CHAPTER 80 Subthalamic Deep Brain Stimulation for Parkinson’s Disease
Surgery was the only effective treatment of PD until the 1960s, when Cotzias introduced treatment with levodopa based on the pioneering work of Arvid Carlson. PD has been known to be related to a deficit of dopamine in the basal ganglia since the report of Ehringer and Hornykiewicz.1 The drawbacks of ablative surgery, when contrasted with the strikingly beneficial effects of levodopa, were responsible for the almost total disappearance of ablative lesions until recognition of the long-term side effects of levodopa therapy, mainly motor fluctuations and dyskinesias, triggered renewed interest in surgical methods, but with no or little tolerance for complications. The need for less invasive techniques generated a series of basic research–based approaches. This was the main motivation for neural transplantation, which is still not considered routine treatment. In this context of a quest for a new procedure, the unexpected observation during intraoperative exploratory electrophysiology that high-frequency stimulation (HFS) was able to mimic in a reversible and adjustable manner the effects of local destruction of functional targets2 serendipitously led to the development of HFS of functional targets as a specific therapeutic tool. VIM-HFS was demonstrated to alleviate the most spectacular symptom of PD—tremor. However, the therapeutic effect was soon clearly recognized as being almost strictly limited to improvement of tremor with no effect on bradykinesia and rigidity. In actuality, although tremor is the most visible of the symptoms of PD, it is not the most disabling; the difficulties of patients with advanced parkinsonism are essentially related to akinesia and rigidity. Reports in the early 1990s by groups led by Mahlon DeLong3 and Crossman4 of the prominent role of the subthalamic nucleus (STN) in the control of motor function and the ability of STN destruction to improve bradykinesia and rigidity in 1-methyl-4-phenyl-1,2,3,6-tetrahydorpyridine (MPTP)-treated monkeys suggested that these symptoms could be also controlled. However, because of its reputation as a source of hemiballismus when destroyed by hemorrhage, the STN was not a very attractive surgical target.5 For this reason, subthalamotomy was not a good procedure to exploit this basic science discovery.
The experience acquired during HFS of the thalamus suggested that the STN could be a target for neuroinhibition-type methods as provided by HFS. This was supported by experiments in MPTP-treated monkeys, but with the use of HFS instead of lesioning.6 The ultimate validation of this target occurred when the first patients with advanced PD underwent implantation; the tremor, rigidity, and bradykinesia were very significantly improved by this method,7 which allowed the dosage of levodopa to be decreased by 60% on average8 and therefore alleviated the levodopa-induced motor fluctuations and dyskinesias.9 Since that time, several thousands of patients have been operated on all over the world and demonstrated improvement, thus making this method the reference surgical procedure for advanced PD.
What is the Subthalamic Nucleus? Anatomy and Physiology
Anatomy
The STN was discovered and described by Luys in 1865.
Shape and Situation
The STN, or corpus Luysi, is oval shaped and lies on the inner surface of the peduncular portion of the internal capsule. Caudally, the medial part of the nucleus overlies the rostral portion of the substantia nigra. Lesions of the STN in primates, in whom it is particularly well developed, generate very distinct clinical symptoms,10–12 and it is extraordinary that the dysfunction of such a small collection of cells, with apparent neurochemical simplicity and connectivity, can disturb normal neurological functions to such an extent.
Texture: Cytoarchitectonics, Histology, Histochemistry
The STN has a high density of dark cells on Nissl-stained sections; its boundaries stand out clearly from the surrounding diencephalic structures. The cells of the STN have been described as having either spindle-shaped, pyramidal, or rounded somata. Each gives rise to several spiny dendritic processes aligned parallel with the rostrocaudal axis of the nucleus. These processes may be very long (up to nearly 1 mm), and they remain within the boundaries of the STN in primates, whereas in nonprimates, they tend to “trespass” onto the territories of adjacent structures, such as the zona incerta. The majority of STN cells project to other neural centers, with only a small population of interneurons. The dominant neurotransmitter within STN cells is glutamate, but other neurochemicals (parvalbumin [calcium-buffering protein] and nitric oxide synthase) have also been reported. Although classically the STN was thought be GABAergic (secreting γ-aminobutyric acid) and inhibitory, most, if not all STN projections have now been revealed, in contradistinction, to be glutamatergic, and they exert extremely powerful excitatory effects on their target structures. For this reason, among others, the STN has been suggested to be a major driving force and central feature of the basal ganglia circuitry.10–13
Connections
The STN connections with other parts of the brain have been well described in nonprimates, as well as in primates. In general, its major connections are with the primary motor cortex, the globus pallidus pars externa (GPe), the substantia nigra pars reticulata (SNr), and the pedunculopontine tegmental nucleus (PPN); its minor connections are with the striatum, intralaminar nuclei of the thalamus, and various brainstem nuclei.10–13 The STN has been reported to have other, generally more sparse connections with various neural centers, including the intralaminar nuclei of the thalamus and the dorsal raphe, as well as a direct projection to the striatum.11–17
Afferents
Cortex
The cortex provides major input to the STN that is rather selective and arises from layer V, mainly from the primary motor cortex and, to a lesser extent, from the prefrontal cortex. The input is primarily via collaterals of axons terminating elsewhere.11 These cortical axons terminate in small boutons on spines and fine dendrites of the STN cells. Through such input the STN is thought to be the pivotal nucleus of the basal ganglia through which the cortex influences the output of the basal ganglia.10–13
Efferents
Substantia Nigra Complex
The STN also projects to the other basal ganglia output nucleus, the SNr. The STN axonal terminals in the SNr have again been shown to be glutamatergic and form asymmetric synapses with dendritic shifts and, to a lesser extent, somata. There have also been reports of a direct but sparse STN projection to the substantia nigra pars compacta (SNc),11,19 which could still generate glutamate excitotoxicity in the parkinsonian condition. In this context, it should be noted that the STN projections to the SNr are thought to be strongly related to the SNr projection to the SNc.11
Pedunculopontine Tegmental Nucleus
In view of recent experimental evidence, this brainstem nucleus has become included by most authors in the basal ganglia scheme and might be a contributing factor in generating the overactivity of the STN in patients with PD.20 Reciprocal connections between the PPN and STN have been documented in both primates and nonprimates. Individual STN cells project directly to the PPN (pars compacta portion) and not elsewhere, whereas a distinct population of STN cells project to the basal ganglia output nuclei, the globus pallidus and SNr.
Physiology
Electrophysiology
STN cells have high spontaneous tonic activity with irregular bursts of activity.21 During a typical electrode track through the brain, the STN is characterized by a sudden increase in background noise (from the relatively quiet of the zona incerta) as a result of the high density of bursting cells. Location of the electrode within the sensorimotor sector of the STN is often verified by the response of cells (increased firing audible through microrecording) to passive movement of the contralateral limbs.
