Chapter 15 Surgical Management of Cerebral Metastases
Magnitude of the Problem
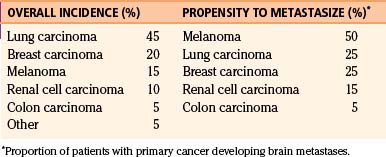
Cerebral metastases are the most common brain tumors in adults.1 Approximately 20% to 40% of patients with cancer develop brain metastases during the course of their illness.2–5 It has been estimated that of more than 560,000 patients in the United States dying each year of cancer, approximately 19%, or more than 100,000 patients, will have brain metastases.6–8 Most brain metastases arise from lung, breast, and renal cell tumors; however, melanoma, followed by lung, breast, and renal cell carcinoma, has the greatest propensity to develop brain metastases (Table 15-1). Characteristically, breast and renal cell carcinomas tend to present as a single metastasis within the brain, while melanoma and lung cancers have an increased incidence of multiplicity.3,9,10 In addition, the interval between the diagnosis of the primary cancer and the brain metastasis depends on the histology of the primary cancer, with breast cancer generally exhibiting the longest interval (mean, 3 years) and lung cancer the shortest (mean, 4 to 10 months).11 The highest incidence of brain metastases is seen in the fifth to seventh decades of life and is equally common among males and females. However, lung carcinoma is the source of most brain metastases in males, and breast carcinomas the most common source of metastases in females. Males with melanoma are more likely to develop brain metastases than are females.10
Treatment Goals: Advantages of Surgical Resection
First, surgery is the only treatment modality that can provide a histologic diagnosis. Although progress in imaging techniques such as magnetic resonance (MR) spectroscopy may allow for precise determination of tumor pathology in the future, surgery remains the only established method for achieving this goal at present. The importance of tissue diagnosis is paramount when the diagnosis of brain metastasis is in question. This occurs most commonly in patients without a diagnosis of primary cancer, or rarely in patients with two known primary tumors. Nevertheless, even for patients with a single known systemic cancer, failure to obtain histologic confirmation may still lead to erroneous diagnosis in 5% to 11% of the cases.12,13 Therefore, it is important not to omit tissue sampling when clinical features raise suspicion of other disease processes such as cerebral abscess or primary lymphoma, whose imaging findings may be indistinguishable from metastatic tumors.
Second, compared with other modalities, surgery is most effective in immediately relieving symptoms caused by the mass effect of the lesion. Although corticosteroids reduce the effects of vasogenic edema, they do not alter the pressure exerted from the lesion itself, and their side effects preclude long-term use. Radiation treatment, including SRS, may reduce the tumor mass, but the effect is delayed.
Patient Selection
Number of Lesions
A primary consideration in deciding to operate is the number of lesions. MR imaging is more sensitive than computed tomography (CT) for the detection of small metastases or those within the posterior fossa14–16 and is thus recommended for definitively establishing the number of intracranial metastases. The term “single” cerebral metastasis is used to describe one metastasis to the brain in the face of other systemic metastases, whereas “solitary” cerebral metastasis indicates that the brain is the only site of metastatic disease within the body.17 Although single cerebral metastases constitute approximately 30% of all cases of patients with brain metastases, solitary metastases are rare.18 To determine management options, patients should be divided into two broad categories: patients with single/solitary metastases or with multiple brain metastases.
Single and Solitary Brain Metastasis
Patients with single/solitary brain metastases are the best candidates for surgery. It has been demonstrated by class I evidence that surgical resection of single or solitary brain metastases is superior to treatment with WBRT alone. The evidence is derived from three randomized controlled trials reported in the 1990s comparing surgical resection plus WBRT with WBRT alone, of which two showed a significant reduction of recurrence and extension of survival with surgical treatment.12,19,20
Patchell and colleagues reported the first study comparing surgical resection plus WBRT with WBRT alone.12 The authors included patients (n = 47) with single brain metastases, good performance status (Karnofsky Performance Scale [KPS] score ≥ 70), and limited extent of disease. They found that the rate of local recurrence was significantly (p < 0.02) lower in the surgical group (20%) compared with the WBRT group (52%). Likewise, the overall length of survival was significantly longer (p < 0.01) following surgical resection plus WBRT (median, 40 weeks) compared with WBRT alone (median, 15 weeks). Importantly, the improved survival was accompanied by maintenance of functional independence (38 weeks in the surgical group vs. 8 weeks in the WBRT group, p < 0.005). A multivariate analysis further indicated that surgical resection (p < 0.0001) and the absence of disseminated disease (p < 0.0004) were predictors of better outcome. These results provided, for the first time, class I evidence in support of surgical resection plus WBRT in lieu of WBRT alone as the gold standard for treatment of single/solitary brain metastases.
In a second prospective randomized study, Vecht and colleagues also compared surgery plus WBRT with WBRT alone in patients with single brain metastases. Like Patchell et al., they included only patients with good performance status and reported a significantly longer median survival time after surgery plus WBRT (43 weeks) compared with WBRT alone (26 weeks, p = 0.04).20 A major difference from the trial of Patchell and colleagues, however, was that the investigators stratified the patients by site (lung cancer vs. non-lung cancer) and by status of extracranial disease (progressive vs. stable). Importantly, they found that the benefits of surgery were most evident in patients with limited systemic disease. Specifically, patients with stable extracranial disease had a more prolonged survival when treated with surgical resection and WBRT (median, 12 months) than when treated with WBRT alone (median, 7 months, p = 0.04).In contrast, patients with progressive extracranial disease generally fared worse and the survival was independent of whether or not surgical resection was performed (median survival time of 5 months in both combined treatment and WBRT alone groups). The tumor type, lung versus non-lung histology, was not a strong predictor of survival.
In a third study, Mintz et al. reported a multicenter prospective trial that randomized 84 patients to either surgery plus WBRT or WBRT alone.19 In contrast to the previous two trials, there was no difference in the median survival time of the surgery plus WBRT group (24 weeks) compared with the WBRT alone group (27 weeks, p = 0.24). Likewise, the duration of time that patients maintained a KPS score ≥ 70 was not different between the two groups. However, the data did support previous findings that extracranial metastases were an important predictor of mortality. One key difference between the study of Mintz et al. and the other two randomized trials was that Mintz et al. included patients with lower performance status (inclusion criterion was KPS score ≥ 50, compared with > 70 in the other studies). Consequently, 21% of their study population had a KPS score < 70, and 45% of patients suffered from extracranial metastases. In contrast, patients with active extracranial disease comprised only 37% of patients in the study of Patchell et al. and 32% of patients in the study of Vecht et al. Because low KPS scores and active extracranial disease are associated with poor survival, the differences between these trials suggest that the benefits of surgery may diminish in patients with more advanced disease as the systemic tumor burden predominates in the clinical course. Such differences also highlight the influence of study populations in altering the overall outcome of clinical trials.
On the basis of these three randomized controlled trials, a Cochrane meta-analysis concluded that for patients with good performance status (KPS score ≥ 70) and controlled systemic disease, surgical resection plus WBRT provides the best outcome for patients with single brain metastases.21 This same conclusion was also reached in recently published guidelines.22 The collective data suggest that the benefits of surgery extend not only to prolongation of overall survival but also to maintenance of functional independence and local disease control, by reducing deaths and disabilities from neurologic causes. For patients with lower performance status (KPS score < 70), the evidence is less clear, as the burden of the extracranial disease is likely to outweigh the influence of the cerebral pathology. However, when considering the implications of these data in clinical practice, it is important to note that the benefits of surgical resection are not limited to the outcome measures examined in these clinical trials, and the role of surgery in reversing neurologic symptoms and deficits by immediate decompression of local mass effects and prevention of death from brain herniation cannot be overemphasized. For example, a drowsy patient harboring a large posterior fossa single metastasis may be unjustly denied a life-saving operation should the decision to operate be based solely on performance status. Therefore, recommendation for surgery requires not only justification from sound literature-based evidence but also the exercise of good clinical judgment, with an ultimate goal of maximizing the clinical outcome of each individual patient.
