Chapter 31 Surgical Airway
I Introduction
A General Principles
Emergency surgical airway management comprises four distinct but related techniques that gain access to the infraglottic airway. These are needle cricothyrotomy, percutaneous cricothyrotomy, surgical cricothyrotomy, and surgical tracheostomy. In emergency situations, cricothyrotomy is greatly preferred over tracheostomy because of its relative simplicity, speed, and lower complication rate. The airway is very superficial at the level of the cricothyroid membrane (CTM), separated from the skin only by the subcutaneous fat and anterior cervical fascia. The trachea moves progressively deeper in the neck as it travels caudally, making anterior access more difficult and introducing additional anatomic barriers (e.g., thyroid isthmus.) Needle cricothyrotomy with percutaneous transtracheal ventilation may provide temporary oxygenation in some patients, but the technique does not provide a secure (protected) airway and cannot support ventilation. Needle cricothyrotomy is reviewed elsewhere in this textbook (see Chapter 30). The emphasis in this chapter is on surgical and percutaneous cricothyrotomy; because the former, and in some cases the latter, places a cuffed endotracheal tube (ETT) in the trachea.
Any discussion of surgical airway management techniques must account for three important concepts:
1 Those Responsible for Surgical Airway Management Have Limited or No Experience
Contemporary emergency department studies using RSI demonstrate high success rates (97% to 99%) and an infrequent need for surgical airway rescue (0.5% to 2.0%) even though the unselected nature of the patients and the large percentage with trauma result in a high proportion of DAs compared with those seen in elective surgery.1–5 Despite increasing familiarity with alternative airway rescue devices (e.g., flexible and semirigid fiberoptic bronchoscopy, video laryngoscopy, retroglottic airways, supraglottic airways, retrograde intubation, lighted stylet) that further reduce the need for cricothyrotomy, the surgical airway remains the final pathway on all failed airway algorithms.6 Therein lies the dilemma. As clinicians embrace new technologies and devices that make the need for surgical airway management increasingly rare, acquisition and maintenance of the skills necessary to perform surgical airway management, which in some cases is the only method capable of sustaining a patient’s life, become increasingly elusive.
B Historical Perspective
The surgical airway as a life-saving procedure has been appreciated for thousands of years. The first depictions of surgical tracheostomy were found on Egyptian tablets dating from 3600 BC.7 In the second century AD, Galen suggested tracheostomy, utilizing a vertical incision, as an emergency treatment for airway obstruction.8–10 Vesalius later published the first detailed descriptions of tracheostomy in the 16th century, using a reed to ventilate the lungs. Ironically, his alleged resuscitation of a Spanish nobleman through tracheostomy and ventilation led to condemnation by the Spanish Inquisition and his ultimate death.11 The first record of a successful tracheostomy performed in the United States was in 1852; the patient later died of airway stenosis, a common complication at that time. A paper from 1886 described a mortality rate of 50% for tracheostomy and a high incidence of stenosis, which accounted for many of the deaths.12
Chevalier Jackson published a landmark paper on tracheostomy in 1909, which enumerated principles still relevant today.13 He described a surgical mortality rate of only 3%, which he attributed to several factors: optimal airway control before surgery, use of local anesthesia rather than sedation, use of a well-designed tube, and meticulous surgical and postoperative care. Jackson achieved international recognition; however, so did his condemnation of “high tracheostomy” as the cause of subglottic stenosis. The high tracheostomy he referred to was a cricothyrotomy, which at that time involved division of the cricoid or thyroid cartilage. Modern cricothyrotomy involves incision of the CTM only. In 1921, Jackson published a study of 200 patients referred to him for postcricothyrotomy stenosis. Aside from the obvious referral bias, the indication for a surgical airway at that time was primarily inflammatory lesions of the upper airway, which probably accounted for the high incidence of subglottic stenosis.14
Although in retrospect it was the technique and the underlying condition that were largely responsible for the high rate of stenosis, fear of this complication condemned the technique of cricothyrotomy for over half a century. In the interest of developing a technique that was safer and quicker than Jackson’s open dissection, Toye and Weinstein described the first percutaneous tracheostomy in 1969.15 However, cricothyrotomy was not widely reconsidered as a surgical airway option until 1976, when Brantigan and Grow published the results of cricothyrotomy for long-term airway management in 655 patients.16 In their series, the rate of stenosis was 0.01%, no major complications were described, and the procedure was found to be faster, simpler, and less likely to cause bleeding than tracheostomy. Subsequent studies have supported their conclusion that cricothyrotomy is a safe and effective surgical airway procedure and should be the preferred technique when emergent surgical airway control is needed.7,17,18 Contemporary case series have demonstrated that cricothyrotomy can be performed with a high success rate and a reasonably low complication rate by hospital-based physicians and other clinicians (i.e., nurses, paramedics) providing prehospital care.13,19–25
C Definitions of the Surgical Airway
The terms surgical cricothyrotomy and surgical tracheostomy refer to the use of a scalpel and other surgical instruments to create an opening in the airway.26,27 This technique allows the creation of a definitive, protected airway by the insertion of a cuffed tracheostomy tube with an internal diameter sufficient for ventilation, oxygenation, and suctioning.
The percutaneous dilational technique utilizes a kit or device that is intended to establish a surgical airway without requiring a formal surgical cricothyrotomy (see Chapter 30 for a detailed discussion). Following a small skin incision, the airway is accessed by a small needle through which a flexible guidewire is passed using the Seldinger technique. The airway device is then introduced over a dilator and passed over the guidewire and into the airway in a manner analogous to that of central line placement. An alternative percutaneous technique has been used that relies on placement of an airway device using a direct puncture into the airway; an example is the Nu-Trake Adult Emergency Cricothyroidotomy Device (Smiths Medical, Keene, NH). A large-bore metal needle or a sharp trocar within the catheter is used to puncture the airway directly, without the use of a guidewire. Direct puncture devices are more hazardous and have fallen out of favor because of a higher incidence of complications and a lower success rate compared with other percutaneous techniques.28–32
Transtracheal catheter ventilation, considered the least invasive surgical technique, involves the direct placement of a moderate-bore catheter through the CTM.33 The small caliber of these devices does not allow adequate oxygenation without attachment to a high-pressure oxygen source or jet ventilator, except in small children, and does not support adequate ventilatory gas exchange. A 6-F reinforced fluorinated ethylene propylene, kink-resistant emergency transtracheal airway catheter (Cook Critical Care, Bloomington, IN) has been designed as a kink-resistant catheter for this purpose.
