Chapter 33 Surgical Approach to Falcine Meningiomas
Falcine meningiomas arise from the falx cerebri and make up approximately 5% to 9% of all intracranial meningiomas.1–2 Falcine meningiomas differ from parasagittal meningiomas in that parasagittal tumors originate from the dura mater enclosing the superior sagittal sinus and are up to 5 to 7 times more common that falcine lesions.3–4 Cushing further distinguished falcine meningiomas from parasagittal tumors by how, from the surgeon’s perspective, falcine tumors are often concealed by overlying cerebral cortex;5 however, large falcine meningiomas can grow superiorly to secondarily invade the superior sagittal sinus.
Symptoms and Presentation
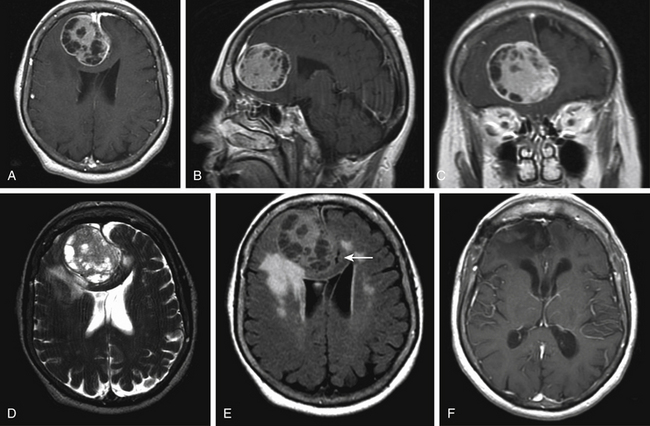
Falcine meningiomas located at the level of the anterior third of the superior sagittal sinus often present somewhat insidiously and result in a frontal lobe syndrome (Fig. 33-1A to F). Slowly progressive symptoms include short attention span, poor short-term memory, personality changes, apathy, and emotional instability; often this complex is confused with age-related dementia, delaying diagnosis. Consequently, anterior third tumors are frequently larger in size at the time of presentation than tumors in other regions. In extreme cases, patients can also present with signs of increased intracranial pressure, including headache, papilledema, or optic atrophy. Of patients with anterior third falcine meningiomas, 25% have seizures, but they are less frequent and less localizing than when tumors abut motor cortex.6
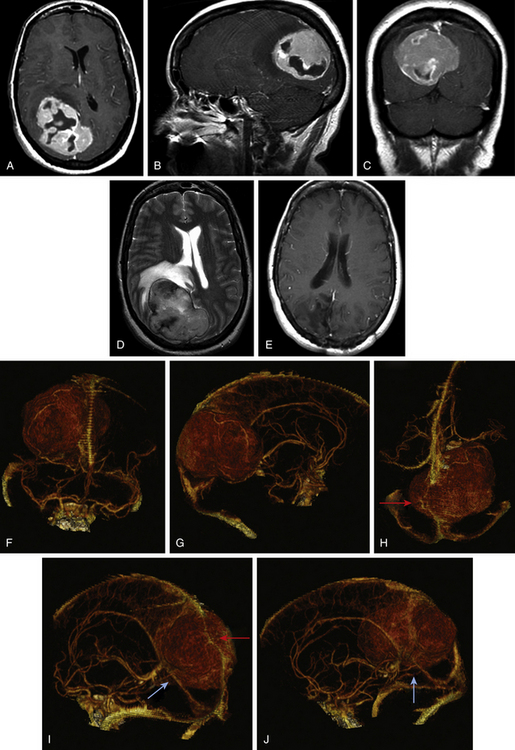
Tumors along the middle third of the superior sagittal sinus are usually located adjacent to the motor and sensory regions and produce more focal and localizable symptoms that cause patients to seek earlier medical intervention. Earlier presentation of symptoms allows for earlier diagnosis, and, thus, tumors in this region rarely attain the same bulk as tumors in the anterior third. While symptoms of increased intracranial pressure are rare, tumors in this region are more likely to produce spastic weakness and focal seizures that involve the contralateral foot and leg. Both motor cortices may be affected if the tumor extends bilaterally from the midline. Patients with meningiomas involving the posterior third of the sagittal sinus usually present with persistent headache and often are found on exam to have a hemianopsia (Fig. 33-2A to E). The degree of visual field involvement depends on the size and location of the tumor. Smaller tumors located above the calcarine fissure can cause anopia to the inferior quadrant. Smaller tumors along the tentorium cerebelli may involve the visual cortex below the calcarine fissure and only produce anopia in the upper quadrants. Larger tumors, on the other hand, can case homonymous hemianopsia with macular sparing. Some patients may present with reports of visual hallucinations. Tumors of the middle and posterior third can occasionally present with bilateral symptoms as the tumor extends across the falx.
