Chapter 29 Surgery of Extramedullary Tumors
INTRODUCTION
Some authors have suggested the classification of the spinal cord tumor based on the foraminal extension.1
SCHWANNOMA WITH FORAMINAL EXTENSION (CAUDA EQUINA LEVEL)
Although most tumors of the thoracic and lumbar spine are readily accessible via standard spinal exposures, there is a group of tumors that cannot be removed with a simple posterior approach. They are large dumbbell tumors with significant intraspinal and paraspinal involvement (Fig. 29-1), paraspinal tumors located in the upper thoracic region (T1–3) or imbedded within the psoas muscle adjacent to the lumbar spine, and extensive unilateral anterior and posterior paraspinal tumors with significant spinal canal and vertebral column involvement.2 Posterior midline approaches provide bilateral exposure of the entire posterior elements (lamina and facets, the dorsal epidural space, the entire intradural space, and the posterior paraspinal region) and lateral spinal elements (pedicle and lateral epidural space); however, access to the anterior element is limited. For tumors with a large extraforaminal portion, the posterolateral (e.g., lateral extracavitary approach) route should be added.
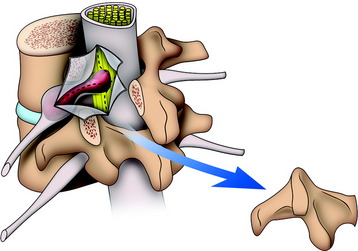
At the cauda equina level, the posterior element is totally removed for exposure of the dural sac and foraminal nerve root. Disarticulation is performed above and below the facet joint. The unilateral complex of superior articular process, transverse process, and inferior articular process is removed from the vertebral body at the point of the pedicle. A part of the spinal canal and unilateral foramen is opened. An underlying mass is seen to originate from the nerve root. The tumor mass–laden nerve root is dissected from the underlying vertebral body. A cottonoid is put under the nerve root. A dural incision is made on the lateral margin of the spinal cord and extended over the tumor mass (Fig. 29-2). The intradural mass is dissected from the surrounding cauda equina, and the origin nerve is cut at the surface of the tumor mass. The distal end of the tumor mass is cut intracapsularly or extracapsularly.
Case I
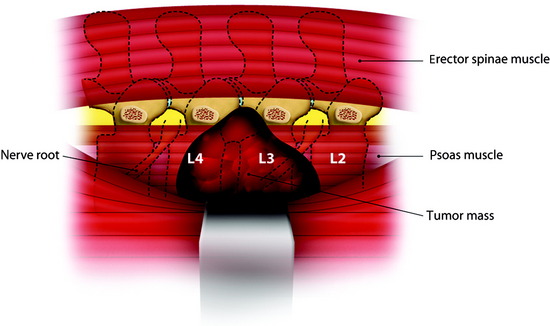
Large Paraspinal Tumor at L3 Level
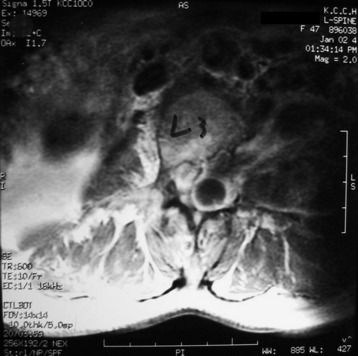
A patient presents with a schwannoma originating from the L3 nerve root that formed a large extraforaminal paraspinal mass at the paraspinal area (Fig. 29-3). The extraforaminal mass has enlarged to expand the left psoas muscle (Fig. 29-4). For the removal of the large extraforaminal portion, extensive paraspinal exposure is required. The lateral extracavitary approach is useful in this situation. This single-staged approach provides excellent exposure of intradural structures as well as extensive access to the anterior and posterior paraspinal region, ventral spinal canal, and vertebral body (Fig. 29-5).
