Chapter 75 Surgical Management of Aneurysms of the Middle Cerebral Artery
The middle cerebral artery (MCA) is a very common site for aneurysm formation. In Finland, MCA aneurysms (MCAAs) represent 40% of all intracranial aneurysms.1–3 MCAAs are more frequent among unruptured aneurysms (48%) than among ruptured aneurysms (34%).4 Despite being so common, surprisingly few reports deal with MCA aneurysms, and especially the overall management outcome of this specific group of patients.5–17 Most MCAAs are located distal to the circle of Willis, and they are often broad based and one or several branches originate from a base.18 When ruptured, they present with intracerebral hematoma (ICH) in nearly half of all cases; many of these hematomas cause severe mass effect.1–3 In his pioneering work on surgery for intracranial aneurysms (IAs), Dandy considered MCAAs hazardous for surgical management, and even inoperable.19 Although currently only a few MCAAs are inoperable, they certainly still present striking problems as compared with other aneurysms in the anterior circulation. The main challenges when operating on MCA aneurysms is the lack of collateral circulation, so that inadvertent occlusion of the MCA or one of its branches can lead to calamitous infarctions and death, especially in acute subarachnoid hemorrhage (SAH). The MCAAs are less suitable for endovascular surgery than other anterior circulation aneurysms,20–25 because of both their anatomy (broad neck with high recanalization rate) and their frequent association with expanding hematomas; thus neurosurgeons should focus on the safe treatment of these lesions.17,26–32
The purpose of this chapter is to review practical anatomy, preoperative planning, and avoidance of complications in the microsurgical dissection and clipping of MCAAs. This review is mainly based on the experience of the senior author (JH) in two of the five Finnish University Hospital neurosurgical departments (Helsinki and Kuopio), which serve, without selection, the catchment area of the entire southern and eastern Finland regions (population 3 million). These two centers have treated nearly 10,000 aneurysm patients since the beginning of the microneurosurgical era in the mid 1970s. Our aim is to present a consecutive, population-based series with as little selection bias as possible. The data presented is not reflective of the senior author’s personal series alone. Most of the data is derived from the Kuopio Cerebral Aneurysm Database (1977–2005), which contains information on all 3005 consecutive patients harboring 4253 aneurysms who were treated at Kuopio University Hospital, Finland, from 1977 to 2005.1–3
Aneurysms of the MCA
Middle cerebral artery aneurysms can be classified into three groups: proximal (M1As), bifurcation (MbifAs), or distal type (MdistAs) aneurysms (Table 75-1). The proximal MCA aneurysms or M1As are located on the main trunk (M1) of the MCA, between the bifurcation of the internal carotid artery (ICA) and the main bifurcation of MCA.1 The MbifAs are located at the main bifurcation of the MCA.2 The MdistAs, originate from the branches of the MCA distal to the main bifurcation inside the sylvian fissure.3 Each of these aneurysms have special features due to anatomic location and general behavior that need to be taken into consideration when planning occlusive treatment. Assigning an MCAA into a particular group can sometimes be difficult since the length and caliber of the M1 segment often varies and there may be two or even three major branching sites along its course. Generally, we consider MCA bifurcation to be the first and major branching site of the MCA where two or more rather similarly sized arterial trunks divide at the limen insula level. Occasionally, a thick frontal or temporal cortical branch of the M1 trunk creates a more proximal “false bifurcation.”33
| Category | Location |
|---|---|
| M1A | Main trunk of MCA, between ICA bifurcation and main MCA bifurcation |
| MbifA | Main MCA bifurcation |
| MdistA | Branches distal to main MCA bifurcation |
ICA, internal carotid artery; MCA, middle cerebral artery.
Incidence of MCA Aneurysms
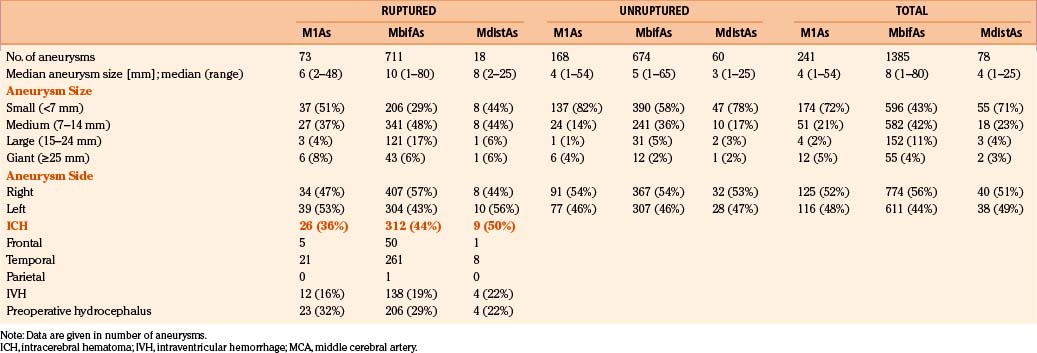
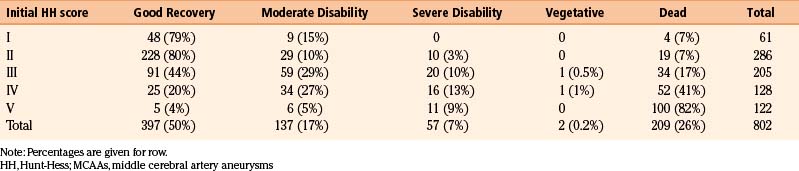
The MCA aneurysms represented 40% of all IAs in a consecutive and population-based series of 3005 patients with 4253 IAs from 1977 to 2005 in the Kuopio Cerebral Aneurysm Data Base.1–4 Tables 75-2 through 75-5 present the clinical data on the 1456 patients with MCA aneurysms in this series. Of the 3005 patients, 1456 (48%) had 1704 MCA aneurysms (Table 75-2). The most frequent location for MCAAs was the MCA bifurcation, and 1166 patients had 1385 MbifAs (33% of all 4253 IAs and 81% of all MCAAs). This breakdown is similar to other MCAA series.6,10–13,15,17,34,35 M1As comprised 14% of the MCAAs and MdistAs were the least frequent ones (5%) (Table 75-2). The right side dominated over the left side (55% vs. 45%) (Table 75-3).
Table 75-2 Patients with MCA Aneurysms in Consecutive and Population-Based Series of 3005 Patients with 4253 IAs from 1977 to 2005 in Kuopio Cerebral Aneurysm Database
| No. Patients | No. Aneurysms | |
|---|---|---|
| Whole series | 3005 | 4253 |
| Patients with primary SAH | 2365 (79%) | 3325 (78%) |
| Patients without primary SAH | 640 (21%) | 928 (22%) |
| MCA aneurysms | 1456 | 1704 |
| M1As | 221 (15%) | 241 (14%) |
| MbifAs | 1166 (80%) | 1385 (81%) |
| MdistAs | 69 (5%) | 78 (5%) |
| Ruptured MCA aneurysms | 802 | 802 |
| M1As | 73 (9%) | 73 (9%) |
| MbifAs | 711 (87%) | 711 (87%) |
| MdistAs | 18 (2%) | 18 (2%) |
| Fusiform MCA aneurysms | 18 | 18 |
| Fusiform M1As | 6 (33%) | 6 (33%) |
| Fusiform MbifAs | 8 (44%) | 8 (44%) |
| Fusiform MdistAs | 4 (22%) | 4 (22%) |
IA, intracranial aneurysm; MCA, middle cerebral artery; SAH, subarachnoid hemorrhage.
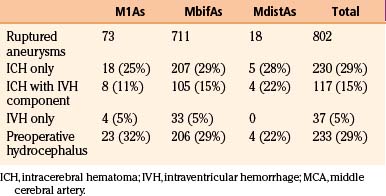
Table 75-4 Intracerebral Hematoma, IVH, and Acute Hydrocephalus Associated with 802 Ruptured MCA Aneurysms
Ruptured and Unruptured MCA Aneurysms
Of the 3005 patients, 2365 (79%) had a primary subarachnoid hemorrhage (SAH) from a ruptured IA. MCAAs were the cause of SAH in 802 (34%) of the 2365 patients. Again the MbifAs were the most frequent, comprising 87% of all the ruptured MCAAs (Table 75-2). M1As represented 9% of the ruptured MCAAs. There were only 18 patients with ruptured MdistA, less than 1% of all the ruptured IAs, and 2% of all the ruptured MCAAs. The median size for ruptured MbifAs was 10 mm (range 1 to 80 mm) (Table 75-3). Both the M1As and MdistAs were smaller than MbifAs in general, with median diameters of 4 mm (range 1–54 mm). Interestingly, 29% of the ruptured MbifAs, and as many as 51% of the ruptured M1As were smaller than 7 mm in diameter. This would indicate, that at least in the Finnish population, even small MCAAs are dangerous and the International Study of Unruptured Intracranial Aneurysms (ISUIA) results are controversial.36 Among the 1704 MCAAs, 69 (4%) were giant, most of them (80%) located at the MCA bifurcation. Of the 69 giant MCAAs, 72% were ruptured. There were 18 fusiform MCAAs, only 1% of all the 1704 MCAAs. Unlike the giant aneurysms, fusiform aneurysms were distributed rather evenly along the whole course of the MCA (Table 75-2). The total number of unruptured IAs in this series was 1888. Among the unruptured IAs, the MCAAs were even more frequent than among the ruptured ones (n = 902, 48%). MbifAs were again the most common (75% of all the unruptured MCAAs). The unruptured MCAAs were smaller in general than their ruptured counterparts, with median size ranging from 3 to 5 mm depending on the aneurysm location (Table 75-3).
Intracerebral Hematoma, Intraventricular Hemorrhage, and Preoperative Hydrocephalus
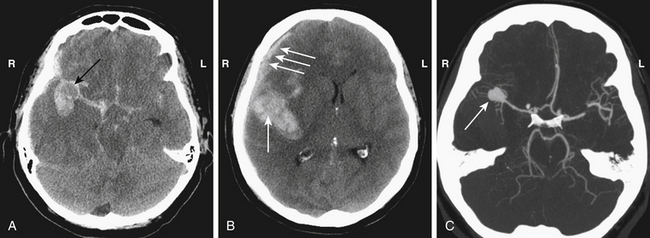
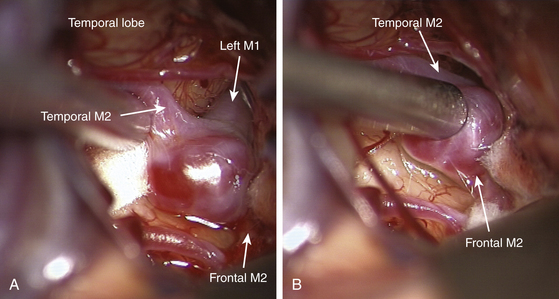
Ruptured MCAAs bleed frequently into the adjacent brain, and as many as 347 (43%) of the 802 patients with ruptured MCAAs presented with a space-occupying ICH (Table 75-4). ICHs were most often seen in MbifAs and MdistAs, 44% and 50%, respectively, and it were less frequently present in ruptured M1As (36%) (Fig. 75-1A to C). The higher risk for ICH in more distal MCAAs is probably due to a tighter cistern with the aneurysm more closely surrounded by the adjacent brain. The ICH was usually located in the temporal lobe (80%) and less frequently in the frontal lobe (20%) (Table 75-3). In the entire series, there was only one patient with a ruptured MCAA and parietal ICH. Intraventricular hemorrhage (IVH) was associated with the ICH in 15%, and isolated IVH without ICH was seen in only 5% of patients (Table 75-4). Rarely, ruptured MCAAs can also present with a subdural hematoma adding to the mass effect of an ICH (0.5% in our series) (Fig. 75-1B). Preoperative hydrocephalus was detected in 29% of the ruptured MCAAs (Table 75-4).
