Chapter 122 Surgery for Intractable Spasticity
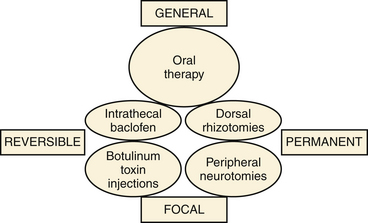
Methods are classified according to whether their impact is general or focal and whether the effects are temporary or permanent (Fig. 122-1). They include intrathecal baclofen (ITB) therapy and botulinum toxin injections, along with lesioning operations aimed at peripheral nerves, dorsal roots, the spinal cord, and the dorsal root entry zone (DREZ).
The guidelines given in the present chapter have been built from personal surgical experience of more than 1000 adult patients and more than 150 children with cerebral palsy over the last 25 years. A strong anatomic–physiologic basis and knowledge of the history and evolution of concepts about surgery for spasticity are important prerequisites before starting to deal with these complex patients.1–4
Surgical Techniques
Intrathecal Baclofen Therapy
The placement of an intrathecal drug pump assures regional delivery of medications in the cerebrospinal fluid (CSF) surrounding the spinal cord for spasticity. Baclofen is a γ-aminobutyric acid B analogue, and direct delivery to the intrathecal CSF avoids the blood–brain barrier.5
ITB therapy can be preceded by a test to screen for adequate response to the medication. The common standard procedure is as follows: The patient receives a bolus of 25 to 50 μg of baclofen via lumbar puncture or via a temporary lumbar catheter connected to a subcutaneous access reservoir. In the absence of a positive response, indicated by a two-point reduction in the patient’s Ashworth score 4 to 8 hours following administration, the bolus dose is increased in 25-μg increments up to a maximum bolus of 100 μg. Once a positive response is observed without unacceptable loss of function, the patient is considered to be a candidate for pump implantation. However, the bolus dose response is a poor guide to the likely daily infusion rate that will be needed subsequently. The “bolus method” can be read as “false-negative responses” in the sense that it may produce a brutal or exaggerated loss of motor power and muscle tone, which might be interpreted by the patient as a decrease in functional status. This holds especially true for the patients with the ability to walk. Therefore, the bolus test should be replaced by a continuous infusion test, using an external automatic injection pump connected to a line implanted into a subcutaneous reservoir. The test should last several days so that functional capabilities can be reliably evaluated. The initial postimplantation infusion dose depends, in part, on the effective screening dose. Typically, the initial starting dose is double the effective screening dose. The dose is then increased daily by 10% to 30% until the desired effect is achieved. The most useful criterion for dose adjustment is effective suppression of the hyperactive reflexes, such as tendon jerk, clonus, spasms, cramps, and decrease of muscle tone. Once the effective dose has been stabilized, the administration of the drug can be fine-tuned. A programmable pump allowing cyclic dose adjustments makes it possible to provide levels that correlate with the daily variability of spastic symptoms. The Synchromed pump (Medtronic, Minneapolis, MN) is the most frequently used. A detailed and well-illustrated description of the surgical technique of implantation can be found in Penn and Kroin’s article.6
Selective Peripheral Neurotomies
Peripheral neurotomies were introduced for treatment of the spastic foot in 1912 by Stoffel.7 Peripheral neurotomies were made more selective by using microsurgical dissection and mapping with intraoperative electric stimulation to better identify the function of individual nerve fascicles8–11 (Fig. 122-2). Neurotomies consist of partial sectioning of one or several motor fascicles corresponding to the muscle or muscles in which spasticity is considered excessive. They act by interrupting the segmental reflex arch on both its afferent and its efferent pathways. Neurotomies must not involve sensory nerve fibers; even their partial section could be responsible for paresthesias and deafferentation pain. Motor branches must be clearly isolated from the nerve trunk; motor fascicles have to be dissected and identified within the nerve trunk proximally to the formation of their corresponding identifiable branch. Empirically, it is agreed that to be effective, neurotomies should section around 50% to 80% of all motor fascicles to the targeted muscles.
Principles
Anesthesia
During surgery, it may be useful to test the efficacy of the procedure by evaluating the stretch reflex (e.g., maneuvers for clonus); this implies that reflexes are not depressed by the anesthetic drugs. Muscles relaxants must be avoided; nitrous oxide and propofol are contraindicated because they modify reflex excitability. General anesthesia has to be performed without long-lasting paralytics so that the motor responses elicited by the bipolar electric stimulation of the motor fascicles can be detected.
Techniques
Surgery at the Lower Limb
Obturator Neurotomy for the Spastic Hip
The incision can be performed along the body of the adductor longus at the proximal part of the thigh or transversely at the hip flexion fold, centered on the prominence of the adductor longus tendon. In addition to its more aesthetic appearance, the later incision facilitates adductor longus tenotomy when necessary (Fig. 122-3A). To rapidly locate the anterior branch of the obturator nerve, which is the target, the dissection is conducted laterally to the adductor longus muscle body. The posterior branch, situated more deeply, should be spared to preserve the hip-stabilizing muscles (Fig. 122-3B).
Hamstring Neurotomy for the Spastic Knee
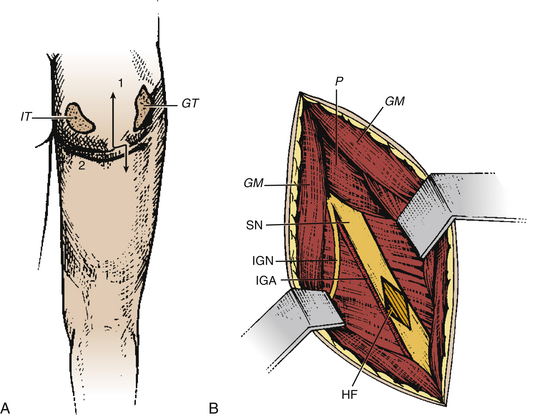
Hamstring neurotomy is indicated in children with spastic diplegia to counter the accentuation of the flexion deformity of the knees observed with growth. The transverse incision is performed at the gluteal fold, centered on the groove between the ischium and the greater trochanter (Fig. 122-4A). After crossing the gluteus maximus, the sciatic nerve is identified in the depth of the incision. The branches to the hamstring muscles are isolated at the border of the nerve, primarily based on responses of the semitendinosus muscle, which is the major muscle responsible for spasticity (Fig. 122-4B).