Radiographic Findings
Magnetic resonance (MR) imaging is the gold standard for preoperative assessment of falcine meningiomas. Falcine meningiomas also frequently extend bilaterally, acquiring a dumbbell or bi-lobed shape with invagination into the medial aspects of both left and right hemispheres. Attempts to use MR imaging to differentiate histologic types of meningiomas have thus far been limited. Although efforts to differentiate benign from atypical meningiomas using MR spectroscopy (MRS) have been attempted, noninvasive measures have been unreliable.7 The majority of tumors appear iso- or slightly hypo-intense relative to the cortex on noncontrast T1-weighted MR images. The T2-weighted characteristics can be variable and are believed to correlate best with histologic subtype, vascularity, and consistency.8 Tumors that are hypo- to iso-intense on T2-weight imaging are found to be more firm and are histologically more fibroblastic or transitional (Fig. 33-1D and Fig. 33-2D). More malignant meningiomas, on the other hand, are more likely to exhibit greater brain edema that manifests as increased hyperintensity on T2-weight imaging and can be suggestive of pial invasion.9–10
For preoperative planning, multi-planar T1-weighted gadolinium enhanced MR images can provide coronal, sagittal and axial views that help in defining the tumor’s anatomic location, size, and cortical involvement (Fig. 33-1A to C and Fig. 33-2A to C). Brain edema adjacent to the tumor can be a sign of pial invasion and can be assessed preoperatively using T2-weighted images and fluid-attenuated inversion recovery (FLAIR) sequences (Fig. 33-1E). Three-dimensional (3D) T1-weighted gadolinium enhanced multiecho, magnetization-prepared, rapid gradient echo images can also be used with neuro-navigational tools to provide surgeons with intraoperative guidance.
Cerebral angiography can provide essential information delineating the tumor’s arterial feeding pattern. Additionally, arterial phase of the angiography can provide crucial information on the course, displacement and possibly the encasement of the anterior cerebral arteries (ACA), pericallosal arteries, and callosomarginal arteries. The venous phase of the angiogram is also vital toward determining the patency of the superior sagittal sinus, understanding the anatomy of the draining cortical veins in relation to the tumor and the degree of collateral circulation.11,12 If the sinus is occluded, the anatomy of the draining collateral vessels needs to be clearly identified during preoperative planning and meticulously preserved during surgery. Inadvertent occlusion of collateral vessels may result in severe postoperative complications such as venous infarct and cerebral edema.
The gold standard for cerebral angiography remains digital subtraction angiography (DSA). DSA provides a dynamic view of both the arterial and venous phases, better resolution of small vessel feeders, better accuracy in identifying sinus obstruction, and areas with reversal of normal venous flow. However, DSA is invasive and associated with a small, but not insignificant, risk for strokes, vessel injury, postdiagnostic hematoma, and possible renal injury from contrast use. Noninvasive means to evaluate the cerebral vasculature include MR angiography and CT angiography. MR angiography/venography capitalizes on its intrinsic sensitivity to flow to detect the patency of vessels. Vessels with high flow give rise to high signal intensity while the absence of flow is characterized by reduced signal intensity.13,14 The technical limitations to MR-angiography include its susceptibility to flow-related artifacts such that patent but obstructed regions of the sinus may appear completely occluded due to decrease in flow and the ability for intracellular deoxyhemoglobin/methemoglobin found in clot can create false signals to suggest a patent vessel. Other practical limitations include longer image acquisition times, and sensitivity to patient movement. CT-angiography/venography (CTA/CTV) is acquired using thin 0.625-mm slices on a helical 64-channel multidetector scanner during the peak arterial and venous enhancements. The acquired images can be viewed using maximum intensity projection (MIP) images, multi-planar reconstruction (MPR) images, and Vitrea 3D reconstruction images (Vital Images, Minnetonka, MN) to provide the surgeon a better sense of the tumor and its relationship to the vasculature (Fig. 33-2F to J). The faster image acquisition times as well as its ability to maintain high spatial resolution has made CTA/CTV the preferred method for routine preoperative vessel assessment at our institution. CTA/CTV has better small-vessel and sinus resolution and fewer motion and flow artifacts seen in MR venography.15–16 In a study by Wetzel and colleagues, CTV was compared to digital subtraction angiography and CTV was found to have an overall sensitivity of 95% and 91% specificity while DSA had 90% sensitivity and 100% specificity.17 Additionally, the shorter image acquisition times allow for less motion artifacts and makes CTA/CTV better suited for uncooperative or sick patients.
