Chapter 191 Surgical Approaches to the Cervicothoracic Junction
Cervicothoracic pathologies are relatively uncommon but include bacterial and tuberculous infections, fractures from primary bone disease, primary bone tumors, meningeal tumors, vascular malformations, congenital connective tissue and skeletal disorders, trauma, and thoracic disc herniations (Table 191-1). Up to 15% of patients with spinal neoplasms have lesions of the upper thoracic vertebrae, and 10% of spinal metastases arise from the T1 to the T4 region.1 One group reported that 8% of patients undergoing tumor resection required posterior instrumentation at the cervicothoracic junction.2 Additionally, as many as 9% of traumatic injuries can involve the cervicothoracic junction.3
TABLE 191-1 Differential Diagnosis of Lesions of the Cervicothoracic Junction
| Metastatic tumors |
| Primary tumors of bone |
| Primary lymphoma |
| Intradural, extramedullar tumors |
| Intradural, intramedullary tumors |
| Bacterial infections |
| Tuberculous infections |
| Vascular malformations |
| Pathologic fracture (primary metabolic disease of bone) |
| Connective tissue and skeletal disorders |
| Traumatic vertebral fractures |
| Disc herniations |
Up to 80% of unstable cervicothoracic pathologies can have neurologic compromise, and many of these lesions require surgical treatment. Unfortunately, injuries to this area are often overlooked on routine radiographic studies.4,5 The surgical goals include neural decompression, immediate stabilization, restoration of anatomic spinal alignment, and early rehabilitation. Appropriate treatment is necessary because acute unstable lesions of the cervicothoracic junction can have severe consequences including poor rates of recovery and significant mortality from cardiopulmonary-related complications.6
Posterior approaches, such as laminectomy and pediculectomy, are common approaches to many diseases of the spine. When treating anterior spinal element diseases, these techniques might not be adequate, can have a higher complication rate, and can disrupt spinal stability.7,8 For these reasons, a variety of anterior and posterolateral surgical approaches have been developed.
The first detailed description of a posterolateral approach to the anterior elements was the costotransversectomy.9 Although this technique gave adequate exposure of the middle and lower thoracic spine, it was less useful in the upper thoracic region. In 1954, Capener10 described a more-extensive posterolateral exposure, the lateral rhachotomy. In 1976, Larson and colleagues11 reported a modification of the lateral rhachotomy, the lateral extracavitary approach, which provided improved exposure of the middle and lower thoracic spine with less morbidity. However, these new additions were still limited at the cervicothoracic junction owing to the shoulder girdle. The lateral parascapular extrapleural operation, a modification of the lateral extracavitary approach, eliminates those obstructions and provides exposure of the thoracic vertebrae up to the inferior end plate of C7.12
Purely anterior, supraclavicular approaches to the cervicothoracic junction were initially described by Jonnesco13 and Brunig14 in 1923 and later used by Royle15 for spastic paralysis, by Gask16 for Raynaud’s disease, and by Ochsner and DeBakey17 for thoracic sympathectomy. However, exposure of the thoracic area was restricted by the clavicle, so the transmanubrial and transclavicular approach was developed by Sundaresan and colleagues18 in 1984 and modified by Birch and colleagues19 in 1990.
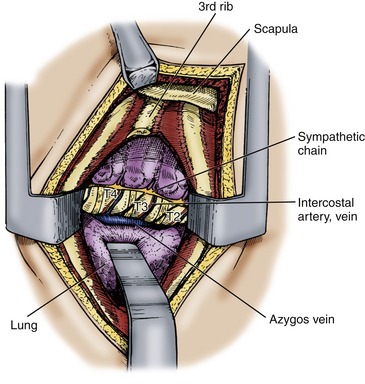
Another approach to the anterior thoracic vertebral elements, the anterolateral thoracotomy, was first described by Hodgson and colleagues.20 This approach involves resection of the third rib and requires transpleural mobilization of the lung with ligation and division of the intercostal arteries, intercostal veins, and the hemiazygos vein.
The following sections discuss the clinical features of cervicothoracic junction diseases, including preoperative evaluation, anesthetic considerations, relevant regional surgical anatomy, biomechanics of the cervicothoracic junction, surgical approaches, and options for surgical reconstruction and stabilization.
Clinical Features
The differential diagnosis for cervicothoracic pathologies is listed in Table 191-1. Disease processes in this region can manifest as pain without neurologic deficit, thoracic myelopathy, C7 or T1 radiculopathy, or a combination of these signs and symptoms. Table 191-2 lists the presenting findings in our series of patients with pathologic processes of the cervicothoracic junction (C7 to T4).1 Of these patients, 93% had decreased sensation, 83% presented with generalized upper thoracic back pain, and 58% had leg weakness. C7 and T1 lesions were less common, and consequently, 33% of patients presented with radicular symptoms and 35% demonstrated hand weakness on examination. Other, less common findings included ataxia (25%) and bowel or bladder dysfunction (17%).
TABLE 191-2 Signs and Symptoms at Presentation of Patients with C7-T4 Pathologic Processes (17 Patients)
| Signs and Symptoms | Percentage of Patients with Signs or Symptoms |
|---|---|
| Back pain | 83 |
| Radicular pain | 33 |
| Leg weakness | 58 |
| Decreased sensation | 92 |
| Hand weakness | 35 |
| Bowel or bladder dysfunction | 17 |
| Ataxia | 25 |
| Babinski sign | 58 |
Preoperative Evaluation
Radiographic Evaluation
Infections such as osteomyelitis or discitis have a variety of characteristics on plain radiographs. The earliest and most consistent finding of infection is narrowing of the disc space, which is present in 74% of patients.21 After 3 to 6 weeks, destructive changes in the body can be noted, which usually begin as lytic areas in the anterior aspect of the body adjacent to the disc and end plate. Active bone formation and sclerosis is also present in 11% of patients.
Metastatic disease of the bone has several classic signs including unilateral erosion of a pedicle, fishmouthing (cephalad and caudad end plate concavity within the vertebral body), osteoblastic changes, and vertebral body collapse. These findings can occur late in the disease process because 30% to 70% of the bone must be destroyed before changes are visible on plain radiographs.22
Radionucleotide bone scintigraphy is a sensitive but not specific method for detecting metastatic disease and infections.23,24 However, false negatives have been reported with lung cancer, renal cell carcinoma, myeloproliferative diseases, and regional ischemia, and in patients with leukopenia.25–27
Magnetic resonance imaging (MRI) is the most important diagnostic tool for evaluating diseases of the thoracic spine. MRI permits early diagnosis of infection and recognition of paravertebral or intraspinal abscesses without the risk associated with myelography.28 MRI is also effective in demonstrating the extent of metastatic disease, primary tumors of bone, primary lymphoma, intradural tumors, thoracic disc herniations, and vertebral fractures. Additionally, this modality may be helpful in spinal vascular malformations, and when assessing metastatic disease, MRI has been found to be at least as sensitive and accurate as gallium and bone scanning combined.29 Finally, MRI provides anatomic detail, especially of soft tissue and nervous structures, not available with any other imaging studies.