D Role of the Surgical Airway
The relative merit of percutaneous dilatational tracheostomy versus open surgical tracheostomy continues to be a subject of debate. Although formal open tracheostomies are primarily performed by surgeons, the percutaneous dilatational tracheostomy is frequently performed by anesthesiologists and other nonsurgical intensivists, particularly in the intensive care setting. This technique was originally described by Toye and Weinstein in 1969, but it did not gain popularity until the results with a modified device were reported by Ciaglia and colleagues in 1985.12,15 There have been no clinical studies to date demonstrating the superiority of any one approach over another or of any of these devices over formal surgical cricothyrotomy.
II Anatomy
A Bones and Cartilages
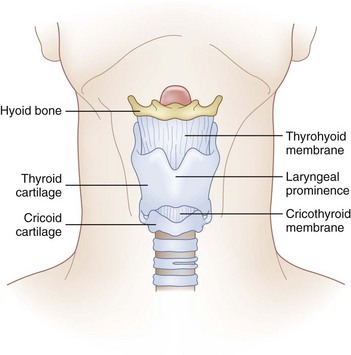
The cricoid cartilage, the only complete cartilaginous ring in the upper airway, defines the inferior aspect of the larynx (Fig. 31-1). It is shaped like a signet ring, with the wider lamina posterior. Superiorly, the lamina has synovial articulations with the arytenoids and thyroid cartilage. Anteriorly, the cricoid ring is attached to the inferior thyroid cartilage by the CTM.
C Vascular Structures
The right and left cricothyroid arteries are branches of the right and left superior thyroid arteries, respectively, and in most patients they cross the superior aspect of the CTM to anastomose in the midline.34 Although they are at risk for injury during a cricothyrotomy, they do not appear to be clinically significant. Bleeding is often self-limited and is easily controlled with gauze packing. There is no venous plexus over the CTM.
E Anatomic Variations
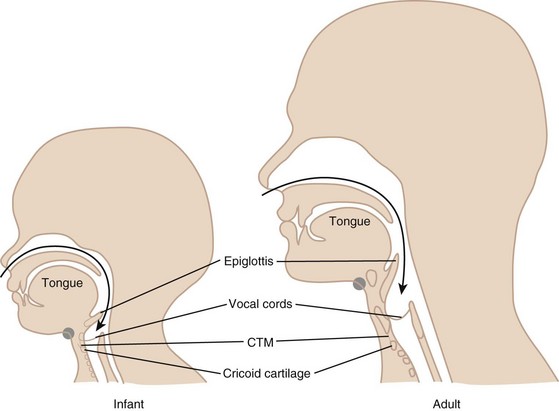
In infants, the hyoid bone and cricoid cartilage are the most prominent structures in the neck. The laryngeal prominence does not develop until adolescence. The larynx also starts higher in the neck of the child; it descends from the level of the second cervical vertebra at birth to the level of the fifth or sixth in the adult.35 The laryngeal prominence is more acute and therefore more prominent in adult males compared with females. This also results in longer vocal cords and accounts for the deeper voices of males.
The CTM varies in size among adults and can be as small as 5 mm in height. This space may narrow further with contraction of the cricothyroid muscle.36 The CTM in the child is disproportionately smaller in area than that in the adult (Fig. 31-2). In an infant, the width of the membrane constitutes only one fourth of the anterior tracheal diameter, as opposed to three fourths in the adult. Because of this smaller area and the difficulty in identifying landmarks in children, emergency surgical cricothyrotomy is difficult and hazardous in small children and is not recommended in those younger than 10 years of age. In this age group, placement of a needle catheter with percutaneous transtracheal ventilation is the preferred method.
III Surgical Cricothyrotomy
A Indications and Contraindictions
The primary indication for an emergent surgical airway (Box 31-1) is the failure of endotracheal intubation or alternative noninvasive airway techniques in a patient who requires immediate airway control. The American Society of Anesthesiologists (ASA) DA algorithm advocates a surgical airway as the final end point for the unsuccessful arm of the emergency pathway.37,38 There are a number of other DA algorithms in the literature, as well as numerous modifications of the ASA guidelines; however, all comprehensive pathways include the surgical airway as the technique of choice when others have failed.38–40 Despite the introduction of numerous alternative rescue devices, the most common error in the management of the DA is persistent attempts at laryngoscopy in a failed airway situation.41–43 This behavior has been associated with increased morbidity and mortality.41 Identification of the CICV scenario should result in immediate consideration of surgical airway access. If alternative methods are tried despite inability to oxygenate and ventilate the patient by bag and mask, precious time may be lost in what are ultimately futile attempts, and by the time cricothyrotomy is undertaken and accomplished, delays in achieving airway control and oxygenation will have led to hypoxic brain injury.
Box 31-1 Indications for Cricothyrotomy
In most circumstances, cricothyrotomy is regarded as an emergency rescue technique when other noninvasive rescue techniques, such as the laryngeal mask airway, have failed, are predicted to fail, or are unavailable.1 There are occasions, however, when a cricothyrotomy is the primary airway of choice. An example is the patient who has such severe facial trauma that nasal or oral approaches to the airway are deemed impossible. There also has been a renewed interest in the role of elective cricothyrotomy in the operative setting. Some cardiothoracic surgeons prefer cricothyrotomy to a tracheostomy in their patients with a median sternotomy, believing that the higher location of the airway wound reduces the potential for contamination of the sternal wound.11 A study described the use of elective cricothyrotomy instead of tracheostomy in the intensive care unit for trauma patients with technically challenging neck anatomy. The procedure was described as simpler with no difference in short- or long-term complications.44
Cricothyrotomy is considered safe in trauma patients with unstable cervical spine injuries provided that cervical spine immobilization is maintained.45,46 Although coagulopathy has been described as a relative contraindication, there are reports of successful cricothyrotomy after systemic fibrinolytic therapy for acute myocardial infarction.47
Contraindications for surgical airway management are few and, with one exception, are relative. That one exception is young age. Children have a small, pliable, mobile larynx and cricoid cartilage, making cricothyrotomy extremely difficult. For children younger than 10 years, unless the larynx and cricoid cartilage are teenage or adult sized, percutaneous transtracheal ventilation should be used as the surgical airway management technique of choice. Two other situations have been proposed as absolute contraindications: tracheal transection and laryngeal fracture.48 In either situation, tracheostomy has been recommended as the preferred method. Although anatomically and philosophically appealing, this assertion is clinically impractical. First, these injuries may not be readily apparent at the bedside. Second, there may be no other means of securing the airway in a dying patient. Third, expert surgical backup may not be readily available, and tracheostomy is a much more complicated procedure than cricothyrotomy. Therefore, these, too, should be considered relative contraindications; cricothyrotomy should be recognized as carrying significant risk in these situations and used when there is thought to be no other method to secure the airway. Relative contraindications also include preexisting laryngeal or tracheal pathology such as tumor, infections, or abscess in the area in which the procedure will be performed; hematoma or other anatomic destruction of the landmarks that would render the procedure difficult or impossible; coagulopathy; and lack of operator expertise.