Operative Technique
Positioning
For tumors located in anterior third of the sagittal sinus, the patient is placed in a supine position with the head slightly elevated. Tumors around the middle third of the sagittal sinus can be approached with the patient supine with the head elevated and flexed, or, alternatively, a semiprone or lateral approach can be used, especially if the tumor is located more posteriorly. A semilateral, semi-sitting position with the head well elevated so that the scalp over the area of the tumor is at the highest point can also be considered for tumors in this area. For tumors involving the posterior third, the patient is placed in the lateral position and the head is elevated and turned to the opposite side so that the center of the tumor is uppermost.18–19 The head is secured with the Mayfield 3-point fixation head rest and the patient’s body is secured to table with tape. Foam padding and gel rolls are used to pad all pressure points.
Dural Opening
A U-shaped dura incision is made starting laterally with the hinge parallel to the falx. Care needs to be taken when elevating the dural flap medially to avoid tearing potentially important bridging veins. Adhesions and bridging veins need to be dissected and freed. If a large cortical vein enters a dural sinus prior to emptying into the superior sagittal sinus, the cortical vein, and the dural sinus need to be incised from the dural flap and be preserved as an attachment to the midline sinus. For bilateral tumors, the dura on the contralateral side is also incised and the free ends of the dura can be sutured together to help with retraction out of the surgical field.
Tumor Resection
For falcine meningiomas that are concealed beneath cortex, it is important to first establish the anterior and posterior limits of the tumor. From these limits, a corridor can be created by gently retracting the medial surface of the hemisphere. The cortex should not be retracted more than 2 cm away from the falx and the sinus. Overlying bridging veins that are within this corridor, again, need to be preserved and can be made slack by being dissected away from the superior sagittal sinus. If necessary, the bridging veins can also be freed from the cortex for a few millimeters to allow for the required retraction of the hemisphere without sacrificing the vessel. In rare cases where the tumor is exceedingly large or where the corridor is compromised by unyielding veins, the surgeon can create additional exposure by resecting some noneloquent cortex and white matter that is well anterior to the primary and supplementary motor areas. Resection of noneloquent cortex is preferable to taking cortical veins. Additionally, a contralateral interhemispheric approach to large deep-seated meningiomas has been reported.20
Once the corridor has been established, the blood supply to the tumor from the falcine arteries is sectioned by cauterizing and sectioning the falx in the inferior-to-superior direction approximately 1 cm anterior and 1 cm posterior to the margins of the tumor. The exposed tumor capsule is then entered using bipolar cautery. Intracapsular enucleation is used to debulk the tumor and this can be accomplished using a combination of bipolar cautery, aspiration, and/or sonic aspiration (e.g., CUSA). Once sufficiently debulked, the rest of the tumor can be delivered by invaginating the capsule into the hollowed tumor cavity. The capsule is peeled away from the cortex using gentle retraction and bipolar cautery. Only small vessels parasitized from the pia arachnoid that are entering the tumor from the brain can be divided. In areas where the capsule has been dissected from the cortex, the brain can be protected with either Gelfoam or Cottonoid pads. It should be noted that a cleavable pial–tumor plane is not always present. Sindou and Alaywan evaluated 150 patients with preoperative angiography.21 Only 34.8% of meningiomas with evidence of pial parasitization on angiogram were found to have a cleavable pial–tumor interface at surgery. Conversely, in meningiomas with primarily a dural supply, 83.6% had a cleavable plane. In addition, in cases where there is pial invasion in areas involving eloquent brain, total resection may result in significant and unacceptable postoperative deficits. In these conditions, it is advisable to leave a thin rim of tumor attached to the cortex rather than risk debilitating neurologic compromise.22 As the tumor is resected, the surgeon should be mindful of the branches of the ACA, including the pericallosal and callosomarginal arteries. These relationships should be identified preoperatively. Smaller arteries directly feeding the tumor can be coagulated and divided; however, traversing branches that are adherent or encased by tumor should be dissected and freed. Once the tumor capsule has been separated from the cortex, the tumor attachment to the falx and sagittal sinus is excised and coagulated. Tumors that extend bilaterally will also require complete resection of the involved falx.
