Chapter 1B Surgical and radiologic anatomy of the liver, biliary tract, and pancreas
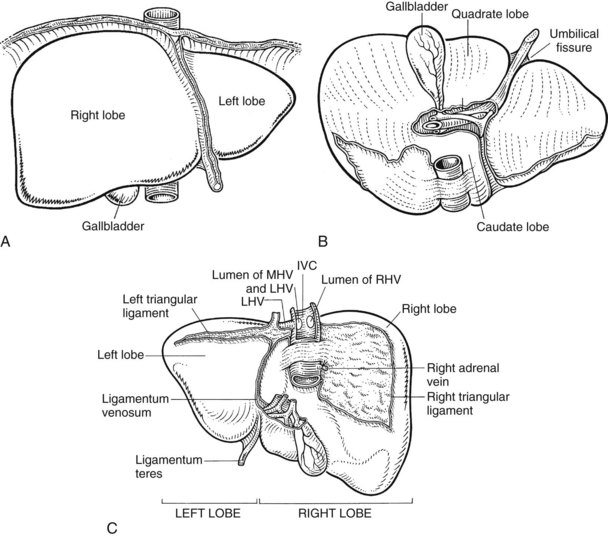
Liver
Retrohepatic Inferior Vena Cava
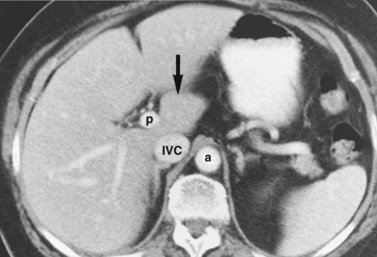
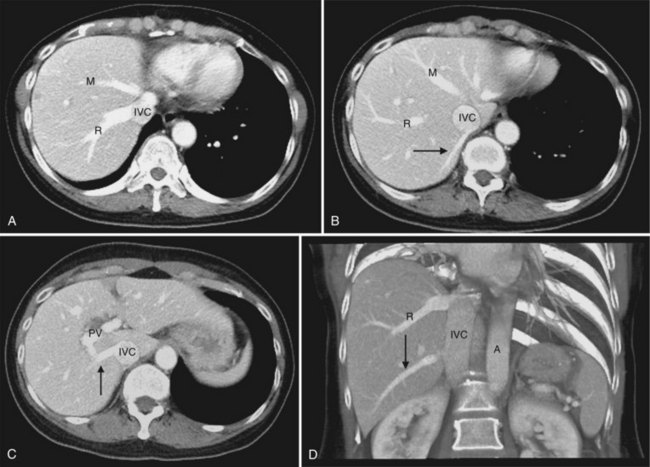
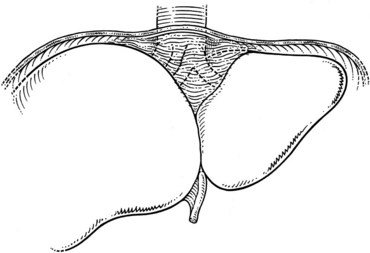
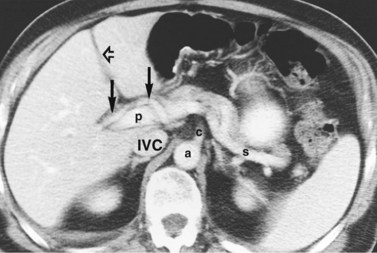
The inferior vena cava (IVC) runs on the right of the aorta on the bodies of the lumbar vertebrae, diverging from the aorta as it passes upward. Below the liver, the IVC lies behind the duodenum and head of the pancreas as a retroperitoneal structure passing upward behind the foramen of Winslow posterior to the right hilar structures of the liver. The renal veins lie in front of the arteries and join the IVC at almost a right angle on the left and obliquely on the right. Behind the liver, the IVC is embraced in a groove on its posterior surface (Figs. 1B.1 and 1B.2). The IVC comes to lie on the right crus of the diaphragm, behind the bare area of the liver; it extends to the central tendon of the diaphragm, which it pierces on a level with the body of T8, behind and higher than the beginning of the abdominal aorta. As the IVC courses upward, it is separated from the right crus of the diaphragm by the right celiac ganglion and, higher up, by the right phrenic artery. The right adrenal vein is a short vessel that enters the IVC behind the bare area (see Fig. 1B.1). There may be a small accessory right adrenal vein on the right that enters into the confluence of the right renal vein and the IVC. The lumbar veins drain posterolaterally into the IVC below the level of the renal veins, but above this level, there are usually no vena caval tributaries posteriorly.
Hepatic Veins
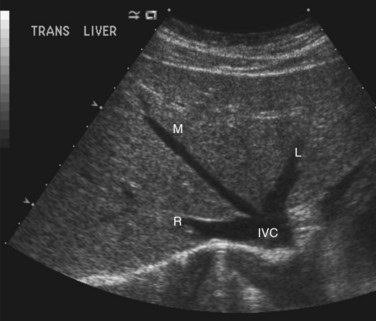
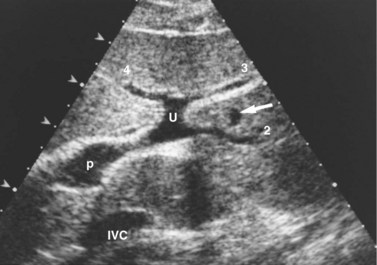
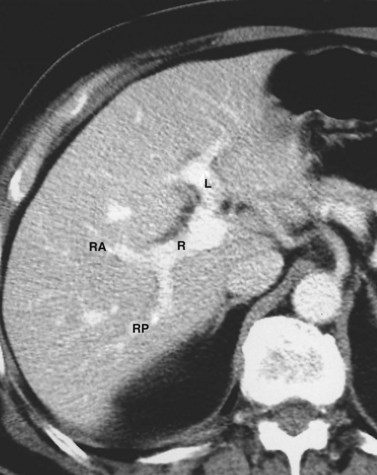
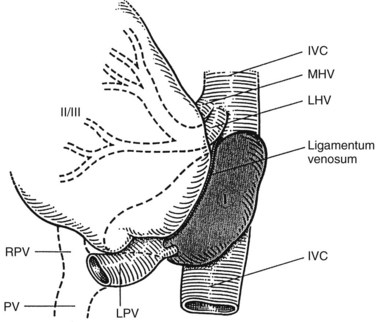
The hepatic veins (Figs. 1B.3 to 1B.5) drain directly from the upper part of the posterior surface of the liver at an oblique angle directly into the vena cava. The right hepatic vein, which is larger than the left and middle hepatic veins, has a short extrahepatic course of approximately 1 cm. The left and middle hepatic veins may drain separately into the IVC but are usually joined, after a short extrahepatic course, to form a common venous channel approximately 2 cm in length that traverses to the left part of the anterior surface of the IVC below the diaphragm (see Figs. 1B.3 and 1B.5). In addition to the three major hepatic veins, there is the umbilical vein, which is single in most cases and runs beneath the falciform ligament between the middle and left hepatic veins; it empties into the terminal portion of the left hepatic vein, although rarely it drains into the middle hepatic vein or directly into the confluence of the middle and left hepatic veins. Additional posteriorly and inferiorly draining hepatic veins, with a short course into the anterior surface of the IVC, are frequent and may be large (see Fig. 1B.4). Hepatic venous drainage of the caudate lobe is directly into the IVC, as described below.
Functional Surgical Anatomy
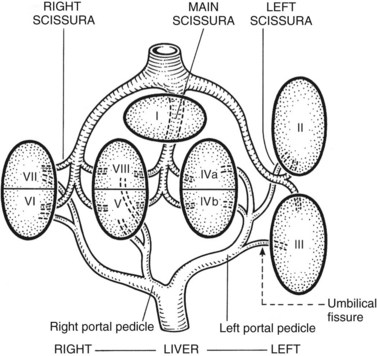
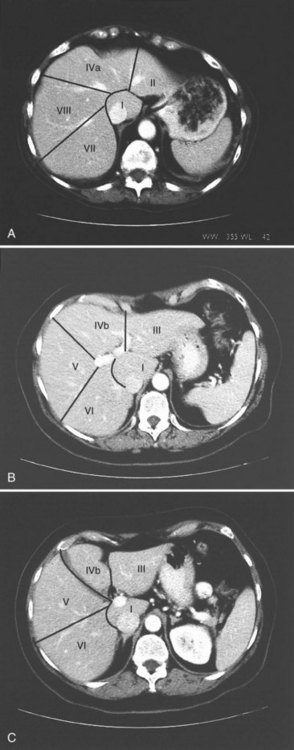
The internal architecture of the liver is composed of a series of segments that combine to form sectors separated by scissurae that contain the hepatic veins (Fig. 1B.6). Together or separately, these constitute the visible lobes described previously. The internal structure has been clarified by the publications of McIndoe and Counseller (1927), Ton That Tung (1939, 1979), Hjörtsjö (1931), Healey and Schroy (1953), Goldsmith and Woodburne (1957), Couinaud (1957), and Bismuth and colleagues (1982); however, the description by Couinaud is the most complete, exact, and useful for the operating surgeon, so it is generally used here. An alternative terminology suggested by a committee of the International Hepato-Pancreatico-Biliary Association (Strasberg et al, 2000) adds little to the understanding or practicality of Couinaud’s description. The main difference is that in the alternative terminology, Couinaud’s sectors are referred to as sections, particularly in describing the anatomy of the left liver (see Chapters 90A to 90F for differences in the terminology of the various hepatic resections).
