CHAPTER 394 Supratentorial and Infratentorial Cavernous Malformations
Cavernous malformations (CMs) account for 5% to 13% of vascular lesions of the central nervous system. Historically, these lesions have been considered quite rare.1–3 With the advent of magnetic resonance imaging (MRI), however, the diagnosis of cerebral CMs has increased. They are now understood to be more common than was appreciated before MRI was available, with an incidence of 0.4% to 0.5% in the general population.4–7
Epidemiology and Clinical Manifestations
As mentioned, CMs account for 5% to 13% of vascular lesions of the central nervous system, with an incidence of 0.4% to 0.5% in the general population.4–7 Most cerebral CMs are supratentorial, and 9% to 35% are found in the brainstem.1–3 Although these lesions are histologically identical, their behavior and management depend on the location of the lesion.
Regardless of location, the defining pathophysiology of CMs is repeated hemorrhage. CMs are formed by endothelin-lined sinusoidal vascular spaces. There is a lack of intervening brain parenchyma inside the collagenous matrix of the lesion. On electron microscopy, the endothelin-lined CMs contain cells that lack tight junctions.8 It has been hypothesized that these “leaky” cell junctions are responsible for the extravasation of blood products seen as extralesional hemosiderin staining on histologic preparations of CMs.
The signs and symptoms of patients with cerebral CMs are highly variable. Many of these lesions are now discovered incidentally (see the section “Imaging”). For superficial supratentorial lesions, a seizure manifestation is typical. For deep-seated supratentorial and infratentorial lesions, symptoms are more dependent on location. In all cases, regardless of whether the onset of symptoms is insidious or apoplectic, the presence of symptoms can be traced back to hemorrhage from the CM. These hemorrhages may be large bleeding episodes that are manifested as apoplectic events, repeated “microhemorrhages” that cause hemosiderin to accumulate in the surrounding brain and subsequently give rise to seizures, or progression of the CM from repeated intralesional hemorrhage and mass effect. With each hemorrhage, symptoms tend to worsen and then improve, but less so after each ictus. In effect, a stepwise progression of “two steps forward, three steps back” is observed. After one hemorrhage, the likelihood of a subsequent hemorrhage is substantially higher than with a silent lesion.
Imaging
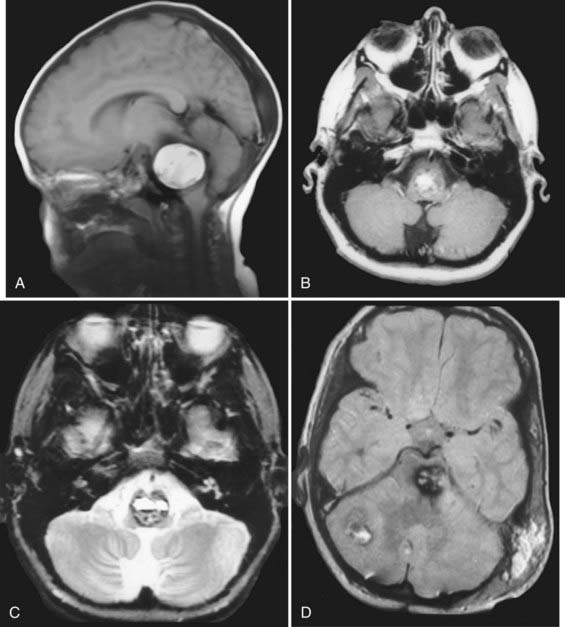
The imaging appearance of CMs is pathognomonic. Hemorrhage in the hyperacute period is isointense with brain tissue on T1-weighted MRI with a short TR (repetition time) and hypointense on T2-weighted MRI. Subacute hematomas (3 weeks to several months old) have a classic “salt and pepper” appearance. They are characterized by a hyperintense center (methemoglobin) on both T1- and T2-weighted images (Fig. 394-1) and by a hypointense surrounding rim of hemosiderin, especially on the latter. Gradient-echo images in particular can be used to screen for small occult lesions.
Natural History
Risk for Hemorrhage
Kondziolka and coauthors reported prospective hemorrhage and rehemorrhage rates of 2.4% to 5% per year, respectively.9 In contrast, in our institutional retrospective review, hemorrhage and rehemorrhage rates were 5% and 30%, respectively.10 Regardless, the timing of a subsequent hemorrhage is impossible to predict, with the interval between hemorrhages ranging from hours to years.