B Procedure
1 Equipment
Tracheostomy tubes are made in a variety of designs and materials.15 They may have a single lumen, but many have an inner cannula that can easily be removed to allow routine cleaning and prevention of mucous obstruction. Most tubes also come with a blunt-tipped obturator that is used to facilitate insertion and reduce trauma. The tubes may be rigid or flexible and kink resistant, and they may have variable curves, angles, and sizes, depending on the anatomic needs of the patient. Tracheostomy tubes are secured with twill or with a padded fastener that goes around the neck and attaches to the neck plate of the tube. Tubes may be cuffed or uncuffed. Cuffs should be kept at inflation pressures of 20 to 25 mm Hg, but over the short term, inflation can be judged by palpation of the reservoir balloon.49 Underinflation can increase aspiration, whereas high cuff pressures can cause mucosal ischemia and subsequent tracheal stenosis or tissue necrosis.
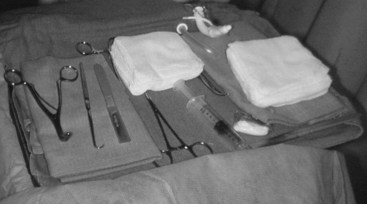
It is critical that whatever surgical technique is chosen, the instrument and its location are familiar to the airway providers. The kit needs to be easily accessible, compact, and ideally located in a DA cart that is positioned in the room or otherwise readily accessible (Fig. 31-3). The operating room tracheostomy surgical trays are not appropriate for this purpose because of their size and complexity. It is recommended that a custom cricothyrotomy kit be assembled with the components listed in Box 31-2 or that a commercial kit designed for this purpose be utilized. A commercial kit has become available that offers both the Seldinger technique and the necessary instruments for a surgical cricothyrotomy with a cuffed tracheostomy tube all in one system (Fig. 31-4).
Box 31-2
Recommended Contents of Cricothyrotomy Kit
D. Cuffed, nonfenestrated No. 4 Shiley tracheostomy tube
E. Several 4 × 4 gauze sponges, 2 small hemostats, surgical drapes
From Walls RM, Luten RC, Murphy MF, Schneider RE: Manual of emergency airway management, ed 2, Philadelphia, 2004, Lippincott Williams & Wilkins.
C Surgical Cricothyrotomy Techniques
1 Traditional Surgical Cricothyrotomy: The “No-Drop” Technique
b Landmark Identification
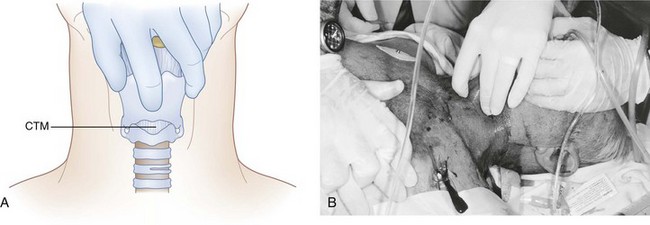
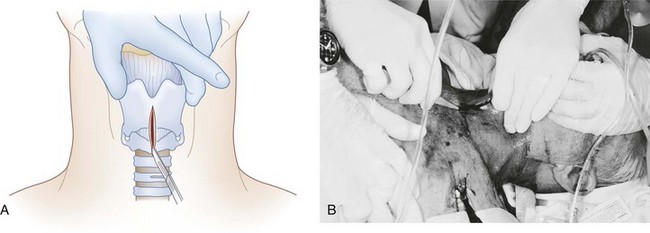
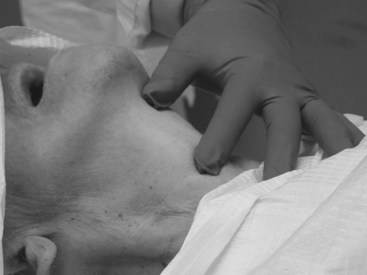
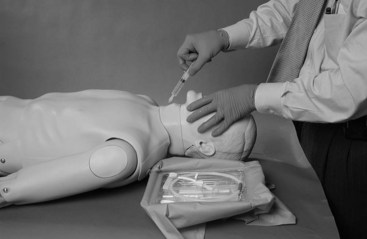
An emergent cricothyrotomy is best thought of as a procedure that is done by touch rather than by visualization. The identification of the external landmarks before initiating the incision and the use of a method to maintain knowledge of the anatomic relationships (see later discussion) are keys to success. The operator should be positioned on the same side of the patient as the operator’s dominant hand (i.e., the hand that will be used to make the incisions); for example, a right-handed operator stands to the patient’s right. After identification of the laryngeal prominence, the superior horns of the thyroid cartilage are firmly grasped between the thumb and long finger of the nondominant hand. This leaves the index finger ideally positioned to localize the CTM and re-identify its location at any time during the procedure by capitalizing on the constancy of the relationship as long as the thyroid cartilage is not released. Therefore, it is critical that control of the larynx with this hand be maintained until the placement of the tracheal hook (Fig. 31-5).
