CHAPTER 43 Laser trabeculoplasty
Introduction
In 1974, Worthen and Wickham first described a laser procedure for the treatment of open angle glaucoma (OAG)1. The laser was directed at the trabecular meshwork and they believed the procedure to be a trabeculotomy. Soon thereafter, Krasnov demonstrated a decrease in intraocular pressure (IOP) using a Q-switched laser to perform ‘goniopuncture’2. In 1979, Wise and Witter introduced the technique and parameters for an enduring procedure called argon laser trabeculoplasty (ALT)3. Since then, different techniques such as trabeculopuncture have been tried with limited long-term success. Interestingly, laser irradiation of the trabecular meshwork (TM) with the neodymium : YAG laser (1064 nm), diode laser (810 nm), and krypton laser (red 647.1 nm or yellow 568.2 nm) also reduce IOP comparably with ALT. Since these lasers interact with the TM in different ways, it suggests the hypotensive effect of laser trabeculoplasty (LTP) is multifactorial. In 1998, Latina published a pilot study using a Q-switched 532-nm Nd : YAG laser to perform trabeculoplasty that lowered IOP without causing coagulative damage to the TM4. This method was coined ‘selective laser trabeculoplasty’ (SLT). SLT and ALT have become the two most common forms of LTP performed for OAG and will be the focus of this chapter.
Epidemiology
The Early Manifest Glaucoma Trial was the first large randomized clinical trial to show that reduction of IOP with a combination of a beta blocker and ALT can slow disease progression in patients with manifest OAG5. Lowering IOP, medically or with laser or filtration surgery, remains the only viable approach to favorably alter the course of OAG. Therefore, the focus of glaucoma treatment remains centered on IOP control at an estimated cost of $623–2511 per patient per year in 20046. In the US, the direct medical cost of glaucoma, including medical treatment, surgical procedures and outpatient visits, was $2.86 billion in 2004. Medical therapy accounts for 38–52% of direct costs7. While real world data on the cost effectiveness of LTP are lacking, studies using simulated models that make assumptions about treatment efficacy suggest that LTP lowers yearly treatment costs for OAG. In one study, the cumulative 5-year cost of treatment with medication alone was $6571, while treatment with LTP reduced the cost to $4838, (including the cost of the procedure), therefore offering a 5-year saving of $1733 or a yearly savings of $346 per patient8. Clinically, the Glaucoma Laser Trial showed that initial treatment with ALT was as effective as initial treatment with topical medication9. Therefore, the use of LTP as treatment, compared with topical medications or in conjunction with medications, may be a reasonable option for selected OAG patients. Using a Medicare database, the numbers of LTPs peaked in 1992 with 176 670 procedures performed10. LTP numbers declined steadily until 2002, when the rates started to increase again to 169 680 in 20067. Given the trend of increased use, along with data regarding clinical efficacy and favorable economic impact, we need to review our understanding of the fundamental principles and describe the techniques of ALT and SLT.
Fundamental principles
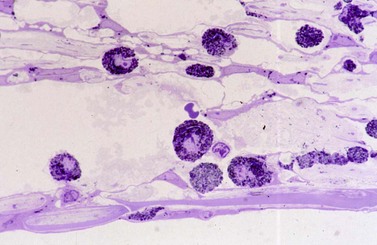
The mechanisms of action of ALT and SLT are not completely understood, but are thought to be different from one another. A comparison of the morphological changes in the TM of eight human eye bank eyes after these procedures offers some insight. After ALT, scanning electron microscopy (SEM) revealed crater formation in the treated areas, measuring approximately 70 µm. Coagulative damage was evident along the base and edges of the craters, with disruption of the collagen scaffolding and scattered debris derived from disrupted trabecular beams. Intracellular changes were evaluated with transmission electron microscopy (TEM). TEM also revealed disrupted trabecular endothelial cells with coagulative damage. In contrast, evaluation with SEM of eyes treated with SLT revealed no evidence of crater formation or coagulative damage. There was minimal evidence of mechanical change, with only rare crack-like defects of the corneo-scleral meshwork identifiable. TEM revealed no coagulative damage to trabecular cells, but revealed disrupted intra-cytoplasmic pigment granules within pigmented TM endothelial cells11. See Figure 43.1.