Multiple Brain Metastases
The traditional treatment of multiple brain metastases is with WBRT, and the presence of multiple metastases has been considered in the past a contraindication to surgery, even when the tumors are surgically accessible.23–26 However, an increasing volume of literature in recent years has suggested that surgery may have a role in the treatment of multiple metastases for a defined patient population. In a retrospective analysis, Bindal et al. reported the outcome of 56 patients who underwent resection for multiple brain metastases. Patients were divided into those who had one or more lesions left unresected (group A, n = 30), and those who had undergone resection of all lesions (group B, n = 26).27 These patients were compared with a group of matched controls who had single metastases that were surgically resected (group C, n = 26). There was no difference in surgical mortality (3%, 4%, and 0% for groups A, B, and C, respectively) or morbidity (8%, 9%, and 8% for groups A, B, and C, respectively) regardless of treatment group. Most importantly, patients with multiple metastases who had all the lesions resected (group B) had a significantly longer survival (median, 14 months) than patients who had some lesions left unresected (group A; median, 6 months; p = 0.003). The survival time of patients who had all lesions removed (group B) was similar to that of patients with resected single metastases (group C; median, 14 months). It was concluded that removal of multiple metastatic lesions is as effective as resection of single metastases, with the important caveat that all lesions had to be removed.27
In support of the above findings, Iwadate and colleagues reported a median survival time of 9.2 months following resection of multiple brain metastases in 61 patients; this was similar to the survival time of 8.7 months following resection of a single brain metastasis in 77 contemporary patients.28 Predictors of shorter survival were age greater than 60 years, KPS score < 70, incomplete surgical resection, and the presence of extensive systemic cancer. Similarly, in a recent single surgeon retrospective series of 208 patients, resection of one or more symptomatic tumors in 76 patients harboring multiple brain metastases achieved a median survival time of 11 months.29 This outcome compared favorably with the median survival time of 8 months in the 132 patients with surgically resected single metastases.29
Location
Resectability (i.e., whether a tumor can be removed with minimal morbidity) is dictated primarily by tumor location. With modern microneurosurgical techniques there are very few, if any, regions within the brain that are inaccessible to the neurosurgeon. However, accessibility and resectability are not the same. The most important features that determine resectability are whether the tumor is deep or superficial and whether the tumor is within or near “eloquent” brain. Stereotactic image-guided surgical techniques and skull base exposures have made previously unreachable tumors resectable. A variety of techniques help to preserve functionally important brain regions during resection. Nevertheless, lesions that are deeply located and within “eloquent areas” are inevitably associated with slightly higher surgical morbidity than those within noneloquent and superficial areas. In this context, Sawaya and colleagues studied 400 consecutive patients undergoing craniotomies for brain tumor resection.30 They found that major neurologic complications occurred in 13% of patients undergoing resection of tumors from “eloquent” brain regions, whereas the incidence was 5% and 3%, respectively, for patients undergoing resection of tumors located within “near-eloquent” and “noneloquent” brain regions. The potential morbidity (hence, recovery time) associated with surgical removal must therefore be weighed against the limited survival expectancy of this patient population. Patients with metastases to the brain stem, thalamus, and basal ganglia are generally not considered surgical candidates, except in rare circumstances. Treatment of lesions in these locations with noninvasive modality such as SRS may be warranted. However, it must be noted that significant morbidity could also develop with SRS when treating lesions near the eloquent brain or cranial nerves, and no study to date has convincingly showed that the morbidity of surgery is more than that of SRS in these circumstances.
Lesion Size
The size of the lesion is another factor that must be considered when choosing therapy. For lesions that are greater than 3 cm in maximum diameter, surgical resection is the primary option because surgery rapidly relieves the mass effect that commonly occurs with these larger, often symptomatic lesions. In contrast, SRS is generally not applicable for tumors >3 cm in diameter because SRS typically results in an unacceptably high radiation dose to the surrounding normal brain due to the limited degree of conformity that can be achieved for large volume tumors.31,32 However, for lesions <1 cm in maximum diameter, radiosurgery is often the ideal treatment because most of these tumors are asymptomatic, and localizing small lesions at surgery, even with MR imaging guidance, may be difficult, especially when deep in the brain.
Clinical Assessment
The status of the patient’s systemic disease (i.e., the extent of the primary tumor and of noncerebral metastases) is a critical consideration in the decision to resect a brain metastasis because advanced systemic disease is a major predictor of short-term survival, whereas limited systemic disease is associated with long survival in patients undergoing surgery for cerebral metastases (see previous).12,20,27,33–35 Indeed, after resection of a single brain metastasis, up to 70% of patients will succumb to their systemic disease and not to their brain disease.12 In this context, most patients with absent systemic disease are surgical candidates, whereas most patients with widely disseminated cancer are not. Decision making in patients with significant systemic cancer burden that is responding to therapy poses a challenge. One practical approach is to determine the expected survival time for the patient, excluding the presence of cerebral metastases. At many centers, patients who are expected to survive for more than 3-4 months are usually candidates for surgical resection.
In addition, the preoperative neurologic status should be considered, because patients with marked neurologic deficits have been shown to have a shorter median survival time than patients who are neurologically intact.35,36 However, as alluded to previously, it is important not to exclude patients from surgery on this basis alone, because there are many patients whose neurologic deficits improve following resection of the offending tumor. One way to determine the potential for recovery is to assess the response of the deficit to corticosteroid administration. Patients whose neurologic deficits are likely to improve after resection usually demonstrate an improvement after treatment with corticosteroids, whereas patients who will not improve postoperatively do not have such a response to corticosteroids. In general, a surgical patient should have an expected survival of at least 3 months, be able to withstand anesthesia, and have a KPS score ≥ 70 (Table 15-2). Patients who have major cardiac, pulmonary, renal, or hematologic diseases may be better suited for nonsurgical treatment.
Table 15-2 Considerations in Patient Selection for Surgical Removal of Brain Metastases
| Factor | Requirement for Surgery |
|---|---|
| Status of Systemic Disease | |
| Control of primary cancer | Expected survival >3 months |
| General medical condition | Able to withstand surgery/anesthesia |
| Neurologic status | KPS score ≥ 70 |
| Resectability | |
| Accessibility | Not brain stem, basal gangila, thalamus |
| Size | >1 cm in maximal diameter |
KPS, Karnofsky Performance Scale.
To assist in treatment decision, several investigators have advocated dividing patients into prognostic categories based on clinical features as determined from prospective clinical trials. One of the most widely recognized predictive models was developed by Gaspar and colleagues, who identified three prognostic groups of patients with brain metastases based on a recursive partitioning analysis (RPA) of 1200 patients enrolled in three consecutive Radiation Therapy Oncology Group (RTOG) trials conducted between 1979 and 1993 that were originally designed to evaluate radiation fractionization paradigms and radiation sensitizers.37 The analysis identified three prognostic categories: class I included patients with a KPS score > 70, age <65 years, controlled primary cancer, and no extracranial metastases; class III was defined by patients with a KPS score < 70; and class II included all other patients. These RPA groups correlated with survival as the median survival time of class I, II, and III patients were 7.1 months, 4.2 months, and 2.3 months, respectively. Based on this analysis, it has been suggested that class I patients are good candidate for aggressive treatment including surgery whereas class III patients are not. Although in practice, these RPA classes have not been adopted into clinical use, they are commonly used as a research tool in designing, stratifying, and assessing treatment results of clinical trials. An understanding of this classification is, therefore, important in critically evaluating the current neuro-oncology literature.
Histologic Assessment
The type of primary cancer, particularly its relative radiosensitivity, is an important consideration in treatment decision making (Table 15-3). In this context, primary treatment with WBRT is strongly considered for patients with highly radiosensitive tumors, such as lymphoma, germ cell tumors, and small cell lung cancer. The most common types of tumors to metastasize to the brain, namely breast and non–small cell lung cancer, are intermediately sensitive to conventional fractionated radiotherapy, and surgery will have a role in many cases. For radioresistant tumors (e.g., melanoma, renal cell carcinoma, and sarcomas), surgical resection is often the treatment of choice. Although this categorization is useful for conventional fractionated radiotherapy, the same does not necessarily hold true for SRS, as melanoma, renal cell carcinoma, and sarcomas may respond well to radiosurgery. The reason behind this difference in response to WBRT and SRS is not entirely clear, but it has been postulated that SRS is tumoricidal because it affects tumor vasculature differently from WBRT.38
Table 15-3 Radiosensitivity of Brain Metastases to Conventional Fractionated Radiotherapy
| Highly Sensitive | Intermediately Sensitive | Poorly Sensitive |
|---|---|---|
| Lymphoma | Breast cancer | Melanoma |
| Germinoma | Lung (non–small cell) cancer | Renal cancer |
| Lung (small cell) cancer | Colon cancer | Sarcoma |
Data from JG Cairncross, JH Kim, JB Posner. Radiation therapy for brain metastases. Ann Neurol. 1980;7:529-541; and FF Lang, R Sawaya. Surgical management of cerebral metastases. Neurosurg Clin North Am. 1996;7:459-484.
