Chapter 9 Surgery for Coronary Artery Disease
Coronary artery bypass graft (CABG) surgery is one of the most commonly performed operations in the United States, and it forms the core of most cardiac surgery programs. CABG surgery provides effective symptom control for angina, and in certain patient groups, it improves survival rates.
INDICATIONS FOR CABG SURGERY
The indications for CABG surgery have recently been reviewed by the American College of Cardiology and the American Heart Association (ACC/AHA) (Table 9-1).1 CABG surgery offers a survival advantage over medical treatment for patients with left main coronary disease (see Fig. 5-4), three-vessel coronary disease, and disease of the proximal left anterior descending (LAD) coronary artery (see Fig. 5-5). This survival advantage is most apparent in patients with impaired left ventricular function. When CABG surgery is compared with PCI, a minimal difference in survival rates is seen2–5—except in diabetic patients, who do better with surgery. Relief from angina and the infrequent need for reintervention are superior with CABG. These trials represent highly selected groups in which patients were considered to be equally treatable by either technique—a feature that may have emphasized the benefits of PCI. The trials were carried out before the introduction of drug-eluting stents, a feature that may have emphasized the benefits of CABG surgery.
| Clinical Subset | Recommendation |
|---|---|
| Mild or Asymptomatic Angina | |
| Left main or left main equivalent CAD (class I) | CABG should be performed |
| 3-vessel CAD (class I) | CABG is useful |
| 1-2 vessel CAD involving the proximal LAD (class IIa, but becomes class I if there is extensive documented ischemia and/or EF < 50%) | CABG can be beneficial |
| 1-2 vessel disease not involving the proximal LAD (class IIb, but becomes class I if there is a large area of viable myocardium and high risk criteria are found on noninvasive testing) | CABG may be considered |
| Stable Angina | |
| Left main, left main equivalent, or 3-vessel CAD (class I) | CABG recommended |
| 2-vessel CAD with proximal LAD stenosis and either EF < 50% or demonstrable ischemia on noninvasive testing (class I) | CABG recommended |
| 1-2 vessel CAD without proximal LAD stenosis, but with a large area of viable myocardium and high risk criteria on noninvasive testing (class I) | CABG is beneficial |
| Disabling angina in patients on maximal medical therapy when surgery can be performed with acceptable risk (class I) | CABG is beneficial |
| 1-vessel CAD with proximal LAD stenosis (class IIa, but becomes class I if extensive ischemia is documented by noninvasive testing and/or EF is < 50%) | CABG is reasonable |
| 1-2 vessel CAD without proximal LAD stenosis, but with a moderate area of viable myocardium and demonstrable ischemia on noninvasive testing | CABG may be useful |
| Unstable Angina/NSTEMI* | |
| Left main, left main equivalent, or 3-vessel CAD (class I) | CABG should be performed |
| Patients with ongoing ischemia not responsive to maximal non-surgical therapy (class I) | CABG is recommended |
| 1-2 vessel CAD with proximal LAD stenosis (class IIa) | CABG probably indicated |
| 1-2 vessel CAD without proximal LAD stenosis when PCI is not optimal or possible (class IIa, but becomes class I if large area of viable myocardium and high risk criteria met on noninvasive testing) | CABG may be considered |
| STEMI* | |
| Indications as for patients with unstable angina/NSTEMI | |
| Emergency CABG in the following circumstances (class I): | CABG recommended |
| Failed PCI with persistent pain or hemodynamic instability in patients with suitable coronary anatomy | |
| Persistent or recurrent ischemia refractory to medical therapy in patients who have suitable coronary anatomy, who have a significant area of myocardium at risk, and who are not candidates for PCI | |
| At the time of surgical repair of postinfarction myocardial rupture or mitral valve regurgitation | |
| Patients with cardiogenic shock* | |
| Patients with life-threatening ventricular arrhythmias who have left main or 3-vessel CAD | |
| Poor Left Ventricular Function | |
| Left main, left main equivalent CAD (class I) | CABG should be performed |
| 2-3 vessel CAD with proximal LAD stenosis (class I) | CABG should be performed |
| Significant viable noncontracting revascularizable myocardium without any of the above anatomic patterns (class IIa) | CABG may be performed |
| Life-Threatening Ventricular Arrhythmias | |
| Left main, left main equivalent, or 3-vessel CAD (class I) | CABG should be performed |
| 1-2 vessel CAD (class IIa, but this becomes class I if the rhythm is resuscitated sudden cardiac death or sustained ventricular tachycardia) | CABG is reasonable |
| Following Failed PCI | |
| Ongoing ischemia or threatened occlusion with significant myocardium at risk (class I) | CABG should be performed |
| Hemodynamic compromise (class I) | CABG should be performed |
| Removal of foreign body in a crucial anatomic position (class IIa) | CABG is reasonable |
| Hemodynamic compromise in patients with impaired coagulation and without previous sternotomy (class IIa) | CABG can be beneficial |
| Hemodynamic compromise in patients with impaired coagulation and with previous sternotomy (class IIb) | CABG can be considered |
| Patients With Previous CABG | |
| Disabling angina despite optimal nonsurgical therapy (class I) | CABG should be performed |
| Occluded bypass grafts and class I indications for surgery for native vessel CAD (class I) | CABG should be performed |
| Bypassable distal vessel(s) with a large area of threatened myocardium by noninvasive studies (class IIa) | CABG is reasonable |
| Atherosclerotic vein grafts with > 50% stenosis supplying LAD or large areas of myocardium (class IIa) | CABG is reasonable |
Criteria for surgery are outlined in text.
CABG, coronary artery bypass graft; CAD, coronary artery disease; EF, ejection fraction; LAD, left anterior descending coronary artery; NSTEMI, non S-T elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, S-T elevation myocardial infarction.
* Mortality rates increase for 3 to 7 days following NSTEMI and STEMI. Ideally, the patients should be medically stabilized and surgery delayed beyond this period.
CABG SURGERY IN HIGH-RISK PATIENTS
The overall mortality risk for patients undergoing isolated CABG surgery is about 3.5%.6 However, operative risk must be stratified according to the presence or absence of various risk factors. In the absence of risk factors, mortality rates are less than 2%, increasing to more than 50% in patients with multiple risk factors. Independent risk factors for adverse outcome following CABG surgery are summarized in Table 9-2.7 Patients with cardiogenic shock, New York Heart Association class III or IV symptoms, a history of previous cardiac surgery, or the need for urgent (in-hospital) or emergency (within 24 hours) surgery have the greatest perioperative risk. These and other factors are incorporated into the various cardiac risk scoring systems outlined in Chapter 41.
