Chapter 110 Surgery for Ocular Trauma
Principles and Techniques of Treatment
Extent of ocular injuries
It is estimated that 1.6 million blinding ocular injuries occur worldwide each year, with an additional 2.3 million sustaining bilateral low vision from trauma, and almost 19 million with unilateral blindness or low vision.1 Ocular trauma is second only to cataract as the most common cause of visual impairment in the USA. Of 2.4 million eye injuries each year, 40 000 are left with significant impairment.2 Overall, approximately 1 million Americans are visually impaired due to ocular trauma, and the annual cost of ocular trauma in the USA is estimated at between US$175 million and US$200 million for hospital care alone.3
According to the United States Eye Injury Registry, ocular injuries are more common among younger people, males, and those with less education and wealth. Statistics from the National Institute for Occupational Safety and Health on work-related diseases and injuries, indicate that over 800 000 work-related ocular injuries are reported annually in the USA. The impact of these injuries is compounded by the personal and national financial burden incurred. Total direct costs of workers’ compensation claims can be estimated at US$924 million, and estimated additional indirect costs (such as time lost by non-injured workers and production slowdowns) exceed US$3 billion.2,4
Ocular trauma classification
In 1996, the Ocular Trauma Classification Group devised a standardized terminology for ocular trauma. The classification has improved communication between physicians and accuracy in clinical practice and in research (Table 110.1),5 and it has been incorporated into many subsequent retrospective studies.
Table 110.1 New standardized classification of ocular trauma terminology
| Term | Definition |
|---|---|
| Eyewall | Sclera and cornea |
| Closed-globe injury | The eyewall does not have a full-thickness wound |
| Open-globe injury | The eyewall does have a full-thickness wound |
| Rupture | Full-thickness eyewall wound caused by a blunt object; the impact results in momentary increase of the IOP and an inside-out injury mechanism |
| Laceration | Full-thickness wound of the eyewall, usually caused by a sharp object; the wound occurs at the impact site by an outside-in mechanism |
| Penetrating injury | Single laceration of the eyewall, usually caused by a sharp object |
| Intraocular foreign body (IOFB) | Retained foreign object(s) causing body injury and entrance laceration(s) |
| Perforating injury | Two full-thickness lacerations (entrance + exit) of the eyewall, usually caused by a sharp object or missile |
Closed-globe injuries
Hyphema
Traumatic hyphema, or anterior chamber hemorrhage, can often manifest after blunt trauma. Most hyphemas clear spontaneously and do not cause residual visual impairment. However, permanent visual impairment can arise from complications of hyphema. These complications include: (1) corneal blood staining;6 (2) ghost cell glaucoma as a result of blocked outflow from clogging of the trabecular meshwork by erythrocytes;7 or (3) central retinal artery occlusion from elevated intraocular pressure.8 All these complications are more common when secondary bleeding occurs, usually 48–72 hours after the injury.
The laboratory workup for traumatic hyphema is tailored to the specific patient. While laboratory evaluation is not indicated in most patients with a hyphema, blood coagulation tests (partial thromboplastin time, prothrombin time, bleeding time, platelet counts, and liver function tests) should be considered in patients with a known bleeding diathesis or pertinent review of systems. African American patients should be questioned about a family or personal history of sickle cell disease or trait. If unknown, a sickle preparation or hemoglobin electrophoresis should be obtained. Patients with sickle cell hemoglobinopathies and traumatic hyphema present special problems because of their decreased ability to tolerate modest rises in intraocular pressure. Goldberg9 reported four patients with sickle cell hyphemas who developed optic atrophy with elevated pressure for short periods. This presumably is due to decreased perfusion pressure, sludging and sickling of erythrocytes and subsequent infarction. In addition, because of its lower likelihood to cause systemic acidosis that promotes sickling, methazolamide (Neptazane) rather than acetazolamide (Diamox) should be used to decrease intraocular pressure in these patients. Mannitol should be used only once to avoid hemoconcentration and the subsequent erythrocyte sludging and vascular obstruction.
Primary management of traumatic hyphema is directed at prevention of rebleeding, which is a significant risk factor for the development of glaucoma.10 Rebleeding has been reported to complicate up to 35% of cases11; the wide range in reported incidence likely relates to variability in patient ethnicity, treatment, and severity of injury. Previous clinical studies have demonstrated the benefits of shielding the injured eye to prevent accidental repeat trauma, using atropine for cycloplegia, and daily checking of visual acuity, corneal status, and intraocular pressure.12,13 On the other hand, no benefit in the prevention of rebleeding has been proven for hospitalization with enforced bed rest or for bilateral patching to reduce eye movement.14
Several pharmacologic measures have been used to prevent rebleeding. Corticosteroids, both topical (prednisolone acetate 1% q.i.d.) and systemic (prednisone 0.5–1.0 mg/kg per day), reduce iritis and ciliary spasm, increase patient comfort, and theoretically stabilize the clot formation, thereby decreasing the rate of rebleed.15 Clinical trials have demonstrated the efficacy of oral epsilon-aminocaproic acid, both systemic (Amicar, Lederle Laboratories, Pearl River NY, given 50 mg/kg every 4 hours for 5 days) and topical (Caprogel, ISTA Pharmaceuticals Irvine, CA, given every 6 hours for 5 days) and tranexamic acid (Cyklokapron, Pfizer, New York, NY) in reducing the incidence of secondary bleeding.16,17 These antifibrinolytic agents reduce clot degradation from traumatized blood vessels by inhibiting the conversion of plasminogen to plasmin, the protein responsible for clot breakdown.
A recent review and meta-analysis examined the use of antifibrinolytic agents, corticosteroids, cycloplegics, miotics, aspirin, conjugated estrogens, unilateral or bilateral patching, elevation of the head, and bed rest. No intervention had a significant effect on visual acuity, whether measured at ≤2 weeks after the trauma or at later time points. All antifibrinolytic agents significantly reduced the incidence of recurrent hemorrhage. The evidence to support an associated reduction in the risk of complications from secondary hemorrhage (i.e., corneal blood staining, peripheral anterior synechiae, elevated intraocular pressure, and development of optic atrophy) by antifibrinolytics was limited by the small number of these events. Use of aminocaproic acid was associated with increased nausea, vomiting, and other adverse events compared with placebo. There was no difference in the number of adverse events with the use of systemic versus topical aminocaproic acid or with standard versus lower drug dose.18
In most cases the hyphema improves with medical management. However, empiric criteria for surgical evaluation have been developed13,19,20: (1) intractably elevated intraocular pressure (IOP) despite medical therapy (>60 mmHg for 2 days in sickle-negative patients; or >24 mmHg for more than 1 day in sickle patients); (2) total hyphema for more than 5 days with IOP >25 mmHg; (3) corneal bloodstaining; (4) persistence of hyphema occupying at least one-half of the anterior chamber volume. Several surgical techniques have been suggested to manage this problem, including paracentesis, anterior chamber washout with a one-needle irrigation or irrigation–aspiration technique,21 washout with a two-needle technique, clot evacuation with a forceps or cryoprobe through a large limbal incision,22 or clot evacuation associated with a trabeculectomy filtering operation.23
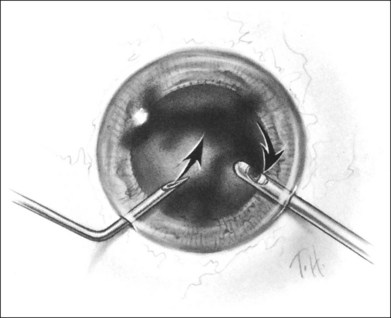
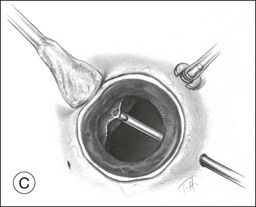
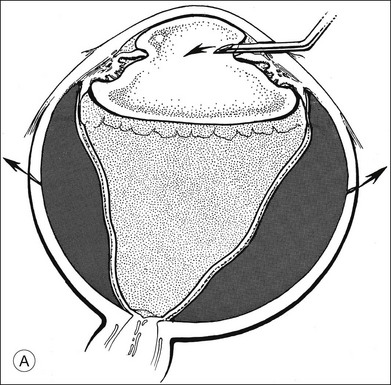
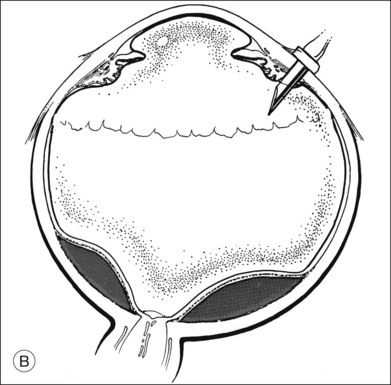
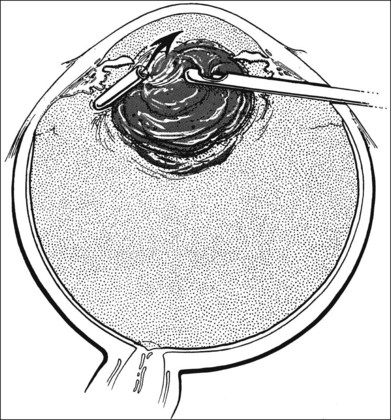
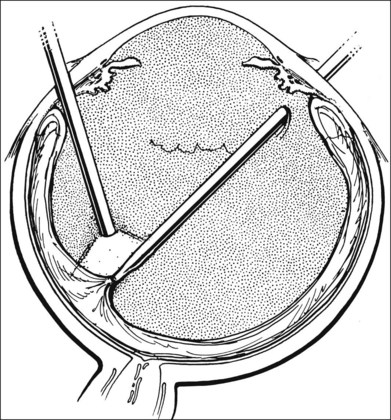
A two-instrument bimanual technique using vitrectomy instrumentation inserted through limbal incisions permits controlled removal of anterior chamber hemorrhage (Fig. 110.1).24,25 The surgeon inserts through one incision a blunt infusion cannula (20-gauge (G), or smaller) or a bent needle (typically 23G) connected to balanced salt solution and a vitrectomy instrument through the other. Using both the aspiration and cutting functions of the instrument, as much of the clot and free blood is removed as possible. Care is taken to avoid damaging the crystalline lens and corneal endothelium. It is not uncommon to leave some residual blood at the end of the procedure. The incisions are closed with “X-type” sutures using 9–0 or 10–0 monofilament nylon with knots buried in the incision. However, this technique may also now be performed with the newer small-gauge vitrectomy instruments. By using a 23G or 25G approach, self-sealing incisions may be possible.
Lens subluxation and dislocation
Blunt trauma can lead to either subluxation or dislocation of the crystalline lens. In lens subluxation, zonular filaments are broken, and the lens is no longer held securely in place but remains in the pupillary aperture. Lens dislocation occurs following complete disruption of the zonular filaments and displacement of the lens from the pupil. Trauma is the leading cause of lens dislocation.26
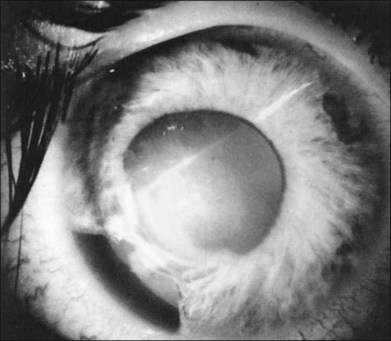
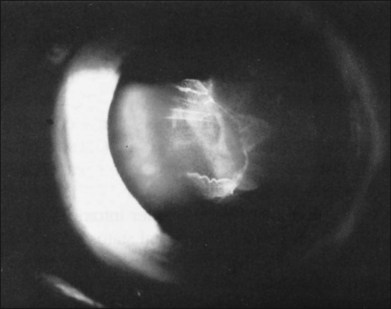
In the evaluation of a patient with a cataractous lens after blunt trauma, it is important to remember that the lens may be subluxated. During the slit-lamp examination, evidence of iridodonesis or phacodonesis must be sought. This usually is more apparent in the undilated state, when the remaining intact zonular filaments are under less tension. Phacodonesis can be detected by having the patient look quickly from side to side or up and down, or by jarring the slit-lamp table. If an associated iridodialysis is present, the absence of zonular fibers can be identified by looking through the area of absent iris (Fig. 110.2). In addition, vitreous sometimes can be identified extending around the lens, either through the pupil or through the iridodialysis. Presence of vitreous in the anterior chamber is proof of zonular rupture and probable lens subluxation.
