CHAPTER 25 Surface ablation
PRK, LASEK, and Epi-LASIK
Epidemiological considerations and terminology
The prevalence of myopia in Western populations is estimated at about 25%1. In some Asian populations it is as high as 70%–90%. According to some epidemiological evidence, the prevalence of myopia is increasing especially in Asia. Although the etiology of myopia is not quite clear, there is substantial evidence that both genetic and environmental factors play a role.
Photorefractive keratectomy (PRK) came into clinical practice at about 19902. In PRK the epithelium is abraded prior to excimer treatment. Toward the end of the decade, laser epithelial keratomileusis (LASEK) began to establish itself as an alternative surface procedure3. In LASEK, an epithelial flap is prepared manually, rolled up before the laser is applied, and then rolled back over the bare stroma after ablation. The most recent variant of surface ablation, epipolis (Greek for surface) LASIK (Epi-LASIK) was introduced in 20034. Epi-LASIK also involves the use of an epithelial flap, but the flap is prepared with a specialized microkeratome. In the course of the evolution of surface ablation techniques, the term advanced surface ablation (ASA), as distinct from the original PRK, has also been used.
Operation techniques
PRK
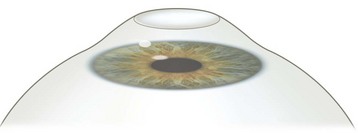
The technician enters the patient data and the treatment protocol into the laser computer and numbers are double-checked by the surgeon. The use of preoperative oral sedatives varies among surgeons and different cultures. Topical anesthetic drops can be started as early as 20 minutes prior to surgery and, unlike in stromal ablation, preservatives are not contraindicated since they may aid in epithelial abrasion by breaking up the hemidesmosomes. With the patient on the operating table and the lid speculum in place, a marker with the desired diameter is used to indicate the area of the planned procedure (usually the central 7–9 mm). Thereafter, abrasion of the epithelium is performed5. This can be done with a blade, with a rotating brush, with 20% ethanol (applied for 20–30 seconds), or with the excimer laser itself (not widely used). Once the epithelium has been removed and Bowman’s membrane displays a smooth aspect, the patient is instructed to focus on a fixation light, the surgeon centers the microscope on the treatment area and initiates the laser treatment (Fig. 25.1). A tracker is used to guard against excessive eye movements. At the end of the procedure an antibiotic ointment and a patch are applied. Some surgeons prefer preservative-free antibiotic drops combined with a soft contact lens.
LASEK
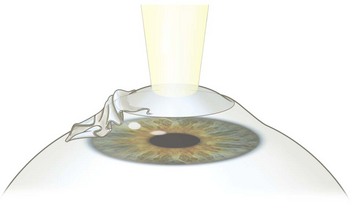
While the initial steps are identical with the PRK procedure, the epithelium is handled differently. The preparation of the epithelial flap is initiated with a dull trephine combined with an alcohol well. The trephine serves to make an approximately 70 µm deep circular epithelial incision, leaving an uncut sector at 12 o’clock serving as a hinge. 20% ethanol is then instilled into the well and allowed to act on the epithelium for 20–30 seconds. The ethanol loosens the epithelium and makes it easier to prepare an intact flap. A special instrument (often termed a ‘hoe’) is then inserted into the circular epithelial incision at 6 o’clock and used to separate the epithelium from Bowman’s layer. The separation is continued until the flap is complete and bunched up at the hinge. The laser procedure is the same as for PRK (Fig. 25.2). At the end of the excimer ablation, a contact lens is applied to prevent flap dislocation. Preservative-free antibiotic drops are given, and the contact lens is usually removed after 3–4 days.
Epi-LASIK
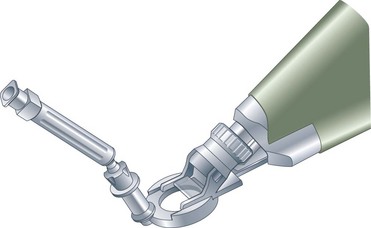
The initial steps are identical to PRK, but preservative-free anesthetic drops are preferred to prevent epithelial damage. The cornea is marked with a dye to make sure the flap can be properly aligned in case it inadvertently separates completely. In contrast to LASEK, the epithelial flap is prepared with a microkeratome that acts as a separator, using a blunt PMMA or metal blade (Fig. 25.3). A suction ring is placed on the eye to achieve an adequate intraocular pressure (IOP) for the creation of the flap. After the suction is applied and the IOP registered, the keratome is activated to create a flap with a nasal hinge. The flap is folded over and protected with a sponge. The ensuing laser procedure is the same as for PRK. Once ablation is completed, the flap is flipped back into place and a contact lens applied. The postoperative regimen is similar to that of LASEK. Some surgeons prefer to discard the flap altogether, choosing to use the keratome for the smooth surface (Bowman’s layer) and the sharp epithelial edges it creates around the ablation zone, believing that healing is faster and less symptomatic in the absence of a flap.