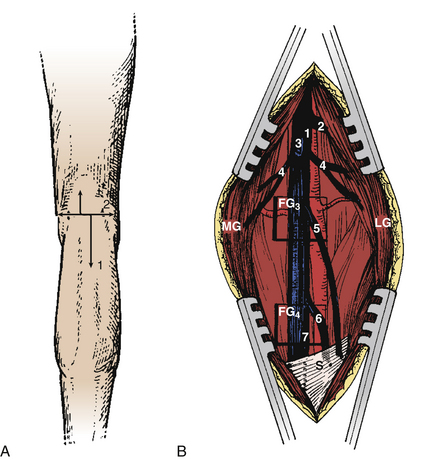
Tibial Neurotomy for the Spastic Foot
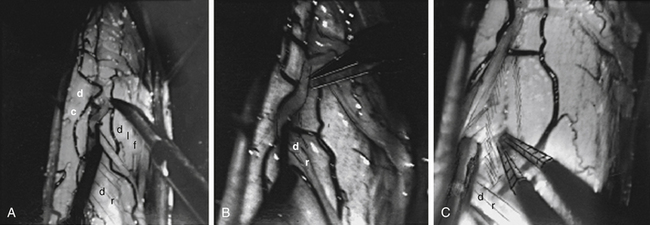
Tibial neurotomy, the most frequently neurotomy used, is indicated for the treatment of varus spastic foot drop with or without claw of toes.10 It consists of exposing all motor branches of the tibial nerve at the popliteal fossa (i.e., the nerves to gastrocnemius and soleus, tibialis posterioris, flexor hallucis longus, and flexor digitorum longus). The soleus has usually been demonstrated to be almost fully responsible for the pathogenesis of spastic foot drop, allowing sparing of gastrocnemius.12 The incision can be vertical on either side of the popliteal fossa or transverse in the popliteal fossa. The latter gives a better aesthetic result and allows tenotomy of the gastrocnemius fascia insertion if necessary (Fig. 122-5A).
The first nerve encountered is the (sensory) medial cutaneous nerve of the leg; situated adjacent to the saphenous vein, it must be spared. More deeply, the tibial nerve trunk, from which the nerves to the gastrocnemius emerge, is easily identifiable. The superior soleus nerve is situated in the midline, just posterior to the tibial nerve. The effect of a soleus neurotomy is assessed by the immediate intraoperative disappearance of ankle clonus. Then by retracting the tibial nerve trunk medially, the other branches can be identified by electric stimulation as they emerge from the lateral edge of the tibial nerve trunk. The most lateral branch is the popliteal nerve, followed by the tibialis posterior nerve and finally by the inferior soleus nerve and the flexor digitorum longus nerves. Some fascicles, often larger, can give a toe flexion response via intrinsic toe flexors (Fig. 122-5B); however, neurotomy of these branches is not recommended if they cannot be clearly individualized at this level—the more so because they may be mixed with sensory fascicles.
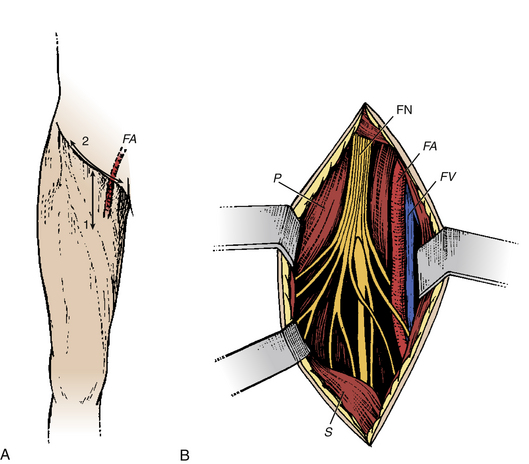
Femoral Neurotomy for the Spastic Quadriceps
The quadriceps muscle is often spastic, which can interfere with gait by limiting knee flexion during the swing phase. Given its “strategic” importance in maintaining upright posture, a motor block is an essential part of the preoperative evaluation. The neurotomy concerns the motor branch to the rectus femoris and vastus intermedius muscles. The incision is horizontal at the hip flexion fold (Fig. 122-6A). The dissection passes medial to the sartorius muscle body and exposes the motor branches of the femoral nerve, first the nerve to the rectus femoris and then, more deeply, the nerve to the vastus intermedius (Fig. 122-6B). Electric stimulation is essential, given the large number of sensory fascicles of this nerve that must be spared.
Surgery at the Upper Limb
Pectoralis Major and Teres Major Neurotomies for the Spastic Shoulder
Neurotomy of collateral branches of the brachial plexus innervating the pectoralis major or the teres major are indicated for spasticity of the shoulder with internal rotation and adduction.13 For the pectoralis major, the skin incision is made at the innermost part of the deltopectoral sulcus and curves along the clavicular axis. Then the clavipectoralis fascia is opened and the upper border of the pectoralis major muscle is reflected downward. Close to the thoracoacromialis artery, the ansa of the pectoralis muscle is identified with the aid of a nerve stimulator. For teres major, the skin incision follows the inner border of teres major, from the lower border of the deltoid muscle posterior head to the lower extremity of the scapula. The lower border of the long portion of brachii triceps constitutes the upper limit of the approach. The dissection goes deeply between teres minor and major muscles. In the vicinity of the subscapularis artery, the nerve ending on teres major is identified. The nerve is surrounded by thick fat when approaching the anterior facet of the muscle body.