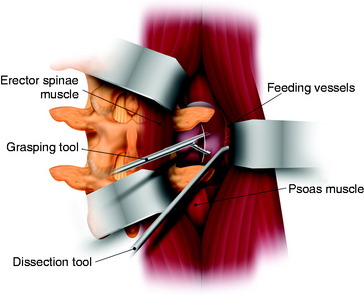
For one-stage removal of the tumor mass, the approach should be performed from both the midline and paramedian route. A hockey-stick incision with the midline long limb centered over the abnormality and the short limb gently curving 8–10 cm toward the tumor side is made. With the midline approach, the intraspinal mass is removed. Once the midline soft tissue dissection is complete, the lateral limb of the skin incision is opened. The thoracodorsal fascia is incised. The lateral margin of the paraspinal muscle is identified and medially elevated to expose the quadratus lumborum and psoas muscles. Detachment of the lateral margin of the paraspinal muscle insertion from the iliac crest and resection of a posterosuperior iliac crest segment facilitate ventral exposure at lower lumbar (i.e., below L4) levels. The paraspinal muscle dissection continues over the facet joints to join the midline dissection. This allows the surgeon to simultaneously or alternatively work on either side of the now completely mobilized paraspinal muscle mass. The L3 and L4 transverse process should be resected to expose the cephalocaudal extent of the tumor mass. The intertransverse ligament and psoas muscle are released from the transverse process to the lateral pedicle margin, at which time the posterior tumor capsule is seen. After the tumor capsule incision is complete, internal debulking is attempted. After the interior of the tumor mass is decompressed, the capsule shrinks. As the tumor capsule is tractioned, the interface between the tumor capsule and psoas muscle is dissected (Fig. 29-6). During dissection, feeding vessels are encountered and should be ligated and cut down. Internal debulking and capsule dissection are repeated. The L2 and L4 nerve roots can hinder ventral exposure because they cross the surgical field and, unlike thoracic nerves, cannot be sacrificed and dorsally displaced. Dissection of lumbar nerves over several centimeters allows for adequate nerve mobilization and prevents a postoperative stretch palsy. Proximal dissection of the segmental nerves identifies the foramen. Vessel loop retraction of these nerves may improve ventral visualization. Depression of the psoas muscle after lumbar nerve dissection provides anterior lumbar paraspinal exposure. Most paraspinal or dumbbell tumors are closely applied to the spine. The psoas muscle is bluntly dissected ventrally off the lateral vertebral body with Cobb elevators. Sharp dissection may be required at the disc space. Dorsal segmental and foraminal branches from the lumbar vessels are cauterized and divided. The margins of the pedicle are sharply defined with curettes. Posterior element (facet joint, pedicle, and transverse process) removal exposes the lateral dural margin, which facilitates dissection and improves ventral canal visualization. Mobilization of the segmental nerves continues to the lateral dural margin. If a two-stage operation is planned, the extraforaminal paraspinal mass is removed with a retroperitoneal anterolateral approach.
Advantages of the Lateral Extracavitary Approach in Spinal Cord Tumor Surgery
Case II
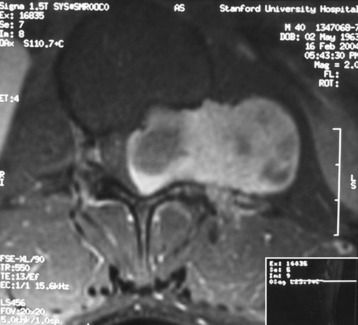
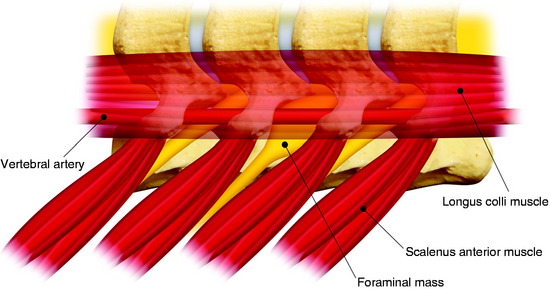
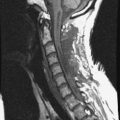
On magnetic resonance imaging (MRI), a well-enhancing intraspinal and extraspinal mass was detected at the L1 level (Figs. 29-7 and 29-8). The operation was performed with a pure posterior approach. The overall appearance of the tumor mass after removal of the laminofacetal complex is shown in Figure 29-9. The intradural mass was removed first, and the foraminal mass was removed later. The intradural portion of the tumor can be safely removed with standard microsurgical techniques via laminectomy with direct visualization of the tumor-spinal cord interface and secure identification of the nerve root attachment. A unilateral facetectomy and a T-shaped lateral dural incision over the root sleeve provide contiguous exposure of the foraminal portion of the tumor. The origin rootlet (dorsal root) was cut and the ventral root was saved (Fig. 29-10). Although the motor root can be preserved with intradural dorsal nerve sheath tumors, sacrifice of the entire spinal nerve is usually required once the tumor extends distal to the dorsal root ganglia. Even though the entire spinal nerve is transected, significant motor deficit is rare.