Postoperative care
After surface ablation, several issues need to be dealt with: pain6, epithelial healing, prophylactic antibiotics, and anti-inflammatory agents.
Postoperative complications
Infectious keratitis is likely the most serious complication after surface ablation. The reported risk of bacterial keratitis is between 0.01% and 0.8%, and the infection typically occurs within 1 week of the procedure7. Since surface ablation patients tend to have discomfort and tearing in the immediate postoperative period, an early diagnosis of bacterial keratitis may be missed if no counseling is given on the signs and symptoms of infection.
While not a genuine complication, delayed visual recovery is a reality that distinguishes surface ablation from LASIK. Re-epithelialization and stromal remodeling make for a slower visual rehabilitation. Most patients achieve functional visual acuity (20/40 or better uncorrected visual acuity [UCVA]) within the first week, but a return to 20/20 or better can take a month or so8.
Assessment of surgery
Long-term studies of PRK have reinforced the excellent safety profile and stability of surface ablation. Even 12-year data from one of the earliest clinical trials showed no significant change in mean spherical equivalent refraction between 1, 6, and 12 years9. In the mean time, lasers and ablation profiles have improved substantially, indications have become more rigorous, and customized treatments have increased the quality of vision.
When LASEK evolved from PRK, and when epi-LASIK developed out of the LASEK experience, each new procedure at first appeared to offer well-defined advantages over the previous method. With time, however, the differences between modern PRK, LASEK, and Epi-LASIK appear to fade away10. Evidence is mounting that the visual and refractive results as well as the problems specifically associated with surface ablation (pain, discomfort, haze, delayed visual recovery) are quite similar for the three procedures. While some studies have shown specific advantages for one method over the other, it seems safe to state that, on the whole, all three deliver excellent outcomes.
1 Kempen JH, Mitchell P, Lee KE, et al. The prevalence of refractive errors among adults in the United States, Western Europe, and Australia. Arch Ophthalmol. 2004;122:495-505.
2 Seiler T, Wollensack J. Myopic photorefractive keratectomy with the excimer laser: one-year follow-up. Ophthalmology. 1991;98:1156-1163.
3 Shahinian L. Laser-assisted subepithelial keratectomy for low to high myopia and astigmatism. J Cataract Refract Surg. 2002;28:1334-1342.
4 Pallikaris IG, Katsanevaki VJ, Kalyvianaki MI, et al. Advances in subepithelial excimer refractive surgical techniques: Epi-LASIK. Curr Opin Ophthalmol. 2003;14:207-212.
5 Lee HK, Lee KS, Kim JK, et al. Epithelial healing and clinical outcomes in excimer laser photorefractive surgery following three epithelial removal techniques: mechanical, alcohol, and excimer laser. Am J Ophthalmol. 2005;139:56-63.
6 Blake CR, Cervantes-Castaneda RA, Macias-Rodriguez Y, et al. Comparison of postoperative pain in patients following photorefractive keratectomy versus advanced surface ablation. J Cataract Refract Surg. 2005;31:1314-1319.
7 Leccisotti A, Bartolomei A, Greco G, et al. Incidence of bacterial keratitis after photorefractive keratectomy. J Refract Surg. 2005;21:96.
8 Zadok D, Barkana Y, Levy Y, et al. Rehabilitation time after simultaneous bilateral photorefractive keratectomy for low to moderate myopia. J Cataract Refract Surg. 2006;32:117-120.
9 Rajan MS, Jaycock P, O’Brart D, et al. A long-term study of photorefractive keratectomy: 12-year follow-up. Ophthalmology. 2004;111:1813-1824.
10 Pirouzian A, Thornton J, Ngo S. One-year outcomes of a bilateral randomized prospective clinical trial comparing laser subepithelial keratomileusis and photorefractive keratectomy. J Refract Surg. 2006;22:575-579.