Approach-Related Surgical Anatomy
Lateral Parascapular Extrapleural Approach and Anterolateral Thoracotomy Approach
The lateral parascapular extrapleural approach is a posterolateral surgery that allows nearly lateral access to the cervicothoracic junction vertebral bodies. The anterolateral thoracotomy is a transthoracic approach through the third rib that allows access to the anterior and lateral aspects of the vertebrae and for control of the mediastinal vasculature. The relevant anatomy may be summarized in three major areas: scapula and parascapular anatomy, posterior thoracic cage, and retromediastinal space and spinal anatomy.
Scapular and Parascapular Anatomy
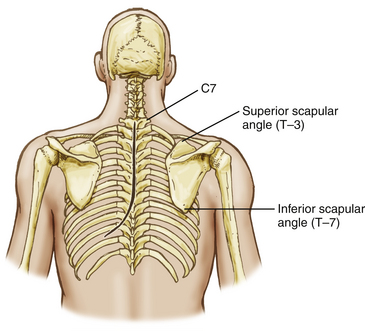
Posterolateral access to the thoracic cage and vertebral elements is hindered by the scapular and the parascapular shoulder musculature (Fig. 191-1). Mobilization of the scapula anterolaterally is necessary and requires the disruption of the posteromedial shoulder musculature.
Posterior Thoracic Cage
The deep or intrinsic muscles including the erector spinae and transversospinalis muscles are next encountered (Fig. 191-2). The erector spinae muscles originate from the sacrum as a dense aponeurotic band and divide into three columns below the last rib as they proceed superiorly. The iliocostalis muscle is located laterally and inserts from the angles of the ribs and into the cervical transverse processes from C4 through C6 as series of related muscular bundles. Each bundle extends over approximately six segments, with the more medial bundles extending more cephalad.
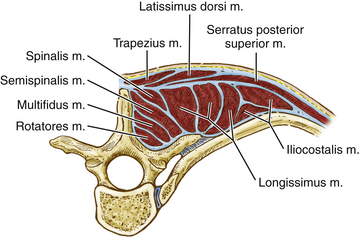
FIGURE 191-2 Cross-sectional anatomy of the deep or intrinsic muscle layer of the upper thoracic spine. M., muscle.
The transversospinalis muscle group passes cephalad from the transverse processes to the spinous processes immediately deep to the erector spinae muscles in three layers. The most superficial layer, the semispinalis, arises from the tips of the transverse processes and inserts at the tips of the spinous processes approximately five vertebral levels cephalad. At the cervicothoracic junction, this muscle is primarily composed of the semispinalis capitis. This muscle passes from the upper thoracic transverse processes and lower cervical articular processes (C4 to T4) to the occipital bone between the superior and inferior nuchal lines. The muscle fibers run vertically and attach to the ligamentum nuchae. The intermediate layer, the multifidus, originates from the erector spinae aponeurosis and from the transverse processes up to C4 and extends to the lower border of each spinous process. This muscle spans approximately three levels. The deepest muscles of this group, the rotatores, are muscles that bridge one interspace. They pass from one transverse process root to the spinous process root immediately above.
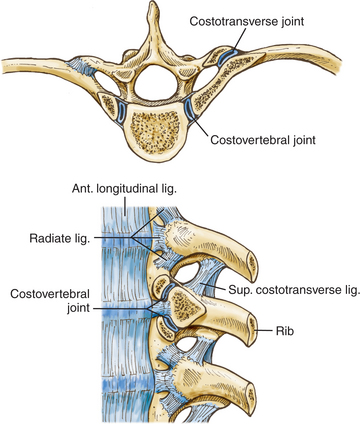
Each rib articulates with its own vertebral body and transverse process, the vertebra above, and the intervertebral disc between them (Fig. 191-3), but the first rib articulates only with its own vertebral body. Each of these articulations forms separate synovial joints, and the joints of the posterolateral vertebral body surface are separated by an intra-articular ligament that attaches to the intervertebral disc. An articular capsule surrounds the joints and attaches to the vertebral body anteriorly via the radiate ligament.
Transmanubrial Approach
The transmanubrial approach is an anterior approach that allows direct access to the vertebral bodies and associated pathologies. This approach consists of three major steps: the thoracic inlet, the visceral and vascular compartments of the superior mediastinum, and the retromediastinal space.
Cervicothoracic Biomechanics
The cervicothoracic junction extends from C7 through T4 and includes the lower brachial plexus, the thoracic outlet, and the superior mediastinum. This region also has a narrow spinal canal and narrow pedicles, and it represents a transition from the lateral masses of the cervical vertebra to the transverse processes of the thoracic spine. The structures affecting spinal stability include the vertebral bodies and intervertebral discs, anterior and posterior longitudinal ligaments, and the interarticulating facet joints and ligamentous complex posteriorly.
The rib cage and its sternal articulations also increase thoracic spine stability. Additionally, biomechanical studies have shown that the costotransverse joints and rib cage play a significant role in lateral bending and axial rotation.30 Berg31 describes the sternal rib complex as the fourth spinal column. At the cervicothoracic junction, disruption to any two spinal columns is unstable and should be treated accordingly.
Trauma, degenerative processes, infection, and neoplastic involvement can alter the biomechanical function of this area and can predispose the cervicothoracic junction to instability. The transition from the mobile, lordotic cervical spine to the rigid, kyphotic thoracic spine exposes this region to unique stress as the transfer of weight occurs between spinal columns.32,33 This stress is amplified by the decrease in vertebral index from C6 to T1.34
Cervicothoracic surgeries can destabilize the region, leading to kyphosis and spinal cord compression. Several authors have reported increasing spinal deformity caused by a previous cervicothoracic junction laminectomy.5,35,36 Spinal fusions ending at the cervicothoracic junction can also be a contributing factor to iatrogenic instability.37 In a series by Steinmetz and colleagues, factors that contributed to fusion failure included laminectomy without instrumentation, multilevel corpectomies, previous cervical surgery, smoking, and deformity correction.38 The increased incidence of neurologic injury may also be related to the smaller spinal canal size and tenuous blood supply.4,5
Few studies have addressed the biomechanics and stability of fixation at the cervicothoracic junction, and the majority of biomechanical studies in this region have focused on the cervical spine.39,40 Biomechanical tests on cervical cadaveric spines have shown superior stability of posterior screw fixation techniques as compared with anterior fixation.41,42 In addition, posterior wiring techniques were found to be inferior to the intact condition and performed poorly in movements other than flexion.41,43 In extension and torsion, lateral mass screws and plating devices provide increased stability relative to posterior wiring alone.44
Biomechanically, transpedicular screw fixation of the lower cervical spine provides the most stability to the unstable cervical segment. Rhee and colleagues demonstrated that C7 pedicle screws were superior to lateral mass screw and wiring techniques, and other studies have shown the importance of increasing screw length as measured by testing of in-line screw pullout strength.45–47 These findings must be weighed against a high rate of pedicle violation with pedicle screw placement in the lower cervical spine. 48 Rhee and colleagues also demonstrated that if C7 pedicle screws were not possible, then C6 and C7 lateral mass fixation was necessary to provide similar stability.47
Bueff and colleagues49 compared three different fixation devices at the cervicothoracic junction following a simulated C7–T1 distractive-flexion injury: an anterior plate, a posterior plate, and a posterior hook-and-rod system. This study showed that the hook-and-rod system provided as much as six times the stiffness of the intact spine, whereas the anterior plate provided stiffness similar to that of the intact condition. Clinically, standalone anterior fixation at the cervicothoracic junction can lead to a high failure rate.50
Vaccaro and colleagues51 examined the use of a novel plate-and-rod construct for the cervicothoracic junction and concluded that their device supported the maximal loading conditions of the native cervical spine. Kreshak and colleagues examined the mean stiffness of three different posterior cervicothoracic fixation systems after a two- or three-column injury.52 These systems included one plate-and-screw system and two rod-and-screw systems. Following a two-column injury, all three constructs were able to stabilize the cervicothoracic junction in flexion and extension, lateral bending, and axial rotation. After a three-column injury, all three systems failed to provide adequate stability, especially in extension. These results suggest that additional anterior stabilization may be needed when a three-column injury occurs at the cervicothoracic junction.