Histological variants of the dumbbell tumor follow:
CERVICAL SCHWANNOMA WITH FORAMINAL EXTENSION
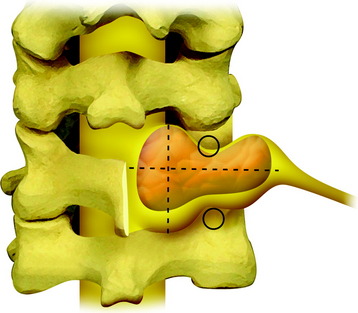
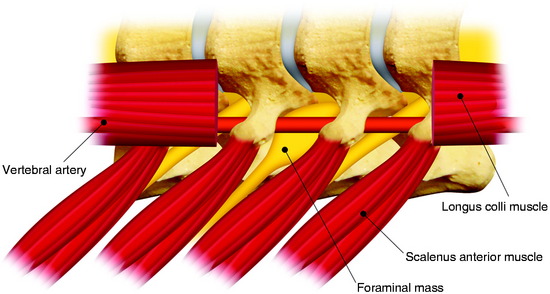
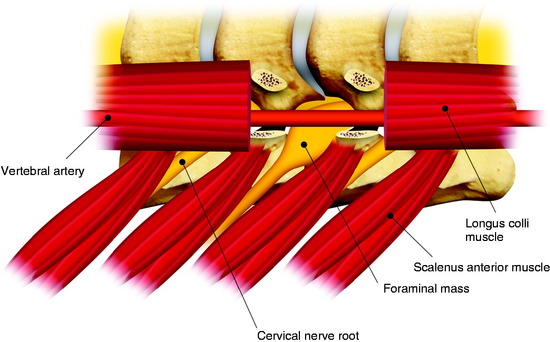
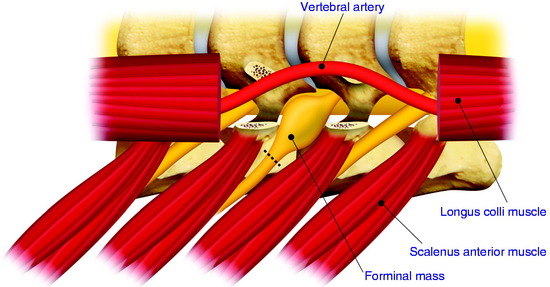
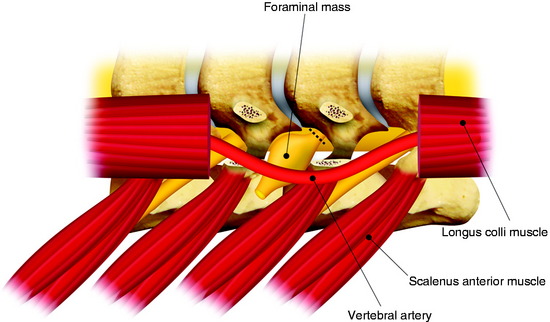
The dumbbell tumor describes a group of spinal tumors that show an hourglass shape in the course of growth as they encounter an anatomic barrier such as the dura mater, intervertebral foramen, or other bony elements (Fig. 29-11). Most schwannomas originate from the dorsal root and grow along the nerve root to the extraforaminal portion. Surgical approaches for cervical dumbbell tumors remain a matter of controversy. Several authors have recommended an anterolateral approach, whereas Lot and George3 reported achieving complete resection of neuromas, including extradural components, with good results using a lateral approach and careful vertebral artery (VA) control. However, tumor removal by a lateral approach still carries a risk of injuring not only the VA but also the phrenic, vagus, accessory, or hypoglossal nerve.
The posterior approach is a classic, standard technique for intraspinal lesions. McCormick2 reported on cervical spine dumbbell tumor patients who underwent resection via a posterior midline approach, including partial laminectomy and complete unilateral facetectomy. He considered these procedures more familiar to surgeons than the anterolateral approach and also emphasized that posterior exposure provided extensive intraspinal access for adequate exposure of large intradural tumor components. The advantages of a combined posterior and anterior approach for a cervical spinal cord tumor have been described by several authors.
