Surface Interactions Between T Cells and Antigen-Presenting Cells
Learning Objectives
• Discuss the clonal selection hypothesis and its relationship to the T cell receptor (TCR)
• Know the structure and functions of the α/β TCRs and γ/δ TCRs
• Define complementarity determining region
• Identify T cell subsets that interact with antigens presented on MHC class II molecules
• Understand the concept of somatic recombination in relationship to the TCR
• Explain the roles of RAG-1 and RAG-2 in the generation of TCR diversity
• Compare and contrast the junctional diversity and N- and P-nucleotide additions
• Recognize the components of the CD3 complex
• Differentiate between the signaling and nonsignaling components of the CD3 complex
• Identify TCR:MHC stabilizing molecules and their ligands
• Recognize the four critical co-stimulatory molecules and their roles in cell activation
• Compare the roles of B7-1 and B7-2 in the activation of T cell subsets
• Compare and contrast the roles of CD28 and CTLA-4 in T cell activation
• Understand how abatacept prevents T cell activation
• Understand how alefacept prevents T cell activation
• Recognize monoclonal antibodies that block TCR–HLA molecule interactions
• Explain the mechanism by which superantigens stimulate T cells
• Compare and contrast the pathophysiology of staphylococcal and streptococcal toxic shock syndrome
• Discuss the relationship between Vβ isoforms and toxic shock syndrome
Key Terms
Abatacept
Alefacept
α/β T cell receptor (TCR)
B7 molecules
Complementarity determining regions (CDRs)
CD2
CD3 complex
CD4
CD8
CD11a
CD28
CD40L
Enterotoxin
γ/δ T cell receptor
Hyper-IgM syndrome
Immuno-receptor tyrosine-based activation motifs (ITAMs)
Junctional diversity
Monoclonal antibodies
Omenn syndrome
Somatic cell recombination
Superantigen
P-nucleotide additions
Introduction
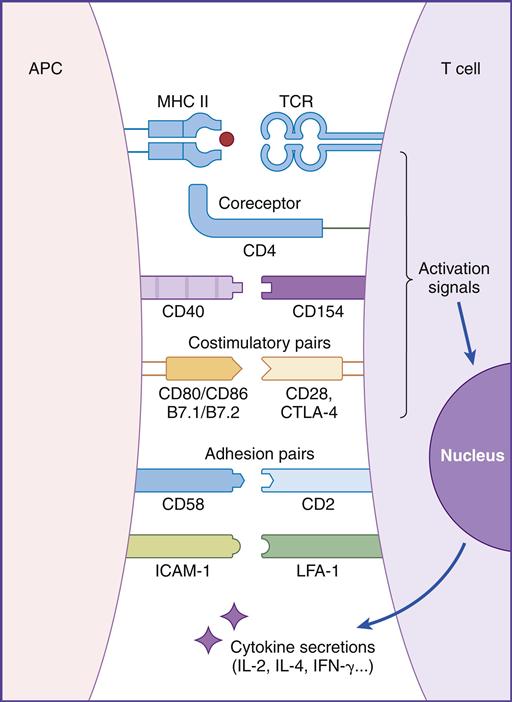
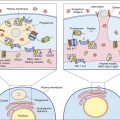
In this chapter, the discussion centers on the roles of receptor interaction in the activation or deactivation of T cells. Much like starting an automobile, several signals must be given in a defined sequence to override interlocking safety systems. For example, T cell activation requires three different steps: (1) The initial step entails interaction between the lymphocyte T cell receptor (TCR) and the antigen-loaded class II molecule. (2) In the second step, a CD4 molecule binds to class II molecules and stabilizes the TCR–class II complex. (3) In the final step of the activation sequence, the CD28 of the T cell interacts with a B7 molecule on the APC (antigen-presenting cell) (Figure 6-1).
If these three interactions do not occur, the T cell becomes nonfunctional for an extended period. In some cases, nonresponsive T cells become apoptotic and die.
T cell activation is central to the elicitation of an immune response. T cells act as effector cells or direct the immune response by influencing the activity of macrophages in the inflammatory response and of B cells in the synthesis of antibodies.
Clonal Selection Hypothesis
In the late 1950s, MacFarland Burnet proposed the clonal selection theory, which states that antigens, rather than lymphocytes, direct the immune response. The five basic tenets of the theory are (1) each lymphocyte has a unique receptor that binds to only one antigen; (2) the receptor must react with the antigen for cellular activation; (3) only selected clones of lymphocytes activated by antigen will divide (clonal expansion); (4) all of the daughter cells will express antigen receptors identical to the parent cell and (5) some of the daughter cells act as effector cells, and others become memory cells.