A biomechanical study by O’Brien and colleagues raised the possibility of posterior-only fixation to treat a three-column injury at the cervicothoracic junction. These investigators used two-level fixation above and below the level of injury, with the addition of two cross-linking connectors to their construct.42 It is worth noting that their injury model did not involve damage to the vertebral body. However, combined anterior and posterior instrumentation for three-column injuries was supported by the biomechnical work of Prybis and colleagues. These authors demonstrated instability with posterior-only constructs and significantly improved stability with the addition of anterior fixation with or without corpectomy.53 This study also showed a trend toward decreased flexibility with increasing levels of thoracic instrumentation.
Choice of Surgical Approach
The choice of surgery depends on the location of the pathologic process. Surgical approaches to the cervicothoracic junction can be divided into four categories: anterior transcervical approaches (supraclavicular, transclavicular/transmanubrial, trans-sternal), anterolateral transthoracic approach, posterolateral approaches (costotransversectomy, lateral extracavitary, lateral parascapular extrapleural), and posterior approaches (laminectomy, transpedicular). The posterior approaches provide limited anterior column exposure and can cause further instability without adequate fixation, and they can also be associated with higher rates of neurologic morbidity.
Kaya and colleagues presented 17 patients who were treated for trauma or pathologic lesions of the cervicothoracic junction and outlined their methods for determining which approach to use. Their system accounts for the type of injury, the level of the lesion, and the time of admission. These authors selected an anterior approach for all pathologies involving the vertebral body and anterior structures and reserved posterior approaches for flexion–dislocation injuries without ventral involvement. The level of the lesion determined the particular anterior exposure, with the supramanubrial approach being used for access to T1, the transmanubrial approach for T2 and T3, and the lateral transthoracic for T3 and below. For patients whose lesions were diagnosed later than 1 month after the initial injury, a combined anterior-posterior approach was needed.54
Surgical Techniques
Lateral Parascapular Extrapleural Approach
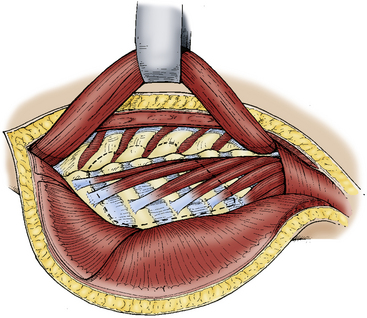
The posterior neck and back from the nuchal line to the sacrum is prepared and draped in standard fashion. The incision begins in the midline three spinous processes above the intended surgical area and continues to three spinous processes below before curving gently to the surgical side of approach. The skin, trapezius muscle, and rhomboid muscles are reflected as a myocutaneous flap toward the medial border of the scapula by developing a plane between these muscles and the paraspinal muscles at the spinous processes (Fig. 191-4). The inferior trapezius fibers are transected to reflect this flap, and an identifiable muscle cuff is left for reapproximation.
At the cervical level, dissection of the levator scapulae muscles also improves exposure of the cervicothoracic junction. This added visualization is achieved by mobilization of the myocutaneous flap and the scapula laterally to expose the lower cervical region, upper dorsal rib cage region, and dorsal vertebral elements.
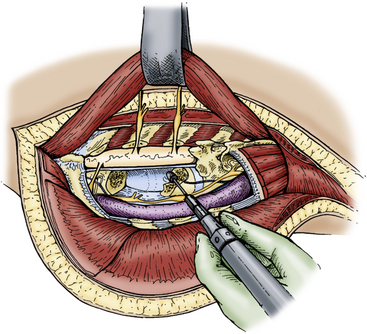
The intercostal nerve can be followed to the vertebral foramen, and the sympathetic chain can then be identified on the lateral vertebral surface. The rami communicantes are then transected, and the sympathetic chain is displaced anterolaterally to expose the vertebral body, pedicle, and foramen. At the level of the intercostal nerve, the transverse process, lamina, and pedicles are removed with a high-speed drill or Kerrison punch to expose the lateral thecal sac. A corpectomy can then be performed using either a high-speed drill or curet with preservation of the anterior and posterior longitudinal ligament to protect the thecal sac and superior mediastinal structures (Fig. 191-5). Vertebral reconstruction is performed using a rib graft or fibular allograft strut. If the pathologic process is malignant, Steinmann pins and methyl methacrylate are used. Alternatively, a metal cage with autologous bone may be used for vertebral body reconstruction. Appropriate posterior spinal fixation is then performed.
Anterolateral Transthoracic Approach
For the anterolateral transthoracic approach, the patient is placed in the lateral decubitus position. The skin is incised from the paraspinous area of T1 to the seventh rib along the medial border of the scapula. This incision is continued laterally, anteriorly, and then medially to the costal cartilage of the third rib (Fig. 191-6). The trapezius, latissimus dorsi, rhomboid major, and serratus posterior muscles are divided to identify the third rib. This identification is aided by locating the attachment of the scalenus anterior or medius muscles on the second rib. The scapula is retracted cephalad and medially using a scapular retractor, and the third rib is resected from its angle to the costal cartilage. The chest spreader can be inserted and opened, exposing the pleura and underlying lungs, aorta, and spine.
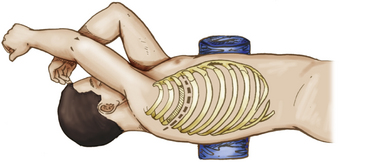
FIGURE 191-6 Patient position and skin incision for right anterolateral transthoracic approach to the upper thoracic spine.