For cases with minimal lens instability, the traditional phacoemulsification techniques through a corneal limbal or scleral tunnel incision may be used, with a thorough hydrodissection and care to minimize the stress on the zonule and vitreous during all steps of the procedure. A capsular support device may increase the safety and ease of phacoemulsification by stabilizing the capsular bag. Alternatively, a larger capsulorrhexis may be used, and the lens may be prolapsed into the anterior chamber for phacoemulsification. Of great concern is vitreous prolapse through the cataract wound, and if vitreous is pulled on, traction transmitted to the vitreous base can cause retinal tears, giant retinal tear, and retinal detachment.27 Consequently, the surgeon must recognize that vitreous prolapse through the wound is probable and be prepared for its management.
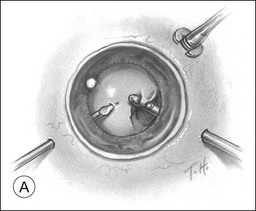
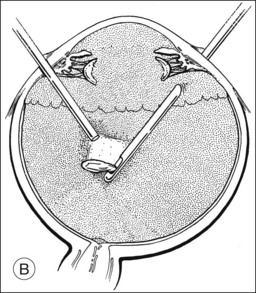
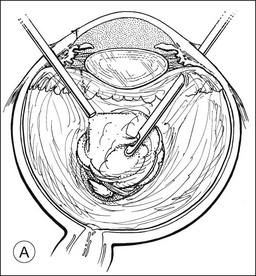
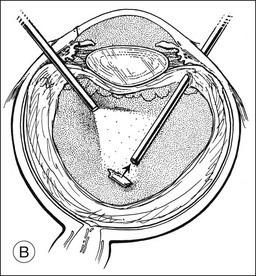
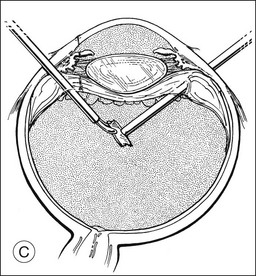
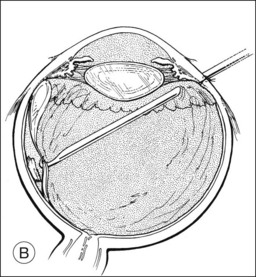
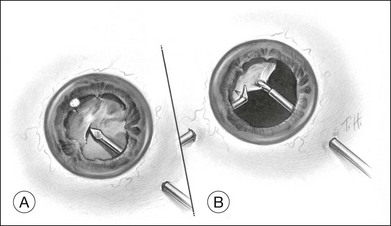
A pars plana approach using vitreoretinal instrumentation avoids many of the risks associated with limbal extraction (Fig. 110.3). Using a two- or three-port system, a myringotomy blade or a bent 21G or 23G butterfly intravenous needle connected to irrigation is inserted through the pars plana into the lens for fixation, and either a vitrectomy or a phacofragmentation instrument is inserted through the opposite pars plana to digest the lens. If the lens is soft, as in children and young adults (the most frequent victims of trauma), the entire lensectomy can be performed with the vitreous cutter. Vitreous around the lens can be managed with high-speed cutting to prevent traction on the vitreous base. Should the lens nucleus be harder and require phacofragmentation, care should be taken to perform either a limited vitrectomy around the lens before introduction of the phacofragmatome or to use the phacofragmatome only when inside the lens capsule. This avoids unintentional aspiration pressure on the vitreous that could lead to retinal damage.
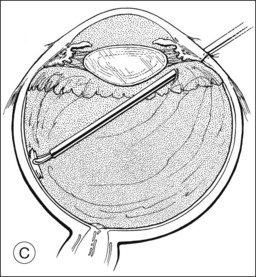
Pars plana vitrectomy and pars plana lensectomy (PPV/PPL) with implantation of IOL can be used for cases of subluxated traumatic cataract with extensive zonular damage. Kazemi et al. reported on nine such eyes, in which an anterior chamber IOL was placed. Complications included retinal detachment in one patient, transient limbal wound leak in one patient, and cystoid macular edema in three patients. All of the complications were managed successfully. Best-corrected visual acuity of better than 20/40 was achieved in six of the nine eyes, and the etiology of poor visual acuity in the remaining three eyes was attributed to the original injury.28
Kodjikian et al. reported on nine consecutive cases of traumatic cataract with zonular dialysis of at least 180° and visual acuity of 20/100 or worse. Following PPV/PPL and implantation of an iris-fixated Artisan IOL, all patients achieved best-corrected visual acuity of 20/30 or better. Postoperative complications included one instance each of iris incarceration into the corneal wound and anterior uveitis, which was medically managed.29 Other authors have reported similar results using PPV/PPL and sutured sulcus IOLs.30 A consistent finding in these studies is that the primary cause of poor visual acuity postoperatively is posterior segment pathology related to the original injury.31
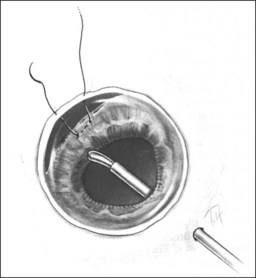
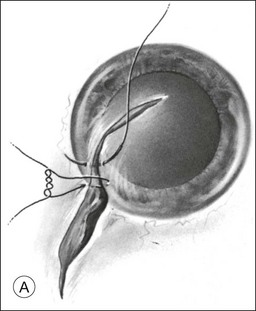
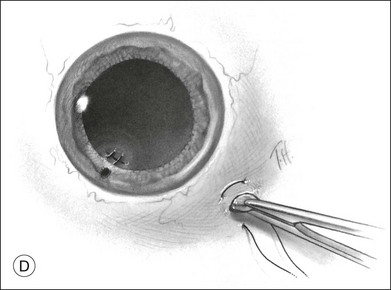
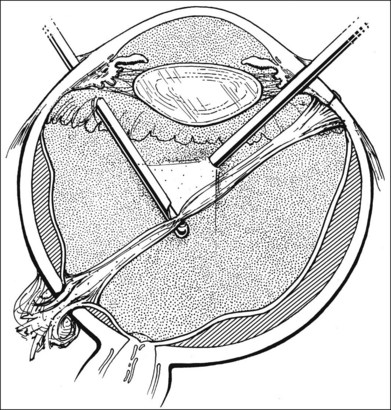
Should an iridodialysis be present, it can be repaired at the end of the case using a 10–0 Prolene suture introduced through the cornea in a modification of the technique described by McCannel32 to fixate IOLs to the pars plana (Fig. 110.4). At the end of the procedure, the peripheral retina should be carefully inspected with scleral depression to identify retinal breaks, dialyses, or areas of retinal detachment. Inspection can be performed either with binocular indirect ophthalmoscopy or through the microscope equipped with wide-field viewing. Should retinal breaks or detachment be present, they should be treated immediately with retinopexy and, if necessary, scleral buckling and endotamponade.
Vitreous hemorrhage
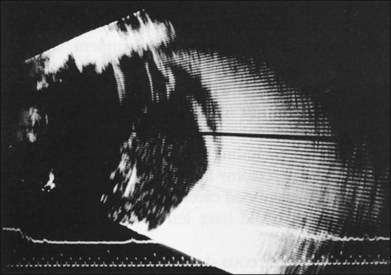
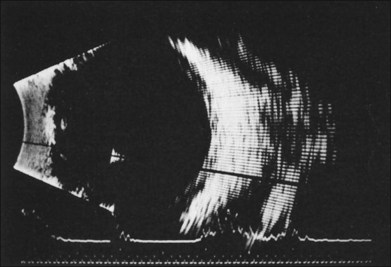
If vitreous hemorrhage obscures examination of the retina, ultrasonography plays a critical role in decision-making. With combined contact A-scan and B-scan techniques, many details of the posterior segment can be determined, including presence of retinal detachment, posterior vitreous detachment, occult scleral rupture, hemorrhagic or serous choroidal detachment, and giant retinal tear (Fig. 110.5). For example, if ultrasound demonstrates vitreous strands emanating from an equatorial location, an occult scleral rupture with vitreous incarcerated in the wound can be inferred, requiring conjunctival peritomy and exploration.
The visual prognosis for eyes with vitreous hemorrhage associated with nonpenetrating trauma depends on associated macular damage (from choroidal rupture, traumatic macular hole, Berlin’s edema, or macular contusion), retinal dysfunction from associated retinal detachment, and occlusional amblyopia in young children. In a study of 33 eyes with severe vitreous hemorrhage associated with closed-globe injury, best-corrected visual acuity following resolution and/or treatment of hemorrhage was <20/200 in 54%. The most common cause of poor visual outcome was macular scar. Poor prognostic factors included presenting visual acuity of light perception or worse, hyphema, traumatic cataract, and age 55 years or younger.33
“Commotio retinae”, avulsion of the vitreous base, and retinal tears
Blunt trauma can damage the retina in many ways, ranging from retinal edema to retinal detachment. “Commotio retinae” is commonly seen after a contusive injury to the globe and appears ophthalmoscopically as retinal whitening. Edema involving the macula (termed Berlin’s edema) can impart an appearance similar to a cherry-red spot. The reasons for this retinal opacification are disputed, with some attributing it to extracellular edema34; however, experimental and histopathologic studies have shown that disruption of the photoreceptor cell outer segment and damage to the retinal pigment epithelium accounts for the retinal whitening.35,36 Vision can be markedly decreased with commotio retinae, but vision most often improves as the swelling resolves over a 3–4-week period. However, the macula can develop an atrophic appearance with granular hyperpigmentation associated with decreased vision. In addition, the cystoid areas can coalesce to form a large cyst, which can lead to macular hole.
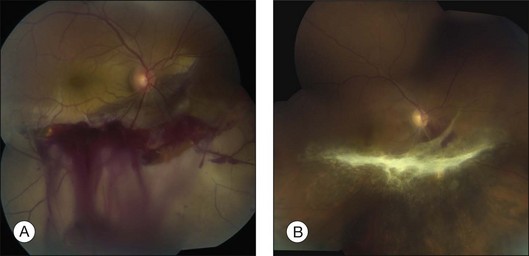
Blunt trauma can also lead to large areas of irregular, jagged retinal holes. These breaks have been observed almost immediately after the contusive injury and are believed to be the result of mechanical disruption and fragmentation of the retina. Similar-appearing breaks have been produced in an experimental model of concussive injury to the globe.37 This syndrome has been given a variety of names, including “acute retinal necrosis”38 and “chorioretinitis sclopetaria.”39 We prefer the latter term to avoid confusion with the acute retinal necrosis inflammatory syndrome of herpetic viral origin.
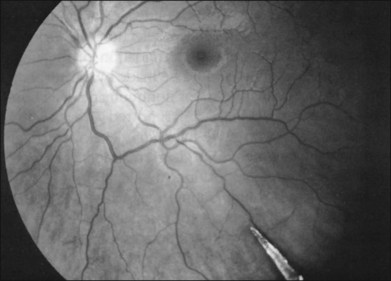
In eyes with chorioretinitis sclopetaria, it is tempting to intervene in some way because of the frightening size and often posterior location of the retinal breaks (Fig. 110.6). However, the retina rarely detaches in this situation, presumably because of the inflammation at the edges of the necrotic retina that leads to a firm chorioretinal adhesion. If a retinal detachment occurs, it is usually from another site. Therefore, it is not necessary to perform prophylactic cryopexy or photocoagulation. Patients with this syndrome often have poor visual acuity because of the effects of the severe blunt force on the macula.
Blunt trauma also can cause retinal breaks by transmission of the force to the vitreous, leading to acute severe vitreoretinal traction. Rapid displacement of the vitreous can tear the retina in various ways, including retinal dialysis with or without avulsion of the vitreous base, operculated retinal tear, macular hole, and horseshoe-shaped retinal tears at the posterior margin of the vitreous base, at the edge of a meridional fold, or at the equator.40,41 A study by Cox37 concluded that the different forms of retinal abnormalities are a result of the point of impact of blunt trauma.
The most common retinal break from trauma is a retinal dialysis. This is seen most commonly in the superonasal and inferotemporal quadrants because of blunt trauma frequently striking the globe inferotemporally.42,43 The blunt force causes equatorial stretching of the globe, leading to traction on the vitreous base at the site of impact and diametrically opposite. Cox et al.40 reviewed 160 patients with retinal detachment caused by contusion. Retinal dialyses larger than one oral bay were found to be the most common retinal break.