Surgical Technique
Surgical Anatomy
Cerebral metastases consist of solid tumor without intervening brain tissue. Although there may be some infiltration of tumor cells into the surrounding brain, this is usually less than 5 mm deep.8 Typically, the mass of tumor cells is surrounded by a gliotic rim that separates the tumor from the surrounding edematous brain. The lesions commonly arise at the gray-white matter junction, where a reduction in blood vessel diameter causes the embolic tumor to become trapped.39
In the supratentorial space, metastases may be classified based on their relationship to adjacent sulci and gyri.39,40 Metastases may occur just under the cortex and fill a gyrus (subcortical), deep within a gyrus adjacent to a sulcus (subgyral), below a sulcus (subsulcal), deep within the hemispheric white matter (lobar), or within the ventricle (intraventricular).41 In the posterior fossa, cerebellar metastases can be categorized as occurring in either deep or hemispheric locations; hemispheric lesions can be considered as lateral or medial. A subset arises directly within the vermis. Knowledge of the relationship to the sulcus is particularly important because this may determine the appropriate surgical path to the tumor (see the following).
Exposure and Operative Approach
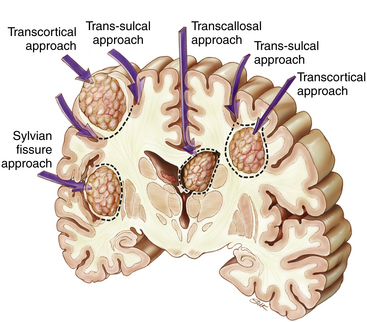
Surgical approaches to a brain metastasis are based on its anatomic location.39 Supratentorial subcortical lesions are best resected by an incision in the apex of the sulcus and circumferential dissection of the tumor (transcortical approach) (Fig. 15-1). Removal of a cortical plug above the lesion improves exposure; this may be problematic when the lesion arises within eloquent cortex. In such a situation, a longitudinal incision dictated by local functional mapping performed with direct brain stimulation (see the following) may minimize injury to the surrounding brain.
Lesions in subgyral or subsulcal locations are best approached by splitting the sulcus leading to the lesion. Subgyral tumors are removed by making an incision in the side of the split sulcus, whereas subsulcal lesions are entered at the sulcal base (transsulcal approach) (see Fig. 15-1). Metastases located deep within the white matter, independent of a single sulcus or gyrus (lobar), may be approached either transcortically or trans-sulcally (see Fig. 15-1). Tumors in the subinsular cortex may be approached by splitting the sylvian fissure. Midline metastases are best approached by splitting the interhemispheric fissure; tumors may then be resected by further splitting or entering a deep gyrus (see Fig. 15-1). Intraventricular lesions may be approached transcallosally or transcortically (Fig. 15-2; see Fig. 15-1).
Lesion Extirpation
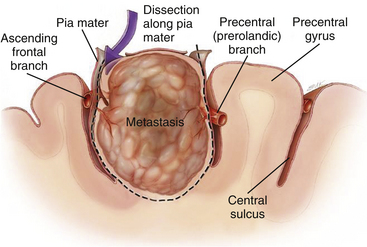
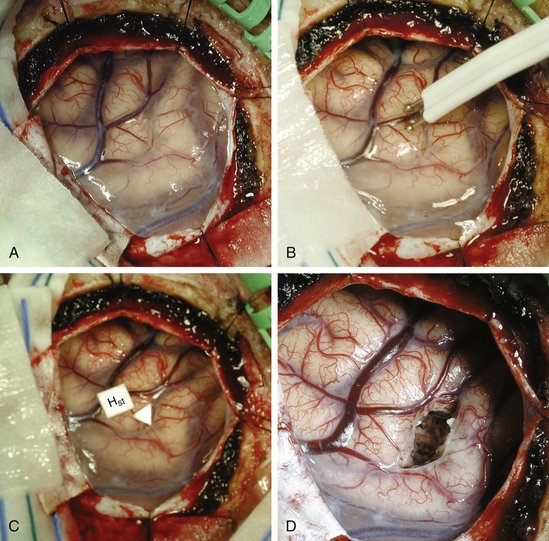
Once the lesion is reached, resection is usually performed in a circumferential, en bloc fashion by dissection in the gliotic pseudocapsule surrounding the lesion (Fig. 15-3).Circumferential dissection is carried out in this gliotic plane without violating the wall of the tumor. Such an approach ensures gross-total resection (because tumor cells rarely infiltrate beyond the gliotic plane) and also reduces spillage of cells into the surrounding area. For lesions located directly in the eloquent brain (e.g., motor strip or speech centers), a longitudinal incision parallel to the orientation of the gyrus can be made and the tumor resected in an “inside-out,” piecemeal fashion, rather than en bloc. Piecemeal removal may also be preferred for very large lesions in difficult areas (e.g., within the ventricle).
The importance of performing an en bloc resection has been highlighted in a retrospective study conducted by Suki et al., in which 260 patients with 1-2 posterior fossa metastases were analyzed based on the type of resection for the development of leptomeningeal disease (LMD, i.e., carcinomatous meningitis).42 Whereas only 6% of 123 patients who underwent en bloc resection developed LMD, 14% of 137 patients who had a piecemeal resection developed LMD. In a related study of 542 patients with supratentorial metastatic tumors from the same investigators, similar results were noted.43 Specifically, of 191 patients who underwent piecemeal resection, 9% developed LMD, whereas LMD developed in only 3% of 351 patients who had en bloc resections. These results remain significant even after controlling for other covariates including tumor volume, tumor type, extent of resection, and patient characteristics such as age, KPS score, and extracranial disease burden. Although these findings have yet to be confirmed in a prospective manner, it is prudent to conclude that en bloc resection should be performed whenever feasible.
Technical Issues in Resecting Multiple Metastases
When resecting multiple brain metastases, special attention must be given to planning the operation. Resection of multiple metastases can be performed via one craniotomy that encompasses all the lesions or via multiple, separate craniotomies. The decision to perform multiple craniotomies is determined by the proximity of the lesions to each other: lesions that are some distance apart generally require separate craniotomies. When multiple craniotomies are required, it is usually possible to perform all the craniotomies simultaneously without having to redrape the patient. The patient may be placed in a neutral position and turned from side to side on the operating table so that the lesion that is being operated on is positioned at the top of the operative field. Linear skin incisions are particularly effective when performing multiple craniotomies, and they also reduce the risk of compromising the vascular supply to the scalp. To maximize efficiency, each step of the operation (i.e., skin incision, bone flap elevation, dural opening, tumor removal, hemostasis, and closure) is performed at each location before the next step is performed. This approach is preferred to removing one lesion at one site and closing that wound and then removing one lesion at another site, not only because it is more efficient, but also because it minimizes the time between hemostasis and patient awakening. Thus any untoward events (e.g., hematoma formation) do not go undetected while the patient is under anesthesia.
Ultrasound
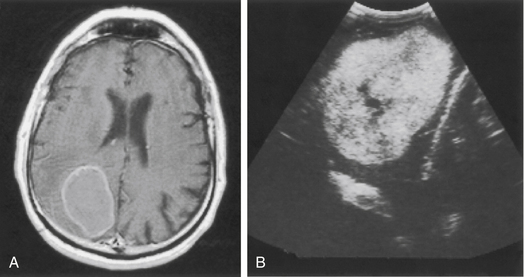
Intraoperative ultrasound is a valuable adjunct available to the surgeon that provides a relatively low-cost method for visualizing tumors below the surface of the brain. Most brain metastases appear homogeneously hyperechogenic, although those with necrotic centers or cysts may be hypoechoic centrally (Fig. 15-4). Compared with other methods of localization, ultrasound has the advantage of real-time imaging; therefore, changes in the tumor, as well as brain shift, are readily identifiable as the resection proceeds. Ultrasound also allows for visualization of the adjacent sulci and other intracranial landmarks (e.g., the ventricle), thus assisting in selection of a corridor of approach to the tumor. Ultrasound can assist in the determination of the extent of tumor resection because gross-total resection corresponds to complete removal of the echogenic mass, in most cases. However, in cases of recurrent tumors after radiotherapy, radiation necrosis may obscure the boundaries of the lesion, making determination of the extent of resection more difficult.44
Stereotaxis
Advances in localization technology have allowed for the evolution of rigid frame-based stereotaxy into frameless systems. These “neuronavigation” devices allow neurosurgeons to navigate the brain based on the preoperative images. These systems are particularly useful for planning the skin incision and craniotomy and the initial trajectory to the lesion. However, they suffer from the inability to compensate for intraoperative changes such as brain shift unless they can be updated with intraoperative imaging (usually MR images or even ultrasonography).45 Thus the authors generally rely on ultrasound as the resection proceeds.
