complications, results and lessons learned. Acta Neurochir (Wien). 2003;145(8):631-642.
46. House W.F. Translabyrinthine approach. In: House W.F., Luetje C.M. Acoustic Tumors II: Management. Baltimore: University Park Press; 1979:43-87.
47. Spetzler R.F., Fukushima T., Martin N., Zabramski J.M. Petrous carotid-to-intradural carotid saphenous vein graft for intracavernous giant aneurysm, tumor, and occlusive cerebrovascular disease. J Neurosurg. 1990;73(4):496-501.
48. Jennett B., Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480-484.
49. Malis L.I. Surgical resection of tumors of the skull base. In: Wilkins R.H., Rengachary S.S. Neurosurgery. New York: McGraw-Hill; 1985:1011-1021.
50. Morrison A.W., King T.T. Experiences with a translabyrinthine-transtentorial approach to the cerebellopontine angle. Technical note. J Neurosurg. 1973;38(3):382-390.
51. Fisch U.. Infratemporal fossa approach for extensive tumors of the temporal bone and base of the skull, Silverstein H., Norell H., editors, Neurological Surgery of the Ear, Birmingham, AL, Aesculapius, 1977;vol. 1:34-53.
52. House W.F., Hitselberger W.E. The transcochlear approach to the skull base. Arch Otolaryngol. 1976;102(6):334-342.
53. Pellet W., Cannoni M., Pech A. The widened transcochlear approach to jugular foramen tumors. J Neurosurg. 1988;69(6):887-894.
54. Al-Mefty O., Fox J.L., Rifai A., Smith R.R. A combined infratemporal and posterior fossa approach for the removal of giant glomus tumors and chondrosarcomas. Surg Neurol. 1987;28(6):423-431.
55. Berg M.R., Symon L. Meningiomas of the clivus and apical petrous bone. Report of 35 cases. J Neurosurg. 1986;65(2):160-167.
56. Samii M., Ammirati M., Mahran A., et al. Surgery of petroclival meningiomas: report of 24 cases. Neurosurgery. 1989;24(1):12-17.
57. Al-Mefty O., Fox J.L., Smith R.R., et al. Petrosal approach for petroclival meningiomas. Neurosurgery. 1988;22(3):510-517.
58. Hammon W.M., Kempe L.G. The posterior fossa approach to aneurysms of the vertebral and basilar arteries. J Neurosurg. 1972;37(3):339-347.
59. de Oliveira E., Rhoton A.L.Jr., Peace D. Microsurgical anatomy of the region of the foramen magnum. Surg Neurol. 1985;24(3):293-352.
60. Sen C.N., Sekhar L.N. Surgical management of anteriorly placed lesions at the craniocervical junction—an alternative approach. Acta Neurochir (Wien). 1991;108(1-2):70-77.