Retinal detachment and macular hole
In treating a retinal detachment in a patient with a history of ocular contusion, the surgeon must remember the types of retinal breaks that are most common in this setting, specifically retinal dialysis. Most traumatic retinal detachments can be treated with conventional scleral buckling techniques. Because of the potential for retinal damage 180° from the impact site, an encircling element is recommended. The selection of chorioretinal adhesive procedure and determination of the need to drain subretinal fluid should be made by the surgeon using the same principles as for nontraumatic detachments. Johnston44 reported 77 eyes with retinal breaks after contusive injury; 65 developed rhegmatogenous detachment. Surgical treatment restored or maintained retinal apposition in 96% of the eyes.
In rare cases, blunt trauma may cause a rhegmatogenous retinal detachment from a giant retinal tear or a macular hole. Although these detachments have been repaired successfully in the past with a variety of scleral buckling techniques, modern vitreoretinal surgery has significantly increased the rate of anatomic reattachment. A giant retinal tear is defined as a retinal tear of greater than 3 clock-hours. Vitreous surgery allows mobilization of the flap by excising adherent vitreous. With the aid of surgical adjuvants, such as perfluorocarbon liquids, and current surgical techniques (as described in Chapter 105, Optimal procedures for retinal detachment repair), the rate of successful retinal reattachment for such cases has greatly improved.
Traumatic macular hole formation is uncommon. Like idiopathic macular holes, traumatic holes can be rehabilitated with vitrectomy. However, a significant number of traumatic macular holes may close spontaneously. In a consecutive series of 18 such cases, spontaneous closure and accompanying visual improvement were seen in eight (44%) and occurred between 1 week and 4 months following injury.45 Although the visual outcome may be limited by associated macular damage (such as a macular choroidal rupture), there are encouraging reports of successful anatomic and functional outcomes with vitrectomy. Since patients with traumatic macular hole are typically younger and phakic, we elect to use long-acting gas endotamponade with 14% perfluoropropane to optimize the chance of hole closure. It has been suggested that surgical adjuvants such as autologous plasmin enzyme46 or removal of internal limiting membrane47,48 may improve the rate of anatomic success. Improvement in visual acuity of at least 2 Snellen lines has been reported in 69–94% of cases.47–49
Open-globe injuries
Preoperative evaluation
It is important to obtain a history from any patient being evaluated for a possible eye injury. Although many trauma patients will be poor historians because of associated shock, intoxication, or neurologic problems, the history can give important clues about the ocular damage. For instance, if a patient felt something flying into his or her eye while hammering a nail but did not note any visual disturbance, the physician must carefully search for evidence of an intraocular foreign body (IOFB), even if there is no evidence of obvious ocular laceration. Furthermore, since many of the medical malpractice cases brought against vitreoretinal specialists involve trauma, careful documentation of the details surrounding the injury may prove valuable in case of litigation later on.50
Careful evaluation is important not only for appropriate management, but can also be helpful in prognostication of visual outcome, when used according to the ocular trauma score (OTS). The OTS was developed by Kuhn et al. following review of over 2500 registered cases of ocular trauma. The OTS provides a single probability estimate that an eye trauma patient will obtain visual acuity within a specific visual range by 6 months after injury.51 In the OTS, a numerical score is derived by adding or subtracting points on the basis of initial visual acuity, presence of rupture, endophthalmitis, globe perforation, retinal detachment, or afferent pupillary defect. Higher OTS scores are associated with a better visual prognosis.51 The OTS can thus be used as an aid in patient counseling and can assist in identifying severe ocular injuries with a poor visual prognosis.
The initial step in examination is determination of visual acuity (VA) with either a near vision card or a Snellen acuity chart. When a retrospective review of penetrating injury patients was subjected to multivariate analysis, VA was the most important determinant of final visual outcome. Patients with initial VA of 5/200 or better had a 28 times greater chance of salvaging acuity at this level than those with vision worse than 5/200.52 Two subsequent prospective studies of penetrating injuries have confirmed this finding. In the first of these series, 94% of patients with initial VA of 20/200 or better achieved a final VA of 20/200 or better.53 In the second series, multivariate analysis revealed that presenting visual acuity of 5/200 or worse was the most important factor contributing to poor visual outcome; statistically insignificant factors were time since injury, cataract, and presence of IOFB.54 In one review of 167 open-globe injuries, only 3% of eyes presenting with vision better than light perception ultimately underwent enucleation, as compared with 39% of eyes presenting with light perception and 89% of eyes presenting with no light perception.55
Others have demonstrated that the presence or absence of an afferent pupillary defect (APD) is a strong predictor of outcome.56,57 A multivariate analysis of 240 traumatized eyes found 69% of eyes without an APD obtained a final VA of 20/200 or better, as compared with 34% of eyes with an APD (P < 0.00001).56
On rare occasions, the surgeon may contemplate primary enucleation when encountering a totally disorganized globe with prolapse of intraocular contents, including retina. In these cases it is important to determine carefully whether the patient can perceive light. This is best done with the uninjured eye covered, with the indirect ophthalmoscope light at maximal intensity, and with the light held at sufficient distance so that the patient cannot perceive the heat of the ophthalmoscope light. Unfortunately, since many patients with injuries of this severity often are either intoxicated or have other injuries affecting their mental status, determination of light perception is questionable. Additionally, there have been rare reports of eyes with preoperative measurement of no light perception achieving visual acuity of light perception or better following reconstructive ocular surgery.51,58 For these reasons, primary enucleation is rarely performed.
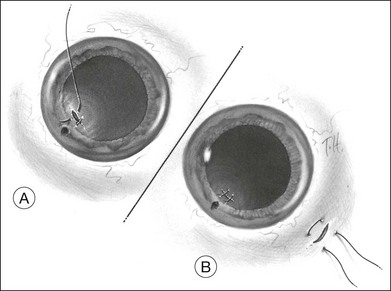
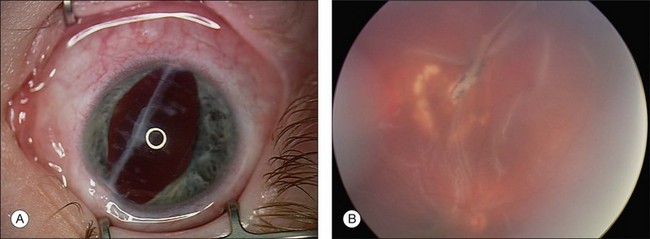
Occasionally, a scleral rupture can be occult (hidden under conjunctiva, Tenon’s capsule, or rectus muscles). Diffuse chemosis or subconjunctival hemorrhage suggests the presence of occult rupture. An open globe often has low IOP, but normal or elevated IOP does not rule out the possibility of a rupture. On occasion, slit-lamp biomicroscopy of the anterior vitreous can be useful, demonstrating vitreous strands directed toward a hidden scleral rupture site (Fig. 110.7). Similarly, ultrasonography can detect this finding. Computed tomography (CT) has also been reported to show occult rupture, with flattening of the posterior contour of the sclera (the “flat tire” sign).59
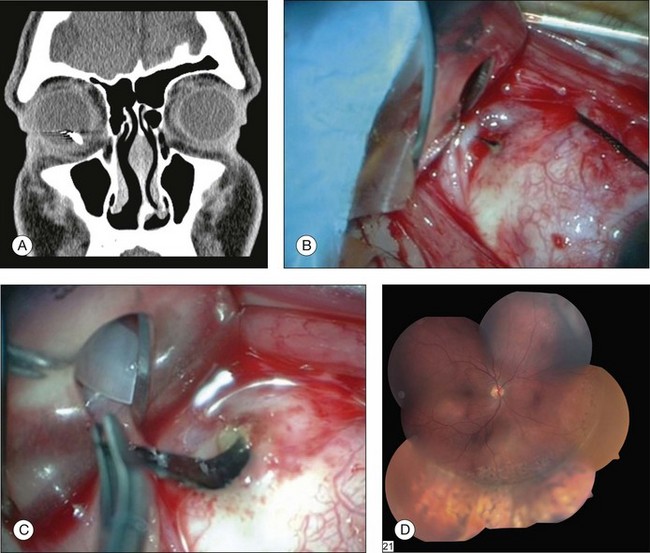
Confirming the presence or absence of a retained IOFB is critical to determining management of a patient with trauma. In many cases, the IOFB can be identified by slit-lamp biomicroscopy or indirect ophthalmoscopy (Fig. 110.8). However, if the ocular media are clouded by corneal damage, hyphema, cataract, or vitreous hemorrhage, ancillary techniques are necessary. For many years, the standard technique of localizing foreign bodies involved special applications of conventional radiographic imaging; however, these methods often were inaccurate and cumbersome, could cause iatrogenic damage to the globe, and were useless in cases involving radiolucent foreign bodies. Consequently, radiographic localization of IOFBs has been replaced by ultrasonography and CT. Ultrasonography can accurately localize foreign bodies, particularly with a systematic approach providing transverse and longitudinal views in all meridians (Fig. 110.9). When the globe is open, however, the resolution of ultrasound may be limited, as the examination often must be performed gently through closed lids. The usefulness of echography is also limited by shadowing caused by highly reflective surfaces such as air, reverberation artifacts created by some IOFBs, and by the user’s skill and familiarity with ocular pathology.60
CT has emerged as the imaging technique of choice in the evaluation of IOFBs. It is a noninvasive technique requiring minimal patient cooperation and can be used to image radiolucent and radiopaque foreign bodies (Fig. 110.10). By assembling many consecutive orbital slices that demonstrate the ocular anatomy and by using computer-generated reconstructions in three dimensions, the presence and location of an IOFB can be determined. In an experimental study to determine the size of CT cuts required to detect small IOFBs, Dass et al. concluded that with modern spiral CT scanning both 3 mm and 1 mm cuts were effective (100% sensitivity) in detecting a 0.5 mm metallic, glass, or stone IOFB.61 The absorption characteristics of the foreign body are quantitated in Hounsfield units and can be compared with the absorptions of various materials already determined experimentally. Zinreich et al.62 have shown that wood is the least dense of the nonmetallic foreign bodies, followed by plastic and then glass. Unfortunately, all metallic foreign bodies have the same absorptions and are impossible to differentiate. CT scanning does have limitations, in that metallic IOFBs often create significant scattering artifact that may obscure their precise location. This can be particularly bothersome when attempting to determine whether a foreign body is intraretinal or intrascleral. In addition, identifying some of the lower-density foreign bodies, such as wood, may be difficult with CT scanning.63
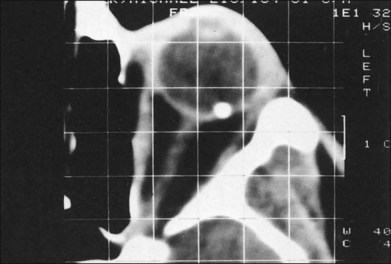
Fig. 110.10 This computed tomography (CT) scan demonstrates an intraretinal shotgun pellet in the patient’s left eye.
Magnetic resonance imaging (MRI) has proved useful for imaging intraocular tumors and other lesions. However, the magnetic fields and heat generated during MRI scanning preclude examination of patients with potential intraocular or intraorbital metallic foreign bodies. Investigators have shown that ferromagnetic foreign bodies implanted in animal eyes can move 7–8 mm in the suprachoroidal space and up to 10 mm in the vitreous space when subjected to the torsional forces of MRI.64 Furthermore, retained intraocular ferromagnetic foreign bodies cause surrounding image distortion, making the globe itself difficult to visualize. Image distortion can similarly occur from the paramagnetic effects in granite, solder, and mascara. Another disadvantage of MRI is its inability to image bone. MRI’s principal advantage over CT is that it can accurately and safely detect wood and plastic IOFBs.
Repair of laceration
A watertight closure can be difficult to achieve with stellate lacerations. In some cases the wound leaks even with multiple sutures criss-crossing the laceration. A purse-string closure may help in these cases. The suture is placed in a circle through the various portions of the cornea involved in the laceration and then tied to itself with the knot buried in the laceration. If this technique does not work, corneal tissue may have been lost. In these situations, use of cyanoacrylate tissue adhesive can seal the leakage site.65 When there is too much tissue loss for glue to work, a corneal patch graft can be used.
When iris is incarcerated in the corneal wound, it can be reposited in most cases. We advocate excision of uveal tissue only if it looks extremely necrotic, usually having been externalized for more than 24 hours. Reposition of incarcerated iris usually can be accomplished with introduction of an iris or cyclodialysis spatula or a 27G cannula connected to viscoelastic through a limbal stab incision made about 90° away from the laceration. This maneuver is best done after the corneal integrity has been partially restored by the first few sutures and the anterior chamber has been reformed with balanced salt solution, air, or hyaluronic acid.66 When there is positive pressure or in the presence of a hyphema, the surgeon may be helped by having the assistant reposit incarcerated iris while the surgeon places the suture.