Management of Sinus Invasion
Falcine meningiomas can grow and extend to involve the superior sagittal sinus. Although occurring less frequently than with parasagittal meningiomas, the surgical considerations for managing tumor infiltration into the sinus are essentially the same. When tumor extends into the sagittal sinus, surgeons are faced with the dilemma of leaving a fragment of invasive tumor and facing a higher rate of recurrence versus attempting to achieve a Simpson I resection but putting the venous circulation at greater risk. As is the case for all meningiomas, the likelihood of recurrence for falcine lesions depends on the Simpson grade at the time of initial resection.3 Most pertinently, the falx and the encroached upon superior sagittal sinus are the dural attachments associated with these tumors. In a recent case review of 68 falcine meningiomas, Chung et al. found that those with Simpson grade I or II resections had a recurrence rate of 3.6% (2 cases out of 56) while those with a grade III or greater had a rate of 44% (4 cases out of 9).3 The imperative for aggressive surgery needs to be tempered by the risks of unexpected and sudden occlusion of the middle or posterior thirds of the sagittal sinus. Such venous injury can lead to significant neurologic morbidity and mortality. Decisions regarding sinus management, therefore, should be tailored to individual patients based on their age, symptoms, tumor location, degree of sinus involvement, and the robustness of the cortical venous collaterals.
Three main surgical strategies can be employed in the management of sinus invasion. The first involves simple resection of the outer dural layer with the tumor and coagulation of the inner layer at the sites of tumor attachment. Simpson’s series from 1957 demonstrated that complete tumor resection and coagulation of attached dura had a recurrence rate of 16% as compared to 29% recurrence rate when gross total resection was performed without dural coagulation.23 Complete tumor resection with excision of attached dural attachments had the lowest rate of recurrence of 6%. The second technique for disease involving the superior sagittal sinus involves resecting the invaded sinus wall(s) and repairing the sinus by one of three techniques:1 resection of the intraluminal tumor and suturing of the sinus edges primarily;2 resection of the infiltrated wall(s) and repair with an autologous patch (e.g., venous or fascia temporalis); or3 resection of the occluded portion with interposition of a bypass graft composed of either autologous vein (e.g., external jugular, internal saphenous vein) or a synthetic tube (e.g., Gore-Tex). The third option for management would be simple coagulation of residual tumor or resection of the involved sinus without venous reconstruction.24
Sindou and Alvernia propose a six-stage classification scheme for progressively tumor invasion into the sinuses.25 Type I lesions involve just the outer surface of the sinus wall. Type II lesions have the tumor extending into the lateral recess of the superior sagittal sinus. Type III tumors infiltrate into the lateral sinus wall and when tumor invaded into the roof of the sinus, they are characterized as type IV lesions. Types V and VI tumors completely occlude the sinus, with and without wall invasion, respectively. For type I lesions, the tumor can be peeled off the wall of the sinus using bipolar cautery. Care is then taken to coagulate the areas of tumor attachment to prevent recurrence. Type II tumors that involve the lateral recess can be managed with opening the sinus edge, removing the residual plaque of tumor, and then progressively closing the edge of the sinus wall primarily with a continuous 5-0 or 6-0 prolene suture. Types III and IV tumors involve to the sinus walls to a greater extent and require more extensive sinus reconstruction. Details for this reconstruction are detailed elsewhere.24,26–28 Type V and VI tumors can be approached on the basis of the location of sinus involvement: anterior, middle, and posterior. A partially or completely occluded superior