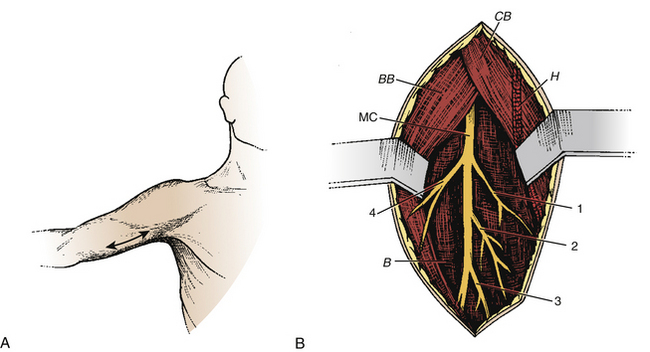
Musculocutaneous Neurotomy for the Spastic Elbow
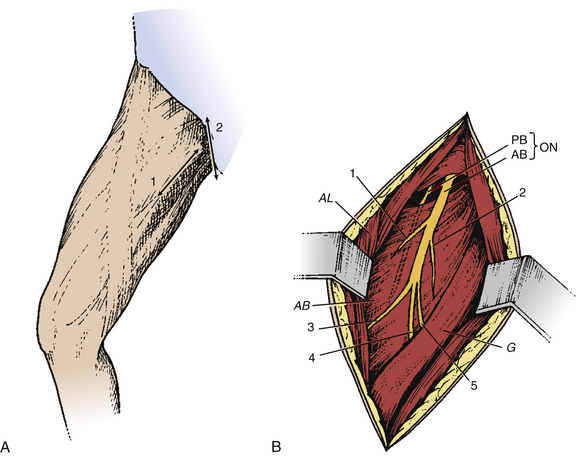
Spasticity of the elbow with flexion depends on the biceps brachii and the brachialis muscles. The skin incision is performed longitudinally, extending from the inferior edge of pectoralis major, medial to the biceps brachii, and down to 5 cm (Fig. 122-7A). The superficial fascia is opened between the biceps laterally and the brachialis medially. The brachial artery and median nerve exit medially. The dissection proceeds in this space where the musculocutaneous nerve lies anterior to the brachialis muscle (Fig. 122-7B). Opening the epineurium allows the fascicles of the nerve to be dissected; the motor fascicles are distinguished from the sensitive ones using nerve stimulation.
Median Neurotomy for Spastic Wrist and Fingers
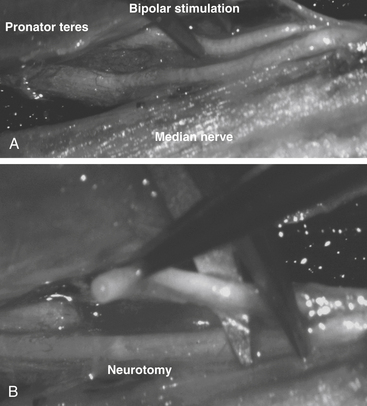
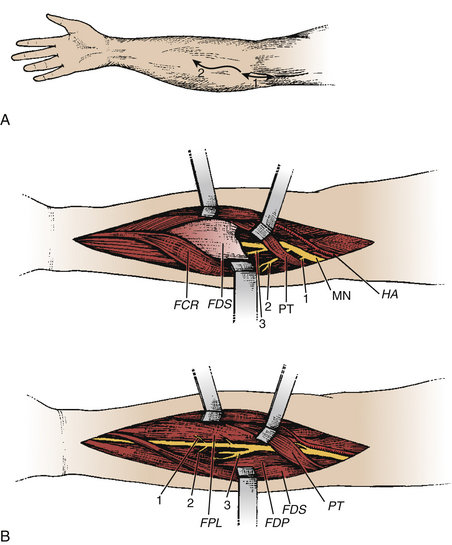
Neurotomy of the median nerve needs to be selective to avoid functional losses and secondary painful phenomena.11,14,15 It is indicated for (1) spasticity of the forearm with pronation, depending on the pronator teres and quadratus muscle; (2) spasticity of the wrist with flexion, depending on the flexor carpi radialis and palmaris longus muscles; and (3) spasticity of the fingers with flexion, depending on the flexor digitorum superficialis (flexion of the proximal interphalangeal and metacarpophalangeal joints) and the flexor digitorum profondus muscle (flexion of the distal interphalangeal, proximal interphalangeal, and metacarpophalangeal joints).
The skin incision begins 2 to 3 cm above the flexion line of the elbow, medial to the biceps brachii tendon; it passes through the elbow and curves toward the junction of the upper and middle thirds of the anterior forearm (the convexity of the curve turns laterally) (Fig. 122-8A). Thereafter, the median nerve is searched medially to the brachial artery and recognized at the elbow, deeply under the lacertus fibrosus, which is cut. Sharp dissection is used to separate the branches of the median nerve. The pronator teres belly with its two heads is retracted medially and distally so that its muscular branches can be inspected. Then this muscle is retracted up and laterally while the flexor carpi radialis is pulled down and medially. The muscular branches to the flexor carpi radialis and to the flexor digitorum superficialis can then be seen. Finally, the latter is retracted medially, uncovering the branches to the flexor digitorum profondus, the flexor pollicis longus, and the pronator quadratus. These latter muscular branches may be individualized as separate branches or remain together in the distal trunk of the anterior interosseous nerve. Sometimes, it may be useful to divide the fibrous arch of the flexor digitorum superficialis muscle to make the dissection easier (Fig. 122-8B).
Ulnar Neurotomy for Spastic Wrist and Fingers
Neurotomy of the ulnar nerve is also indicated (1) for spasticity of the wrist with flexion and with ulnar deviation, both depending on the flexor carpi ulnaris; (2) for spasticity of the fingers with flexion, depending on the flexor digitorum profondus muscle (flexion of the distal interphalangeal, proximal interphalangeal, and metacarpophalangeal joints) partly innervated by the ulnar nerve; and (3) for spasticity with adduction/flexion, depending on the adductor pollicis muscle.11,15 Ulnar neurotomy can also be performed in combination with median neurotomy for swan-neck deformation of the fingers.