POSTERIOR APPROACH
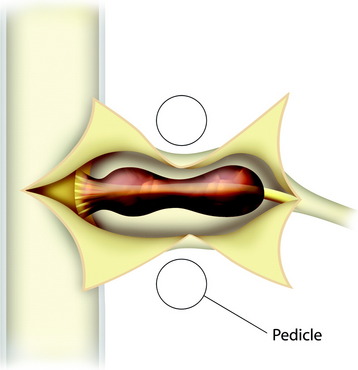
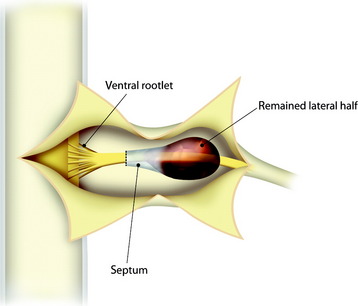
The patient is placed in the prone position with skeletal fixation. The midline skin is incised and the paraspinal muscle dissected to expose the lamina and facet margin. Unilateral hemilaminectomy and facetectomy are performed. Dural incision is made in the paramedian way, and then another incision is added perpendicular to the first incision, following the foraminal portion of the mass (Fig. 29-12).4 The foraminal mass is severely adhered to the overlying dura and perineurium (Fig. 29-13). When the dura overlying the foraminal portion is incised, severe bleeding is observed (Fig. 29-14). At first, the intraspinal portion is removed and the nerve root from which the tumor originated is resected under microscope. The lateral end of the mass is so deep and associated with surrounding vessels that surgeons may have difficulty in dissection. When the tumor mass is removed completely, the vertebral artery can be seen under the thin connective tissue membrane. The membrane divides the ventral root and dorsal root in a nerve root (Fig. 29-15).1 Most neurogenic tumors originate from dorsal root, and the ventral root is compressed. The interface between the tumor and VA is smooth, so the dissection is relatively easy. After the complete removal of the tumor mass, the dura should be closed with watertight sutures. In cases of large tumors, the dura may be redundant. Some portion of the dura is resected and adjusted to the margin (Fig. 29-16).
ANTERIOR APPROACH3
A longitudinal skin incision is made along the medial border of the sternocleidomastoid (SCM) muscle at the level of the vertebral bodies to expose them. The natural space between the SCM and the internal jugular vein is opened by sharp dissection, and control of the accessory nerve is maintained for exposure above C4. The SCM muscle is retracted laterally, while the great vessels, trachea, and esophagus are kept undissected medially and protected by a blade. The transverse processes can be identified with the finger. They are covered with the prevertebral muscles (Fig. 29-17). The aponeurosis overlying the prevertebral muscles is cut longitudinally medial to and along the sympathetic chain. The aponeurosis and sympathetic chain are then retracted laterally. The longus colli and longus capitis muscles are divided along the transverse processes and vertebral bodies at the decided levels and then removed (Fig. 29-18). After prevertebral muscle resection, the anterior surface of the transverse processes is clean (Fig. 29-19). The anterior branches of the transverse processes situated above and below the tumor level are resected with great care to preserve the periosteal sheath surrounding the VA and its venous plexus. The limit of the tumor capsule is not always obvious and may be confused with the periosteal sheath. The VA is displaced anteriorly or medially. The tumor is seen under the VA and at the tip of the transverse process. The distal nerve root is cut if there is no response after stimulation (Fig. 29-20). Then the tumor is debulked from the lateral to the proximal part. Tumor debulking takes place on either side of the VA, which may be displaced medially or laterally. In cases of positive reaction to stimulation, either the functional rootlets are preserved or a part of tumor must be left along the root. Removal of the intradural mass requires additional drilling of the enlarged foramen. With limited drilling of the lateral aspect of the upper vertebral body, the anterolateral surface of the dural sac can be seen. After the dura is reached, a longitudinal incision of the dura is performed. The intradural part can then be pulled out and resected after the proximal part of the root has been divided (Fig. 29-21). In cases of tumors above C2, the posterior approach is preferred.
