Chapter 27 Surgical Approaches to Lateral and Third Ventricular Tumors
The majority of tumors of the lateral and third ventricles are benign or low-grade lesions. Because of their relatively slow growth rate, these lesions may reach several centimeters in size before they present to medical attention. The most common clinical manifestations of these tumors include headaches, memory loss, gait disorders, and cognitive changes.1
Lateral Ventricles
Surgical Anatomy
The lateral ventricles are divided into five areas: frontal horns, bodies, atria, occipital horns, and temporal horns (Fig. 27-1).
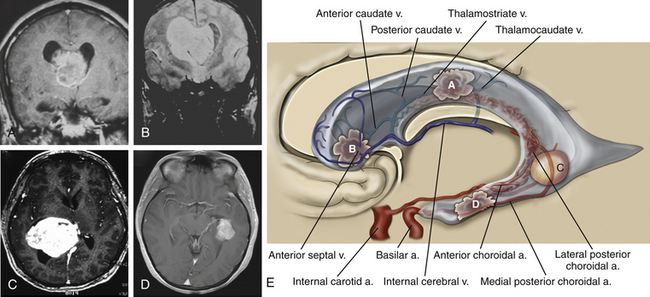
The trigone or atrium of the lateral ventricles is a confluence of the body and temporal and occipital horns. The atrium begins as a continuation of the body at the posterior edge of the thalamus and ends further posteriorly as the corpus callosum blends into the occipital lobe. The splenium (superiorly) and the tapetum of the corpus callosum (more posteriorly) make up the roof of the atrium. Because the roof bends into the lateral wall posteriorly, the tapetum covers this lateral wall segment. More anteriorly, the caudate tail covers the lateral wall as it curves downward, on its way toward the temporal lobe. The anterior boundary of the atrium starts just medial to the caudate tail with the pulvinar eminence. Medial to the pulvinar, covered by choroid, is the crus of the fornix. At the atrial level of the choroid plexus, two choroidal arteries can often be seen, one curving with the choroid medially and the anterior choroidal artery, which can course into the body of the ventricle. More laterally, the lateral posterior choroidal artery, which may have several branches, runs to supply the atrium and body of the choroid. The triangular enlargement of the choroid plexus at the trigone is called the glomus. The medial wall of the atrium has two prominences. The upper prominence consists of the forceps major fibers and is called the bulb of the corpus callosum. The lower prominence is called the calcar avis and is simply the ventricular protrusion of the calcarine sulcus. The floor similarly consists of the upward protrusion of the collateral sulcus forming the collateral trigone.
The temporal horn is an extension of the lateral ventricles into the medial temporal lobe. The floor displays two prominences: (1) laterally the collateral eminence formed by the underlying deep collateral sulcus and (2) medial to that, the hippocampus, which protrudes prominently into the floor. The lateral wall, which angles into the roof of the temporal horn, is lined by the tapetum. In the medial part of the roof, the tail of the caudate projects anteriorly toward the amygdaloid nucleus. Medial to the caudate tail, forming the medial wall of the temporal horn, is the thalamus and, inferior to it, the fimbria of the fornix. The choroid fissure separates the thalamus from the fornix. The choroid plexus is attached as it continues anteriorly to end just posterior to the amygdaloid nucleus at the inferior choroidal point. The anterior choroidal artery enters the choroidal fissure at approximately this point and courses posteriorly in the plexus. More posteriorly, the lateral posterior choroidal artery enters the fissure and is seen more laterally in the choroid plexus. The temporal horn ends in the amygdaloid nucleus.2–5
Surgical Options
Anterior Transcallosal Approach
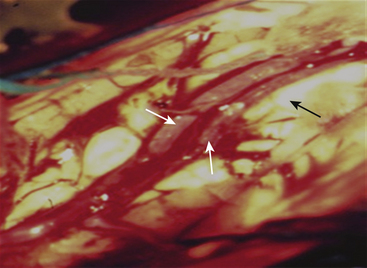
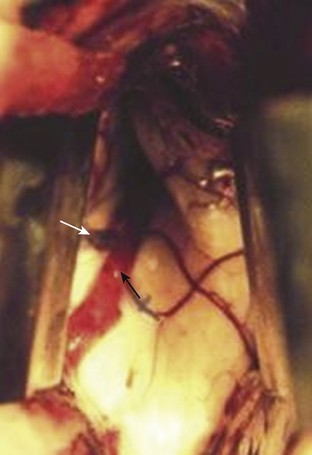
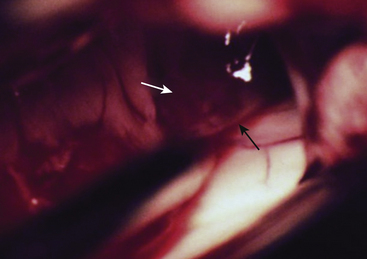