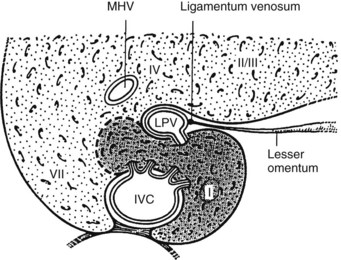
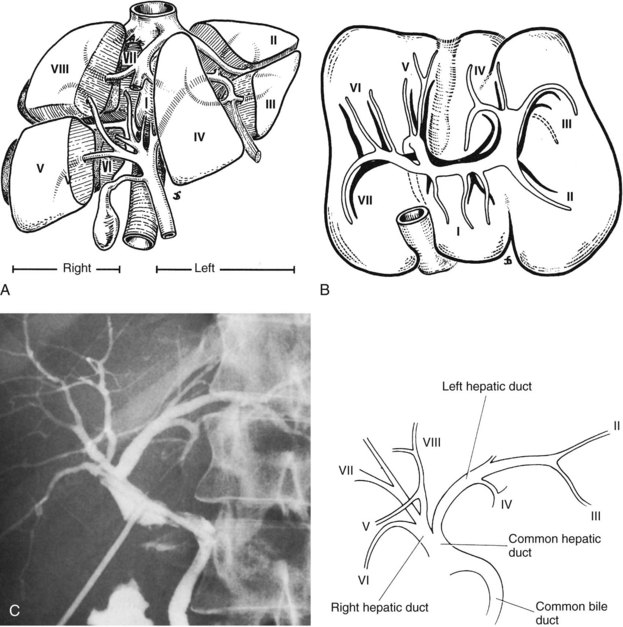
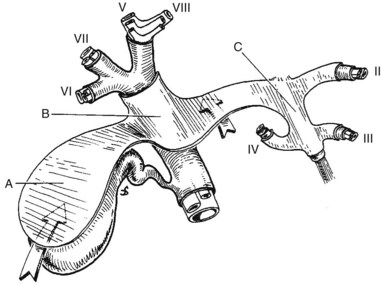
Essentially, the three main hepatic veins within the scissurae divide the liver into four sectors, each of which receives a portal pedicle, with alternation between the hepatic veins and portal pedicles. The main portal scissura contains the middle hepatic vein and progresses from the middle of the gallbladder bed anteriorly to the left of the vena cava posteriorly. The right and left parts of the liver, demarcated by the main portal scissura, are independent in terms of portal and arterial vascularization and biliary drainage (Fig. 1B.7). These right and left livers are themselves divided into two by the remaining portal scissurae. These four subdivisions are referred to as segments in the description of Goldsmith and Woodburne (1957), but in Couinaud’s nomenclature (1957), they are termed sectors (see Figs. 1B.6 and 1B.7).
The right portal scissura separates the right liver into two sectors: anteromedial (anterior) and posterolateral (posterior). With the body supine, this scissura is almost in the frontal plane. The right hepatic vein runs within the right scissura. The left portal scissura divides the left liver into two sectors, but the left portal scissura is not within the umbilical fissure, because this fissure is not a portal scissura and contains a portal pedicle. The left portal scissura is located posterior to the ligamentum teres and within the left lobe of the liver, along the course of the left hepatic vein. The anterior sector of the left liver—the left medial section, in the terminology of Strasberg and others (2000)—is composed of a part of the right lobe (segment IV), to the left of the main portal scissura, and of the anterior part of the left lobe (segment III; see Figs. 1B.6 and 1B.7). The left posterior sector is the only sector composed of one segment (segment II; the left posterior section in terminology of Strasberg et al).
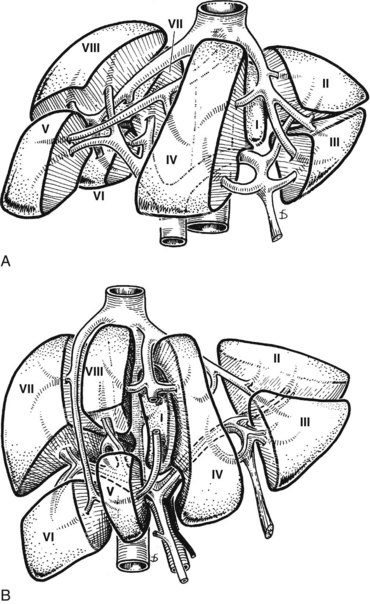
At the hilus of the liver, the right portal triad pursues a short course of approximately 1 to 1.5 cm before entering the substance of the right liver (Fig. 1B.8A). In some cases, the right anterior and posterior pedicles arise independently, and their origins may be separated by 2 cm (see Fig. 1B.8B). In some cases, it appears as if the left portal vein arises from the right anterior branch (see also Fig. 1B.40). On the left side, however, the portal triad crosses over approximately 3 to 4 cm beneath the quadrate lobe, embraced in a peritoneal sheath at the upper end of the gastrohepatic ligament and separated from the undersurface of the quadrate lobe by connective tissue (hilar plate). This prolongation of the left portal pedicle turns anteriorly and caudally within the umbilical fissure, giving branches of supply to segments II and III and recurrent branches to segment IV (Fig. 1B.9; see also Fig. 1B.6). Beneath the quadrate lobe, the pedicle is composed of the left branch of the portal vein and the left hepatic duct, but it is joined at the base of the umbilical fissure by the left branch of the hepatic artery.
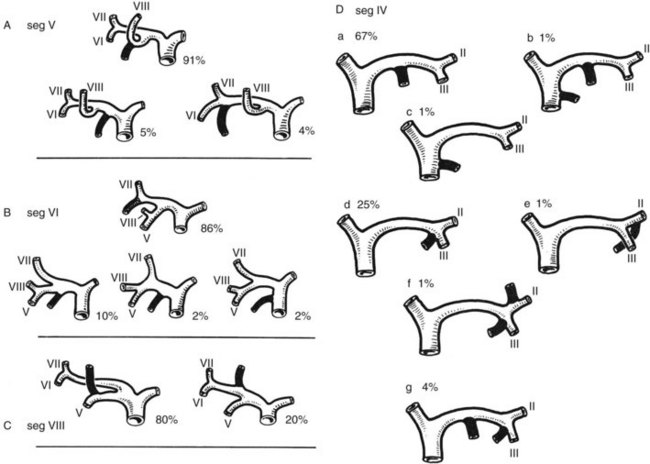
The branching of the portal pedicle at the hilus (Fig. 1B.10; see also Figs. 1B.6, 1B.8, and 1B.9), the distribution of the branches to the caudate lobe (segment I) on the right and left sides, and the distribution to the segments of the right (segments V through VIII) and left (segments II through IV) hemiliver follow a remarkably symmetric pattern and, as described by Scheele (1994), allow separation of segment IV into segment IVa superiorly and segment IVb inferiorly (see Fig. 1B.6). This arrangement of subsegments mimics the distribution to segments V and VIII on the right side. The umbilical vein provides drainage of at least parts of segment IVb after ligation of the middle hepatic vein, and it is important in the performance of segmental resection.
The caudate lobe (segment I) is the dorsal portion of the liver lying posteriorly and embracing the retrohepatic IVC. This lobe lies between major vascular structures: On the left, the caudate lies between the IVC posteriorly and the left portal triad inferiorly and the IVC and the middle and left hepatic veins superiorly (Figs. 1B.11 and 1B.12). This portion of the caudate is sometimes referred to as segment IX.
The portion of the caudate on the right varies but is usually quite small. The anterior surface within the parenchyma is covered by the posterior surface of segment IV, the limit being an oblique plane slanting from the left portal vein to the left hepatic vein. Thus, there is a caudate lobe (segment I) with a constantly present left portion and a right portion of variable size (see Figs. 1B.11 and 1B.12).
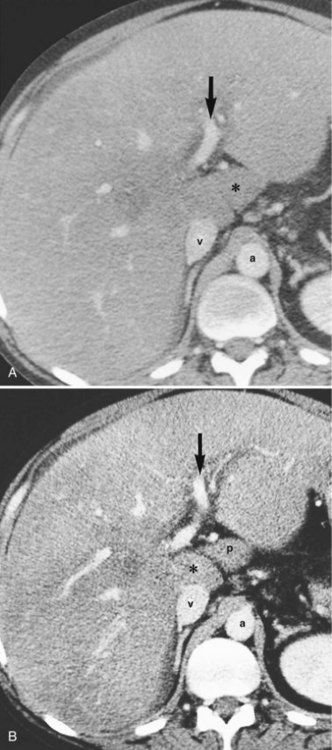
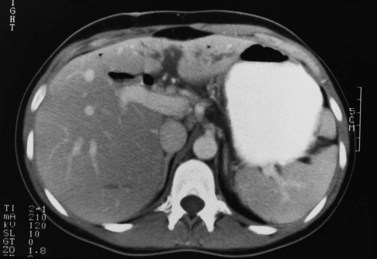
In the usual and common circumstance, the posterior edge of the caudate lobe on the left has a fibrous component, which fans out and attaches lightly to the crural area of the diaphragm; but it extends posteriorly, behind the vena cava, to link with a similar component of fibrous tissue that protrudes from the posterior surface of segment VII and embraces the vena cava (see Figs. 1B.1C and 1B.11). In 50% of patients, this ligament is replaced by hepatic tissue, in whole or in part, and the caudate may completely encircle the IVC and may contact segment VII on the right side; a significant retrocaval component may prevent a left-sided approach to the caudate veins. The caudal margin of the caudate lobe has a papillary process that occasionally may attach to the rest of the lobe via a narrow connection. It is bulky in 27% of cases and can be mistaken for an enlarged lymph node on computed tomography (CT) scan (Fig. 1B.13).