The parietal pleura is incised from the costochondral cartilage to the midvertebral body. The lung is deflated and retracted to expose the spine (Fig. 191-7). The pleura is incised cephalad and caudad to expose the pathologic vertebrae and the intervertebral discs, as well as the intercostal arteries and veins that lie over the midvertebral body. The appropriate intercostal arteries and veins are dissected, ligated, and cut prior to vertebrectomy.
Transmanubrial and Transclavicular Approach
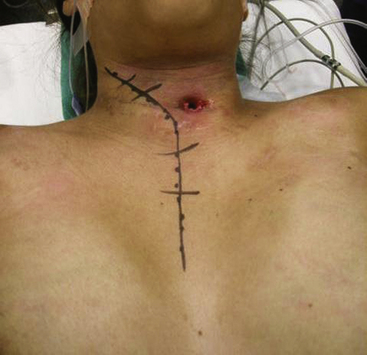
The patient is positioned supine with the head turned slightly to the side contralateral to the approach. An incision is made 2 cm above the clavicle from the contralateral sternocleidomastoid to 2 to 3 cm lateral to the ipsilateral sternocleidomastoid (Fig. 191-8). A T incision is then created in the midline, extending to the midpoint of the sternal body. This incision may also be modified depending on the patient’s specific anatomy and pathology (Fig. 191-9). The platysma muscle is divided and mobilized cephalad and caudad, and the external jugular vein is divided. The supraclavicular nerves should be identified and protected as much as possible, but the medial branches are often sacrificed.
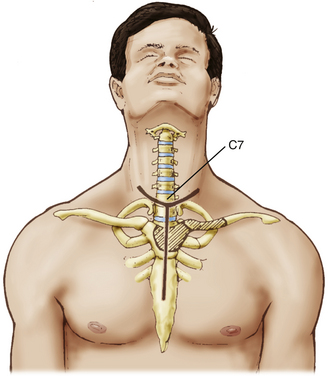
FIGURE 191-8 Patient position and skin incision for a transmanubrial/transclavicular approach to the cervicothoracic junction.
The suprasternal space of Burns (a suprasternal midline space created by a leaflet split in the superficial layer of deep fascia) is opened while leaving the periosteum and anterior transthoracic fascia for protection of the brachiocephalic veins. Next, the omohyoid muscle is marked and divided prior to identifying the internal jugular vein and common carotid artery.
The alar and mediastinal fascia are exposed via blunt dissection medial to the carotid sheath, and incision of these fasciae exposes the retromediastinal space and the prevertebral fascia covering the vertebral bodies and longus colli muscles (Fig. 191-10). Retractors are placed, and the pathologic process can be removed via curettage and suction or via a high-speed drill and Kerrison punch.
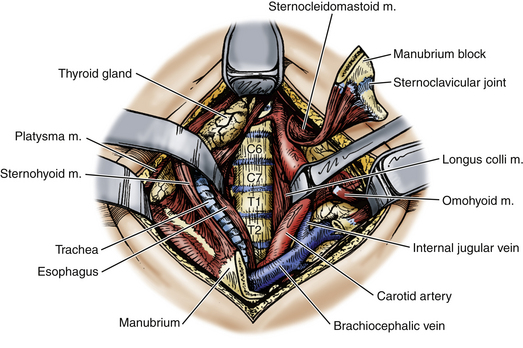
FIGURE 191-10 Operative exposure of the cervicothoracic junction via transmanubrial/transclavicular approach. M., muscle.
Following decompression of the spinal cord, reconstruction is begun using either autologous or allograft bone (tricortical iliac crest or fibula) or by synthetic implant (Fig. 191-11). After bony reconstruction, stabilization is achieved by an anterior plate screwed to the intact vertebrae above and below the pathologic levels or by placement in a halo brace.
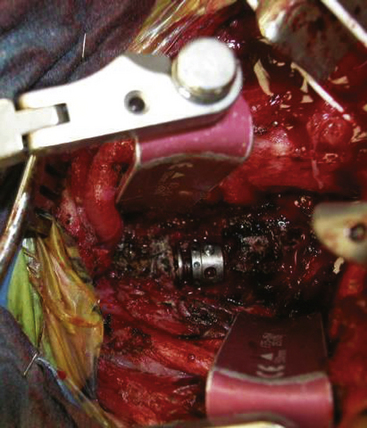
FIGURE 191-11 Intraoperative photograph of the cervicothoracic junction after corpectomy and placement of implant.
Variations have been reported in an attempt to reduce the sternal and clavicular dissection. Liu and colleagues reported the “trans–upper sternal approach,” which involves splitting the sternum to a point 2 cm distal to the sternal angle followed by cutting the two parts of the sternum transversely between the second and third rib.55 This procedure does not involve disruption of the clavicle. Pointillart and colleagues reported resection of lesions to T1–2 without resection of the sternum and to T5 with removal of a portion of the manubrium without violating the sternoclavicular joints.56 Teng and colleagues also described a preoperative method for determining the type of surgery based on the cervicothoracic angle (an angle formed between the axial plane of the suprasternal notch and the C7–T1 intervertebral disc). Lesions within this region were treated with the suprasternal approach, with occasional need for sternal splitting.57
Reconstruction and Stabilization
Operations at the cervicothoracic junction often require reconstruction and spinal stabilization because this region experiences significant biomechanical forces caused by the transition from the mobile, lordotic cervical spine to the fixed, kyphotic thoracic segment. Anterior reconstruction and stabilization techniques resemble that of the midcervical spine, but special anatomic issues arise for posterior instrumented fixation. A wide variety of options for instrumented constructs currently exist, with frequent new developments (Table 191-3).
| Anterior | Posterior |
|---|---|
| Structural Graft | |
| Iliac crest autograft | Iliac crest autograft |
| Rib | Laminectomy bone |
| Fibular allograft | |
| Vascularized fibular autograft | |
| Methyl methacrylate | |
| Instrumentation | |
| Anterior cervical plate | Interspinous wires |
| Harms cage | Luque rectangle/sublaminar wires |
| Steinmann methyl methacrylate pins | Pediatric CD hooks and rods |
| AO plates/lateral mass or pedicle screws | |
AO, Arbeitsgemeinschaft Osteosynthese; CD, Cotrel-Dubousset.