Functions
As with other major nuclei of the basal ganglia, the STN can be divided into distinct functional sectors: (1) a large sensorimotor sector that occupies the dorsolateral regions of the nucleus—STN cells in this sector respond to somatosensory stimulation by changing discharge rates during movement of the contralateral limbs (in fact, there is a segregated map of the body found within the STN); (2) a small associative territory that occupies the ventromedial region of the nucleus—cells here are activated during visual oculomotor tasks; and (3) a limbic sector that occupies the medial tip of the nucleus—this sector receives input from the limbic cortex and ventral regions of the globus pallidus. In primates, these sectors involve distinct cell groups that respond to the different types of stimuli; in nonprimates, however, the sectors are less clear, perhaps because individual STN cells tend to have multiple projection sites and input.10–13
Dysfunction of the Subthalamic Nucleus Leads to Motor Disorders
Overexcitation
Conversely, overactivity of the STN (oscillatory bursts and synchronicity) induces PD symptoms (akinesia, rigidity, and tremor).11 In PD patients, as well as in two well-known animal models of PD—6-hydroxydopamine–lesioned rats and MPTP-treated monkeys—there is an increase in the firing rate of STN cells. Simultaneously, STN cells change their pattern of firing and exhibit an increase in oscillatory bursts and synchronicity,11,12,14,15,22 and MPTP-treated monkeys have an increase in glucose metabolism and mitochondrial enzyme activity in the STN. In fact, STN cells enhance their activity during the course of MPTP treatment, even before the first appearance of clinical signs.24 Furthermore, in PD patients and MPTP-treated monkeys, both lesioning and high-frequency deep brain stimulation (DBS) of the STN alleviate the parkinsonian symptoms, presumably by reducing the activity of the basal ganglia output streams. For these reasons, the STN is viewed as a centerpiece of pathophysiology in PD and has become a popular surgical target for the relief of motor symptoms.25,26
Chemical Modulation
Because the SNr sends projections to the SNc, the STN may “switch off” the dopaminergic SNc cells via its heavy projection to the SNr. Hence, during DBS of the STN, which inhibits the abnormally overactive STN cells in PD, there is an increase in the activity of SNc dopaminergic neurons27 and dopaminergic levels in the striatum.28 This may counteract the direct STN projection to the SNc, which is weaker than the SNr projection,19 but may not necessarily prevent the release of glutamate onto SNc cells by the overactive STN in patients with PD (and hence not prevent glutamate excitotoxicity).
Rationale for Using the Subthalamic Nucleus as a Target
Animal Experiments
Improvement of MPTP-induced parkinsonism has been achieved by inducing lesions of the STN electrolytically3,4 and by STN-HFS in the same model.6
Strategic Situation
The STN is functionally situated at a crossroad on the output of the basal ganglia, which may explain its major role in the control of motricity; the basal ganglia are closely involved in the control of movements and subsequently in the genesis of movement disorders when a component of this network is altered by a pathologic process, such as the nigrostriatal degeneration that is at the basis of PD. This results in dysregulation of the function of these networks by disrupting the various nodes of the networks and most often results in an abnormal firing pattern consisting of a combination of hyperactivity and bursting, thereby generating irregular activity, the most demonstrative being associated with tremor and observable in several nuclei of the network, such as the thalamus, the STN, and the GPi. A simplistic approach toward therapeutic application is to consider that alleviation of symptoms could be achieved by altering these abnormal activities, either by suppression (via ablative surgery) or by functional inhibition, such as by delivering a sort of “blank noise” via HFS that would jam, mask, or disrupt the abnormal burst-like hyperactivity. Because the STN projects to both the globus pallidus and SNr, it is thought to influence the activity of cells that constitute the output systems of the basal ganglia. These STN efferents may control the initiation of movement by setting the physiologic conditions (e.g., membrane potential) of the pallidal and SNr cells to appropriate levels before arrival of the striatal signals.11 Thus, the STN is in a position to influence the whole net output of the basal ganglia. In particular, the cortex is thought to drive the basal ganglia circuitry, not only through its classic input to the striatum but also through the STN.
How to Interfere with the Target
Lesioning
Lesioning is the most classic approach, as suggested by clinical observation and the aforementioned animal experiments, but lesioning of the STN in humans induces hemiballismus.29
Chemical Modulation
Chemical modulation by glutamate antagonists such as MK-801 has been used to induce temporary inactivation,30 and neurotoxins (kainic acid, ibotenic acid) are used to generate a permanent lesion in neurons or fibers, or both.
Electrical Modulation
Rationale
The rationale for using STN-HFS to treat PD comes from the application of HFS for the treatment of tremor, both parkinsonian tremor, which has been performed since 1987,31 and essential tremor.32 The fact that replication of the effects of lesions in the STN with HFS for the treatment of MPTP-induced parkinsonism in monkeys3,4 proved to be reversible, titratable, and safe in treating parkinsonian and essential tremor provided experimental support to attempt it in humans, which was done in 1993.7,33
Mode of Action
HFS mimics the effects of ablative lesions in all the targets that have been used thus far (thalamus, pallidum, STN, accumbens nucleus, hypothalamus, and others). However, HFS does not create a lesion; all effects are created by stimulation, and all improvements are only temporary and last as long as the duration of the stimulation. Another difference from lesioning is that HFS probably induces specific effects by excitation or inhibition of structures at a distance, such as the motor or sensory side effects obtained by diffusion to the motor pyramidal tract or to the lemniscus medialis fibers. The mechanism of action of DBS at high frequency is still completely unknown 22 years after its introduction. It is independent of the target because DBS mimics the effects of ablation in all targets used thus far, but it does not create a lesion and all of its effects occur only during stimulation. A large series of papers have been published on the mechanism of action of HFS, and there is an important trend in basic research aimed at this goal. One can surmise that several submechanisms are probably involved in combination to produce functional inhibition34: (1) jamming of the neuronal message transiting through the stimulated structure32 is highly probable, and we have suggested that it might take place in the very early stages of the procedure2,32,34 and could be responsible for desynchronizing abnormal oscillations expressed by the bursting patterns.14,35 This could be consistent with the observation that pathologic states such as PD create synchronization of neuronal activities, which are expressed by bursting patterns visible during microrecording, as shown in the pallidum in monkeys.14 This is supported by experimental data obtained in monkeys showing that STN-HFS resets the subthalamic firing and reduces the abnormal oscillations of STN neurons.35,36 (2) The hypothesis of extinction or strong inhibition of neuronal firing is also supported by direct observation of a decrease in the discharge rate during stimulation.37–39 (3) Dual effects could also be the mechanism, such as associated excitation and induction of high-frequency bursts.40 (4) Finally, it has been shown in our laboratory that HFS inhibits the production or release, or both, of certain neurotransmitters and hormones in culture,41 thus suggesting that this phenomenon could participate in the functional inhibition induced by HFS. Neuronal mechanisms that could be at the origin of the paradoxical inhibitory-like effect of HFS have recently been reviewed.40
Materials and Methods
Surgery
The procedure varies among teams, depending on their equipment and usual practices.