1 The liver is divided into two hemilivers by the main hepatic scissura, within which runs the middle hepatic vein.
2 The left liver is divided into two sectors by the left portal scissura, within which the left hepatic vein runs (see Fig. 1B.6). The posterior sector comprises only one segment, segment II, which is the posterior part of the left lobe. This is the only sector that comprises one segment, referred to as the left posterior section by Strasberg and others (2000). The anterior sector is divided by the umbilical fissure into two segments: a medial segment, the quadrate lobe (segment IV), and a lateral segment (segment III), which is the anterior part of the left lobe.
3 The right liver is divided into two sectors by the right portal scissura, containing the right hepatic vein. Each of these sectors is further divided into two segments: an anterior segment V inferiorly and segment VIII superiorly, and a posterior segment, segment VI inferiorly and segment VII superiorly (see Figs. 1B.6 and 1B.7).
4 Segment I, the caudate lobe, lies posteriorly and embraces the vena cava, its intraparenchymal anterior surface abutting the posterior surface of segment IV and merging with segments VI and VII on the right (Fig. 1B.14; see Fig. 1B.11).
Further details of segmental anatomy important in sectoral or segmental resection are described in Chapter 90A, Chapter 90B, Chapter 90C, Chapter 90D, Chapter 90E, Chapter 90F, Chapter 92 .
Surgical Implications and Exposure
All methods for precise partial hepatectomy depend on control of the inflow vasculature and draining bile ducts and of the outflow hepatic veins of the portion of liver to be excised, which may be a segment, a subsegment, or an entire lobe. The remnant remaining after partial hepatectomy must be provided with an excellent portal venous and hepatic arterial supply and biliary drainage and an unimpeded hepatic venous outflow. Under such circumstances, hepatic regeneration is usually prompt. The classification of the various partial hepatic resection procedures, incisions and exposure, necessary mobilization of the liver, and the methods of control of the structures within the portal triads and of the hepatic veins are described in detail in Chapter 90A, Chapter 90B, Chapter 90C, Chapter 90D, Chapter 90E, Chapter 90F, Chapter 92 .
Biliary Tract
Intrahepatic Bile Duct Anatomy
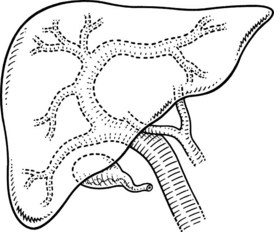
The right and left livers are drained by the right and the left hepatic ducts, whereas the dorsal lobe (caudate lobe) is drained by several ducts that join both the right and left hepatic ducts. The intrahepatic ducts are tributaries of the corresponding hepatic ducts, which form part of the major portal triads that penetrate the liver, invaginating the Glisson capsule at the hilus. Bile ducts usually are located above the corresponding portal branches, whereas hepatic arterial branches are situated inferiorly to the veins. Each branch of the intrahepatic portal veins corresponds to one or two bile duct tributaries that join to form the right and left hepatic ductal systems, converging at the liver hilus to constitute the common hepatic duct. The umbilical fissure divides the left liver, passing between segment III and segment IV, where it may be bridged by a tongue of liver tissue. The ligamentum teres passes through the umbilical fissure to join the left branch of the portal vein (see Fig. 1B.1).
The left hepatic duct drains the three segments—II, III, and IV—that constitute the left liver (Fig. 1B.15). The duct that drains segment III is located slightly behind the left horn of the umbilical recess. It is joined by the tributary from segment IVb to form the left duct, which is similarly joined by the duct of segment II and the duct of segment IVa, where the left branch of the portal vein turns forward and caudally. The left hepatic duct traverses beneath the left liver at the base of segment IV, just above and behind the left branch of the portal vein; it crosses the anterior edge of that vein and joins the right hepatic duct to constitute the hepatic ductal confluence. In its transverse portion, it receives one to three small branches from segment IV.
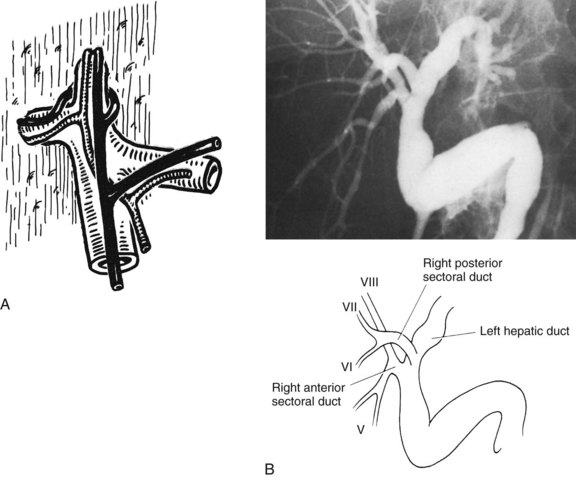
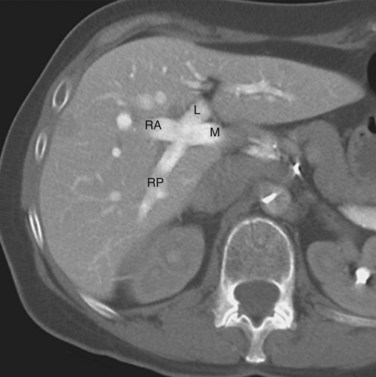
The right hepatic duct drains segments V, VI, VII, and VIII and arises from the junction of two main sectoral duct tributaries (Fig. 1B.16). The posterior or lateral duct and the anterior or medial duct are each accompanied by a corresponding vein. The right posterior sectoral duct has an almost horizontal course and constitutes the confluence of the ducts of segments VI and VII (see Figs. 1B.15 and 1B.16). The duct then runs to join the right anterior sectoral duct, as it descends in a vertical manner. The right anterior sectoral duct is formed by the confluence of the ducts draining segments V and VIII. Its main trunk is located to the left of the right anterior sectoral branch of the portal vein, which pursues an ascending course. The junction of these two main right biliary channels usually occurs above the right branch of the portal vein. The right hepatic duct is short and joins the left hepatic duct to constitute the confluence lying in front of the right portal vein and forming the common hepatic duct.
The caudate lobe (segment I) has its own biliary drainage (Healey & Schroy, 1953). The caudate lobe is divided into right and left portions and a caudate process. In 44% of individuals, three separate ducts drain these three parts of the lobe, whereas in 26% a common duct lies between the right portion of the caudate lobe proper and the caudate process and an independent duct that drains the left part of the caudate lobe. The site of drainage of these ducts varies. In 78% of cases, drainage of the caudate lobe is into the right and left hepatic ducts, but in 15% drainage is by the left hepatic ductal system only. In about 7%, the drainage is into the right hepatic system.
Extrahepatic Biliary Anatomy and Vascular Anatomy of the Liver and Pancreas
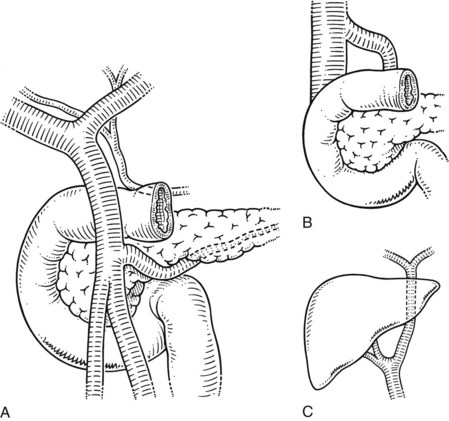
The extrahepatic bile ducts are represented by the extrahepatic segments of the right and left hepatic ducts, joining to form the biliary confluence and the main biliary channel draining to the duodenum. The accessory biliary apparatus, which constitutes a reservoir, comprises the gallbladder and cystic duct (Figs. 1B.17 and 1B.18). The confluence of the right and left hepatic ducts occurs at the right of the hilar fissure of the liver, anterior to the portal venous bifurcation and overlying the origin of the right branch of the portal vein (see Fig. 1B.17). The extrahepatic segment of the right duct is short, but the left duct has a much longer extrahepatic course. The biliary confluence is separated from the posterior aspect of the quadrate lobe of the liver by the hilar plate, which is the fusion of connective tissue enclosing the biliary and vascular elements with the Glisson capsule (Fig. 1B.19). Because of the absence of any vascular interposition, it is possible to open the connective tissue constituting the hilar plate at the inferior border of the quadrate lobe and, by elevating it, to display the biliary confluence and left hepatic duct (Fig. 1B.20).