Anterior Reconstruction
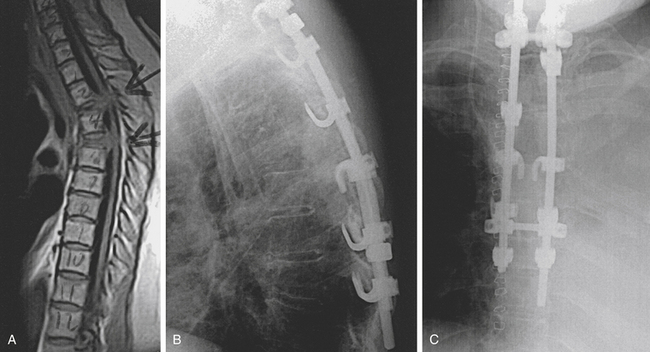
Bony fusion is desirable for trauma, infection, degenerative disease, and benign tumors. Most neurosurgeons have experience with anterior cervical stabilization, and following the approach, anterior cervicothoracic fusions are not significantly different. Fibular strut grafts or iliac crest autografts supplemented with an anterior cervical plate and screws provide excellent structural results but require a straight anterior approach (Fig. 191-12).
A Harms cage packed with autograft is another option for anterior support and eventual fusion, and recent additions of expandable cages and metal spacers can help facilitate reconstruction. Also, the use of free vascularized fibular strut grafts has been increasingly reported, particularly in correcting deformities.58
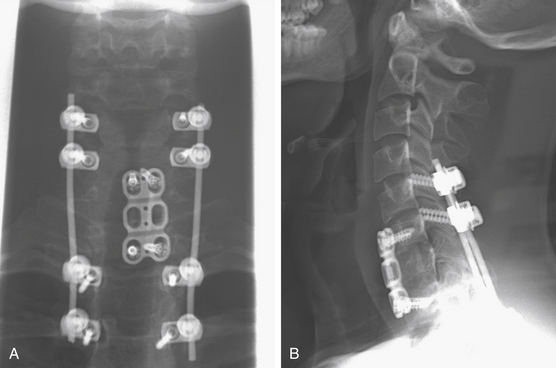
Posterior Stabilization
Posterior cervicothoracic instrumentation ranges from a simple tension band to complex hook, rod, and screw constructs. Because the cervicothoracic junction is under significant biomechanical stress, surgical fixation should incorporate several segments above and below the level of injury (Fig. 191-13). Tension bands resist flexion and distraction and can be used for pure ligamentous disruption. These tension bands are usually interspinous wiring, which can exert significant resistive forces and are simple, safe, fast, and a useful adjunct to other techniques.
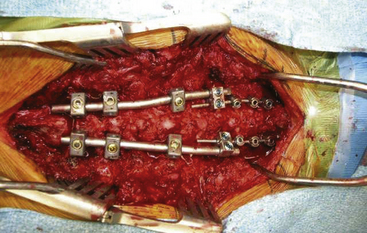
FIGURE 191-13 Posterior rod-and-screw construct spanning above and below the cervicothoracic junction.
Standalone anterior constructs have been reported to fail more commonly than posterior instrumentation, which affirms the mechanical advantage of posterior instrumentation.1 O’Brien and colleagues used two-column injury models to demonstrate the feasibility of biomechanical stabilization from posterior instrumentation alone.42
The Luque rectangle with sublaminar wires or Songer cables was a mainstay of internal fixation at the cervicothoracic junction. This relatively rigid construct combines segmental fixation with resistance to flexion, torsion, and some translation. This technique requires intact and healthy posterior elements and carries the risk of spinal cord injury with passage of the sublaminar wires. The construct should cover at least two levels above and two below the region of instability. However, recent biomechanical data demonstrate that posterior wiring might not increase stability when a lateral mass or pedicle screw construct has been used.47
Pediatric Cotrel-Dubousset systems with laminar hooks and rods have been applied at the cervicothoracic junction (Fig. 191-14). This construct is stable, but it requires intact posterior elements at the points of fixation and may be limited by the narrow cervicothoracic spinal canal.
Other options exist when the posterior elements have been disrupted by trauma or have been removed surgically. Beginning with the lateral mass plates of Roy-Camille and colleagues,59 new plate-and-rod–based fixation methods have emerged.60 Some additions include minipolyaxial screws, dual-diameter rods, cervical cross-links, and interconnectors. Current stabilization techniques require experience with cervical lateral mass or pedicle screw placement, upper and lower thoracic pedicle screw placement, and thoracic transverse process, pedicle, and laminar hooks. When the lamina are intact, sublaminar wiring techniques can add to the construct’s stability and are especially useful in the cervical area where screw pullout can often occur. Also, quadrilateral stability can be achieved by using two cross-links. Placentonakis and colleagues demonstrated the evolution of posterior instrumentation techniques in tumor surgery from sublaminar hook-and-rod constructs to lateral mass and pedicle screw constructs with improved stabilization.2
At C7, the vertebral artery is on average 18 mm from midline and 17 mm from the posterior cortex of the lateral mass.61 Vertebral artery injury can occur if a lateral mass screw is too long or is directed less than 14 degrees laterally from the midpoint of the lateral mass of C7. At T1, the vertebral artery is 22 mm from the midline and 22 mm from the posterior cortex of the transverse process. These relationships can change with significant kyphotic or scoliotic deformity and can increase the risk of injury. Additionally, a caudally placed lateral mass screw can lead to spinal nerve injury.
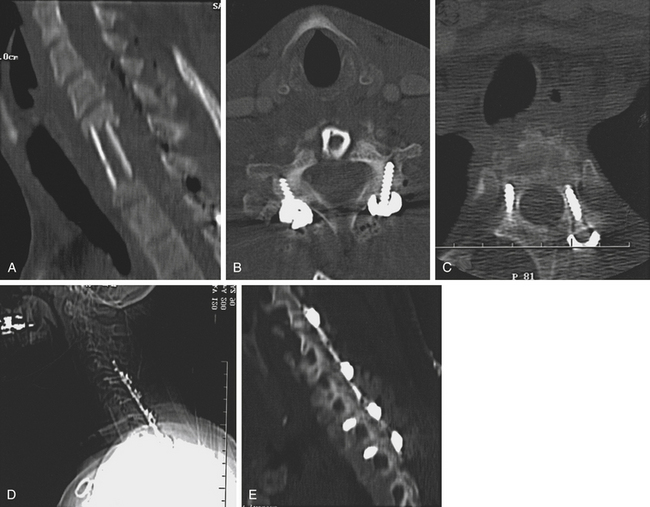
At C7, the facet and lateral mass is thinner and can provide inadequate screw purchase, and as a result, lateral mass plating might not produce a biomechanically stable construct at the cervicothoracic junction. Pedicle screw constructs, however, have shown excellent biomechanical stability in this region (Fig. 191-15).
A number of studies have reported the unique anatomic constraints of the cervicothoracic pedicles. An and colleagues62 found it difficult to determine pedicle alignment in a cadaveric study. Stanescu and colleagues63 and Panjabi and coworkers64 both found highly variable pedicle dimensions and angles in the lower cervical and upper thoracic spine. This work has shown that the mean pedicle width increases from 5 mm at C5 to 8 mm at T1 and then decreases again to 4.5 mm at T5. Based on these data, the pedicles at C7, T1, and T2 are often large enough to permit placement of a 3.5-mm screw.