Functional Mapping
When resecting metastases within eloquent areas of the brain, mapping the location of the critical functions is vital to safe tumor resection. Preoperative identification of sensory, motor, and language cortices is possible with functional MR imaging,46 and diffusion-tensor imaging allows for identification of important white matter tracts (e.g., internal capsule).47 These preoperative studies, however, are only a general guide, and precise localization of function usually requires verification during the surgical resection. Consequently, functional mapping methods have been used to precisely define eloquent brain regions intraoperatively.
Neurophysiologic techniques, such as SSEPs, can be utilized to identify the reversal of phase that occurs between the motor and sensory cortices. A strip electrode is placed on the cortical surface, and stimulation of median, ulnar, or posterior tibial nerves results in cortical potentials that are detected by the electrodes. A “reversal of phase” is seen when the electrode covers both the motor and the sensory cortex, because the motor potentials are typically positive, and sensory potentials are typically negative. This permits an intraoperative indirect identification of motor and sensory cortices. The technique has also been used to continuously monitor the potentials throughout the operative procedure so as to guide resections adjacent to the primary somatosensory cortex.48
Direct cortical electrical stimulation can be used to identify eloquent cortex and is particularly useful in the localization of language areas. The technique involves stimulation of the cerebral cortex at a frequency of 60 Hz for 1 millisecond with biphasic square wave pulses and a current of 1 to 15 mA (Fig. 15-5).49–51 Stimulation of the motor cortex elicits a motor response in the patient, thus resulting in an objective response, which is an advantage over the SSEP technique. Stimulation of subcortical motor pathways can also be performed in a similar manner; however, the results are somewhat less reliable than with cortical stimulation.52
Although motor mapping can be performed with patients under general anesthesia, language mapping requires an awake patient. The authors’ current method of awake craniotomy employs intubation with a laryngeal mask and short-duration anesthetics, along with a local anesthetic scalp block prior to placement of the three-point head fixator.53 The muscle (if exposed) and dura are carefully infiltrated with local anesthetic. Once the craniotomy is completed, the patient is awakened, and the laryngeal mask is removed. Cortical stimulation with mapping of speech may then commence. Speech areas are usually defined as sites where electrical stimulation elicits speech arrest. In addition, patients can freely converse during the resection of the tumor in order to avoid loss of function as the resection proceeds. Once the resection is completed, the laryngeal mask may be replaced and the patient anesthetized, if required; or a short-acting sedative, along with a narcotic, may be given during the closure. This awake method allows for the safe removal of the tumor from eloquent brain and provides the patient with maximal comfort.
Outcomes after Surgical Resection
Surgical Mortality
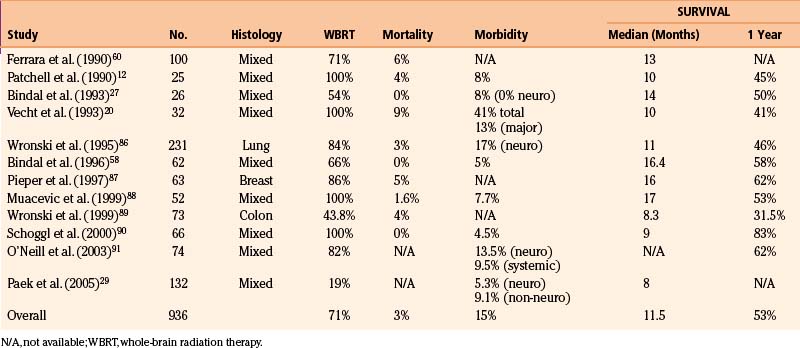
Most studies define surgical mortality as death that occurs within 30 days of operation, although some of the earlier surgeons used shorter intervals.54–56 Other series include deaths after 30 days if the patient did not leave the hospital.34,57 Surgical mortality has decreased dramatically since the earliest reports. For example, Cushing found that the mortality after resection of brain metastases was quite high (38%).57 In contrast, over the last two decades, using modern techniques, surgical mortalities of 3% or less have often been reported (Table 15-4). In fact, some of the more recent series report no mortality after surgery for brain metastases.33,44,58,59 In the randomized trial of Patchell and colleagues,12 the 30-day operative mortality and the 30-day postradiotherapy mortality were both 4%. In a comprehensive analysis of 400 craniotomies for all types of brain tumors, the overall 30-day mortality for patients with cerebral metastases was 2% (4/194), with the cause of death being sepsis in two patients and progressive leptomeningeal carcinomatosis in two others.30
Surgical Morbidity
Postoperative morbidity after surgery for brain metastases includes those related to neurologic changes and those related to non-neurologic problems (e.g., postoperative hematoma, wound infection, deep venous thrombosis, pneumonia, and pulmonary embolism). Some studies separate these two aspects of morbidity,27,29,33,35 others consider them together,12,20,24 a few report only neurologic morbidity,36,54,60–62 and many do not report morbidity at all (see Table 15-4).26,34,55,56,63,64
One of the most comprehensive analyses of postoperative complications for brain metastases was conducted by Sawaya and colleagues.30 This series from a large tertiary cancer center reviewed the complications that occurred after 194 craniotomies for brain metastases performed using all the modern technologies described above. Importantly, complications were categorized as either neurologic (directly producing neurologic compromise), regional (at the surgical site), or systemic (more generalized medical problems). Complications were considered to be minor (not life threatening and not prolonging the length of the hospital stay) when they resolved within a few days to 30 days without surgical intervention. They were considered to be major when they persisted for more than 30 days (reducing the quality of life) or required aggressive treatment because of their life-threatening nature. The rates of major neurologic, regional, and systemic complications were 6%, 3%, and 6%, respectively. In a critical analysis of factors contributing to complications, the authors reported that the most important variable affecting the frequency of neurologic complications was the relationship of the tumor to functional (eloquent) brain. Specifically, tumors located within or near eloquent brain had more neurologic complications than did those in noneloquent areas. Nevertheless, the risk of major neurologic complications, even when the lesion was within eloquent areas, was low (13%). Based on their extensive data, the authors used a statistical model to predict the risk of major complications from any source. They found that patients who were relatively young (age 40 years), with a KPS score of 100 and a metastasis in noneloquent brain, had a 5% risk of a major complication, whereas, at the opposite extreme, for a relatively old patient (age 65 years) with a low KPS score (of 50) and a tumor in eloquent brain, this risk rose to 23%.
Recurrence
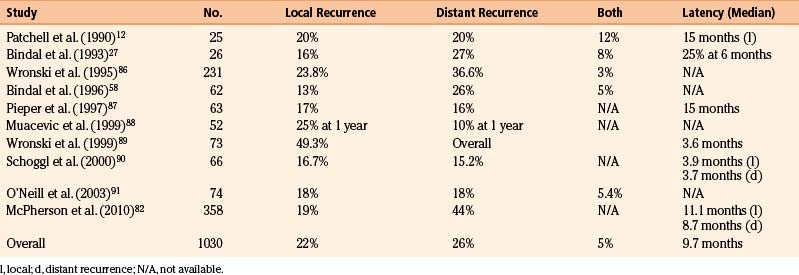
Recurrence is fairly easily measured after resection because surgery typically removes the entire gadolinium contrast-enhancing tumor mass (as visualized by MR imaging) and causes regression of the secondary brain edema. Thus, reappearance of a contrast-enhancing mass and edema on an MR image can be determined, although minimal postoperative contrast enhancement may be present for up to 3 months after surgery. In addition, one must distinguish between recurrence at the surgical site (i.e., local recurrence) and the development of new lesions in the brain at sites outside the initial resection site (i.e., distant recurrence). These events represent two distinct biologic processes. Local recurrence represents regrowth of microscopic residual disease after surgery, whereas distant recurrence is believed to arise from hematogenous dissemination of tumor cells to the brain from the primary site. When evaluating rates of local and distant tumor recurrence, it is important to know whether the patients received adjunctive WBRT. In the prospective study by Patchell and colleagues of patients with single brain metastases who were then randomized either to receive or not to receive WBRT after surgery,65 the local recurrence rate after surgery alone was 46% (21 of 46 patients), whereas the distant recurrence rate was 37% (17 of 46 patients). This high rate of local recurrence is not consistent with the results of other studies, which suggest recurrence rates of 10% to 15%.66 Table 15-5 lists the local and distant recurrence rates in recent surgical series of brain metastases.