Postulated mechanisms of action of ALT include: mechanical, cellular, and biochemical. The mechanical theory suggests that coagulative damage causes shortening or ‘tightening’ of the inner trabecular lamellae. This trabecular tightening may increase outflow by preventing trabecular beams from collapsing. In 1972, Nesterov performed perfusion studies on 20 cadaver eyes and proposed that collapse of the trabecular lamellae upon each other and onto Schlemm’s canal may contribute to primary open angle glaucoma (POAG)12. Thus, the mechanical theory of ALT proposes that the tightening of the trabecular lamellae prevents or delays collapse of the lamellae or Schlemm’s canal allowing for increased outflow. The efficacy of a new procedure, canaloplasty, provides some construct validity for the mechanical theory of ALT. In canaloplasty, a 10-0 suture is threaded into Schlemm’s canal for 360° and a knot is tied to tighten the trabecular ring. This vaults Schlemm’s canal inwards and in one study the degree of this effect was proportional to the magnitude of IOP lowering13. The mechanical theory explains IOP lowering based on changes in anatomic conformations.
The cellular theory, or the ‘repopulation’ theory, suggests that LTP stimulates TM cell division and repopulates the trabecular beams with healthy cells that contribute to an improved outflow facility. In support, a histological study that measured TM cellularity in patients with POAG compared with that of non-glaucomatous individuals found that the TM from POAG patients had lower cellularity than age-matched normals14. Laser may stimulate repopulation by cells that are derived from a stem cell population in the anterior, non-filtering region of the TM15. LTP may also trigger macrophage infiltration of the TM aiding removal of cellular debris and thereby increase outflow facility16.
The cellular theory links to the biochemical theory due to the altered biochemical profile of healthier cells that repopulate the TM. Furthermore, infiltrating macrophages may also degrade extracellular matrix, further improving outflow facility. The biochemical theory focuses primarily on the alterations in the extracellular matrix, particularly the juxtacanalicular tissue, which allows aqueous to enter Schlemm’s canal. Such changes are of particular interest for the mechanism of SLT, since SLT produces minimal mechanical and coagulative changes in the TM, yet results in a significant IOP lowering effect. The trabecular surface and intratrabecular spaces are lined with mucinous glycosaminoglycans (GAGs). GAGs are important contributors to aqueous outflow resistance in the juxtacanalicular connective tissue and regulate fluid outflow by forming gel-like solutions. Laser treatment has been shown to alter the expression of GAGs by the TM cells17. Whether the modulation GAGs directly changes the extracellular matrix to allow for more outflow, or if it allows the TM cells to respond to biophysical cues and further modulate the matrix to then increase outflow, is yet to be elucidated18,19. Studies of other factors released by the TM that regulate Schlemm’s canal permeability are also contributing to the understanding of the mechanisms of LTP. SLT results in the release of interleukin-1α, 1β, and tumor necrosis factor-α into the aqueous humor. These cytokines bind to TM cells near the juxtacanalicular tissue and increase flow across the extracellular matrix tissues by inducing the release of matrix metalloproteinases19. Therefore, the IOP lowering effect is not due to one factor but likely due to the interactions of many mechanisms and enzyme cascades.
Preoperative assessment
Determining which patients are good candidates for LTP requires assessment of multiple factors. These include age, race, angle anatomy, TM pigmentation, and the mechanism of glaucoma. Some secondary glaucomas such as pigmentary20, exfoliative21, or steroid-induced22 can be treated successfully with LTP, while others such as uveitic, angle-recession23, and those associated with irido-corneal endothelial syndrome show minimal benefit with LTP. Congenital or juvenile glaucomas or glaucomas associated with angle dysgenesis and malformations also do not respond well to LTP.