Associated Aneurysms
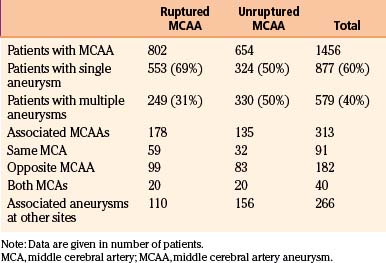
Middle cerebral artery aneurysms are often associated with other aneurysms, accounting for 40% of cases in our series (Table 75-5). Of the 579 patients who had at least one associated aneurysm, 313 (54%) had an MCAA as an associated aneurysm and 46% had associated aneurysms at locations other than the MCA. The most common associated aneurysm was MbifA. The associated MCAAs were more often seen at the opposite MCA than at the same MCA as the primary aneurysm (58% vs. 29%); 13% of patients with multiple MCAAs had the associated MCAAs on both MCAs (“mirror aneurysms”) (Table 75-5) (Fig. 75-2). MbifA was also the most frequently associated aneurysm among all 2365 patients with ruptured IAs in this series, and 12% had at least one associated MbifA.
Microsurgically Relevant Anatomy
Middle Cerebral Artery
The middle cerebral artery (MCA) is the major terminal branch of the ICA supplying a large part of the cerebral hemisphere along with the insula, lentiform nucleus, and internal capsule.37 The MCA is the most complex major cerebral artery owing to its anatomic and hemodynamic features. Microneurosurgical anatomy details of the MCA have been described by Yaşargil13,33 and others.37–42
The MCA is generally divided into four segments: M1 (sphenoidal), M2 (insular), M3 (opercular), and M4 (cortical).43 The M1 segment, the most proximal segment of the MCA, begins at the carotid bifurcation and extends to the bifurcation of the MCA, which is usually at the level of limen insula where it splits into two, sometimes three, major M2 branches. The M2s give rise to 8 to 12 branches before becoming the M3s at the peri-insular sulcus.37 The M3s continue to the surface of the sylvian fissure at the lateral surface of the brain. The M4 segments are located on the parasylvian surface of the brain and supply the lateral cortical surface of the cerebral hemisphere.13,33,37,38,43
M1 Segment
The M1 starts in the sylvian cistern at the carotid bifurcation, supralateral to the optic chiasm, inferior to the anterior perforated substance, and posterior to the division of olfactory tract. Thick arachnoid covers the M1 origin and bridging arachnoid fibers surround its proximal part. M1 travels laterally in the sylvian fissure until the bifurcation at the insular apex.37 At the MCA bifurcation, the M1 splits usually into two (bifurcation) branches (M2s), the superior (frontal) and the inferior (temporal).33,37 Türe et al. divided M1 branches into (1) the cortical branches (often named as temporopolar, frontotemporal, and orbitofrontal branches) and (2) the lateral lenticulostriate branches. In the surgical trajectory to the sylvian cistern, the cortical branches (one to three) mainly project toward the temporal lobe (75%) and less often toward the frontal lobe (25%). Variations include temporal only, temporal and frontal, frontal only, and no major cortical branches.37,43 Lateral lenticulostriate arteries originate mainly from the M1 trunk (see below), and identification of their origin should help to distinguish the true MCA bifurcation. The preservation of M1 branches is of paramount importance in the occlusive therapy for M1As.
Lateral Lenticulostriate Arteries
The lateral lenticulostriate arteries (LLAs) are quite variable in number (up to 20) and in sites of origin.33,37,38,40,41,44 Lateral lenticulostriate arteries mainly arise from the frontal aspect or cortical branches of the M1. However, LLAs may also arise, in up to 23%, from the MCA bifurcation, the M2s, or an accessory M2.37,40 The more proximal the bifurcation, the greater the number of postbifurcational LLA branches.43 In the surgical trajectory, LLAs mainly arise from the frontal aspect of the M1 and they mainly turn toward the frontal lobe. LLAs enter the brain via central and lateral parts of the anterior perforating substance and supply the substantia innominata, putamen, globus pallidus, head and body of the caudate nucleus, internal capsule and adjacent corona radiata, and the central portion of the anterior commissure.37 M1As and MbifAs may more or less involve LLAs at their branching sites,13,40,43 and LLAs may be displaced, compressed, distorted, or stretched by M1As.44 During dissection and clipping of M1As, the site and pattern of origin of the LLAs are of special concern. LLAs may arise from a single-stem branch of M1, and severing the stem branch causes infarct in the entire LLA supply area.44 The arachnoid adhesions together with cortical and lateral lenticulostriate branches as well as very small pial branches, also originating from M1, limit the mobilization of M1 in the sylvian fissure.33,37,41
MCA Bifurcation and M2 Segments
The location of the bifurcational complex in the sylvian fissure varies considerably depending on the length of the M1, as well as the angioarchitecture of the bifurcation complex.33,37,38,43 Occasionally, a thick frontal or temporal cortical branch of the M1 trunk creates a “false bifurcation” more proximal.33 After their origin at the MCA bifurcation, the M2s run somewhat parallel and supply the insula.37,41,43 The M2s are seldom of equal diameter (15%), and usually, the inferior (temporal) trunk is dominating (50%). In 55% of the hemispheres studied by Türe et al., the dominant M2 trunk bifurcated soon after the main bifurcation.37 This gave an impression of trifurcation in 12.5%, and quadrifurcation was seen in 2.5% when both M2s bifurcated immediately. Umansky et al.40 reported bifurcation in 66%, trifurcation in 26%, and quadrifurcation in 4%, and Gibo et al.38 reported bifurcation in 78%, trifurcation in 12%, and multiple trunks in 10%.
The M2s give rise to 8 to 12 branches, mainly arising from the superior trunk, before becoming the M3s.37 The superior (frontal) M2 is the origin of the prefrontal, precentral, and central arteries. Furthermore, 23% the anterior and posterior parietal arteries have their origin from the superior M2.37 They mainly supply the inferior frontal cortex, the frontal opercular cortex, and also the cortex in parietal and central sulcus areas.33,37,38,43 The inferior (temporal) M2 is the main origin of the posterior and middle temporal arteries, supplying mainly the middle and posterior temporal cortex and temporo-occipital, angular, and posterior parietal regions.33,37,38,43
Distal MCA Branches (M3 and M4)
The M3 (opercular) segments start at the peri-insular sulci, from where they rise toward the lateral surface of the brain at the surface of the sylvian fissure. The M3 branches run on either side (temporal or frontal) of the sylvian fissure, they do not generally cross over. The M3s mainly supply the medial opercular surface and, to a lesser extent (25%), the superior or inferior peri-insular sulcus.37 The M4 segments are located on the cerebral cortex rising from inside the sylvian fissure.33,37,38,43 They supply the 12 previously documented arterial territories of the lateral surface of the cerebral hemisphere: (1) the lateral orbitofrontal, (2) the prefrontal, (3) the precentral, (4) the central, (5) the anterior parietal, (6) the posterior parietal, (7) the angular, (8) the temporo-occipital, (9) the posterior temporal, (10) the middle temporal, (11) the anterior temporal, and (12) the temporopolar areas.33,37,38,43
Cisternal Anatomy
The MCA (M1–M3) travels inside the sylvian fissure for most of its course. Only the proximal portion of the M1 segment is found inside the carotid cistern, which is limited by the proximal sylvian membrane from its lateral border.45 After passing the proximal sylvian membrane the M1 enters into the anterior compartment of the sylvian cistern. It is usually in the anterior compartment of the sylvian cistern where most of the LLAs can be found.45 The borderline between the anterior and posterior compartment of the sylvian cistern is the limen insula. The posterior compartment of the sylvian cistern is located behind the limen insulae where MCA, before or after bifurcating, makes a relatively sharp, almost 90-degree turn (“the genu of MCA”).46 The posterior compartment is further divided into the medial and lateral compartments by the intermediate sylvian membrane. The medial compartment contains the M2 trunks, whereas the M3 segments passing toward the cortical surface run for most of their course in the lateral compartment.45 The width, depth, and folding of the sylvian fissure vary considerably.33,46 In general, the portions of MCA that are the most difficult to reach are on the M1 segment once it has entered the sylvian cistern, as the cisternal space here is very deep and narrow and there is high risk of injuring the lateral lenticulostriate arteries.1 The other challenging region is the very distal part of sylvian fissure, which is also narrow and there is risk of damage to cortical MCA branches. Fortunately, most MCA aneurysms are located at the MCA bifurcation, which can be found in most cases at the border of the anterior and posterior compartments of the sylvian cistern where the cistern is wider.