Survival
Most series from the modern neurosurgical era that include metastases with different tumor histologies indicate a median patient survival time of 11 months and a 1-year survival rate of 53% (see Table 15-4). Kelly and colleagues39 reported a 1-year survival rate of 63% using computer-assisted stereotactic craniotomy. Studies from a large tertiary cancer center reported a median survival time of 14 months, with a 1-year survival rate of 50% for patients with single brain metastases.27,33 Similar median (14 months) and 1-year (55%) survival values were observed in patients with multiple metastases in whom all the lesions were removed.27 In most studies, variables associated with poor survival are the presence of multiple metastases, extensive and progressive systemic cancer, and a poor KPS score.
Role of Stereotactic Radiosurgery
Stereotactic radiosurgery in the form of gamma knife or linear accelerator methods delivers a single large dose of focused radiation, with rapid falloff in the surrounding tissue through cross-firing from many directions, to destroy lesions localized by stereotaxy. A major advantage of this technique over conventional surgery is that it can treat surgically inaccessible tumors, especially where eloquent brain would be transgressed during surgery to reach the lesion. Many brain metastases that in the past could only be treated with WBRT can now be directly targeted by SRS. Other advantages compared with surgery are that SRS is less costly, less invasive (requiring only placement of a stereotactic head frame under local anesthesia), requires shorter hospital stays because only a single fraction of radiation is given, and can be offered to patients who cannot tolerate surgery. Based on early retrospective reports, SRS has been shown to be effective in treating cerebral metastases, with local control rates ranging from 85% to 95% and a patient median survival time of 7 to 13 months.63–65 These results compare favorably with a local recurrence rate of 10% to 15% reported after surgical resection.66 Nevertheless, SRS has several important limitations compared with surgery. First, due to aforementioned dosing limitations, treatment is restricted to small lesions, usually to those not exceeding 3 cm in maximum diameter (volume <10–12 cm3).28,29 Second, no histologic verification of the metastatic nature of the lesion can be obtained with SRS, which is important considering that 5% to 11% of patients with systemic cancer are found to have nonmetastatic brain lesions (e.g., primary brain tumors or abscesses).8,12,13 Third, because SRS does not have the immediate effect of tumor extirpation, patients may have to remain on high steroid doses for longer intervals, and the mass effects of tumors (e.g., neurologic deficits and raised intracranial pressure) are not immediately relieved.
To define the role of SRS in the management of cerebral metastases, several randomized controlled trials have been conducted in recent years. In order to determine the benefits of adding SRS to standard WBRT, Kondziolka et al. randomized 27 patients with two to four cerebral metastases (≤2.5 cm) to receiving either WBRT alone or WBRT plus SRS.67 They found that the addition of SRS (16 Gy) to the standard 30 Gy WBRT dose significantly prolonged the median time to local control failure from 6 months to 36 months (p = 0.0005). However, the median survival time of patients receiving WBRT plus SRS (11 months) was not found to be significantly longer than in those having WBRT alone (7.5 months, p = 0.22). Notably, the power of this study was grossly limited by its small sample size.
In another prospective study, Andrews et al. addressed the same question, comparing the treatment outcome of WBRT with or without an SRS boost.68 In this multicenter study carried out under the auspices of the RTOG, 333 patients with one to three brain metastases (≤4 cm in maximal diameter) were randomized to receive either WBRT alone or WBRT plus SRS. In the primary analysis of all 333 patients, there was no difference in survival between the patients who received WBRT plus SRS and those who received WBRT alone (6.5 months for WBRT plus SRS vs. 5.7 months for WBRT alone, p = 0.14). However, when analyzing the data in terms of secondary outcomes, patients treated with the combined approach were more likely to have stable or improved performance status (43% for WBRT plus SRS vs. 27% for WBRT alone, p = 0.03) and decreased steroid use at the 6-month follow-up evaluation. Most importantly, in a subset analysis that included only patients with single metastases (n = 186), there was a small but significant improvement in median survival time in the WBRT plus SRS group (6.5 months) when compared with the WBRT alone group (4.9 months, p = 0.04). This study represents the first large-scale randomized trial of SRS as an adjuvant to WBRT and has provided vital data in support of the positive impact of SRS in the treatment of single, small brain metastases.
The minimally invasive nature of SRS has led to the important question of whether SRS should replace surgery as the primary treatment of brain metastases, particularly single metastases. To date there is only one published randomized trial69 that has attempted to compare SRS with conventional surgery in treatment efficacy. Specifically, Muacevic et al. conducted a multicenter randomized clinical trial in which patients with small (<3 cm maximal diameter) single metastases, good performance status (KPS score ≥ 70), and stable systemic disease were randomized to receive either SRS or conventional surgery plus WBRT. This study was initially designed to recruit 240 patients in order to achieve enough statistical power to detect a 15% difference in 1-year survival. However due to poor patient accrual, the study was aborted, and only 64 patients were randomized in this trial. The results showed that the median survival time was 9.5 months in the surgery plus WBRT group and 10.3 months in the SRS group, the difference between which did not reach statistical significance (p = 0.8). Although the investigators concluded that the results supported SRS and surgery as being essentially equivalent treatments, because the study was aborted before the accrual goal was reached, it did not have the power to demonstrate equivalence. Because of this weakness, the validity of the statistical evaluation is questionable, and the claim for equivalence based on the negative comparisons was unsound.70
In a separate effort to address the same question, the group at The University of Texas MD Anderson Cancer Center (MD Anderson) has also undertaken a prospective randomized trial comparing SRS with conventional surgery, but preliminary results have only been reported in abstract form.71 In this clinical trial, patients >16 years old, with newly diagnosed, single brain metastases, and receive a KPS score > 70were randomly assigned to receive conventional surgery or SRS. Similar to the trial of Muacevic et al., this MD Anderson trial also suffered from poor accrual. However, in the MD Anderson trial eligible patients who refused randomization were allowed to choose their treatment and were then followed identically to patients who accepted randomization. Thus this trial included both randomized and nonrandomized arms. Outcome measures were local recurrence, distant recurrence, and overall survival. Fifty-nine patients were entered in the randomized arm (30 received surgery and 29 received SRS), and 155 patients were entered in the nonrandomized arm (89 chose surgery and 66 chose SRS). In the preliminary analysis, treatment with SRS had a statistically significant increased risk of local recurrence compared with surgery in both the randomized and nonrandomized arms of the trial. Based on multivariate analyses which took into account the randomized and nonrandomized populations, and adjusted for confounding covariates (age, gender, WBRT, primary tumor type, extent of disease, tumor volume and location, KPS score, and RPA class) and randomization effects, SRS was associated with a nearly threefold increased risk of local recurrence compared with surgery. For overall survival, the smaller randomized trial showed no difference between surgery and SRS, but there was a significant advantage of surgery over SRS in the larger nonrandomized arm. As a measure of baseline heterogeneity, there was no difference in distant recurrence rates between the groups in both the randomized and nonrandomized arms of the trial, suggesting that the patients in the SRS group and the surgery group were similar in both arms of the trial. Therefore, in this prospective trial characterized by the inclusion of randomized and nonrandomized arms, conventional surgery appeared to provide a significant advantage over SRS in terms of local recurrence and suggests that surgery may also provide an advantage in terms of overall survival. On the basis of these data, the investigators recommended that surgery should be the treatment of choice for brain metastases that are amenable to either treatment. SRS, on the other hand, can be adopted as the primary modality for treatment of small (<1 cm) lesions, especially if they are multiple, surgically inaccessible, and located deep within the brain. Patients with multiple medical comorbidities who cannot tolerate conventional surgery are also candidates for SRS.