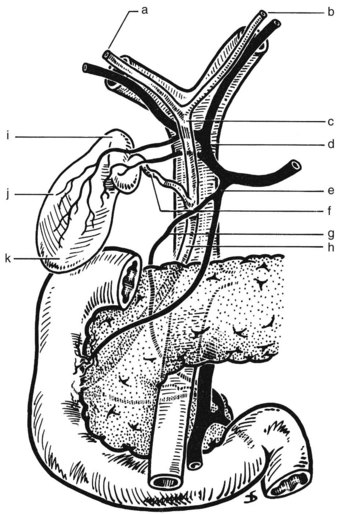
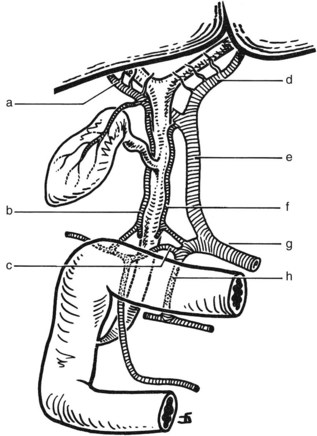
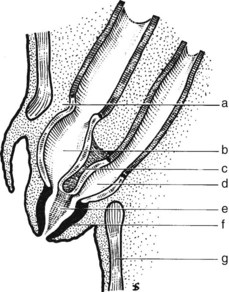
FIGURE 1B.17 Anterior aspect of the biliary anatomy and of the head of the pancreas: right hepatic duct (a); left hepatic duct (b); common hepatic duct (c); hepatic artery (d); gastroduodenal artery (e); cystic duct (f); retroduodenal artery (g); common bile duct (h); neck of the gallbladder (i); body of the gallbladder (j); fundus of the gallbladder (k). Note particularly the position of the hepatic bile duct confluence anterior to the right branch of the portal vein, the posterior course of the cystic artery behind the common hepatic duct, and the relationship of the neck of the gallbladder to the right branch of the hepatic artery. Note also the relationship of the major vessels (portal vein, superior mesenteric vein, and artery) to the head of the pancreas (see also Figs. 1B.29, 1B.35, and 1B.45).
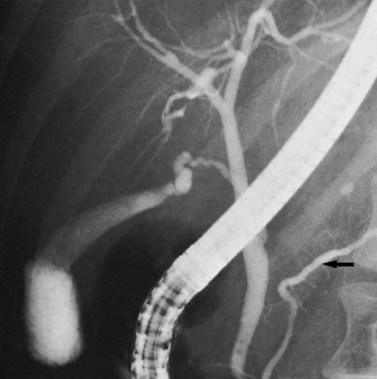
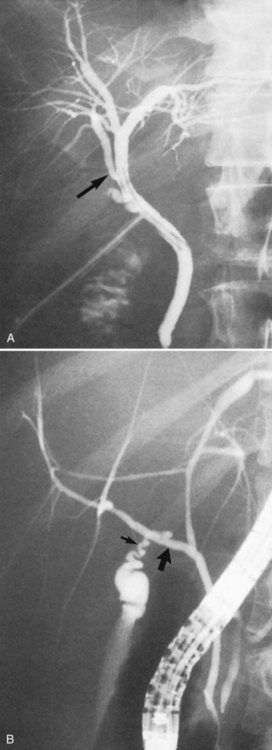
FIGURE 1B.18 Endoscopic retrograde choledochopancreatogram showing the pancreatic duct (arrow), gallbladder, and biliary tree.
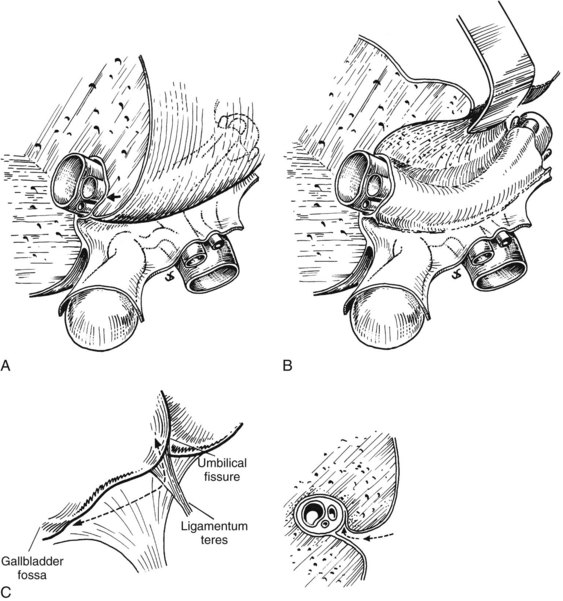
FIGURE 1B.20 A, Relationship between the posterior aspect of the quadrate lobe and the biliary confluence. The hilar plate (arrow) is formed by the fusion of the connective tissue enclosing the biliary and vascular elements with the Glisson capsule. B, Biliary confluence and left hepatic duct exposed by lifting the quadrate lobe upward after incision of the Glisson capsule at its base. This technique, lowering of the hilar plate (Hepp & Couinaud, 1956), generally is used to display a dilated bile duct above an iatrogenic stricture or hilar cholangiocarcinoma. C, Line of incision (left) to allow extensive mobilization of the quadrate lobe. This maneuver is of particular value for high bile duct stricture and in the presence of liver atrophy or hypertrophy. The procedure consists of lifting the quadrate lobe upward (see A and B), then not only opening the umbilical fissure but also incising the deepest portion of the gallbladder fossa. Right, Incision of the Glisson capsule to gain access to the biliary system (arrow).
Main Bile Duct and Sphincter of Oddi
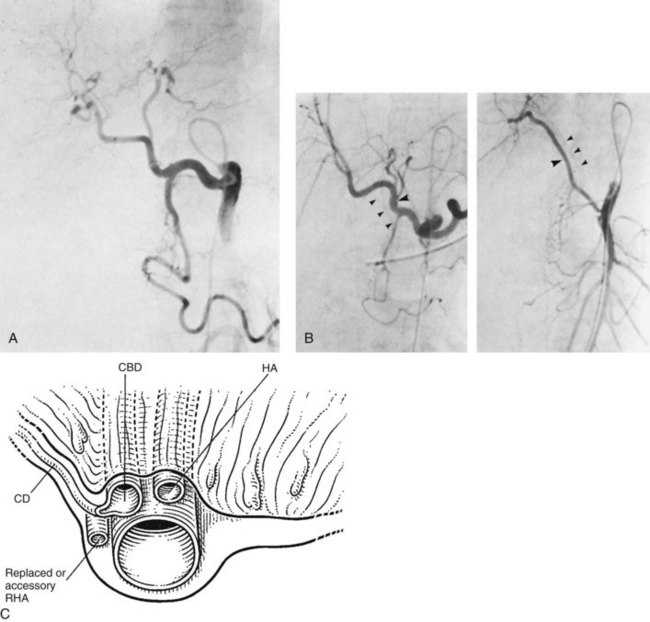
The main bile duct (see Fig. 1B.17), the mean diameter of which is about 6 mm, is divided into two portions: the upper is called the common hepatic duct and is situated above the cystic duct, which joins it to form the lower portion, the common bile duct (see Fig. 1B.18). The common duct courses downward anterior to the portal vein, in the free edge of the lesser omentum; it is closely applied to the hepatic artery, which runs upward on its left, giving rise to the right branch of the hepatic artery, which crosses the main bile duct usually posteriorly, although in about 20% of cases, it crosses anteriorly. The cystic artery, arising from the right branch of the hepatic artery, may cross the common hepatic duct posteriorly or anteriorly. The common hepatic duct constitutes the left border of the triangle of Calot, the other corners of which were originally described as the cystic duct below and the cystic artery above (Rocko et al, 1981). The commonly accepted working definition of the triangle of Calot recognizes, however, the inferior surface of the right lobe of the liver as the upper border and the cystic duct as the lower border (Wood, 1979). Dissection of the triangle of Calot is of key significance during cholecystectomy, because in this triangle runs the cystic artery, often the right branch of the hepatic artery, and occasionally a bile duct, which should be displayed before cholecystectomy. If there is a replaced or accessory common or right hepatic artery, it usually runs behind the cystic duct to enter the triangle of Calot (Fig. 1B.21).
The common variations in the relationship of the hepatic artery and origin and course of the cystic artery to the biliary apparatus are shown in Fig. 1B.22. Ignorance of these variations may provoke unexpected hemorrhage or biliary injury (Champetier et al, 1982) during cholecystectomy and may result in bile duct injury during efforts to secure hemostasis. The union between the cystic duct and the common hepatic duct may be located at various levels. At its lower extrahepatic portion, the common bile duct traverses the posterior aspect of the pancreas, running in a groove or tunnel. The retropancreatic portion of the common bile duct approaches the second portion of the duodenum obliquely, accompanied by the terminal part of the pancreatic duct of Wirsung.
Gallbladder and Cystic Duct
The gallbladder is a reservoir located on the undersurface of the right lobe of the liver, within the cystic fossa; it is separated from the hepatic parenchyma by the cystic plate, which is composed of connective tissue closely applied to the Glisson capsule and elongating the hilar plate (see Fig. 1B.19). Sometimes the gallbladder is deeply embedded in the liver, but occasionally it occurs on a mesenteric attachment and may be susceptible to volvulus. The gallbladder varies in size and consists of a fundus, a body, and a neck (Fig. 1B.23). The tip of the fundus usually, but not always, reaches the free edge of the liver and is closely applied to the cystic plate. The cystic fossa is a precise anterior landmark to the main liver incisura. The neck of the gallbladder makes an angle with the fundus and creates the Hartmann pouch, which may obscure the common hepatic duct and constitute a real danger point during cholecystectomy.