Venous Anatomy
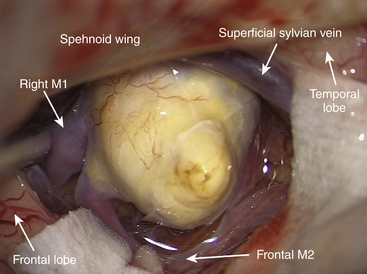
The most important vein encountered during surgery for MCA aneurysms is the superficial sylvian vein. It usually arises at the posterior end of the sylvian fissure as one or several trunks, and courses anteriorly and inferiorly along the fissure. The separate trunks often merge into a single large channel before emptying into the venous sinuses along the sphenoid ridge.47 The superficial sylvian vein receives the frontosylvian, parietosylvian, and temporosylvian veins and commonly anastomoses with the veins of Trolard and Labbé. It penetrates the arachnoid covering of the anterior portion of the sylvian fissure and joins the sphenoparietal sinus as it courses just below the medial part of the sphenoid ridge, or it may pass directly to the cavernous sinus.47 Anomalies of the venous configuration are common and sometimes the superficial sylvian vein may be absent altogether.33,47 Most of the time the superficial sylvian vein courses mainly on the temporal side of the sylvian fissure so that arachnoid opening of the frontotemporal arachnoid membrane should be planned on the frontal-lobe side of the sylvian fissure. Venous crossover branches from one side of the sylvian fissure to the other are more frequent than in arteries. The main trunk of the superficial sylvian vein should always be left intact to prevent postoperative venous infarcts. Small crossover branch may need to be coagulated and cut to provide sufficient exposure of the deeper parts of the sylvian fissure. Deeper, inside the sylvian fissure the deep middle sylvian vein can be encountered. This collects venous outflow mainly from veins of the insular cortex and it terminates in the basal vein of Rosenthal.48
Location and Orientation of MCA Aneurysms
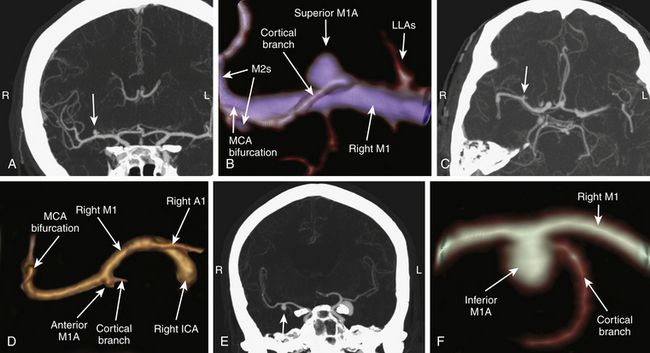
M1As can be found along the entire M1 segment, most often at the distal portion of the M1 segment at the origin of one of the cortical branches. On angiograms, the M1As are oriented with their dome pointing anterior, inferior, superior, or posterior (Fig. 75-3A to F). The superior or posterior projecting M1As, also called frontally projecting, project toward the frontal lobe. They are considered the most challenging M1As for three main reasons: (1) heavy involvement with LLAs, (2) in the surgical view the M1 trunk is partially or completely obstructing the view toward the aneurysm base and the origin of the cortical branch(s), and (3) the dome is buried inside the inferior portion of the frontal lobe in the deepest and narrowest part of the proximal sylvian fissure. The M1As with anterior or inferior projection, also called temporally projecting, project toward the temporal lobe. They are usually easier to expose during dissection than the frontally projecting ones.
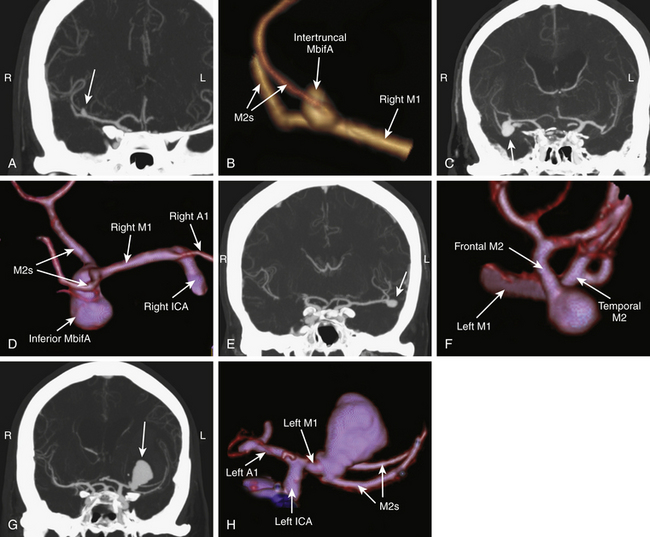
The orientation of MbifAs in the sylvian fissure depends on the depth of the fissure, the length and course of the M1, and the projection of the MbifA dome. We classify MbifAs into five groups based on their orientation (Fig. 75-4A to H):
1. Intertruncal MbifA: The dome projects superiorly in the coronal (AP) plane and posteriorly in the axial plane. Intertruncal MbifAs lie between the M2s, the base often more on the thicker M2, and the M2s are more or less involved in the base.
2. Inferior MbifA: The dome projects inferiorly in the coronal (AP) plane and anteriorly (toward the sphenoid ridge) in the axial plane.
3. Lateral MbifA: The dome projects laterally in the coronal (AP) plane and laterally in the axial plane, in the same direction as M1.
4. Insular MbifA: The dome projects medially (toward the insula) in the coronal (AP) plane and medially in the axial plane.
5. Complex MbifA: In some dysmorphic and large or giant aneurysms, the growth of the dome may be multidirectional and the relation with M1 and M2s may be a combination of the aforementioned types (Fig. 75-5). Types 2, 3, and 4 are not intertruncal and do not principally involve the M2s. The orientation may be distorted by a space-occupying ICH.
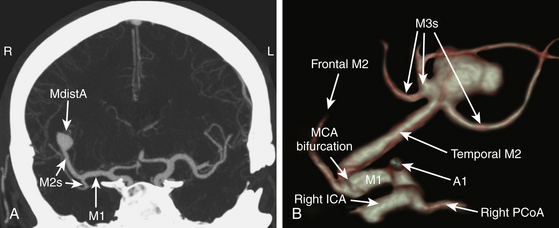
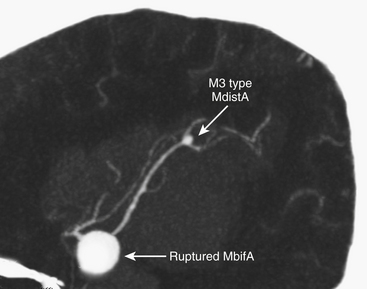
We divide MdistAs into aneurysms of the M2 trunk or at the M2–M3 junction (Fig. 75-6A and B), and those distal to the M2–M3 junction or of peripheral (M3) branches (Fig. 75-7). Location is more important than the dome orientation in MdistAs.
Imaging
1. Noninvasive and quick imaging technique
2. Shows anatomy of the adjacent bony structures to help to plan the approach
3. Comparable sensitivity and specificity to DSA in aneurysms larger than 2 mm49–56
4. Disclosure of calcifications in the walls of arteries and aneurysm57,58
5. Quick reconstruction of three-dimensional (3D) images that, for example, show the surgeon’s view of the origin of the MCA aneurysm
Some MCAAs may be difficult to visualize by routine 3D CTA,50,57 usually due to very small size, so that subsequent rotational 3D DSA is required.
At the workstation, 3D CTA images can be rotated accordingly to evaluate the surgeon’s view to the MCA and the bifurcation, which is not standard but is tailored according to the aneurysm dome projection and relation to the MCA and its branches. The prime concern is to find a view that best helps to preserve the perforators around the base and the dome of the aneurysm.
Principles of Neuroanesthesia
A review of our neuroanesthesiologic principles in treatment of SAH patients has been published previously.59 Here we present only some key points.
Tranexamic acid (1 g intravenously every 6 hours for 3 days) is administered to prevent rebleeding until clipping.60 Nimodipine (oral or intravenous) is given to all patients with ruptured aneurysms to prevent vasospasm.61,62
Postoperatively, all SAH patients are treated at the neurointensive care unit (NICU). Our general principles of postoperative treatment and monitoring are summarized in Table 75-6.
Table 75-6 Postoperative Care of Patients with Aneurysmal SAH at Neurosurgical ICU in Helsinki
| Prevention/Treatment of Vasospasm |
|---|
| Nimodipine oral/I.V. Magnesium: 60 mmol/day (only in detected vasospasm) Hypertension: phenylephrine, norepinephrine, or dopamine/dobutamine Hemodilution: Hct 0.3. Ringer’s acetate (+NaCl)/tetrastarch Prevention of vasospasm: HH: 1-2; Fischer: 1-2; sBP > 110–120 mm Hg, normovolemia HH: 1-2; Fischer: 3-4; sBP > 140 mm Hg, normovolemia HH: 3-5: Fischer: 3-4; sBP > 140–160 mm Hg, slight hypervolemia Treatment of vasospasm: “Triple H” (hypertension; hypervolemia; hemodilution): sBP > 160–180 mm Hg |
| Pulmonary/Airway Management |
| Oxygen/ventilatory support as needed: Normoventilation, SaO2 > 95%, PaO2 > 13 kPa Pneumonia, aspiration: Antibiotics Pulmonary edema: Noncardiogenic/cardiogenic, PEEP, furosemide, dobutamine |
| Seizures |
| Previous antiepileptic drugs (lorazepam or lavetiracetam) No routine prophylaxis |
| Electrolytes and Glucose |
| Correct abnormalities Hyponatremia: SIADH, CSW syndrome B-gluc 5-8 mmol/L |
| Sedation, Postoperative Pain and Fever |
| Propofol and/or dexmedetomidine patients under mechanical ventilation Benzodiatzepines Opioids: Oxycodone Paracetamol Active cooling as needed NSAIDs: 5–7 days post SAH |
| Thromboembolism |
| Antiembolic or pneumatic compression stockings Individually LMWH 5–7 days postcraniotomy |
CSW, cerebral salt wasting; HH, Hunt-Hess; LMWH, low molecular weight heparin; PEEP, positive end-expiratory pressure; SAH, subarachnoid hemorrhage; sBP, systolic blood pressure; SIADH, syndrome of inappropriate antidiuretic hormone hypersecretion.
Patient Positioning and Craniotomy
All except those rare distal MCAAs can be reached through a standard pterional approach as described by Yaşargil.13 At our institution we prefer the lateral supraorbital (LSO) approach, a more frontal and less invasive modification of the pterional approach.63 The LSO approach has been used by the senior author (JH) in microsurgery of more than 3000 anterior circulation aneurysms over the past 20 years. The pterional approach is reserved for selected cases with space occupying ICH or giant aneurysms. A detailed description of the LSO approach has been described elsewhere.1,63
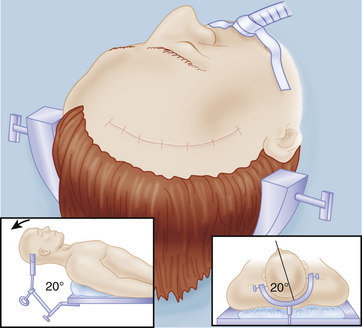
Briefly, the head fixed to the head frame is elevated clearly above the cardiac level, rotated 20 degrees to 30 degrees toward the opposite side, tilted somewhat laterally for optimal visualization of the MCA and the aneurysm base, and minimally extended (Fig. 75-8). The lateral tilt is used to have the proximal part of the sylvian fissure almost vertical. It is an error to overturn the head so that the temporal lobe “covers” the sylvian fissure and the aneurysm in the surgeon’s view requiring unnecessary retraction of the temporal lobe. It is our practice to adjust the position of the fixed head and body during the operation as needed.64 We prefer to use a Sugita head frame with four-point fixation. Besides providing good retraction force by its fishhooks, the frame allows to be rotated during surgery. If this feature is not available, the table can be rotated as needed.