Preoperative gonioscopy is essential when LTP is considered as a treatment modality. This not only determines the status of the angle structures but allows assessment patient cooperation as well, since the procedure will require adequate intraoperative patient positioning and lens placement. Anecdotally, we have found that patients with severe blepharospasm and small lid fissures may require a pediatric Goldmann lens for successful LTP. It is advantageous to recognize such challenges preoperatively rather than in the laser suite. During gonioscopy note the amount of TM pigmentation, as this dictates the power necessary at the time of LTP. For both ALT and SLT, increased pigmentation likely leads to increased absorption of laser energy by pigmented TM cells. Eyes with increased TM pigmentation are at higher risk of post-LTP IOP spikes (particularly SLT24). In order to reduce this risk, use lower energy settings or fewer laser applications.
Procedure
The preoperative preparations for both ALT and SLT require the following:
The operative eye should be treated with a drop of topical anesthetic. Some surgeons place a drop in both eyes to maximize patient comfort. The use of pilocarpine to constrict the pupil and facilitate the view of the angle structures is optional and should be considered if the angle appeared slightly narrow on preoperative gonioscopy. Application of either apraclonidine or brimonidine prior to laser treatment reduces the likelihood of a post-treatment IOP spike25.
Operative procedure
Argon laser trabeculoplasty (ALT)
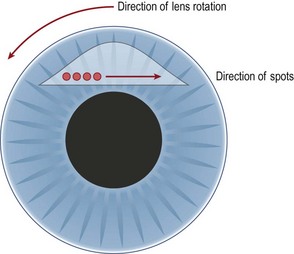
The application should bridge the area between the pigmented and non-pigmented trabecular meshwork. The spots should be evenly spaced, so there are approximately 25 spots per quadrant. The space between spots should be about three to four times the size of the spot itself. Use of a consecutive pattern of applying spots between areas where the lens is rotated prevents confusion that may lead to re-treatment or omission of an area. To apply consecutive spots the lens should be turned in the direction opposite to that in which the laser spots are applied. For example, if the laser spots are applied in a clockwise fashion, the lens should be rotated counterclockwise (Fig. 43.3).
For both ALT and SLT there is no consensus whether it is best to treat 180° initially or 360°. Generally, there is a higher risk of inflammation and postoperative pressure spikes if 360° are treated26. Several studies report equal efficacy of IOP lowering with treatment of 180° versus 360°27, while others report greater IOP lowering with treatment of 360°. In one study, there was less inter-visit IOP fluctuation when 360° were treated versus treating 180°28.
Selective laser trabeculoplasty
The patient should receive all the same preoperative drops as ALT including either apraclonidine or brimonidine prior to the procedure and a drop of an anesthetic agent. Either a Goldmann three-mirror lens, Ritch lens or a Latina SLT lens can be used and should be coupled with methylcellulose. The patient should then be placed in the laser–slit-lamp system and the lens placed on the eye. The spot size for SLT is 400 µm and therefore encompasses the width of the entire TM; 50–100 adjacent, but not overlapping spots over 180–360° are applied. The typical starting power is 0.8 mJ and it should be adjusted according to tissue response. Unlike ALT, there is no notable blanching; the endpoint is minimal release of tiny ‘champagne’ bubbles with some, but not all, laser applications. Precautions should be taken so as to not involve the iris in the spot application as this may increase the amount of inflammation and risk of postoperative IOP spike. Similar to ALT, starting at the 12 o’clock mirror position and turning the lens opposite to the direction of laser applications is the least confusing and easiest way to ensure proper treatment without skipped or overlapped spots. Overlapping spots may reduce the efficacy of the SLT29. It is not clear if this is due to the effect of overlapping the spots or of the total area treated since the study compared 100 overlapping spots in 180° versus 100 non-overlapping spots over 360°. See Table 43.1 for a comparison of ALT and SLT treatment parameters.
Table 43.1 Comparison of treatment parameters for argon laser trabeculoplasty (ALT) and selective laser trabeculoplasty (SLT)
| ALT | SLT | |
|---|---|---|
| Wavelength | 488 nm | 532 nm |
| Power | 400–800 mW | 0.2–1.2 mJ |
| Time | 0.1 s | 3 ns |
| Spot size | 50 µm | 400 µm |
Complications
Less common, but reported complications of LTP include hyphema30, cystoid macular edema31, corneal edema32, and choroidal effusion33.