The cystic duct arises from the neck or infundibulum of the gallbladder and extends to join the common hepatic duct. Its lumen usually measures about 1 to 3 mm, and its length varies, depending on the type of union with the common hepatic duct. The mucosa of the cystic duct is arranged in spiral folds known as the valves of Heister (Wood, 1979). Although the cystic duct joins the common hepatic duct in its supraduodenal segment in 80% of cases, it may extend downward to the retroduodenal or retropancreatic area. Occasionally, the cystic duct may join the right hepatic duct or a right hepatic sectoral duct (Fig. 1B.24).
Biliary Ductal Anomalies
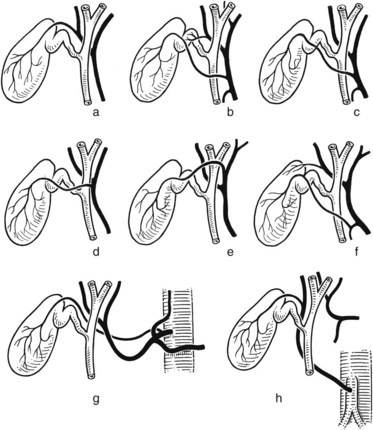
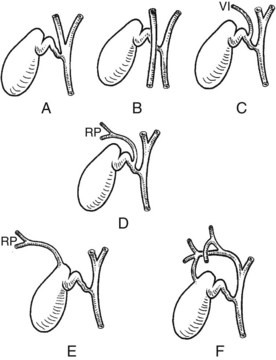
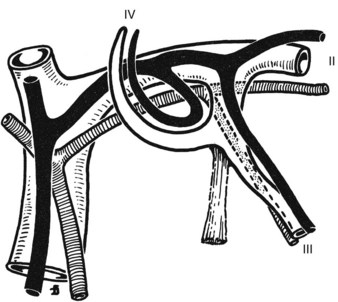
Full knowledge of the frequent variations from the described normal biliary anatomy is required when performing any hepatobiliary procedure. The constitution of a normal biliary confluence by union of the right and left hepatic ducts as described previously is reported in 72% of patients (Healey & Schroy, 1953). There is a triple confluence of the right anterior and posterior sectoral ducts and the left hepatic duct in 12% of individuals (Couinaud, 1957), and a right sectoral duct joins the main bile duct directly in 20%. In 16% the right anterior sectoral duct, and in 4% the right posterior sectoral duct, may approach the main bile duct in this fashion (Fig. 1B.25A). In 6%, a right sectoral duct may join the left hepatic duct (the posterior duct in 5% and the anterior duct in 1%; see Fig. 1B.16B). In 3%, there is an absence of the hepatic duct confluence, and the right posterior sectoral duct may join the neck of the gallbladder, or it may be entered by the cystic duct in 2% (Couinaud, 1957). In any event, these multiple biliary ductal variations at the hilus are important to recognize in resection and reconstructive surgery of the biliary tree at the hilus and during partial hepatectomy.
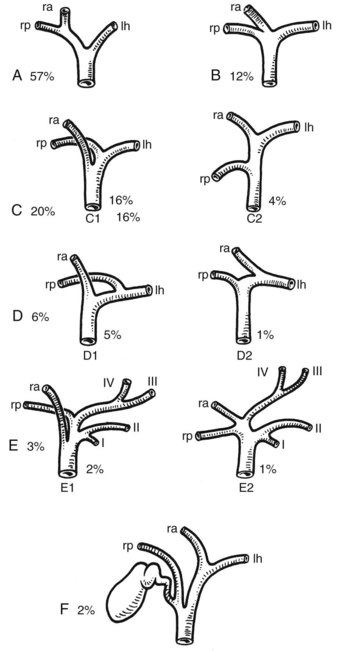
FIGURE 1B.25 Main variations of the hepatic duct confluence (Couinaud, 1957). A, Typical anatomy of the confluence. B, Triple confluence. C, Ectopic drainage of a right sectoral duct into the common hepatic duct (C1, Right anterior [ra] duct draining into the common hepatic duct; C2, Right posterior [rp] duct draining into the common hepatic duct). D, Ectopic drainage of a right sectoral duct into the left hepatic ductal system (D1, Right posterior sectoral duct draining into the left hepatic [lh] ductal system; D2, Right anterior sectoral duct draining into the left hepatic ductal system). E, Absence of the hepatic duct confluence. F, Absence of right hepatic duct and ectopic drainage of the right posterior duct into the cystic duct.
Intrahepatic bile duct variations also are common (Fig. 1B.26; Healey & Schroy, 1953). The main right intrahepatic duct variations are represented by an ectopic drainage of segment V in 9%, of segment VI in 14%, and of segment VIII in 20%. In addition, a subvesical duct has been described in 20% to 50% of cases. This duct, sometimes deeply embedded in the cystic plate, joins either the common hepatic duct or the right hepatic duct. It does not drain any specific liver territory, never communicates with the gallbladder, and is not a satellite of an intrahepatic branch of the portal vein or hepatic artery. Although not of major anatomic significance, injury may occur during cholecystectomy if the cystic plate is not preserved, which may lead to a postoperative biliary leak.
In 67% of patients (Healey & Schroy, 1953) a classic distribution of the main left intrahepatic biliary ductal system exists. The main variation in this region is represented by a common union between the ducts of segments III and IV in 25%, and in only 2% does the duct of segment IV join the common hepatic duct independently. Several anomalies of drainage of the intrahepatic ducts into the neck of the gallbladder or cystic duct have been reported (Fig. 1B.27; Albaret et al, 1981; Couinaud, 1957), and these must be kept in mind during cholecystectomy (see Chapter 33).
Anomalies of the Accessory Biliary Apparatus
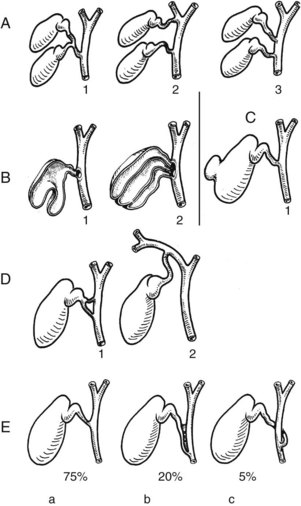
Many anomalies of the accessory biliary apparatus have been described (Fig. 1B.28A; Gross, 1936). Although rare, agenesis of the gallbladder (Boyden, 1926; Rachad-Mohassel et al, 1973; Rogers et al, 1975), bilobar gallbladders with a single cystic duct but two fundi (Hobby, 1970), and duplication of the gallbladder with two cystic ducts all have been described. A double cystic duct may drain a unilocular gallbladder (Perelman, 1961), and congenital diverticulum of the gallbladder with a muscular wall may also be found (Eelkema et al, 1958). More frequently reported are anomalies of position of the gallbladder, which may be in an intrahepatic position, completely surrounded by normal liver tissue, or it may be found on the left of the liver (Newcombe & Henley, 1964).
The mode of union of the cystic duct with the common hepatic duct may be angular, parallel, or spiral (see Fig. 1B.28B). An angular union is the most frequent and is found in 75% of patients (Kune, 1970). The cystic duct may run a parallel course to the common hepatic duct in 20%, with connective tissue ensheathing both ducts. Finally, the cystic duct may approach the common bile duct in a spiral fashion. The absence of a cystic duct is probably an acquired anomaly, representing a cholecystocholedochal fistula.
Bile Duct Blood Supply
The bile duct may be divided into three segments: hilar, supraduodenal, and retropancreatic (lower common bile duct). The blood supply of the supraduodenal duct is essentially axial (Fig. 1B.29; Northover & Terblanche, 1979). Most vessels to the supraduodenal duct arise from the superior pancreaticoduodenal artery, the right branch of the hepatic artery, the cystic artery, the gastroduodenal artery, and the retroduodenal artery. On average, eight small arteries, each measuring about 0.3 mm in diameter, supply the supraduodenal duct. The most important of these vessels run along the lateral borders of the duct and have been called the 3 o’clock and 9 o’clock arteries. Of the blood vessels vascularizing the supraduodenal duct, 60% run upward from the major inferior vessels, and only 38% of arteries run downward, originating from the right branch of the hepatic artery and other vessels. Only 2% of the arterial supply is nonaxial, arising directly from the main trunk of the hepatic artery, as it courses up parallel to the main biliary channel. The hilar ducts receive a copious supply of arterial blood from surrounding vessels, forming a rich network on the surface of the ducts in continuity with the plexus around the supraduodenal duct. The source of blood supply to the retropancreatic common bile duct is from the retroduodenal artery, which provides multiple small vessels running around the duct to form a mural plexus.