sagittal sinus in the anterior third can usually be resected without risk of neurologic consequences. Traditionally, middle and posterior segments of the sinus that are found to be occluded on digital subtraction angiography can be resected without the need for venous reconstruction or bypass. Some author advocate testing with temporary occlusion prior to excision.22 Sekhar temporarily occludes the sinus and measures proximal venous pressure for over 10 minutes.22 If there is no change in sinus pressure and if there is evidence of brain swelling, the sinus can be resected. However, some authors warn that cerebral edema, venous infarction and subcutaneous cerebrospinal fluid collection can still occur and suggest the need for a venous reconstruction or sinus bypass. The 1955 study by Hoessly and Olivecrona evaluated 196 parasagittal meningiomas treated without venous reconstruction and reported perioperative morbidity of 12.3%, of which more than half was associated with venous drainage.29 A more recent examination study of 108 cases of falcine or parasagittal meningiomas invading the superior sagittal sinus described 9 cases of severe cerebral edema, of which 3 involved en bloc resection without venous reconstruction.30 However despite these risks, surgeons are still advised to consider each case individually and weigh the options which include adjuvant radiosurgery and radiotherapy for residual tumor.
Summary
The surgical approach to falcine meningiomas is dictated by the tumor’s location relative to the superior sagittal sinus. Preoperative and intraoperative high-resolution gadolinium-enhanced MR images can aide in understanding tumor size and location and can be helpful in planning patient positioning, skin incision, and the size of the bone flap craniotomy. Standard digital subtraction and noninvasive CT and MR cerebral angiography can provide critical insight on the course of the arterial feeders. Additionally, for cases where tumor infiltrates the superior sagittal sinus, cerebral angiography can reveal the degree of sinus occlusion and the anatomy of the collateral draining veins. Intraoperatively, the surgeon needs be judicious in the degree of cortex retraction and be meticulous in preserving surrounding cortical veins in order to avoid potentially devastating neurologic injuries. Occasionally falcine meningiomas can secondarily invade the superior sagittal sinus and in such cases, the surgeon needs to consider the patient’s age, symptoms, degree of tumor infiltration, and the role of adjuvant radiosurgery and radiotherapy. The options for sinus repair and/or venous bypass should be discussed at length before pursuing tumor within the sinus. Intraoperative verification of completely occluded sinuses using manometry and temporary clipping is recommended prior to sinus resection. Finally, the surgeon needs to be aware of the risks for delayed sinus thrombosis and to keep patients well hydrated postoperatively.
Al-Mefty O., Origitano T.C., Harkey H.L. Controversies in Neurosurgery. New York: Thieme, 1996.
Barajas R.F.Jr., Sughrue M.E., McDermott M.W. Large falcine meningioma fed by callosomarginal branch successfully removed following contralateral interhemispheric approach. J Neurooncol. 2009;97:127-131.
Casey S.O., Alberico R.A., Patel M., et al. Cerebral CT venography. Radiology. 1996;198:163-170.
Chung S.B., Kim C.Y., Park C.K., et al. Falx meningiomas: surgical results and lessons learned from 68 cases. J Korean Neurosurg Soc. 2007;42:276-280.
Demir M.K., Iplikcioglu A.C., Dincer A., et al. Single voxel proton MR spectroscopy findings of typical and atypical intracranial meningiomas. Eur J Radiol. 2006;60(1):48-55.
DiMeco F., Li K.W., Casali C., et al. Meningiomas invading the superior sagittal sinus: surgical experience of 108 cases. Neurosurgery. 2004;55:1263-1274.
Hoessly G.F., Olivecrona H. Report on 280 cases of verified parasagittal meningioma. J Neurosurg. 1955;12:614-626.
Kim C.Y., Jung H.W. Falcine meningiomas. In: Lee J.H., editor. Meningiomas: Diagnosis, Treatment, and Outcome. London: Springer-Verlag, 2008.