Table 9-2 Risk factors for Mortality in the Short-Term Following CABG Surgery
| Variable | Odds Ratio for Mortality in the Short Term (95% Confidence Interval) |
|---|---|
| Age (per 10-year increase) | 1.44 (1.37-1.53) |
| Chronic obstructive pulmonary disease | 1.22 (1.11-1.35) |
| Peripheral vascular disease | 1.35 (1.23-1.49) |
| Cerebral vascular disease | 1.37 (1.23-1.51) |
| Serum creatinine ≥1.5 mg/dl (0.13 mmol/l) | 1.72 (1.56-1.90) |
| Prior heart surgery | 2.69 (2.40-3.01) |
| Canadian cardiovascular society anginal class III or IV | 1.27 (1.12-1.44) |
| Prior myocardial infarction | 1.28 (1.16-1.42) |
| Preoperative ST segment depression on ECG | 1.37 (1.24-1.51) |
| Urgent or emergency surgery | 1.83 (1.66-2.02) |
| New York Heart Association class III or IV | 1.77 (1.59-1.96) |
| Left main coronary stenosis ≥ 50% | 1.20 (1.09-1.32) |
| Left ventricular ejection fraction < 45% | 1.26 (1.15-1.39) |
Adapted from Gardner SC, Grunwald GK, Rumsfeld JS, et al: Comparison of short-term mortality risk factors for valve replacement versus coronary artery bypass graft surgery. Ann Thorac Surg 77:549-556, 2004.
Acute Coronary Syndromes
The effectiveness of CABG in patients with severe or unstable angina has been clearly demonstrated. However, mortality rates are substantially increased if CABG surgery is undertaken within 48 hours of an ST segment elevation myocardial infarction (STEMI).8 Increased operative risk is less pronounced following non-ST segment elevation myocardial infarction (NSTEMI).8
In the first few days following a myocardial infarction, myocardial stunning occurs and a systemic inflammatory response develops—the latter characterized by fever, leukocytosis, and increases in inflammatory markers. Myocardial stunning results in a reduced ejection fraction, segmental wall motion abnormalities (SWMAs), and increased left ventricular end-diastolic pressure. Patients may develop acute heart failure or even frank cardiogenic shock. Following STEMI or NSTEMI, CABG surgery should be delayed by 3 to 7 days or until such time as the clinical features of congestive cardiac failure have resolved.1
In patients with critical coronary anatomy, particularly left main or left main equivalent coronary artery disease, the decision regarding the timing of surgery is difficult. Despite a large area of threatened myocardium, it is probably still beneficial to attempt to delay surgery for at least 48 hours following an acute myocardial infarction. Following an anteroseptal STEMI, there may be benefit in waiting several weeks until a scar has developed and then performing CABG surgery in conjunction with a remodeling procedure (see later discussion). Following an inferior myocardial infarction with right ventricular involvement, right ventricular function may take as long as 4 weeks to recover,9 and CABG surgery is best delayed beyond this period.
Preoperative Optimization
Prior to urgent CABG surgery, some patients benefit from a short period of aggressive medical optimization in the ICU. While in the ICU, invasive hemodynamic monitoring can be established and, if not already performed, an echocardiogram can be obtained. A pulmonary artery catheter provides important information but is contraindicated in patients with recurrent arrhythmias. Preoperative treatment with an intraaortic balloon pump (IABP) may improve outcome in high-risk patients undergoing CABG surgery11 and should be considered in all patients with acute ischemia, arrhythmias, pulmonary edema, or shock. For patients with profound cardiogenic shock secondary to myocardial infarction, primary treatment with a ventricular assist device may be preferable to CABG surgery (see Chapters 19 and Chapter 22).12
Tight glycemic control has been shown to improve outcome in diabetic patients undergoing cardiac surgery (see Diabetes in subsequent discussion), and in one randomized trial, maintenance of serum glucose between 80 and 110 mg/dl (4.4 and 6.1 mmol/l) improved survival rates in unselected patients (diabetic and nondiabetic; cardiac surgery and noncardiac surgery) admitted to a surgical ICU. Furthermore, treatment with glucose-insulin infusions (as opposed to simply maintaining glycemic control) may improve outcome after CABG surgery in patients with left ventricular failure13 and acute myocardial ischemia.14
Chronic Left Ventricular Dysfunction
Patients with depressed left ventricular function who have demonstrable hibernating myocardium derive substantial benefit from CABG surgery in terms of improved ejection fraction, relief of heart failure symptoms, and enhanced survival rates.15 Hibernating myocardium may be identified by a number of myocardial viability studies, as outlined in Chapter 5. A patient with low ejection fraction (<35%) whose symptoms are predominantly those of heart failure should undergo a myocardial viability study; if hibernating myocardium is demonstrated, and the coronary anatomy is suitable, CABG surgery should be considered. A patient with a low ejection fraction whose symptoms are predominantly those of angina and only minimal heart failure should be offered CABG surgery only if indicated on the basis of their coronary artery disease.1 Patients with extensive anteroseptal scar formation following a STEMI may be suitable for ventricular remodeling surgery (see Surgery to Remodel the Left Ventricle, in subsequent material).
Valvular Heart Disease
In general, any valve pathology graded as moderate or severe should be considered for repair or replacement at the time of CABG surgery. In deciding whether to perform valve surgery, the additive risk of a combined valve-coronary procedure must be weighed against the risk of leaving the patient with an uncorrected valve lesion.
Mitral Regurgitation
The decision to repair or replace a regurgitant mitral valve is difficult and depends on the mechanism of the regurgitation, the likely ease of valve repair, and the degree to which the regurgitation is due to hibernating myocardium. A preoperative transesophageal echocardiogram can help to define the severity and mechanism of the mitral regurgitation. A patient with moderate or severe ischemic mitral regurgitation who undergoes isolated CABG surgery is at risk for important postoperative mitral regurgitation.16 In general, a patient with moderate or severe ischemic mitral regurgitation should undergo mitral valve surgery at the time of CABG surgery. However, combined CABG and mitral valve surgery substantially increases the operative risk,7 particularly in the presence of impaired ventricular function.
Carotid Disease
Carotid disease is common in patients presenting for cardiac surgery. In two studies, the incidence of severe (≥80%) carotid stenosis in unselected patients undergoing cardiac surgery was 8.5% and 12%, with an incidence of postoperative stroke of 18.2% and 5.3%, respectively.17,18 All patients who have carotid bruit or a history of transient ischemic attack, stroke, or previous carotid endarterectomy should undergo ultrasound-flow imaging of the carotid arteries prior to surgery. As age above 65 years, left main coronary disease, smoking, and peripheral vascular disease are all associated with carotid atherosclerosis, routine carotid ultrasonography is justified in these patient groups. Because of the increased risk for postoperative stroke associated with high-grade carotid stenosis in patients undergoing cardiac surgery, carotid endarterectomy is indicated when unilateral or bilateral carotid stenoses are greater than 80%, irrespective of symptoms. The outcomes of staged (with carotid endarterectomy performed first) or combined procedures are similar.19
Chronic Obstructive Pulmonary Disease
Patients with severe chronic obstructive pulmonary disease (COPD), as evidenced by a forced expiratory volume over 1 second (FEV1) <1.25 l, have significantly higher mortality rates following CABG surgery—mainly due to cardiac arrhythmias—than do patients without severe COPD.20 In patients with end-stage COPD (FEV1 <0.5l to 1.0 l), cardiac surgery may be considered to be contraindicated. Patients with moderate to severe COPD are likely to benefit from preoperative optimization, including smoking cessation, incentive spirometry, nutritional support, and effective treatment of the bronchospastic or infective components of their pulmonary diseases.