Anatomy of Biliary Exposure
Biliary-Vascular Sheaths and Exposure of the Hepatic Bile Duct Confluence
Fusion of the Glisson capsule with the connective tissue sheaths surrounding the biliary and vascular elements at the inferior aspect of the liver constitute the plate system (see Figs. 1B.19 and 1B.20), which includes the hilar plate above the biliary confluence, the cystic plate related to the gallbladder, and the umbilical plate situated above the umbilical portion of the left portal vein (Couinaud, 1957). Hepp and Couinaud (1956) described a technique whereby lifting the quadrate lobe upward and incising the Glisson capsule at its base offers a good exposure of the hepatic hilar structures (see Fig. 1B.20A and B). This technique was referred to as lowering of the hilar plate. It can be carried out safely because only exceptionally (in 1% of cases) is there any vascular interposition between the hilar plate and the inferior aspect of the liver. This maneuver is of particular value in exposing the extrahepatic segment of the left hepatic duct, because it has a long course beneath the quadrate lobe. It is not as effective in exposing the extrahepatic right duct or its secondary branches, which are short. The technique is of major importance for the identification of proximal biliary mucosa during bile duct repair after injury. Basically, an incision is required at the posterior edge of the quadrate lobe, where the Glisson capsule is attached to the hilar plate (see Fig. 1B.20B and Chapter 29). The upper surface of the hilar plate can be separated from the hepatic parenchyma and, by lifting the quadrate lobe upward, display of the hepatic duct convergence, which is always extrahepatic, is effected. Bile duct incision allows performance of a mucosa-to-mucosa anastomosis. Rarely, it may be hazardous to approach the biliary confluence in this manner, especially when anatomic deformity has been created by atrophy or hypertrophy of liver lobes and in patients in whom there appears to be a very deep hilus that is displaced upward and rotated laterally. Frequently, by a simultaneous opening of the deepest portion of the gallbladder fossa and the umbilical fissure (see Fig. 1B.20C), good exposure of the biliary duct confluence, and especially the right hepatic duct, can be obtained without the necessity for full hepatotomy.
Umbilical Fissure and Segment III (Ligamentum Teres) Approach
The round ligament, which is the remnant of the obliterated umbilical vein, runs through the umbilical fissure to connect with the left branch of the portal vein. The round ligament is sometimes deeply embedded in the umbilical fissure. At the junction of the round ligament and the termination of the left portal vein, elongations containing channels that are elements of the left portal system course into the liver. The bile ducts of the left lobe of the liver (Figs. 1B.30 and 1B.31A) are located above the left branch of the portal vein and lie behind these elongations, whereas the corresponding artery is situated below the vein. Dissection of the round ligament on its left side and division of one or two vascular elongations of segment III allow display of the pedicle or anterior branch of the duct of segment III (see Fig. 1B.31B). In the event of biliary obstruction with intrahepatic biliary ductal dilatation, the segment III duct is generally easily located above the left branch of the portal vein. It is often preferable to split the normal liver tissue just to the left of the umbilical fissure to widen the fissure further, which allows access to the ductal system without having to divide any elements of the portal blood supply to segment III (Fig. 1B.32; see Chapter 29).
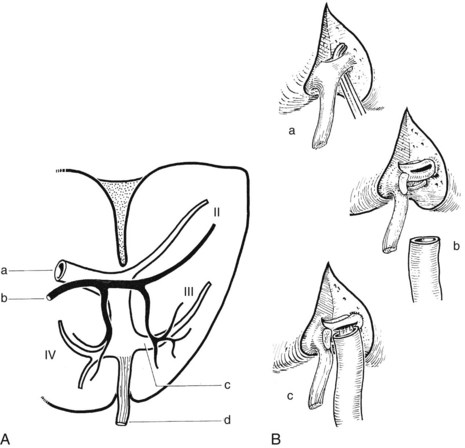
FIGURE 1B.31 A, The biliary and vascular anatomy of the left liver. Note the relationship of the left horn of the umbilical recess with the segment III ductal system: left portal vein (a); left hepatic duct (b); segment III system—note that the duct (black) lies adjacent to the portal venous branch indicated (c); ligamentum teres (d). B, Segment III ductal approach: exposure of the left horn of the umbilical recess (a); division of the left horn of the umbilical recess, including segment III portal vein branches (b); exposure and opening of segment III duct: hepaticojejunostomy to the segment III ductal system (c; see also Chapter 29).
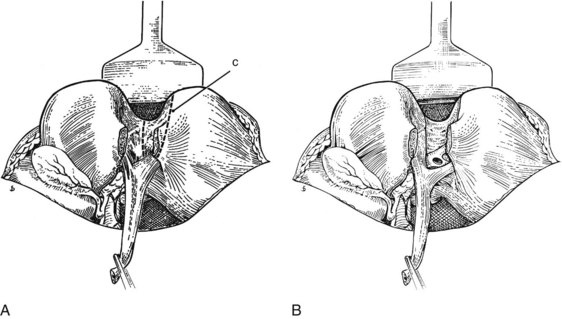
FIGURE 1B.32 A, The liver is split to the left of the ligamentum teres in the umbilical fissure. It may be necessary to remove a small wedge of liver tissue (c). B, Segment III duct is exposed at the base of the liver split, above and behind its accompanying vein, and is ready for anastomosis (see also Chapter 29, Chapter 42A, Chapter 42B ).
Surgical Approaches to the Right Hepatic Biliary Ductal System
Because of the lack of precise anatomic landmarks, exposure of the right intrahepatic ductal system is much more hazardous and imprecise than that of the left. In some cases of hilar cholangiocarcinoma, the planned surgical procedure—partial hepatectomy or segment III duct bypass—seems impossible at operation. In such a critical operative situation, intrahepatic right ductal system drainage is an option. Anatomically, the anterior sectoral duct and its branches run on the left side of the corresponding portal vein. In essence, the end of the liver scissura, within which lies the right branch of the portal vein, is opened over a short distance. The anterior sectoral duct is displayed on the left aspect of the vein, and the dilated duct is opened longitudinally and anastomosed to a Roux-en-Y loop of jejunum (Fig. 1B.33). Although this technique is rarely used, it may be valuable in selected cases. An alternative method is to open into the segment V duct through the gallbladder fossa, although we seldom use this approach and prefer the anterior sectoral duct approach.
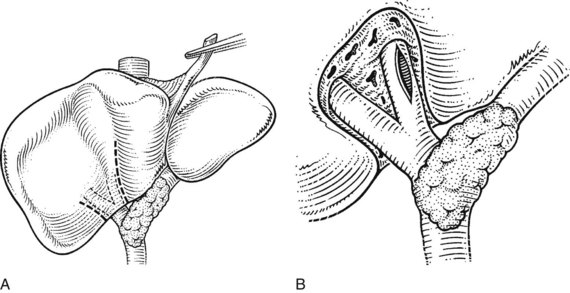
FIGURE 1B.33 A, Anterior sectoral approach. If necessary, the liver substance is opened over a short distance in the line of the right anterior sectoral pedicle. B, The duct is displayed anterior and to the left side of the corresponding vein. This can be facilitated using a posterior pedicular approach as described by Launois (see Chapters 90A to 90F).
Extrahepatic Vasculature
Celiac Axis and Blood Supply of Liver, Biliary Tract, and Pancreas
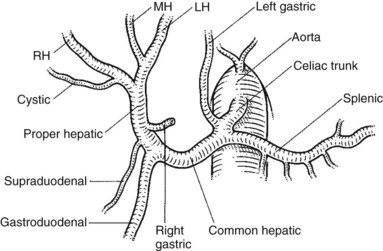
The usual classic description of the arterial blood supply of the liver, biliary system, and pancreas is found in only about 60% of specimens (Figs. 1B.34 to 1B.36). The right and the left hepatic arteries, the former in the right of the hilus of the liver and the latter in the left at the base of the umbilical fissure, become enclosed in the sheath of peritoneum, forming the right and left portal triads. In this sheath, further branching to the right anterior and posterior sectors of the liver and on the left to segments II, III, and IV occurs within the respective pedicles, which also come to enclose the portal vein branches and the tributary bile ducts from these sectors and segments. The arterial supply of the common bile duct was described earlier; it arises from branches of the hepatic artery, the gastroduodenal artery, and the pancreaticoduodenal arcades.
For practical surgical issues, the most important relationships in the anatomy of the pancreas concern the arterial blood supply and the venous drainage. The dorsal pancreatic artery is a major branch, usually arising from the splenic artery, but it can arise directly from the hepatic artery. When splenectomy is performed, it is important to establish the site of origin of the distal pancreatic artery by proximal arterial ligation to avoid distal pancreatic ischemia. The superior mesenteric artery (SMA) arises from the aorta posteriorly behind the pancreas and runs forward and upward to run first behind and then to the left of the superior mesenteric vein (see Fig. 1B.35).
Variations in the Hepatic Artery
As a result of the complex embryologic development of the celiac axis and SMA, wide variations in the arterial supply of the liver are found (Fig. 1B.37). These variations are important to the angiographer and the surgeon. Failure to show all arteries feeding the liver at angiography may not only result in errors of diagnosis but may also seriously mislead the surgeon or the interventional radiologist. In most cases, the hepatic artery arises from the celiac axis as described earlier, but it may be entirely replaced by a common hepatic artery taking origin from the SMA. In this instance, the hepatic artery passes lateral to the portal vein and lies posterior and lateral to the common bile duct in the hepatoduodenal ligament, where it is susceptible to operative injury if not recognized. This applies to a replaced or an accessory hepatic artery. Other variations in the origin of the common hepatic artery include its origin from the aorta and the persistence of a primitive embryologic link between the celiac and superior mesenteric systems. These variations are of considerable importance in controlling the arterial blood supply to the liver during hepatic resection or enucleative procedures, in the performance of devascularization of the liver, in the placement of intraarterial hepatic infusion devices, and in the resection of the head of the pancreas.