A separate arched skin incision is made to expose the ulnar nerve at the medial part of the elbow (Fig. 122-9), where it enters between the two heads of the flexor carpi ulnaris. There, the motor branches to this latter muscle are identified. More distally the branches to the medial half of the flexor digitorum profondus are found.
Complications and Recurrences
Sensory disturbances such as paresthesias and dysesthesias, or deafferentation pain, might be observed if section would inappropriately include sensory fascicles.4 This might be particularly severe and disabling with neurotomy of the median nerve (hand) or the tibial nerve (plantar sole). Hypesthesia (more often transient) of the anterior part of the forearm or of the lateral aspect of the foot may happen because of a surgical approach with inappropriate lesion of subcutaneous sensitive branches, rather than of the neurotomy itself.
Improvement in Function
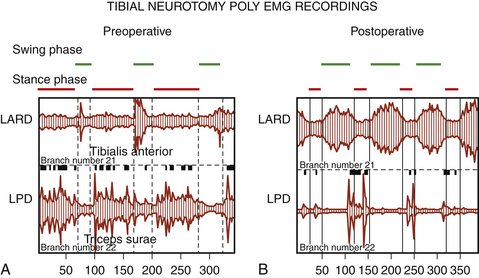
The mandate of neuroablative procedures is that excessive hypertonia be reduced without suppressing useful muscular tone or impairing any residual motor and/or sensory functions. In patients who retain masked voluntary motility, the re-equilibration of the balance between paretic agonist muscles and spastic antagonist muscles results in improvement in (or reappearance of) voluntary motility (Fig. 122-10).
Surgery in the Spinal Roots and Spinal Cord
On Sherrington’s experimental data,16 Foerster performed in 1913 the first dorsal rhizotomies, for lower limb spasticity in cerebral palsy patients.17 Sectioning was from L1 to S2—excluding L4, the root of the quadriceps.
To treat irreducible spasticities with severe spasms, Munro18 suggested sectioning lumbar ventral roots, on the rationale that such spastic phenomena are associated with spontaneous hyperactivity of the motor neurons. Such clinical manifestations are seen following anoxia. In such cases, sectioning dorsal roots was ineffective, whereas sectioning ventral root abolishes spasms.
Bischof described longitudinal myelotomy19 with the aim of interrupting the spinal reflex arch between the ventral and the dorsal horns by a vertical coronal incision performed laterally from one side of the spinal cord to the other, from L1 to S1. Indicated patients are totally paraplegic patients with triple flexion and severe sphincter disturbances.
Intrathecal chemical rhizotomies, originally introduced for the treatment of pain associated with cancerous lesions, were then used for the treatment of severe spasticity in bed-ridden patients; alcoholic solution was the first chemical agent used, followed by phenol, an hyperbaric solution.20 Percutaneous radiofrequency rhizotomies, also introduced to treat chronic pain, were then applied to certain spasticities. Indications are neurogenic detrusor hyperreflexia, for which the target is the sacral roots, or hip spasticity in flexion–adduction, for which the target is lumbar roots L2-L3.21
To reduce the incidence of harmful effects of dorsal rhizotomies on the postural tone, especially in patients with cerebral palsy capable of walking, a number of attempts were made to render dorsal rhizotomies more selective.22–25
In 1972, Sindou observed that his technique of microsurgical lesioning of the ventrolateral part the DREZ, developed for treating pain,26–28 led to marked hypotonia in the muscles corresponding to the operated spinal cord segments.29,30 Subsequently, the technique was applied not only to treat hyperspasticity in severely affected paraplegic patients30,31 but also to treat excess of spasticity in the upper limb of hemiplegic patients.32
Dorsal Rhizotomies
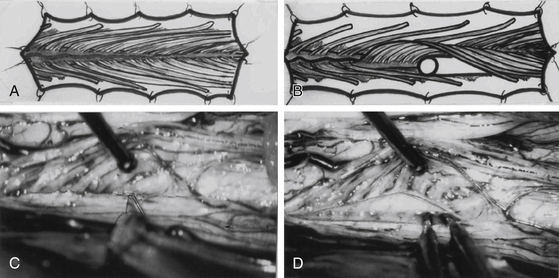
Surgical approaches for dorsal rhizotomies are significantly different from one team to another. The classical technique—the one described first by Fasano et al.25 and then by Peacock and Arens33 and Abbott et al.34—can be summarized as follows: The one piece laminotomy from L1 through S1 is performed using a power saw, which allows repositioning at end of the procedure. Bipolar electric stimulation (using the Nimbus i-Care neurostimulator) of the sensory roots is carried out using multichannel EMG recordings, in addition to palpation of the leg muscles for evidence of contraction. Roots that, when stimulated, cause either activity lasting after cessation of the stimulus or muscle activity outside of its myotome are deemed abnormal and are separated into their rootlets. The rootlets are in turn stimulated, and the same criteria are used to judge their normality. Abnormally responsive rootlets are candidates to be cut. Changes in excitability due to long exposure and extensive manipulation of the rootlets are major drawbacks of the method.