T Cell Receptors
The unique antigen binding receptor on T cells is called the T cell receptor (TCR). It is expressed on all T cells and exists in either α/β or γ/δ forms. The α/β TCR is expressed on peripheral blood T cells. γ/δ expressing T cells are rare in peripheral blood but are found in abundance in mucosal tissue. Each T cell has a homogeneous TCR population that recognizes a single antigen or epitope.
α/β T Cell Receptors
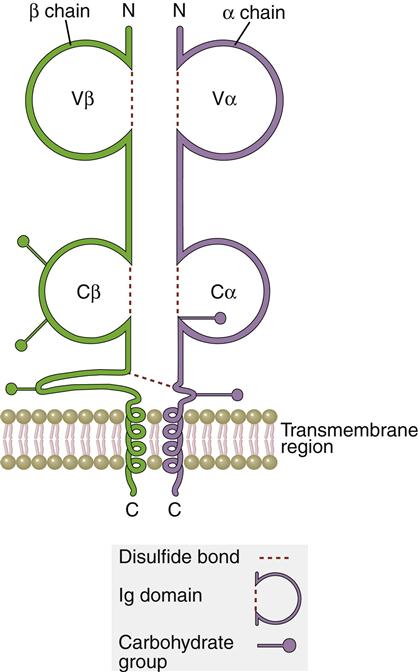
The TCR is a dual chain α/β heterodimer that consists of variable (V) and constant (C) regions. The structure of an α/β TCR is shown in Figure 6-2.
The V regions of the α/β-chains determine antigenic specificity by creating a three-dimensional pocket that recognizes immunogenic epitopes. The dual-chain V region pocket is created through the association of several different genes. The Vα pocket is the result of Vα genes joining with a junctional (Jα) gene. In the β-chain, a functional Vβ is created by linking Vβ, diversity (Dβ), and Jβ-domains.
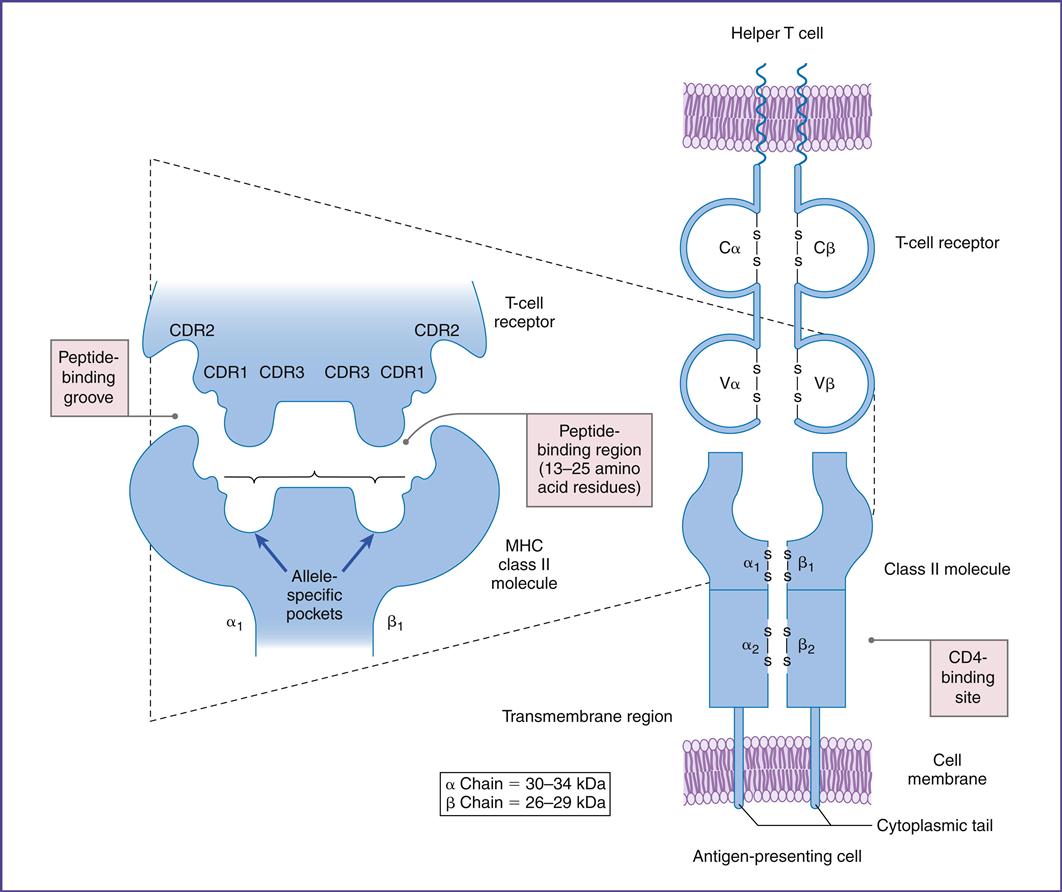
Complementarity determining regions (CDRs), which are hypervariable regions in the Vα/β-chains have actual contact with antigens. Class II molecules have six CDRs (three on each chain). The role of CDRs in binding to major histocompatibility complex (MHC) class II molecules is shown in Figure 6-3.