Several factors have been proposed to predispose a CM to rupture, including its location,7,11 a history of previous rupture, its size,12,13 and the presence of an associated developmental venous anomaly.14 The factor most consistently associated with increased risk for rupture across series is location. The hemorrhage rate of infratentorial lesions may be 30 times that of lesions in the supratentorial compartment. Both retrospective and prospective studies undertaken to define risk factors for hemorrhage from CMs have consistently identified the location of a lesion as having a significant impact on the rate of rupture. Brainstem CMs consistently have a higher rate of symptomatic hemorrhage than those at other locations. Hemorrhage rates as high as 60% have been reported for brainstem CMs.15
The mechanism for such a disparity in rupture rates, however, remains obscure. Most authors attribute this difference, at least partially, to the sensitivity of the brainstem to hemorrhage. In the literature, a history of previous rupture is strongly associated with as much as a sevenfold increase in the risk for prospective rupture.9,16
Some authors have attempted to link the presence or absence of an associated venous malformation with a higher rate of rupture.14 In our experience, though, CMs have universally been associated with venous anomalies, whether supratentorial, infratentorial, or even extra-axial (e.g., for CMs of the cranial nerves). Venous malformations are completely benign, but abnormal constellations of veins that drain normal brain tissue. They are the most frequent form of vascular malformation and are a common incidental finding on MRI.
It is important to emphasize that venous malformations, per se, do not rupture; however, they are frequently associated with CMs that do.17 Thus, given the association between CMs and venous anomalies, it is currently thought that any hemorrhage in the vicinity of a venous anomaly is the result of rupture of an associated CM, regardless of whether it is visualized on imaging studies (some CMs may be small enough to be missed on routine imaging studies). Unfortunately, a complete consensus on this point is lacking. Because of the association between CMs and venous anomalies, the older literature is replete with suggestions that venous anomalies may occasionally hemorrhage.18
Treatment Options
Radiation Therapy
Radiation therapy, including stereotactic irradiation, has not been shown to confer a protective benefit from hemorrhage in the treatment of CMs, partially because the natural history of CMs has remained difficult to define and because CMs treated with radiosurgery do not disappear on subsequent imaging studies. Furthermore, the hemorrhage rate never approaches zero. In fact, radiation has been implicated in the pathogenesis of these lesions. Moreover, for CMs in eloquent regions such as the brainstem and basal ganglia, new neurological deficits related to a treatment effect have been reported in 17% to 59% of patients.19,20 At present, therefore, most authors do not recommend radiation therapy for the treatment of deep-seated CMs.
Operative Procedure
Goals of Surgery and Patient Counseling
To determine the best surgical approach, we use the “two-point method” (Fig. 394-2).21 One point is placed in the center of the lesion and a second is placed where the lesion most closely reaches a pial surface. The two points are connected, and the resultant straight line through the least eloquent tissue dictates the most appropriate surgical approach. Preoperative permanent neurological deficits, such as seventh or eighth cranial nerve palsies, can also influence the choice of approach. Such deficits, for example, may make a translabyrinthine or transcochlear approach more attractive.
Surgical Technique
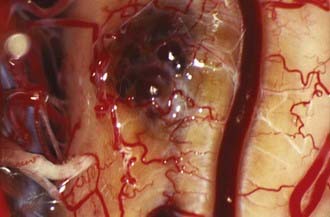
In general and regardless of location, CMs are accessed through minimal cortical openings. The CM is removed sharply and in piecemeal fashion. If intrinsic lesions fail to reach a pial surface of the brainstem, normal brainstem tissue will be violated during surgery. In this case, an opening is made by using hemosiderin staining or a bulge in the brainstem as a guide. Alternatively, the two-point method may be applied in conjunction with frameless stereotactic guidance. Entry into the brainstem is well tolerated, even in the case of deep-seated intrinsic lesions, if the cortical opening is small and the fibers of the brainstem are gently stretched to allow resection of the lesion. In contrast, exophytic lesions are readily apparent, assuming that the correct surgical approach was chosen. Lesions usually have a characteristic “mulberry” appearance with a thin layer of arachnoid (Fig. 394-3).