In some cases, the scleral laceration extends posterior to the ora serrata and may have an associated retinal laceration. Because of associated vitreous hemorrhage, it is rare to be able to identify a retinal laceration at the time of primary repair. Cryopexy should not be performed prophylactically unless a specific retinal tear can be visualized, since cryotherapy may cause excessive inflammation that may promote the development of later traction retinal detachment.67
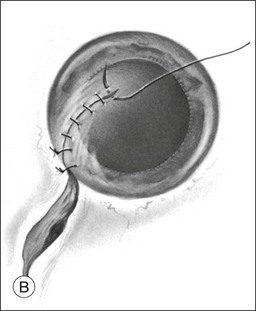
In cases of lacerations involving both the cornea and sclera, the extent of the laceration must be determined first. In an effort to restore structural integrity, the limbus is the best place to start, as it provides a recognizable anatomic landmark. Limbal wounds are closed using 9–0 nylon sutures (Fig. 110.11). After the limbal portion of the laceration is closed, it is easier to repair the corneal laceration first, then the scleral laceration. The same techniques and principles described for lacerations limited to the cornea or sclera should be employed.
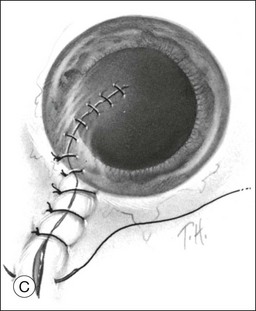
The value of scleral buckling at the time of primary open-globe repair has been revisited by two retrospective studies.68,69 The justification for prophylactic scleral buckling (typically only considered in eyes with lacerations extending >5 mm posterior to the limbus) is that support of the vitreous base may reduce vitreoretinal traction and subsequent retinal tears or detachment, which occurs in up to two-thirds of cases.70 Placement of a buckling element at the time of primary repair, however, is easier because the rectus muscles are often already isolated during exploration of the open globe, and there is no scarring of the wound, Tenon’s fascia, or conjunctiva. In one comparison of 18 eyes treated with prophylactic placement of a 3.5 or 4 mm encircling band and 33 eyes treated without buckling, the rate of subsequent retinal detachment (30–39%) and mean final VA (20/200–20/400) were statistically similar. Importantly, a worse outcome was seen in eyes with opaque media at presentation, independent of primary treatment.68 This institutional study was limited, however, by small sample size and selection bias, since more severely injured eyes may have been more likely to receive prophylactic buckling. Arroyo et al. employed a matched cohort study from two institutions and found significantly better visual and anatomic outcomes in eyes treated with prophylactic buckling than in matched controls. These results may be explained by a trend toward a lower incidence of retinal detachment in prophylactically treated eyes (26% compared with 53%).69
Management of intraocular foreign body
In general, we recommend removal of an IOFB at the time of repair of the entry site or soon afterwards. In certain cases, IOFB removal must be delayed, because of inaccessibility to appropriate specialized resources or because of systemic medical instability. Because of the inflammation caused by IOFBs, they often are rapidly surrounded by a fibrous capsule that can make delayed surgical removal more difficult. Foreign bodies may contain copper, which can cause immediate severe inflammatory changes, or iron, which can cause chronic siderotic damage to the eye.71 In addition, traumatic endophthalmitis, particularly associated with the Bacillus cereus, is more commonly seen with IOFBs than with other forms of penetrating injuries.66,72 Traditionally, IOFB removal within 24 hours of injury has been advocated because of the increased risk of endophthalmitis.73,74 In these series, however, it was not clear that systemic antibiotics were administered. By contrast, in a recent report of military soldiers sustaining ocular trauma with IOFB, none of 79 eyes developed endophthalmitis despite a median delay of 38 days in IOFB removal. Since 97% of patients received antibiotics (most commonly by systemic administration, and most commonly levofloxacin), the authors contended that prompt antibiotic use was helpful in reducing the chance of endophthalmitis.75
Three instruments are commonly available for IOFB extraction: external magnets, intraocular forceps, and intraocular magnets. External magnets are best reserved for cases in which extraction can be performed immediately (success rates fall when the IOFB becomes encapsulated76) and when there is good fundus visualization with minimal associated posterior segment injury. (In a study of eyes with comparable damage, significantly better anatomical and functional results were achieved in eyes managed with vitrectomy and intraocular magnet.77)
Our surgical approach to IOFBs is summarized in Table 110.2. When a magnetic intravitreal foreign body can be well visualized, extraction with an external electromagnet is usually effective (Fig. 110.12). This should be done through a pars plana sclerotomy after repair of the entry site. It is important to make the sclerotomy large enough so that the foreign body can pass out of the globe without becoming incarcerated in the pars plana. If the scleral laceration site is used for extraction, then it should be enlarged, since the elastic sclera and ocular tissues will have stretched after initial IOFB entry and made the wound smaller than the longest dimension of the IOFB. A magnetic extraction is best performed with an assistant holding the magnet over the sclerotomy while the surgeon visualizes the IOFB with indirect ophthalmoscopy and controls the magnet’s foot pedal. If a large sclerotomy is needed, it is helpful to pre-place a mattress suture so that it can be closed quickly after removal of the IOFB to decrease the period of hypotony. We do not advocate prophylactic cryopexy or scleral buckle in these patients.
| Well visualized | Poorly visualized | |
|---|---|---|
| Intravitreal | ||
| Magnetic | External magnet | Vitrectomy, forceps/REM |
| Nonmagnetic | Vitrectomy/forceps | Vitrectomy/forceps |
| Intraretinal | ||
| Magnetic | Transscleral “trap door” or vitrectomy, forceps/REM | Vitrectomy, forceps/REM |
| Nonmagnetic | Transscleral “trap door” or vitrectomy, forceps | Vitrectomy/forceps |
REM, rare earth magnet.
When the magnetic intravitreal foreign body is poorly visualized because of cataract or vitreous hemorrhage, a pars plana vitrectomy and/or lensectomy needs to be performed (Fig. 110.13). The foreign body can then be removed by a number of techniques. The IOFB can be removed with greatest control using IOFB forceps inserted through the pars plana sclerotomy site or with the rare earth intraocular magnet.78 If the intraocular magnet is used to grasp the foreign body and bring it into the anterior vitreous, it often helps to transfer the IOFB to foreign body forceps to pull it through the sclerotomy.
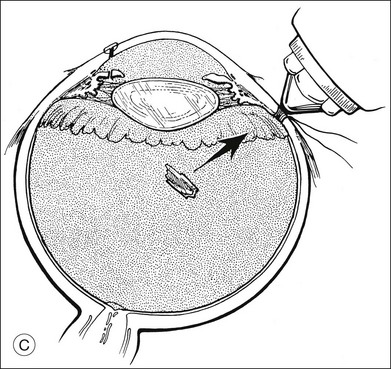
Removal of intraretinal foreign bodies is more difficult and more hazardous because of the risk of retinal detachment (Fig. 110.14). If the ocular media are clear and the IOFB can be localized by indirect ophthalmoscopy, it can be removed by a transscleral approach (Fig. 110.15). A trapdoor scleral flap is created, the choroidal bed is treated with external diathermy, the choroid is incised, and the foreign body is removed with either forceps or an external magnet. If retinal incarceration occurs, the area can be treated with a localized scleral buckle. Because the surrounding area has already been treated with diathermy, it is unnecessary to add cryopexy.
Intraretinal foreign bodies can also be removed via a transvitreal approach, particularly when they cannot be well visualized (Fig. 110.16). After a pars plana vitrectomy is performed, the foreign body should be gently mobilized with the foreign body forceps. In many cases the foreign body will be somewhat adherent to the retina or choroid. If the IOFB has been surrounded by a fibrous capsule, the capsule may be incised with a myringotomy blade before removal. All these manipulations are made to avoid further tearing of the retina. After the IOFB is freed, it can then be removed with either the forceps or intraocular magnet using the method described above.
When it is necessary to perform a vitrectomy to remove an intraretinal foreign body, it is important to remove the posterior hyaloid overlying the incarceration site. In some cases a PVD will be present; otherwise one must be created using powered suction either with a soft-tipped extrusion cannula or the vitrectomy instrument. After the posterior hyaloid is engaged, a pick can be inserted between the hyaloid and retina, and hyaloid is gently lifted off the retina. If the posterior hyaloid is left, there is potential for the postoperative development of vitreoretinal traction or formation of epiretinal proliferation along the posterior hyaloid (Fig. 110.17). In an early report describing the use of pars plana surgical techniques for removal of intraretinal foreign bodies, 90% of eyes developed either macular pucker or proliferative vitreoretinopathy.79 The authors believe the frequency of this complication can be decreased by careful attention to removal of the posterior hyaloid.
There has also been controversy over the optimal management of a retinal tear caused by the foreign body. Some surgeons emphasize the necessity of creating a chorioretinal adhesion at this site with either cryopexy or photocoagulation. In our experience the inflammation caused by the foreign body impaction has been adequate to prevent retinal detachment, particularly if the surgeon pays close attention to proper management of the posterior hyaloid. There has also been discussion of the need to place photocoagulation around the intraretinal foreign body preoperatively to decrease the risk of retinal detachment at the time of foreign body removal. Again, it has been our experience that this is unnecessary. Ambler and Meyers80 reported five eyes with intraretinal foreign bodies managed without retinopexy or fluid–air exchange without developing retinal detachment or preretinal fibrosis. If, however, a retinal detachment does occur at the time of extraction, it is particularly important to attempt removal of adherent posterior cortical vitreous before fluid–gas exchange to reattach the retina. In these cases, endophotocoagulation should be placed around the retinal break. If the surgeon is unable to remove all adherent vitreous, then the retinal tear should be supported on a scleral buckle. We perform prophylactic encircling scleral buckles in all eyes receiving vitrectomy for management of penetrating injuries, as discussed later in this chapter.
Nonmagnetic intravitreal foreign bodies require extraction with intraocular forceps whether they are well visualized or partially hidden by vitreous hemorrhage or cataract.81,82 In these cases, we perform a vitrectomy with or without a lensectomy, depending on the lens clarity. The foreign body is grasped with the IOFB forceps and removed through the pars plana sclerotomy site. Some nonmagnetic foreign bodies may be quite inert, such as glass or plastic, and can be observed without immediate removal. However, it is difficult to know whether they are infected, and with rare exceptions, we advocate immediate removal of IOFBs, regardless of their type.
Intralenticular foreign bodies usually must be removed in concert with lens extraction. However, if the anterior capsule tear is small and the foreign body is composed of a nontoxic material, the capsular wound may fibrose, resulting in a localized, visually insignificant cataract that can be observed.83 If cataract formation has occurred, or if the object is metallic, then lens and foreign body extraction should be performed using standard extracapsular cataract extraction methods. We also recommend lensectomy for small capsular defects when endophthalmitis is suspected or if the foreign body was dirty (e.g., rural trauma).
Overall, patients with IOFBs can have an excellent outcome, with approximately one-half of eyes achieving visual acuity of 20/50 or better. Worse outcomes are typically associated with IOFBs of greater mass, uveal prolapse, and injury with a BB or pellet.84,85
Perforating injury
Perforating injuries represent a small subset of ocular trauma, occurring in only 4.4% of lacerated globes.86 Until the introduction of vitreoretinal surgery, these eyes had a uniformly poor prognosis because of ingrowth of fibrovascular tissue through the posterior exit site, leading to severe retinal detachment. Our current management of this type of injury results from the experimental studies of Topping et al.87 They reported that by day 7 after an experimental perforating injury, the scleral wounds had self-sealed. The degree of transvitreal proliferation increased dramatically after day 7. Contraction of the transvitreal proliferation can lead to tractional retinal detachment. Pars plana vitrectomy is performed to intercede in this process, removing the scaffold for proliferation and contraction.88
Ideally, vitrectomy would be performed early enough to prevent transvitreal proliferation. However, vitrectomy is impossible unless the scleral rupture sites are sealed. The entry site can be repaired by the surgeon using standard techniques described earlier in this chapter. However, attempts to close posterior rupture sites can be difficult as well as hazardous, causing excessive traction on the globe and optic nerve and possibly leading to extrusion of intraocular contents. Having learned from the experimental studies that the scleral wounds seal by day 7, we routinely delay vitrectomy until this time or later. We do advocate suturing the anterior “entry site” promptly after injury (see Chapter 98, Pathophysiology of trauma).