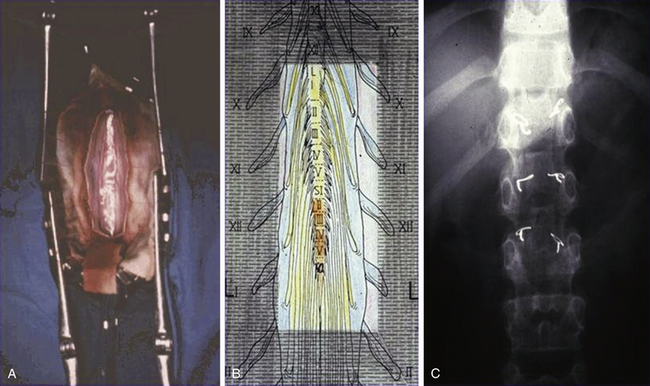
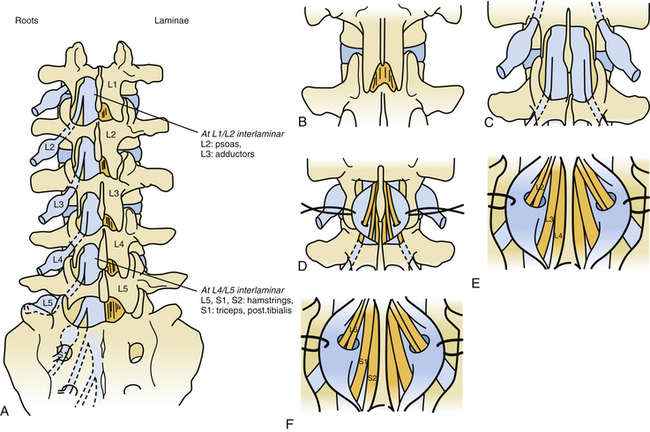
To limit the extent of the approach, we prefer to use a limited osteoplastic laminotomy from T11 to L135 (Fig. 122-11). Through this approach, L2 and L3 ventral (and corresponding dorsal) L1 roots can be identified by their muscular responses to electric stimulation performed intradurally just before entry into their respective dural sheaths. The dorsal lumbosacral rootlets can be identified at their entry into the dorsolateral sulcus at the conus medullaris. The landmark between S1 and S2 medullary segments is located approximately 30 mm from the exit of the tiny coccygeal root from the conus. The medullary segments S1, L5, and L4 can be identified by their evoked motor responses to stimulation (in triceps surae, dorsal flexors of the foot, and quadriceps, respectively). The roots for bladder (S2-S3) can be identified by monitoring vesical pressure, and those for the anal sphincter (S3-S4) can be identified by rectomanometry (or simply using a protected finger introduced into the anus canal) or EMG recordings.
To further reduce invasiveness, in 2001 we started using a “staged interlaminar (IL) approach,”35 the level or levels depending on the roots to be targeted (Fig. 122-12). The lumbar spine is approached posteriorly on the midline so that the preoperated selected IL spaces can be reached. The spinous processes, as well as interspinous ligaments, are preserved. After resecting the flavum ligament, the chosen IL spaces are enlarged by resecting the lower half of the upper lamina and the upper half of the lower laminae. Then the dura is opened in the midline over 2 cm.
Surgery in the DREZ
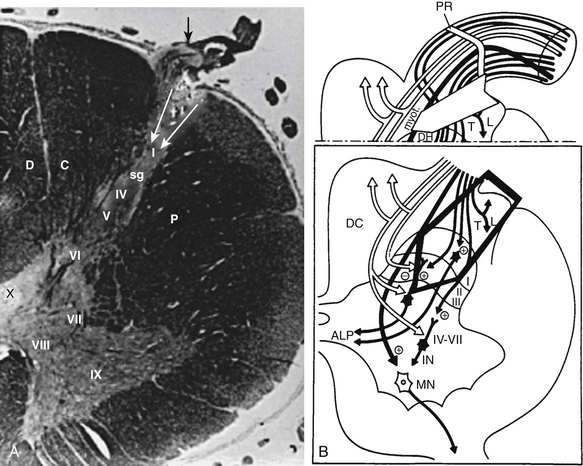
Microsurgical DREZotomy (MDT)26,27 aims at preferentially interrupting both the small-caliber (nociceptive) and the large-caliber (myotatic) fibers of the dorsal roots, both considered tonigenic and situated laterally and in the middle of the entry zone, respectively.
Surgical lesioning must at least partially preserve the medial bundle of large-caliber fibers that project to the dorsal horn and are located medially into the entry zone to reach the dorsal column. The surgical target also includes the dorsalmost layers of the dorsal horn, where the circuits and neurons, responsible for activation of the segmental circuitry of the spinal cord, are located (Fig. 122-13). The techniques for the cervical and the lumbosacral cord segments are detailed and illustrated in Figs. 122-14 and 122-15, respectively. In brief, the procedure consists of a 3-mm-deep microsurgical incision—at the level of the dorsolateral sulcus—with a 35-degree angle at the cervical level and a 45-degree angle at the lumbosacral level. Bipolar coagulations in a dotted fashion are performed ventrolaterally at the entrance of the rootlets into the dorsolateral sulcus and penetrate approximately of 3 mm inside the gray matter of the dorsal horn, along all the spinal cord segments selected for undergoing the operation. Each microcoagulation is performed under high magnification of the microscope. The intensity starts at the minimum of the bipolar coagulator and remains at a low value. The duration of each microcoagulation is 1 to 3 seconds. The degree and extent of the coagulation are controlled by vision. For performing MDT, special instruments have been designed by Sindou (Strycker-Leibinger, Freiburg, Germany), as indicated in the legend of Figs. 122-14 and 122-15).
Decision Making in Adults
Generally, patients do not complain about spasticity; they are more likely to be aware of stiffness, deformity, and limitations in functional abilities. “Stiffness” is a useful term because it is widely understood by both clinicians and patients and does not imply a specific cause. After time, the patients have a mixture of spasticity and muscle shortening or contractures. In any discussion of management, an agreed terminology is important in recognizing two principal components of muscle stiffness: The first is “dynamic” shortening of muscles caused by spasticity; such patients exhibit hyperreflexia, clonus, and velocity-dependent resistance to passive joint motion. The second, “fixed” shortening of muscles, is described as contracture, which is much less velocity dependent and remains present under local blocks or anesthesia.
Methods for control of spasticity can be classified according to whether they are focal or general in effect and whether the effects are permanent or temporary. Within this four-way matrix, practical clinical guidelines may be derived, as illustrated in Fig. 122-1.
Intrathecal Baclofen Therapy
ITB therapy has also been used to treat cerebral palsy patients, especially ones at an adult age. In patients with the capacity to walk, adequate doses (i.e., ones effective on the excess of tone that do not produce motor weakness) are difficult to find.
Neuroablative Procedures
Peripheral Neurotomies
Peripheral neurotomies are indicated when spasticity is localized to a few muscular groups, supplied by a single or a few peripheral nerves. To decide, a local anesthetic block of the nerve (with long-lasting bupivacaine) is useful and gives the patient an idea of what to expect from the operation. Botulinum toxin injections may also act as a “prolonged” test.