In the α-chains, CDR1 interacts with the N-terminal portion of the epitope. β-chain CDR1 reacts with the C-terminal portion of the peptide. CDR2s interact with the class II molecules but are not involved in antigen recognition. CDR3s are responsible for binding to the epitope presented by class II molecules. Most of the sequence variability responsible for TCR diversity is found in CDR3.
In addition to V regions, each TCR has an invariant constant (C) region. The C region consists of 140 to 180 hydrophilic amino acids and continues to a short hinge region with cysteine residues that link the α-chain and the β-chain A transmembrane anchor unit, which consists of highly charged hydrophobic amino acids, is connected to a short cytoplasmic region.
γ/δ T Cell Receptors
The Tγ/δ TCR structure is similar to the α/β TCRs. These T cells are only found in the mucosa and function as a bridge between innate and adaptive immunity. CD4γ/δ T cells recognize microbial phosphorylated molecules such as isopentylpyrophosphate (IDP) and dimethylallylpyrophosphate (DMAP). Direct interaction between these molecules and the γ/δ TCR activates the T cell. Antigen processing by APCs or presentation by MHC molecules is not required for activation. A vigorous γ/δ T cell response localizes the microbe in the mucosa until an adaptive response is mounted.
Origin of T Cell Receptor Diversity
In the generation of TCR diversity, two factors must be considered. First, each lymphocyte has a TCR that recognizes a single epitope. Second, 1013 different epitopes must be recognized. If a single lymphocyte TCR recognizes only one epitope, it follows that 1013 lymphocyte clones are present in each of the CD4 (Th1 and Th2) and CD8 (Tc1 and Tc2) populations.
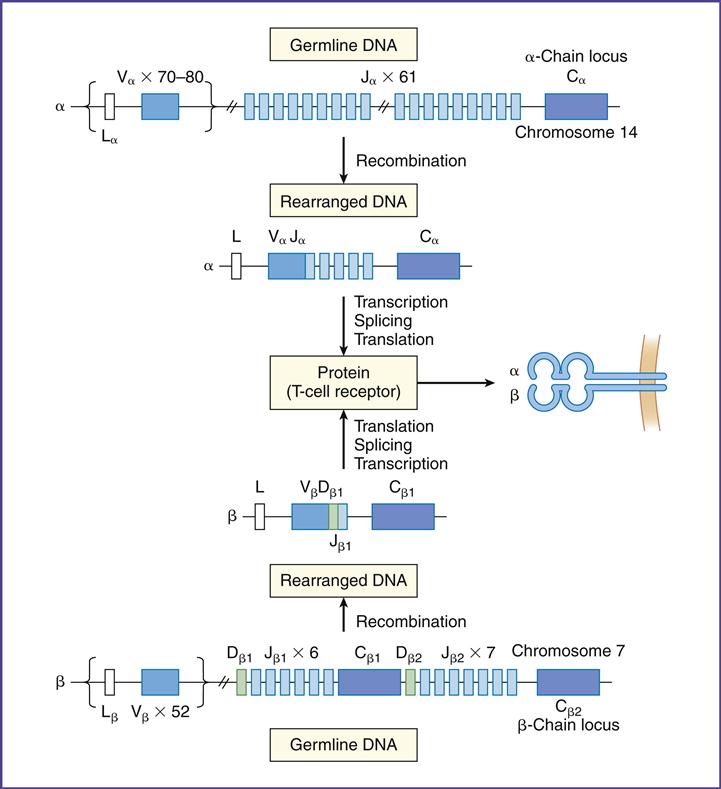
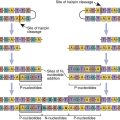
To create a repertoire of antigen-specific TCRs, alternative forms of genes present in somatic cells are rearranged in a process, called somatic cell recombination, by using RAG-1 and RAG-2 recombinase activating enzymes (Figure 6-4).
In the α-chain, 70 to 80 different Vα gene products can be linked to 61 different J-chains. Simple recombination in the α-chain can result in approximately 4.9 × 103 antigen-specific TCRs. β-chains have 52 variable gene segments, two diversity gene segments, and 13 J region genes. Recombination in the β-chain results in 1.3 × 103 unique TCRs.