Another consideration in deciphering the role of SRS compared with surgery in the management of brain metastases is that local tumor control rates after SRS fall sharply when the maximum diameter of the tumor exceeds 1 to 1.5 cm, as demonstrated in two retrospective series.72,73 Consequently, it has been argued that the upper size limit of a 3-cm maximal diameter may be too high for adequate SRS treatment,74 and it has been suggested that a lower threshold (e.g., 2–2.5 cm maximal diameter) may be more appropriate as the upper limit for applying SRS. More careful studies of the issue of tumor size and the efficacy of radiosurgery are needed.
Role of Whole-Brain Radiation Therapy
Whole-brain radiation therapy has traditionally been considered the standard treatment for patients with cerebral metastases, particularly multiple brain metastases. Based on the results of multiple RTOG trials conducted in the 1970s and 1980s, it is established that patient survival is prolonged from 1 to 2 months with supportive care alone to 3-6 months with a standard regime of 30 Gy delivered in 10 fractions.62,75,76
After surgery or SRS, adjuvant WBRT is often recommended on a routine basis in an effort to eradicate residual cancer cells at the resection site and to eliminate microscopic foci at distant sites within the brain.22 However, the benefit of this practice has been questioned in recent years as improvement in systemic cancer treatment has resulted in more patients surviving long enough to be exposed to the debilitating neurocognitive side effects of WBRT. This has particularly become an issue for patients with good KPS scores as these patients are most affected by radiation-induced neurocognitive sequelae emerging 6 to 12 months post treatment, which may critically outweigh the intended therapeutic benefits of WBRT.77 Several authors have, therefore, suggested that WBRT should be withheld, especially for radioresistant tumors such as melanoma and renal cell carcinoma, and only be considered when local treatment fails.78,79
In order to clarify whether routine adjuvant WBRT is of benefit after surgical resection of single brain metastases, Patchell and colleagues randomized 95 patients with single metastases to WBRT or observation after conventional surgery.65 The patients who received WBRT showed a significant reduction in tumor recurrence, both at the site of the surgery and at distant sites in the brain, compared with the observation group. Specifically, whereas the overall recurrence rate was 70% in the observation group, it was only 18% in the adjuvant WBRT group (p < 0.001). However, this decrease in recurrence in the WBRT group did not translate into a statistically significant improvement in survival, as the median survival time of the adjuvant WBRT group was 12 months and that of the observation group was 10.8 months (p = 0.39). However, it must be noted that the trial was not sufficiently powered to detect a difference in survival between the groups. In addition, 61% of the patients in the observation group eventually crossed over to the WBRT group and received salvage WBRT for recurrence. Therefore, the lack of a significant difference in overall survival between the groups must be interpreted with caution. Also, because neurocognitive function was not assessed, the functional effects of routine adjuvant WBRT were not addressed in this study.
In a related study, Aoyama and coworkers from the Japanese Radiation Oncology Study Group investigated the impact of withholding adjuvant WBRT following SRS.80 In this randomized trial, patients with one to four cerebral metastases were randomized to either WBRT or observation after SRS. Similar to the study by Patchell et al.,65 in this study of 132 patients, the rate of local tumor control at 1 year was superior in the WBRT group (88.7%) compared with the observation group (72.5%). However, the median survival times were similar in both groups (7.5 months in the WBRT group vs. 8 months in the observation group, p = 0.42), with only 16% of patients in the observation group crossing over to receive salvage WBRT. Of patients who survived for more than 12 months, functional assessment was performed using KPS scores. The rate of functional status preservation (KPS score ≥ 70) was not significantly different at 1 year between the two groups (33.9% in the adjuvant WBRT group vs. 26.9% in the observation group). Nevertheless, it should be noted that the KPS score is a measure primarily of physical functions, not of cognitive functions and the preservation in KPS score cannot be interpreted as preservation of neurocognitive abilities, such as learning and verbal skills.
In a recently reported prospective randomized trial, Chang et al. investigated the neurocognitive effects of WBRT based on serial examinations of cognitive functions across several domains using validated assessment tools.81 In this trial, patients with one to three newly diagnosed brain metastases were randomized to receive either SRS plus WBRT (n = 28) or SRS alone (n = 30). Patients were evaluated before treatment and then monthly afterwards. By 4 months, patients receiving WBRT suffered a significantly greater decline in memory and learning ability compared with patients receiving SRS alone. These results, obtained from an interim analysis, were so significant that the study was terminated before the targeted enrollment of 90 patients was reached. Interestingly, although the 1-year local tumor control rate was lower in patients in the observation group (67%) when compared with the WBRT group (100%, p = 0.012), the overall median survival time in the observation group (15.2 months) was superior to that of the WBRT group (5.7 months, p = 0.003). As a secondary finding, such a marked difference in survival between the two groups is difficult to account for post hoc, but as suggested by the authors, significant differences in the use of salvage and systemic therapies between the two groups, which were not stratified and controlled a priori, may play a role.
Given that adjuvant WBRT reduces recurrence but is not innocuous, it has become increasingly desirable to define subsets of patients who are at higher risk of experiencing early local or distant recurrence after surgical resection or SRS and who would, therefore, potentially benefit from adjuvant WBRT, despite the potential risk for developing neurocognitive dysfunction in the long term. To this end, McPherson et al.82 analyzed the outcomes of 358 patients with single brain metastases originating from a wide range of primary sites, all of whom were treated with microsurgical resection without or with the standard dose adjuvant WBRT. In a multivariate analysis, it was found that tumors >3 cm in maximal diameter that did not receive adjuvant WBRT had a significantly increased risk of recurring locally (HR = 3.14, CI 1.02–9.69, p = 0.05). Additionally, patients whose primary disease was progressing and who did not receive WBRT had a significantly increased risk of distant recurrence (HR = 2.16, CI 1.01–4.66, p = 0.05). There was no effect of WBRT based on tumor type. Based on this retrospective report, it has been suggested that after surgical resection of a single metastasis, adjuvant WBRT may be most beneficial in patients in whom the resected metastasis was large (>3 cm) or in whom the systemic disease is active. Conversely, adjuvant WBRT may be withheld in patients whose resected metastasis was small and in whom the systemic disease is controlled.
As a substitute for adjuvant WBRT after resection of a single metastasis, it has been suggested that SRS can be applied to the tumor resection cavity in order to decrease the chance for local tumor recurrence following surgery, while avoiding the adverse effects of WBRT.83,84 Supported by the observation in melanoma brain metastases that the presence of intratumoral hemorrhage was associated with increased recurrence,85 the role of treating the resection cavity with SRS has been particularly advocated in tumors that have hemorrhaged, as it is hypothesized that during hemorrhage the normally defined tumor–brain interface characteristic of cerebral metastases are inevitably violated, resulting in widely dispersed micrometastases that are not amenable to surgical resection alone. Despite the theoretical appeal of this approach, the current data supporting postresection SRS are limited to few small retrospective series.83,84 Although favorable local disease control rates (>94%) have been reported, further validation from clinical trials is required. In light of this, a randomized controlled trial was opened at MD Anderson in August 2009 to evaluate the efficacy of postoperative SRS treatment the resection bed in reducing the risk of local tumor recurrence. This trial, expected to be completed in 2014, will provide important data to clarify the proper use of this adjunctive treatment.
Andrews D.W., Scott C.B., Sperduto P.W., et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665-1672.
Aoyama H., Shirato H., Tago M., et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs. stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483-2491.
Chang E.L., Hassenbusch S.J.3rd, Shiu A.S., et al. The role of tumor size in the radiosurgical management of patients with ambiguous brain metastases. Neurosurgery. 2003;53:272-280. discussion 280-281
Chang E.L., Wefel J.S., Hess K.R., et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037-1044.
DeAngelis L.M., Delattre J.Y., Posner J.B. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789-796.
Gaspar L., Scott C., Rotman M., et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745-751.
Hart M.G., Grant R., Walker M., et al. Surgical resection and whole brain radiation therapy versus whole brain radiation therapy alone for single brain metastases. Cochrane Database Syst Rev. 2005:CD003292.
Kondziolka D., Patel A., Lunsford L.D., et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427-434.
Lang FF: Conventional surgery versus stereotactic radiosurgery in the treatment of single brain metastases: a prospective study with both randomized and nonrandomized arms. (clinicaltrails.gov: NCT00460395), Seventy-sixth Annual Meeting of the American Association of Neurological Surgeons (AANS), 2008.