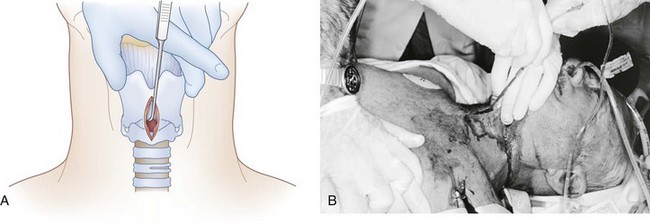
c Vertical Skin Incision
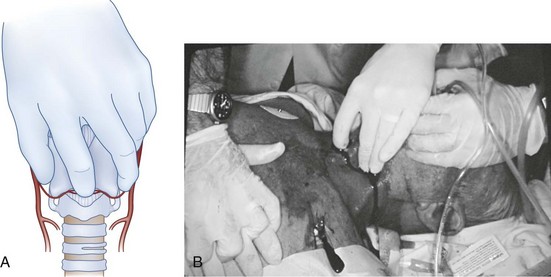
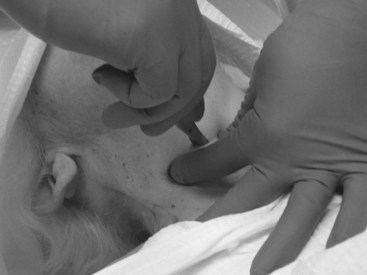
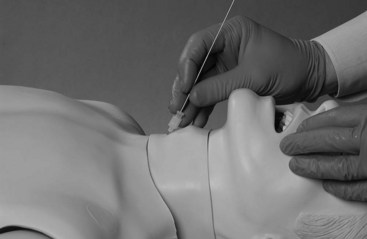
The operator uses the dominant hand to make a generous vertical incision in the midline, with its center over the CTM. The incision should be at least 2 cm in length and may need to be extended in patients with significant obesity or if identification is difficult. The incision should go through subcutaneous tissues down to, but not into, the thyroid and cricoid cartilages (Fig. 31-6).
d Confirmation of Membrane Location
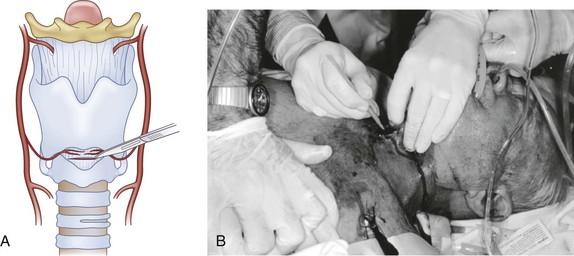
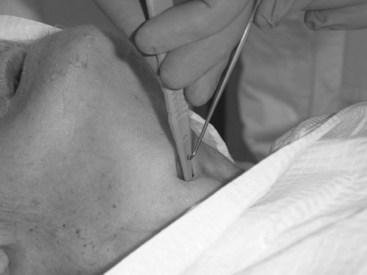
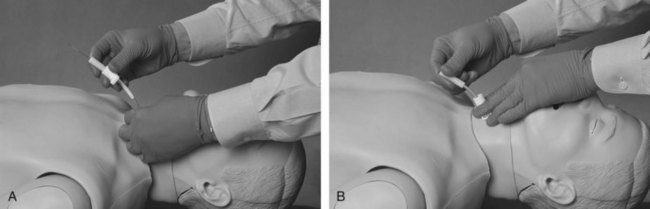
With the thumb and long finger maintaining control of the thyroid cartilage, the index finger may now be inserted into the incision. Without interposed skin and tissues, it is much easier to appreciate the structures of the anterior neck and to confirm the location of the CTM. The index finger can be left in the wound, resting on the inferior aspect of the anterior larynx, indicating the superior limit of the CTM (Fig. 31-7).
e Horizontal Incision of the Cricothyroid Membrane
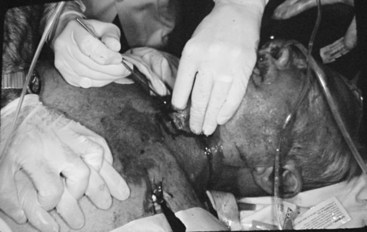
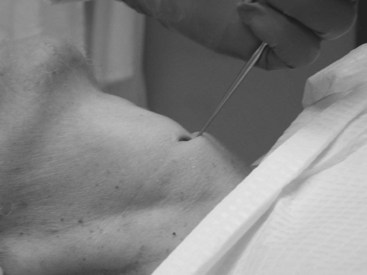
The index finger may be withdrawn just prior to incision or left in the wound to serve as a guide. The CTM should be incised horizontally for a distance of 1 to 2 cm (Fig. 31-8). It is recommended that an attempt be made to incise the lower half of the membrane to avoid the superiorly placed cricothyroid artery and vein. This can be difficult to achieve, and inadvertent injury to these vessels is rarely clinically significant. Despite the exposure obtained with the skin incision, bleeding from the skin and various vascular structures eliminates a clear view of the field in most cases. Time does not permit the operator to attempt to deal with bleeding so as to achieve a bloodless field. Maintenance of the anatomic relationships outlined previously allows the procedure to be completed expeditiously without the need for direct visualization of the structures of interest. If significant bleeding occurs, it can be dealt with (usually by simple wound packing) after the airway has been secured.
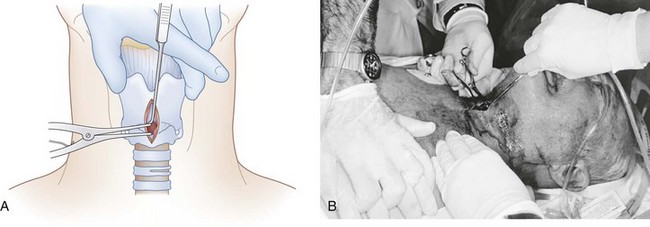
f Insertion of the Tracheal Hook
Laryngeal control should be maintained with the thumb and long finger of the nondominant hand. The hook is held in the dominant hand, turned so that it is oriented transversely, and then inserted into the trachea through the surgical opening (Fig. 31-9). The index finger of the nondominant hand can serve as a guide to help place the hook into the incision. Once through the CTM, the device hook is rotated so that the hook is oriented in the cephalad direction. The hook is firmly applied to the inferior border of the thyroid cartilage, and gentle upward and anterior traction with the hook handle at a 45-degree angle to the anterior neck skin can be used to bring the airway up to the skin (Fig. 31-10). The hook is then passed to an assistant to maintain immobilization and control of the larynx. The assistant will not release the hook under any circumstance until a definitive airway has been successfully inserted and placement is confirmed; thus the term “no drop technique.” The operator’s nondominant hand may then be released for completion of the procedure, with the proviso that the tracheal hook is maintaining control and should not be removed until confirmation of tube placement has been obtained.