Diabetes
CABG surgery in patients with diabetes and multivessel coronary disease results in improved long-term survival rates compared to those seen after medical treatment or PCI.21,22 However, diabetic patients suffer a higher rate of perioperative complications, such as renal failure, stroke, and wound infection, and have increased early mortality rates compared to nondiabetic patients.23 Patients with diabetes often have silent myocardial ischemia, diffuse coronary disease, renal dysfunction, and peripheral vascular disease.
In diabetic patients undergoing cardiac surgery, including CABG surgery, perioperative control of serum glucose by means of intravenous insulin improves survival rates and reduces the incidence of various complications such as sternal wound infection.24–26 Management strategies for perioperative glucose control are outlined in Chapter 36.
Renal Dysfunction
A number of factors are associated with developing postoperative renal failure (Chapter 33), the most important of which is preoperative renal dysfunction. A preoperative creatinine level of greater than 2.5 mg/dl (0.22 mmol/l) is strongly associated with the need for long-term dialysis following cardiac surgery.27 Postoperative acute renal failure is associated with a greatly increased mortality rate. In one large series, the mortality rate was 63% in patients who developed dialysis-dependent renal failure following CABG surgery compared to only 0.9% in patients whose renal function remained normal.28
If an elevated creatinine level is discovered prior to cardiac surgery it is essential to know whether it is acute or chronic. An acute deterioration in renal function should be carefully investigated and all potential causes eliminated. In particular, drugs such as spironolactone, nonsteroidal antiinflammatory drugs, and nephrotoxic antibiotics should be discontinued; consideration should also be given to stopping ACE inhibitors and angiotensin receptor blockers. If possible, CABG surgery should be delayed until renal function has returned to baseline following administration of radiocontrast agents.
End-Stage Renal Failure
Patients with dialysis-dependent renal failure have a perioperative mortality rate in excess of 10% following cardiac surgery.29 Such patients frequently have coexisting hypertension, diabetes, anemia, abnormal hemostasis, and electrolyte abnormalities. They are often anuric or oliguric; thus, any fluid that is administered must be removed by dialysis. Immediately following dialysis, patients tend to be hypovolemic and hypokalemic; immediately prior to dialysis, patients tend to be hypervolemic and hyperkalemic. Dialysis should be performed on the day prior to surgery, not on the morning of surgery, to allow postdialysis fluid shifts to equilibrate. Following surgery, dialysis is performed, ideally, on the first postoperative day (see Chapter 33).
Body Mass Index
Underweight patients (body mass index [BMI] <18 kg/m2) undergoing cardiac surgery are at increased risk for adverse outcomes, such as postoperative infection and prolonged ICU stay.30 This finding probably reflects poor nutrition in the context of severe cardiac disease. Such patients may benefit from a period of preoperative supplemental nutrition under the supervision of a dietitian.
Moderate obesity (BMI 35 to 39.9 kg/m2) is associated with only a slightly increased operative risk compared to normal or mildly obese patients (BMI 18.5 to 34.9 kg/m2) undergoing CABG surgery. In contrast, extreme obesity (BMI >40 kg/m2) is associated with an increased incidence of mediastinitis, renal failure, prolonged hospital stay, and mortality.31
Peripheral Vascular Disease
There is controversy about whether coronary revascularization should be undertaken prior to major noncardiac surgery in patients with severe coronary artery disease. Furthermore, it is not certain what form of revascularization should be offered, because patients undergoing PCI are known to have high rates of early stent occlusion if subsequently subjected to major surgery. A recent trial found no difference in outcome between preoperative revascularization and no preoperative revascularization (PCI or CABG surgery) prior to major vascular surgery.32
Other Medical Problems
A patient with a history of adrenocortical disease (including chronic treatment with corticosteroids) or thyroid disease (including chronic treatment with amiodarone) should undergo preoperative testing of adrenal and thyroid function. Guidelines for perioperative treatment with corticosteroids and thyroxine are provided in Chapter 36.
Reoperation
Up to 10% of patients who undergo CABG surgery present for reoperation.1 The early mortality rate in revision CABG surgery is higher than that in revision valve surgery and in first-time CABG surgery, but long-term survival is good.
A range of intraoperative problems may be encountered with revision CABG surgery that are not seen with first-time surgery. Patent grafts may be damaged during resternotomy. A patent mammary graft may allow continued myocardial perfusion during aortic cross-clamp, making effective cardioplegia difficult. Atherosclerosis within diseased saphenous vein grafts can result in coronary emboli during surgical manipulations. Surgery is often prolonged because of the requirement for extensive dissection and division of adhesions. With revision CABG surgery, the entire heart may have to be mobilized. (In contrast, the whole heart does not usually require mobilization for revision valve surgery.) Thus, bleeding, impaired ventricular function, and a pronounced systemic inflammatory response often complicate the postoperative course.
OPERATIVE DECISION MAKING
Coronary Grafts
Internal Mammary Arteries
The figures for patency vary among studies,33,34 but a LIMA-LAD graft consistently has the highest long-term patency of any graft, typically being greater than 90% at 10 years. Patency rates for the RIMA graft are slightly lower than those for the LIMA-LAD graft.35 There is some evidence of an incremental benefit of using both internal mammary arteries rather than only a LIMA-LAD graft.36
Radial Arteries
The radial artery has recently been reintroduced as a conduit, and it has patency rates superior to those of saphenous vein grafts37 and approaching those of the internal mammary grafts. Because the potential for digital ischemia or hand claudication exists following resection of the radial artery, the graft is usually taken from the nondominant arm. In addition to preoperative clinical examination, a Doppler ultrasound study of the arterial supply to the hand may be carried out prior to surgery. The radial artery should be used only if ulnar arterial supply to the deep and superficial palmar arches is satisfactory. Resection of the radial artery is occasionally associated with radial nerve damage or a brachial plexus injury (due to excessive abduction at the shoulder during positioning).
Saphenous Vein Grafts
Saphenous vein grafts have historically been the most commonly used conduit for CABG surgery, and their use remains widespread today. Reported patency rates of 80% at 3 years are typical.38 However, recently a 5-year patency rate of 95% has been reported,33 possibly related to aggressive use of statin therapy postoperatively.
Graft Placement
Graft placement is determined primarily on the basis of the coronary angiogram (see Chapter 5). In patients with impaired ventricular function, additional information from a myocardial viability study may be sought to help determine optimal graft placement.