The anterior transcallosal route is useful for lesions arising within the body of the lateral ventricle (Fig. 27-1A). Cortical veins draining into the sagittal sinus can be a significant obstacle to interhemispheric access and a preoperative cerebral angiogram or magnetic resonance venogram can be important for surgical planning. Furthermore, the ventricular venous and arterial structures can be distorted by the tumor and should be noted preoperatively. The patient is placed in a supine position, with the head slightly flexed. Alternatively, the patient’s head is fixed in a lateral position with the affected hemisphere toward the floor to use gravity to assist with retraction. A bicoronal incision is made anterior to the coronal suture. The craniotomy is also centered anterior to the coronal suture. Midline exposure should extend up to the superior sagittal sinus. The dura is opened and reflected medially up to the sagittal sinus. Often, cortical draining veins enter the dura before reaching the midline. These veins may be preserved by opening the dura on all sides around the veins, leaving the dura covering the venous access to the sagittal sinus intact. If exuberant arachnoid granulations are encountered, they can be divided with sharp dissection and bipolar cautery. Once the midline is reached, the falx is followed to its depth. At this point, the operating microscope is used. The arachnoid below the falx may be adherent, and this must be carefully divided to avoid injury to the cingulate gyrus on either side. Once the corpus callosum is reached, the two pericallosal arteries are visualized (Fig. 27-2). Ventricular access between the two arteries helps to prevent vascular injury. The callosum midline is often demarcated by a very small callosal artery. The callosotomy can be started just posterior to the genu and developed 2 to 3 cm posteriorly to gain access to the lateral ventricle. By performing the callosotomy off midline and toward the ventricle of interest, opening the contralateral ventricle can be avoided. Proper orientation is confirmed by locating the choroid plexus and the thalamostriate vein entering into the foramen of Monro. If the vein is to the right of the choroid plexus, the surgeon is in the right ventricle, if the vein is to the left of the choroid, the surgeon is in the left ventricle (Fig. 27-3). Throughout the course of tumor resection, the interface between the tumor and the ependymal surface (Fig. 27-4) should be identified and preserved as this prevents losing the plane of dissection. Since many lateral ventricular tumors can reach a very large size, an internal decompression may be required prior to isolating the tumor capsule away from surrounding ventricular structures.2,6–9
Anterior Trans-Sulcal Approach
The anterior trans-sulcal approach (superior frontal sulcus) is used commonly for lesions in the anterior lateral ventricles, especially when associated with hydrocephalus. In addition, this approach is preferred over the transcallosal route for large, midline-draining cortical veins. The craniotomy is similar to the one used for the transcallosal approach and uses a bicoronal incision lateral to the midline, measuring 4 to 6 cm in length. The superior and middle frontal gyri need to be exposed. Intraoperative ultrasound or stereotaxis can provide guidance for direct ventricular access. The sulcus is dissected open to its depths (or a 2 to 3 cm gyral incision is made) and developed down into the ventricle. The operative microscope is used after the ventricular chamber is opened. Tumor removal is achieved by delivering the lesion to the area of exposure while maintaining the tumor interface with the ependymal surface.
Combined Approaches
Very large tumors that extend into the frontal horn and body of the ventricle may require both a trans-sulcal and transcallosal approach (see Fig. 27-1B). This is necessitated by the limitations of each operative exposure. Entering the ventricle through the corpus callosum cannot provide access to the frontal horn without excessive retraction. Similarly, an anterior trans-sulcal exposure limits access to the posterior body of the ventricle. In those tumors that fill the lateral ventricle, an initial decompression through a trans-sulcal corridor can generate adequate relaxation of the hemisphere to permit interhemispheric dissection. Once this is achieved, the transcallosal corridor can be opened to complete the tumor resection without excessive retraction to either region.