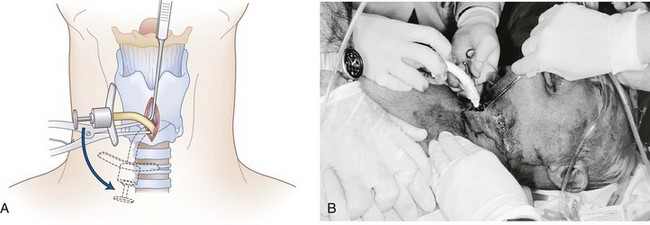
g Dilation of the Opening with Dilator
The Trousseau dilator is then inserted using the dominant hand. It is our preference to insert the dilator so that the prongs are oriented to dilate the opening in the rostral-caudal direction rather than transversely (Fig. 31-11). Although the dilator appears to be designed to insert directly into the airway and dilate transversely, it is the rostral-caudal dimension of the CTM that provides the most resistance to cannulation, so it is desirable to dilate in this plane. The Trousseau dilator is inserted only a couple of millimeters into the airway and then opened to dilate the airway. With the airway thus opened, the dilator, in situ, is transferred from the operator’s dominant hand to the nondominant hand, where it can be held from underneath (see Fig. 31-11). This frees the dominant hand to insert the tracheostomy tube.
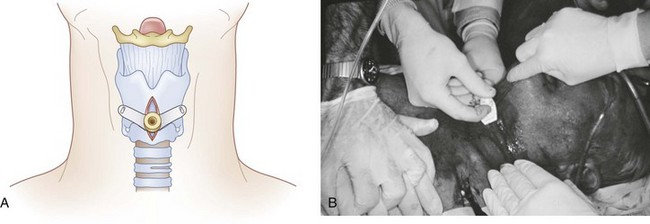
h Insertion of the Tracheostomy Tube
The tracheostomy tube is then inserted with the obturator in place (Fig. 31-12). To avoid unnecessary delays at this juncture, the tube should have been removed from its packaging before initiation of the procedure and the blunt-tipped obturator should already have been inserted. The tube is oriented along the handle of the dilator (i.e., at a 90-degree angle to the patient) and inserted between the dilator blades, following their natural curve. As the tracheostomy tube gains the airway and is passing between the prongs of the Trousseau dilator, it is helpful to rotate the dilator handle counterclockwise 90 degrees (see Fig. 31-12) so that the dilation occurs in the transverse rather than the rostral-caudal dimension. The reason is that when the tracheostomy tube has gained access into the airway, the prongs of the Trousseau dilator may themselves inhibit successful passage of the tip of the tracheostomy tube down the trachea. Through rotation of the Trousseau dilator, the prongs are moved out of the way to either side of the tube, but dilation is continued to assist in placing the tracheostomy tube until it is firmly seated against the anterior neck. The dilator is gently removed as the tube is being advanced, just before it is seated in its final position (Fig. 31-13).
2 Rapid Four-Step Technique
The rapid four-step technique (RFST) is an attempt to simplify the cricothyrotomy procedure by using a horizontal stab incision through the skin and membrane simultaneously, followed by tracheal hook traction applied caudad at the cricoid ring.16 Cadaveric studies have shown RFST to be simple to learn and faster in obtaining a surgical airway than the open technique just described.50–52 Other advantages include the following: (1) less equipment is necessary (it is performed with a scalpel, hook, and tracheostomy tube); (2) it may be performed independently; and (3) the operator is positioned at the head of the bed, similar to the stance for performance of orotracheal intubation.
Acute complications from RFST may be more common than with the traditional “no-drop” method. The stab technique may increase the incidence of trauma to the posterior trachea and anterior aspect of the esophagus. In cadaveric models, an increase in damage to the cricoid ring due to direct traction on the ring with the tracheal hook was found.1,50–52 This may be remedied through the use of a double-hook device that disperses the forces across the cricoid ring.1,51
RFST may not be as desirable in patients in whom landmark identification is difficult. In this circumstance, we recommend beginning with a vertical incision, similar to the traditional technique described previously.16 There are no clinical studies that report the success rates and associated acute and delayed complications of RFST compared with traditional methods in live patients. The choice between the RFST and the traditional technique is an individual one, because there is no clear evidence that either method is superior or more likely to bring success than the other.
a Landmark Identification
As in traditional cricothyrotomy, the airway is accessed through the CTM. Therefore, the identification of landmarks is exactly the same as described previously (Fig. 31-14; see Fig. 31-5). Because of the horizontal stab incision, however, it is even more critical to be confident of the location of the membrane. If there is uncertainty, it is recommended to begin with a vertical incision first, as described earlier.
b Horizontal Stab Incision
A single horizontal stab incision using a no. 20 scalpel is made directly through the skin, subcutaneous tissues, and CTM. The incision should be approximately 1.5 cm wide, and because of the size of the no. 20 blade, widening of the opening is rarely required (Fig. 31-15). If the anatomy is not readily palpable through the skin, an initial vertical incision should be created to allow subsequent palpation of the anatomy and identification of the CTM. Once the CTM has been incised, the no. 20 blade is maintained in the airway until the tracheal hook is secured.
c Stabilization of the Larynx with the Tracheal Hook
A tracheal hook is placed parallel to the scalpel on the caudal side of the blade (Fig. 31-16). The hook is rotated to secure and control the cricoid ring, and the scalpel is then removed from the airway. The tracheal hook is used to apply gentle traction on the cricoid ring to lift the airway up toward the surface of the skin and to provide modest stoma dilation (Fig. 31-17). The direction of force on the hook is reminiscent of the “up and away” direction employed with laryngoscopy. The amount of traction force required for easy intubation (18 newtons) is significantly lower than the force that is associated with breakage of the cricoid ring (54 newtons); however, the chance of trauma may be further reduced by using a double-tined hook.1 Use of the hook in this direction usually provides sufficient widening of the incision to obviate the need for further dilation (i.e., Trousseau dilator). Placement of the hook on the cricoid ring may also reduce the possibility of intubating the pretracheal potential space, which is essentially eliminated due to the apposition of the airway and the subcutaneous fat by the traction on the hook.
d Insertion of the Tracheostomy Tube
With adequate control of the airway using the hook placed on the cricoid ring, a tracheostomy tube is gently inserted through the cricothyroid space into the trachea (Fig. 31-18). The cuff is then inflated, the tube is secured, and its location is confirmed by the same methods described earlier.