Patency rates are better for grafts to the left coronary artery than to the right coronary artery and are better when larger (>2 mm internal diameter) vessels are grafted, irrespective of the conduit used.34 The ACC/AHA guidelines recommend that a LIMA-LAD graft be considered in all patients undergoing CABG surgery. Although arterial conduits are associated with high long-term patency, they are more prone to postoperative spasm than are saphenous vein grafts—particularly the radial artery.39 Also, there is an increased risk for early occlusion when arterial grafts are used to bypass coronary stenoses of less than 70%. Thus, arterial conduits tend to be favored in younger patients with high-grade, discrete stenoses, whereas saphenous vein grafts are more likely to be used in older patients with lower grade (50% to 70%) stenoses or diffuse disease.
Techniques of Coronary Artery Surgery
On-Pump CABG Surgery
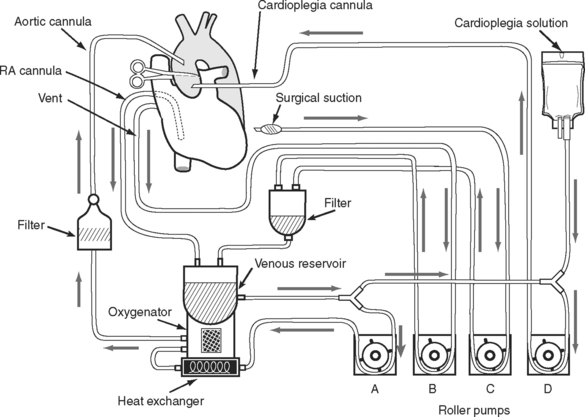
The circuit for the CPB machine is shown in Fig. 9-1. Deoxygenated blood is drained by gravity to the venous reservoir via a large-bore cannula placed in the patient’s right atrium. For CABG surgery, this is usually a single venous cannula that drains both the superior and inferior vena cavae. This is distinct from tricuspid and mitral valve surgery in which selective cannulation of the vena cavae (i.e., bicaval cannulation) is usually performed to allow the right atrium to be opened (in tricuspid valve surgery) or to improve surgical access (in mitral valve surgery). From the reservoir, blood is pumped, typically using a roller or constrained vortex pump, through a heat exchanger to allow precise temperature control, and through a hollow-fiber membrane oxygenator where gas exchange takes place. Oxygenated blood then passes through a 20- to 40-μm filter and is returned to the patient via a cannula (usually) placed in the proximal ascending aorta. In this way the heart and lungs are “bypassed,” allowing the heart to be stopped to facilitate surgery.
The fluid load associated with the CPB prime and the cardioplegia solution (along with the use of mannitol in the prime fluid, as occurs in some centers) can result in marked polyuria during the early postoperative period.
OPCAB Graft Surgery
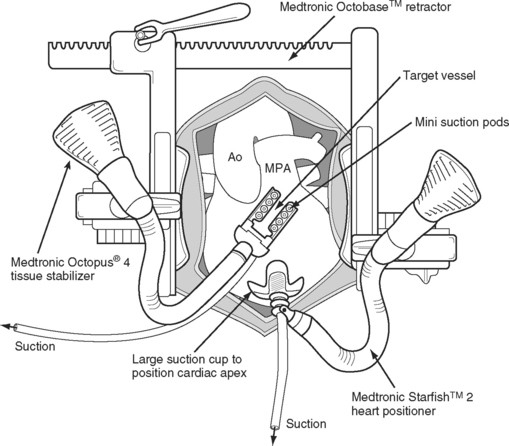
During OPCAB graft surgery, coronary grafting is performed on a beating heart. Various stabilizing devices are used to keep the heart still during the coronary anastomoses (Fig. 9-2), and temporary intracoronary shunts may be used to provide a relatively bloodless surgical field and distal vessel perfusion. To place grafts to the obtuse marginal and posterior descending arteries, the heart must be partially lifted out of the chest and rotated, with the possibility of hemodynamic instability and, occasionally, cardiovascular collapse. Proximal aortic anastomoses are performed by using a partial occlusion side-biting aortic clamp. Alternatively, proximal anastomoses may be attached to a pedicle LIMA graft. Some surgeons perform most or all of their coronary surgery off-pump; others limit the technique to grafts to the LAD coronary artery and its branches; and other surgeons do not use the technique at all.
The main advantage of OPCAB surgery is the avoidance of CPB and its deleterious effects. A disadvantage of OPCAB is that concomitant intracardiac surgery (e.g., valve or ventricular remodeling surgery) cannot be performed without the use of CPB. OPCAB graft surgery has been the subject of an enormous amount of research and debate over the past 10 years. For surgeons and institutions experienced in off-pump surgery, reduced perioperative morbidity and mortality rates can be achieved.40,41 A recent metaanalysis of randomized controlled trials demonstrated a nonsignificant trend toward improved outcome with OPCAB,42 but a subsequent large randomized trial demonstrated almost identical short-term outcomes with the two techniques.43 There is some evidence that early graft patency may be reduced with OPCAB surgery.44
A 2003 survey of Canadian practice found that 16% of all coronary revascularizations were performed off-pump, with only 23% of respondents indicating that their use of the technique would increase over the next 5 years.45
POSTOPERATIVE CARE FOLLOWING CABG SURGERY
In most respects, the care of patients following CABG surgery is the same as that of any patient undergoing heart surgery, as outlined in Chapter 17. However, two issues specific to patients undergoing CABG surgery warrant special mention here: (1) pharmacotherapy following CABG surgery; and (2) the diagnosis and treatment of postoperative myocardial ischemia.
PHARMACOTHERAPY FOLLOWING CABG SURGERY
The pharmacology and use of the drugs listed subsequently are discussed in Chapter 3.
Drugs to Prevent Coronary Graft Spasm
Vasodilators are widely used after CABG surgery to prevent spasm within coronary grafts. Both nitrates and calcium channel blockers are effective for this purpose.46 In the early postoperative period, an intravenous agent such as nitroglycerin or nicardipine may be used. Milrinone is also a potent dilator of arterial vessels and, in patients with impaired ventricular function, has been reported to reduce myocardial injury more than nifedipine.47
The radial artery has a greater propensity for spasm than other grafts.39 Consequently, in most centers, a dihydropyridine calcium channel blocker (e.g., felodopine, 2.5 to 5 mg daily) or a long-acting nitrate is continued for 3 to 6 months following surgery if a radial artery conduit has been used. The use of calcium channel blockers has been associated with improved in-hospital survival following CABG surgery,48 but there is no benefit in continuing treatment beyond 1 year.49
Drugs to Prevent Coronary Graft Thrombosis or Atherosclerosis
All patients should receive aspirin (100 mg/daily) indefinitely because it has been shown to improve the patency of saphenous vein grafts.50 Unless the patient is bleeding excessively, aspirin should be given within 7 hours of surgery; if it is started at 48 hours after surgery, the beneficial effect on saphenous vein patency may not occur.51 If aspirin is contraindicated because of allergy, clopidogrel can be used as an alternative.52 Clopidogrel and aspirin are sometimes used in combination following OPCAB surgery.