Assessment
There are varied reports regarding the success of LTP, in part due to different criteria used to define success. The Glaucoma Laser Trial reported a mean reduction in IOP of 9 mmHg in eyes undergoing primary ALT versus 7 mmHg at 1 year, in eyes initially treated with medications9. The various other clinical studies on ALT reveal an average decrease in IOP at 1 year between 6 and 9 mmHg. One 10-year follow-up study reported the average IOP decrease to be 8.9 mmHg at 1 year, 10.0 mmHg at 5 years, and 8.9 mmHg at 10 years34. Success rates in this study were reported at 77% at 1 year, 49% at 5 years, and 32% at 10 years. Success was defined as a decrease in IOP of 3 mmHg or greater, IOP of 19 mmHg or less, and stable visual fields and optic nerve with no other laser or surgical intervention. The failure rate according to the aforementioned success criteria was found to be highest in the first year, at about 23%, and then ranged from 5 to 9% per year thereafter. Another 10-year study by Wise reported that the percentage of eyes with IOP less than 21 mmHg was 61% at 5 years and 70% at 10 years35.
Latina et al. published the first study demonstrating the efficacy of SLT in 19984. This multicenter, pilot study showed 70% success in lowering IOP by at least 3 mmHg. The mean IOP reduction at 26 weeks was 5.8 mmHg. One year postoperative data from clinical trials comparing ALT with SLT found the mean decrease in IOP to be around 6 mmHg for both procedures with no significant differences in the rate of complications36. There are no 10-year data available comparing SLT with ALT, but studies with up to 5-year follow-up for both forms of LTP demonstrated equal success rates. Success rates, defined as a decrease in IOP of 3 mmHg or more, were 68%, 46%, and 32% at 1, 3, and 5 years respectively for SLT. The comparable success rates for ALT in the same study were 54%, 30%, and 31%37. See Table 43.2.
| ALT | SLT | |
|---|---|---|
| Approximate total power used | 4000 mJ | 40 mJ |
| Success rates at 1 year | 50–80% | 50–80% |
| Repeatability | Limited efficacy | Yes |
| % of patients with IOP spikes | Up to 50% | Up to 30% |
| PAS formation | Yes | No |
The only statistically significant difference in success rates of ALT and SLT was in repeat laser procedures after previous ALT. SLT appears to be better than ALT in lowering IOP in eyes with prior ALT38.
There are some interesting trends in the response to treatment for the various OAG subtypes. For example, young patients with POAG may not have as much of a response to ALT as older patients. In contrast, pigmentary glaucoma patients younger than 40 years of age may have a better response to the treatment than older patients with the same condition20. Also, patients with exfoliation glaucoma have a high success rate with ALT (perhaps even higher than patients with POAG), though over time the success rate regresses to a level comparable with that of POAG patients21. Furthermore, there are isolated reports of success in lowering IOP in steroid induced glaucoma. LTP may be a good temporizing measure in these cases where the IOP spike may be temporary and thus may spare the patient an incisional procedure22.
There have been some theories that the effect of SLT may be dampened if the patient is already on a prostaglandin analog. This was thought to be true if the pathway of prostaglandin analogs lowering IOP overlapped with the mechanism of SLT lowering IOP. Studies that compare the effect of SLT on patients using a prostaglandin analog versus not using an analog have not supported this theory. They have found that in fact the effects may be additive and that the IOP is lower with SLT in conjunction with prostaglandin analog treatment39.
In conclusion, LTP provides an option for the treatment of many kinds of OAG. The success rates of SLT and ALT are comparable in POAG. The choice of which laser to use varies according to the individual situation. ALT may be preferable in patients with pigmentary glaucoma or exfoliation glaucoma. In contrast, SLT may be the procedure of choice for a fairly young patient with POAG. Furthermore, in patients undergoing a repeat LTP treatment, SLT may be more favorable. Patients may feel less discomfort with SLT than ALT during the treatment itself40. SLT remains an easier procedure to master due to the larger spot size but the investment in a separate laser for a single procedure still keeps ALT a popularly performed procedure. Optimization of the indications for LTP, preoperative preparation, laser surgical technique, and postoperative care has much to offer OAG patients worldwide.