Selective neurotomies are able not only to reduce excess of spasticity and prevent deformity but also to improve motor function by re-equilibrating the tonic balance between agonist and antagonist muscles. This is especially true for the spastic foot with equinovarus deformity, as shown in Fig. 122-10. With regard to the spastic hand, which is a difficult problem to deal with, a functional benefit in prehension can only be achieved if patients retain a residual motor function in the extensor and supinator muscles, together with a sufficient residual sensory function. If these conditions are not present, only better comfort and a better cosmetic aspect can be achieved.
Surgery in the DREZ
Surgery in the DREZ is preferred when severe spasticity affects an entire limb so as to render it functionally useless. For patients with paraplegia, the L2-S5 segments are approached through a T11-L2 laminectomy (Fig. 122-15). For the hemiplegic upper limb, a C4-C7 hemilaminectomy with conservation of the spinous processes is sufficient to reach the C5-T1 segments (Fig. 122-14). MDT is indicated in paraplegic patients, especially when they are bedridden as a result of disabling flexion spasms. In hemiplegic patients, MDT is indicated when the upper limb is affected with irreducible and/or painful hyperspasticity. MDT also can be applied to treat a neurogenic bladder with uninhibited detrusor contractions resulting in voiding around a catheter.
Decision Making in Children
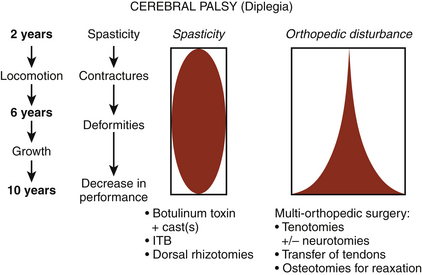
Spasticity in children, as in adults, can be useful to function or may increase disability. Indications for surgery are more difficult to define in children than in adults because the child is in constant development; thus, orthopedic status changes with increase of growth, in particular during puberty. For deciding which treatment to use for mitigating spasticity, when to propose it, and with which orthopedic procedure to combine it, the medical team has to project into the future, extrapolate the potential for musculoskeletal contractures and their harmful consequences, and extrapolate the positive functional evolution with spontaneous psychomotor development.37
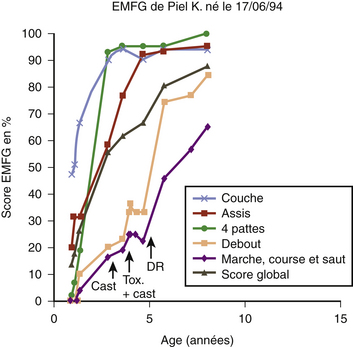
The first stage for assessment is clinical observation to have a global idea of the child’s function. The second stage is measurement of the range of motion to detect contractures that would not be accessible to neurosurgical treatment. The third stage is quantification of the assessment of spasticity with the Ashworth scale or the Tardieu scale. The final stage is grading the child on the Gross Motor Function Measure (EMFG) and observing the child’s evolution with time (Fig. 122-17).38,39
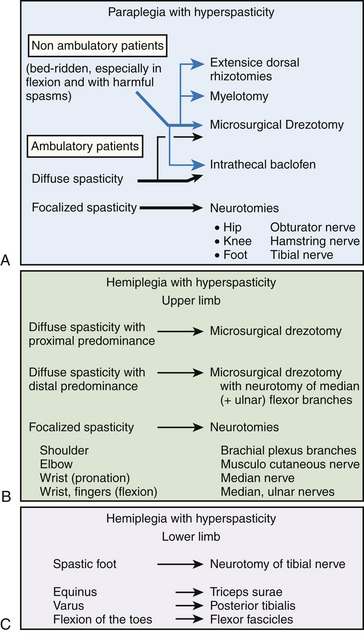
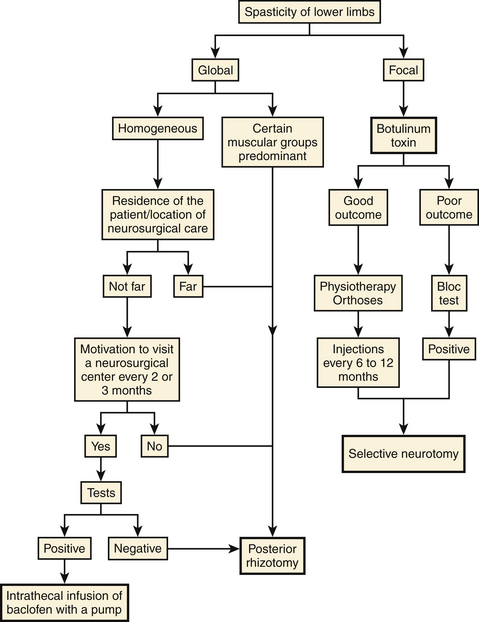
There are several effective neurosurgical treatments for spasticity in children with cerebral palsy (Fig. 122-18). For diffuse spasticity of lower limbs, dorsal rhizotomy or ITB administration may be considered. For focal spasticity, botulinum toxin injection permits delay of surgery until the child is old enough to undergo a selective neurotomy or a dorsal rhizotomy (Fig. 122-19).
• If spasticity in the two lower limbs is global, dorsal rhizotomy or ITB therapy can be considered. The latter imposes a visit every 6 months to fill the pump; thus, the child and family must be motivated to comply. The volume of the implanted pump is a major obstacle in young children. Dorsal rhizotomy is generally preferred before 6 years of age, especially when a definitive action is desired on certain and well-defined muscular groups.
• If spasticity is focal and involves the gastrocnemius and soleus muscles, botulinum toxin can be proposed as a good complement to physiotherapy and a plaster cast. This approach enables neurosurgical treatment to be delayed until the child reaches the age for selective neurotomy. When spasticity is focal on the adductors, botulinum toxin is not always sufficient to avoid an obturator neurotomy, often coupled with a tenotomy of the adductors, to prevent dislocation of the hip.