We employ the standard three-port technique for vitrectomy, proceeding from anterior to posterior, clearing vitreous opacities (Fig. 110.18). As in vitrectomies for penetrating injuries, it is important to identify the posterior hyaloid and, if it is still adherent to the retina, create a PVD. The stump of proliferation growing through the exit site should be reduced but not eliminated, so that the posterior exit site is not reopened. Retinal breaks are managed with air–fluid exchange and endophotocoagulation or scleral buckling with cryopexy. We routinely advocate an encircling scleral buckle, even in eyes without retinal detachment.
Overall, the prognosis for these eyes appears to be improved by this management (Fig. 110.19). In a case–control study of penetrating injuries, a beneficial trend was demonstrated for those eyes managed with vitrectomy.89 Martin et al.90 achieved anatomic success in 41 of 51 eyes, with functional success (visual acuity better than 5/200) in 32 eyes (63%). Although the authors of these series have investigated a number of factors that may correlate with prognosis, the location of the exit site has profound influence on the final visual acuity. If the exit site is through the macula or optic nerve, the final vision is obviously limited. The location of the exit site also affects the surgeon’s ability to complete removal of the posterior hyaloid. This is more easily achieved when the exit site is located posterior to the vitreous base.
More recently, it has been demonstrated that chorioretinectomy (removal of the choroid and/or retina at the impact or perforation site) may improve final visual acuity and increase the chance of globe salvage.91 In this technique, first advanced by Zivojnovic nearly three decades ago,92 any retinal or uveal tissue incarcerated into the perforation site is removed using the vitrector, with diathermy destruction of the retina/choroid in a 1 mm ring around the exit wound/impact site. High cutting speeds (2500 cpm) with low vacuum are typically used. Bleeding, though uncommon from necrotic incarcerated tissue, is controlled with endodiathermy or transient elevation of infusion pressure. In a small pilot series of such a “proactive technique” reported by Kuhn et al. in 2004, chorioretinectomy was performed in five consecutive eyes within 100 hours of perforating globe injury. Their strategy involved limited vitrectomy (performed while looking through the binocular indirect ophthalmoscope) at the time of primary repair, intensive topical corticosteroid therapy, and complete vitrectomy, localized retinectomy, evacuation of subretinal blood, laser retinopexy, and placement of silicone oil 3 days later.93 While no substantive conclusions or definitive management recommendations can be made on the basis of a small uncontrolled series, the absence of proliferative vitreoretinopathy or traction retinal detachment in this series suggests proof of concept. Such a technique should only be considered, however, by surgeons with the requisite experience and in cases with adequate visualization of the posterior segment and confirmation that there is minimal retinal or choroidal detachment.
Vitreous hemorrhage and retinal detachment
Despite advances in the management of eyes with penetrating injuries, a large group of patients still have a poor prognosis. These more severe injuries most often have posterior segment involvement. A seminal study evaluating the outcome of penetrating ocular injuries identified several factors other than initial visual acuity that correlate with a poor final visual outcome. These include presence of an afferent pupillary defect, wounds involving the sclera or extending posterior to the insertion of rectus muscles, wounds >10 mm, and vitreous hemorrhage.57 The study emphasized that the prognosis after a penetrating injury is strongly influenced by the nature of the injury and the location and extent of initial damage. Functional loss of these eyes is caused by either inoperable retinal detachment or damage to ciliary body function. These changes, in most cases, result from intravitreal fibrovascular and fibroglial proliferation, which leads to traction on the ciliary body and the retina. This proliferation seems to occur more commonly in injuries with lacerations of the ciliary body and retina and in injuries with vitreous hemorrhage. These initial conclusions were recently corroborated in a prospective observational study of 69 eyes with penetrating injury.54
Beginning in 1979, Cleary and Ryan94 published a series of papers in which they described an experimental model for penetrating ocular injury with vitreous hemorrhage. Invariably, a traction retinal detachment resulted, appearing similar to the retinal detachment seen clinically after penetrating ocular injuries. In subsequent studies, they demonstrated that vitrectomy performed 1–14 days after injury could significantly reduce the risk of traction retinal detachment.95 Although several previous studies suggested the efficacy of vitreous surgery in management of severe penetrating ocular injury,96 Cleary and Ryan’s experimental studies supported the clinical impression that by removing the scaffold for intraocular proliferation, vitrectomy may reduce the incidence of severe visual loss after penetrating injuries. However, the precise role and timing of vitrectomy in penetrating injuries has not been clarified.
In eyes with vitreous hemorrhage secondary to penetrating injury, vitrectomy has been suggested to minimize intraocular fibrous proliferation and progressive vitreoretinal traction, as well as to provide visualization of the retina for identification and treatment of possible retinal detachment. A series of patients with severe penetrating ocular injury with vitreous hemorrhage and no retinal detachment treated with vitrectomy had an 82% functional success rate.97 This uses the Ryan and Allen98 definition of functional success. However, only 17 of 33 (52%) eyes with vitreous hemorrhage and concurrent retinal detachment were successfully repaired using vitrectomy.
Vitrectomy for penetrating ocular trauma was first advocated by Coles and Haik99 in 1972 when, on examining several eyes enucleated after trauma, they noted fibroblastic tissue in areas of vitreous hemorrhage within several hours of injury. Primary vitrectomy at the time of closure is thought to decrease the risk of this intraocular proliferation. Coleman,100 re-expressing this rationale for early vitrectomy, speculated that development of severe inflammatory changes may be prevented by performing vitrectomy within 72 hours of injury. At the same time, the surgeon can decrease the risk of fibroblastic proliferation. Faulborn et al.101 also advocated early surgery, suggesting primary vitrectomy (albeit open-sky) at the time of primary repair of the corneoscleral laceration. They believed that this approach improves visual prognosis by permitting examination of the fundus and immediate retinal surgery when indicated. In addition, it prevents the complications from vitreous incarceration in the laceration. Another consideration favoring early vitrectomy is that it can be combined with primary wound repair, thereby obviating the need for a second hospitalization and second operation.
In addition, several authors have noted the problem of severe hemorrhage when attempting early vitrectomy,96,98,102 presumably secondary to uveal congestion associated with acute penetrating injury. Certainly, hemorrhagic choroidal detachment can accompany a penetrating injury, making it extremely difficult to insert an infusion cannula or other vitrectomy instruments properly without damaging the retina. Delaying surgery may decrease the risk of uncontrollable intraoperative hemorrhage, as well as allow choroidals to recede or to be drained more easily.
Most authors believe that vitrectomy for severe penetrating injuries should not be delayed beyond 14 days.98,101 First, if the vitrectomy is unsuccessful, these eyes may be at risk for developing sympathetic ophthalmia and require enucleation to prevent its development. Evidence suggests that the risk of sympathetic ophthalmia may be significantly reduced if enucleation is performed within 14 days of injury. Second, histologic studies of penetrating eye injuries show intraocular cellular proliferation within 1 week of injury with membrane formation already occurring by 2 weeks.103
In most cases, general anesthesia with endotracheal intubation is employed for vitrectomy following trauma. Peribulbar or retrobulbar anesthesia has the risk of increasing orbital pressure leading to extrusion of intraocular contents, but has been used successfully in selected patients, typically those with short corneal or limbal wounds and a formed anterior chamber.104 Conventional wisdom has it that succinylcholine chloride should be avoided during induction. However, in an experimental study, succinylcholine administration resulted only in forward displacement of the iris and lens without extrusion of intraocular contents, suggesting that some of the historical fears of its use may be unwarranted.105 In most cases, the wound will have been closed prior to vitrectomy, but the globe should be inspected, and open or leaking wounds should be resutured to make the eye watertight.
At the onset of surgery, the surgeon must make several critical decisions that can have profound influence on the procedure’s outcome. First, the presence or absence of hemorrhagic choroidal detachment must be determined. This can best be done by preoperative ultrasonography. If present, the blood can often be drained through scleral incisions while simultaneously infusing air or balanced salt solution through a cannula inserted through the limbus in aphakic eyes and pars plana in phakic eyes to maintain normal IOP (Fig. 110.20). After drainage of the choroidal hemorrhage, a long pars plana infusion cannula (6 mm) should be inserted, but infusion should not be turned on until the cannula tip can be visualized in the vitreous cavity. It is essential that all sclerotomies be deep enough to ensure penetration through the uveal tissue.
Second, the surgeon must decide whether surgery can begin through the pars plana or whether it must be started through limbal incisions. For instance, in the presence of total hyphema, where the status of the retina is unknown, the hyphema can be evacuated through a limbal incision with an irrigating infusion needle inserted through a second incision (Fig. 110.21). On the other hand, the same problem can be managed in aphakic eyes by inserting a long irrigating infusion needle through a pars plana incision into the anterior chamber where it can be visualized and then turned on. Similarly, the vitrectomy instrument can be inserted through the pars plana and brought anteriorly to evacuate the hyphema.
After the anterior segment is cleared, the vitrectomy can be performed, proceeding from anterior to posterior. It is critical to remove the posterior cortical vitreous (Fig. 110.22). This is easily achieved in the presence of a PVD, which is present in many eyes with vitreous hemorrhage by 10 days after the injury. If the cortical vitreous is adherent to the retina, a soft-tipped extrusion cannula or pic should be used to create a PVD. This can be quite difficult and, possibly, hazardous, in areas of detached retina. In recent years, the introduction of smaller-gauge instrumentation has resulted in a significant percentage of vitreoretinal procedures being performed with 23G or 25G instruments. However, due to the complexity of trauma cases and the likelihood of unanticipated intraoperative findings, we still perform the majority of these procedures using the conventional 20G instruments.
When retinal detachment is present, it is essential that all tangential and anteroposterior vitreoretinal traction on the retinal break be relieved either by membrane peeling, delamination, or segmentation. If this traction cannot be relieved, it will be necessary to place a scleral buckle to support the break or to perform a relaxing retinectomy. Subretinal fluid usually is drained transvitreally during a fluid–gas exchange either through a posterior retinal break or through a posterior retinotomy created superonasal to the optic nerve in an area free of traction. In some cases, extrusion can be used to flatten the retina by drainage of posterior subretinal fluid through a peripheral retinal break. Long-acting gases, such as sulfur hexafluoride or perfluoropropane, can be injected to provide a longer-lasting internal tamponade to the detached retina. In selected cases, silicone oil may be used in primary vitrectomies for trauma.106
Retinal incarceration in the scleral laceration site can produce difficult retinal detachments. In many cases, this situation can be managed with vitrectomy and scleral buckling, particularly when the incarceration is located anteriorly. However, when the incarceration is more posterior, scleral buckling is more difficult, and retinotomy may be necessary. Han et al.107 reviewed their experience with this problem and recommended retinectomy when a scleral buckle is insufficient to relieve the traction or when the incarceration is located too posteriorly to be treated with a scleral buckle. In the former situation, they performed a circumferential relaxing retinectomy. In the latter situation, they performed retinotomies circumscribing the retinal incarceration site. Endodiathermy was placed to provide hemostasis before the retinotomy. With these techniques, they achieved anatomic success in 11 of 15 eyes (73%), but only six eyes regained visual acuity of 5/200 or better. Eyes with traumatic retinal detachment associated with retinal incarceration appear to carry a poor prognosis even when managed aggressively.