Posterior Trans-Sulcal Approach
The interparietal sulcus approach (superior parietal lobule) is the preferred route to the atrium of the lateral ventricle and allows access to both medial and lateral segments of this part of the ventricle (see Fig. 27-1C). The patient is positioned in the three-quarter prone position with the parietal region at the highest point in the field. The craniotomy extends over the superior parietal lobule. A preoperative magnetic resonance venogram or cerebral angiogram is helpful in determining the position of major draining veins. The craniotomy does not cross midline. Once the cortex is exposed (or cortical incision is made), dissection proceeds along the interparietal sulcus. The atrium is more lateral at this location, and the dissection can be guided by ultrasound or stereotaxis. Once inside the atrium, the surgeon can visualize the thalamus anteriorly, the choroid plexus more medially, and the crus of the fornix. It should be remembered that the optic radiations define the lateral wall of the atrium, and the surgeon should avoid manipulation of that area. When tumors compress the lateral wall of the atrium, the tumor should be decompressed before separating it from this lateral ependymal surface. The vascular pedicle of the tumor should be identified and coagulated at the earliest possible time to avoid excessive bleeding.2,10
Posterior Transcallosal Approach
The posterior transcallosal approach gains access to the roof and medial part of the atrium of the lateral ventricles. This is achieved by splitting the splenium of the corpus callosum and is contraindicated for patients with a preoperative homonymous hemianopia contralateral to the dominant hemisphere due to a risk of alexia. Because the lateral ventricle extends laterally in this region, the lateral part of the atrium is not well visualized by this route. Preoperatively, as in the anterior approaches, a magnetic resonance venogram or cerebral angiogram helps to guide the placement of the craniotomy by visualizing the dominant cortical draining vessels. The patient is positioned in the three-quarter prone position, with the parietal area of the operated side in the dependent position. The craniotomy begins at the posterior edge of the postcentral gyrus and extends approximately 4 cm posteriorly. The craniotomy exposes the superior sagittal sinus and extends laterally 3 to 4 cm. The dura is reflected medially, and care is taken to maintain the large draining veins. The parietal lobe is gently retracted (approximately 2 cm) away from the falx. Once the arachnoid adhesions are opened, the distal pericallosal arteries and the splenium are identified. Below the splenium, the ICVs join Galen’s vein, and these can be seen once the splenium is cut. The splenium is incised with a bipolar cautery, and this incision must be made lateral to the midline because the atrium of the lateral ventricle deviates laterally. Access into the atrium is now achieved; however, tumors not found in the medial part of the atrium will be hard to resect by this route, and the surgeon should consider the posterior transcortical route for lateral atrial tumors.2,11,12
Inferior Temporal Approach
This approach is used to gain access to temporal horn lesions (see Fig. 27-1D). The patient is placed supine, with the head tilted away by 45 degrees and extended. A reverse question mark incision is made starting at the level of the zygoma just anterior to the ear, then curving posteriorly over the ear and anteriorly toward the forehead. The temporalis muscle is mobilized anteriorly, and the craniotomy is extended inferiorly to the level of the zygoma. The dura is opened with its base positioned anteriorly. Access to the ventricle is achieved by making a cortical incision in the middle or inferior temporal gyrus or traversing the middle temporal sulcus. The more inferior temporal approaches are often used for lesions residing in the temporal horn or lateral atrium of the dominant hemisphere. Decompression of the tumor is followed by dissection away from surrounding ependyma.2
The Third Ventricle
Surgical Anatomy
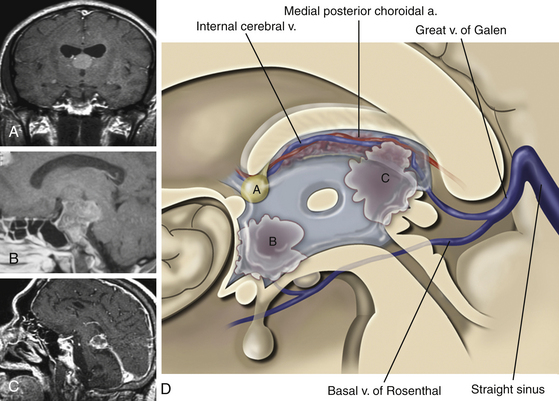
The third ventricle communicates with the lateral ventricles via the foramen of Monro and drains posteriorly into the aqueduct of Sylvius. Approximately one-third of the third ventricle is located anterior to the foramen of Monro and extends to the optic chiasm inferiorly. The anterior wall consists mainly of the lamina terminalis, a thin sheet of pia and gray matter that runs from the optic chiasm to the rostrum of the corpus callosum. The columns of the fornix are found at the superior lateral margins, and the anterior commissure crosses the anterior wall at its upper end. The lateral wall of the third ventricle is formed inferiorly by the hypothalamus and superiorly and posteriorly by the thalamus. In 75% of cases the massa intermedia, a thalamic projection, bridges the third ventricle at the superior-posterior end. The floor of the third ventricle starts at the optic chiasm at its anterior pole, dips into the infundibular recess before slanting superiorly and posteriorly over the tuber cinereum, the two mammillary bodies and the posterior perforated substance, located anterior to the cerebral peduncles. Posterior to the level of the peduncles is the aqueduct, which is surrounded by the tegmentum of the midbrain. The roof of the third ventricle starts anteriorly at the foramen of Monro and ends posteriorly in the suprapineal recess. The roof is separated from the lateral wall by the choroidal fissure, which runs in the cleft between the upper part of the thalamus and the fornix. Over the anterior part of the roof, the fornices run in parallel and are often attached into the body of the fornix, whereas over the posterior roof, the fornices separate into the forniceal crura, and the roof is draped in interforniceal-connecting white matter called the hippocampal commissure. However, the fornices and hippocampal commissure in the roof of the third ventricle are covered by a loose trabecular pial tissue that forms a double layer called the tela choroidea. Between these two layers of tela choroidea is a space, the velum interpositum, through which the internal cerebral veins (ICVs) and the medial posterior choroidal arteries course. The ICVs start at the posterior edge of the foramen of Monro and run posteriorly to exit the velum interpositum just above the pineal body. The third ventricular choroid plexus is attached to the roof by the tela choroidea, which communicates through the choroidal fissure with the lateral ventricular tela choroidea. The posterior wall of the third ventricle begins at Sylvius’ aqueduct anteriorly and inferiorly. Proceeding in a posterior and superior direction, the posterior wall of the third ventricle contains the posterior commissure, the pineal body, the habenular commissure, and the suprapineal recess above.13–15
Surgical Options
The Anterior Third Ventricle
Transforaminal and Interforniceal Approaches