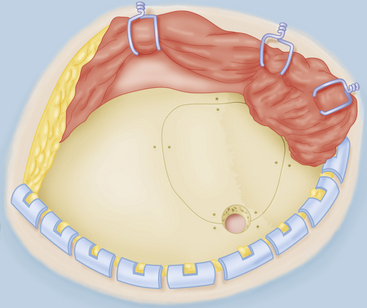
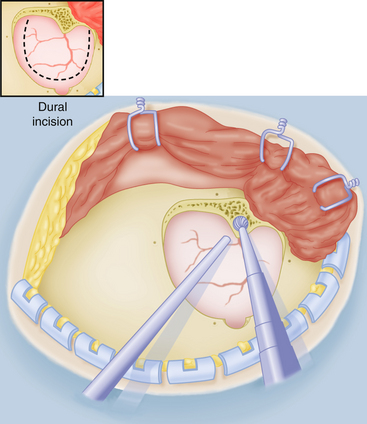
After minimal shaving, an oblique frontotemporal skin incision is made behind the hair line. The incision is short and stops 2 to 3 cm above the zygomatic arch. The incision is partially opened by frontal spring hooks. The temporal muscle is split vertically by a short incision, and a single spring hook is placed in the incision to retract the muscle toward the zygomatic arch. The one-layer skin–muscle flap is retracted frontally by spring hooks until the superior orbital rim and the anterior zygomatic arch are exposed (Fig. 75-9). The extent of craniotomy depends on the surgeon’s experience and preferences. Usually, a small LSO craniotomy is all that is necessary (the keyhole principle). A single burr hole is placed just under the temporal line in the bone, the superior insertion of the temporal muscle. The bone flap of 3×3 cm is detached mostly by the side-cutting drill, and the basal part can be drilled before lifting. In case of ICH or giant MCAA, a larger craniotomy is performed toward the zygomatic arch such as the classic pterional craniotomy. The vertical bone ridge and lateral sphenoid ridge are drilled to create an optimal view of the sylvian cistern (Fig. 75-10). Anterior clinoid is not removed.
For MdistAs, the exposure of aneurysms on the M2s or at the M2–M3 junction depends on the location of the main bifurcation and the presence of ICH or associated aneurysms. These aneurysms are operated on using the LSO approach with little less of sphenoid ridge removal. Aneurysms located on the inferior (temporal) M2 trunk or its branches are usually hidden under the temporal operculum, so the head should be rotated less with minimal or no lateral tilt. Aneurysms located on the superior (frontal) M2 trunk are hidden under frontal operculum, so lateral tilt is usually helpful. The exposure in aneurysms distal to the M2–M3 junction depends on how distally they are in the sylvian fissure. Positioning of the head, and placement of the craniotomy and approach, should be tailored according to anatomic localization and projection of the aneurysm, and the presence of ICH or associated aneurysms. Usually, these aneurysms can be approached through a temporal craniotomy with the patient in lateral (park bench) position.65 Neuronavigation may be of help in planning the craniotomy in these cases.
Intracerebral Hematoma
MbifAs are the most frequent cause of aneurysmal ICH that requires emergency removal (Fig. 75-1A to C).17 In the Kuopio series, as many as 44% of the 711 ruptured MbifAs had bled into the adjacent brain tissue (Table 75-4). In our practice, patients with massive ICHs are transferred directly to the operating room from emergent CT/CTA for immediate evacuation of the ICH and clipping of the aneurysm(s). Early surgical removal of massive ICH is believed to improve the outcome with ruptured MCA aneurysms.10,17,30,31,66–71 The propensity for ICH may explain higher-than-average management morbidity and mortality of patients with MbifA compared to other anterior circulation aneurysms.17
Dissection Toward the Aneurysm
The best place to enter the sylvian fissure is usually where transparent arachnoid is present. The venous anatomy on the surface of the sylvian fissure is highly variable. Multiple large veins often follow the course of the sylvian fissure, draining into the sphenoparietal or cavernous sinuses. These veins are generally running on the temporal side of the sylvian fissure. Generally, we prefer to open the arachnoid covering the sylvian fissure on the frontal lobe side. However, in the presence of multiple large veins or anatomic variations the dissection plan should be tailored accordingly. Dissection of the sylvian fissure is more difficult with swollen brain tissue in acute SAH or with adhesions from previous SAH or microsurgery. Preservation of the dissection plane is mandatory.
The whole opening of the sylvian fissure should be performed under very high magnification (7–9×) of the microscope. First, we usually open a small window in the arachnoid with a pair of jeweler forceps or a sharp needle acting like an arachnoid knife. Then we expand the sylvian fissure by injecting saline using a handheld syringe, that is, the water dissection technique of Toth.72 The idea is to get relatively deep into the sylvian fissure to enter the sylvian cistern from this small arachnoid opening. There are two arachnoid membranes that need to be opened, a superficial one covering the cortex and a deeper one inside the fissure limiting the sylvian cistern (Fig. 75-11). Once inside the sylvian cistern, the dissection proceeds proximally by gently spreading the fissure in an inside-out manner. In our experience, this technique allows easier identification of the proper dissection plane. Bipolar forceps and suction act both as dissection instruments and delicate microretractors.64 Cottonoids applied at the edges of the dissected space act as soft retractors, and pressure applied gently on both the walls of the fissure will stretch the overlying bridging tissues, facilitating their sharp dissection. All arachnoid attachments and strands are cut with microscissors, which can be also used as a dissector when the tips are closed. In order to preserve larger veins, some small bridging veins may have to be coagulated and cut.
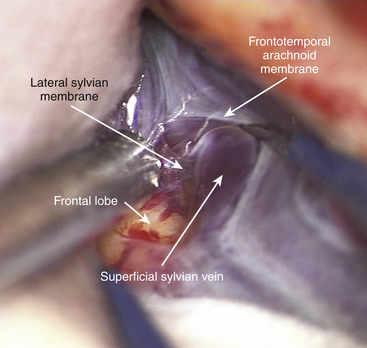
FIGURE 75-11 Intraoperative photograph showing the course of superficial sylvian vein between the two arachnoid membranes.
Inside the sylvian cistern, the M3s and M2s are identified and followed proximally. The M2s should be covered by the intermediate sylvian membrane, another arachnoid membrane, which in some patients can be rather prominent and in others hardly identifiable. By following the M2s proximally, one should arrive at the MCA bifurcation where the most difficult task is to identify the M1 trunk for proximal control (Fig. 75-12A). In the surgical view the M1 is often hidden by the bifurcation and its course is often along the visual axis of the microscope making its identification quite difficult during the initial dissection of the MCA bifurcation. One of the M2 trunks with medial course is easily confused with the M1 unless one keeps this in mind. The M1 can be often more easily reached from behind and below the bifurcation than from in front and above. A more distal opening of the sylvian fissure provides a better angle to visualize and obtain control of the M1 just beneath the bifurcation. Also, both M2s should be prepared for temporary clips. For M1As, the dissection continues proximally along the M1 trunk in the deepest and often the narrowest part of the proximal sylvian fissure. Care is needed to avoid severing the LLAs during the various stages of dissection. To better expose the origin of the cortical branches and the M1A base, gentle retraction of the M1 trunk frontally by suction or bipolar may be necessary. Numerous arachnoid trabeculations around the proximal M1 trunk make dissection demanding, and we advocate sharp dissection.
Dissection and Clipping of MCA Aneurysms
Usually, dissecting the dome completely free before applying the so-called “pilot” clip is not recommended. Instead, the arteries around and adjacent to the base should be dissected free and the base cleared thoroughly. The M1, M2s, and adjacent and perforating branches should be unhurriedly, clearly, and painstakingly visualized before final clipping of the MCAA neck (Fig. 75-12B).
Aneurysm Rupture before Clipping
Middle cerebral artery aneurysms may rupture while opening the sylvian fissure or dissecting the aneurysm base. The risk of rupture is highest for the aneurysms attached to the temporal lobe, where extensive manipulation and retraction of the temporal lobe may stretch the dome and cause intraoperative rupture of the aneurysm. This is why retraction of the temporal lobe should be avoided during the initial steps of the dissection. In case of rupture, control should be first attempted via suction and compression of the bleeding site with Cottonoids. One should not try to clip the aneurysm in haste directly as this could easily end up in tearing the aneurysm base or even the parent artery. Instead, the aneurysm should be isolated with temporary clips applied both proximally and distally. With the bleeding under control, the aneurysm neck is dissected free and the pilot clip applied. Short and sudden hypotension by cardiac arrest, induced by intravenous adenosine,59 can be used to facilitate quick dissection and application of a pilot clip in case of uncontrolled bleeding. A small and thin walled MCAA may rupture at its neck during dissection. Under temporary clipping of arteries, reconstruction of the base by involving a part of the parent artery in the clip should be attempted. One option, hindered by the deep location inside the sylvian fissure, is to suture the rupture site with 8-0 or 9-0 running sutures or to repair the site using anastoclips,73 followed by clipping and augmented by glue.
Acute Hydrocephalus and Cerebrospinal Fluid Drainage
In the Kuopio series, 29% of the SAH patients had hydrocephalus early in the treatment (Table 75-4). In few cases, an emergent extraventricular drain (EVD) can be life-saving to reduce the ICP, and thereby to lower the risk of brain damage. In most cases we do this after securing an acutely ruptured aneurysm, but we have not seen iatrogenic ruptures due to puncturing of the ventricles, either. Nowadays, leaving drainage via lamina terminalis after clipping of the aneurysm has almost completely replaced conventional EVDs.74 In most unruptured MCAAs, we directly open the sylvian fissure. In ruptured MCAAs and in some unruptured ones, carotid and chiasmatic cisterns are first opened to gradually let cerebrospinal fluid (CSF) drain. In acute SAH, we usually continue the dissection subfrontally to open the lamina terminalis for additional CSF removal before clipping.74 A catheter can be inserted into the third ventricle through the same opening in the lamina terminalis for postoperative ICP monitoring and CSF drainage. Intraoperative ventricular puncture is rarely adopted and may be technically challenging.
Special Considerations for Different Locations and Orientations of MCA Aneurysms
M1A
M1As are located deep and proximal inside the sylvian fissure where there is much less space for dissection than in more distal MCAAs. M1As are intimately connected to one or more branching arteries of the M1 and often in close proximity to LLAs (Fig. 75-3A to F). They are often wide-necked, small, and thin-walled, which makes their proper clipping tedious. There are few reports of M1A microsurgery.1,8,9,13,75,76
MbifA
Of the MCA aneurysms, MbifAs are by far the most frequent (Table 75-2). In population-based services, MbifAs are frequent targets for elective surgery (unruptured), acute surgery (ruptured), emergency surgery (large ICH), and even advanced approaches (giant) (see Hernesniemi, MCA Video 5).2 MbifAs are often broad-necked and may be dysmorphic in shape involving one or both branches of the bifurcation (M2s). Other branches may be attached to their wall, and, less frequently, perforators may be at risk when originating from the bifurcational region. Exposure in MbifA surgery depends on the length of the M1, the size and projection of the aneurysm dome, and the existence of an ICH or associated aneurysms.
Intertruncal MbifAs project superiorly in the coronal (AP) and posteriorly in the axial plane (Fig. 75-4A and B). The dome projects to the same direction as the M2 trunks and lies between them. The base is often broad and involves the origin of one M2 (the thicker one) or both. The attachment of the M2s to the base and the proximal part of the dome makes intertruncal MbifAs demanding to clip adequately. The head is rotated 25 to 30 degrees with minimal extension and some lateral tilt. As the dome of the aneurysm lies between M2s, we prefer distal opening of the sylvian fissure and careful exposure of the frontal M2 at the beginning. Dissection is continued at the frontal side of the bifurcation, so as not to expose the aneurysm dome, and then turned below to search the M1. In intertruncal MbifAs, painstaking dissection of the base is required, with visualization of the M1 in the early phase of dissection for temporary clip placement.