C2 NEUROMA
The incision is vertical on the midline and then curves laterally below the occipital protuberance. The lower part of the occipital bone, the posterior arch of the atlas, and the laminae of C2 are exposed (Fig. 29-22). At the tumor side, the exposure is extended laterally as far as the transverse foramina. The neurogenic tumor may involve the two anterior and posterior branches of the C2 nerve root that run respectively before and behind the C1–2 VA segment. Most tumors originate from the posterior branch. For C2 neurogenic tumors, because they rarely extend beyond the VA, the distal part of the C2 nerve root is exposed in the first step (Fig. 29-23).5 The C1–2 interspace is very wide; it is not necessary for surgeons to resect the C2 lamina. If the neuroma is large, the posterior arch of the atlas may be eroded, and the superior part of the tumor comes close to the above C1 VA segment. Consequently, exposure of the VA in the groove of the atlas with resection of the arch of the atlas may be necessary.
SURGERY OF SPINAL CORD MENINGIOMA
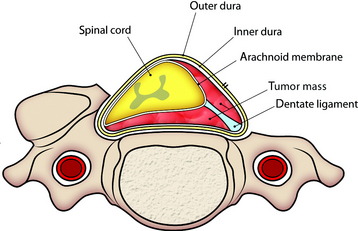
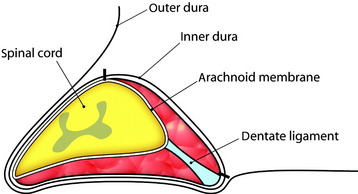
Meningiomas grow from the arachnoid cap cell in the dura mater. Dorsal and dorsolateral lesions are removed from the spinal cord with traction on the open dural margins, after which a circumscribing excision of the dural origin completes the removal. For lateral and ventral tumors, the arachnoid over the exposed portion of the tumor is incised and reflected so that the dissection may proceed directly on the tumor surface.6 Large tumors are bisected and debulked through a central trough. The tumor segment apposing the spinal cord is then delivered into the resection cavity with gentle traction and surface dissection. The remaining dual-based tumor is amputated from the dural attachment. The attachment is then extensively coagulated. Alternatively, the dural base may be excised and replaced with a patch graft.
CASE ILLUSTRATION
This intradural extramedullary tumor shows dense tumor attachment at C7 and the T1 and T2 levels, nearly circumscribing the spinal cord (Figs. 29-24 and 29-25).
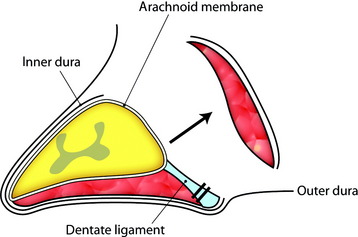
Total laminectomy and right side facet removal are performed to expose the tumor mass. A dural incision is made in the paramedian direction on the surface of the normal dura (Fig. 29-26). Care should be taken to make an incision only within the outer layer of the dura. The spinal dura can be easily divided into two layers, inner and outer. The small incision in the outer layer is slowly extended; this layer is then stripped away from the inner layer with a pair of microforceps and microdissectors in a rostral-to-caudal direction. At this point, the tumor base can be seen through the inner layer of the dura. After the outer layers are retracted bilaterally with stay sutures, a small incision is made with a scalpel in the inner layer of the dura (Fig. 29-27). At this time, care should be taken not to make an incision in the arachnoid membrane. The incision in the inner layer is continued around the dorsolateral base of the tumor. The dorsolateral portion of the tumor becomes free from the surrounding dura, and the inner dura should be lifted up carefully to release tumor mass from the arachnoid membrane (Fig. 29-28).