Portal Vein
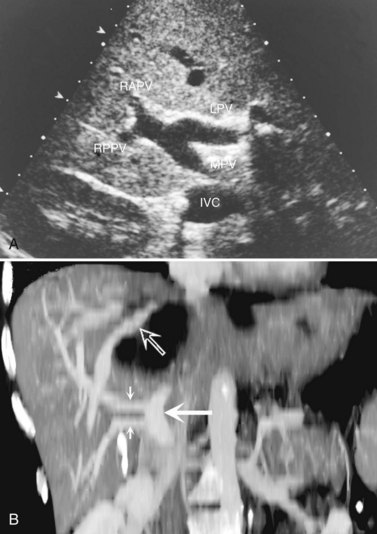
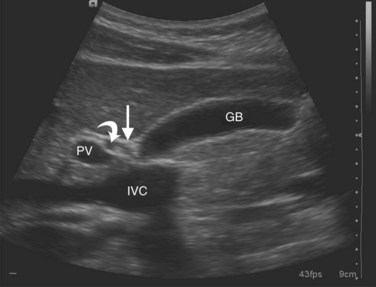
The portal vein (Fig. 1B.38) is formed behind the neck of the pancreas by confluence of the superior mesenteric and splenic veins (Fig. 1B.39). The venous drainage of the pancreas runs in the main parallel to the arterial supply. There are anterior and posterior and superior and inferior pancreaticoduodenal veins that drain to the portal vein and the superior mesenteric vein (SMV). The left gastric vein and the inferior mesenteric vein (IMV) usually drain into the splenic vein, but they can drain directly into the portal vein, whereas the various small splenic tributaries drain directly to the splenic vein.
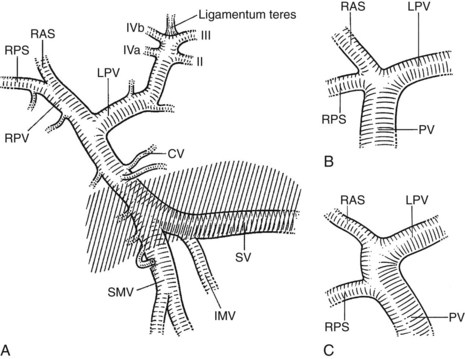
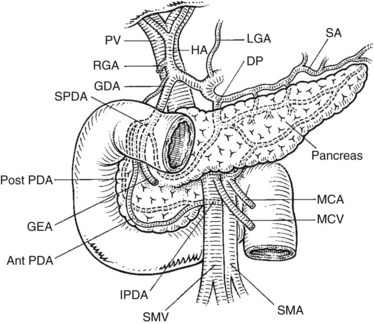
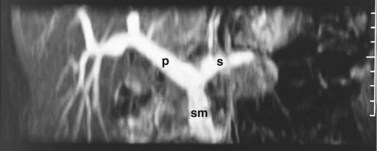
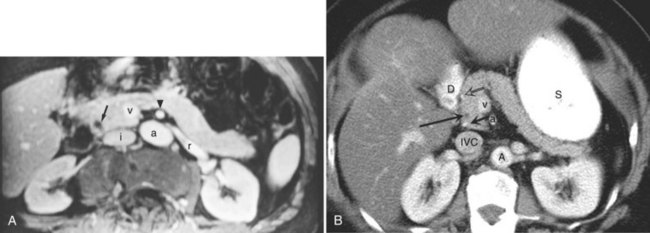
FIGURE 1B.38 A, The superior mesenteric vein (SMV) at the root of the lesser omentum is usually a single trunk; two or sometimes even three branches may unite as the vessel enters the tunnel beneath the neck of the pancreas (shaded) to form a superior mesenteric trunk. This trunk ascends behind the neck of the pancreas and is joined by the splenic vein (SV), which enters it from the left to form the portal vein (PV), which emerges from the retroperitoneal upper border of the neck of the pancreas and ascends toward the liver within the free edge of the lesser omentum, lying behind the bile duct and the hepatic artery and surrounded by the lymphatics and nodes of the lesser omentum. During this course, it receives blood through the coronary vein (CV), which communicates with esophageal venous collaterals, which connect with the gastric vein and the esophageal plexus. Sometimes a separate right gastric vein enters the PV in this area. A superior pancreaticoduodenal vein often enters the PV just above the level of the pancreas, and several smaller veins enter the SMV and PV from the right side beneath the neck of the pancreas. As the PV ascends behind the common bile duct and common hepatic duct, it approaches the hilus of the liver and bifurcates into two branches, a larger right (RPV) and a smaller left portal vein (LPV). The branch on the left courses below the left hepatic duct to enter the umbilical fissure, in company with the left hepatic artery, and subsequently branches to supply the left liver segments (II-IV). Just before its entry into the umbilical fissure, it gives off a major caudate vein, segment I, which runs posteriorly and laterally to the left. Sometimes this vein consists of two or more branches; the right portal branch, which is much shorter in length before its entry into the liver, divides at the extremity of the hilus into the right anterior (RAS) and posterior (RPS) sectoral branches and is accompanied by the respective arterial branches and biliary tributaries. B, The division of the portal vein may arise more proximally, however, and the right anterior and posterior sectoral portal veins may arise independently from the portal venous trunk. C, See also Figs. 1B.8B, 1B.40, and Chapters 90 A through F.
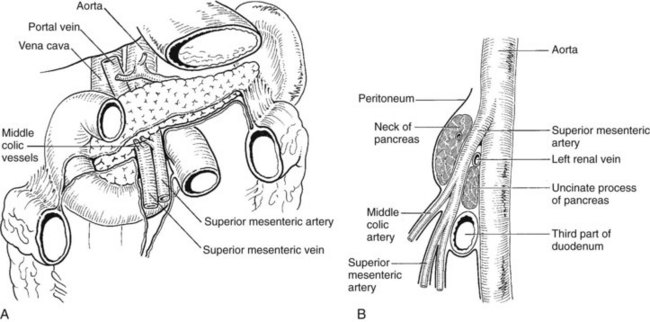
The anatomic relationship of the pancreas to the SMV, the splenic vein, and the portal vein (see Fig. 1B.38) is important in pancreaticoduodenectomy (see Chapter 62A, Chapter 62B ). The uncinate process can extend behind the SMV to well behind the SMA (see Fig. 1B.35). Access to the portal vein behind the pancreas usually is obtained from below by elevating the pancreas from the surface of the SMV just before it joins the splenic vein. With the exception of the inferior pancreaticoduodenal veins, which enter the SMV at the inferior border of the pancreas, it is uncommon to see branches from the pancreas run directly posteriorly into the SMV. Fixation here is usually by some inflammatory or neoplastic process. Superiorly, the portal vein runs behind the pancreas and is identified first in the gap between the curvature of the splenic vein, splenic artery, common hepatic artery, and gastroduodenal artery. Division of the gastroduodenal artery provides much greater access to the superior surface of the portal vein. If difficulty is encountered in this area, division of the common bile duct, usually above the cystic duct, can provide excellent access to the right lateral aspect and anterior surface of the portal vein. The SMA can be approached behind the pancreas above the point at which it is embraced by the uncinate process at the origin from the aorta. This allows dissection of the most proximal part of the SMA from behind.
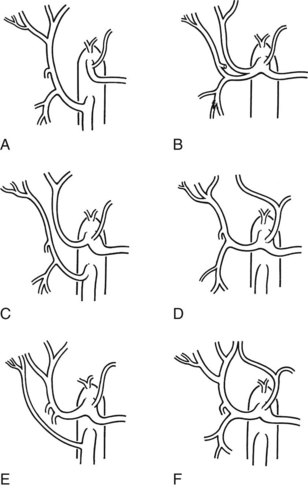
Several variations in anatomy and rare congenital anomalies of the portal vein are of surgical significance (Figs. 1B.40 to 1B.43). For example, performance of right hepatic resection, with division of what appears to be the right portal vein in a patient with absence of the left portal vein (Fig 1B.42), can deprive the entire liver of blood; this surgical error is usually fatal. Agenesis of the right branch of the portal vein is associated with agenesis of the right hemiliver and left liver hypertrophy. This may be associated with biliary and hepatic venous anatomic changes, which can compromise surgical approaches to the liver and to biliary repair (Fields et al, 2008).
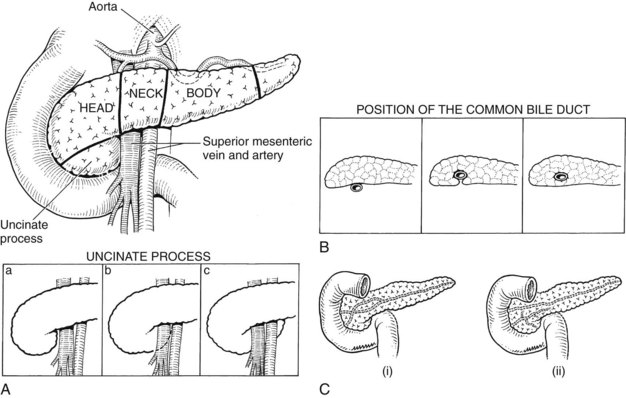
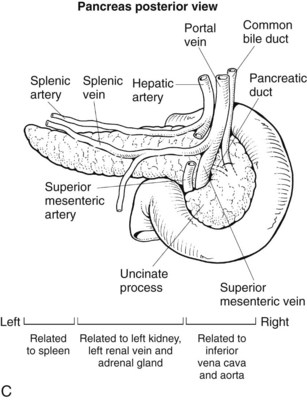
Pancreas
The pancreas is a posteriorly situated retroperitoneal organ draped across the major vessels and the vertebral column (Fig. 1B.44). The organ is composed of a head, neck, and body; the head is encompassed by the duodenum, and the tail rests in the splenic hilum (Figs. 1B.45 and 1B.46). A portion of the head inferiorly is termed the uncinate process and is intimately related to the SMV and SMA (see Figs. 1B.45 and 1B.46). Posteriorly, the pancreas is related to the IVC, aorta, left renal vein and kidney, and spleen (see Figs. 1B.44 and 1B.46B). Usually 4 to 6 inches in length, the relative dimensions of the pancreas have been evaluated more recently. The mean weight is 91.8 g (range, 40.9 to 182 g). To the right of the common bile duct, the average weight of the pancreas is 11.4% of its total weight; the portion lateral to the portal vein averages 56.4% of the total weight. The pancreatic capsule is loosely attached to the surface of the pancreas and is contiguous with the anterior layer of the mesocolon, such that it can be dissected in continuity if necessary. The mesenteric attachments to the pancreas tend to be contiguous (see Fig. 1B.46). The arterial blood supply and venous drainage and the relationships to the common bile duct were described and illustrated earlier (see Figs. 1B.17, 1B.29, 1B.34, 1B.35, and 1B.38).