Karaikovic and colleagues65 studied 53 European-American cervical spines and found outer pedicle widths equal to or smaller than 4 mm in 13.2% of C5 and C6 and 6.6% of C7 pedicles. Miller and coworkers66 used the technique of Jeanneret and colleagues67 to attempt 3-mm pedicle screws in cadaveric cervical spines blindly or with a small laminotomy. They found potentially dangerous violations of the pedicle in 10 of 38 blinded placements and one of 40 placements after the advent of a laminotomy. In the thoracic spine, this group compared the Roy-Camille technique with the open lamina technique. They found grade II and III pedicle violations in 48 of 95 screws and 10 of 94 screws, respectively. In general, the T3 to T5 levels often have small, narrow pedicles that make screw placement challenging, often necessitating the use of thoracic hooks.
Despite anatomic concerns, cervicothoracic pedicle screws have been successfully employed in clinical practice. Abumi and colleagues68 used pedicle screws in 13 patients with fracture-dislocations of the lower cervical spine with good results and no of complications. In another series,69 these authors reported placement of 191 cervical pedicle screws (most by landmarks) with one transient screw thread–related radiculopathy.
Chapman and colleagues40 published a series of 23 patients instrumented posteriorly using AO plates, as well as cervical lateral mass and thoracic pedicle screws. In this series, they reported a 100% fusion rate, no neurovascular complications, and no hardware complications requiring reoperation. Albert and colleagues39 reviewed 21 patients who were treated with pedicle screws at C7 with the open lamina technique. They found no neurovascular complications and no instrument failures at 1-year follow-up. Lenoir and colleagues achieved fusion in all patients (n = 30) using posterior instrumentation with two delayed mechanical failures.6
New advances in intraoperative image guidance have led to improved accuracy in pedicle screw placement. Bledsoe and colleagues reported the use of three-dimensional image guidance for the placement of 150 pedicle screws. These authors noted 93% accuracy in screw placement with rare grade I pedicle violations based on postoperative CT scans.70 Lee and colleagues compared the placement of thoracic pedicle screws using the open laminotomy technique against computer-assisted techniques and found improved screw placement using the image-guided systems.71 These findings demonstrate that a combination of an extensive anatomic understanding and a knowledge of current technologies offers the best outcome for patients.
Discussion and Conclusion
Among these approaches, the transmanubrial/transclavicular approach of Sundaresan and colleagues18 provides the best approach to a combination of the lower cervical and upper thoracic regions to T3. The transthoracic approach through the third rib exposes the vertebral bodies anterolaterally by opening the intrapleural cavity to reach the paramedian compartment of the retromediastinal space. Exposure of the T3 and T4 vertebral bodies is generally adequate, but access to T1 and T2 is limited by the narrowing of the thoracic inlet.
Surgical approaches to the cervicothoracic junction can be divided into these three groups, and the choice of procedure depends on the location and extent of pathology. Considerations for selecting the most appropriate surgical approach are listed in Table 191-4. Pathology that extends from the lower cervical to the upper thoracic region above T3 is most easily approached through a transmanubrial/transclavicular approach. Pathology limited to T3 and T4 can be easily accessed through either the lateral parascapular extrapleural or the anterolateral thoracotomy approaches. Pathology that includes T3 or T4 and extends cephalad to (but no farther than) the C7–T1 disc space is more easily approached through the lateral parascapular extrapleural approach. Thus, a knowledge of this region’s anatomy, surgical approaches, and instrumentation is essential for the successful treatment of the diverse pathologies of the cervicothoracic junction (Fig. 191-16).
| Approach | Pathology |
|---|---|
| Transmanubrial/transclavicular | Pathology extends from C6 or C7 to T1 or T2 but not below T2 |
| Lateral parascapular extrapleural or anterolateral thoracotomy | Pathology limited to T3 and/or T4 |
| Lateral parascapular extrapleural | Pathology extends up to but not beyond C7–T1 disc |
An H.S., Vaccaro A., Cotler J.M., Lin S. Spinal disorders at the cervicothoracic junction. Spine. 1994;19:2557-2564.
Birch R., Bonney G., Marshall R.W. A surgical approach to the cervicothoracic spine. J Bone Joint Surg Br. 1990;72:904-907.
Brunig F. Technik der kombinierten Resektionsmethode samtlicher sympathischen Nervenbaum am Halse. Zentralbl Chir. 1923;50:1056-1059.
Butler E.G., Dohrmann P.J., Stark R.J. Spinal subdural abscess. Clin Exp Neurol. 1988;25:67-70.
Capener N. The evolution of lateral rachotomy. J Bone Joint Surg Br. 1954;36:173-179.
Edelstyn G.A., Gillespie P.J., Grebbell F.S. The radiological demonstration of osseous metastases. Clin Radiol. 1967;18:158-162.
Fessler R.G., Dietze D.D.Jr., MacMillan M., Peace D. Lateral parascapular extrapleural approach to the upper thoracic spine. J Neurosurg. 1991;75:349-355.
Galasko C.S. Skeletal metastases. Clin Orthop Relat Res. 1986;210:18-30.
Gask G.E. The surgery of the sympathetic nervous system. Br J Surg. 1933;21:113-130.
Hodgson A.R., Stock F.E., Fang H.S.Y., Ong G.B. Anterior spinal fusion: The operative approach and pathological findings in 412 patients with Pott’s disease of the spine. Br J Surg. 1960;48:172-178.
Jonnesco T. Le Sympathique Cervico-thoracico. Paris: Masson; 1923.
Larson S.J., Holst R.A., Hemmy D.C., Sances A. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45:628-637.
Le H., Balabhadra R., Park J., Kim D. Surgical treatment of tumors involving the cervicothoracic junction. Neurosurg Focus. 2003;15:E3.
Lenoir T., Hoffmann E., Thevenin-Lemoine C., et al. Neurological and functional outcome after unstable cervicothoracic junction injury treated by posterior reduction and synthesis. Spine J. 2006;6:507-513.
Menard V. Etude Pratique sur le Mal de Pott. Paris: Masson; 1910.
Nichols C.G., Young D.H., Schiller W.R. Evaluation of cervicothoracic junction injury. Ann Emerg Med. 1987;16:640-642.
Ochsner A., DeBakey M. Peripheral vascular disease. Surg Gynecol Obstet. 1940;70:1058-1072.
O’Mara R.E. Bone scanning in osseous metastatic disease. JAMA. 1974;229:1915.
Placantonakis D.G., Laufer I., Wang J.C., et al. Posterior stabilization strategies following resection of cervicothoracic junction tumors: review of 90 consecutive cases. J Neurosurg Spine. 2008;9:111-119.
Royle N.D. Observations on the alteration of the circulation of the brain by surgical means in diseases of the central nervous system. Br Med J. 1932;1:1063-1068.
Sapico B.L., Montgomerie J.Z. Vertebral osteomyelitis in intravenous drug abusers: Report of three cases and review of the literature. Rev Infect Dis. 1980;2:196-206.