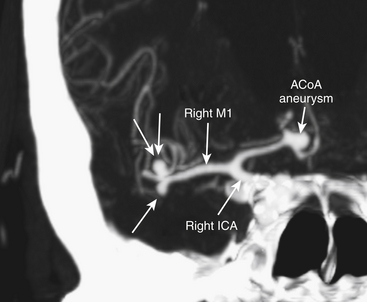
Inferior MbifAs project inferiorly in the coronal (AP) and anteriorly toward the sphenoid ridge, in the axial plane (Fig. 75-4C and D). Consequently, the dome is projecting to the temporal aspect of the surgeon’s view toward the surface of the sylvian fissure (Fig. 75-13). Minor flexion of the head plus normal rotation and increased lateral tilt provide a good view of the sphenoid ridge and proximal part of the M1. After proximal opening of the sylvian fissure, dissection is continued on the frontal side of the bifurcation, and, with slight retraction of the frontal lobe, the M1 and the frontal M2 are visualized and dissected (see Hernesniemi, MCA Video 3 and MCA Video 4). Any retraction on the temporal side would risk a rupture of the aneurysm. After sharp dissection of both the M1 and the frontal M2, the base of the aneurysm is exposed. Visualization of the temporal M2 requires further careful dissection on the distal side of the base. The base of this aneurysm type is usually free of perforating arteries or branches, and the pilot clip can be placed easily under the control of temporary clips. Special attention must be paid to the origin of the temporal M2 trunk which easily becomes pinched or occluded by the distal tips of the clip blades.
Lateral MbifAs project laterally in the coronal (AP) plane and in the axial plane (Fig. 75-4E and F). In the surgeon’s view, lateral MbifAs follow the same direction as the M1 trunk. Minor flexion of the head together with normal rotation (25–30 degrees) and more pronounced lateral tilt provide the best possible view of the base of the aneurysm and direct the tip of the aneurysm away from surgical trajectory. Lateral MbifAs are frequently attached to the arachnoid coverings of the sylvian fissure, risking premature rupture if the dissection of the coverings is started improperly. Sylvian dissection is started distally to find the frontal M2 which is then followed toward the bifurcation and the base of the aneurysm (see Hernesniemi, MCA Video 6). To prepare for a premature rupture, first the base of the aneurysm is carefully prepared for a pilot clip placement over the dome, and then the dissection is continued toward the M1 to find a proper place for proximal temporary clip. Special care must be taken to visualize the origin of the temporal M2. Coagulation and reshaping must be done with respect to the origins of the M2s. A final clip is placed along the largest diameter of the base.
Insular MbifAs project medially in the coronal (AP) plane and medially in the axial plane (Fig. 75-4G and H). In the surgeon’s view, insular MbifAs project behind the bifurcation, toward the insular surface. The head is rotated more than normal (>25 to 30 degrees) so that the bifurcation is exposed to the surgeon, making proximal control and clipping most feasible. However, overturning may cause hiding of the sylvian fissure by the temporal lobe. Because the aneurysm dome projects behind the bifurcation, distal to proximal dissection of the M2s, the bifurcation and the M1 is safe. When the M1 and M2s are free, two to three temporary clips are applied, and with complete isolation of the blood flow, the base of the aneurysm is carefully dissected in its anterior and lateral parts. The shortest possible pilot clip is placed on the base and the temporary clips are removed. The position of the pilot clip is carefully checked with particular care for small perforating branches, which might easily be occluded, in the same way as during clipping of ICA and basilar tip aneurysms.
Complex MbifAs are a group of dysmorphic and large or giant aneurysms, where the growth of the dome is usually multidirectional and the relation of the base with the M1 and the M2s may be a combination of the other MbifA types (Fig. 75-5). Head positioning and craniotomy should be tailored according to the 3D relation of aneurysm with the MCA bifurcation. Proper clipping of the aneurysm usually requires a combination of several clips.
MdistA
MdistAs are the least frequent of MCA aneurysms (Table 75-2). The greatest challenge in microneurosurgical treatment of MdistAs is to localize them, as they lie deep in the sylvian cistern, among the distal branches of the MCA.3 Intraoperative navigation may be further complicated by the presence of SAHs and ICHs. Furthermore, MdistAs can be mycotic, inflammatory, or dissecting. The lack of collateral circulation makes occlusion more challenging, necessitating bypass and revascularization techniques. There are only few reports on management of MdistAs.3,17,77–79
The exposure in aneurysms on the M2 or at the M2–M3 junction depends on the location of the main bifurcation and the presence of ICH or associated aneurysms (Fig. 75-6A and B). In the proximity of the main MCA bifurcation, the same approach as for MbifAs will do. To expose more distal MdistAs, the approach must be more occipital over the sylvian fissure (see Hernesniemi, MCA Video 7). We measure the distance between the ICA and the MCA bifurcation (length of M1 segment) in CTA images, in both coronal and axial planes. This is important when selecting the proper site for the arachnoid opening and for intrasylvian orientation. The more distal the MdistAs are, the more difficult they are to localize. Bony landmarks in the CTA are of less value in the localization of MdistAs than in M1As or MbifAs. The sylvian fissure is opened at a proper site proximal to the aneurysm, and the dissection is performed from proximal to distal toward the aneurysm. After finding the aneurysm, the rest of the dissection and subsequent clipping are often straightforward. When the aneurysm site is identified, the parent artery is dissected free and a temporary clip is applied to allow final dissection of the aneurysm dome and clipping of its neck.
The exposure in aneurysms distal to the M2–M3 junction depends on how distally they are located in the sylvian fissure (Fig. 75-7). Positioning and placement of the craniotomy and approach should be tailored according to the anatomic localization and projection of the aneurysm, and the presence of an ICH or associated aneurysms. Typically, these aneurysms can be approached through a temporal craniotomy with the patient in lateral (park bench) position. When MdistAs originate from the branches distal to the M2–M3 junction, they are more difficult to find, especially when the sylvian fissure is filled with fresh blood in acute SAH. The parent artery and the aneurysm are usually deep and behind a corner in the T-shaped sylvian fissure, hidden behind the frontal (superior) or the temporal (inferior) operculum of the insula. Neuronavigation, intraoperative noninvasive indocyaninen green angiography (ICG),80 or color Doppler ultrasound, should be considered for localizing the aneurysm. The actual clipping of the aneurysm is seldom a problem. A small and light final clip should be used when possible to prevent kinking of the arteries.
Postoperative Care and Imaging
Anesthesiologic principles for our postoperative management of patients after aneurysm surgery are summarized in Table 75-6. Postoperative imaging is performed in all patients; the aim is quality control to identify unexpected neck remnants and branch occlusions. In a previous analysis, we saw unexpected neck remnants in 10 (3%) and unexpected major branch occlusions in 11 (4%) of 306 clipped MCA aneurysms.81 We perform CT and CTA routinely on all patients on the first postoperative day. Even though CTA has been considered less accurate than DSA for controlling neck remnants of clipped aneurysms, with modern devices and the use of titanium clips, accuracy has increased dramatically, and DSA can be reserved for complex aneurysms or situations where multiple clips prevent proper interpretation of the CTA images.82 In general, the risk for rerupture of a previously clipped aneurysm is low and late imaging follow-up is not used routinely.83,84 In young patients with familial history or multiple aneurysms, we recommend a more cautious approach with long-term angiographic follow-up.85,86
Associated Aneurysms
Half of all patients with unruptured MCAAs and 31% of those with ruptured MCAAs have at least one additional aneurysm (Table 75-5). Multiple MCAAs were seen in as many as 54% of the patients with multiple aneurysms (Fig. 75-2). Our strategy is to clip all the aneurysms that can be exposed through the same craniotomy. This may not be advisable if the clipping of the ruptured aneurysm is difficult or the brain is swollen owing to an acute SAH.87,88 We do not advocate multiple craniotomies in the acute stage of an SAH. The remaining unruptured aneurysms can be treated depending on the estimated rupture risk and patient’s wishes in separate session(s) once the patient has recovered, such as 3 to 12 months after the initial SAH.
Contralateral Approach for MCA Aneurysms
Contralateral M1As close to the ICA bifurcation can be clipped via the contralateral approach irrespective of their orientation.13,87,89–91 A contralateral MbifA can also be reached, but only if it projects downward in the sylvian fissure and the length of the M1 is reasonable (<20 mm). The contralateral approach for bilateral MCA aneurysms is not recommended at an early learning curve. Extensive dissection above the chiasm along the contralateral M1 risks overstretching of the medial lenticulostriate arteries, LLAs, and perforators to the chiasm, hypothalamus, and anterior perforating substance. A contralateral approach should be avoided if the brain is swollen due to an acute SAH. In addition, extensive venous structures in the ICA bifurcation region and along the M1 may prevent proper visualization of the contralateral M1.