3 Percutaneous Cricothyrotomy Techniques
Numerous commercial cricothyrotomy devices are available, many of which use a modified Seldinger technique to assist in the placement of a tracheal airway (see Fig. 31-4). There are aspects of this technique that may make it appealing to the anesthesiologist. This method is similar to the one commonly used for placement of central venous catheters and offers some familiarity to the operator who is uncomfortable or inexperienced with the surgical cricothyrotomy technique described earlier. This technique can be learned quickly. By the fifth practice attempt on a mannequin model, 96% of anesthesiologists achieved success within 40 seconds.6 However, the technique still requires knowledge of the anatomy and the ability to localize the membrane and has several steps, so it approaches the open technique in complexity. When compared with the standard open technique in cadavers and dog models, there were no differences in performance times or complications.53–55 It is estimated that the procedure may be accomplished in 40 to 100 seconds.6,27,53–55 Also, the percutaneous cricothyrotomy kits are preassembled and commercially available, whereas at present the surgical tools used in the traditional approach are not (except in the combination kit shown in Fig. 31-4). It seems intuitive that the percutaneous technique should result in less bleeding, but no studies have been performed to assess the significance of any difference. One of the limitations is the relatively smaller lumen, which is of no real concern in an emergency, and in some models the absence of a cuff, which could be an issue if airway protection from emesis or hemorrhage is needed. It is suggested that a device with a cuff be selected, because many of these procedures are performed on patients who have not been fasted or are actively hemorrhaging and require further airway protection. The Melker Cuffed Emergency Cricothyrotomy Catheter Set and the Melker Universal Cricothyrotomy Set are available with a cuffed airway catheter (Cook Critical Care).
b Insertion of Locator Needle
The introducer needle is then inserted through the skin and the CTM in a slightly caudal direction. The needle is attached to a syringe and advanced with the dominant hand while negative pressure is maintained on the syringe. The sudden aspiration of air indicates placement of the needle into the tracheal lumen (Fig. 31-19).
c Insertion of the Guidewire
The syringe is then removed from the needle, and a soft-tipped guidewire is inserted through the needle into the trachea in a caudal direction. The needle is then removed, leaving the wire in place. As with most Seldinger techniques, control of the wire must be maintained at all times (Fig. 31-20).
e Insertion of the Airway
The airway catheter provided in the kit (3 to 6 mm internal diameter), with an introducing dilator in place, is then inserted over the wire into the trachea. If resistance is met, the skin incision should be deepened and a gentle twisting motion applied to the airway device (Fig. 31-21). After the airway device is firmly seated against the skin, the wire and obturator are removed together, leaving the tracheostomy tube in place. Tube location should then be confirmed, as described previously, and secured properly. The devices are radiopaque.
IV Training Issues
A survey published in 1995 found that although 80% of anesthesiology programs taught cricothyrotomy as part of their curriculum, most of them did so through lectures only, with no practical experience.21 In a survey published in 2003, only 21% of anesthesiologists claimed skills to perform cricothyrotomy.56 Most anesthesiology graduates have never performed a cricothyrotomy during their training.6
The advent of emergency medicine as a specialty with its own airway expertise has resulted in a significant decrease in exposure to emergency airways for anesthesiology trainees. The success of RSI in the emergency setting, as well as advances in airway management techniques in anesthesiology, have prompted editorials concerned with the problem of gaining and maintaining competence in invasive airway management.57–59 Studies suggest a current cricothyrotomy rate of approximately 1% of all emergency airways and a dramatically lower rate in the operating suite. Regardless of the setting, this incidence is too low to ensure adequate training, but it highlights the probability that most airway managers will be called on to perform an invasive airway at some point in their career.
The practice required to obtain familiarity with the equipment and technique must take place outside the clinical setting. Of anesthesiology programs that instruct their residents on cricothyrotomy, 60% use lectures only, which is a poor teaching technique for developing proficiency in manual skills.21 One study using a mannequin model determined that five cricothyrotomies were necessary to reach a steady performance state in which the procedure could be completed in 40 seconds.25
No studies have identified the optimal interval between training episodes for retention, although one small report suggested increased retention when training was repeated monthly versus every 3 months.38 Studies in cadavers performed primarily to compare different surgical airway techniques incidentally identified a similar rapid learning curve.60,61 There are no studies examining the clinical correlation of these training techniques, but the high success rate of emergency cricothyrotomy suggests that retention and competence have occurred.
V Choosing the Right Technique
Acknowledging this limitation, several generalizations can be made:
• Dilational techniques that rely on direct puncture into the airway are associated with an unacceptably high rate of complications, including injury to airway structures and catheter misplacement.28–31 These techniques cannot be recommended unless other options are unavailable.
• The success rates of open surgical and percutaneous guidewire (Seldinger) cricothyrotomy are roughly comparable.62
• The complications associated with open surgical and Seldinger cricothyrotomy are distinctly different. When complications arise with open surgical cricothyrotomy, bleeding or injury to airway structures is the predominant problem. With Seldinger cricothyrotomy, the most common complication is misplacement of the airway into the pretracheal or paratracheal spaces.62
VI Complications
With these limitations in mind, the complication rate after surgical cricothyrotomy is highly variable, ranging from 14% to 50% depending on the technique, clinical setting, definition of complications, and experience of the operator.2,9,18,52,63–67
VIII Clinical Pearls
• New technology and improved skill in performing direct laryngoscopy have reduced the need for emergent surgical cricothyrotomy, and this has implications for skill retention among physicians managing airways in the emergency setting.
• Surgical cricothyrotomy is indicated in the “cannot intubate, cannot ventilate” (CICV) situation, in which direct laryngoscopy and rescue techniques have failed.
• Surgical cricothyrotomy can be performed via a percutaneous Seldinger technique or an open technique, and there is few data on which method is optimal.
• The presence or absence of adequate anatomic landmarks can help guide the clinician with technique selection. If the cricothyroid membrane is easily identifiable, either method can be used. If landmarks are difficult to identify (e.g., in obese patients), the open technique is more likely to be successful.