1 Worthen DM, Wickham MG. Argon laser trabeculotomy. Trans Am Acad Ophthalmol Otolayngol. 1974;78:371-375.
2 Krasnov MM. Q Switched laser goniopuncture. Archs Ophthal. 1974;92:37.
3 Wise JB, Witter SL. Argon laser therapy for open-angle glaucoma; a pilot study. Arch Ophthalmol. 1979;97:319-322.
4 Latina MA, Sibayan SA, Shin DH, et al. Q-switched 532-nm Nd:YAG laser trabeculoplasty (Selective Laser Trabeculoplasty); A multicenter, pilot, clinical study. Ophthalmology. 1998;105:2082-2090.
5 Leske MC, Heijl A, Hyman L, et al. Early manifest glaucoma trial: Design and Baseline Data. Ophthalmology. 1999;106:2144-2153.
6 Fiscella RG, Lee J, Davis EJH, et al. Cost of illness of glaucoma: A critical and systematic review. Pharmacoeconomics. 2009;27(3):189-198.
7 Schmier JK, Covert DW, Lau EC, et al. Trend in annual medicare expenditures for glaucoma surgical procedures from 1997–2006. Arch Ophthalmol. 2009;127(7):900-905.
8 Cantor LB, Katz LJ, Cheng JW, et al. Economic evaluation of medication, laser trabeculoplasty and filtering surgeries in treating patients with glaucoma in the US. Curr Med Res Opin. 2008;24(10):2905-2918.
9 Glaucoma Laser Trial Research Group. The glaucoma laser trial (GLT) and glaucoma laser trial follow-up Study: 7. Results. Am J Ophthalmol. 1995;120:718-731.
10 Albright CD, Schuman SG, Netland PA. Usage and cost of laser trabeculoplasty in the United States. Ophthal Surg Lasers. 2002;23(4):334-336.
11 Kramer TR, Noecker RJ. Comparison of the morphologic changes after selective laser trabeculoplasty and argon laser trabeculoplasty in the human eye bank eyes. Ophthalmology. 2001;108(4):773-779.
12 Nesterov AP, Batmanov YE. Study on morphology and function of the drainage area of the eye of man. Acta Ophthalmol. 1972;50:337-350.
13 Lewis RA, von Wolff K, Tretz K, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm’s canal using a flexible microcatheter for the treatment of open angle glaucoma in adults. Interim clinical study analysis. J Cataract Refract Surg. 2009;33:1217-1226.
14 Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564-579.
15 Kelley MJ, Rose AY, Keller KE, et al. Stem cells in the trabecular meshwork: Present and future promises. Exp Eye Res. 2009;88:747-751.
16 Melamed S, Pei J, Epstein DL. Short-term effect of argon laser trabeculoplasty in monkeys. Arch Ophthalmol. 1985;103:1546-1552.
17 Alexander RG, Church W. The effect of argon laser trabeculoplasty upon the normal human trabecular meshwork. Graefe’s Arch Clin Exp Ophthalmol. 1989;227:72-77.
18 Amelinckx A, Castello M, Arrieta-Quintero E, et al. Laser trabeculoplasty induces changes in the trabecular meshwork glycoproteome: A pilot study. J Proteome Res. 2009;8:3727-3736.
19 Alvarado JA, Yeh R, Franse-Carman L, et al. Interactions between endothelia of the trabecular meshwork and of Schlemm’s canal: a new insight into the regulation of aqueous outflow in the eye. Trans Am Ophthalmol Soc. 2005;103:148-163.
20 Ritch R, Liebmann J, Robin A, et al. Argon laser trabeculoplasty in pigmentary glaucoma. Ophthalmology. 1993;100:909-913.
21 Threlkend AB, Hertzmark E, Sturm RT. Comparative study of the efficacy of argon laser trabeculoplasty for exfoliation and primary open-angle glaucoma. J Glaucoma. 1996;5:311-316.