Surgical approaches
Midline Suboccipital and Telovelar Approaches
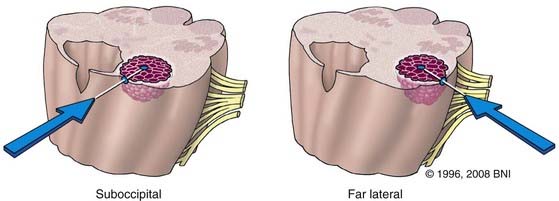
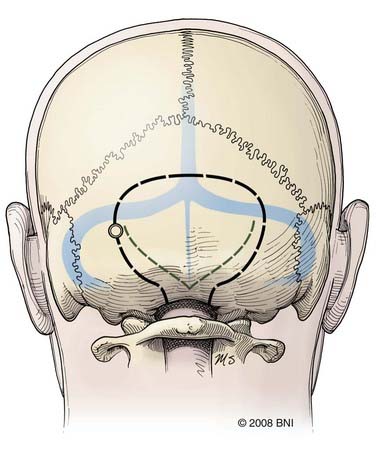
The midline suboccipital approach (Fig. 394-4) is used to reach lesions situated in the cerebellum, the posterior cervicomedullary junction, and the midline floor of the fourth ventricle. In this approach, the patient is placed prone on chest rolls or a laminectomy frame, and the neck is flexed to open the space between the foramen magnum and C1. After a strip shave, a midline skin incision is made that extends from approximately C3 to the inion. The fascia is opened such that a Y-shaped cuff with its base attached to the inion is created. This technique helps identify the avascular midline plane between the semispinalis capitis, trapezius, and splenius capitis muscles and provides a watertight fascial closure at the end of the procedure. The posterior cervical musculature is elevated from the suboccipital bone by subperiosteal dissection and retracted laterally with fishhooks. A suboccipital craniotomy is fashioned with a pneumatic drill that has a side-cutting bit and footplate. A burr hole can be made over the cerebellar hemisphere. Alternatively, the footplate can be placed under the foramen magnum. The dura is opened in a Y-shaped fashion, with its base along the torcular. The telovelar variation of the suboccipital approach is ideal for lateral fourth ventricular lesions, especially those involving the middle cerebellar peduncle. In this approach, the patient is similarly positioned prone on the operating table. The surgeon sits at the head of the operating table. Maximal flexion, particularly at the occipitocervical articulation (i.e., capital flexion), is even more important than with a routine suboccipital approach because the angle of approach to the lesion is much more obtuse. For this reason also, the head of the bed may need to be lowered considerably. After performing the same soft tissue dissection as for a conventional suboccipital approach, the posterior arch of C1 is removed piecemeal with a rongeur or with a side-cutting bit and footplate attachments on a pneumatic drill. The suboccipital craniotomy and dural opening are fashioned as described earlier. A retractor is useful to elevate the cerebellar vermis or hemisphere (or both) during dissection of the cerebellomedullary fissure, which is done sharply, until the cerebellar peduncle is visualized.
If a lesion is to be approached through the floor of the fourth ventricle, the location of cranial nerve nuclei becomes relevant. In 1993, Kyoshima and colleagues described “safe entry zones” above and below the facial colliculus.22 Bogucki and associates later modified the zones to access intrapontine lesions.23 The infrafacial zone has a line 2 mm lateral to the median sulcus on its medial border, the hypoglossal triangle on its inferior border, the facial colliculus on its superior border, and the vestibular area laterally. The suprafacial zone has the following boundaries: laterally, the superior cerebellar peduncle; medially, a vertical line 2 mm lateral to the median sulcus; superiorly, the frenulum veli; and inferiorly, the facial colliculus. Tumors, however, can distort normal anatomy and obscure these landmarks. In such cases, the facial colliculus can be stimulated directly while the facial nerve is monitored. Typically, however, lesions of the floor of the fourth ventricle are not treated surgically unless the patient has a complete neurological deficit such as internuclear ophthalmoplegia, abducens palsy, or facial paresis.