Access to the anterior part of the third ventricle can be accomplished through an enlarged foramen of Monro. This transforaminal approach is particularly useful for tumors that dilate the foramen (Fig. 27-5A). Colloid cysts can be removed in this fashion with minimal manipulation of the foramen and the encircling fornix. The use of angled view endoscopes has allowed visualization of third ventricular structures. However, access to the third ventricle is limited by the size of the foramen of Monro, and when this limitation prohibits further removal of the tumor, alternative approaches to the third ventricle can be developed from this vantage point. The first method is enlargement of the foramen of Monro by unilateral transection of the fornix. This allows anterior access into the third ventricle. This approach, however, has been associated with potential significant morbidity in memory impairment. Thus it is not a recommended route.16–20
A second approach is the interforniceal route to the third ventricle, which gains access by splitting the fornices in the sagittal plane, along the direction of their fibers. In this approach, the septum pellucidum is opened and used as a guide to the midline. The great advantage of this approach is that posterior dissection can be carried out to expose the entire third ventricle. The ICVs have no reported branches between them and must be separated. The disadvantage of this method is the potential bilateral damage to the fornices. Consequently, the interforniceal approach is best used for patients who have a cavum septum pellucidum.21–23
Lateral Subfrontal Approach
This approach is useful for midline suprasellar and anterior third ventricular lesions (Fig. 27-5B). The patient is positioned supine and a bicoronal incision is used. The unilateral craniotomy starts laterally at the pterion and runs just above the orbital ridge. The brain is relaxed with mannitol and CSF drainage, while the frontal lobe is retracted gently. To reduce retraction and increase the upward angle of vision, the orbital ridges can be removed. Care should be taken to coagulate small draining veins because they may rupture during retraction. Past the planum sphenoidale, the optic nerves, the chiasm, and both internal carotid arteries are visualized. The tumor is generally evident at this stage. The A1 branches must be identified bilaterally to the level of the anterior communicating artery. If the tumor has a cystic component, such as in a craniopharyngioma, it is useful to decompress the cyst at this point. Care should be taken not to allow cystic contents to escape into the ventricle or subarachnoid space because they can cause aseptic meningitis. If the tumor does not contain a cystic component, then internal decompression is highly beneficial in reducing tension on the surrounding structures during dissection of the capsule. The resection can be performed through the prechiasmatic space, the opticocarotid triangle, or the retrocarotid space. The latter two routes are the reasons for a wide craniotomy extending to the pterion. In patients with a prefixed chiasm, resection is particularly difficult to accomplish, and opening of the lamina terminalis may be required. The lamina is opened above the chiasm up to the anterior commissure. In suprasellar tumors that extend upward into the anterior third ventricle, the anterior floor of the third ventricle is pushed upward. Thus, on opening the lamina, the tumor is covered by a thin third ventricular floor, which every effort should be made to save. Occasionally, the tumor can be accessed and delivered through either the prechiasmatic space or the opticocarotid triangle.24–27
Pterional Approach
This approach is a common one to suprasellar tumors that extend into the anterior third ventricle (Fig. 27-5B). The weakness of this approach is the poor visualization of the ipsilateral third ventricular extension and contralateral opticocarotid and retrocarotid space. The positioning is supine, with the head tilted approximately 45 degrees to the left and in approximately 20 degrees of extension. The incision follows a hairline curve from the zygoma anterior to the ear to the frontal region. It is important to stay flush with the pterional base; alternatively, an orbital osteotomy maximizes the upward angle. The Sylvian fissure may need to be opened. Once CSF is released and brain relaxation is achieved, gentle retraction is applied to the frontal lobe. The tumor is accessed through the retrocarotid space, the opticocarotid triangle, and the prechiasmatic space. In addition, the lamina terminalis can be accessed and opened.28
Endoscopic Approaches
The endoscope offers a surgical approach that is useful for intraventricular tumor surgery (Fig. 27-5A). The improvement of endoscopic techniques and instruments has established endoscopic approaches as an alternative to microsurgical techniques in selected cases.29 The fluid-filled space of the ventricular system can be a very suitable region for endoscopy, including tumor biopsy under direct visualization and restoration of CSF flow by opening obstructed pathways.30 The endoscope is also useful for inspecting regions not easily accessed with the operative microscope. The most commonly treated lesions using an endoscopic approach are colloid cysts of the third ventricle. Through a burr hole, a working sleeve is stereotactically inserted. A video unit is connected to a rigid endoscope with variable-degree angled optical systems. Endoscopic instruments and coagulation can be inserted through working ports and controlled in the visual field of the scope. This provides enough space and flexibility to separate tumor tissue or membranes and remove small intraventricular tumors, stereotactically aspirate the cyst contents, and relieve associated hydrocephalus by CSF diversion. The limitations of endoscopic techniques prohibit removal of tumors with high vascularity or fibrous consistency. Furthermore, the lesion can be engulfed by neural or vascular structures, such as the choroid plexus, or covered by large veins that cannot be mobilized during endoscopic surgery. In experienced hands, the endoscopic approach using optimal instrumentation may permit complete or near complete removal of the colloid cyst wall and should result in a low recurrence rate, mostly without the consequences described. Bleeding is a potential risk in endoscopic surgery. Since this problem may not be controlled without wide exposure, the surgeon should be prepared to perform a craniotomy when necessary.31,32
Endoscopy can be used in selected cases in ventricular surgery, but even in appropriate cases, the risks of incomplete tumor removal, bleeding, and a small operative corridor must be considered against the three-dimensional visualization of the operative microscope, wider operative field, greater chance of total resection, and better hemostasis. Additional expertise and improved instrumentation will continue to increase the usefulness of this technique in the future.