Pancreatic Duct
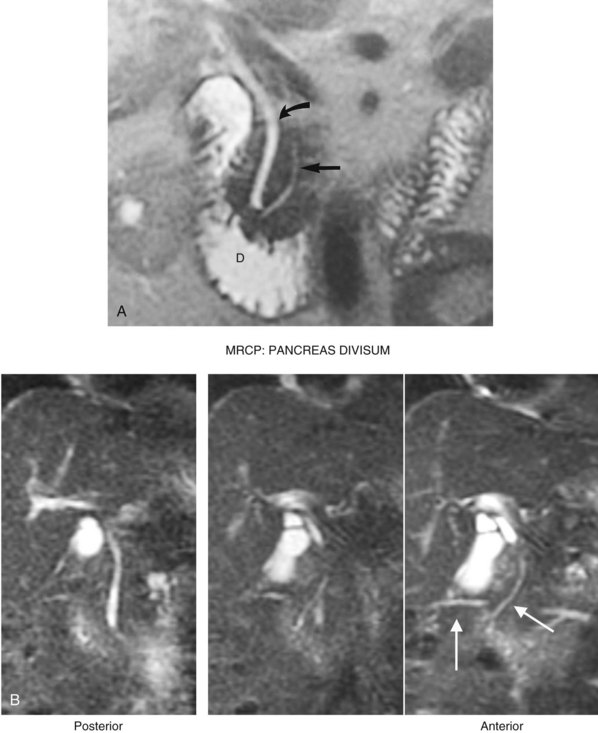
The duct of Wirsung, beginning in the distal tail as a confluence of small ductules, runs through the body to the head, where it usually passes downward and backward in close juxtaposition to the common bile duct (see Fig. 1B.45). The sphincter of Oddi (Fig. 1B.47) has been thoroughly studied (Boyden, 1957; Delmont, 1979) and consists of a unique cluster of smooth muscle fibers distinguishable from the adjacent smooth muscle of the duodenal wall. The papilla of Vater at the termination of the common bile duct is a small, nipplelike structure that protrudes into the duodenal lumen and is marked by a longitudinal fold of duodenal mucosa. The duct of Wirsung runs downward and parallel to the common bile duct for approximately 2 cm and joins it within the sphincter segment in 70% to 85% of patients; it enters the duodenum independently in 10% to 13% of patients and is replaced by the duct of Santorini in 2% of patients (Fig. 1B.48; see also Fig. 1B.45). Rarely, the duct of Santorini and the duct of Wirsung are separate, which is known as pancreas divisum (see Figs. 1B.45Cii and 1B.48B). The islands or islets of Langerhans, which provide the endocrine component of the gland, are scattered throughout the pancreas.
Lymphatic Drainage
Liver and Pancreas
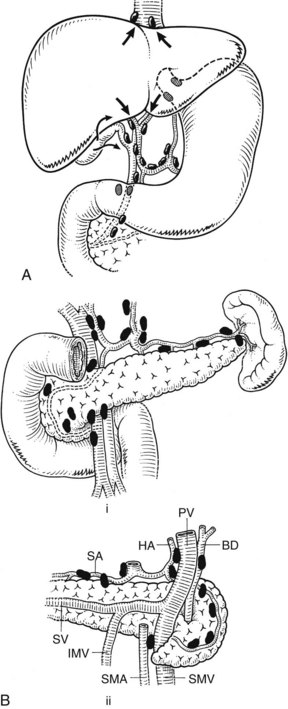
The lymphatic drainage of the liver and gallbladder is mainly to nodes in the hepatoduodenal ligament and along the hepatic artery; this is shown in Fig. 1B.49A. The lymphatic drainage of the pancreas is predominantly to the nodes that lie in juxtaposition to the arteries and veins (see Fig. 1B.50B).
Nerve Supply to the Liver and Pancreas
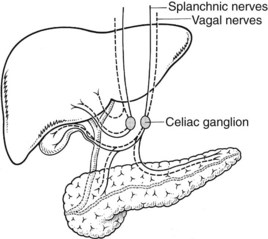
The nerve supply to the liver and pancreas (Fig. 1B.50) is from branches of the celiac ganglion. It is composed of sympathetic and parasympathetic elements.
Albaret P, et al. A propos des caneaux hépatiques directement abouchés dans la voie biliaire accessorie. Ann Chir. 1981;35:88-92.
Bismuth H, et al. Major and minor segmentectomies—“réglées”—in liver surgery. World J Surg. 1982;6:10-24.
Boyden EA. The accessory gallbladder: an embryological and comparative study of aberrant biliary vesicles occurring in man and the domestic mammals. Am J Anat. 1926;38:177-231.
Boyden EA. The anatomy of the choledochoduodenal junction in man. Surg Gynecol Obstet. 1957;104:641-652.
Champetier J, et al. Aberrant biliary ducts (vasa aberrantia): surgical implications. Anat Clin. 1982;4:137-145.
Couinaud C. Le Foi: Études Anatomogiques et Chirurgicales. Paris: Masson; 1957.
Delmont J. Le sphincter d’Oddi: anatomie traditionelle et fonctionnelle. Gastroentrol Clin Biol. 1979;3:157-165.
Eelkema HH, et al. Partial duplication of the gallbladder, diverticulum type: report of a case. Radiology. 1958;70:410-412.
Fields RC, et al. Biliary injury after laparoscopic cholecystectomy in a patient with right liver agenesis: case report and review of the literature. J Gastrointest Surg. 2008;12(9):1577-1581.
Goldsmith NA, Woodburne RT. Surgical anatomy pertaining to liver resection. Surg Gynecol Obstet. 1957;195:310-318.
Gross RE. Congenital abnormalities of the gallbladder: a review of 148 cases with report of a double gallbladder. Arch Surg. 1936;32:131-162.
Healey JE, Schroy PC. Anatomy of the biliary ducts within the human liver: analysis of the prevailing pattern of branchings and the major variations of the biliary ducts. Am Med Assoc Arch Surg. 1953;66:599-616.
Hepp J, Couinaud C. L’abord et l’utilisation du canal hepatique gauche dans les reparations de la voie biliare principale. Presse Med. 1956;64:947-948.
Hjörtsjö CH. The topography of the intrahepatic duct systems. Acta Anat. 1931;11:599-615.
Hobby JAE. Bilobed gallbladder. Br J Surg. 1970;57:870-872.
Kune GA. The influence of structure and function in the surgery of the biliary tract. Ann R Coll Surg Engl. 1970;47:78-91.
McIndoe AH, Counseller VX. A report on the bilaterality of the liver. Arch Surg. 1927;15:589.
Newcombe JF, Henley FA. Left-sided gallbladder: a review of the literature and a report of a case associated with hepatic duct carcinoma. Arch Surg. 1964;88:494-497.
Northover JMA, Terblanche J. A new look at the arterial blood supply of the bile duct in man and its surgical implications. Br J Surg. 1979;66:379-384.
Perelman H. Cystic duct reduplication. JAMA. 1961;175:710-711.
Rachad-Mohassel MA, et al. Duplication de la vésicule biliaire. Arch Fr Mal App Dig. 1973;62:679-683.
Rocko JM, et al. Calot’s triangle revisited. Surg Gynecol Obstet. 1981;153:410-414.
Rogers HI, et al. Congenital absence of the gallbladder with choledocholithiasis: literature review and discussion of mechanisms. Gastroenterology. 1975;48:524-529.
Scheele J, Stangl R. Segment-orientated anatomical liver resections. In: Blumgart, LH, editor. Surgery of the Liver and Biliary Tract. 2nd ed. New York: Churchill Livingstone; 1994:1557-1578.
Strasberg SM, et al. Terminology of liver anatomy and resections (Terminology Committee of the International Hepato-Pancreato-Biliary Association). St Louis: Washington University; 2000.
Ton That Tung. La vascularisation veineuse du foie et ses applications aux resections hepatiques. Hanoi: Thèse; 1939.
Ton That Tung. Les Resections Majeures et Mineures du Foie. Paris: Masson; 1979.
Wood D. Eponyms in biliary tract surgery. Am J Surg. 1979;138:746-754.