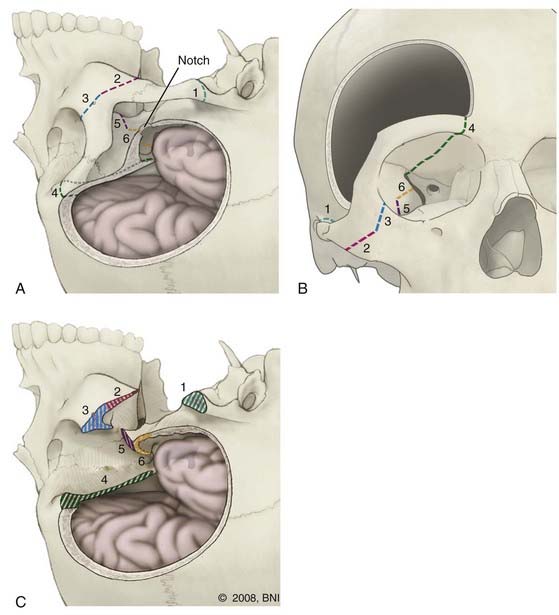
Orbitozygomatic Approach
The orbitozygomatic approach (Fig. 394-5) is used to access the anterior and lateral midbrain, interpeduncular region, rostral pons, pontomesencephalic junction, optic chiasm, hypothalamus, and the area caudal to the midthird ventricle. In 1986, Hakuba and coworkers first described this approach for lesions of the parasellar region, interpeduncular fossa, medial sphenoid wing, Meckel’s cave, and basilar tip.24 The main advantage is that it permits downward retraction of the globe of the eye to gain an upward and oblique view of the interpeduncular fossa and third ventricle. The technique, as performed at our institution, has been detailed elsewhere25 and is reviewed here briefly.
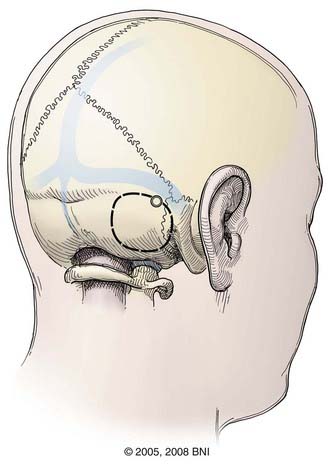
Retrosigmoid Approach
The retrosigmoid approach (Fig. 394-6) offers access to the posterolateral pons, lateral middle cerebellar peduncle, superior lateral medulla, and cerebellopontine angle. The patient can be positioned lateral, prone, or supine with a sandbag beneath the ipsilateral shoulder. In the supine position, the head is turned flat, parallel with the floor, and the neck is flexed such that a finger can be placed between the mandible and clavicle. Excessive rotation or flexion during positioning can cause neurovascular compromise, especially in patients with extracranial carotid disease or degenerative cervical spondylosis. Baseline SSEPs should therefore be recorded before and after positioning. If the SSEPs change, the patient should be repositioned. Alternatively, the patient can be placed prone or in the lateral decubitus position.
Far Lateral Approach
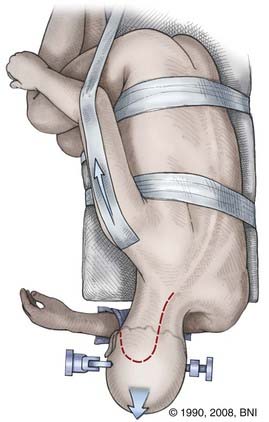
The far lateral or transcondylar approach has several modifications and variations. It basically involves a partial condylectomy, with or without resection of the lateral mass of C1. This approach allows the surgeon to achieve an anterolateral trajectory to the brainstem, and it eliminates the need to traverse contaminated mucosal structures through the transoral or transfacial route. Thus, the risk for meningitis, CSF leakage, or both can be minimized. First described by Heros26 and later modified by Spetzler and Grahm,27 this approach provides access to lesions of the vertebrobasilar junction, inferolateral pons, anterolateral medulla, and upper cervical spinal cord.
The clivus is brought perpendicular to the floor by performing four maneuvers on the neck: (1) flexion in the anteroposterior plane until the chin is one fingerbreadth from the clavicle, (2) rotation 45 degrees contralateral to the side of the lesion, (3) lateral flexion 30 degrees down toward the opposite shoulder (also the floor), and (4) slight distraction to increase the interval between the foramen magnum and C1 so that the surgeon can look down the axis of the brainstem and work between the horizontally oriented cranial nerves. The ipsilateral shoulder should also be retracted inferiorly with cloth tape to allow greater freedom of movement with the microscope (Fig. 394-7). SSEPs should be monitored before and after positioning to avoid inflicting a stretch injury on the brachial plexus. The patient should be taped securely to the bed to permit frequent and extreme rotation.