Lawton M.T., Golfinos J.G., Spetzler R.F. The contralateral transcallosal approach: experience with 32 patients. Neurosurgery. 1996;39(4):729-735.
Liauw L., van Buchem M.A., Split A., et al. MR angiography of the intracranial venous system. Radiology. 2000;214:678-682.
Longstreth W.T., Dennis L.K., McGuire V.M. Epidemology of intracranial meningiomas. Cancer. 1993;72:639-648.
Maiuri F., Iaconetta G., de Divitiis O., et al. Intracranial meningiomas: correlations between MR imaging and histology. Eur J Radiol. 1999;31(1):69-75.
Ojemann R.G. Management of cranial and spinal meningiomas. In: Selman W., editor. Clinical Neurosurgery. Baltimore: Williams and Wilkins, 1992.
Ojemann R.G., Ogilvy C.S. Convexity, parasagittal and parafalcine meningiomas. In: Apuzzo M.L.J., editor. Brain Surgery: Complication Avoidance and Managment. New York: Churchill Livingstone, 1993.
Ozsvath R.R., Casey S.O., Lustrin E.S., et al. Cerebral venography: comparison of CT and MR projection venography. AJR Am J Roentgenol. 1997;169:1699-1707.
Sekhar L.N., de Oliveira E., Riedel C.J. Parasagittal, falx meningiomas. Cranial Microsurgery: Approaches and Techniques. New York: Thieme; 1997.
Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22-39.
Sindou M. Meningiomas invading the sagittal or transverse sinuses, resection with venous reconstruction. J Clin Neurosci. 2001;8(suppl 1):8-11.
Sindou M., Alaywan M. Most intracranial meningiomas are not cleavable tumors: anatomic-surgical evidence and angiographic predictability. Neurosurgery. 1998;42:476-480.
Sindou M., Auque J. The intracranial venous system as a neurosurgeon’s perspective. Adv Tech Stan Neurosurg. 2000;26:131-216.
Sindou M., Hallacq P. Venous reconstruction in surgery of meningiomas invading the sagittal and transverse sinuses. Skull Base Surg. 1998;8:57-64.
Sindou M.P., Alvernia J.E. Results of attempted radical tumor removal and venous repair in 100 consecutive meningiomas involving the major dural sinuses. J Neurosurg. 2006;105:514-525.
Sindou M.P., Bokor J., Brunon J. Bilaeral thrombosis of the transverse sinuses: microsurgical revascularization with venous bypass. Surg Neurol. 1980;13:215-220.
Tamiya T., Ono Y., Matsumoto K., Ohmoto T. Peritumoral brain edema in intracranial meningiomas: effects of radiological and histological factors. Neurosurgery. 2001;49(5):1046-1051.
Wetzel S.G., Kirsh E., Stock K.W., et al. Cerebral veins: comparative study of CT venography with intraarterial digital subtraction angiography. Am J Neuroradiol. 1999;20:249-255.
1. Lawton M.T., Golfinos J.G., Spetzler R.F. The contralateral transcallosal approach: experience with 32 patients. Neurosurgery. 1996;39(4):729-735.
2. Longstreth W.T., Dennis L.K., McGuire V.M. Epidemology of intracranial meningiomas. Cancer. 1993;72:639-648.
3. Chung S.B., Kim C.Y., Park C.K. Falx meningiomas: surgical results and lessons learned from 68 cases. J Korean Neurosurg Soc. 2007;42:276-280.
4. Kim C.Y., Jung H.W. Falcine meningiomas. In: Lee J.H., editor. Meningiomas: Diagnosis, Treatment, and Outcome. London: Springer-Verlag, 2008.
5. Cushing H., Eisenhardt L. Meningiomas: their Classification, Regional Behavior, Life History, and Surgical End Results. Springfield, IL: Charles C. Thomas; 1938.
6. Lund M. Epilepsy in association with intracranial tumours. Acta Pscyhiatr Scand. 81(suppl), 1952.
7. Demir M.K., Iplikcioglu A.C., Dincer A. Single voxel proton MR spectroscopy findings of typical and atypical intracranial meningiomas. Eur J Radiol. 2006;60(1):48-55.