Intraocular bleeding can be a frequent intraoperative hazard of vitrectomy for penetrating injuries. Massive uncontrollable intraoperative hemorrhage has been noted as a significant problem precluding successful vitrectomy in some eyes injured by trauma. This complication was noted, however, in only two of 106 vitrectomies for trauma reported by Brinton et al.70 Intraoperative bleeding can be managed by a number of maneuvers, including raising the infusion pressure to increase the IOP and endodiathermy to areas of active hemorrhage. In some cases, a fluid–air exchange can help tamponade active bleeding. If all of these maneuvers fail, control of bleeding by infusion of a thrombin solution at a concentration of 100 U/mL may be attempted.108
Visualization of the posterior structures can be a problem for reasons other than bleeding. When the cornea becomes cloudy because of epithelial edema, the epithelium can be carefully removed with a surgical blade. Folds in Descemet’s membrane can be overcome by coating the corneal endothelial surface with sodium hyaluronate.109 In some cases, visualization through a scarred cornea can be improved with the use of a wide-angle viewing system. In cases in which the cornea is grossly edematous, markedly distorted by a stellate laceration with multiple sutures, or damaged with tissue loss requiring a patch graft, vitreoretinal surgery can be performed with a temporary keratoprosthesis. The first temporary keratoprosthesis, the Landers–Foulks device, is a clear cylindrical polymethylmethacrylate lens that fits into a trephinated corneal bed and permits excellent visualization of the vitreous and retina without an additional contact lens.110 Though a great advance, this keratoprosthesis has a concave lens that minimizes the retinal image and a long optical cylinder that obstructs visualization of the anterior retina and vitreous base where much of the significant pathology in traumatized eyes exists. Many of these limitations were eliminated with the Eckardt keratoprosthesis.111 This device has normal magnification and a short optical cylinder, allowing anterior vitreoretinal dissections and better visualization of posterior segment pathology. In a series of 11 traumatized eyes surgically rehabilitated with the aid of an Eckardt keratoprosthesis, five (45%) obtained a visual acuity of 20/400 or better.112
Penetrating keratoplasty (PKP) can be combined with vitrectomy and temporary keratoprosthesis to reconstruct severely traumatized eyes. However, functional outcomes are usually disappointing, due in large part to graft failure and hypotony from ciliary body dysfunction. In a recent series of 34 eyes managed with combined PKP and vitrectomy, fewer than one-third achieved vision of better than hand motions at any time postoperatively, and fewer than 15% of grafts remained clear more than 1 year postoperatively.113
An alternative to temporary keratoprosthesis in eyes with impaired visualization due to corneal opacity, is endoscopy.114 This approach may be particularly useful in cases in which a donor graft or corneal surgeon may not be readily available or in cases in which silicone oil placement is planned and there is concern of corneal graft failure. Disadvantages of endoscopy include the need for specialized equipment, mandatory additional surgery for corneal transplantation, and a steep learning curve.
No prospective controlled study to date has evaluated the efficacy of vitrectomy in penetrating ocular injury. Large series of patients treated with vitrectomy have been published, with the comment that favorable results would not have been possible without vitreoretinal surgical techniques. The frequency of obtaining functional visual acuity of 5/200 or better ranged from 52% (in the series of 106 patients of Brinton et al.70) to 78% (in the 112-patient series of Coleman).100 Most results from other authors were also in this range. 98,101,102 A 1984 case–control study matched eyes managed with vitrectomy with similar eyes that did not undergo vitrectomy.89 When visual results were compared, no statistical advantage from vitrectomy was found. However, a beneficial trend was demonstrated in severe injuries in which initial visual acuity was worse than 5/200. Further analysis of data from this study demonstrates that case–control matching was not done according to preoperative visual acuity and that significantly more patients had poor vision in the vitrectomy group than in the nonvitrectomy group. The shift in the distribution of visual acuity indicated a greater benefit for vitrectomy in the group with preoperative visual acuity worse than 5/200.
Endophthalmitis
Endophthalmitis is an uncommon feature of a penetrating injury and occurs in 1–7% of penetrating injuries.115,116
In one retrospective investigation, risk factors for endophthalmitis after penetrating ocular trauma were subjected to univariate and multivariate analysis.117 Factors such as scleral wounds, hyphema, uveal prolapse, retinal detachment, suprachoroidal and vitreous hemorrhage, lens rupture, and IOFB were investigated. Only lens rupture was independently significant for the development of endophthalmitis, carrying a relative risk of infection of 15.8. In a more recent comparative cohort study, patients developing post-traumatic endophthalmitis were ascertained prospectively and compared with historical controls of patients with open globe injury. Delay in primary repair, ruptured lens capsule, and dirty wound were each independently associated with the development of post-traumatic endophthalmitis.118 The presence of an IOFB had a relative risk of infection of 1.9, but this did not reach statistical significance. The National Eye Trauma Study found a statistically significant increased incidence of endophthalmitis for eyes with retained IOFBs, particularly if surgical intervention occurred more than 24 hours after the injury (13.4% versus 3.5%, P < 0.0001).119,120
Two recent series have reported lower rates of endophthalmitis, and the authors attributed this reduction in part to the systematic use of antibiotics.116,121 In the series from the Massachusetts Eye and Ear Infirmary, intravenous vancomycin (or clindamycin if allergic) and ceftazidime (or a fluoroquinolone if allergic) were administered promptly and continued for at least 48 hours.116
While the majority of cases of postoperative endophthalmitis are caused by less virulent coagulase-negative staphylococci, post-traumatic endophthalmitis is frequently caused by virulent organisms such as Bacillus, Streptococcus, and Gram-negative organisms. Bacillus cereus is most commonly associated with penetrating ocular injuries that occur in rural areas and, while rarely seen in postoperative endophthalmitis, accounts for 25-46% of cases of posttraumatic endophthalmitis.121 The multiple enzymes and exotoxins elaborated by this organism lead to blindness or enucleation in the vast majority of eyes.121 Several cases of retained useful vision have been reported after successful management of B. cereus endophthalmitis.122 Gram-negative bacteria are responsible for up to 20% of cases of posttraumatic endophthalmitis.72 Similarly, these cases also carry a poor prognosis, with one-third of eyes ending in no light perception or enucleation.123
We recommend antibiotic delivery via systemic, subconjunctival, intraocular, and topical routes. Although the Endophthalmitis Vitrectomy Study (EVS) did not show a benefit to using intravenous ceftazidime or aminoglycosides, it should be noted that the EVS studied only patients with acute endophthalmitis following cataract extraction or lens exchange.124 In post-traumatic endophthalmitis, we still consider systemic antibiotic use the standard of care. We prefer a third- or fourth-generation fluoroquinolone (levofloxacin 500–750 mg or moxifloxacin 400 mg daily for 5 days) because of its broad coverage of most likely pathogens and its excellent vitreous penetration following intravenous or oral administration.
Prophylactic intraocular injection of antibiotics to prevent endophthalmitis in patients with penetrating injury is controversial. However, in settings where there is high suspicion of injury from a contaminated foreign body, or early signs of endophthalmitis are present (e.g., significant anterior chamber cells, perivascular retinal sheathing), prophylactic treatment with intravitreal antibiotics should be considered. In a prospective study of eyes with penetrating injury, patients were randomized to receive either intraocular injection of antibiotics (40 µg of gentamicin sulfate and 45 µg of clindamycin sulfate) or balanced saline solution. The rate of endophthalmitis within 2 weeks was significantly lower in the group treated with antibiotics (2.3% versus 0.3%), but this difference was driven by the high incidence of infection in eyes with IOFB (28% in the control group versus 0% in the antibiotic group).125
Prognostication for cases of traumatic endophthalmitis is difficult because of the compounding effect of the penetrating injury. Prognosis also relates to the specific organism and to the interval to diagnosis. Overall, Brinton et al.115 reported 42% of cases obtaining final VA of 20/300 or better and 26% with 20/30 or better. Affeldt et al.126 showed only 22% of their patients with 20/400 or better. Lieb et al. reported final VA of 20/400 or better in only 50% of open globes with a positive bacterial culture and overt signs of endophthalmitis.127
Cataract
When the decision has been made to perform a primary lensectomy, the surgeon should first repair the laceration using the techniques and principles described earlier in this chapter. At that point, it must be decided whether the cataract extraction can be performed through a limbal incision or whether a pars plana approach is necessary. When the laceration is limited to the cornea and the posterior lens capsule is believed to be intact, a limbal incision should be created and lens aspiration, phacoemulsification, or standard extracapsular cataract surgery performed. If, during the course of the surgery, it becomes apparent that the posterior capsule has been damaged, it is critical to manage the vitreous loss properly, making sure there is no vitreous incarcerated in either the laceration site or the limbal incision. Primary placement of an IOL has been shown to be well tolerated in selected cases and does not appear to increase the risk of endophthalmitis or other postoperative complications. However, the IOL power selection may be inaccurate and the surgeon may misjudge the intactness of the posterior capsule. In one series, 64% of patients who underwent lensectomy and IOL implantation at the time of primary repair of a penetrating ocular injury obtained 20/40 or better visual acuity.128 Therefore, if capsular support allows, one can proceed with IOL placement. If too little capsule remains, an anterior chamber IOL, or sutured posterior chamber IOL should be deferred as a second procedure. Implantation of an IOL in children has also become an accepted means of visual rehabilitation. In children 4–17 years of age, up to 87% of penetrating ocular injuries primarily managed with lens aspiration and IOL implantation obtained 20/40 or better visual acuity.129 IOL implantation in infants, however, is still controversial and should probably be deferred to a secondary procedure.
Late complications of penetrating injury
Fibrotic membranes result from organization of residual lens material after a traumatic cataract. In this situation, the material is often quite difficult to cut with the vitrectomy instrument alone (Fig. 110.23). However, after segmentation with a myringotomy blade or scissors, the material may be more easily removed with the vitrectomy probe. When this fails, the segments of tissue can be grasped with intraocular microforceps, cut with microscissors, and removed from the eye through the sclerotomy. If the tissue has become vascularized, it may be necessary to use endodiathermy for hemostasis. With these techniques, a clear pupillary space can be achieved in nearly all cases. Final vision depends on the status of the remainder of the eye, as well as amblyopia in children.
Some aphakic eyes develop an occluded pupil (Fig. 110.24). This often is caused by residual lens material but can also result from contraction of a vitreous sheet incarcerated in a corneal or limbal laceration. In the latter situation, the pupillary space can be recreated by freeing the vitreous from the wound and excising it, although cutting of the iris with the vitrectomy instrument may be necessary in some instances.
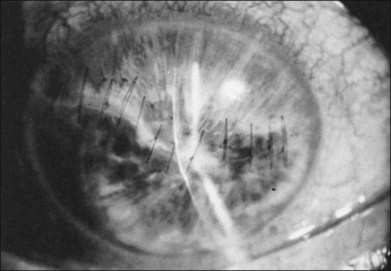
Fig. 110.24 This patient developed an occluded pupil after an extensive corneal laceration with extrusion of the lens.
Macular pucker can develop after penetrating injuries and is managed in an identical manner to an idiopathic macular pucker. Retinal detachment, either traction or rhegmatogenous, can also be a late complication of a penetrating injury. Pars plana vitrectomy and prophylactic scleral buckling have been described as decreasing the risk of this complication. However, even in eyes with ocular lacerations without vitreous hemorrhage, retinal detachments can be seen in a higher percentage than in uninjured eyes,130 since vitreous is incarcerated in the wound site in virtually all cases of scleral laceration. Repair of late retinal detachments in patients with a history of penetrating trauma should be managed similar to nontraumatic detachments. However, additional care should be taken when one is performing the conjunctival peritomy and isolating the rectus muscle because of the possibility of areas of scleral thinning associated with the laceration.
Sympathetic ophthalmia
Sympathetic ophthalmia is a bilateral inflammation of the uveal tract characterized by nodular or diffuse infiltration with lymphocytes and epithelioid cells. Its onset is insidious, and its course is characterized by exacerbations and remissions. Although the cause is unknown, almost all reported cases are associated with a penetrating injury to the globe.131 The risk of sympathetic ophthalmia following trauma is quoted to be between 0.3% and 1.9%.132 In a prospective surveillance study for sympathetic ophthalmia performed in the UK and Ireland, an estimated incidence of 0.03/100 000 was calculated. Interestingly, the most common cause in the 23 cases reported was retinal surgery.133
Evidence suggests that the risk of sympathetic ophthalmia is extremely small if the injured eye is enucleated within 2 weeks of the injury, hence the recommendation for secondary vitreoretinal surgery within this time frame to allow determination of whether an injured eye is salvageable. If it is believed that the eye has no visual potential after vitrectomy, enucleation is performed. However, isolated cases have been reported at days 5, 8, and 10 after injury in which inflammation was noted in the sympathizing eye.134
Corticosteroids remain the treatment of choice for sympathetic ophthalmia, though steroid-sparing immunomodulating agents may be necessary because of side effects of corticosteroids or the need for long-term use. High doses of prednisone (up to 200 mg/day) over the first 7–10 days may be particularly critical to the patient’s prognosis. Frequent topical corticosteroids should be used in cases with anterior segment inflammation. In patients whose disease does not appear to respond to corticosteroids, cytotoxic agents may be effective. Use of corticosteroids from the time of injury does not appear to prevent development of sympathetic ophthalmia.135
Although there is little consensus on the optimal method of treatment, it is generally agreed that prompt recognition and treatment of sympathetic ophthalmia are crucial for preservation of vision. Lubin et al.134 reported 13 of 18 having final vision of 20/50 or better. Kilmartin et al. reported 1-year visual acuity of 20/40 or better in 12 of 16 patients treated initially with systemic corticosteroids, although 11 received additional immunosuppression.133
Prevention
As the vast majority of ocular trauma is preventable, physicians should reinforce the importance of eye protection for all occupational or recreational activities that may lead to eye injury. The primary occupational risks include metalworking and use of powered tools. Sports with a higher risk of injury included basketball, baseball, pool sports, and racquet sports.136 In a joint policy statement, the American Academy of Ophthalmology and the American Academy of Pediatrics recommend the mandatory use of protective eyewear for all young athletes with best-corrected vision worse than 20/40 in one eye or those who have undergone eye surgery.137 Various styles of goggles, face shields, face guards and masks tailored to individual sports are readily available. Sports eyewear should conform to the requirements of the American Society for Testing and Materials (ASTM), which provides standards of protection appropriate for specific sports. In the USA, the Protective Eyewear Certification Council (PECC) has begun certifying protectors that comply with the ASTM requirements. Lenses made of polycarbonate material (or CR-39, an allyl/resin plastic, for stronger prescriptions) are recommended.