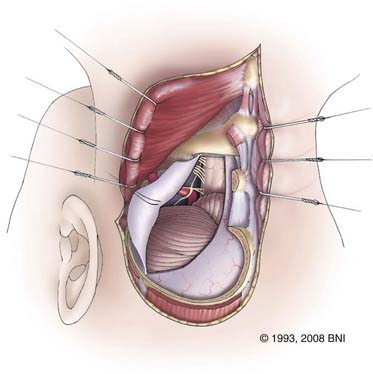
The posterior arch of C1 is removed with a Kerrison rongeur or drill (Fig. 394-8). The lateral aspect of the ipsilateral C1 posterior arch is further removed with a rongeur to the lateral aspect of the dura. The extradural vertebral artery, which lies on top of the sulcus arteriosus, can be followed into the foramen transversarium with a Woodson or dental instrument. The venous plexus surrounding the artery can be the source of profuse bleeding, which can be controlled with Nu-Knit gauze and bipolar coagulation. The foramen transversarium can be unroofed by sliding the footplate of a Kerrison rongeur into it. Alternatively, a diamond burr can be used to skeletonize the vertebral artery. The C2 nerve root between C1 and C2 should be preserved to avoid occipital numbness. The occipitoatlantal membrane is dissected off the foramen magnum with a curved curet to prepare for the craniotomy.
Supracerebellar Infratentorial Approach and Variations
In 1936, Dandy first described a supratentorial parafalcine approach to the pineal/tectal region.28 He used the semisitting position and in some cases sectioned the corpus callosum, which probably damaged the deep galenic venous system and resulted in cerebral edema. He lacked the benefit of the operating microscope, MRI, frameless stereotaxy, and advanced neuroanesthetic techniques. Thus, not surprisingly, complication rates were high. As techniques evolved, it became apparent that the infratentorial route would more safely access the same area by dissecting below the galenic venous system.
This approach was initially described by Krause29 and later popularized by Stein.30 The supracerebellar infratentorial approach permits exposure of malformations involving the midline tectum and pineal region. Positioning is the same as for the suboccipital approach. However, the angle of the tentorium must be considered. In the ideal position the tentorium is perpendicular to the floor. This positioning can be checked preoperatively with frameless stereotactic guidance. The craniotomy should extend above and below the transverse sinus to expose the junction of the traverse sinus and torcular. This craniotomy permits greater retraction of the tentorium superiorly than possible with a pure suboccipital craniotomy. It can be performed safely by placing a single burr hole lateral to the superior sagittal sinus with a pneumatic drill, footplate, and drill bit. Before the sinus is crossed, the surgeon should reverse the footplate and irrigate through the craniotomy line to confirm that the plate is extradural.
The dura is opened in an inverted V shape with the base on the edge of the transverse sinuses (Fig. 394-9). Bridging veins from the superior aspect of the cerebellum that drain into the transverse sinus are coagulated and divided to permit downward retraction of the cerebellum. If these veins are not coagulated early in the procedure, severe venous bleeding can occur during retraction. They must be coagulated as close to the surface of the cerebellum as possible to leave a pedicle on the surface of the tentorium. If avulsed at the interface of the tentorium, coagulation is not only futile but may enlarge the hole in the sinus and cause disastrous bleeding. If this occurs, a piece of Nu-Knit larger than the defect should be patched over the hole. A cotton pad is then placed over the hemostatic agent and can be held in place with a retractor during the remainder of the procedure. At the end of the procedure, the retractor is removed and the Nu-Knit will adhere to the venous opening.
Clinical Outcomes
We have reported our experience with resection of brainstem CMs in 86 patients. In our initial report, 87% of the surgical patients were the same or better, 9% were worse, and 3.5% had died. In the nonsurgical group, 58% were the same or better, 32% were worse, and 8% had died.10 We have subsequently reviewed our results with an additional 141 patients (Lekovic and colleagues, unpublished data, 2009) and have confirmed our initial findings that surgery for brainstem CMs can be performed safely and can be an effective cure. Importantly, since our initial report, no patients have died. We attribute this outcome to the lessons learned in our initial experience, such as the importance of preserving the associated developmental venous anomaly and minimizing the use of morbid approaches. These changes have increased the safety of surgery without compromising its effectiveness.
Abdulrauf SI, Kaynar MY, Awad IA. A comparison of the clinical profile of cavernous malformations with and without associated venous malformations. Neurosurgery. 1999;44:41-46.
Bogucki J, Gielecki J, Czernicki Z. The anatomical aspects of a surgical approach through the floor of the fourth ventricle. Acta Neurochir (Wien). 1997;139:1014-1019.