Aggressive treatment with statins improves the long-term patency of saphenous vein grafts and reduces the need for revascularization.53 Additionally, preoperative statin therapy has been associated with a reduction in early postoperative mortality following CABG surgery.54 Statins should be commenced in all patients following CABG surgery, aiming for a low-density lipoprotein cholesterol less than 100 mg/dl or less than 70 mg/dl in high-risk patients.1
Other Drugs
In patients undergoing CABG surgery, preoperative use of β blockers is associated with reduced mortality rates.55 β Blockers reduce the incidence of postoperative atrial fibrillation and other arrhythmias and are useful as antihypertensive agents. In patients with left ventricular dysfunction, β blockers help prevent ventricular remodeling and, over time, improve ejection fraction. Acute cessation of therapy at the time of surgery in patients chronically treated with β blockers may precipitate arrhythmias or, in patients who are incompletely revascularized, myocardial ischemia. Thus, β blockers should be continued up until the morning of surgery and reinstituted on the first postoperative day. In the absence of contraindications, routine treatment with β blockers should be considered in all patients after CABG surgery. However, in patients with impaired ventricular function or histories of congestive cardiac failure, β blockers should be introduced cautiously.
ACE inhibitors improve survival rates in patients with impaired left ventricular function and in patients with normal left ventricular function at risk for ischemic cardiac events.56 Thus, in the absence of contraindications, routine treatment with ACE inhibitors should be considered in all patients after CABG surgery. ACE inhibitors should be introduced cautiously in patients with recent renal dysfunction (see Chapter 3).
Anticoagulation with warfarin is indicated in patients with recent anteroapical myocardial infarction and a persistent wall motion abnormality after CABG surgery. Treatment should be continued for 3 to 6 months.1 Warfarin is also indicated for atrial fibrillation that lasts longer than 24 to 48 hours. Other routine postoperative drug therapy, not specific for CABG surgery, is discussed in Chapter 17.
POSTOPERATIVE MYOCARDIAL ISCHEMIA AND INFARCTION
Perioperative myocardial infarction, as identified by new Q waves on the ECG, occurs in 2% to 4% of patients after CABG surgery and is predictive of increased likelihood of mortality.57,58 However, the diagnoses of postoperative myocardial ischemia and infarction are not straightforward. In the early period, the critical issue is whether there is acute ischemia secondary to a problem with a coronary graft or native coronary vessel that warrants urgent intervention.
Diagnosis of Myocardial Ischemia and Infarction
Some degree of myocardial injury virtually always occurs after CABG surgery. At one end of the spectrum, this myocardial injury is manifested as a small troponin release with no clinical sequelae. Troponin release occurs for a variety of reasons, including direct myocardial trauma resulting from surgical incisions, occlusion or resection of small coronary vessels, and imperfect intraoperative myocardial protection, a particular problem in patients with hypertrophied ventricles and tight proximal coronary stenoses. At the other end of the spectrum is severe myocardial ischemia or infarction that is associated with hypotension, low cardiac output, and ventricular arrhythmias. This latter situation demands urgent investigation because it may represent an acute obstruction (spasm, kinking, or thrombosis) of a coronary graft or native coronary vessel. Timely intervention may be lifesaving.
Clinical Assessment
If awake, patients may complain of angina-type pain. However, pain caused by myocardial ischemia is very difficult to distinguish from wound pain. Most patients are sedated and ventilated during their early postoperative period and cannot report their symptoms. Hemodynamic instability has many causes, but one of the most important to consider in patients after CABG surgery is myocardial ischemia. Acute ischemia of severity sufficient to cause hypotension or low cardiac output implies a large region of threatened myocardium and warrants urgent intervention and treatment. If a pulmonary artery catheter is in place, there may be elevations in the pulmonary artery and pulmonary artery wedge pressures, but these findings are nonspecific. A systematic approach to hemodynamic instability is outlined in Chapter 20.
ECG Diagnosis
New significant Q waves on an ECG (≥ 0.04 second duration in any two leads except III and aVR) may be indicative of full-thickness myocardial infarction, but they take 24 to 48 hours to develop and are therefore not useful in the assessment of suspected ischemia (see also Chapters 8 and Chapter 18). In contrast, ST segment deviation (≥ 1 mm, measured 0.06 sec after the J point), if it occurs, develops concurrently with myocardial ischemia. ST segment deviation, particularly elevation, is associated with the subsequent development of new Q waves after CABG surgery59 and therefore may provide a useful marker of acute ischemia. However, ST segment and T-wave abnormalities, particularly the “pericarditic” pattern of upsloping ST segment elevation across multiple leads, are common following cardiac surgery and do not necessarily imply ischemia. New left bundle branch or atrioventricular block may indicate acute ischemia, but they too are common following cardiac surgery. However, ventricular arrhythmias, particularly recurrent ventricular tachycardia or fibrillation, are strongly suggestive of severe acute ischemia. Despite the limitations of ECG analysis, the finding of ST segment depression or elevation that is limited to a specific coronary territory, particularly if associated with hemodynamic instability or ventricular arrhythmias, is strongly suggestive of acute ischemia.
Echocardiography
All patients with suspected myocardial ischemia after CABG surgery should undergo urgent echocardiograms—preferably a transesophageal echocardiogram examination— looking for segmental wall motion abnormalities (SWMAs). SWMAs are more sensitive and specific for myocardial ischemia than ECG changes, but they can be difficult to interpret in postoperative patients. In addition to acute ischemia, SWMAs may be caused by myocardial infarction, stunning, hibernation, and the effects of pacing. Any SWMAs identified should be compared with findings in the preoperative or intraoperative echocardiograms and with previously known patterns of myocardial ischemia and infarction. The characteristics of SWMAs that are suggestive of acute ischemia are outlined in Chapter 7.
Biochemical Markers
As noted earlier, a small rise in serum troponin is a normal finding after CABG surgery. However, large increases are indicative of perioperative myocardial infarction and are associated with adverse outcome. For troponin I, a value greater than 20 μg/l is associated with prolonged hospital stay60 and indicative of early graft failure.61 For troponin T, a value above 1.58 μg/l at 18 to 24 hours after surgery is predictive of adverse outcome, including death.62
A limitation of making judgments based on troponins is that peak levels occur at about 24 hours after an ischemic event; troponin T peaks a little later than troponin I (see Fig. 18-11). Thus, these markers are not ideal for the evaluation of acute ischemia soon after surgery.