Legislation in a given country has a major impact on the incidence of serious eye injuries caused by a particular object. For example, firearms and BB guns are responsible for a very high rate of injury (12% in the United States Eye Injury Registry), whereas the rate is less where their use is illegal (<1% in the Hungarian Registry). Another typical example is fireworks, a major source of injury if legalized: in the Eye Injury Registry of Alabama, fireworks are responsible for 4.4% of all serious eye injuries; in Hungary, where fireworks are illegal for individual use, the rate is 0.1%.138 Thus, legislation directed at regulation of behaviors or restriction of items known to be associated with ocular injury may reduce incidence and severity of ocular trauma.
1 Negrel AD, Thylefors B. The global impact of eye injuries. Ophthalmic Epidemiol. 1998;5:143–169.
2 Prevent Blindness America. The scope of the eye injury problem (brochure). Chicago: IL. 2008.
3 Anon. Leads from the MMWR: leading work-related diseases and injuries – United States. JAMA. 1984;251:2503–2504.
4 National Institute for Occupational Safety and Health. updated February 27, 2008 http://www.cdc.gov/niosh/topics/eye/
5 Kuhn F, Morris R, Witherspoon CD, et al. A standardized classification of ocular trauma. Ophthalmology. 1996;103:240–243.
6 Broadrick JD. Corneal blooding staining after hyphema. Br J Ophthalmol. 1972;56:589–592.
7 Campbell DG. Ghost cell glaucoma following trauma. Ophthalmology. 1981;38:1151–1158.
8 Radius RL, Finkelstein D. Central retinal artery occlusion (reversible) in sickle trait with glaucoma. Br J Ophthalmol. 1976;60:428–430.
9 Goldberg MF. Sickled erythrocytes, hyphema, and secondary glaucoma. I. The diagnosis and treatment of sickled erythrocytes in human hyphemas. Ophthalmic Surg. 1979;10:17–31.
10 Kitazawa Y. Management of traumatic hyphema with glaucoma. Int Ophthalmol Clin. 1981;21:167–181.
11 Kearns P. Traumatic hyphema: a retrospective study of 314 cases. Br Ophthalmol. 1991;75:137–141.
12 Gilbert HD, Jensen AD. Atropine in the treatment of traumatic hyphema. Am J Ophthalmol. 1973;5:1297–1300.
13 Read J, Goldberg MF. Comparison of medical treatment for traumatic hyphema. Trans Am Acad Ophthalmol Otolaryngol. 1974;78:799–806.
14 Spoor TC, Hammer M, Belloso H. Traumatic hyphema: failure of steroids to alter its course. Arch Ophthalmol. 1980;98:116–119.
15 Granelli-Piperno A, Vassalli JD, Reich E. Secretion of plasminogen activator by human polymorphonuclear leukocytes: modulation by glucocorticoids and other effects. J Exp Med. 1977;146:1693.
16 Palmer DJ, Goldberg MF, Frenkel, et al. A comparison of two dose regimens of epsilon aminocaproic acid in the prevention and management of secondary traumatic hyphemas. Ophthalmology. 1986;93:102–108.
17 Rahmani B, Jahadi HR. Comparison of tranexamic acid and prednisolone in the treatment of traumatic hyphema. A randomized clinical trial. Ophthalmology. 1999;106:375–379.
18 Gharaibeh A, Savage HI, Scherer RW, et al. Medical interventions for traumatic hyphema. Cochrane Database of Systematic Reviews. 1, 2011. CD005431
19 Read J. Traumatic hyphema: surgical versus medical management. Ann Ophthalmol. 1975;7:659–670.
20 Deutsch TA, Weinreb RN, Goldberg MF. Indications for surgical management of hyphema in patients with sickle cell trait. Arch Ophthalmol. 1984;102:566–569.
21 Belcher CD, Brown SVL, Simmons RJ. Anterior chamber washout for traumatic hyphema. Ophthalmology. 1979;16:475–479.
22 Sears ML. Surgical management of blackball hyphema. Trans Am Acad Ophthalmol Otolaryngol. 1970;74:820–826.
23 Weiss JS, Parrish RK, Anderson DR. Surgical therapy of traumatic hyphema. Ophthalmic Surg. 1983;14:343–345.
24 McCuen BW, Fung WE. The role of vitrectomy instrumentation in the treatment of severe traumatic hyphema. Am J Ophthalmol. 1979;88:930–934.
25 Stern WH, Monclal KM. Vitrectomy instrumentation for surgical evacuation of total anterior chamber hyphema and control of recurrent anterior chamber hemorrhage. Ophthalmic Surg. 1979;10:34–37.
26 Jarrett WH. Dislocation of the lens: a study of 166 hospitalized cases. Arch Ophthalmol. 1979;78:289–296.
27 Aaberg TM, Jr., Rubsamen PE, Flynn HW, Jr., et al. Giant retinal tear as a complication of attempted removal of intravitreal lens fragments during cataract surgery. Am J Ophthalmol. 1997;24:222–226.
28 Kazemi S, Wirostko WJ, Sinha S, et al. Combined pars plana lensectomy-vitrectomy with open-loop flexible anterior chamber intraocular lens (AC IOL) implantation for subluxated lenses. Trans Am Ophthalmol Soc. 2000;98:247–251. discussion 251–253
29 Kodjikian L, Beby F, Spire M, et al. Combined pars plana phacofragmentation, vitrectomy, and Artisan lens implantation for traumatic subluxated cataracts. Retina. 2006;26:909–916.
30 Chaudhry NA, Belfort A, Flynn HW, Jr., et al. Combined lensectomy, vitrectomy and scleral fixation of intraocular lens implant after closed-globe injury. Ophthalmic Surg Lasers. 1999;30:375–381.
31 Greven CM, Collins AS, Slusher MM, et al. Visual results, prognostic indicators, and posterior segment findings following surgery for cataract/lens subluxation-dislocation secondary to ocular contusion injuries. Retina. 2002;22:575–580.
32 McCannel MA. A retrievable suture idea for anterior uveal problems. Ophthalmic Surg. 1976;7:98–103.
33 Yeung L, Chen TL, Kuo YH, et al. Severe vitreous hemorrhage associated with closed-globe injury. Graefes Arch Clin Exp Ophthalmol. 2006;244:52–57.
34 Gregor Z, Ryan SJ. Blood-retinal barrier after blunt trauma to the eye. Graefes Arch Clin Exp Ophthalmol. 1982;219:205–208.
35 Mansour AM, Green WR, Hogge C. Histopathology of commotio retinae. Retina. 1992;12:24–28.
36 Sipperley JO, Quigley HA, Gass JDM. Traumatic retinopathy in primates: the explanation of commotio retinae. Arch Ophthalmol. 1978;96:2267–2273. 178
37 Cox MS. Retinal breaks caused by blunt nonperforating trauma at the point of impact. Trans Am Ophthalmol Soc. 1980;78:414–465.
38 Bloome MA, Ruiz RS, Russo CE, et al. Acute retinal necrosis. Ann Ophthalmol. 1979;11:723–728.
39 Richards RD. Chorioretinitis sclopetaria. Trans Am Ophthalmol Soc. 1966;66:214–232.
40 Cox MS, Schepens CL, Freeman HM. Retinal detachment due to ocular contusion. Arch Ophthalmol. 1966;76:678–685.
41 Weidenthal DT, Schepens CL. Peripheral fundus changes associated with ocular contusion. Am J Ophthalmol. 1966;62:465–477.
42 Ross WH. Traumatic retinal dialysis. Arch Ophthalmol. 1981;99:1371–1374.
43 Zion VM, Burton TC. Retinal dialysis. Arch Ophthalmol. 1980;98:1971–1974.
44 Johnston PB. Traumatic retinal detachment. Br J Ophthalmol. 1991;75:18–21.
45 Yamashita T, Uemara A, Uchino E, et al. Spontaneous closure of traumatic macular hole. Am J Ophthalmol. 2002;133:230–235.
46 Wu W, Drenser KA, Trese MT, et al. Pediatric traumatic macular hole: results of autologous plasmin enzyme-assisted vitrectomy. Am J Ophthalmol. 2007;144:668–672.
47 Johnson RN, McDonald HR, Lewis H, et al. Traumatic macular hole: observations, pathogenesis, and results of vitrectomy surgery. Ophthalmology. 2001;108:853–857.
48 Kuhn F, Morris R, Mester V, et al. Internal limiting membrane removal for traumatic macular hole. Ophthalmic Surg Lasers. 2001;32:308–315.
49 Chow DR, Williams GA, Trese MT, et al. Successful closure of traumatic macular holes. Retina. 1999;19:405–409.
50 Kraushar MF. Medical malpractice experiences of vitreoretinal specialists: risk prevention strategies. Retina. 2003;23:523–529.
51 Kuhn F, Maisiak R, Mann L, et al. The ocular trauma score (OTS). Ophthalmol Clin N Am. 2002;15:163–165.
52 Sternberg P, Jr., de Juan E, Michels RG. Multivariate analysis of prognostic factors in penetrating ocular injuries. Am J Ophthalmol. 1984;98:467–472.
53 Gilbert CM, Soong HK, Hirst LW. A two-year prospective study of penetrating ocular trauma at the Wilmer Ophthalmological Institute. Ann Ophthalmol. 1987;19:104–106.
54 Rao LG, Ninan A, Rao KA. Descriptive study on ocular survival, visual outcome and prognostic factors in open globe injuries. Indian J Ophthalmol. 2010;58:321–323.
55 Esmaeli B, Elner SG, Schork MA, et al. Visual outcome and ocular survival after penetrating trauma: a clinicopathologic study. Ophthalmology. 1995;102:393–400.
56 Ahmadieh H, Soheilian M, Sajjadi H, et al. Vitrectomy in ocular trauma: factors influencing final visual outcome. Retina. 1993;13:107–113.
57 De Juan E, Sternberg P, Jr., Michels RG. Penetrating injuries: types of injuries and visual results. Ophthalmology. 1983;90:1318–1322.
58 Weichel ED, Colyer MH, Ludlow SE, et al. Combat ocular trauma visual outcomes during operations Iraqi and enduring freedom. Ophthalmology. 2008;115:2235–2245.
59 Sevel D, Krausz H, Ponder T, et al. Value of computed tomography for the diagnosis of a ruptured eye. J Comput Assist Tomogr. 1983;7:870–875.
60 Kramer M, Hart L, Miller JW. Ultrasonography in the management of penetrating ocular trauma. Int Ophthalmol Clin. 1995;35:181–192.
61 Dass AB, Ferrone PJ, Chu RY, et al. Sensitivity of spiral computed tomography scanning for detecting intraocular foreign bodies. Ophthalmology. 2001;108:2326–2328.
62 Zinreich SJ, Miller NR, Aguayo JB, et al. Computed tomographic three-dimensional localization and compositional evaluation of intraocular and orbital foreign bodies. Arch Ophthalmol. 1986;104:1477–1482.
63 Topilow HW, Ackerman AL, Zimmerman RD. Limitations of computerized tomography in the localization of intraocular foreign bodies. Arch Ophthalmol. 1986;104:1477–1482.
64 Gunenc U, Maden A, Kayak S, et al. Magnetic resonance imaging and computed tomography in the detection and location of intraocular foreign bodies. Doc Ophthalmol. 1992;81:369–378.
65 Erdey RA, Lindahl KJ, Temnycky GO, et al. Techniques for application of tissue adhesive for corneal perforations. Ophthalmic Surg. 1991;22:352–354.