Lang F.F., Sawaya R. Surgical management of cerebral metastases. Neurosurg Clin N Am. 1996;7:459-484.
Lang E.F., Slater J. Metastatic brain tumors. Results of surgical and nonsurgical treatment. Surg Clin N Am. 1964;44:865-872.
McPherson C., Suki D., Feiz-Erfan I., et al. Adjuvant whole brain radiation therapy after surgical resection of single brain metastases. Neuro-oncology E-pub ahead of print. 2010.
Mintz A.H., Kestle J., Rathbone M.P., et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996;78:1470-1476.
Muacevic A., Wowra B., Siefert A., et al. Microsurgery plus whole brain irradiation versus gamma knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol. 2008;87:299-307.
Paek S.H., Audu P.B., Sperling M.R., et al. Reevaluation of surgery for the treatment of brain metastases: review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery. 2005;56:1021-1034. discussion 1021-1034
Patchell R.A., Tibbs P.A., Walsh J.W., et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494-500.
Patchell R.A., Tibbs P.A., Regine W.F., et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485-1489.
Sawaya R. Surgical treatment of brain metastases. Clin Neurosurg. 1999;45:41-47.
Sawaya R., Bindal R., Lang F. Metastatic brain tumors. In: Kaye A.H., Laws E.R. Brain tumors: an encyclopedic approach. ed 2nd. New York: Churchill Livingstone; 2001:999-1026.
Sawaya R., Hammoud M., Schoppa D., et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044-1055. discussion 1055-1056
Sawaya R., Wildrick D.M. Metastatic brain tumors: surgery perspective. In: Chin L.S., Regine W.F. Principles and practice of stereotactic radiosurgery. New York: Springer; 2008:193-199.
Selek U., Chang E.L., Hassenbusch S.J.3rd, et al. Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int J Radiat Oncol Biol Phys. 2004;59:1097-1106.
Suki D., Abouassi H., Patel A.J., et al. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg. 2008;108:248-257.
Suki D., Hatiboglu M.A., Patel A.J., et al. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery. 2009;64:664-674. discussion 674-676
Vecht C.J., Haaxma-Reiche H., Noordijk E.M., et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583-590.
1. Wingo P.A., Tong T., Bolden S. Cancer statistics, 1995. CA Cancer J Clin. 1995;45:8-30.
2. Cairncross J.G., Kim J.H., Posner J.B. Radiation therapy for brain metastases. Ann Neurol. 1980;7:529-541.
3. Delattre J.Y., Krol G., Thaler H.T., et al. Distribution of brain metastases. Arch Neurol. 1988;45:741-744.
4. Patchell R.A. Brain metastases. Neurol Clin. 1991;9:817-824.
5. Posner J.B., Chernik N.L. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579-592.
6. Jemal A., Tiwari R.C., Murray T., et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8-29.
7. Landis S.H., Murray T., Bolden S., et al. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6-29.
8. Sawaya R., Bindal R., Lang F. Metastatic brain tumors. In: Kaye A.H., Laws E.R. Brain tumors: an encyclopedic approach. 2nd ed. New York: Churchill Livingstone; 2001:999-1026.
9. Byrne T.N., Cascino T.L., Posner J.B. Brain metastasis from melanoma. J Neurooncol. 1983;1:313-317.
10. Madajewicz S., Karakousis C., West C.R., et al. Malignant melanoma brain metastases. Review of Roswell Park Memorial Institute experience. Cancer. 1984;53:2550-2552.
11. Black P. Surgical and radiotherapeutic management of brain metastasis, 4th ed. Philadelphia: W.B. Saunders; 2000.
12. Patchell R.A., Tibbs P.A., Walsh J.W., et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494-500.
13. Voorhies R.M., Sundaresan N., Thaler H.T. The single supratentorial lesion. An evaluation of preoperative diagnostic tests. J Neurosurg. 1980;53:364-368.
14. Kuhn M.J., Hammer G.M., Swenson L.C., et al. MRI evaluation of “solitary” brain metastases with triple-dose gadoteridol: comparison with contrast-enhanced CT and conventional-dose gadopentetate dimeglumine MRI studies in the same patients. comput Med Imaging Graph. 1994;18:391-399.
15. Nomoto Y., Miyamoto T., Yamaguchi Y. Brain metastasis of small cell lung carcinoma: comparison of Gd-DTPA enhanced magnetic resonance imaging and enhanced computerized tomography. Jpn J Clin Oncol. 1994;24:258-262.
16. Sze G., Milano E., Johnson C., et al. Detection of brain metastases: comparison of contrast-enhanced MR with unenhanced MR and enhanced CT. AJNR Am J Neuroradiol. 1990;11:785-791.
17. Macchiarini P., Buonaguidi R., Hardin M., et al. Results and prognostic factors of surgery in the management of non–small cell lung cancer with solitary brain metastasis. Cancer. 1991;68:300-304.
18. Gavrilovic I.T., Posner J.B. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75:5-14.
19. Mintz A.H., Kestle J., Rathbone M.P., et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996;78:1470-1476.
20. Vecht C.J., Haaxma-Reiche H., Noordijk E.M., et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583-590.
21. Hart M.G., Grant R., Walker M., et al. Surgical resection and whole brain radiation therapy versus whole brain radiation therapy alone for single brain metastases. Cochrane Database Syst Rev. 2005:CD003292.
22. Kalkanis S.N., Kondziolka D., Gaspar L.E., et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:33-43.
23. Elvidge A.R., Baldwin M. Clinical analysis of 88 cases of metastatic carcinoma involving the central nervous system; with an outline of therapeutic principles. J Neurosurg. 1949;6:495-502.
24. Haar F., Patterson R.H.Jr. Surgery for metastatic intracranial neoplasm. Cancer. 1972;30:1241-1245.
25. Oldberg E. Surgical considerations of carcinomatous metastases to the brain. JAMA. 1933;101:1458-1461.
26. Ransohoff J. Surgical management of metastatic tumors. Semin Oncol. 1975;2:21-27.
27. Bindal R.K., Sawaya R., Leavens M.E., et al. Surgical treatment of multiple brain metastases. J Neurosurg. 1993;79:210-216.
28. Iwadate Y., Namba H., Yamaura A. Significance of surgical resection for the treatment of multiple brain metastases. Anticancer Res. 2000;20:573-577.
29. Paek S.H., Audu P.B., Sperling M.R., et al. Reevaluation of surgery for the treatment of brain metastases: review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery. 2005;56:1021-1034. discussion 1021-1034
30. Sawaya R., Hammoud M., Schoppa D., et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044-1055. discussion 1055-1056
31. Kondziolka D., Lunsford L. Brain metastases. In: Apuzzo M.L.J., editor. Brain surgery: complication avoidance and management. New York: Churchill Livingstone; 1993:615-641.
32. Sturm V., Kimmig B., Engenhardt R., et al. Radiosurgical treatment of cerebral metastases. Method, indications and results. Stereotact Funct Neurosurg. 1991;57:7-10.
33. Bindal R.K., Sawaya R., Leavens M.E., et al. Reoperation for recurrent metastatic brain tumors. J Neurosurg. 1995;83:600-604.
34. Galicich J.H., Sundaresan N., Arbit E., et al. Surgical treatment of single brain metastasis: factors associated with survival. Cancer. 1980;45:381-386.
35. Sundaresan N., Galicich J.H. Surgical treatment of brain metastases. Clinical and computerized tomography evaluation of the results of treatment. Cancer. 1985;55:1382-1388.
36. Winston K.R., Walsh J.W., Fischer E.G. Results of operative treatment of intracranial metastatic tumors. Cancer. 1980;45:2639-2645.
37. Gaspar L., Scott C., Rotman M., et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745-751.
38. Brown P.D., Brown C.A., Pollock B.E., et al. Stereotactic radiosurgery for patients with “radioresistant” brain metastases. Neurosurgery. 2002;51:656-665. discussion 665-667
39. Kelly P.J., Kall B.A., Goerss S.J. Results of computed tomography-based computer-assisted stereotactic resection of metastatic intracranial tumors. Neurosurgery. 1988;22:7-17.