Elevation of the creatine kinase (CK) muscle and brain subunits (MB) fraction is also associated with adverse outcome following CABG surgery63 but is less sensitive and specific than the troponins.61 CKMB takes nearly 24 hours to reach peak levels. Myoglobin levels peak within 6 to 12 hours of ischemic injury but are poorly predictive of outcome.61
Interventions for Suspected Postoperative Myocardial Ischemia
If myocardial ischemia is suspected on the basis of hemodynamic instability or ECG changes, an urgent transesophageal echocardiogram should be performed and a serum troponin level obtained. The ECG and echocardiographic findings must be integrated into the surgical findings. For instance, an anterior SWMA may fit with surgical concern regarding the LAD graft. If SWMAs consistent with acute myocardial ischemia are confirmed by echocardiography, aggressive treatment for presumed graft spasm should be commenced. If the patient is hypertensive, an intravenous vasodilator such as nitroglycerin or nicardipine should be commenced, and the dose should be increased to the maximum level tolerated, while avoiding hypotension. In the hypotensive patient, hypovolemia should be corrected and, if necessary, a vasopressor such as norepinephrine used to support blood pressure. The reduction in blood flow through arterial conduits due to hypotension greatly exceeds the adverse effects of α1-receptor-mediated vasoconstriction of the conduit.46 An IABP should be considered in patients refractory to pharmacologic therapy or in cardiogenic shock.
Interventions for Postoperative Myocardial Infarction
A proportion of patients, many of whom will have had uneventful postoperative courses, demonstrate either ECG or biochemical evidence of myocardial infarction on routine postoperative tests. Such patients are at increased risk for adverse events and should be kept in a highly monitored environment (ICU or high-dependency unit) during their early postoperative periods. Prior to discharge to the ward, such patients should be medically optimized with β blockers, ACE inhibitors, antiplatelet agents (aspirin ± clopidogrel), and statins.1
SURGERY FOR THE COMPLICATIONS OF MYOCARDIAL INFARCTION
Surgery may be required for acute or chronic complications of myocardial infarction. Acute complications include ventricular septal rupture, free wall rupture, and acute mitral regurgitation. Patients are typically critically unwell and have cardiogenic shock. Mortality rates are very high. The major chronic complications of myocardial infarction are congestive cardiac failure and mitral regurgitation. A number of procedures to remodel the left ventricle and therefore improve the outcome of congestive cardiac failure have been developed. The causes and treatment of chronic ischemic mitral regurgitation are discussed in Chapter 10.
Acute Mitral Regurgitation
Acute mitral regurgitation64 causing shock is usually caused by rupture of the posteromedial papillary muscle and is associated with inferior myocardial infarction. The posteromedial papillary muscle is prone to infarction because it is supplied entirely by branches of the right coronary artery. In contrast, the anterolateral papillary muscle has dual blood supply from branches of the LAD and circumflex coronary arteries. The abrupt onset of shock and pulmonary edema 2 to 7 days following a myocardial infarction is the most useful diagnostic clue. The precipitating myocardial infarction may be clinically insignificant. The murmur of mitral regurgitation may be soft and limited to early systole; in the presence of shock, it may be inaudible. The diagnosis is best confirmed by echocardiography. A pulmonary artery catheter typically demonstrates pulmonary hypertension and elevated pulmonary artery wedge pressure by revealing large V waves.
Ventricular Free Wall Rupture
Ventricular free wall rupture65 occurs in 0.8% to 6.2% of patients after myocardial infarction. Risk factors include female sex, increased age, and large, full-thickness infarction with persisting ST segment elevation. Late thrombolysis (>11 hr) increases the risk, whereas early thrombolysis (<7 hr) and PCI diminish the risk.
The majority of ruptures occur within 24 hours of infarction. The classic presentation is an abrupt onset of pulseless electrical activity or asystole in association with distended neck veins in a patient not previously shocked. Complete rupture is rapidly fatal, but in about 30% of patients, the presentation is subacute, showing signs of hypotension, syncope, recurrent chest pain, and arrhythmias. Pulsus paradoxus and distended neck veins are classic signs of tamponade but are absent in a significant proportion of cases of subacute rupture. Evidence of pericardial fluid and signs of tamponade should be sought by means of echocardiography. However, of the 20 patients who developed cardiac rupture in the SHOCK trial registry, only 15 had evidence of a pericardial collection on echocardiography.66 Furthermore, pericardial fluid can be identified echocardiographically in 25% of patients following myocardial infarction without evidence of rupture.
Following subacute rupture, a small proportion of patients will survive and go on to develop a contained rupture (ventricular pseudoaneurysm),67 but for the majority of patients, early surgery is the only hope for survival. Pericardiocentesis (see Chapter 40) may be performed as a temporizing measure prior to surgery. Mortality rates are very high.
Ventricular Septal Rupture
Ventricular septal rupture68 occurs in less than 2% of patients after myocardial infarction. It may occur within a few hours following a myocardial infarction but more commonly presents within a few days to a week. The condition is associated with the sudden onset of pulmonary edema, hypotension, a harsh pansystolic murmur best heard over the left sternal edge and, possibly, recurrence of angina. The ECG may show a new rightward axis and conduction defects such as right bundle branch or atrioventricular block.
Surgery to Remodel the Left Ventricle
STEMI may be associated with significant scar formation and ventricular remodeling within the surrounding (noninfarcted) myocardium. Ventricular remodeling results in the loss of the normal cylindrical shape of the left ventricle and the development of a more spherical conformation. Left ventricular dilatation, specifically a left ventricular end-systolic volume index greater than 40 ml/m2 is an independent predictor of mortality following myocardial infarction.69
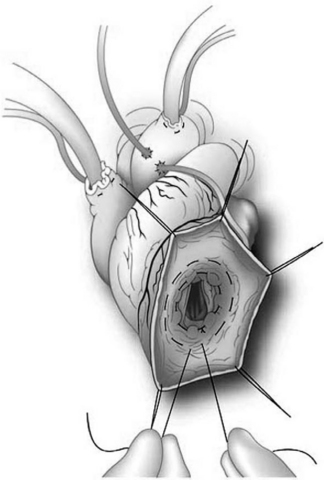
These observations have led to the development of a number of operations to remodel the left ventricle, of which the most widely employed is the Dor operation (Fig. 9-3). CABG surgery, mitral repair, or resection of endocardium and cryotherapy for ventricular arrhythmias may be performed at the same time. The aim of the surgery is to return the ventricle to a more physiologic shape and to reduce the left ventricular end-systolic volume index >60 ml/min/m2. The ideal candidate for this type of operation has a dilated left ventricle (endsystolic volume index >60 ml/min/m2), an anteroseptal scar with evidence of remote viability, graftable coronary disease, and minimal mitral regurgitation.
Dor has demonstrated an average increase in ejection fraction from 20% to 40% at 1 year following surgery and a perioperative mortality of 12% with this procedure.70 Despite the fact that the procedure involves a ventriculotomy and concomitant CABG surgery (and possibly also mitral valve surgery), patients do not usually have stormy postoperative courses. A period of support with inotropic therapy and possibly an IABP may be required. A feared complication of this procedure is an excessive reduction in ventricular volume, resulting in persisting postoperative congestive cardiac failure due to diastolic dysfunction.