8. Maiuri F., Iaconetta G., de Divitiis O. Intracranial meningiomas: correlations between MR imaging and histology. Eur J Radiol. 1999;31(1):69-75.
9. Tamiya T., Ono Y., Matsumoto K., Ohmoto T. Peritumoral brain edema in intracranial meningiomas: effects of radiological and histological factors. Neurosurgery. 2001;49(5):1046-1051.
10. Nakano T., Asano K., Miura H. Meningiomas with brain edema: radiological characteristics on MRI and review of the literature. Clin Imaging. 2002;26(4):243-249.
11. Al-Mefty O., Origitano T.C., Harkey H.L. Controversies in Neurosurgery. New York: Thieme, 1996.
12. Rossitti S. Preoperative embolization of lower-falx meingiomas with ethylene vinyl alcohol copolymer: technical and anatomical aspects. Acta Radiol. 2007;48(3):321-326.
13. Khandelwal N., Agarwal A., Kochhar R., et al. Comparison of CT venography with MR venography in cerebral sinovenous thrombosis. AJR Am J Roentgenol. 2006;187:1637-1643.
14. Liauw L., van Buchem M.A., Split A., et al. MR angiography of the intracranial venous system. Radiology. 2000;214:678-682.
15. Casey S.O., Alberico R.A., Patel M., et al. Cerebral CT venography. Radiology. 1996;198:163-170.
16. Ozsvath R.R., Casey S.O., Lustrin E.S. Cerebral venography: comparison of CT and MR projection venography. AJR Am J Roentgenol. 1997;169:1699-1707.
17. Wetzel S.G., Kirsh E., Stock K.W. Cerebral veins; comparative study of CT venography with intraarterial digital subtraction angiography. Am J Neuroradiol. 1999;20:249-255.
18. Ojemann R.G., Ogilvy C.S. Convexity, parasagittal and parafalcine meningiomas. In: Apuzzo M.L.J., editor. Brain Surgery: Complication Avoidance and Managment. New York: Churchill Livingstone; 1993:187-202.
19. Ojemann R.G. Management of cranial and spinal meningiomas. In: Selman W., editor. Clinical Neurosurgery. Baltimore: Williams and Wilkins; 1991:321-383. 40
20. Barajas R.F.Jr., Sughrue M.E., McDermott M.W. Large falcine meningioma fed by callosomarginal branch successfully removed following contralateral interhemispheric approach. J Neurooncol. 2009;97:127-131.
21. Sindou M., Alaywan M. Most intracranial meningiomas are not cleavable tumors: anatomic-surgical evidence and angiographic predictability. Neurosurgery. 1998;42:476-480.
22. Sekhar L.N., de Oliveira E., Riedel C.J. Parasagittal, falx meningiomas. Cranial Microsurgery: Approaches and Techniques. New York: Thieme; 1997.
23. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22-39.
24. Sindou M., Auque J. The intracranial venous system as a neurosurgeon’s perspective. Adv Tech Stan Neurosurg. 2000;26:131-216.
25. Sindou M.P., Alvernia J.E. Results of attempted radical tumor removal and venous repair in 100 consecutive meningiomas involving the major dural sinuses. J Neurosurg. 2006;105:514-525.
26. Sindou M. Meningiomas invading the sagittal or transverse sinuses, resection with venous reconstruction. J Clin Neurosci. 2001;8(suppl 1):8-11.
27. Sindou M., Hallacq P. Venous reconstruction in surgery of meningiomas invading the sagittal and transverse sinuses. Skull Base Surg. 1998;8:57-64.
28. Sindou M., Mercier P., Bokor J., Brunon J. Bilateral thrombosis of the transverse sinuses: microsurgical revascularization with venous bypass. Surg Neurol. 1980;13:215-220.
29. Hoessly G.F., Olivecrona H. Report on 280 cases of verified parasagittal meningioma. J Neurosurg. 1955;12:614-626.
30. DiMeco F., Li K.W., Casali C., et al. Meningiomas invading the superior sagittal sinus: surgical experience of 108 cases. Neurosurgery. 2004;55:1263-1274.