Additional TCR diversity is created by alternate joining of Dβ-chains, junctional flexibility, and N- and P-nucleotide additions. Alternate joining of D domains creates 5 × 103 diverse TCRs. Adding six or more amino acids to the J domains (junctional diversity) creates more TCR diversity. Random insertion of palindromic sequences at single strand breaks (P-nucleotides) or nontemplated amino acids (N-nucleotides) into the TCR coding regions also occurs. Junctional diversity and P- and N-nucleotides generate an additional 1011 possible TCRs. The sum of the gene re-arrangements (4.9 × 103 + 1.3 × 103 + 5.3 × 10 3 + 1.0 × 1011) allows the immune system to respond to all known antigens.
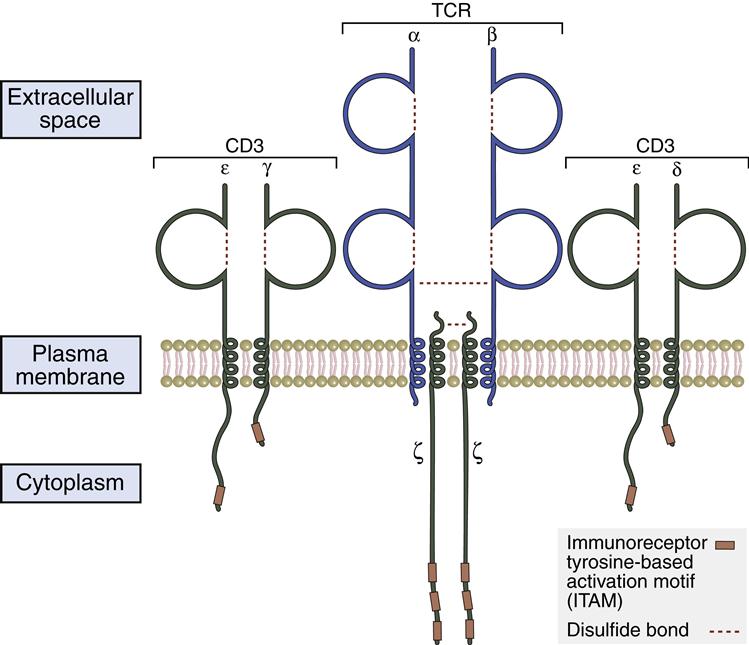
CD3–T Cell Receptors Protein Complex
The TCR lacks intracellular signaling capability because of short cytoplasmic tails. To transduce activation signals to the nucleus, the TCR associates with a number of signaling proteins to create the CD3 complex. The pan T cell maker CD3 is composed of five subunits (α, β, ζ, ε, and γ) proteins. In the assembly of the CD3 complex, two copies of of ζ-chains which form disulfide-linked homodimers (ζ–ζ) are present. Signal transduction is associated with the intracellular domains of ε-, γ-, and ζ-chains. These chains contain 44 to 81 amino acid sequences called immunoreceptor tyrosine–based activation motifs (ITAMs), which are essential to the signaling process. TCR ζ-chains have a short, nine-amino-acid transmembrane sequence and a long (113 amino acids) cytoplasmic tail with three ITAMs (Figure 6-5).
Stabilizing Molecules
The TCR binds to the class II molecules with low affinity. The dissociation constant (Kd) is approximately 10–5 to 10–7M. Because of the low affinity, additional molecules are necessary to stabilize the complex (see Figure 6-1).
CD4 molecules stabilize the interaction between the TCR and the APCs expressing HLA molecules. CD4 molecules are expressed on CD4Th1 and CD4Th2 cells, macrophages, and dendritic cells. The CD4 molecule has a small transmembrane region and a cytoplasmic tail and is capable of transducing signals to the nucleus. Phosphorylation of serine residues in the cytoplasmic tail activates the lck or p56 kinases. These kinases play a critical role in the activation of HLA class II restricted T helper cells.
CD8 is a dual-chain heterodimer that stabilizes the interaction between CD8 cells and antigen-loaded HLA class I molecules. Extracellular CD8 domains bind to the α3 regions on class I molecules. The role of CD8 and endogenous antigens will be discussed in Chapter 18.