Brown AP, Thompson BG, Spetzler RF. The two-point method: evaluating brain stem lesions. BNI Q. 1996;12:20-24.
Dandy W. Operative experience in cases of pineal tumor. Arch Surg. 1936;33:19-46.
Del Curling OJr, Kelly DLJr, Elster AD, et al. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991;75:702-708.
Hakuba A, Liu S, Nishimura S. The orbitozygomatic infratemporal approach: a new surgical technique. Surg Neurol. 1986;26:271-276.
Hasegawa T, McInerney J, Kondziolka D, et al. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery. 2002;50:1190-1197.
Heros RC. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg. 1986;64:559-562.
Jellinger K. The morphology of centrally-situated angiomas. In: Pia H, Gleave J, Grote E, et al, editors. Cerebral Angiomas: Advances in Diagnosis and Therapy. New York: Springer Verlag; 1975:9-20.
Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83:820-824.
Krause F. Operative Freilegung der Vierhügel, nebst Beobachtungen über Hirndruch und Dekompression. Zentralbl Chir. 1926;53:2812-2819.
Kupersmith MJ, Kalish H, Epstein F, et al. Natural history of brainstem cavernous malformations. Neurosurgery. 2001;48:47-53.
Kyoshima K, Kobayashi S, Gibo H, et al. A study of safe entry zones via the floor of the fourth ventricle for brain-stem lesions. Report of three cases. J Neurosurg. 1993;78:987-993.
Malik GM, Morgan JK, Boulos RS, et al. Venous angiomas: an underestimated cause of intracranial hemorrhage. Surg Neurol. 1988;30:350-358.
Mathiesen T, Edner G, Kihlstrom L. Deep and brainstem cavernomas: a consecutive 8-year series. J Neurosurg. 2003;99:31-37.
McCormick WF. Pathology of vascular malformations of the brain. In: Wilson C, Stein B, editors. Intracranial Arteriovenous Malformations. Baltimore: Williams & Wilkins; 1984:44-63.
McCormick WF, Hardman JM, Boulter TR. Vascular malformations (“angiomas”) of the brain, with special reference to those occurring in the posterior fossa. J Neurosurg. 1968;28:241-251.
min-Hanjani S, Ogilvy CS, Candia GJ, et al. Stereotactic radiosurgery for cavernous malformations: Kjellberg’s experience with proton beam therapy in 98 cases at the Harvard cyclotron. Neurosurgery. 1998;42:1229-1236.
Mizoi K, Yoshimoto T, Suzuki J. Clinical analysis of ten cases with surgically treated brain stem cavernous angiomas. Tohoku J Exp Med. 1992;166:259-267.
Moriarity JL, Clatterbuck RE, Rigamonti D. The natural history of cavernous malformations. Neurosurg Clin N Am. 1999;10:411-417.
Otten P, Pizzolato GP, Rilliet B, et al. 131 cases of cavernous angioma (cavernomas) of the CNS, discovered by retrospective analysis of 24,535 autopsies [in French]. Neurochirurgie. 1989;35:82-83, 128-131.
Pollock BE, Garces YI, Stafford SL, et al. Stereotactic radiosurgery for cavernous malformations. J Neurosurg. 2000;93:987-991.
Porter RW, Detwiler PW, Spetzler RF, et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg. 1999;90:50-58.
Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg. 1991;75:709-714.
Sarwar M, McCormick WF. Intracerebral venous angioma. Case report and review. Arch Neurol. 1978;35:323-325.
Spetzler RF, Grahm TW. The far-lateral approach to the inferior clivus and the upper cervical region: technical note. BNI Q. 1990;6:35-38.
Stein BM. The infratentorial supracerebellar approach to pineal lesions. J Neurosurg. 1971;35:197-202.
Topper R, Jurgens E, Reul J, et al. Clinical significance of intracranial developmental venous anomalies. J Neurol Neurosurg Psychiatry. 1999;67:234-238.
Wong JH, Awad IA, Kim JH. Ultrastructural pathological features of cerebrovascular malformations: a preliminary report. Neurosurgery. 2000;46:1454-1459.
Zabramski JM, Kiris T, Sankhla SK, et al. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998;89:336-341.
1 Jellinger K. The morphology of centrally-situated angiomas. In: Pia H, Gleave J, Grote E, et al, editors. Cerebral Angiomas: Advances in Diagnosis and Therapy. New York: Springer Verlag; 1975:9-20.