• For spasticity in the upper limb, the first stage of treatment is botulinum toxin injections. The muscles of the upper limbs are small. Even if quite a few must be injected, the maximum allowable dose is rarely too small to be insufficient. These injections can be considered a treatment and can be repeated every 6 to 12 months. However, the multiple visits required are a constraint. Also, patients may develop an immunoresistance that decreases the effectiveness of treatment with time. If so, botulinum injections, because they simulate the outcome of a selective neurotomy of the involved nerve, allow the patient and family to appreciate the potential benefit that could follow a selective neurotomy.
Conclusion
Neurosurgery for spasticity needs—by essence—to be practiced within the frame of a multidisciplinary approach.1,3,4 Teams dealing with “spastic patients” should at least have most of the technical modalities on hand to offer the best option to every patient.
Abbott A., Forem S.L., Johann M. Selective posterior rhizotomy for the treatment of spasticity. Childs Nerv Syst. 1989;5:337-346.
Bischof W. Die longitudinal myelotomie. Zbl. Neurochir. 1951;11:79-88.
Brunelli G., Brunelli F. Selective microsurgical denervation in spastic paralysis. Ann Chir Main. 1983;2:277-280.
Decq P., Filipetti P., Feve A., et al. Peripheral selective neurotomies of the brachial plexus branches for the spastic shoulder. Anatomical study and clinical results in 5 patients. J Neurosurg. 1997;86:648-653.
Decq P., Mertens P., et al. La neurochirurgie de la spasticité. Neurochirurgie. 2003;49:135-416.
Fasano V.A., Barolat-Romana G., Ivaldi A., Squazzi A. La radicotomie postérieure fonctionnelle dans le traitement de la spasticité cérébrale. Neurochirurgie. 1976;22:23-34.
Foerster O. On the indications and results of the excision of posterior spinal nerve roots in men. Surg Gynecol Obstet. 1913;16:463-474.
Fraioli B., Guidetti B. Posterior partial rootlet section in the treatment of spasticity. J Neurosurg. 1977;46:618-626.
Gros C.. Spasticity—Clinical classification and surgical treatment, Krayenbühl, editor, Advances and Technical Standards in Neurosurgery, Advances and Technical Standards in Neurosurgery, Wien, New York, Springer-Verlag, 1979;Vol 6:55-97.
Gros C., Ouaknine G., Vlahovitch B., Frerebeau P.H. La radicotomie sélective postérieure dans le traitement neurochirurgical de l’hypertonie pyramidale. Neurochirurgie. 1967;13:505-518.
Maarrawi J., Mertens P., Luaute J., et al. Long-term functional results of selective peripheral neurotomy for the treatment of spastic upper limb: prospective study in 31 patients. J. Neurosurg. 2006;104:215-225.
Munro D. The rehabilitation of patients totally paralysed below waist: anterior rhizotomy for spastic paraplegia. Engl J Med. 1945;233:456-461.
Nathan P.W. Intrathecal phenol to relieve spasticity in paraplegia. Lancet. 1959:1099-1102. ii
Peacock W.J., Arens L.J. Selective posterior rhizotomy for the relief of spasticity in cerebral palsy. S Afr Med J. 1982;62:119-124.
Penn R.D., Kroin J.S. Intrathecal baclofen alleviates spinal cord spasticity. Lancet. 1984;1:1078.
Privat J.M., Benezech J., Frerebeau P., Gros C. Sectorial posterior rhizotomy, a new technique of surgical treatment for spasticity. Acta Neurochir. 1976;35:181-195.
Sherrington C.S. Decerebrate rigidity and reflex coordination of movements. J Physiol. 1898;22:319-332.
Sindou M.. Neurosurgical Management of Disabling Spasticity, Spetzler R.F., editor, Operative Techniques in Neurosurgery, Operative Techniques in Neurosurgery, Philadelphia, Elsevier, 2004;Vol 7:95-174.
Sindou M., Jeanmonod D. Microsurgical DREZotomy for the treatment of spasticity and pain in the lower limbs. Neurosurgery. 1989;24:655-670.
Sindou M., Mertens P. Selective neurotomy of the tibial nerve for the treatment of the spastic foot. Neurosurgery. 1988;23:738-744.
Sindou M., Mifsud J.J., Boisson D., Goutelle A. Selective posterior rhizotomy in the dorsal root entry zone for treatment of hyperspasticity and pain in the hemiplegic upper limb. Neurosurgery. 1986;18:587-595.
Sindou M., Quoex C., Baleydier C. Fiber organization at the posterior spinal cord–rootlet junction in man. J Comp Neurol. 1974 (a);153:14-26.
Sindou M., Simon F., Mertens P., Decq P. Selective peripheral neurotomy for spasticity in childhood. Childs Nerv Syst. 2007;23:957-970. (contains 30 references for SNP)
Sindou M., Abbott A., Keravel Y. Neurosurgery for spasticity: A multidisciplinary Approach. Wien, New York: Springer-Verlag, 1991;218.
Stoffel A. The treatment of spastic contractures. Am J Orthop Surg. 1912;10:611-644.
1. Sindou M., Abbott A., Keravel Y. Neurosurgery for spasticity: A multidisciplinary Approach. Wien, New York: Springer-Verlag, 1991;218.
2. Mertens P., Sindou M. Surgical management of spasticity. In: Barnes M.P., Johnson G.R. Clinical Management of Spasticity. Cambridge: Cambridge University Press; 2001:239-265.
3. Decq P., Mertens P., et al. La Neurochirurgie de la Spasticité. Neurochirurgie. 2003;49:135-416.
4. Sindou M.. Neurosurgical Management of Disabling Spasticity, Spetzler R.F., editor, Operative Techniques in Neurosurgery, Operative Techniques in Neurosurgery, Philadelphia, Elsevier, 2004;Vol 7:95-174.