Co-stimulatory Molecules
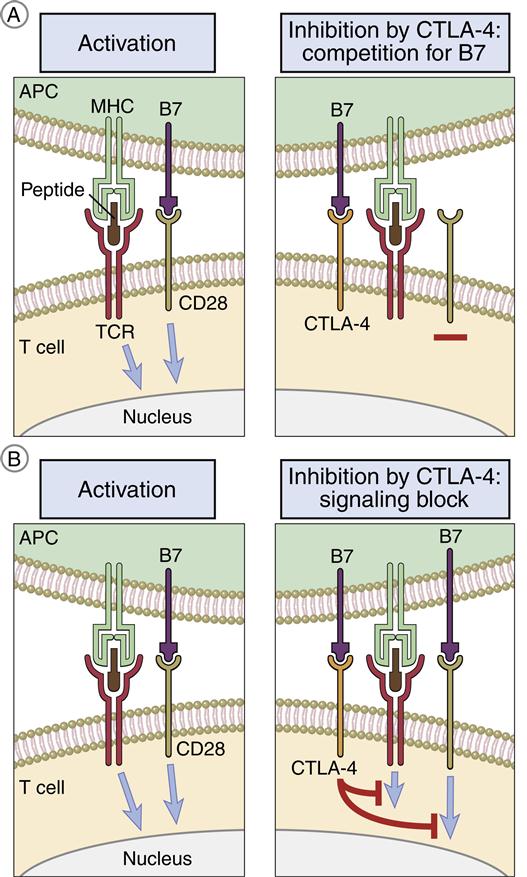
CD28/B7 Interaction
T cells can be activated or downregulated following interaction with B7 molecules on APCs. B7-1 and B7-2 molecules are expressed on monocytes, macrophages, interstitial dendritic cells, and epithelial dendritic cells. After antigen processing, one of the two B7 isoforms is expressed, which determines the nature of the immune response. For example, expression of B7-1 activates CD4Th2 (helper cells in antibody production) and B7-2 expression activates CD4Th1 (inflammatory response).
T cells have two ligands for B7. Interactions between CD28 and B7 activate T cells. The second ligand, which binds to B7 with a high affinity, is called cytotoxic T lymphocyte protein 4 (CTLA-4). Interaction between CTLA-4 and B7 downregulates T cell signaling and prevents T cell activation (Figure 6-6).
CD40 Ligand/CD40
CD40 ligand (CD40L) is a 261-amino-acid membrane glycoprotein expressed on activated CD4 lymphocytes. Expression occurs shortly after T cell activation. The natural ligand for CD40L is CD40, which is a transmembrane protein present on B cells, follicular dendritic cells, and macrophages. CD40–CD40L interactions play an important role in the amplification of the immune response and the production of antibodies. Ligation stimulates the secretion of interleukin 12 (IL-12) from the APCs. In turn, IL-12 activates CD4Th1, CD8, and natural killer (NK) cells and amplifies the immune response.
In the production of antibodies, T and B cells are brought into close proximity. CD40/CD40L ligation between T and B cells induces B cell activation, differentiation, isotypic switching, and the generation of memory cells.
CD2/CD58
CD2 is a glycoprotein present on 90% to 95% of mature T cells and NK cells. Lymphocyte function-associated antigen (LFA-3 or CD58) on APCs is the principal ligand for CD2. Both molecules are adhesion factors that strengthen the interactions between T cells and APCs. Ligation results in the production of proteins that regulate the cellular response to IL-2.
CD11a/CD18/CD54
Integrins are a family of molecules that facilitate the adhesion of T cells to molecules on APCs. CD11a is an α1integrin subunit that associates with an β2-integrin subunit (CD18) to form LFA-1. LFA-1 is a glycoprotein expressed on all leukocytes. The LFA-1 ligand is intercellular adhesion molecule 1 (ICAM-1) on APCs. Interaction between these adhesion factors plays a critical role in T cell activation, T cell mobility, and control of autoimmune diseases.
Agents That Block T Cell Receptor–Antigen Presenting Cell Interactions
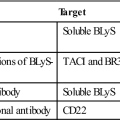
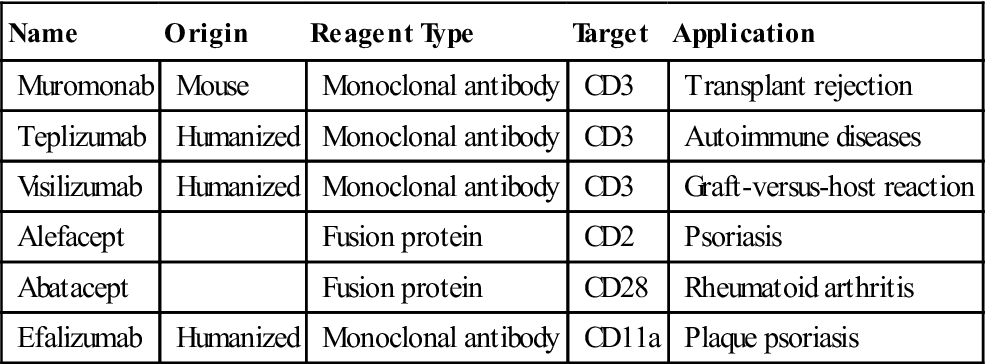
Biotechnology-derived immunotherapeutic agents disrupt or prevent interactions between the TCR or co-stimulatory molecules and the receptors on APCs. Monoclonal antibodies and fusion proteins that prevent T cell activation are shown in Table 6-1.