Operative Techniques
The procedure detailed in this chapter is the one followed in Grenoble and describes the state of the art of the method by our team, as applied to all targets (STN, GPi, thalamic VIM, PPN, subgenual cortex CG25). There are obviously other ways to perform it, as reported by other teams, and depending on future technical improvements, such methods might be undertaken by our own team. For instance, the implantation session is generally performed with the patient under local anesthesia, whereas some teams are using general anesthesia to decrease the stress and pain42 but lose intraoperative observation of the clinical benefits of DBS.
Stereotactic Frame and Robotized Arm
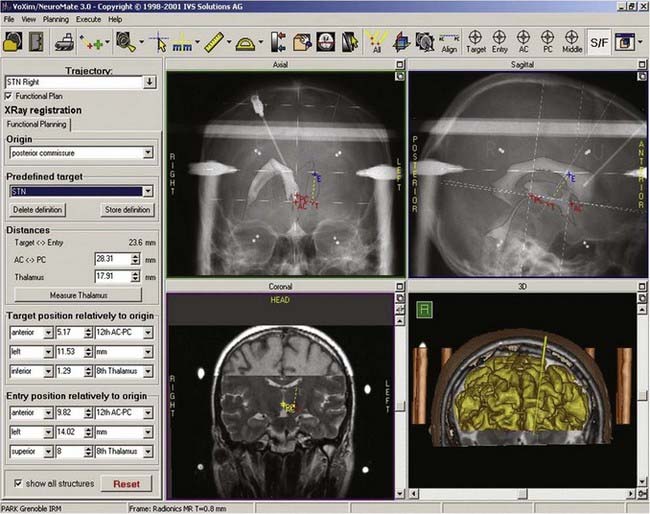
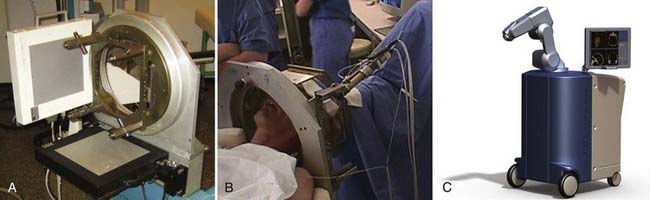
At our institution we use a robotized arm that allows high-precision positioning of the guide tube along the trajectory. The number and types of these devices are slowly increasing, but until recently the NeuroMate (Schaerer-Mayfield, Lyon, France) and more recently the Rosa (Medtech, Montpellier, France) were the only ones available (Fig. 80-1).
Preoperative Imaging and Planning
Stereotactic x-Ray Ventriculography
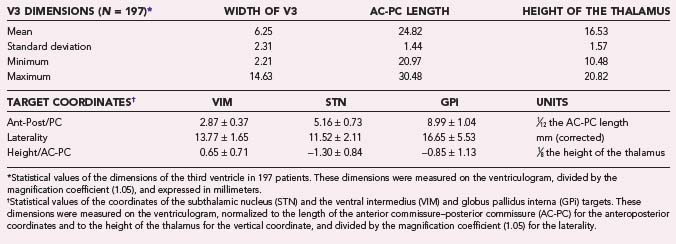
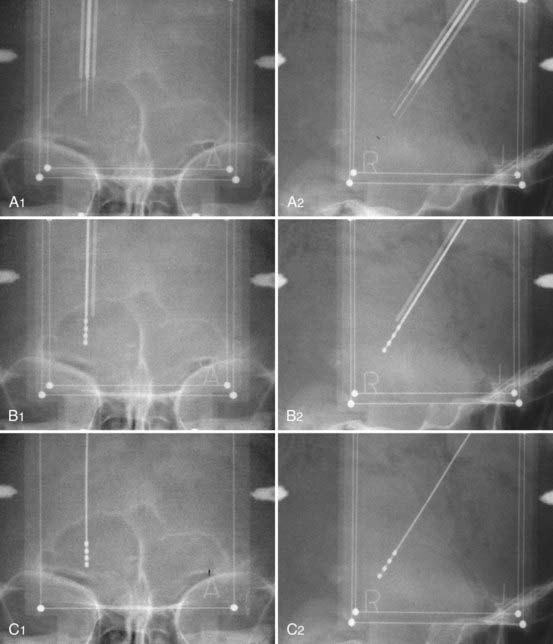
Stereotactic ventriculography (Fig. 80-2) is still performed by some teams under general anesthesia,43 as by ours, although a large number of centers do not use it for fear of complications or because they consider MRI localization to be satisfactory. The problem of MRI distortion, which is the major reason to continue performing ventriculography, is still rarely addressed. After a small skin incision, a hole through the skull, 9 cm from the nasion and 2.5 cm from the midline, is created with a twist drill. The right frontal horn of the ventricle is tapped with a Cushing cannula at a depth of 6.5 cm from the skin surface. A 2-mL air bubble is injected to check for correct placement of the tip of the cannula. During the injection of 6.5 mL of contrast medium (Iopamiro, Schering), a 12-second sequence of x-ray images is recorded in the lateral direction, immediately followed by radiographs taken in the anteroposterior direction. These x-ray images provide internal landmarks for the third ventricle, to which various atlases and coordinates of targets can be related.
Magnetic Resonance Imaging and Atlas Fusion
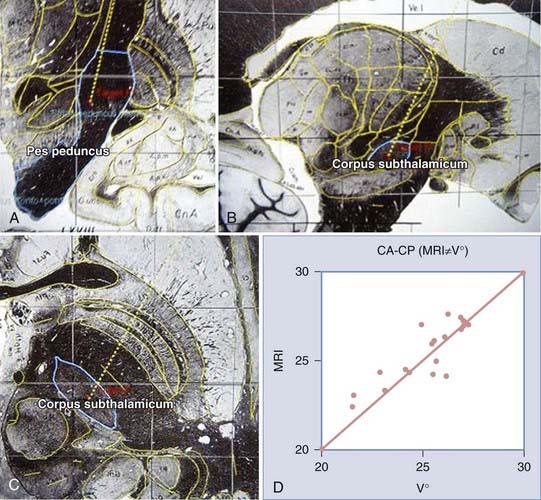
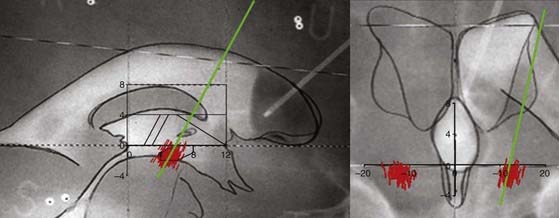
Stereotactic MRI is performed on awake patients with an MRI-compatible stereotactic head holder and localizer. This provides direct visualization of the STN target, which is visible on T2-weighted sequences as a hypointense signal surrounded by white matter (zona incerta above and fields of Forel bundles below) separating the STN from the SNr (Fig. 80-3). MRI, eventually merged to the ventriculographic images, is used to plan the procedure. The STN stereotactic target can be also constructed with graphic tools included in the navigation software.