2 McCormick WF, Hardman JM, Boulter TR. Vascular malformations (“angiomas”) of the brain, with special reference to those occurring in the posterior fossa. J Neurosurg. 1968;28:241-251.
3 Sarwar M, McCormick WF. Intracerebral venous angioma. Case report and review. Arch Neurol. 1978;35:323-325.
4 Otten P, Pizzolato GP, Rilliet B, et al. 131 cases of cavernous angioma (cavernomas) of the CNS, discovered by retrospective analysis of 24,535 autopsies [in French]. Neurochirurgie. 1989;35:82-83, 128-131.
5 McCormick WF. Pathology of vascular malformations of the Brain. In: Wilson C, Stein B, editors. Intracranial Arteriovenous Malformations. Baltimore: Williams & Wilkins; 1984:44-63.
6 Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg. 1991;75:709-714.
7 Del Curling OJr, Kelly DLJr, Elster AD, et al. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991;75:702-708.
8 Wong JH, Awad IA, Kim JH. Ultrastructural pathological features of cerebrovascular malformations: a preliminary report. Neurosurgery. 2000;46:1454-1459.
9 Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83:820-824.
10 Porter RW, Detwiler PW, Spetzler RF, et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg. 1999;90:50-58.
11 Moriarity JL, Clatterbuck RE, Rigamonti D. The natural history of cavernous malformations. Neurosurg Clin N Am. 1999;10:411-417.
12 Hasegawa T, McInerney J, Kondziolka D, et al. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery. 2002;50:1190-1197.
13 Kupersmith MJ, Kalish H, Epstein F, et al. Natural history of brainstem cavernous malformations. Neurosurgery. 2001;48:47-53.
14 Abdulrauf SI, Kaynar MY, Awad IA. A comparison of the clinical profile of cavernous malformations with and without associated venous malformations. Neurosurgery. 1999;44:41-46.
15 Mizoi K, Yoshimoto T, Suzuki J. Clinical analysis of ten cases with surgically treated brain stem cavernous angiomas. Tohoku J Exp Med. 1992;166:259-267.
16 Mathiesen T, Edner G, Kihlstrom L. Deep and brainstem cavernomas: a consecutive 8-year series. J Neurosurg. 2003;99:31-37.
17 Topper R, Jurgens E, Reul J, et al. Clinical significance of intracranial developmental venous anomalies. J Neurol Neurosurg Psychiatry. 1999;67:234-238.
18 Malik GM, Morgan JK, Boulos RS, et al. Venous angiomas: an underestimated cause of intracranial hemorrhage. Surg Neurol. 1988;30:350-358.
19 min-Hanjani S, Ogilvy CS, Candia GJ, et al. Stereotactic radiosurgery for cavernous malformations: Kjellberg’s experience with proton beam therapy in 98 cases at the Harvard cyclotron. Neurosurgery. 1998;42:1229-1236.
20 Pollock BE, Garces YI, Stafford SL, et al. Stereotactic radiosurgery for cavernous malformations. J Neurosurg. 2000;93:987-991.
21 Brown AP, Thompson BG, Spetzler RF. The two-point method: evaluating brain stem lesions. BNI Q. 1996;12:20-24.
22 Kyoshima K, Kobayashi S, Gibo H, et al. A study of safe entry zones via the floor of the fourth ventricle for brain-stem lesions. Report of three cases. J Neurosurg. 1993;78:987-993.
23 Bogucki J, Gielecki J, Czernicki Z. The anatomical aspects of a surgical approach through the floor of the fourth ventricle. Acta Neurochir (Wien). 1997;139:1014-1019.
24 Hakuba A, Liu S, Nishimura S. The orbitozygomatic infratemporal approach: a new surgical technique. Surg Neurol. 1986;26:271-276.
25 Zabramski JM, Kiris T, Sankhla SK, et al. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998;89:336-341.
26 Heros RC. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg. 1986;64:559-562.
27 Spetzler RF, Grahm TW. The far-lateral approach to the inferior clivus and the upper cervical region: technical note. BNI Q. 1990;6:35-38.
28 Dandy W. Operative experience in cases of pineal tumor. Arch Surg. 1936;33:19-46.
29 Krause F. Operative Freilegung der Vierhügel, nebst Beobachtungen über Hirndruch und Dekompression. Zentralbl Chir. 1926;53:2812-2819.
30 Stein BM. The infratentorial supracerebellar approach to pineal lesions. J Neurosurg. 1971;35:197-202.