Table 6-1
Biologic Reagents That Block Interactions between T Cells and Antigen-Presenting Cells
< ?comst?>
| Name | Origin | Reagent Type | Target | Application |
| Muromonab | Mouse | Monoclonal antibody | CD3 | Transplant rejection |
| Teplizumab | Humanized | Monoclonal antibody | CD3 | Autoimmune diseases |
| Visilizumab | Humanized | Monoclonal antibody | CD3 | Graft-versus-host reaction |
| Alefacept | Fusion protein | CD2 | Psoriasis | |
| Abatacept | Fusion protein | CD28 | Rheumatoid arthritis | |
| Efalizumab | Humanized | Monoclonal antibody | CD11a | Plaque psoriasis |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Blockade of T Cell Receptor Interactions
Laboratory-derived monoclonal antibodies (muromomab, teplizumab, and visilizumab) bind to CD3 on activated T cells. Blocking antibodies prevent the interaction between CD3 and the class I or II molecules and are thus useful in preventing recipient graft rejection and, in rare cases, the graft rejecting the host.
Blockade of Co-Stimulatory Molecule Interactions
The interaction between co-stimulatory factors on T cells and APCs can be blocked by biotechnology-derived fusion proteins or monoclonal antibodies. To create a fusion protein, two genes are fused in the laboratory and inserted into an expression vector such as a bacterium or yeast. When translated, the fusion protein contains the active portions of both proteins. Currently, two fusion proteins are used to block the interaction between co-stimulator molecules on T cells and APCs.
Abatacept is a co-stimulator inhibitor in a new class of drugs called disease modifying antirheumatic drugs (DMARDs). In patients with rheumatoid arthritis, T cell activation is the major cause of the inflammatory response in the synovium. Abatacept consists of an Fc region from the immunoglobulin G (IgG) antibody and the extracellular domain of CTLA-4. The CTLA-4 fusion protein physically blocks the interaction between CD28 and the B7 on APCs. Failure to engage CD28 drives activated T cells into apoptosis.
Alefacept is another fusion protein indicated for psoriasis vulgaris, an autoimmune skin disease. Activated CD4, CD8, and memory cells are found in the skin lesions. Alefacept prevents T cell activation and reduces the severity of the lesions. The fusion protein consists of LFA-3 (CD58) linked to the distal end of an antibody molecule. Interactions between the soluble alefacept and the T cell CD2 molecule block the binding of CD2 to the CD58 on APCs.
Monoclonal antibodies, which inhibit co-stimulatory molecules, are also indicated for the treatment of psoriasis. For example, efalizumab blocks interactions between the CD11a component of LFA-1 and the adhesion factors on APCs. Again, downregulation of T cells reduces the severity of skin lesions.
Aberrant Interactions Between TCR And Class II Molecules
Superantigens
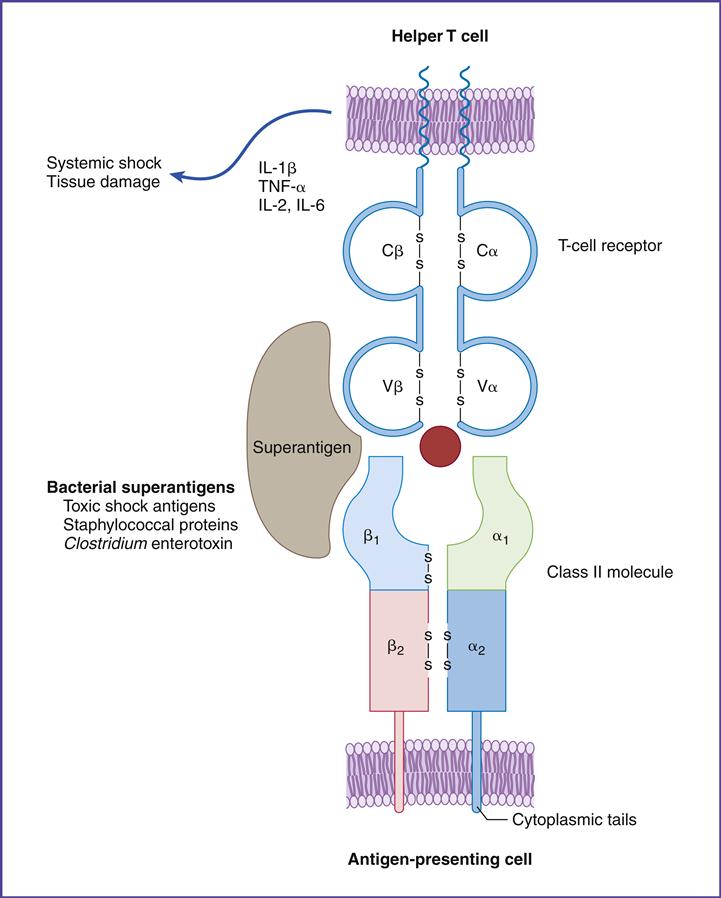
Superantigens are molecules that indiscriminately stimulate up to 20% of all T lymphocytes (normal response to antigen stimulates only 0.01% of T cells), which release massive amounts of proinflammatory cytokines such as tumor factor–α (TNF-α). When released into blood, high levels of TNF-α cause life threatening hypovolemic shock and organ failure.