66 Rashid ER, Waring GO. Use of Healon in anterior segment trauma. Ophthalmic Surg. 1982;13:201–203.
67 Campochiaro PA, Kaden IH, Vidaurri-Leal J, et al. Cryotherapy enhances intravitreal dispersion of viable retinal pigment epithelial cells. Arch Ophthalmol. 1985;103:434–436.
68 Stone TW, Siddiqui N, Arroyo JG, et al. Primary scleral buckling in open-globe injury involving the posterior segment. Ophthalmology. 2000;107:1923–1926.
69 Arroyo JG, Postel EA, Stone T, et al. A matched study of primary scleral buckle placement during repair of posterior segment open globe injuries. Br J Ophthalmol. 2003;87:75–78.
70 Brinton GS, Aaberg TM, Reeser FH, et al. Surgical results in ocular trauma involving the posterior segment. Am J Ophthalmol. 1982;94:271–278.
71 Neubauer H. Ocular metallosis. Trans Ophthalmol Soc UK. 1979;99:502–510.
72 Parrish CM, O’Day DM. Traumatic endophthalmitis. Int Ophthalmol Clin. 1987;27:112–119.
73 Thompson JT, Parver LM, Enger CL, et al. Infectious endophthalmitis after penetrating injuries with retained intraocular foreign bodies. National Eye Trauma System. Ophthalmology. 1993;100:1468–1474.
74 Chaudhry IA, Shamsi FA, Al-Harthi E, et al. Incidence and visual outcome of endophthalmitis associated with intraocular foreign bodies. Graefes Arch Clin Exp Ophthalmol. 2008;246:181–186.
75 Colyer MH, Weber ED, Weichel ED, et al. Delayed intraocular foreign body removal without endophthalmitis during Operations Iraqi Freedom and Enduring Freedom. Ophthalmology. 2007;114:1439–1447.
76 Percival SPB. Late complications from posterior segment intraocular foreign bodies. Br J Ophthalmol. 1972;56:462–468.
77 Mester V, Kuhn F. Ferrous intraocular foreign bodies retained in the posterior segment: management options and results. Int Ophthalmol. 1998;22:355–362.
78 Crock GW, Janakiraman P, Reddy P. Intraocular magnet of Parel. Br J Ophthalmol. 1986;70:879–885.
79 Slusher MM, Sarin LK, Federman JL. Management of intraretinal foreign bodies. Ophthalmology. 1982;89:369–373.
80 Ambler JS, Meyers SM. Management of intraretinal metallic foreign bodies without retinopexy in the absence of retinal detachment. Ophthalmology. 1991;98:391–394.
81 Hutton WL, Snyder WB, Vaiser A. Surgical removal of nonmagnetic foreign bodies. Am J Ophthalmol. 1975;80:838–843.
82 Michels RG. Surgical management of nonmagnetic intraocular foreign bodies. Arch Ophthalmol. 1975;93:1003–1006.
83 Pieramici DJ, Capone A, Jr., Rubsamen PE, et al. Lens preservation after intraocular foreign body injury. Ophthalmology. 1996;103:1563–1567.
84 Woodcock MG, Scott RA, Huntbach J, et al. Mass and shape as factors in intraocular foreign body injuries. Ophthalmology. 2006;113:2262–2269.
85 Ehlers JP, Kunimoto DY, Ittoop S, et al. Metallic intraocular foreign bodies: characteristics, interventions, and prognostic factors for visual outcome and globe survival. Am J Ophthalmol. 2008;146:427–433.
86 Muller-Jensen K. [Doppelt perforierende augenverletzungen.]. Klin Monatsbl Augenheilkd. 1964;145:754–758.
87 Topping TM, Abrams GW, Machemer R. Experimental double perforating injury of the posterior segment in rabbit eyes. Arch Ophthalmol. 1979;97:735–742.
88 Abrams GW, Topping TM, Machemer R. Vitrectomy for injury. Arch Ophthalmol. 1979;97:743–748.
89 De Juan E, Sternberg P, Jr., Michels RG. Evaluation of vitrectomy in penetrating ocular trauma: a case control study. Arch Ophthalmol. 1984;102:1160–1163.
90 Martin DF, Meredith TA, Topping TM, et al. Perforating (through-and-through) injuries of the globe: surgical results with vitrectomy. Arch Ophthalmol. 1991;109:951–956.
91 Weichel ED, Bower KS, Colyer MH. Chorioretinectomy for perforating or severe intraocular foreign body injuries. Graefes Arch Clin Exp Ophthalmol.. 2010;248:319–330.
92 Zivojnovic R. Silicone oil in vitreoretinal surgery. Chapter 5: Surgical techniques. Dordrecht: Marinus Nijhoff; 1987. p. 45–103
93 Kuhn F, Mester V, Morris R. A proactive treatment approach for eyes with perforating injury. Klin Monbl Augenheilkd. 2004;221:622–628.
94 Cleary PE, Ryan SJ. Method of production and natural history of experimental posterior penetrating eye injury in the rhesus monkey. Am J Ophthalmol. 1979;88:212–220.
95 Cleary PE, Ryan SJ. Vitrectomy in penetrating eye injury: results of a controlled trial of vitrectomy in an experimental posterior penetrating eye injury in the rhesus monkey. Arch Ophthalmol. 1981;99:287–292.
96 Benson WE, Machemer R. Severe perforating injuries treated with pars plana vitrectomy. Am J Ophthalmol. 1976;81:728–732.
97 Meredith TA, Gordon PA. Pars plana vitrectomy for severe penetrating injury with posterior segment involvement. Am J Ophthalmol. 1987;103:549–554.
98 Ryan SJ, Allen AW. Pars plana vitrectomy in ocular trauma. Am J Ophthalmol. 1979;88:483–491.
99 Coles WH, Haik GM. Vitrectomy in intraocular trauma. Arch Ophthalmol. 1972;87:621–628.
100 Coleman DJ. Early vitrectomy in the management of the severely traumatized eye. Am J Ophthalmol. 1982;93:543–551.
101 Faulborn J, Atkinson A, Olivier C. Primary vitrectomy as a preventive surgical procedure in the treatment of severely injured eyes. Br J Ophthalmol. 1977;61:202–208.
102 Cupples HP, Whitmore PV, Wertz FD, III., et al. Ocular trauma treated by vitreous surgery. Retina. 1983;3:103–107.
103 Winthrop SR, Cleary PE, Minckler DS, et al. Penetrating eye injuries: a histopathological review. Br J Ophthalmol. 1980;64:809–817.
104 Scott IU, Gayer S, Voo I, et al. Regional anesthesia with monitored anesthesia care for surgical repair of selected open globe injuries. Ophthalmic Surg Lasers Imaging. 2005;36:122–128.
105 Moreno RJ, Kloess P, Carlson DW. Effect of succinylcholine on the intraocular contents of open globes. Ophthalmology. 1991;98:636–638.
106 Spiegel D, Nasemann J, Nawrocki J, et al. Severe ocular trauma managed with primary pars plana vitrectomy and silicone oil. Retina. 1997;17:275–285.
107 Han DP, Mieler WF, Abrams GL, et al. Management of traumatic retinal incarceration with vitrectomy. Arch Ophthalmol. 1988;106:640–645.
108 De Bustros S, Glaser BM, Johnson MA. Thrombin infusion for the control of intraocular bleeding during vitreous surgery. Arch Ophthalmol. 1985;103:837–839.
109 Landers MB, III. Sodium hyaluronate (Healon) as an aide to intravitreal fluid/gas exchange. Am J Ophthalmol. 1982;94:557–559.
110 Landers MB, III., Foulks GN, Landers, et al. Temporary keratoprosthesis for use during pars plana vitrectomy. Am J Ophthalmol. 1981;91:615–619.
111 Eckardt C. A new temporary keratoprosthesis for pars plana vitrectomy. Retina. 1987;7:34–37.
112 Gallemore RP, Bokosky JE. Penetrating keratoplasty with vitreoretinal surgery using the Eckardt temporary keratoprosthesis: modified technique allowing use of larger corneal grafts. Cornea. 1995;14:33–38.
113 Roters S, Szurman P, Hermes S, et al. Outcome of combined penetrating keratoplasty with vitreoretinal surgery for management of severe ocular injuries. Retina. 2003;23:48–56.
114 Sabti KA, Raizada S, Kandari JA, et al. Applications of endoscopy in vitreoretinal surgery. Retina. 2008;28:159–166.
115 Brinton GS, Topping TM, Hyndiuk RA, et al. Posttraumatic endophthalmitis. Arch Ophthalmol. 1984;102:547–550.
116 Andreoli CM, Andreoli MT, Kloek CE, et al. Low rate of endophthalmitis in a large series of open globe injuries. Am J Ophthalmol. 2009;147:601–608.
117 Thompson WS, Rubsamen PE, Flynn HW, et al. Endophthalmitis after penetrating trauma: Risk factors and visual acuity outcomes. Ophthalmol. 1995;102:1696–1701.
118 Essex RW, Yi Q, Charles PG, et al. Post-traumatic endophthalmitis. Ophthalmology. 2004;111:2015–2022.
119 Dannenberg AL, Parver LM, Brechner RJ, et al. Penetrating eye injuries in the workplace: the National Eye Trauma System Registry. Arch Ophthalmol. 1992;110:843–848.
120 Dannenberg AL, Parver LM, Fowler CJ. Penetrating eye injuries related to assault: the National Eye Trauma System Registry. Arch Ophthalmol. 1992;110:849–852.
121 Boldt HC, Pulido JS, Blodi CF, et al. Rural endophthalmitis. Ophthalmology. 1989;96:1722–1726.
122 Foster RE, Martinez JA, Murray TG, et al. Useful visual outcomes after treatment of Bacillus cereus endophthalmitis. Ophthalmology. 1996;103:390–397.
123 Irvine WD, Flynn HW, Jr., Miller D, et al. Endophthalmitis caused by gram-negative organisms. Arch Ophthalmol. 1992;110:1450–1454.
124 Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479–1496.
125 Soheilian M, Rafati N, Mohebbi MR, et al. Prophylaxis of acute posttraumatic bacterial endophthalmitis: a multicenter, randomized clinical trial of intraocular antibiotic injection, report 2. Arch Ophthalmol. 2007;125:460–465.
126 Affeldt JC, Flynn HW, Jr., Forster RK, et al. Microbial endophthalmitis resulting from ocular trauma. Ophthalmology. 1987;94:407–413.
127 Lieb DF, Scott IU, Flynn HW, Jr., et al. Open globe injuries with positive intraocular cultures. Factors influencing final visual acuity outcomes. Ophthalmology. 2003;110:1560–1566.
128 Rubsamen PE, Irvin WD, McCuen BW, II., et al. Primary intraocular lens implantation in the setting of penetrating ocular trauma. Ophthalmology. 1995;102:101–107.
129 Koenig SB, Ruttum MS, Lewandowski MF, et al. Pseudophakia for traumatic cataracts in children. Ophthalmology. 1993;100:1218–1224.
130 Cox MS, Freeman HM. Retinal detachment due to ocular penetration. I. Clinical characteristics and surgical results. Arch Ophthalmol. 1978;96:1354–1361.
131 Lewis ML, Gass JDM, Spencer WH. Sympathetic uveitis after trauma and vitrectomy. Arch Ophthalmol. 1978;96:263–267.
132 Gasch AT, Foster CS, Grosskreutz CL, et al. Postoperative sympathetic ophthalmia. Int Ophthalmol Clin. 2000;40:69–84.
133 Kilmartin DJ, Dick AD, Forrester JV. Prospective surveillance of sympathetic ophthalmia in the UK and Republic of Ireland. Br J Ophthalmol. 2000;84:259–263.
134 Lubin JR, Albert DM, Weinstein M. Sixty-five years of sympathetic ophthalmia: a clinicopathologic review of 105 cases (1913–1978). Ophthalmology. 1980;87:109–121.
135 Kay ML, Yanoff M, Katowitz JA. Development of sympathetic uveitis in spite of corticosteroid therapy. Am J Ophthalmol. 1974;78:90–94.
136 Larrison WI, Hersh PS, Kunzweiler T, et al. Sports-related ocular trauma. Ophthalmology. 1990;97:1265–1269.
137 American Academy of Pediatrics Committee on Sports Medicine and Fitness. Protective eyewear for young athletes. Pediatrics. 2004;113:619–622.
138 Kuhn F, Morris R, Witherspoon CD, et al. Serious fireworks-related eye injuries. Ophthalmic Epidemiol. 2000;7:139–148.