The Posterior Third Ventricle
Transcallosal Transvelum Interpositum Approaches
Following access to the lateral ventricle, the choroidal dissection is performed medial to the lateral ventricular choroid, through the tenia fornicis, separating the choroid from the body of the fornix. The choroid plexus is either removed or reflected laterally. This minimizes contact with the posterior choroidal artery and the superficial thalamic veins. Once the choroid is reflected laterally, the velum interpositum, the ICVs, and the medial posterior choroidal arteries are visualized (Fig. 27-6). At this point, the ICVs are separated and a plane is developed between them. Alternatively, the ICV is dissected from the ipsilateral fornix to expose the third ventricle. There are no reported bridging veins between these vessels; nevertheless, because they run closely together, they require manipulation and intermittent compression to gain access to the third ventricle. The last layer in this approach to be split is the third ventricular choroid plexus, which must also be separated in the midline. Finally, one should keep in mind that the anatomy is often distorted; in this case, the preoperative radiographic studies are particularly useful.4,13,33,34
Infratentorial Supracerebellar Approach
This approach is well suited for midline tumors in the pineal region and avoids retraction or manipulation of the cerebral hemispheres (Fig. 27-5C). The approach is not optimally suited if the tumor infiltrates laterally or superiorly above the tentorium. The patient can be placed in the sitting position, the three-quarter prone position, or the prone position. The sitting position is optimal for brain relaxation; the cerebellum falls away, while venous drainage is optimized. If this position is chosen, the patient is susceptible to air embolus; thus a central line, carbon dioxide monitor, PEEP, and compression stockings are advised. The incision is midline, and a wide suboccipital craniotomy is performed, thus exposing the transverse sinus and torculum. The dura is opened with its base superiorly. Retraction may be applied superiorly to the underside of the tentorium in the midline, while gentle inferior retraction can be placed on the vermis. Care should be taken to coagulate and divide cerebellar bridging veins as they drain superiorly into the tentorium. The arachnoid is thick and should be divided before the parapineal vessels and the tumor are visualized. The precentral cerebellar vein, connecting the vermis to Galen’s vein, can be sacrificed to gain access to the pineal region. Resection of the tumor proceeds inferior to Galen’s vein, the ICVs, and Rosenthal’s basal vein. The quadrigeminal plate should be well visualized. After tumor resection, communication of the posterior third ventricle can be confirmed by direct visualization.35,36
Occipital Transtentorial Approach
This approach is used for pineal and posterior third ventricular lesions with either supratentorial or infratentorial components (Fig. 27-5C). The patient can be placed in either the sitting or semiprone position. However, whereas the sitting position helps in the infratentorial approach by allowing the cerebellum to fall away, the three-quarter prone position helps the occipital lobe to fall away, thus reducing need for retraction. The trapdoor incision is made with its base inferiorly and across the midline. The occipital craniotomy can expose the midline and the transverse sinus. The dura is opened with its base on the sinuses. Minimal retraction on the occipital lobe is necessary with adequate CSF drainage and brain relaxation. There are rarely significant draining veins from the medial occipital lobe into the tentorium. The transection of the tentorium proceeds in a posterior to anterior direction by first making an incision proximally and then proceeding in a line approximately 1 cm off midline toward the tentorial edge. The tentorium is well vascularized, and hemostasis may require a bipolar cautery and clips. The thick arachnoid should be separated at the edge of the tentorium to avoid undue bleeding of small vessels. The deep veins around the pineal are surrounded in a thick arachnoid; this needs to be opened widely to expose the local anatomic landmarks. If necessary, the precentral cerebellar vein should be sacrificed to increase the working space. Tumor resection can proceed between the ICVs and Rosenthal’s basal vein. The falx can be cut approximately 1 cm anterior to the insertion of the vein of Galen into the sinus, after coagulating or clipping the inferior sagittal sinus. Retraction on the falx allows further exposure. The splenium does not have to be cut but can be gently retracted upward to allow extra exposure.37,38
Complications
Mortality