40. Yasargil M.G. Topographic anatomy for microsurgical approaches to intrinsic brain tumors. In: Yasargil M.G., Adamson T.E. Microneurosurgery. CNS tumors: surgical anatomy, neuropathology, neuroradiology, neurophysiology, clinical considerations, operability, treatment options. New York: Thieme Medical Publishers, 1994. 2–114
41. Lang F.F., Sawaya R. Surgical management of cerebral metastases. Neurosurg Clin N Am. 1996;7:459-484.
42. Suki D., Abouassi H., Patel A.J., et al. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg. 2008;108:248-257.
43. Suki D., Hatiboglu M.A., Patel A.J., et al. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery. 2009;64:664-674. discussion 674-676
44. Hammoud M.A., Ligon B.L., elSouki R., et al. Use of intraoperative ultrasound for localizing tumors and determining the extent of resection: a comparative study with magnetic resonance imaging. J Neurosurg. 1996;84:737-741.
45. Unsgaard G., Ommedal S., Muller T., et al. Neuronavigation by intraoperative three-dimensional ultrasound: initial experience during brain tumor resection. Neurosurgery. 2002;50:804-812. discussion 812
46. Heilbrun M.P., Lee J.N., Alvord L. Practical application of fMRI for surgical planning. Stereotact Funct Neurosurg. 2001;76:168-174.
47. Witwer B.P., Moftakhar R., Hasan K.M., et al. Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J Neurosurg. 2002;97:568-575.
48. Grant G.A., Farrell D., Silbergeld D.L. Continuous somatosensory evoked potential monitoring during brain tumor resection. Report of four cases and review of the literature. J Neurosurg. 2002;97:709-713.
49. Berger M.S., Ojemann G.A. Intraoperative brain mapping techniques in neuro-oncology. Stereotact Funct Neurosurg. 1992;58:153-161.
50. Matz P.G., Cobbs C., Berger M.S. Intraoperative cortical mapping as a guide to the surgical resection of gliomas. J Neurooncol. 1999;42:233-245.
51. Taylor M.D., Bernstein M. Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: a prospective trial of 200 cases. J Neurosurg. 1999;90:35-41.
52. Skirboll S.S., Ojemann G.A., Berger M.S., et al. Functional cortex and subcortical white matter located within gliomas. Neurosurgery. 1996;38:678-684. discussion 684-685
53. Toms S.A., Ferson D.Z., Sawaya R. Basic surgical techniques in the resection of malignant gliomas. J Neurooncol. 1999;42:215-226.
54. Raskind R., Weiss S.R., Manning J.J., et al. Survival after surgical excision of single metastatic brain tumors. Am J Roentgenol Radium Ther Nucl Med. 1971;111:323-328.
55. Stortebecker T.P. Metastatic tumors of the brain from a neurosurgical point of view; a follow-up study of 158 cases. J Neurosurg. 1954;11:84-111.
56. Vieth R.G., Odom G.L. Intracranial metastases and their neurosurgical treatment. J Neurosurg. 1965;23:375-383.
57. Cushing H. Intracranial Tumours; Notes upon a Series of Two Thousand Verified Cases with Surgical-Mortality Percentages Pertaining Thereto. Springfield, IL, and Baltimore, MD: C.C. Thomas; 1932.
58. Bindal A.K., Bindal R.K., Hess K.R., et al. Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg. 1996;84:748-754.
59. Brega K., Robinson W.A., Winston K., et al. Surgical treatment of brain metastases in malignant melanoma. Cancer. 1990;66:2105-2110.
60. Ferrara M., Bizzozzero L., Talamonti G., et al. Surgical treatment of 100 single brain metastases. Analysis of the results. J Neurosurg Sci. 1990;34:303-308.
61. Lang E.F., Slater J. Metastatic brain tumors. Results of surgical and nonsurgical treatment. Surg Clin N Am. 1964;44:865-872.
62. Sause W.T., Crowley J.J., Morantz R., et al. Solitary brain metastasis: results of an RTOG/SWOG protocol evaluation surgery + RT versus RT alone. Am J Clin Oncol. 1990;13:427-432.
63. Simionescu M.D. Metastatic tumors of the brain: a follow-up study of 195 patients with neurosurgical considerations. J Neurosurg. 1960;17:361-373.
64. White K.T., Fleming T.R., Laws E.R.Jr. Single metastasis to the brain. surgical treatment in 122 consecutive patients. Mayo Clin Proc. 1981;56:424-428.
65. Patchell R.A., Tibbs P.A., Regine W.F., et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485-1489.
66. Sawaya R. Surgical treatment of brain metastases. Clin Neurosurg. 1999;45:41-47.
67. Kondziolka D., Patel A., Lunsford L.D., et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427-434.
68. Andrews D.W., Scott C.B., Sperduto P.W., et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665-1672.
69. Muacevic A., Wowra B., Siefert A., et al. Microsurgery plus whole brain irradiation versus gamma knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol. 2008;87:299-307.
70. Costa L.J., Xavier A.C., del Giglio A. Negative results in cancer clinical trials—equivalence or poor accrual? Control Clin Trials. 2004;25:525-533.
71. Lang FF: Conventional surgery versus stereotactic radiosurgery in the treatment of single brain metastases: a prospective study with both randomized and nonrandomized arms. (clinicaltrails.gov: NCT00460395), Seventy-sixth Annual Meeting of the American Association of Neurological Surgeons (AANS), 2008.
72. Selek U., Chang E.L., Hassenbusch S.J.3rd, et al. Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int J Radiat Oncol Biol Phys. 2004;59:1097-1106.
73. Chang E.L., Hassenbusch S.J.3rd, Shiu A.S., et al. The role of tumor size in the radiosurgical management of patients with ambiguous brain metastases. Neurosurgery. 2003;53:272-280. discussion 280-281
74. Sawaya R., Wildrick D.M. Metastatic brain tumors: surgery perspective. In: Chin L.S., Regine W.F. Principles and Practice of Stereotactic Radiosurgery. New York: Springer; 2008:193-199.
75. Phillips T.L., Scott C.B., Leibel S.A., et al. Results of a randomized comparison of radiotherapy and bromodeoxyuridine with radiotherapy alone for brain metastases: report of RTOG trial 89-05. Int J Radiat Oncol Biol Phys. 1995;33:339-348.
76. Komarnicky L.T., Phillips T.L., Martz K., et al. A randomized phase III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG-7916). Int J Radiat Oncol Biol Phys. 1991;20:53-58.
77. DeAngelis L.M., Delattre J.Y., Posner J.B. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789-796.
78. Sneed P.K., Lamborn K.R., Forstner J.M., et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43:549-558.
79. Deinsberger R., Tidstrand J. LINAC radiosurgery as single treatment in cerebral metastases. J Neurooncol. 2006;76:77-83.
80. Aoyama H., Shirato H., Tago M., et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs. stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483-2491.
81. Chang E.L., Wefel J.S., Hess K.R., et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037-1044.
82. McPherson C., Suki D., Feiz-Erfan I., et al. Adjuvant whole brain radiation therapy after surgical resection of single brain metastases. Neurooncology. 2010;12:711-719.
83. Jagannathan J., Yen C.P., Ray D.K., et al. Gamma knife radiosurgery to the surgical cavity following resection of brain metastases. J Neurosurg. 2009;111:431-438.
84. Kim P.K., Ellis T.L., Stieber V.W., et al. Gamma knife surgery targeting the resection cavity of brain metastasis that has progressed after whole-brain radiotherapy. J Neurosurg. 2006;105(suppl):75-78.
85. Mathieu D., Kondziolka D., Cooper P.B., et al. Gamma knife radiosurgery in the management of malignant melanoma brain metastases. Neurosurgery. 2007;60:471-481. discussion 481-482
86. Wronski M., Arbit E., Burt M., et al. Survival after surgical treatment of brain metastases from lung cancer: a follow-up study of 231 patients treated between 1976 and 1991. J Neurosurg. 1995;83:605-616.
87. Pieper D.R., Hess K.R., Sawaya R.E. Role of surgery in the treatment of brain metastases in patients with breast cancer. Ann Surg Oncol. 1997;4:481-490.
88. Muacevic A., Kreth F.W., Horstmann G.A., et al. Surgery and radiotherapy compared with gamma knife radiosurgery in the treatment of solitary cerebral metastases of small diameter. J Neurosurg. 1999;91:35-43.
89. Wronski M., Arbit E. Resection of brain metastases from colorectal carcinoma in 73 patients. Cancer. 1999;85:1677-1685.
90. Schoggl A., Kitz K., Reddy M., et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien). 2000;142:621-626.
91. O’Neill B.P., Iturria N.J., Link M.J., et al. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55:1169-1176.