5. Penn R.D., Kroin J.S. Intrathecal baclofen alleviates spinal cord spasticity. Lancet. 1984;1:1078.
6. Penn R.D. Intrathecal baclofen therapy. Operative Techniques in Neurosurgery. 2004;vol:7:124-127.
7. Stoffel A. The treatment of spastic contractures. Am J Orthop Surg. 1912;10:611-644.
8. Gros C. La chirurgie de la spasticité. Neurochirurgie. 1972;23:316-388.
9. Gros C.. Spasticity—Clinical classification and surgical treatment, Krayenbühl, editor, Advances and Technical Standards in Neurosurgery, Advances and Technical Standards in Neurosurgery, Wien, New York, Springer-Verlag, 1979;Vol 6:55-97.
10. Sindou M., Mertens P. Selective neurotomy of the tibial nerve for the treatment of the spastic foot. Neurosurgery. 1988;23:738-744.
11. Mertens P., Sindou M. Selective peripheral neurotomies for the treatment of spasticity. In: Sindou M., Abbott R., Keravel Y. Neurosurgery for Spasticity. A Multidisciplinary Approach. Wien, New York: Springer-Verlag; 1991:119-132.
12. Decq P., Cuny E., Filipetti P., Keravel Y. Role of soleus muscle in spastic equinus foot. Lancet. 1998;11:118.
13. Decq P., Filipetti P., Feve A., et al. Peripheral selective neurotomies of the brachial plexus branches for the spastic shoulder. Anatomical study and clinical results in 5 patients. J Neurosurg. 1997;86:648-653.
14. Brunelli G., Brunelli F. Selective microsurgical denervation in spastic paralysis. Ann Chir Main. 1983;2:277-280.
15. Maarrawi J., Mertens P., Luaute J., et al. Long-term functional results of selective peripheral neurotomy for the treatment of spastic upper limb: prospective study in 31 patients. J Neurosurg. 2006;104:215-225.
16. Sherrington C.S. Decerebrate rigidity and reflex coordination of movements. J Physiol. 1898;22:319-332.
17. Foerster O. On the indications and results of the excision of posterior spinal nerve roots in men. Surg Gynecol Obstet. 1913;16:463-474.
18. Munro D. The rehabilitation of patients totally paralysed below waist: anterior rhizotomy for spastic paraplegia. Engl J Med. 1945;233:456-461.
19. Bischof W. Die longitudinal myelotomie. Zbl Neurochir. 1951;11:79-88.
20. Nathan P.W. Intrathecal phenol to relieve spasticity in paraplegia. Lancet. 1959:1099-1102. ii
21. Segnarbieux F., Frerebeau P.h. The different (open surgical, percutaneous thermal, and intrathecal chemical) rhizotomies for the treatment of spasticity. In: Sindou M., Abbott R., Keravel Y. Neurosurgery for spasticity: a multidisciplinary approach. Wien, New York: Springer-Verlag; 1991:133-139.
22. Gros C., Ouaknine G., Vlahovitch B., Frerebeau P.H. La radicotomie sélective postérieure dans le traitement neurochirurgical de l’hypertonie pyramidale. Neurochirurgie. 1967;13:505-518.
23. Privat J.M., Benezech J., Frerebeau P., Gros C. Sectorial posterior rhizotomy, a new technique of surgical treatment for spasticity. Acta Neurochir. 1976;35:181-195.
24. Fraioli B., Guidetti B. Posterior partial rootlet section in the treatment of spasticity. J Neurosurg. 1977;46:618-626.
25. Fasano V.A., Barolat-Romana G., Ivaldi A., Squazzi A. La radicotomie postérieure fonctionnelle dans le traitement de la spasticité cérébrale. Neurochirurgie. 1976;22:23-34.
26. Sindou M. Anatomical study of the dorsal root entry zone (DREZ). Surgery in the DREZ for pain. M D Thesis: University of Lyon; 1972. 182
27. Sindou M., Quoex C., Baleydier C. Fiber organization at the posterior spinal cord–rootlet junction in man. J Comp Neurol. 1974 (a);153:14-26.
28. Sindou M., Fischer G., Goutelle A., Mansuy L. La radicellotomie postérieure sélective. Premiers résultats dans la chirurgie de la douleur. Neurochirurgie. 1974(b);20:391-408.
29. Sindou M., Fischer C., Gouttelle A., et al. La radicellotomie postérieure sélective dans le traitement des spasticités. Rev Neurol. 1974©;30:201-205.
30. Sindou M., Millet M.F., Mortamais J., Eyssette M. Results of selective posterior rhizotomy in the treatment of painful and spastic paraplegia secondary to multiple sclerosis. Appl Neurophysiol. 1982;45:335-340.
31. Sindou M., Jeanmonod D. Microsurgical DREZotomy for the treatment of spasticity and pain in the lower limbs. Neurosurgery. 1989;24:655-670.
32. Sindou M., Mifsud J.J., Boisson D., Goutelle A. Selective posterior rhizotomy in the dorsal root entry zone for treatment of hyperspasticity and pain in the hemiplegic upper limb. Neurosurgery. 1986;18:587-595.
33. Peacock W.J., Arens L.J. Selective posterior rhizotomy for the relief of spasticity in cerebral palsy. S Afr Med J. 1982;62:119-124.
34. Abbott A., Forem S.L., Johann M. Selective posterior rhizotomy for the treatment of spasticity. Childs Nerv Syst. 1989;5:337-346.
35. Sindou M. Radicotomies dorsales chez l’enfant. Neurochirurgie. 2003;49:312-323.
36. Sindou M., Mertens P. Decision-making for neurosurgical treatment of disabling spasticity in adults. Operative Techniques in Neurosurgery. 2004;vol. 7:113-118.
37. Hodgkinson I., Berard C. Assessment of spasticity in pediatric patients. Operative Techniques in Neurosurgery. 2004;vol. 7:109-111.
38. Hodgkinson I., Sindou M. Decision-making for treatment of disabling spasticity in children. Operative Techniques in Neurosurgery. 2004;vol. 7:120-123.
39. Sindou M., Simon F., Mertens P., Decq P. Selective peripheral neurotomy for spasticity in childhood. Childs Nerv Syst. 2007;23:957-970. (contains 30 references for SNP)