1 Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation. 2004;110:340-437.
2 de Feyter PJ, Serruys PW, Unger F, et al. Bypass surgery versus stenting for the treatment of multivessel disease in patients with unstable angina compared with stable angina. Circulation. 2002;105:2367-2372.
3 Serruys PW, Unger F, Sousa JE, et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001;344:1117-1124.
4 SoS Investigators. Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the Stent or Surgery trial): a randomised controlled trial. Lancet. 2002;360:965-970.
5 Morrison DA, Sethi G, Sacks J, et al. Percutaneous coronary intervention versus repeat bypass surgery for patients with medically refractory myocardial ischemia: AWESOME randomized trial and registry experience with post-CABG patients. J Am Coll Cardiol. 2002;40:1951-1954.
6 Edwards FH, Grover FL, Shroyer AL, et al. The Society of Thoracic Surgeons National Cardiac Surgery Database: current risk assessment. Ann Thorac Surg. 1997;63:903-908.
7 Gardner SC, Grunwald GK, Rumsfeld JS, et al. Comparison of short-term mortality risk factors for valve replacement versus coronary artery bypass graft surgery. Ann Thorac Surg. 2004;77:549-556.
8 Braxton JH, Hammond GL, Letsou GV, et al. Optimal timing of coronary artery bypass graft surgery after acute myocardial infarction. Circulation. 1995;92:II66-II68.
9 Bowers TR, O’Neill WW, Grines C, et al. Effect of reperfusion on biventricular function and survival after right ventricular infarction. N Engl J Med. 1998;338:933-940.
10 Lotfi M, Mackie K, Dzavik V, et al. Impact of delays to cardiac surgery after failed angioplasty and stenting. J Am Coll Cardiol. 2004;43:337-342.
11 Christenson JT, Simonet F, Badel P, et al. Evaluation of preoperative intra-aortic balloon pump support in high risk coronary patients. Eur J Cardiothorac Surg. 1997;11:1097-1101.
12 Dang NC, Topkara VK, Leacche M, et al. Left ventricular assist device implantation after acute anterior wall myocardial infarction and cardiogenic shock: a two-center study. J Thorac Cardiovasc Surg. 2005;130:693-698.
13 Coleman GM, Gradinac S, Taegtmeyer H, et al. Efficacy of metabolic support with glucose-insulin-potassium for left ventricular pump failure after aortocoronary bypass surgery. Circulation. 1989;80:I91-I96.
14 Lazar HL, Philippides G, Fitzgerald C, et al. Glucose-insulin-potassium solutions enhance recovery after urgent coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1997;113:354-360.
15 Pagley PR, Beller GA, Watson DD, et al. Improved outcome after coronary bypass surgery in patients with ischemic cardiomyopathy and residual myocardial viability. Circulation. 1997;96:793-800.
16 Aklog L, Filsoufi F, Flores KQ, et al. Does coronary artery bypass grafting alone correct moderate ischemic mitral regurgitation ? Circulation. 2001;104:I68-I75.
17 Salasidis GC, Latter DA, Steinmetz OK, et al. Carotid artery duplex scanning in preoperative assessment for coronary artery revascularization: the association between peripheral vascular disease, carotid artery stenosis, and stroke. J Vasc Surg. 1995;21:154-160.
18 Schwartz LB, Bridgman AH, Kieffer RW, et al. Asymptomatic carotid artery stenosis and stroke in patients undergoing cardiopulmonary bypass. J Vasc Surg. 1995;21:146-153.
19 Naylor AR. A critical review of the role of carotid disease and the outcomes of staged and synchronous carotid surgery. Sem Cardiothorac Vasc Anesth. 2004;8:37-42.
20 Medalion B, Katz MG, Cohen AJ, et al. Long-term beneficial effect of coronary artery bypass grafting in patients with COPD. Chest. 2004;125:56-62.
21 Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. N Engl J Med. 1996;335:217-225.
22 Pell JP, Pell AC, Jeffrey RR, et al. Comparison of survival following coronary artery bypass grafting vs. percutaneous coronary intervention in diabetic and non-diabetic patients: retrospective cohort study of 6320 procedures. Diabet Med. 2004;21:790-792.
23 Woods SE, Smith JM, Sohail S, et al. The influence of type 2 diabetes mellitus in patients undergoing coronary artery bypass graft surgery: an 8-year prospective cohort study. Chest. 2004;126:1789-1795.
24 Lazar HL, Chipkin SR, Fitzgerald CA, et al. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109:1497-1502.
25 Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007-1021.
26 Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Prac. 2004;10:21-33.
27 Samuels LE, Sharma S, Morris RJ, et al. Coronary artery bypass grafting in patients with chronic renal failure: a reappraisal. J Card Surg. 1996;11:128-133.
28 Mangano CM, Diamondstone LS, Ramsay JG, et al. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194-203.
29 Horst M, Mehlhorn U, Hoerstrup SP, et al. Cardiac surgery in patients with end-stage renal disease: 10-year experience. Ann Thorac Surg. 2000;69:96-101.
30 Potapov EV, Loebe M, Anker S, et al. Impact of body mass index on outcome in patients after coronary artery bypass grafting with and without valve surgery. Eur Heart J. 2003;24:1933-1941.
31 Prabhakar G, Haan CK, Peterson ED, et al. The risks of moderate and extreme obesity for coronary artery bypass grafting outcomes: a study from the Society of Thoracic Surgeons ’ database. Ann Thorac Surg. 2002;74:1125-1130.
32 McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795-2804.
33 Tatoulis J, Buxton BF, Fuller JA. Patencies of 2127 arterial to coronary conduits over 15 years. Ann Thorac Surg. 2004;77:93-101.
34 Khot UN, Friedman DT, Pettersson G, et al. Radial artery bypass grafts have an increased occurrence of angiographically severe stenosis and occlusion compared with left internal mammary arteries and saphenous vein grafts. Circulation. 2004;109:2086-2091.
35 Chow MS, Sim E, Orszulak TA, et al. Patency of internal thoracic artery grafts: comparison of right versus left and importance of vessel grafted. Circulation. 1994;90:II129-III132.
36 Rizzoli G, Schiavon L, Bellini P. Does the use of bilateral internal mammary artery (IMA) grafts provide incremental benefit relative to the use of a single IMA graft ? A meta-analysis approach. Eur J Cardiothorac Surg. 2002;22:781-786.
37 Desai ND, Cohen EA, Naylor CD, et al. A randomized comparison of radial-artery and saphenous-vein coronary bypass grafts. N Engl J Med. 2004;351:2302-2309.
38 Goldman S, Copeland J, Moritz T, et al. Long-term graft patency (3 years) after coronary artery surgery: effects of aspirin: results of a VA Cooperative study. Circulation. 1994;89:1138-1143.