Sapkas G., Papadakis S., Katonis P., et al. Operative treatment of unstable injuries of the cervicothoracic junction. Eur Spine J. 1999;8:279-283.
Siegal T. Surgical decompression of anterior and posterior malignant epidural tumors compressing the spinal cord: A prospective study. Neurosurgery. 1985;17:424-432.
Sundaresan N., DiGiacinto G.V., Hughes J.E.O., Krol G. Spondylectomy for malignant tumors of the spine. J Clin Oncol. 1989;7:1485-1491.
Sundaresan N., Shah J., Foley K.M., Rosen G. An anterior surgical approach to the upper thoracic vertebrae. J Neurosurg. 1984;61:686-690.
1. Le H., Balabhadra R., Park J., Kim D. Surgical treatment of tumors involving the cervicothoracic junction. Neurosurg Focus. 2003;15:E3.
2. Placantonakis D.G., Laufer I., Wang J.C., et al. Posterior stabilization strategies following resection of cervicothoracic junction tumors: review of 90 consecutive cases. J Neurosurg Spine. 2008;9:111-119.
3. Nichols C.G., Young D.H., Schiller W.R. Evaluation of cervicothoracic junction injury. Ann Emerg Med. 1987;16:640-642.
4. Sapkas G., Papadakis S., Katonis P., et al. Operative treatment of unstable injuries of the cervicothoracic junction. Eur Spine J. 1999;8:279-283.
5. An H.S., Vaccaro A., Cotler J.M., Lin S. Spinal disorders at the cervicothoracic junction. Spine. 1994;19:2557-2564.
6. Lenoir T., Hoffmann E., Thevenin-Lemoine C., et al. Neurological and functional outcome after unstable cervicothoracic junction injury treated by posterior reduction and synthesis. Spine J. 2006;6:507-513.
7. Sundaresan N., DiGiacinto G.V., Hughes J.E.O., Krol G. Spondylectomy for malignant tumors of the spine. J Clin Oncol. 1989;7:1485-1491.
8. Siegal T. Surgical decompression of anterior and posterior malignant epidural tumors compressing the spinal cord: A prospective study. Neurosurgery. 1985;17:424-432.
9. Menard V. Etude Pratique sur le Mal de Pott. Paris: Masson; 1910.
10. Capener N. The evolution of lateral rachotomy. J Bone Joint Surg Br. 1954;36:173-179.
11. Larson S.J., Holst R.A., Hemmy D.C., Sances A. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45:628-637.
12. Fessler R.G., Dietze D.D.Jr., MacMillan M., Peace D. Lateral parascapular extrapleural approach to the upper thoracic spine. J Neurosurg. 1991;75:349-355.
13. Jonnesco T. Le Sympathique Cervico-thoracico. Paris: Masson; 1923.
14. Brunig F. Technik der kombinierten Resektionsmethode samtlicher sympathischen Nervenbaum am Halse. Zentralbl Chir. 1923;50:1056-1059.
15. Royle N.D. Observations on the alteration of the circulation of the brain by surgical means in diseases of the central nervous system. Br Med J. 1932;1:1063-1068.
16. Gask G.E. The surgery of the sympathetic nervous system. Br J Surg. 1933;21:113-130.
17. Ochsner A., DeBakey M. Peripheral vascular disease. Surg Gynecol Obstet. 1940;70:1058-1072.
18. Sundaresan N., Shah J., Foley K.M., Rosen G. An anterior surgical approach to the upper thoracic vertebrae. J Neurosurg. 1984;61:686-690.
19. Birch R., Bonney G., Marshall R.W. A surgical approach to the cervicothoracic spine. J Bone Joint Surg Br. 1990;72:904-907.
20. Hodgson A.R., Stock F.E., Fang H.S.Y., Ong G.B. Anterior spinal fusion: The operative approach and pathological findings in 412 patients with Pott’s disease of the spine. Br J Surg. 1960;48:172-178.
21. Sapico B.L., Montgomerie J.Z. Vertebral osteomyelitis in intravenous drug abusers: Report of three cases and review of the literature. Rev Infect Dis. 1980;2:196-206.
22. Edelstyn G.A., Gillespie P.J., Grebbell F.S. The radiological demonstration of osseous metastases. Clin Radiol. 1967;18:158-162.
23. O’Mara R.E. Bone scanning in osseous metastatic disease. JAMA. 1974;229:1915.
24. Butler E.G., Dohrmann P.J., Stark R.J. Spinal subdural abscess. Clin Exp Neurol. 1988;25:67-70.
25. Galasko C.S. Skeletal metastases. Clin Orthop Relat Res. 1986;210:18-30.
26. Schlaeffer F., Mikolich D.J., Mates S.M. Technetium Tc 99m diphosphonate bone scan: False normal findings in elderly patients with hematogenous vertebral osteomyelitis. Arch Intern Med. 1987;147:2024-2026.
27. Staab E.V., McCartney W.H. Role of gallium 67 in inflammatory disease. Semin Nucl Med. 1978;8:219-234.
28. Bruns J., Maas R. Advantages of diagnosing bacterial spondylitis with magnetic resonance imaging. Arch Orthop Trauma Surg. 1989;108:30-35.
29. Paushter D.M., Modic M.T., Masaryk T.J. Magnetic resonance imaging of the spine: Applications and limitations. Radiol Clin North Am. 1985;23:551-562.
30. Oda I., Abumi K., Lu D., et al. Biomechanical role of the posterior elements, costovertebral joints, and rib cage in the stability of the thoracic spine. Spine. 1996;21:1423-1429.
31. Berg E.E. The sternal-rib complex: A possible fourth column in thoracic spine fractures. Spine. 1993;18:1916-1919.
32. An H.S., Wise J.J., Xu R. Anatomy of the cervicothoracic junction: A study of cadaveric dissection, cryomicrotomy, and magnetic resonance imaging. J Spinal Disord. 1999;12:519-525.
33. Pal G.P., Routal R.V. A study of weight transmission through the cervical and upper thoracic regions of the vertebral column in man. J Anat. 1986;148:245-261.
34. Boyle J.J., Singer K.P., Milne N. Morphological survey of the cervicothoracic junctional region. Spine. 1996;21:544-548.
35. Yasuoka S., Peterson H.A., MacCarty C.S. Incidence of spinal column deformity after multilevel laminectomy in children and adults. J Neurosurg. 1982;57:441-445.
36. Inoue A., Ikata T., Katoh S. Spinal deformity following surgery for spinal cord tumors and tumorous lesions: Analysis based on an assessment of the spinal functional curve. Spinal Cord. 1996;34:536-542.
37. Drennan J.C., King E.W. Cervical dislocation following fusion of the upper thoracic spine for scoliosis: A case report. J Bone Joint Surg Am. 1978;60:1003-1005.
38. Steinmetz M.P., Miller J., Warbel A., et al. Regional instability following cervicothoracic junction surgery. J Neurosurg Spine. 2006;4:278-284.