Superantigens stimulate CD4 cells by a unique mechanism. T cells and APCs are brought into direct contact by the bridging of the constant region of class II molecules and the variable segments of the TCR β-chain (Vβ). Superantigen binding is unique, however, in that it occurs outside the normal binding cleft (Figure 6-7).
Staphylococcal and streptococcal superantigens have been implicated in food poisoning, exfoliative dermatitis in infants (scalded skin syndrome), cellulitis, scarlet fever, and toxic shock syndrome. Staphylococcus aureus secretes five enterotoxins (SEA, SEB, SEC2, SEE, and TSST-1). Enterotoxins are similar to exotoxins but usually only cause moderate to severe diarrhea. All staphylococcal enterotoxins can cause the symptoms of food poisoning, but only SEA and SEB are involved in exfoliative dermatitis. Toxic shock syndrome is associated with the TSST-1, SEB, or SEC2 superantigens.
In the last 20 years, an increase has been seen in the incidence of streptococcal toxic shock syndrome associated with necrotizing fasciitis or myositis. The etiologic agents are invasive Group A streptococcal strains such as Streptococcus pyogenes. These strains produce three different superantigens (SPE-A, SPE-B, and SPE-C) and numerous pyogenic toxins. SPE-A is specifically associated with streptococcal toxic shock syndrome (sTSS). Streptococcal and staphylococcal superantigens act in a similar manner.
Toxic Shock Syndrome and Tampon Use
An association between superabsorbent tampons and toxic shock syndrome has been reported in the literature. In 1979, a tampon manufacturer changed the normal tampon cotton matrix to a highly absorbent polyacrylate. The new matrix concentrated water, magnesium, iron, and oxygen and enhanced the growth of Staphylococcus or Streptococcus, which are part of the normal vaginal flora. Increased magnesium concentrations triggered the production of TSST-1 or SPE-A. The toxin enters the blood by two mechanisms. (1) First, vaginal epithelial cells have TSST-1 receptors that translocate the toxin across the vaginal wall and into the bloodstream. (2) Second, high-absorbency tampons decrease moisture in the vaginal walls. During tampon removal, the vaginal wall is often torn allowing TSST-1 and SPE-A direct access to the bloodstream. In the 12 months following the release of the product into the market, 1200 cases of toxic shock syndrome and significant mortality occurred. Subsequently, the polyacrylate tampon was removed from the market in 1983, and thereafter the incidence of toxic shock syndrome has decreased significantly.
Toxic Shock Syndrome
Staphylococcal toxic shock syndrome is characterized by rapid onset (within 8–12 hours) of fever, vomiting, diarrhea, hypovolemic shock, and multi-organ failure. Death usually occurs within 24 to 36 hours. Superantigen-activated T cells produce high levels of cytokines such as TNF-α and IL-1. These cytokines plus TSST-1 promote vascular permeability and fluid leakage into tissue and severe diarrhea. As a result of electrolyte and fluid loss, less blood is available for the heart to pump. The significant reduction in cardiac output results in hypovolemic shock. Reduced tissue perfusion eventually damages the kidneys, heart, and lungs. Cardiac failure results in death.
Streptococcal superantigens and pyogenic toxins evoke different toxic shock symptomology. sTSS is characterized by adult respiratory distress syndrome, shock, and renal failure. Between 30% and 70% of patients die in spite of aggressive medical treatment.