Coordinates of the Target and Entry Point
Construction of the target is based on ventriculographic landmarks—the anterior commissure (AC), posterior commissure (PC), height of the thalamus (floor of the lateral ventricle), and midline of the third ventricle (Fig. 80-4, Table 80-1)—which allows automatic drawing of the intended target (in the present situation the STN, but the coordinates of any other stereotactic target can be introduced in the software). This x-ray target is fused with the MRI scans (three-dimensional axial T1- and coronal T2-weighted, with injection of contrast material) that are imported into the Voxim software of the robotized arm (NeuroMate) through the hospital image network. Importation of both the MRI and x-ray images obtained stereotactically in the same patient on the same day allows one to check the coherence of x-ray and MRI data by matching two anatomic structures, AC and PC, which are clearly visible with both modalities (Fig. 80-5). They usually match, but there is often a non-negligible and unpredictable discrepancy of up to 2 to 3 mm between AC images, for instance, thus stressing the still unresolved problem of MRI-based localization. If it is satisfactory, one checks for matching between the “theoretical” ventriculography-based STN target and the T2-weighted MRI actual scan of the STN. If there is a significant mismatch, particularly in the lateral direction, the laterality is corrected, which is not helped by the use of atlases because they are still not always coherent in the three spatial dimensions (Fig. 80-6). When MRI/ventriculography coherence is not satisfactory, the theoretical target is used alone. Planning of the entry point with MRI must avoid the cortical vessels, deep vessels in the sulci, the ventricle if possible, and the caudate nucleus. The planning data are then exported to the NeuroMate robotized arm controller through the neuronavigation software (see Figs. 80-1 and 80-5).
Targeting and Electrode Implantation
The implantation session is performed 3 days after pretargeting with the patient under local anesthesia. The patient is reinstalled on the frame and the pins reinserted into the hollow screws in accordance with the previous four vernier readings. Correct placement is checked on radiographs. Using the preplanning data, which have been stored in the neuronavigation software, the NeuroMate is launched and reaches the preplanned position on the first side to be operated (in general, the side contralateral to the worst clinical side). An arciform skin incision centered on the entry point is made. The skin, subcutaneous tissue, and periosteum are retracted en bloc from the skull by a rugination, and a 6- or 9-mm burr hole is made through the NeuroMate tool holder. The electrode guide tool (Ben Gun with five parallel channels, distant 2-mm axis-to-axis accommodating guide tubes, and long-term DBS electrodes 1.27 mm in diameter) is inserted down to the dura, which is not opened. The microelectrode guide tubes are introduced by perforating the dura matter with sharp stylets and then lowered into the brain with blunt stylets. When the stylets are removed, they are replaced by microelectrodes (tip diameter, 1 µm; impedance, 1 to 10 MΩ; FHC Bowdoinham, ME) in their own guide tubes and inserted 15 mm above the target point under radiographic guidance (Fig. 80-7). They are connected to the electronic stages of the data acquisition and processing system (Alpha Omega NeuroTrek) and moved toward the target with a micromanipulator.
Electrophysiology
Electrophysiologic Exploration
Two types of electrophysiologic investigation are performed:
The electrophysiologic signature of the STN units, as reported in literature,44,45 is typically composed of asymmetric spikes at rather high frequency, and it exhibits burst patterns in PD (Fig. 80-8). They respond to passive contralateral limb movements and proprioceptive input, such as muscle pressure, and exhibit synchronous contralateral tremor activity. Recording length in the STN varies from 5 to 6 mm between two silent zones corresponding to white matter, the first one between 0 and 2 to 3 mm below the AC-PC plane (subthalamic area and anterior zona incerta) and the other one corresponding to the white matter between 9 and 11 mm just above the SNr. SNr neurons fire in regular, symmetrical, large-amplitude spikes that are generally unresponsive to external stimuli (see Fig. 80-8).
Microstimulation: Looking for Beneficial Effects and Side Effects
Microstimulation can be performed with the microelectrode that has been used to record neuronal activity at current intensities of up to 10 mA for short (10 to 30 seconds) periods. Microstimulation is essential because it allows observation of the beneficial effects (improvement of PD symptoms) inside the target and the side effects (limiting factors for efficient stimulation) outside it.46
Final Choice: Synthesis of Benefits and Side Effects
When the best track (best beneficial effects, least side effects, largest security margin between the threshold for improvement and side effects) has been identified, the corresponding microelectrode is removed and replaced with a final lead (DBS 3389, 1.5-mm contact length, 0.5-mm spacing, 1.27-mm diameter) sutured to the rim of the bur hole (made with a small oblique twist drill) and, after removing the other microelectrodes, embedded in dental cement to prevent leakage of cerebrospinal fluid (CSF) and infection back along the track. The external part of the electrode is folded under the periosteum and the skin is sutured (Fig. 80-9). The NeuroMate is then aimed at the other side for a similarly preplanned procedure. At the end of the procedure, final x-ray images are taken for use in determination of electrode position and statistical evaluation of the most effective contacts (Fig. 80-10). The question of unilateral STN-DBS, which is often raised because of the alledgedly lower morbidity47 in symptomatically asymmetric patients and the observation of ipsilateral effects of unilateral STN stimulation,48 still needs definitive resolution through a large and well-conducted and controlled clinical trial.
Patients
Clinical Indications
Good Prognostic Factors: Who Should Undergo Surgery?
Idiopathic Parkinson’s Disease
The patients most responsive to STN-HFS have clinically diagnosed PD (defined as the presence of at least one of the triad of symptoms and levodopa responsiveness) and in whom it can be predicted that the main symptoms of the triad (bradykinesia, rigidity, and tremor) will be improved significantly.7–9,49,50 Higher baseline Unified Parkinson’s Disease Rating Scale (UPDRS) III “off “ scores and higher baseline levodopa responsiveness are independent predictors of greater change in motor scores after surgery.