Special Subgroups of MCA Aneurysms
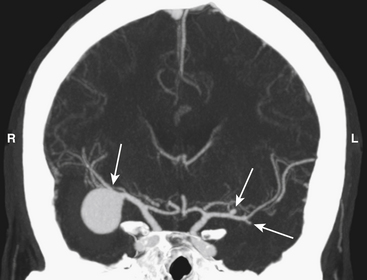
Giant MCA Aneurysms
The MCA is the most frequent site for giant aneurysms. In the Kuopio series, 4% of all MCAAs and 6% of ruptured MCAAs were giant (≥25 mm) (Table 75-3). Combined 3D DSA, CTA, and MRI data are necessary for a complete view on the vascular anatomy, intraluminal thrombus, and thickness and calcifications of the wall.92–94 In published series, direct clipping was possible in the majority of cases (38%–71%).9,95–100 Cases considered for EC–IC or IC–IC bypass surgery are increasing.101
The head position is adjusted for a better view of the proximal M1. Classical pterional approach with a large enough bone flap, also to the medial frontobasal direction, is undertaken to allow specific neurovascular techniques. For adequate visualization of the aneurysm base, an extensive exposure of the sylvian fissure is needed. The ICA bifurcation and the proximal M1 and the M2s (distal to the aneurysm) should be exposed and prepared for temporary clipping. In patients with ICH, we prefer a combination of trans-sylvian and superior temporal approaches. Here, besides the removal of a part of the hematoma, a narrow cortical incision and subpial resection may provide a better view of the aneurysm base and branches. Lamina terminalis is opened to let CSF drain. Clips of proper lengths and configurations are selected. Temporary clips are inserted onto the proximal M1 and the M2s, and the aneurysm dome is incised with a knife for internal decompression, performed usually by suction or, in case of major thrombus, by ultrasonic aspirator. Intraluminal thrombus is carefully removed, and the decompressed dome, between the neck and the incision, is clamped by a DeBakey vascular clamp. The vascular clamp softens the base for aneurysm clips and also prevents slipping of the intraluminal thrombus inside the M2s.102 The lumen is irrigated copiously by saline. Then the dome is usually reduced to allow for final dissection of the neck anatomy before deciding how to perform the final clipping. In case of extensive atheroma, it is dangerous to remove it down to the base and some part of it is left out of the clip so as not to occlude the origins of the M2s. Thick-walled and calcified large aneurysms usually require several clips (Fig. 75-13). If the first clip slides off from a broad base to occlude the parent artery or distally to leave a large neck remnant, a ring clip can be first inserted to compress a part of the neck, and a straight second clip is placed proximally to close the remaining neck inside the ring of the first clip (Drake’s tandem-clipping technique).103
Fusiform and Dissecting MCA Aneurysms
Fusiform, dissecting, and mycotic aneurysms are longitudinal dilations of the cerebral arteries. We prefer microsurgery over endovascular procedures when there is a SAH or, in particular, a large ICH. With the new intra-arterial flow diverters, the situation might change in the future, but even with them there is the problem of postprocedural anticoagulation. In some fusiform aneurysms, it may be possible to find the so-called beer belly, which is tangentially clipped with a small straight or curved clip under temporary occlusion of the parent artery. In some distal aneurysms with good retrograde flow, it is often possible to sacrifice the parent artery without a bypass. In more proximal fusiform MCAAs bypass and proximal/distal occlusion or trapping is our treatment of choice. Wrapping may be reasonable in the absence of alternatives.104
Bypass Surgery for MCAAs
Preoperative high-flow EC–IC or IC–IC bypass using the high-flow or low-flow bypass may be considered in large or giant MCAAs, when the exclusion of the neck from the parent and branching arteries cannot be performed.105,106 Each case must be evaluated individually and optimal configuration for the bypass depends on the angioarchitecture of the entire MCA. We prefer to use the most simple bypass strategy, utilizing the superficial temporal artery (STA) to the MCA direct arterial bypass whenever possible. Complex bypasses and proximal high-flow bypass with the ELANA technique107 are limited to situations where other options are not possible. A comprehensive neurovascular team should be prepared to perform intraoperative arteriotomies—for example, to remove coils or thrombi—and intraoperative EC–IC or IC–IC bypasses, and in case of emergency as well. In clinical practice, bypass surgeries are relatively infrequent and they should be channeled into high-volume, dedicated neurovascular centers.
Outcome of Ruptured MCA Aneurysms
The outcome for ruptured MCAAs depends very much on the referral system of the particular institution. In centers with population responsibility where also poor-grade patients are also routinely admitted and treated, the management outcome is always going to be worse than in referral centers that treat mainly good-grade patients. In Suzuki’s series (413 patients), 94% of the patients were in good or excellent condition 6 months after treatment.11 Half of these patients had late surgery or their aneurysms were unruptured and/or had good grades (0 to I). In a Hungarian series of 289 patients with MCAAs, only 18% had poor outcome after surgery in a long-term follow-up.10 Yaşargil reported unfavorable results in only 6% of his 231 patients with MCAAs who were not operated in the acute setting.13 Excellent results were published also by Sundt et al., with a 14% frequency for poor results after surgery for MCAAs.108 All these are surgical series, and reflect not only excellent surgical skills but also selection and referral bias. Early surgery is now advocated to prevent early reruptures with high morbidity and mortality.109
Here we present results from the Kuopio Aneurysm Database in a patient population that is much less selected and where large numbers of poor-grade patients are admitted and treated. The department of neurosurgery at Kuopio is the only neurosurgical center for the entire eastern region of Finland with a catchment population of about 1 million. Between 1977 and 2005, there were 802 patients with ruptured MCAAs as mentioned earlier (see Incidence of MCA Aneurysm section). On admission, 346 (43%) of these patients were Hunt and Hess (HH) grade 1 or 2, 204 (26%) were HH 3, and 250 (31%) were HH grade 4 or 5. Of the 802 patients, 67% had favorable outcome (Glasgow Outcome Score [GOS] ≥ 4) at 1 year after the SAH, 7% were severely disabled (GOS 3), only two patients were in a vegetative state (GOS 2), and 26% were dead (GOS 1). These results represent total management outcome irrespective of the treatment method. Of the 346 HH 1 to 2 patients, 313 (90%) had favorable outcome at 1 year. In contrast, only 70 (28%) of the 250 HH 4 to 5 patients had favorable outcome, and 152 (61%) of them were dead (Table 75-7). These results show how management outcome strongly depends on patient selection and why comparison of different series is very difficult.
When compared to ruptured aneurysms at other locations, patients with MCAAs had slightly worse results. Among the 1562 patients with ruptured aneurysm other than MCAAs, 70% had a favorable outcome, 6% were severely disabled, and 23% were dead at 1 year. The overall management outcome was almost equal for all MCAA sites: 34% poor outcome (GOS ≤ 3) in patients with ruptured M1A, 32% with ruptured MbifA, and 30% for ruptured MdistA. Our earlier analysis of 561 MCAAs showed that in ruptured MCAAs, the patient’s condition on admission, vasospasm, postoperative hematoma, and age are the most significant independent contributors to outcome.17 Temporal ICHs, together with vasospasm and inadvertent occlusion of main vessel(s) or thalamostriate perforators, explain specific late disabilities seen in patients with MCAAs (see below).
Specific Late Disabilities Associated with Ruptured MCAAs
Epilepsy. The incidence of late epilepsy after SAH varies from 7% to 25% in different studies,110–115 the risk depending on the exact aneurysm location, presence or absence of a temporal ICH, brain ischemia, and hypertension. Also, the existence of multiple IAs increases the risk for late epilepsy. In our series, late epilepsy occurred in 18% of the long-term survivors with a single MCAA. The frequencies were even higher in patients with MIA and one MCAA (20%) and with multiple MCAAs (27%). This is significantly higher than with any other ruptured aneurysms. Frequencies for late epilepsy were not significantly different according to MCAA location: M1A 22%, MbifA 24%, and MdistA 30%. ICH was a significant risk factor for the development of epilepsy. Half of the long-term survivors with ruptured MCAAs and epilepsy had an ICH on their initial CT scan, most often in the temporal lobe.
Dashti R., Rinne J., Hernesniemi J., et al. Microneurosurgical management of proximal middle cerebral artery aneurysms. Surg Neurol. 2007;67:6-14.
Dashti R., Hernesniemi J., Niemela M., et al. Microneurosurgical management of middle cerebral artery bifurcation aneurysms. Surg Neurol. 2007;67:441-456.
Dashti R., Hernesniemi J., Niemela M., et al. Microneurosurgical management of distal middle cerebral artery aneurysms. Surg Neurol. 2007;67:553-563.
Dashti R., Laakso A., Niemela M., et al. Microscope-integrated near-infrared indocyanine green videoangiography during surgery of intracranial aneurysms: the Helsinki experience. Surg Neurol. 2009;71:543-550.
Heiskanen O., Poranen A., Kuurne T., et al. Acute surgery for intracerebral haematomas caused by rupture of an intracranial arterial aneurysm. A prospective randomized study. Acta Neurochir (Wien). 1988;90:81-83.
Hernesniemi J., Ishii K., Niemela M., et al. Lateral supraorbital approach as an alternative to the classical pterional approach. Acta Neurochir Suppl. 2005;94:17-21.
Hernesniemi J., Niemela M., Karatas A., et al. Some collected principles of microneurosurgery: simple and fast, while preserving normal anatomy: a review. Surg Neurol. 2005;64:195-200.
Heros R.C. Aneurysms in the middle cerebral artery. In: Symon L., Thomas D.G., Clarke K. Rob & Smith’s Operative Surgery: Neurosurgery. New York: Chapman and Hall; 1994:171-179.
Heros R.C. Middle cerebral artery aneurysms. In: Wilkins R.H., Rengachary S.S. Neurosurgery. New York: McGraw-Hill; 1985:1376-1382.
Inoue K., Seker A., Osawa S., et al. Microsurgical and endoscopic anatomy of the supratentorial arachnoidal membranes and cisterns. Neurosurgery. 2009;65:644-665.
Koivisto T., Vanninen R., Hurskainen H., et al. Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms. A prospective randomized study. Stroke. 2000;31:2369-2377.
Lawton M.T., Spetzler R.F. Surgical management of giant intracranial aneurysms: experience with 171 patients. Clin Neurosurg. 1995;42:245-266.
Lehecka M., Laakso A., Hernesniemi J. Helsinki Microneurosurgery: Basics and Tricks. Balgheim: Druckerei Hohl GmbH; 2011.
Lehto H., Dashti R., Karatas A., et al. Third ventriculostomy through the fenestrated lamina terminalis during microneurosurgical clipping of intracranial aneurysms: an alternative to conventional ventriculostomy. Neurosurgery. 2009;64:430-435.
Nagy L., Ishii K., Karatas A., et al. Water dissection technique of Toth for opening neurosurgical cleavage planes. Surg Neurol. 2006;65:38-41.
Ogilvy C.S., Crowell R.M., Heros R.C. Surgical management of middle cerebral artery aneurysms: experience with transsylvian and superior temporal gyrus approaches. Surg Neurol. 1995;43:15-24.
Randell T., Niemela M., Kytta J., et al. Principles of neuroanesthesia in aneurysmal subarachnoid hemorrhage: the Helsinki experience. Surg Neurol. 2006;66:382-388.
Regli L., Dehdashti A.R., Uske A., et al. Endovascular coiling compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: an update. Acta Neurochir Suppl. 2002;82:41-46.
Rhoton A.L.Jr. The supratentorial arteries. Neurosurgery. 2002;51:S53-S120.
Rhoton A.L.Jr. The cerebral veins. Neurosurgery. 2002;51:S159-S205.
Rinne J., Hernesniemi J., Niskanen M., et al. Analysis of 561 patients with 690 middle cerebral artery aneurysms: anatomic and clinical features as correlated to management outcome. Neurosurgery. 1996;38:2-11.
Suzuki J., Yoshimoto T., Kayama T. Surgical treatment of middle cerebral artery aneurysms. J Neurosurg. 1984;61:17-23.
Türe U., Yaşargil M.G., Al-Mefty O., et al. Arteries of the insula. J Neurosurg. 2000;92:676-687.
Wen H.T., Rhoton A.L.Jr., de Oliveira E., et al. Microsurgical anatomy of the temporal lobe: part 2—sylvian fissure region and its clinical application. Neurosurgery. 2009;65:1-36.
Yaşargil M.G.. Operative anatomy, Yaşargil M.G., editor, Microneurosurgery, Stuttgart, Georg Thieme Verlag, 1984;vol. 1:165-168.
Yaşargil M.G.. Middle cerebral artery aneurysms, Yaşargil M.G., editor, Microneurosurgery, Stuttgart, Thieme Verlag, 1984;vol. 2:124-164.