Surgery of the lateral and third ventricle has carried an extremely high mortality rate in the past (as high as 75%). With advances in microsurgery and improved understanding of anatomic pathways, the 30-day postoperative mortality at present is 5% to 12%. Among the immediate causes of the mortality were cerebral hemorrhage, infarction, brain swelling, and pulmonary embolus.11,39–42
Cognitive Deficits
Intraventricular surgery can cause postoperative impairment of cognitive functions. Some of these symptoms are related to the corpus callosum disconnection and include disturbed consciousness, a transient state of mutism, memory impairment and apathy, contralateral leg weakness, incontinence, and disinhibition. The severity of these symptoms correlate with the length of the callosotomy and can be seen in as many as 75% of patients but tend to resolve within 3 weeks. Permanent changes in cognition are reported in 5% to 10%. Neuropsychological testing is useful in these cases. Persistent focal neurologic deficits such as impairment of motor function or a visual field cut are reported in 8% to 30% of cases.9,11,41,42
Seizures
Postoperative seizures are more common in patients who undergo transcortical procedures, and previous reports have indicated that approximately one-third of patients have seizures in the postoperative period. In patients undergoing transcallosal procedures, the incidence is unknown, although it is presumed to be lower. Although most approaches avoid transversing cortical tissue, retraction injuries to the brain may also cause postoperative seizures.11,15,40
Hydrocephalus
It is common for lateral and third ventricular tumors to cause hydrocephalus. It should be remembered that following resection of the tumor, hydrocephalus persists in as many as 33% of patients. These patients require shunting. Furthermore, these shunts often (>20% of cases) malfunction, likely because of the higher protein content in the CSF of these postoperative patients, and there is also an increased risk of shunt infection. Overshunting can add to the problem of postoperative subdural hematoma collections, which are found in approximately 40% of patients. Only one fourth of these require surgery for drainage of the subdural collection; nevertheless, this implies that approximately 10% of patients who under-go ventricular surgery will require drainage of a subdural collection later on. The more pronounced the preoperative ventriculomegaly, the higher the risk of this complication.43–45
Asgari S., Engelhorn T., Brondics A., et al. Transcortical or transcallosal approach to ventricle-associated lesions: a clinical study on the prognostic role of surgical approach. Neurosurg Rev. 2003;26:192-197.
Bellotti C., Pappada G., Sani R., et al. The transcallosal approach for lesions affecting the lateral and third ventricles: surgical considerations and results in a series of 42 cases. Acta Neurochir (Wien). 1991;111:103-113.
Bruce D.A. Complications of third ventricular surgery. Pediatr Neurosurg. 1991;17:325-330.
Bruce J.N., Stein B.M. Surgical management of pineal region tumors. Acta Neurochir (Wien). 1995;134:130-135.
Clark W.K., Batjer H.H. The occipital transtentorial approach. In: Apuzzo M.L.J., editor. Surgery of the Third Ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998:721-741.
Dandy W.E. Operative experience in cases of pineal tumor. Arch Surg. 1936;33:19-46.
Ehni G., Ehni B.L. Considerations in transforaminal entry. In: Apuzzo M.L.J., editor. Surgery of the Third Ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998:391-419.
Fuji K., Lenkey C., Rhoton A.L.Jr. Microsurgical anatomy of the choroidal arteries: lateral and third ventricle. J Neurosurg. 1980;52:165-188.
Hellwig D., Bauer B.L., Schulte M., et al. Neuroendoscopic treatment for colloid cysts of the third ventricle: the experience of a decade. Neurosurgery. 2003;52:525-533.
Nagata S., Rhoton A.L.Jr., Barry M. Microsurgical anatomy of the choroidal fissure. Surg Neurol. 1988;30:3-59.
Ono M., Rhoton A.L.Jr., Peace D., et al. Microsurgical anatomy of the deep venous system of the brain. Neurosurgery. 1984;15:621-657.
Oppel F., Hoff H.J., Pannek H.W. Endoscopy of the ventricular system: indications, operative procedure and technical aspects. In: Hellwig D., Bauer B.L. Minimally Invasive Techniques for Neurosurgery. Berlin: Springer; 1998:97-100.
Patterson R.H. Jr: The subfrontal transsphenoidal and trans-lamina terminalis approaches. In: Apuzzo M.L.J., editor. Surgery of the Third Ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998:471-487.
Piepmeier J., Spencer D., Sass K., et al. Lateral ventricular masses. In: Apuzzo M.L.J., editor. Brain Surgery: Complications Avoidance and Management. New York: Churchill Livingstone; 1993:581-600.