22 Rubin B, Taglienti A, Rothman RF, et al. The effect of selective laser trabeculoplasty on intraocular pressure in patients with intravitreal steroid-induced elevated intraocular pressure. J Glaucoma. 2008;17:287-292.
23 Robin AL, Pollack IP. Argon laser trabeculoplasty in secondary forms of open-angle glaucoma. Arch Ophthalmol. 1983;101:382-384.
24 Harasymowycz PJ, Papamatheakis DG, Latima M, et al. Selective laser trabeculoplasty (SLT) complicated by intraocular pressure elevation with heavily pigmented trabecular meshworks. Am J Ophthalmol. 2005;139:1110-1113.
25 Chen TC, Ang RT, Grosskreutz CL, et al. Brimonidine 0.2% versus apraclonidine 0.5% for prevention of intraocular pressure elevations after anterior segment laser surgery. Ophthalmology. 2001;108:1033-1038.
26 Thomas JV, Simmons RJ, Belcher CD, et al. Laser trabeculoplasty: Technique, indications, results, and complications. Int Ophthalmol Clin. 1984;24(3):97-120.
27 Schwartz LW, Spaeth GL, Traverso C, et al. Variation of techniques on the results of argon laser trabeculoplasty. Ophthalmology. 1983;90:78-84.
28 Prasad N, Murthy S, Dagianis JJ, et al. A comparison of the intervisit intraocular pressure fluctuation after 180 and 360 degrees of selective laser trabeculoplasty (SLT) as a primary therapy in primary open angle glaucoma and ocular hypertension. J Glaucoma. 2009;18:157-160.
29 George MK, Emerson JW, Cheema SA, et al. Evaluation of a modified protocol for selective laser trabeculoplasty. J Glaucoma. 2008;17:197-202.
30 Rhee DJ, Krad O, Pasquale LR. Hyphema following selective laser trabeculoplasty. Ophthalmic Surg Lasers Imaging. 2009;40(5):493-494.
31 Wechsler DZ, Wechsler IB. Cystoid macular oedema after selective laser trabeculoplasty. Eye. 2010;24(6):1113.
32 Moubayed SP, Hamid M, Choremis J, et al. An unusual finding of corneal edema following selective laser trabeculoplasty. Can J Ophthalmol. 2009;44(3):337-338.
33 Kim DY, Singh A. Severe iritis and choroidal effusion following selective laser trabeculoplasty. Ophthalmic Surg Lasers Imaging. 2008;39(5):409-411.
34 Shingleton BJ, Richter CU, Dharma SK, et al. Long term efficacy of argon laser trabeculoplasty: A 10-year follow-up study. Ophthalmology. 1993;100:1324-1329.
35 Wise JB. Ten year results of laser trabeculoplasty. Does laser avoid glaucoma surgery or merely defer it? Eye. 1987;1:45-50.
36 Damji KF, Bovell AM, Hodge WG, et al. Selective laser trabeculoplasty versus argon laser trabeculoplasty: results from a 1-year randomized clinical trial. Br J Ophthalmol. 2006;90:1490-1494.
37 Juzych MS, Chopra V, Banitt MR, et al. Comparison of Long-term outcomes of selective laser trabeculoplasty versus argon laser trabeculoplasty in open angle glaucoma. Ophthalmology. 2004;111:1853-1859.
38 Russo V, Barone A, Cosma A, et al. Selective laser trabeculoplasty versus argon laser trabeculoplasty in patients with uncontrolled open-angle glaucoma. Eur J Ophthalmol. 2009;19(3):429-434.
39 Scherer WJ. Effect of topical prostaglandin analog use on outcome following selective laser trabeculoplasty. J Ocul Pharmacol Ther. 2007;23(5):503-512.
40 Martinez-de-la-Casa JM, Garcia-Feijoo J, Castillo A, et al. Selective vs argon laser trabeculoplasty: hypotensive efficacy, anterior chamber inflammation, and postoperative pain. Eye. 2004;18:498-502.