Levodopa Responsiveness
It has been shown that the percentage of improvement with the best optimal adjustment of antiparkinsonian medication or a suprathreshold dose of levodopa (e.g., 300 mg in a single dose) is highly predictive of a similar improvement after optimal placement of electrodes in the subthalamic target.44,52 Moreover, the levodopa response is one of the mandatory criteria for the diagnosis of idiopathic PD. Age and response to levodopa are among the factors predictive of motor outcome.51
Motor Fluctuations
Severe levodopa-related motor complications despite optimal adjustment of antiparkinsonian medication are usually improved significantly after surgery, which plays a major role in improvement of the patient’s quality of life (QOL).49,52 This is explained by the mechanism of induction of dyskinesia related to the pulsatile administration of levodopa.53 The decrease in or arrest of these pharmacologic adverse effects achieved by the beneficial effects of STN stimulation restores a more normal pharmacokinetic regimen of the striatal dopaminergic receptors.
Poor Prognostic Factors: Who Should or Should Not Undergo Surgery?
This is difficult to establish.
Age
Age, as in all surgical endeavors, is more or less negatively related to the general outcome. However, this must be carefully evaluated for each patient, in whom the effects of age vary considerably. Although STN-HFS reduces motor complications equally in all patients, QOL improves postoperatively only in patients younger than 65 years.54
Gait Disturbance
This topic has to considered seriously during the decision-making process. Gait disturbances need to be carefully assessed by the neurological team before surgery. Freezing is part of the patterns of akinesia and usually responds to levodopa treatment. When freezing of gait occurs, persists, and is not improved in the on-medication period, it is not usually improved by STN stimulation55 (but might be improved by low-frequency stimulation of a new target, such as the PPN56–58), which should therefore be taken into account during the decision-making process or at least the patient, family, and caregivers should be clearly informed.55
Speech Problems
Speech is usually improved, but much less than the other motor symptoms.59,60 When patients are hypophonic before surgery, the hypophonia might be impaired or worsened afterward, particularly when the doses of medication are significantly decreased, which might not be compensated for by the improvement from DBS; the severe hypophonia that results generally elicits complaints from the patient and family. Such deterioration in speech is one of the reasons for reintroducing a certain amount of levodopa as an additional treatment to DBS. This possibility should also be taken into account during the decision-making process.
Contraindications: Who Should Not Undergo Surgery?
Specific Situations
Previous surgery, either ablative (thalamotomy, pallidotomy, subthalamotomy, and so on) or nondestructive (previous DBS in the STN or in other targets), is not a contraindication to STN-DBS, provided that the target itself has not been destroyed.61,62
Implantation of an electrode into the target does not destroy it; therefore, when previous DBS in any target has failed to produce clinical improvement and all selection criteria for predicting a beneficial effect were fulfilled, it is always possible to reimplant the target. Furthermore, it is not even necessary to remove an inefficient electrode that had not been placed in the right target, provided that it is at least at 2 mm apart. In several patients in whom placement of the electrode was not optimal, we have reoperated and found that in all cases the new electrode was more efficient and beneficial.63
Results: Desired Versus Undesired
Since the first application of bilateral STN-HFS in 1993, several thousands of patients have undergone implantation around the world, and their clinical results have been reported. The following data summarize the results from the literature.9,49,64 In addition, complications in the complete series of 325 STN patients at our institution have also been evaluated in a retrospective, unblinded study (unpublished data).
Complications: “What Should Not Happen”
We grouped complications and adverse effects into three quantitative categories:
Qualitative Results
Hemorrhage
Hemorrhages (8.4% of all STN-DBS patients: 3.4% asymptomatic and only visible on MRI, 4.4% transient, and 0.6% permanent) occurred mostly at the entry point or subcortically, rarely in the target, and more often in patients with hypertension.9,65–75 Of these hemorrhages, 93.2% were either asymptomatic or transiently symptomatic and just 6.8% were responsible for permanent symptoms. Asymptomatic hemorrhages (40.5%) were seen only on postoperative MRI, which enhances the importance of MRI in the preoperative stage to avoid superficial vessels and penetration of a sulcus, as well as the ventricle and caudate nucleus.
Infection
Infections (4.4% of all STN-DBS patients: 1.1% severe, 1.3% significant, 1.9% mild or benign) were mostly superficial and related to the hardware.76
Neurological Symptoms
Three patients died: 1 died 3 years after a postoperative intracerebral hemorrhage that was evacuated but left the patient bedridden, 1 died of myocardial infarction 11 months after surgery, and 1 committed suicide 6 months after surgery. Forty-one of 42 patients gained weight (mean, 3 kg; maximum, 5 kg).77
Some adverse effects related to the antiparkinsonian action of STN-DBS are expected but are generally reversible if the parameters are adjusted to an acceptable compromise between the absence of side effects and suboptimal benefit. This situation often occurs when electrode placement is also suboptimal. These adverse effects vary according to the anatomic location of the stimulated fibers or neuronal structure and include dysarthria or hypophonia in 4% to 17% of patients,61,64,78–81 dysphagia, motor contraction, paraesthesias, eye deviation, gaze deviation, visual flashes, nausea, dizziness, sweating, flushes, imbalance, dyskinesias, and termination of the effect of levodopa with resultant worsening of the akinesia. Thirty-one percent of patients experienced eye lid opening apraxia in the first 3 months,82,83 which remained a problem in 19% after 5 years. Stimulation-induced dyskinesias can be a good sign of accurate placement and are reversible by decreasing the voltage or the drug dosage, or both.9,49,56,61,84
Neurocognition and Behavior
Depression and Suicidality
Patients who are depressed after surgery are already depressed before it.85 Depression is a frequent finding in the patient population seeking surgery,82 and patients with suicidal ideation are at risk and thus require optimal treatment and very close psychiatric follow-up. Preoperative depression, although transiently improved in the first postoperative year, does not change in the long term with STN-DBS and was found to be a risk factor for postoperative suicide; it also points to selection bias.86 Postoperative depression occurred in 12.4% of the patients and lasted several weeks to several months; it always receded either spontaneously or with the use of antidepressant drugs. Transient depressive episodes were observed in 17% with longer follow-up. The cause of the induced depression and suicidality was multifactorial and included changes in treatment, which was purely medical before surgery but changed to a combination of stimulation and drugs after surgery. They are not specifically related to the target.56 Reports of suicide after STN surgery,8,82,86 although rare (1.3% attempted suicide but only 0.2% committed suicide in our series87) and currently estimated at 0.5%,88 have raised concern. Depression and suicide have also been observed after all major surgeries, even though they provide the patient with significant improvement, as well as recovery of freedom from long-term incapacitation,89–94 and are related to societal issues.
Behavior and Neuropsychiatric Complications
The majority of the neuropsychiatric symptoms observed are considered to be transient, treatable, and potentially preventable.83 Patients displaying behavioral abnormalities after surgery generally had the same problems before surgery, and the reported abnormal behavior is not clearly specific to the target.82 They occur in 25% of cases overall.51 In the postoperative period, transient hypomania in 8%,9,49,85 acute sadness,95 impulsive aggressive behavior,9,96 or hilarity97 or mania98,99 may develop, frequently because of the added effects of drugs and STN surgery,100 and are rapidly reversible.