Treatment of Toxic Shock Syndrome
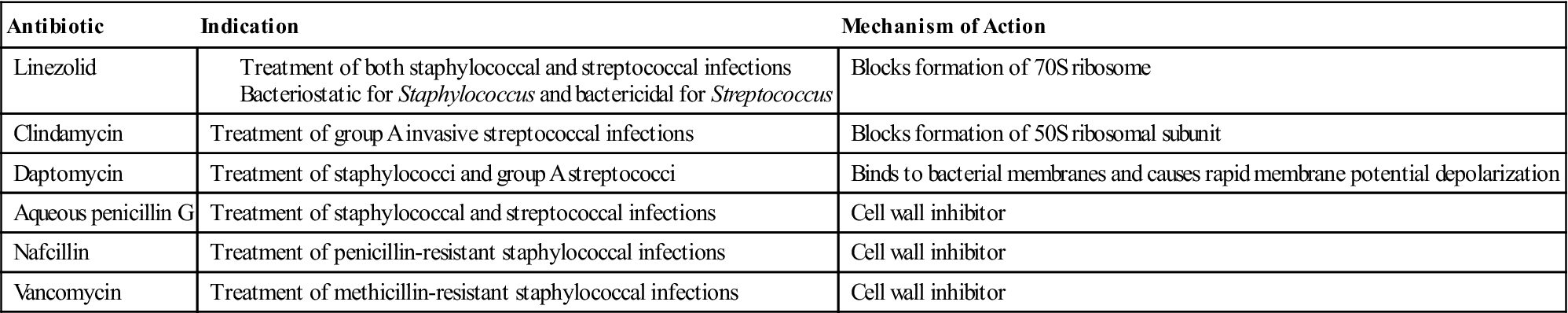
The goal of pharmacotherapy is to eradicate the infective agent. Careful consideration must be given to the identification of the etiologic agent and the antibiotic resistance patterns. Antibiotics used in the treatment of toxic shock are shown in Table 6-2.
Table 6-2
Antibiotics Indicated for Treatment of Toxic Shock Syndrome
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Vβ Isoforms and Superantigen-Induced Clinical Symptoms
Only select individuals are at risk for the development of superantigen-induced clinical symptoms. Risk is related to the presence of genetically determied TCR Vβ isoforms. Fifty-two possible Vβ genes exist. Only individuals with a Vβ2 TCR have are at high risk for developing staphylococcal toxic shock syndrome. Similarly, an increased risk of streptococcal toxic shock is present in individuals expressing Vβ8, 12, or 14 TCRs (Table 6-3).
Table 6-3
Staphylococcal and Streptococcal Superantigens
| Staphylococcal Superantigens | TCR Vβ |
| SEA | Vβ3,11 |
| SEB | Vβ3,12,14,15,17,20 |
| SEC2 | Vβ12,13,14,15,17,20 |
| SEE | Vβ5.1,6.1,6.3, 8.18 |
| Staphylococcal toxic shock syndrome (TSST-1) | Vβ2 |
| Streptococcal Superantigens | TCR Vβ |
| Streptococcal toxic shock syndrome SPE-A | Vβ8,12,14 |
| Streptococcal erythrogenic toxin SPE-B | Vβ8 |
| SPE-C | Vβ1,2,5.1,10 |
Immunodeficiencies
X-Linked Immunodeficiency with Hyper-Immunoglobulin M
The original syndrome was named Hyper-IgM (HIGM). However, the most common form (XHIGM or HGM1) is inherited as an X-linked recessive trait. Individuals with HIGM lack a functioning CD40 ligand (CD40L) on activated T cells. CD40L activation is necessary for switching between antibody classes. These individuals have elevated levels of the high-molecular-weight IgM antibody, but cannot form other antibody types necessary for protection against bacteria and viruses. CD4Th1 immunity also is impaired because CD40L is necessary for interaction with monocytes and dendritic cells. Affected individuals have repeated infections with Haemophilus influenzae and Streptococcus pneumoniae infections. Reduced cellular immunity is reflected in opportunistic infections with Pneumocystis carinii and Cryptosporidium.
Treatment of Hyper-Immunoglobulin M Syndrome
The goal of pharmacotherapy is to prevent infections. Intravenous or subcutaneous immunoglobulin replacement remains the mainstay of therapy. Treatment significantly decreases the incidence of lower respiratory tract infections such as bacterial pneumonia but has no effect on upper respiratory tract infections.
Omenn Syndrome
Omenn syndrome is an autosomal recessive form of severe combined immunodeficiency (SCID). Neonates with Omenn syndrome have a missense mutation in RAG-1 and RAG-2 genes that assemble TCR VDJ and VJ genes. This defect blocks the generation of mature T cells and some B cells. Infants lack CD3 and α/β TCR expressing cells. However, large numbers of γ/δ T cells are found in skin. Desquamation of skin is often evident. Affected children usually do not survive without bone marrow transplantation.