39. Albert T.J., Klein G.R., Joffe D., Vaccaro A.R. Use of cervicothoracic junction pedicle screws for reconstruction of complex cervical spine pathology. Spine. 1998;23:1596-1599.
40. Chapman J.R., Anderson P.A., Pepin C., et al. Posterior instrumentation of the unstable cervicothoracic spine. J Neurosurg. 1996;84:552-558.
41. Ulrich C., Arand M., Nothwang J. Internal fixation on the lower cervical spine: biomechanics and clinical practice of procedures and implants. Eur Spine J. 2001;10:88-100.
42. O’Brien J.R., Dmitriev A.E., Yu W., et al. Posterior-only stabilization of 2-column and 3-column injuries at the cervicothoracic junction: a biomechanical study. J Spinal Disord Tech. 2009;22:340-346.
43. Wellman B.J., Follett K.A., Traynelis V.C. Complications of posterior articular mass plate fixation of the subaxial cervical spine in 43 consecutive patients. Spine. 1998;23:193-200.
44. Ulrich C., Woersdoerfer O., Kalff R., et al. Biomechanics of fixation systems to the cervical spine. Spine. 1991;16(suppl 3):S4-S9.
45. Heller J.G., Estes B.T., Zaouali M., Diop A. Biomechanical study of screws in the lateral masses: Variables affecting pull-out resistance. J Bone Joint Surg Am. 1996;78:1315-1321.
46. Kotani Y., Cunningham B.W., Abumi K., McAfee P.C. Biomechanical analysis of cervical stabilization systems: An assessment of transpedicular screw fixation in the cervical spine. Spine. 1994;19:2529-2539.
47. Rhee J.M., Kraiwattanapong C., Hutton W.C. A comparison of pedicle and lateral mass screw construct stiffnesses at the cervicothoracic junction: a biomechanical study. Spine. 2005;30:E636-E640.
48. Miller R.M., Ebraheim N.A., Xu R., Yeasting R.A. Anatomic consideration of transpedicular screw placement in the cervical spine: An analysis of two approaches. Spine. 1996;21:2317-2322.
49. Bueff H.U., Lotz J.C., Colliou O.K., et al. Instrumentation of the cervicothoracic junction after destabilization. Spine. 1995;20:1789-1792.
50. Boockvar J.A., Philips M.F., Telfeian A.E., et al. Results and risk factors for anterior cervicothoracic junction surgery. J Neurosurg. 2001;94(suppl 1):12-17.
51. Vaccaro R., Conant R.F., Hilibrand A.S., Albert T.J. A plate–rod device for treatment of cervicothoracic disorders: Comparison of mechanical testing with established cervical spine in vitro load testing data. J Spinal Disord. 2000;13:350-355.
52. Kreshak J.L., Kim D.H., Lindsey D.P., et al. Posterior stabilization at the cervicothoracic junction: A biomechanical study. Spine. 2002;27:2763-2770.
53. Prybis B.G., Tortolani P.J., Hu N., et al. A comparative biomechanical analysis of spinal instability and instrumentation of the cervicothoracic junction: an in vitro human cadaveric model. J Spinal Disord Tech. 2007;20:233-238.
54. Kaya R.A., Türkmenoglu O.N., Koç O.N., et al. A perspective for the selection of surgical approaches in patients with upper thoracic and cervicothoracic junction instabilities. Surg Neurol. 2006;65:454-463.
55. Liu Y.L., Hao Y.J., Li T., et al. Trans-upper sternal approach to the cervicothoracic junction. Clin Orthop Relat Res. 2009;467:2018-2024.
56. Pointillart V., Aurouer N., Gangnet N., Vital J.M. Anterior approach to the cervicothoracic junction without sternotomy: a report of 37 cases. Spine. 2007;32:2875-2879.
57. Teng H., Hsiang J., Wu C., et al. Surgery in the cervicothoracic junction with an anterior low suprasternal approach alone or combined with manubriotomy and sternotomy: an approach selection method based on the cervicothoracic angle. J Neurosurg Spine. 2009;10:531-542.
58. Kaneda K., Kurakami C., Minami A. Free vascularized fibular strut graft in the treatment of kyphosis. Spine. 1988;13:1273-1277.
59. Roy-Camille R., SG, Mazel C. Internal fixation of the unstable cervical spine by a posterior osteosynthesis with plates and screws. In: Sherk H.H., Dunn E.J., Eismont F.J., et al. The Cervical Spine. 2nd ed. Philadelphia: JB Lippincott; 1989:390-403.
60. Xu R., Ebraheim N.A., Ou Y., Yeasting R.A. Anatomic considerations of pedicle screw placement in the thoracic spine: Roy-Camille technique versus open-lamina technique. Spine. 1998;23:1065-1068.
61. Xu R., Ebraheim N.A., Tang G., Stanescu S. Location of the vertebral artery in the cervicothoracic junction. Am J Orthop. 2000;29:453-456.
62. An H.S., Gordin R., Renner K. Anatomic considerations in plate–screw fixation of the cervical spine. Spine. 1981;16(Suppl):548-551.
63. Stanescu S., Ebraheim N.A., Yeasting R., et al. Morphometric evaluation of the cervico-thoracic junction. Spine. 1994;19:2082-2088.
64. Panjabi M.M., Duranceau J., Goel V., et al. Cervical human vertebrae: Quantitative three-dimensional anatomy of the middle and lower regions. Spine. 1991;16:861-869.
65. Karaikovic E.E., Daubs M.D., Gaines R.W.Jr. Morphologic characteristics of human cervical pedicles. Spine. 1997;22:493-500.
66. Miller R.C., Bonner J.A., Wenger D.E., et al. Spinal cord localization in the treatment of lung cancer: Use of radiographic landmarks. Int J Radiat Oncol Biol Phys. 1998;40:347-351.
67. Jeanneret B., Gebhard J.S., Magerl F. Transpedicular screw fixation of articular mass fracture–separation: Result of an anatomical study and operative technique. J Spinal Disord. 1994;7:222-229.
68. Abumi K., Itoh H., Taneichi H., Kaneda K. Transpedicular screw fixation for traumatic lesions of the middle and lower cervical spine: Description of the techniques and preliminary report. J Spinal Disord. 1994;7:19-28.
69. Abumi K., Kaneda K. Pedicle screw fixation for nontraumatic lesions of the cervical spine. Spine. 1997;22:1853-1863.
70. Bledsoe J.M., Fenton D., Fogelson J.L., Nottmeier E.W. Accuracy of upper thoracic pedicle screw placement using three-dimensional image guidance. Spine J. 2009;9:817-821.
71. Lee G.Y., Massicotte E.M., Rampersaud Y.R. Clinical accuracy of cervicothoracic pedicle screw placement: a comparison of the “open” lamino-foraminotomy and computer-assisted techniques. J Spinal Disord Tech. 2007;20:25-32.