Apathy
Transient apathy was observed in 5% of patients and responded to medication in 88%. Apathy is part of PD101 and is a frequent finding in patients with PD managed with STN-DBS.9,85 Severe apathy can occur as a result of postoperative withdrawal of dopaminergic medication, especially in patients addicted to levodopa,102 and responds to resumption of dopaminergic medication.82,85 These changes in mood might be related to stimulation of surrounding structures, but they could represent behavioral patterns liberated by an abrupt change in STN limbic activity.97
Confusion
Transient postoperative confusion developed in 10.3% of patients, possibly related to bilateral trauma to the caudate nucleus by the guide tubes during electrophysiologic exploration. These neuropsychological and behavioral complications occurred in 24.3% of the patients overall and in a more restricted series of 42 patients observed as a cohort for 5 years.103 We analyzed the neurocognitive complications more precisely. There was a high prevalence (24% of the patients) of confusion (from temporospatial disorientation to psychosis) and behavioral side effects during the first few postoperative days, but in the long term they were relatively rare.104
Neurocognitive Changes
The most frequently observed change was a decline in word fluency.105 Under STN-DBS, patients manifest a form of impulsivity.106 There was no short-term global cognitive deterioration in nondemented patients.88,107–109 The minor changes in neuropsychological test results83,110 have limited impact on cognitive function110; there were no significant changes in the Beck depression inventory or the Mattis dementia rating. No major modifications in personality structure took place.111 The average score for frontal lobe function becomes slightly impaired with time,9 and patients with reduced cognitive reserve or preoperative cognitive decline are at risk for decompensation.9,11 In the long term, progression of the dysexecutive syndrome leads to dementia, as in nonoperated PD patients.9 Therefore, aside from the clinically valuable improvement in motor function, the benefit-to-risk ratio for DBS seems favorable in severely disabled but carefully selected patients.
Causality
Related to Target
In comparing the occurrence of severe and significant adverse effects between the main targets, they were significantly more frequent with stimulation of the STN (22.2% of 325 patients) than with VIM (8.6% of 138 patients) and GPi (7.9% of 63 patients) stimulation. Even though the populations and symptoms are different in terms of the target-specific indications, complications and adverse events seem more frequent with STN as the target than with other targets in some series49,112,113 but not in others.66,114 It is, however, difficult to evaluate because very few randomized controlled trials have been designed to compare the various targets.114
Related to Implantation, Including Hardware
With respect to the phases of surgery, 7.4% of complications were related to ventriculography and frame setting, 20.9% to electrode implantation, and 13.5% to the IPG and hardware. Severe adverse effects leading to permanent neurological aftereffects are mainly due to intracranial hemorrhage, which occurred in 2% to 4% of patients.81,106 Other transient or benign complications are frequent and do not lead to aftereffects. Transient postoperative confusion occurred in 10.3% of patients (range, 1% to 36%).9,85,88,105,109,110,116–125 General surgical complications, including aspiration pneumonia, pulmonary or urinary infection, thrombophlebitis, and pulmonary embolism, can occur in the most severe PD patients. The duration of surgery, microelectrode recording, and the number of passes have been poorly related to clinical outcome and the complication rate,126,127 but the influence of microrecording is still debated.66,126–129 As a rule, when the indication is correct, poor-outcome DBS for parkinsonism is related to either incorrect implantation or hardware failure.61,130
Complications Related to Hardware After the Operative Period
Hardware-related complications have the highest incidence, with rates ranging from 2.7% to 50%.73,103,121,131–134 Reported infection rates for DBS surgery vary widely, from less than 1% to as high as 15%.64–69,71–75,115,128,129,135–146 Infections are mostly superficial and related to the hardware and occur in about 4.4% of patients; 1.1% are severe, 1.3% are significant, and 1.9% are mild or benign. They develop within 3 months after surgery, most often at the IPG.73,75,115,129,139,144,147 Complications related to the implant, such as infection, skin erosion, lead breakage, extension wire failure, and consumption or malfunction of the IPG, are common.58,73,129,142,145,148–151 Such complications led to discontinuation of treatment in 6.1% of patients in a multicenter study with 4 years’ follow-up.151 A 9% incidence of adverse effects related to the hardware (infections, lead and pulse generator problems) has been reported.152 These side effects can generally be managed without permanent morbidity, but in the event of infection, the implanted hardware almost always has to be removed.
Related to Stimulation
The percentage of complications related to stimulation was 31.1%. From this point of view, the STN was again mostly involved; the reason might be that most STN patients have a long history of PD and they are older with severe symptoms. Permanent neurological impairment is relatively rare (≈3%). The occurrence of adverse events was not significantly different, but serious adverse events were significantly more common with neurostimulation than with medication alone in studies comparing stimulated patients and those treated only medically.61
How to Avoid Complications
Precision of Electrode Placement is Crucial
The complications related to stimulation can be improved by precise positioning of the electrode so that diffusion to neighboring structures is avoided, which is responsible for adverse effects such as muscular contractions from spreading of current to the pyramidal tract.153 This may be a limiting factor and therefore prevent optimal realization of the benefits of stimulation.
Drawbacks
Drawbacks are related to hardware at the time of implantation and replacement.154 In 49 patients bilaterally implanted with the Itrel II and undergoing continuous stimulation at the usual parameters (amplitude, 3.2 ± 0.3 V; pulse width, 65 ± 10 µsec; frequency, 145 ± 16 Hz), replacement had to be anticipated in 25% because of unilateral depletion, and the average duration of life of the IPG was 83 ± 14 months (range, 40 to 113).
The electrodes, extensions, and the IPG must be purchased together at the time of implantation at a price of several thousand dollars. Comparison between implanted and medically treated patients155 has clearly shown that the cost of the hardware and all of the expenses related to the surgery, including hospitalization, are lower than the cost of medications, caregivers, and accessories such as wheelchairs during a period equivalent to the duration of life of the IPG.