Piepmeier J.M., Sass K.J., et al. Surgical management of lateral ventricular tumors, Paoletti P., Takakura K., Walker M., Neurooncology. Kluwer: Dordrecht, 1991:333-335
Rhoton A.L.Jr., Yamamoto I., Pease D.A. Microsurgery of the third ventricle. Part 2: Operative approaches. Neurosurgery. 1981;8:357-373.
Rhoton A.L.Jr. Microsurgical anatomy of the third ventricular region. In: Apuzzo M.L.J., editor. Surgery of the Third Ventricle. 2nd ed. Baltimore:: Williams & Wilkins; 1998:89-158.
Sass K., Novelly R., Spencer D., et al. Amnestic and attention impairments following corpus callosotomy section for epilepsy. J Epilepsy. 1988;1:61-66.
Stein B.M., Bruce J.N. Surgical management of pineal region tumors. Clin Neurosurg. 1992;39:509-532.
Sugita K., Kobayashi S., Yokoo A. Preservation of large bridging veins during brain retraction. J Neurosurg. 1982;57:856-860.
Timurkaynak E., Rhoton A.L.Jr., Barry M. Microsurgical anatomy and operative approaches to the lateral ventricles. Neurosurgery. 1986;19:685-723.
Ture U., Yasargil M.G., Al-Mefty O. The transcallosal-transforaminal approach to the third ventricle with regard to the venous variations in this region. J Neurosurg (Wien). 1997;87:706-715.
Wen W.T., Rhoton A.L.Jr., de Oliveira E. Transchoroidal approach to the third ventricle: an anatomic study of the choroidal fissure and its clinical application. Neurosurgery. 1998;42:1205-1219.
Yasargil M.G., Abdulrauf S.I. Surgery of intraventricular tumors. Neurosurgery. 2008;62:1029-1040.
Yasargil M.G., Curcic M., Kis M., et al. Total removal of craniopharyngiomas: approaches and long term results in 144 patients. J Neurosurg. 1990;73:3-11.
1. Yasargil MG, Abdulrauf SI. Surgery of intraventricular tumors. Neurosurgery. 2008;62:1029-1040.
2. Timurkaynak E, Rhoton ALJr, Barry M. Microsurgical anatomy and operative approaches to the lateral ventricles. Neurosurgery. 1986;19:685-723.
3. Ono M, Rhoton ALJr, Peace D, et al. Microsurgical anatomy of the deep venous system of the brain. Neurosurgery. 1984;15:621-657.
4. Nagata S, Rhoton ALJr, Barry M. Microsurgical anatomy of the choroidal fissure. Surg Neurol. 1988;30:3-59.
5. Fuji K, Lenkey C, Rhoton ALJr. Microsurgical anatomy of the choroidal arteries: lateral and third ventricle. J Neurosurg. 1980;52:165-188.
6. Sugita K, Kobayashi S, Yokoo A. Preservation of large bridging veins during brain retraction. J Neurosurg. 1982;57:856-860.
7. Shucart WA, Stein BM. Transcallosal approach to the anterior ventricular system. Neurosurgery. 1978;3:339-343.
8. Geffen G, Walsh A, Simspson D, et al. Comparison of the effects of transcortical removal of intraventricular tumors. Brain. 1980;103:773-788.
9. Sass K, Novelly R, Spencer D, et al. Amnestic and attention impairments following corpus callosotomy section for epilepsy. J Epilepsy. 1988;1:61-66.
10. Rhoton ALJr, Yamamoto I, Pease DA. Microsurgery of the third ventricle. Part 2: Operative approaches. Neurosurgery. 1981;8:357-373.
11. Piepmeier J, Spencer D, Sass K, et al. Lateral ventricular masses. In: Apuzzo MLJ, editor. Brain Surgery: Complications Avoidance and Management. New York: Churchill Livingstone; 1993:581-600.
12. Dandy WE. Operative experience in cases of pineal tumor. Arch Surg. 1936;33:19-46.
13. Wen WT, Rhoton ALJr, de Oliveira E. Transchoroidal approach to the third ventricle: an anatomic study of the choroidal fissure and its clinical application. Neurosurgery. 1998;42:1205-1219.
14. Rhoton AL. Jr: Microsurgical anatomy of the third ventricular region. In: Apuzzo MLJ, editor. Surgery of the Third Ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998:89-158.
15. Piepmeier JM, Sass KJ, et al. Surgical management of lateral ventricular tumors, Paoletti P, Takakura K, Walker M, Neurooncology. Kluwer: Dordrecht, 1991:333-335
16. Ehni G, Ehni BL. Considerations in transforaminal entry. In: Apuzzo MLJ, editor. Surgery of the Third Ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998:391-419.
17. Garcia-Bengochea F, Friedman WA. Persistent memory loss following section of the anterior fornix in humans: a historical review. Surg Neurol. 1987;27:361-364.