39 Chardigny C, Jebara VA, Acar C, et al. Vasoreactivity of the radial artery: comparison with the internal mammary and gastroepiploic arteries with implications for coronary artery surgery. Circulation. 1993;88:II115-II127.
40 Cleveland JCJr, Shroyer AL, Chen AY, et al. Off-pump coronary artery bypass grafting decreases risk-adjusted mortality and morbidity. Ann Thorac Surg. 2001;72:1282-1288.
41 Plomondon ME, Cleveland JCJr, Ludwig ST, et al. Off-pump coronary artery bypass is associated with improved risk-adjusted outcomes. Ann Thorac Surg. 2001;72:114-119.
42 Parolari A, Alamanni F, Cannata A, et al. Off-pump versus on-pump coronary artery bypass: meta-analysis of currently available randomized trials. Ann Thorac Surg. 2003;76:37-40.
43 Legare JF, Buth KJ, King S, et al. Coronary bypass surgery performed off pump does not result in lower in-hospital morbidity than coronary artery bypass grafting performed on pump. Circulation. 2004;109:887-892.
44 Khan NE, de Souza A, Mister R, et al. A randomized comparison of off-pump and on-pump multivessel coronary-artery bypass surgery. N Engl J Med. 2004;350:21-28.
45 Desai ND, Pelletier MP, Mallidi HR, et al. Why is off-pump coronary surgery uncommon in Canada ? Results of a population-based survey of Canadian heart surgeons. Circulation. 2004;110:II7-II12.
46 Rosenfeldt FL, He GW, Buxton BF, et al. Pharmacology of coronary artery bypass grafts. Ann Thorac Surg. 1999;67:878-888.
47 Mollhoff T, Schmidt C, van Aken H, et al. Myocardial ischaemia in patients with impaired left ventricular function undergoing coronary artery bypass grafting—milrinone versus nifedipin. Eur J Anaesthesiol. 2002;19:796-802.
48 Wijeysundera DN, Beattie WS, Rao V, et al. Calcium antagonists are associated with reduced mortality after cardiac surgery: a propensity analysis. J Thorac Cardiovasc Surg. 2004;127:755-762.
49 Gaudino M, Glieca F, Luciani N, et al. Clinical and angiographic effects of chronic calcium channel blocker therapy continued beyond first postoperative year in patients with radial artery grafts: results of a prospective randomized investigation. Circulation. 2001;104:I64-I67.
50 Lorenz RL, Schacky CV, Weber M, et al. Improved aortocoronary bypass patency by low-dose aspirin (100 mg daily): effects on platelet aggregation and thromboxane formation. Lancet. 1984;323:1261-1264.
51 Sharma GV, Khuri SF, Josa M, et al. The effect of antiplatelet therapy on saphenous vein coronary artery bypass graft patency. Circulation. 1983;68:II218-II221.
52 Stein PD, Schunemann HJ, Dalen JE, et al. Antithrombotic therapy in patients with saphenous vein and internal mammary artery bypass grafts: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:600S-608S.
53 Post Coronary Artery Bypass Graft Trial Investigators. The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. The Post Coronary Artery Bypass Graft Trial Investigators. N Engl J Med. 1997;336:153-162.
54 Pan W, Pintar T, Anton J, et al. Statins are associated with a reduced incidence of perioperative mortality after coronary artery bypass graft surgery. Circulation. 2004;110:II45-II49.
55 Ferguson TBJr, Coombs LP, Peterson ED, Society of Thoracic Surgeons National Adult Cardiac Surgery D. Preoperative beta-blocker use and mortality and morbidity following CABG surgery in North America. JAMA. 2002;287:2221-2227.
56 Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145-153.
57 Schaff HV, Gersh BJ, Fisher LD, et al. Detrimental effect of perioperative myocardial infarction on late survival after coronary artery bypass. Report from the Coronary Artery Surgery Study —CASS. J Thorac Cardiovasc Surg. 1984;88:972-981.
58 Chaitman BR, Alderman EL, Sheffield LT, et al. Use of survival analysis to determine the clinical significance of new Q waves after coronary bypass surgery. Circulation. 1983;67:302-309.
59 Jain U, Laflamme CJ, Aggarwal A, et al. Electrocardiographic and hemodynamic changes and their association with myocardial infarction during coronary artery bypass surgery: a multicenter study. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Anesthesiology. 1997;86:576-591.
60 Salamonsen RF, Schneider HG, Bailey M, et al. Cardiac troponin I concentrations, but not electrocardiographic results, predict an extended hospital stay after coronary artery bypass graft surgery. Clin Chem. 2005;51:40-46.
61 Thielmann M, Massoudy P, Marggraf G, et al. Role of troponin I, myoglobin, and creatine kinase for the detection of early graft failure following coronary artery bypass grafting. Eur J Cardiothorac Surg. 2004;26:102-109.
62 Januzzi JL, Lewandrowski K, MacGillivray TE, et al. A comparison of cardiac troponin T and creatine kinase-MB for patient evaluation after cardiac surgery. J Am Coll Cardiol. 2002;39:1518-1523.
63 Costa MA, Carere RG, Lichtenstein SV, et al. Incidence, predictors, and significance of abnormal cardiac enzyme rise in patients treated with bypass surgery in the arterial revascularization therapies study (ARTS). Circulation. 2001;104:2689-2693.
64 Birnbaum Y, Chamoun AJ, Conti VR, et al. Mitral regurgitation following acute myocardial infarction. Coron Artery Dis. 2002;13:337-344.
65 Birnbaum Y, Chamoun AJ, Anzuini A, et al. Ventricular free wall rupture following acute myocardial infarction. Coron Artery Dis. 2003;14:463-470.
66 Slater J, Brown RJ, Antonelli TA, et al. Cardiogenic shock due to cardiac free-wall rupture or tamponade after acute myocardial infarction: a report from the SHOCK Trial Registry. Should we emergently revascularize occluded coronaries for cardiogenic shock ? J Am Coll Cardiol. 2000;36:1117-1122.
67 Frances C, Romero A, Grady D. Left ventricular pseudoaneurysm. J Am Coll Cardiol. 1998;32:557-561.
68 Chaux A, Blanche C, Matloff JM, et al. Postinfarction ventricular septal defect. Sem Thorac Cardiovasc Surg. 1998;10:93-99.
69 Migrino RQ, Young JB, Ellis SG, et al. End-systolic volume index at 90 to 180 minutes into reperfusion therapy for acute myocardial infarction is a strong predictor of early and late mortality. The Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO)-I Angiographic Investigators. Circulation. 1997;96:116-121.
70 Dor V, Sabatier M, Di Donato M, et al. Efficacy of endoventricular patch plasty in large postinfarction akinetic scar and severe left ventricular dysfunction: comparison with a series of large dyskinetic scars. J Thorac Cardiovasc Surg. 1998;116:50-59.