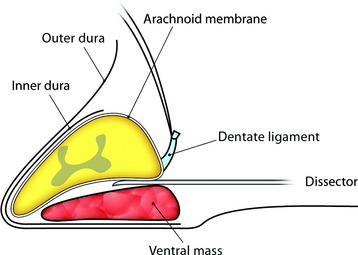
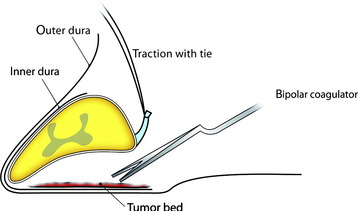
For removal of the ventral portion, the dentate ligament is resected and tied with silk. The spinal cord is retracted with the tied silk to the left side for the easy visualization of the ventral mass. The dissection is performed between the arachnoid over the tumor mass and ventral surface of the spinal cord (Fig. 29-29). With surface dissection and traction, the hidden portion of the ventral mass is brought into view in the operative field. The ventral mass is removed piecemeal, and the remaining dual-based tumor is amputated from the dural attachment. The attachment is then extensively coagulated (Fig. 29-30). Some specialists advocate extensive coagulation of the tumor base, whereas others stress the need for complete resection of the involved dura. However, when the dural defect cannot be primarily closed or grafted, the patient may suffer from the formation of a pseudomeningocele. In a comparison of radical dural resection with dural coagulation, Solero et al7 reported recurrence rates of 8% vs. 5.6%. For the resection of isolated intradural meningiomas with expansion lateral or posterior to the spinal cord, this method can be a substitute for complete dural resection. After removal of the tumor, the preserved outer layer is continuously sutured to make it watertight, followed by a lay-by-lay wound closure in the standard fashion.
SURGICAL TREATMENT FOR SYNOVIAL CYST
Synovial cysts occur more frequently in the lumbar spine (90%) than in the cervical or thoracic regions8 and are commonly found at the L4–5 level.
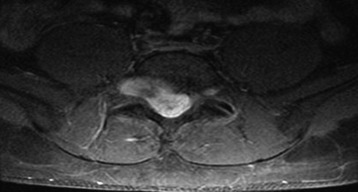
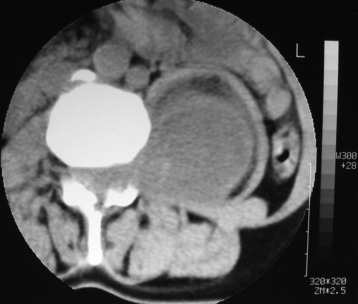
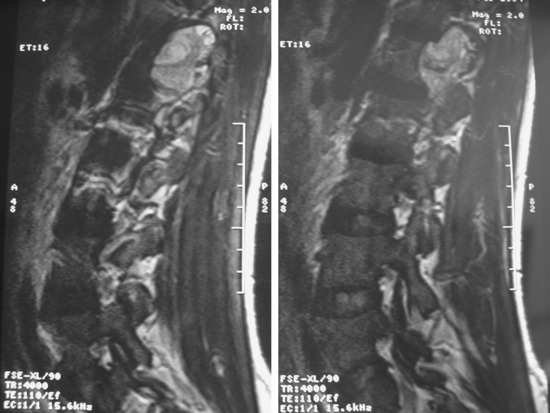
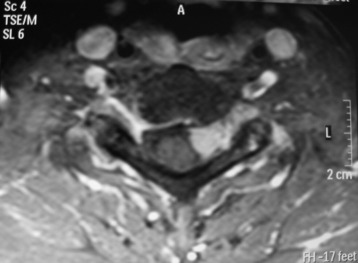
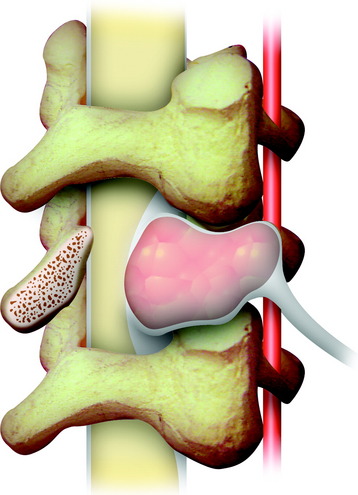
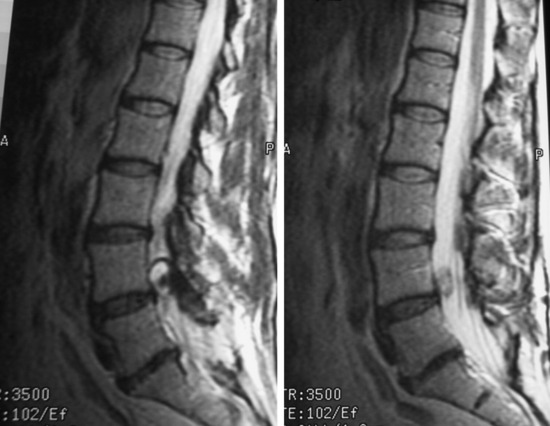
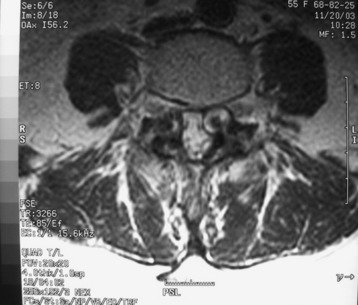
Lumbar synovial cysts are known to cause various symptoms and signs such as painful radiculopathy, neurogenic claudication, and cauda equina syndrome. On MRI, synovial cysts appear well-circumscribed, smooth, extradural in location, and adjacent to facet joints. The proteinaceous content of the cyst can demonstrate greater signal intensity than the surrounding cerebrospinal fluid on both T1- and T2-weighted images (Fig. 29-31 and Fig. 29-32). MRI is considered the diagnostic imaging of choice. The predilection for cysts to occur adjacent to this facet joint level has been attributed to the amount of degenerative spondylosis and spinal instability. The distinction between synovial cysts (with a synovial lining) and ganglion cysts (without a lining) is a histological finding.9 These cysts can, on rare occasion, hemorrhage and bleed into surrounding soft tissues and/or the spinal canal, causing acute compression of the spinal cord. The etiology is still unclear, but underlying spinal instability has a strong association for formation of spinal cysts and worsening symptoms.