1. Dashti R., Rinne J., Hernesniemi J., et al. Microneurosurgical management of proximal middle cerebral artery aneurysms. Surg Neurol. 2007;67:6-14.
2. Dashti R., Hernesniemi J., Niemela M., et al. Microneurosurgical management of middle cerebral artery bifurcation aneurysms. Surg Neurol. 2007;67:441-456.
3. Dashti R., Hernesniemi J., Niemela M., et al. Microneurosurgical management of distal middle cerebral artery aneurysms. Surg Neurol. 2007;67:553-563.
4. Huttunen T., Fraunberg M., Frosen J., et al. Saccular intracranial aneurysm disease—distribution of site, size and age suggest different etiologies for aneurysm formation and rupture in 316 familial and 1454 sporadic eastern Finnish patients. Neurosurgery. 2010;66:631-638.
5. Aydin I.H., Takci E., Kadioglu H.H., et al. The variations of lenticulostriate arteries in the middle cerebral artery aneurysms. Acta Neurochir (Wien). 1996;138:555-559.
6. Fox J. Technique of aneurysm surgery. IV: middle cerebral artery aneurysms. In: Intracranial aneurysms. New York: Springer-Verlag; 1983:1012-1023.
7. Heros R.C. Middle cerebral artery aneurysms. In: Wilkins R.H., Rengachary S.S. Neurosurgery. New York: McGraw-Hill; 1985:1376-1382.
8. Hosoda K., Fujita S., Kawaguchi T., et al. Saccular aneurysms of the proximal (M1) segment of the middle cerebral artery. Neurosurgery. 1995;36:441-446.
9. Ogilvy C.S., Crowell R.M., Heros R.C. Surgical management of middle cerebral artery aneurysms: experience with transsylvian and superior temporal gyrus approaches. Surg Neurol. 1995;43:15-24.
10. Pasztor E., Vajda J., Juhasz J., et al. The surgery of middle cerebral artery aneurysms. Acta Neurochir (Wien). 1986;82:92-101.
11. Suzuki J., Yoshimoto T., Kayama T. Surgical treatment of middle cerebral artery aneurysms. J Neurosurg. 1984;61:17-23.
12. Heros R.C. Aneurysms in the middle cerebral artery. In: Symon L., Thomas D.G., Clarke K. Rob & Smith’s Operative Surgery: Neurosurgery. New York: Chapman and Hall; 1994:171-179.
13. Yaşargil M.G. Middle cerebral artery aneurysms. In: Yaşargil M.G., editor. Microneurosurgery. Stuttgart: Thieme Verlag; 1984:124-164.
14. Weir B., Findlay J.M., Disney L. Middle cerebral artery aneurysms. In: Apuzzo M.L.J., editor. Brain Surgery. New York: Churchill Livingstone; 1993:983-1008.
15. Peerless S.J. The surgical approach to middle cerebral and posterior communicating aneurysms. Clin Neurosurg. 1974;21:151-165.
16. Park D.H., Kang S.H., Lee J.B., et al. Angiographic features, surgical management and outcomes of proximal middle cerebral artery aneurysms. Clin Neurol Neurosurg. 2008;110:544-551.
17. Rinne J., Hernesniemi J., Niskanen M., et al. Analysis of 561 patients with 690 middle cerebral artery aneurysms: anatomic and clinical features as correlated to management outcome. Neurosurgery. 1996;38:2-11.
18. Kumar M.V., Karagiozov K.L., Chen L., et al. A classification of unruptured middle cerebral artery bifurcation aneurysms that can help in choice of clipping technique. Minim Invasive Neurosurg. 2007;50:132-139.
19. Dandy W.E. Surgical treatment of aneurysms of the middle cerebral artery: intracranial aneurysms. Ithaca, NY: Comstock Publishing Company; 1945.
20. Vendrell J.F., Menjot N., Costalat V., et al. Endovascular treatment of 174 middle cerebral artery aneurysms: clinical outcome and radiologic results at long-term follow-up. Radiology. 2009;253:191-198.
21. Suzuki S., Tateshima S., Jahan R., et al. Endovascular treatment of middle cerebral artery aneurysms with detachable coils: angiographic and clinical outcomes in 115 consecutive patients. Neurosurgery. 2009;64:876-889.
22. Regli L., Dehdashti A.R., Uske A., et al. Endovascular coiling compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: an update. Acta Neurochir Suppl. 2002;82:41-46.
23. Quadros R.S., Gallas S., Noudel R., et al. Endovascular treatment of middle cerebral artery aneurysms as first option: a single center experience of 92 aneurysms. AJNR Am J Neuroradiol. 2007;28:1567-1572.
24. Doerfler A., Wanke I., Goericke S.L., et al. Endovascular treatment of middle cerebral artery aneurysms with electrolytically detachable coils. AJNR Am J Neuroradiol. 2006;27:513-520.
25. Bracard S., Abdel-Kerim A., Thuillier L., et al. Endovascular coil occlusion of 152 middle cerebral artery aneurysms: initial and midterm angiographic and clinical results. J Neurosurg. 2009;112:703-708.
26. Papo I., Bodosi M., Doczi T. Intracerebral haematomas from aneurysm rupture: their clinical significance. Acta Neurochir (Wien). 1987;89:100-105.
27. Raftopoulos C., Goffette P., Vaz G., et al. Surgical clipping may lead to better results than coil embolization: results from a series of 101 consecutive unruptured intracranial aneurysms. Neurosurgery. 2003;52:1280-1290.
28. Tokuda Y., Inagawa T., Katoh Y., et al. Intracerebral hematoma in patients with ruptured cerebral aneurysms. Surg Neurol. 1995;43:272-277.
29. Koivisto T., Vanninen R., Hurskainen H., et al. Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms. A prospective randomized study. Stroke. 2000;31:2369-2377.
30. Heiskanen O., Poranen A., Kuurne T., et al. Acute surgery for intracerebral haematomas caused by rupture of an intracranial arterial aneurysm. A prospective randomized study. Acta Neurochir (Wien). 1988;90:81-83.
31. Tapaninaho A., Hernesniemi J., Vapalahti M. Emergency treatment of cerebral aneurysms with large haematomas. Acta Neurochir (Wien). 1988;91:21-24.
32. Vanninen R., Koivisto T., Saari T., et al. Ruptured intracranial aneurysms: acute endovascular treatment with electrolytically detachable coils—a prospective randomized study. Radiology. 1999;211:325-336.
33. Yaşargil M.G.. Operative anatomy, Yaşargil M.G., editor, Microneurosurgery, Stuttgart, Georg Thieme Verlag, 1984;vol. 1:165-168.
34. Rasmussen P., Busch H., Haase J., et al. Intracranial saccular aneurysms. Results of treatment in 851 patients. Acta Neurochir (Wien). 1980;53:1-17.
35. Rosenorn J., Eskesen V., Schmidt K., et al. The risk of rebleeding from ruptured intracranial aneurysms. J Neurosurg. 1987;67:329-332.
36. Wiebers D.O., Whisnant J.P., Huston J.3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110.
37. Türe U., Yaşargil M.G., Al-Mefty O., et al. Arteries of the insula. J Neurosurg. 2000;92:676-687.
38. Gibo H., Carver C.C., Rhoton A.L.Jr., et al. Microsurgical anatomy of the middle cerebral artery. J Neurosurg. 1981;54:151-169.
39. Rosner S.S., Rhoton A.L.Jr., Ono M., et al. Microsurgical anatomy of the anterior perforating arteries. J Neurosurg. 1984;61:468-485.
40. Umansky F., Gomes F.B., Dujovny M., et al. The perforating branches of the middle cerebral artery. A microanatomical study. J Neurosurg. 1985;62:261-268.
41. Umansky F., Juarez S.M., Dujovny M., et al. Microsurgical anatomy of the proximal segments of the middle cerebral artery. J Neurosurg. 1984;61:458-467.
42. Tanriover N., Rhoton A.L.Jr., Kawashima M., et al. Microsurgical anatomy of the insula and the sylvian fissure. J Neurosurg. 2004;100:891-922.
43. Rhoton A.L.Jr. The supratentorial arteries. Neurosurgery. 2002;51:S53-120.
44. Marinkovic S., Gibo H., Milisavljevic M., et al. Anatomic and clinical correlations of the lenticulostriate arteries. Clin Anat. 2001;14:190-195.
45. Inoue K., Seker A., Osawa S., et al. Microsurgical and endoscopic anatomy of the supratentorial arachnoidal membranes and cisterns. Neurosurgery. 2009;65:644-665.
46. Wen H.T., Rhoton A.L.Jr., de Oliveira E., et al. Microsurgical anatomy of the temporal lobe: part 2—sylvian fissure region and its clinical application. Neurosurgery. 2009;65:1-36.
47. Rhoton A.L.Jr. The cerebral veins. Neurosurgery. 2002;51:S159-S205.
48. Ono M., Rhoton A.L.Jr., Peace D., et al. Microsurgical anatomy of the deep venous system of the brain. Neurosurgery. 1984;15:621-657.
49. Gonzalez-Darder J.M., Pesudo-Martinez J.V., Feliu-Tatay R.A. Microsurgical management of cerebral aneurysms based in CT angiography with three-dimensional reconstruction (3D-CTA) and without preoperative cerebral angiography. Acta Neurochir (Wien). 2001;143:673-679.
50. Kangasniemi M., Makela T., Koskinen S., et al. Detection of intracranial aneurysms with two-dimensional and three-dimensional multislice helical computed tomographic angiography. Neurosurgery. 2004;54:336-341.
51. Karamessini M.T., Kagadis G.C., Petsas T., et al. CT angiography with three-dimensional techniques for the early diagnosis of intracranial aneurysms. Comparison with intra-arterial DSA and the surgical findings. Eur J Radiol. 2004;49:212-223.
52. Siablis D., Kagadis G.C., Karamessini M.T., et al. Intracranial aneurysms: reproduction of the surgical view using 3D-CT angiography. Eur J Radiol. 2005;55:92-95.
53. Tipper G., U-King-Im J.M., Price S.J., et al. Detection and evaluation of intracranial aneurysms with 16-row multislice CT angiography. Clin Radiol. 2005;60:565-572.
54. Villablanca J.P., Hooshi P., Martin N., et al. Three-dimensional helical computerized tomography angiography in the diagnosis, characterization, and management of middle cerebral artery aneurysms: comparison with conventional angiography and intraoperative findings. J Neurosurg. 2002;97:1322-1332.
55. Wintermark M., Ko N.U., Smith W.S., et al. Vasospasm after subarachnoid hemorrhage: utility of perfusion CT and CT angiography on diagnosis and management. AJNR Am J Neuroradiol. 2006;27:26-34.
56. Wintermark M., Uske A., Chalaron M., et al. Multislice computerized tomography angiography in the evaluation of intracranial aneurysms: a comparison with intraarterial digital subtraction angiography. J Neurosurg. 2003;98:828-836.