18. Sweet WH, Talland GA, Ervin FR. Loss of recent memory following section of fornix. Trans Am Neurol Assoc. 1959;84:76-82.
19. Tucker DM, Roeltgen DP, Tully R, et al. Memory dysfunction following unilateral transection of the fornix: a hippocampal disconnection syndrome. Cortex. 1988;23:465-472.
20. Ture U, Yasargil MG, Al-Mefty O. The transcallosal-transforaminal approach to the third ventricle with regard to the venous variations in this region. J Neurosurg (Wien). 1997;87:706-715.
21. Little JR, MacCarty CS. Colloid cysts of the third ventricle. J Neurosurg. 1974;40:230-235.
22. Wang AM, Power TC, Rumbaugh CL. Lateral ventricular meningioma. Comput Radiol. 1985;9:355-358.
23. Nitta M, Symon L. Colloid cyst of the third ventricle: a review of 36 cases. Acta Neurochir (Wien). 1985;76:99-104.
24. Patterson RH. Jr: The subfrontal transsphenoidal and trans-lamina terminalis approaches. In: Apuzzo MLJ, editor. Surgery of the Third Ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998:471-487.
25. Choux M, Lena G, Genitori L. Craniopharyngioma in children. Neurochirurgie. 1991;37:1-174.
26. Tomita T, McLone D. Radical resection of childhood meningiomas. Pediatr Neurosurg. 1993;19:6-14.
27. King TT. Removal of intraventricular craniopharyngiomas through the lamina terminalis. Acta Neurochir (Wien). 1979;45:277-286.
28. Yasargil MG, Curcic M, Kis M, et al. Total removal of craniopharyngiomas: approaches and long term results in 144 patients. J Neurosurg. 1990;73:3-11.
29. Hellwig D, Bauer BL, Schulte M, et al. Neuroendoscopic treatment for colloid cysts of the third ventricle: the experience of a decade. Neurosurgery. 2003;52:525-533.
30. Souweidane MM, Sandberg DI, Bilsky MH, et al. Endoscopic biopsy for tumors of the third ventricle. Pediatr Neurosurg. 2000;33:32-37.
31. Oppel F, Hoff HJ, Pannek HW. Endoscopy of the ventricular system: indications, operative procedure and technical aspects. In: Hellwig D, Bauer BL. Minimally Invasive Techniques for Neurosurgery. Berlin: Springer; 1998:97-100.
32. King WA, Ullman JS, Frazee JG, et al. Endoscopic resection of colloid cysts: surgical considerations using the rigid endoscope. Neurosurgery. 1999;44:1103-1109.
33. Lavyne MH, Patterson RHJr. Sub-choroidal trans-velum interpositum approach to mid third ventricular tumors. Neurosurgery. 1983;12:86-94.
34. Petrucci RJ, Bucheit WA, Woodruff GC, et al. Transcallosal paraforniceal approach for third ventricle tumors: neurophysiological consequences. Neurosurgery. 1987;20:457-464.
35. Stein BM. The infratentorial supracerebellar approach to pineal lesions. J Neurosurg. 1971;35:197-202.
36. Bruce JN, Stein BM. Surgical management of pineal region tumors. Acta Neurochir (Wien). 1995;134:130-135.
37. Reid WS, Clark WK. Comparison of the infratentorial and transtentorial approaches to the pineal region. Neurosurgery. 1978;3:1-8.
38. Clark WK, Batjer HH. The occipital transtentorial approach. In: Apuzzo MLJ, editor. Surgery of the Third Ventricle. 2nd ed. Baltimore: Williams & Wilkins; 1998:721-741.
39. Hassaneen W, Suki D, Salaskar AL, et al. Surgical management of lateral-ventricle metastases: report of 29 cases in a single-institution experience. J Neurosurg. 2010;112:1046-1055.
40. Asgari S, Engelhorn T, Brondics A, et al. Transcortical or transcallosal approach to ventricle-associated lesions: a clinical study on the prognostic role of surgical approach. Neurosurg Rev. 2003;26:192-197.
41. Villani R, Papagno C, Tomei G, et al. Transcallosal approach to tumors of the third ventricle. Surgical results and neuropsychological evaluation. J Neurosurg Sci. 1997;41:41-50.
42. Bellotti C, Pappada G, Sani R, et al. The transcallosal approach for lesions affecting the lateral and third ventricles: surgical considerations and results in a series of 42 cases. Acta Neurochir (Wien). 1991;111:103-113.
43. Bruce DA. Complications of third ventricular surgery. Pediatr Neurosurg. 1991;17:325-330.
44. Stein BM, Bruce JN. Surgical management of pineal region tumors. Clin Neurosurg. 1992;39:509-532.
45. Camacho A, Abernathey CD, Kelly PJ, et al. Colloid cysts: experience with the management of 84 cases since the introduction of computer tomography. Neurosurgery. 1989;24:693-700.