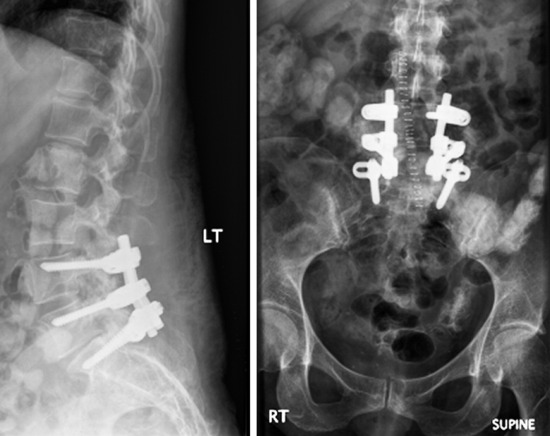
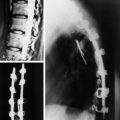
In 70–80% of patients with synovial cyst, spondylolisthesis is found.10 Synovial cysts resistant to conservative therapy should be treated surgically. Patients with spinal segment fusion tended to have better outcomes, with 80% having excellent or good outcomes vs. approximately 70% without fusion.9 Optimal surgical management may well include not only laminectomy but also simultaneous fusion in cases in which disruption of the facet and the joint capsule, particularly in the presence of spondylolisthesis, renders patients’ spines unstable.11
CASE ILLUSTRATION
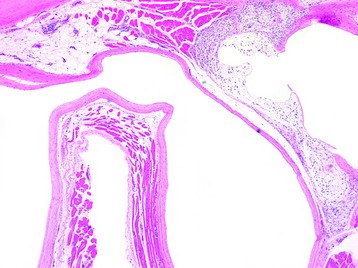
To remove a unilateral synovial cyst in a stenotic spinal canal at the L4–5 level, usually a partial laminectomy of L3, full laminectomy of L4, and partial laminectomy of L5 are performed. Superiorly, ventrally, and foraminally, the exiting L4 nerve root and the thecal sac must be differentiated from the synovial cyst, which often fills the lateral gutter, extending toward the cephalad L3–4 interspace. This is followed by removal of hypertrophied yellow ligament in the lateral and foraminal regions. Ipsilateral dissection with identification of the superior nerve root and the thecal sac follows, allowing for resection of the synovial cyst, often in a layered fashion. The cyst may be decompressed, removing the often thick, viscous contents. Then it is ascertained whether a clean dural plane exists ventrally between the capsule of the cyst and underlying theca. If a plane is identified, the synovial cyst may be entirely removed. However, if there is ossification or marked adhesion of the ventral aspect of the cyst to the dura, decompression alone without excision should suffice, while also avoiding or risking a cerebrospinal fluid fistula. When the facet joint is destroyed with mass removal, concomitant fusion is performed (Fig. 29-33). In this case, severe degeneration of the L5–S1 disc space is observed. L4–L5–S1 posterolateral fusion is performed. The pathological finding shows that the synovial lining cells are arrayed along the cyst wall (Fig. 29-34).