57. Schuknecht B. High-concentration contrast media (HCCM) in CT angiography of the carotid system: impact on therapeutic decision making. Neuroradiology. 2007;49(Suppl 1):S15-S26.
58. Forsting M. CTA of the ICA bifurcation and intracranial vessels. Eur Radiol. 2005;15(Suppl 4):D25-D27.
59. Randell T., Niemela M., Kytta J., et al. Principles of neuroanesthesia in aneurysmal subarachnoid hemorrhage: the Helsinki experience. Surg Neurol. 2006;66:382-388.
60. Hillman J., Fridriksson S., Nilsson O., et al. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg. 2002;97:771-778.
61. Öhman J., Servo A., Heiskanen O. Long-term effects of nimodipine on cerebral infarcts and outcome after aneurysmal subarachnoid hemorrhage and surgery. J Neurosurg. 1991;74:8-13.
62. Öhman J., Heiskanen O. Effect of nimodipine on the outcome of patients after aneurysmal subarachnoid hemorrhage and surgery. J Neurosurg. 1988;69:683-686.
63. Hernesniemi J., Ishii K., Niemela M., et al. Lateral supraorbital approach as an alternative to the classical pterional approach. Acta Neurochir Suppl. 2005;94:17-21.
64. Hernesniemi J., Niemela M., Karatas A., et al. Some collected principles of microneurosurgery: simple and fast, while preserving normal anatomy: a review. Surg Neurol. 2005;64:195-200.
65. Drake C.G., Peerless S.J., Hernesniemi J. Surgery on vertebrobasilar aneurysms. New York: Springer-Verlag Wien; 1996.
66. Bailes J.E., Spetzler R.F., Hadley M.N., et al. Management morbidity and mortality of poor-grade aneurysm patients. J Neurosurg. 1990;72:559-566.
67. Baskaya M.K., Menendez J.A., Yuceer N., et al. Results of surgical treatment of intrasylvian hematomas due to ruptured intracranial aneurysms. Clin Neurol Neurosurg. 2001;103:23-28.
68. Brandt L., Sonesson B., Ljunggren B., et al. Ruptured middle cerebral artery aneurysm with intracerebral hemorrhage in younger patients appearing moribund: emergency operation? Neurosurgery. 1987;20:925-929.
69. Shimoda M., Oda S., Mamata Y., et al. Surgical indications in patients with an intracerebral hemorrhage due to ruptured middle cerebral artery aneurysm. J Neurosurg. 1997;87:170-175.
70. Wheelock B., Weir B., Watts R., et al. Timing of surgery for intracerebral hematomas due to aneurysm rupture. J Neurosurg. 1983;58:476-481.
71. Yoshimoto Y., Wakai S., Satoh A., et al. Intraparenchymal and intrasylvian haematomas secondary to ruptured middle cerebral artery aneurysms: prognostic factors and therapeutic considerations. Br J Neurosurg. 1999;13:18-24.
72. Nagy L., Ishii K., Karatas A., et al. Water dissection technique of Toth for opening neurosurgical cleavage planes. Surg Neurol. 2006;65:38-41.
73. Romani R., Kivisaari R., Celik O., et al. Repair of an alarming intraoperative intracavernous carotid artery tear with anastoclips: technical case report. Neurosurgery. 2009;65:E998-E999.
74. Lehto H., Dashti R., Karatas A., et al. Third ventriculostomy through the fenestrated lamina terminalis during microneurosurgical clipping of intracranial aneurysms: an alternative to conventional ventriculostomy. Neurosurgery. 2009;64:430-435.
75. Iwama T., Yoshimura S., Kaku Y., et al. Considerations in the surgical treatment of superior-wall type aneurysm at the proximal (M1) segment of the middle cerebral artery. Acta Neurochir (Wien). 2004;146:967-972.
76. Lipovsek M. Ruptured aneurysms of the proximal middle cerebral artery. J Neurosurg. 1973;39:498-502.
77. Horiuchi T., Tanaka Y., Takasawa H., et al. Ruptured distal middle cerebral artery aneurysm. J Neurosurg. 2004;100:384-388.
78. Piepgras D.G., McGrail K.M., Tazelaar H.D. Intracranial dissection of the distal middle cerebral artery as an uncommon cause of distal cerebral artery aneurysm. Case report. J Neurosurg. 1994;80:909-913.
79. Sakamoto S., Ikawa F., Kawamoto H., et al. Acute surgery for ruptured dissecting aneurysm of the M3 portion of the middle cerebral artery. Neurol Med Chir (Tokyo). 2003;43:188-191.
80. Dashti R., Laakso A., Niemela M., et al. Microscope-integrated near-infrared indocyanine green videoangiography during surgery of intracranial aneurysms: the Helsinki experience. Surg Neurol. 2009;71:543-550.
81. Kivisaari R.P., Porras M., Ohman J., et al. Routine cerebral angiography after surgery for saccular aneurysms: is it worth it? Neurosurgery. 2004;55:1015-1024.
82. Dehdashti A.R., Binaghi S., Uske A., et al. Comparison of multislice computerized tomography angiography and digital subtraction angiography in the postoperative evaluation of patients with clipped aneurysms. J Neurosurg. 2006;104:395-403.
83. Lehecka M., Niemela M., Seppanen J., et al. No long-term excess mortality in 280 patients with ruptured distal anterior cerebral artery aneurysms. Neurosurgery. 2007;60:235-241.
84. Molyneux A.J., Kerr R.S., Birks J., et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. 2009;8:427-433.
85. Bilguvar K., Yasuno K., Niemela M., et al. Susceptibility loci for intracranial aneurysm in European and Japanese populations. Nat Genet. 2008;40:1472-1477.
86. Ronkainen A., Hernesniemi J., Puranen M., et al. Familial intracranial aneurysms. Lancet. 1997;349:380-384.
87. de Oliveira E., Tedeschi H., Siqueira M.G., et al. Anatomical and technical aspects of the contralateral approach for multiple aneurysms. Acta Neurochir (Wien). 1996;138:1-11.
88. Rinne J., Hernesniemi J., Niskanen M., et al. Management outcome for multiple intracranial aneurysms. Neurosurgery. 1995;36:31-38.
89. Vajda J., Juhasz J., Pasztor E., et al. Contralateral approach to bilateral and ophthalmic aneurysms. Neurosurgery. 1988;22:662-668.
90. Lynch J.C., Andrade R. Unilateral pterional approach to bilateral cerebral aneurysms. Surg Neurol. 1993;39:120-127.
91. de Sousa A.A., Filho M.A., Faglioni W.Jr., et al. Unilateral pterional approach to bilateral aneurysms of the middle cerebral artery. Surg Neurol. 2005;63(Suppl 1):S1-S7.
92. Sundt T.M.Jr., Piepgras D.G. Surgical approach to giant intracranial aneurysms. Operative experience with 80 cases. J Neurosurg. 1979;51:731-742.
93. Symon L., Vajda J. Surgical experiences with giant intracranial aneurysms. J Neurosurg. 1984;61:1009-1028.
94. Heros R.C., Fritsch M.J. Surgical management of middle cerebral artery aneurysms. Neurosurgery. 2001;48:780-786.
95. Lawton M.T., Spetzler R.F. Surgical strategies for giant intracranial aneurysms. Neurosurg Clin N Am. 1998;9:725-742.
96. Gewirtz R.J., Awad I.A. Giant aneurysms of the anterior circle of Willis: management outcome of open microsurgical treatment. Surg Neurol. 1996;45:409-421.
97. Gewirtz R.J., Awad I.A. Giant aneurysms of the proximal anterior cerebral artery: report of three cases. Neurosurgery. 1993;33:120-124. discussion 124-125
98. Lawton M.T., Spetzler R.F. Surgical strategies for giant intracranial aneurysms. Acta Neurochir Suppl. 1999;72:141-156.
99. Lawton M.T., Spetzler R.F. Surgical management of giant intracranial aneurysms: experience with 171 patients. Clin Neurosurg. 1995;42:245-266.
100. Piepgras D.G., Khurana V.G., Whisnant J.P. Ruptured giant intracranial aneurysms. Part II. A retrospective analysis of timing and outcome of surgical treatment. J Neurosurg. 1998;88:430-435.
101. Bojanowski W.M., Spetzler R.F., Carter L.P. Reconstruction of the MCA bifurcation after excision of a giant aneurysm. Technical note. J Neurosurg. 1988;68:974-977.
102. Navratil O., Lehecka M., Lehto H., et al. Vascular clamp-assisted clipping of thick-walled giant aneurysms. Neurosurgery. 2009;64:113-121.
103. Drake C.G. Giant intracranial aneurysms: experience with surgical treatment in 174 patients. Clin Neurosurg. 1979;26:12-95.
104. Sadasivan B., Ma S., Dujovny M., et al. Use of experimental aneurysms to evaluate wrapping materials. Surg Neurol. 1990;34:3-7.
105. Sanai N., Zador Z., Lawton M.T. Bypass surgery for complex brain aneurysms: an assessment of intracranial-intracranial bypass. Neurosurgery. 2009;65:670-683.
106. van Doormaal T.P., van der Zwan A., Verweij B.H., et al. Treatment of giant middle cerebral artery aneurysms with a flow replacement bypass using the excimer laser-assisted nonocclusive anastomosis technique. Neurosurgery. 2008;63:12-22.
107. Tulleken C.A., Verdaasdonk R.M., Mansvelt Beck H.J. Nonocclusive excimer laser-assisted end-to-side anastomosis. Ann Thorac Surg. 1997;63:S138-S142.
108. Sundt T.M.Jr., Kobayashi S., Fode N.C., et al. Results and complications of surgical management of 809 intracranial aneurysms in 722 cases. Related and unrelated to grade of patient, type of aneurysm, and timing of surgery. J Neurosurg. 1982;56:753-765.
109. Hernesniemi J., Vapalahti M., Niskanen M., et al. One-year outcome in early aneurysm surgery: a 14 year experience. Acta Neurochir (Wien). 1993;122:1-10.
110. Baker C.J., Prestigiacomo C.J., Solomon R.A. Short-term perioperative anticonvulsant prophylaxis for the surgical treatment of low-risk patients with intracranial aneurysms. Neurosurgery. 1995;37:863-871.
111. Cabral R.J., King T.T., Scott D.F. Epilepsy after two different neurosurgical approaches to the treatment of ruptured intracranial aneurysm. J Neurol Neurosurg Psychiatry. 1976;39:1052-1056.
112. Fabinyi G.C., Artiola-Fortuny L. Epilepsy after craniotomy for intracranial aneurysm. Lancet. 1980;1:1299-1300.
113. Keranen T., Tapaninaho A., Hernesniemi J., et al. Late epilepsy after aneurysm operations. Neurosurgery. 1985;17:897-900.
114. Kotila M., Waltimo O. Epilepsy after stroke. Epilepsia. 1992;33:495-498.
115. Ukkola V., Heikkinen E.R. Epilepsy after operative treatment of ruptured cerebral aneurysms. Acta Neurochir (Wien). 1990;106:115-118.