• Needle cricothyrotomy is preferred in children younger than 10 years of age because of the unique anatomic differences (i.e., small cricothyroid membrane) in this patient population.
All references can be found online at expertconsult.com.
1 Bair AE, Filbin MR, Kulkarni RG, et al. The failed intubation attempt in the emergency department: Analysis of prevalence, rescue techniques, and personnel. J Emerg Med. 2002;23:131–140.
35 Davis DP, Bramwell KJ, Hamilton RS, et al. Safety and efficacy of the rapid four-step technique for cricothyrotomy using a Bair Claw. J Emerg Med. 2000;19:125–129.
52 Holmes JF, Panacek EA, Sackles JC, Brofeldt BT. Comparison of 2 cricothyrotomy techniques: Standard method versus rapid 4-step technique. Ann Emerg Med. 1998;32:442–447.
57 Chang RS, Hamilton RJ, Carter WA. Declining rate of cricothyrotomy in trauma patients with an emergency medicine residency: Implications for skills training. Acad Emerg Med. 1998;5:247–251.
61 Eisenberger P, Laczika K, List M, et al. Comparison of conventional surgical versus Seldinger technique emergency cricothyrotomy performed by inexperienced clinicians. Anesthesiology. 2000;92:687–690.
1 Bair AE, Filbin MR, Kulkarni RG, et al. The failed intubation attempt in the emergency department: Analysis of prevalence, rescue techniques, and personnel. J Emerg Med. 2002;23:131–140.
2 Francois B, Clavel M, Desachy A, et al. Complications of tracheostomy performed in the ICU: Subthyroid tracheostomy versus surgical cricothyroidotomy. Chest. 2003;123:151–158.
3 Rosenblatt WH, Wagner PJ, Ovassapian A, et al. Practice patterns in managing the difficult airway by anesthesiologists in the United States. Anesth Analg. 1998;87:153–157.
4 Salvino CK, Dries D, Gamelli R, et al. Emergency cricothyrotomy in trauma victims. J Trauma. 1993;34:503–505.
5 Tayal VS, Riggs RW, Marx JA, et al. Rapid-sequence intubation at an emergency medicine residency: Success rates and adverse events during a two-year period. Acad Emerg Med. 1999;6:31–37.
6 Walls RM, Gibbs MA. Surgical airway. In: Katz RL, Patel RV. Seminars in anesthesia, perioperative medicine and pain: The difficult airway. Philadelphia: Saunders, 2000.
7 Mace SE. Cricothyrotomy. J Emerg Med. 1988;6:309–319.
8 Boyle MF, Hatton D, Sheets C. Surgical cricothyrotomy performed by air ambulance flight nurses: A 5-year experience. J Emerg Med. 1993;11:41–45.
9 Bradby M. History of tracheostomy. Nurs Times. 1966;62:1548–1550.
10 Mace SE. Blunt laryngotracheal trauma: The recognition and management of acute laryngeal fracture. Ann Emerg Med. 1986;15:836–839.
11 Morain WD. Cricothyroidostomy in head and neck surgery. Plast Reconstr Surg. 1980;65:424–428.
12 Ciaglia P, Firsching R, Syneic C. Elective percutaneous dilatational tracheostomy: A new simple bedside procedure—Preliminary report. Chest. 1985;87:715–719.
13 Jackson C. Tracheotomy. Laryngoscope. 1909;18:285.
14 Jackson C. High tracheostomy and other errors: The chief causes of chronic laryngeal stenosis. Surg Gynecol Obstet. 1921;32:392.
15 Toye FJ, Weinstein JD. A percutaneous tracheostomy device. Surgery. 1969;65:384–389.
16 Brantigan CO, Grow JB. Cricothyrotomy: Elective use in respiratory problems requiring tracheostomy. J Thorac Cardiovasc Surg. 1976;71:72–81.
17 Benumof JL. Transtracheal jet ventilation via percutaneous catheter and high-pressure source. In: Benumof JL, ed. Airway management: Principles and practice. St. Louis: Mosby; 1996:455–474.
18 Gillespie MB, Eisele DW. Outcomes of emergency surgical airway procedures in a hospital-wide setting. Laryngoscope. 1999;109:1766–1769.
19 Boyd AD, Romita MC, Conlan AA. A clinical evaluation of cricothyrotomy. Surg Gynecol Obstet. 1979;149:365–368.
20 Gerling MC, Davis DP, Hamilton RS, et al. Effect of surgical cricothyrotomy on the unstable cervical spine in a cadaver model of intubation. J Emerg Med. 2001;20:1–5.
21 Koppel J, Reed A. Formal instruction in difficult airway management. Anesthesiology. 1995;83:1343–1346.
22 Murphy MF, Walls RM. The difficult and failed airway. In: Walls RM, ed. Manual of emergency airway management. Philadelphia: Lippincott Williams & Wilkins; 2000:31–39.
23 Pierce WS, Tyers GFO, Walhausen JA. Effective isolation of a tracheostomy from a sternal wound. J Thorac Cardiovasc Surg. 1973;96:178–193.
24 Sakles JC, Laurin EG, Ranatapaa AA, et al. Airway management in the emergency department: A one year study of 610 tracheal intubations. Ann Emerg Med. 1998;31:398–400.
25 Wong DT, Prabhu AJ, Coloma M, et al. What is the minimum training required for successful cricothyrotomy? Anesthesiology. 2003;98:349–353.
26 Mace SE. Cricothyrotomy and translaryngeal jet ventilation. In: Roberts JR, Hedges JR. Clinical procedures in emergency medicine. ed 3. Philadelphia: WB Saunders; 1998:57–74.
27 Walls RM, Pollack CV. Successful cricothyrotomy after thrombolytic therapy for acute myocardial infarction: A report of two cases. Ann Emerg Med. 2000;35:188–191.
28 Abbrecht PH, Kyle RR, Reams WH, Brunette J. Insertion forces and risk of complications during cricothyroid cannulation. J Emerg Med. 1992;10:417–426.
29 Benkhadra M, Lenfant F, Nemetz W, et al. A comparison of two emergency cricothyoidotomy kits in human cadavers. Anesth Analg. 2008;106:182–185.
30 Mariappa V, Stachowski E, Balik M, et al. Cricothyroidotomy: Comparison of three different techniques on a porcine model. Anaesth Intensive Care. 2009;37:961–967.