Benefits
Improvement of Symptoms
The main instrument for analyzing the intensity of symptoms in patients with PD is the UPDRS. This scale has been validated by evidence-based medicine studies156 and is considered the reference standard in comparison to other less specific and global scales157,158 or those specially aimed at QOL (e.g., Parkinson’s Disease Questionnaire [PDQ-39]).8,49,71,80,107,112,115,159–170 The estimated decreases in absolute UPDRS-II (activities of daily living) and UPDRS-III (motor) scores after surgery in the stimulation-on/medication-off state versus the preoperative medication-off state were 50% and 52%, respectively.9,49,71 Neurostimulation results in significantly greater improvements than medication alone in the PDQ-39 and the UPDRS-III. The mean UPDRS-III score improves by 41% in the medication-off state and by 23% in the medication-on state.49 The STN-DBS–associated improvement in UPDRS-III, as compared with baseline values, is stable over time, 66% and 54% at 1 and 5 years, respectively; in additional studies with follow-up periods ranging from 2 to 4 years, improvement of 43% to 57% was reported.79,80,165,171–173 The improvement was 70% to 75% for rigidity and tremor and 50% for akinesia. STN-DBS has a direct effect on off-period dystonia, which was observed in 71% of the patients preoperatively and in only 19% and 33% at 1 and 5 years, respectively. Postural stability and gait also improve, but speech improves only during the first year and then progressively returns to baseline at 5 years. UPDRS-II improves similarly, but with significant worsening over time. The average postoperative reduction in dopaminergic drugs was 50%49 to 56%.129 As a result, levodopa-induced dyskinesias, the ensuing disability, and their duration are decreased by 69%, 58%, and 71%, respectively, which has a major impact on QOL.49,52 This finding mainly reflects desensitization secondary to both long-term stimulation-induced neuronal plasticity and levodopa withdrawal.174–176 This is explained by the mechanism of induction of dyskinesia related to the pulsatile administration of levodopa.53 As stated earlier, the decrease or arrest of these pharmacologic adverse effects achieved by the beneficial effects of STN stimulation restores a more normal pharmacokinetic regimen of the striatal dopaminergic receptors. On-period motor symptoms are moderately112,163 or not8 improved by STN-DBS. Moreover, these UPDRS-III data neglect the temporal dimension of the improvement in that the fluctuating benefit after drug intake is replaced by a stable improvement reflected by an increase in “on” time of about 47% to 71%.9,49,52,71,112,160,166
Speech is generally less improved with STN-DBS8,9,112 than other parkinsonian signs. Hypophonia may improve, or dysarthria may be aggravated because of diffusion of current to the corticobulbar fibers.177 As a consequence, patients’ satisfaction, particularly regarding hypophonia and the ability to communicate with their family, can decline after surgery. Improvement in sleep architecture178 and quality179 have been reported, with the increase in total sleep time (by as much as 47%) resulting indirectly from improvement in nighttime akinesia and early morning dystonia.178 STN stimulation can also be effective in improving voiding control by decreasing detrusor hyperreflexia.180,181
Progression of symptoms over time closely resembles the natural history of PD with medical treatment but without the motor complications. Therefore, these changes are believed to represent progression of the disease rather than side effects of stimulation. This is compatible with a longitudinal positron emission tomography (PET) study showing a continuous decline in dopaminergic function in patients with advanced PD managed with clinically effective bilateral STN-DBS, and the rates of progression were within the range of previous studies in nonstimulated patients.182
Improvement in Quality of Life
QOL is a direct index of what patients expect from treatment. A large randomized controlled multicenter study involving 156 patients compared bilateral STN-DBS in combination with medical treatment versus best medical therapy alone over a 6-month period.49 Neurostimulation resulted in improvements of 24% to 38% in the PDQ-39 subscales for mobility, activities of daily living, emotional well-being, stigmata, and body discomfort and a 22% improvement in the physical summary score of the 36-item short-from health survey (SF-36) versus practically no change in the medication group. The mean improvement in the PDQ-39 summary index score was 24%, and the dyskinesia score in off-medication patients was improved by 54%. The total number of adverse events was higher in medication-only patients. This result confirmed previous uncontrolled studies on QOL after STN-DBS183,184 that consistently reported greater improvements in subscores of mobility, activities of daily living, stigmata, emotional well-being, and body discomfort than in social support, cognition, and communication.183 The QOL of caregivers was also improved.130
Medications and Stimulation Settings
With 5 years of follow-up after STN-DBS, levodopa was still arrested in a third of the patients, the decrease in levodopa-equivalent dose was 67%, similar to that at 1 year,9 and less than 1% took any dopaminergic drugs. A dramatic and early reduction in medication intake may have accounted for some of the complications, such as dysarthria, apathy, and cognitive problems.9,185
The amplitude of stimulation was 2.9 ± 0.6 V, the frequency was 139 ± 18 Hz, and the pulse duration was 63 ± 7.7 µsec. Monopolar stimulation was used in the majority of patients in most studies, with comparable values.112 There is no indication of tolerance in that the effects were stable over a 5-year period, with no increase in stimulation parameters after the first year.9 STN-DBS is mostly bilateral because candidates for surgery usually exhibit bilateral motor symptoms and the effects of unilateral stimulation are primarily contralateral7 and do not provide maximal improvement in walking,7,186 except in some patients with asymmetric motor symptoms.187 Postoperative management of dopaminergic drugs may be difficult after unilateral STN-DBS. The batteries (Itrel II) may last up to 7 years.154
Neuroprotection?
It has been demonstrated that in parkinsonian patients, as well as in animal models, the neuronal activity of the STN is profoundly altered, with the appearance of a rhythmic pattern composed of bursts, in addition to a general increase in the firing rate. STN neurons are glutamatergic, and glutamate is an excitatory amino acid that has excitotoxic effects on the dopaminergic neurons of the projection area of the STN. The increased glutamate output of the STN on the dopaminergic neurons of the SNc participate in their degeneration, which led to the idea that decreasing output by antagonists such as MK-801 would slow down the degeneration process involving the dopaminergic neurons.30 Because one of the mechanisms of HFS might be a decrease in the firing rate of neurons submitted to this type of stimulation, one can suppose that this (as well as the ablation of this structure) would play a similar role and would be slowing down the neurodegenerative process involving the nigral dopaminergic system.
Several studies in rodent and primate models of PD have provided experimental evidence that manipulation of the STN, by either lesions or long-term stimulation, can protect dopaminergic neurons in the SNc and significantly decrease the cell loss induced by the neurotoxins used in these models.31,35,38,142,188–192
In humans, the only study using PET scanning did not confirm these experimental data, but it was performed in patients with a very advanced stage of the disease.182 One may also consider that in the early stages, the putative neuroprotective effect might not only slow down the neurodegeneration but also allow the dopaminergic neurons that had lost their dopaminergic production but were still alive193 to recover sufficiently to again produce dopamine, which would stabilize but even improve the patient’s condition. To clarify this important issue, there is an urgent need for controlled randomized clinical trials of patients investigated with both clinical and nonclinical tests (such as PET) and operated on early enough in the course of the disease.