31 Metterlein T, Frommer M, Ginzkey C, et al. A randomized trial comparing two cuffed emergency cricothyrotomy devices using a wire-guided and a catheter-over-needle technique. J Emerg Med. 2011;41:326–332.
32 Practice guidelines for the management of the difficult airway: A report by the American Society for Anesthesiologists Task Force on the Management of the Difficult Airway. Anesthesiology. 1993;78:597–602.
33 Benumof JL. Management of the difficult adult airway with special emphasis on awake tracheal intubation. Anesthesiology. 1991;75:1087–1110.
34 Leibovici D, Fredman B, Gofrit ON, et al. Prehospital cricothyrotomy by physicians. Am J Emerg Med. 1997;15:91–93.
35 Davis DP, Bramwell KJ, Hamilton RS, et al. Safety and efficacy of the rapid four-step technique for cricothyrotomy using a Bair Claw. J Emerg Med. 2000;19:125–129.
36 Jacobson LE, Gomez GA, Sobieray RJ, et al. Surgical cricothyrotomy in trauma patients: Analysis of its use by paramedics in the field. J Trauma. 1996;41:15–20.
37 ASA Task Force on the Management of the Difficult Airway. Practice guidelines for the management of the difficult airway. Anesthesiology. 2003;98:1269–1277.
38 Prabhu AJ, Correa R, Wong DT, et al. What is the optimal training interval for a cricothyrotomy? Can J Anaesth. 2001;48:A59.
39 Bair AE, Panacek EA, Wisner DH, et al. Cricothyrotomy: A 5-year experience at one institution. J Emerg Med. 2003;24:151–156.
40 Morch ET. History of mechanical ventilation. In: Kirby RR, Banner MJ, Downs JB. Clinical applications of ventilatory support. New York: Churchill Livingstone; 1990:1–61.
41 Rehm CG, Wanek SM, Gagnon EB, et al. Cricothyroidotomy for elective airway management in critically ill trauma patients with technically challenging neck anatomy. Crit Care. 2002;6:531–535.
42 Rose DK, Cohen MM. Has the practice of airway management changed? Can J Anaesth. 1997;44:A51B.
43 Rose DK, Cohen MM. The airway: Problems and predictions in 18,500 patients. Can J Anaesth. 1994;41:372–383.
44 Ravlo O, Bach V, Lybecker H, et al. A comparison between two emergency cricothyroidotomy instruments. Acta Anaesthesiol Scand. 1987;31:317–319.
45 American College of Surgeons Committee on Trauma. Advanced trauma life support providers’ manual. Chicago, IL: ACS; 2003.
46 Gerich TG, Schmidt U, Hubrich V, et al. Prehospital airway management in the acutely injured patient: The role of surgical cricothyrotomy revisited. J Trauma. 1988;45:312–314.
47 Vissers RJ. Tracheostomy issues. In: Meldon S, ed. Geriatric emergency medicine. New York: McGraw-Hill; 2004:485–489.
48 Little CM, Parker MG, Tarnopolsky R. The incidence of vasculature at risk during cricothyroidostomy. Ann Emerg Med. 1986;15:805–807.
49 Greisz H, Qvarnstorm O, Willen R. Elective cricothyrotomy: A clinical and histopathological study. Crit Care Med. 1982;10:387–389.
50 Colles CJ. On stenosis of the trachea after tracheostomy for croup, diphtheria. Ann Surg. 1886;3:499.
51 Heffner JE, Hess D. Tracheostomy management in the chronically ventilated patient. Clin Chest Med. 2001;22:55–69.
52 Holmes JF, Panacek EA, Sackles JC, Brofeldt BT. Comparison of 2 cricothyrotomy techniques: Standard method versus rapid 4-step technique. Ann Emerg Med. 1998;32:442–447.
53 Bainton CR. Cricothyrotomy. Int Anesthesiol Clin. 1994;32:95–108.
54 Brofeldt BT, Panacek EA, Richards JR. An easy cricothyrotomy approach: The rapid four-step technique. Acad Emerg Med. 1996;3:1060–1063.
55 Dickinson AE. The normal and abnormal pediatric upper airway: Recognition and management of obstruction. Clin Chest Med. 1987;8:583–596.
56 Ezri T, Szmuk P, Warters RD, et al. Difficult airway management practice patterns among anesthesiologists practicing in the United States: Have we made any progress? J Clin Anesth. 2003;15:418–422.
57 Chang RS, Hamilton RJ, Carter WA. Declining rate of cricothyrotomy in trauma patients with an emergency medicine residency: Implications for skills training. Acad Emerg Med. 1998;5:247–251.
58 Knopp RK. Practicing cricothyrotomy on the newly dead. Ann Emerg Med. 1995;25:694–695.
59 Knopp RK, Waeckerle JF, Callaham ML. Rapid sequence intubation revisited. Ann Emerg Med. 1998;31:398–400.
60 Chan TC, Vilke GM, Bramwell KJ, et al. Comparison of wire-guided cricothyrotomy versus standard surgical cricothyrotomy technique. J Emerg Med. 1999;17:957–962.
61 Eisenberger P, Laczika K, List M, et al. Comparison of conventional surgical versus Seldinger technique emergency cricothyrotomy performed by inexperienced clinicians. Anesthesiology. 2000;92:687–690.
62 Schaumann N, Lorenz V, Schellongowski P, et al. Evaluation of Seldinger technique emergency cricothyroidotomy versus standard surgical cricothyroidotomy in 200 cadavers. Anesthesiology. 2005;102:7–11.
63 Bair AE, Laurin EG, Karchin A, et al. Cricoid ring integrity: Implications for cricothyrotomy. Ann Emerg Med. 2003;41:331–337.
64 Isaacs JHJr. Emergency cricothyrotomy: Long-term results. Am Surg. 2001;67:349–350.
65 Kirchner JA. Cricothyroidotomy and subglottic stenosis. Plast Reconstr Surg. 1981;68:828–829.
66 Nugent WL, Rhee KJ, Wisner DH, et al. Can nurses perform surgical cricothyrotomy with acceptable success and complication rates? Ann Emerg Med. 1991;20:367–370.
67 Xeropotamos NS, Coats TJ, Wilson AW. Prehospital surgical airway management: 1 year’s experience from the Helicopter Emergency Medical Service. Injury. 1993;24:222–224.