Future Perspectives
Improvements
New Paradigms
There is no reason that the current waveform might be the best one. New waveforms and paradigms of stimulation must be tested.194
Additional Targets
The lower morbidity associated with HFS of brain structures has allowed investigation of the effects of its application to targets suggested by the results of basic research. This was the case of the STN for PD, for the accumbens nucleus for psychosurgery, for the posterior hypothalamus for cluster headaches, and for the PPN for the freezing of gait.55,176,195 The preliminary results tend to support the basic science assumptions. Improvement in gait dysfunction and postural instability has been reported in both the “on” and “off “ medication states.57,58,196 The effects on gait improve the benefits obtained by STN-DBS but cannot replace them. The exact anatomic structure to be stimulated is still debated,197,198 and determination of the target might benefit from new imaging procedures.199
Alternatives
Other Methods of Manipulation of the Basal Ganglia System
Gene Therapy
There are encouraging but very preliminary data, either experimental, with the use of adeno-associated virus glutamic acid decarboxylase (AAV GAD) gene therapy in rats,201 or clinical, with reports of clinical improvement, as well as PET-based evidence of metabolic improvement, after AAV GAD gene therapy in human STN202 or after the administration of neurturin in the human striatum.203
Infusion Therapy
Continuous infusion of dopamine agonists such as apomorphine204 or lisuride205 produces a more stable and regular dopamine concentration in the brain and clearly decreases the dyskinesias, but it induces local complications such as cutaneous nodules at the site of injection on the abdominal wall, which restricts the use of this method. In the same spirit, another route of continuous administration is being tested. Intraduodenal administration of levodopa (Duodopa) with an intragastric catheter through a duodenogastrostomy has been attempted, and satisfactory results are being reported despite the invasiveness and discomfort of this method.206
Infusion of Growth Factors
Chronic infusion of glial-derived nerve factor into the striatum has been performed in several patients, and highly significant improvement was reported.207–209 Such improvement, however, was not confirmed by an international multicenter double-blind controlled study.210
Cortical Stimulation
The results reported thus far for cortical stimulation have been disappointing, depending on the indications,211,212 although experimental chronic cortical stimulation of the motor area in monkeys had demonstrated encouraging data.213
Grafting Methods (e.g., Mesencephalic Fetal Cells, Stem Cells, Retinal Epithelial Pigmented Cells, Encapsulated Cells)
For several decades, an impressive amount of work in basic science and in animal models has been performed in highly expert laboratories around the world, as well as several transfers of the method to parkinsonian patients in controlled trials of grafting. Various types of cells have been used (adrenal gland, mesencephalic fetal grafts, and more recently, epithelial retinal cells). Stem cells are also being investigated as a potential material that would be much more immunologically tolerated, but it raises its own (oncologic) problems. This approach is ultimately the most elegant but is still experimental and cannot be part of a therapeutic panel.214
Conclusion
These advantages are also counteracted by drawbacks for the same reasons:
Aziz TZ, Peggs D, Sambrook MA, et al. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Disord. 1991;6:288-292.
Benabid AL, Pollak P, Louveau A, et al. Combined (thalamotomy and stimulation) stereotactic surgery of the Vim thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344-346.
Benazzouz A, Gross C, Feger J, et al. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci. 1993;5:382-389.
Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436-1438.
Castelli L, Perozzo P, Zibetti M, et al. Chronic deep brain stimulation of the subthalamic nucleus for Parkinson’s disease: Effects on cognition, mood, anxiety and personality traits. Eur Neurol. 2006;55:136-144.
Charles PD, Van Blercom N, Krack P, et al. Predictors of effective bilateral subthalamic nucleus stimulation for PD. Neurology. 2002;59:932-934.
Deuschl G, Schade-Brittinger C, Krack P, et al. for the German Parkinson Study Group, Neurostimulation Section. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896-908. Erratum in N Engl J Med. 2006;355:1289
Filali M, Hutchison WD, Palter VN, et al. Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp Brain Res. 2004;156:274-281.
Funkiewiez A, Ardouin C, Caputo E, et al. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:834-839.
Gill SS, Patel NK, Hotton GR, et al. Direct brain infusion of glial cell line–derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589-595.
Hamani C, Richter E, Schwalb JM, et al. Bilateral subthalamic nucleus stimulation for Parkinson’s disease: a systematic review of the clinical literature. Neurosurgery. 2005;56:1313-1321.
Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349:1925-1934.
Krack P, Limousin-Dowsey P, Benabid AL, et al. Chronic stimulation of subthalamic nucleus improves levodopa-induced dyskinesias in Parkinson’s disease. Lancet. 1997;350:1676.
Limousin P, Krack P, Pollak P, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 1998;339:1105-1111.
Mazzone P, Lozano A, Stanzione P, et al. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson’s disease. Neuroreport. 2005;16:1877-1881.
Meissner W, Leblois A, Hansel D, et al. Subthalamic high frequency stimulation resets subthalamic firing and reduces abnormal oscillations. Brain. 2005;128:2372-2382.
Piallat B, Benazzouz A, Benabid AL. Subthalamic nucleus lesion in rats prevents dopaminergic nigral neuron degeneration after striatal 6-OHDA injection: behavioural and immunohistochemical studies. Eur J Neurosci. 1996;8:1408-1414.
Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. Neuroreport. 2005;16:1883-1887.
Stefani A, Lozano AM, Peppe A, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130:1596-1607.
Videnovic A, Verhagen Metman L. Deep brain stimulation for Parkinson’s disease: prevalence of adverse events and need for standardized reporting. Mov Disord. 2008;23:343-349.
Voon V, Moro E, Saint-Cyr JA, et al. Psychiatric symptoms following surgery for Parkinson’s disease with an emphasis on subthalamic stimulation. Adv Neurol. 2005;96:130-147.
Wallace BA, Ashkan K, Heise CE, et al. Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain. 2007;130:2129-2145.
Xia R, Berger F, Piallat B, et al. Alteration of hormone and neurotransmitter production in cultured cells by high and low frequency electrical stimulation. Acta Neurochir (Wien). 2007;149:67-73.
1 Ehringer H, Hornykiewicz O. Verteilung von Noradrenalin und Dopamin (3-hydroxytryptamin) im Gehirn des Menschen und ihr Verhaltenbei Erkranktungendes extrapyramidalen Systems. Klein Worschenschr. 1960;38:1236-1239.
2 Benabid AL, Wallace B, Mitrofanis J, et al. A putative generalized model of the effects and mechanism of action of high frequency electrical stimulation of the central nervous system. Acta Neurol Belg. 2005;105:149-157.
3 Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436-1438.
4 Aziz TZ, Peggs D, Sambrook MA, et al. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Diserd. 1991;6:288-292.
5 Aebischer P, Goddard M. Treating Parkinson’s disease with lesions of the subthalamic nucleus. Science. 1991;252:133-134.
6 Benazzouz A, Gross C, Feger J, et al. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci. 1993;5:382-389.
7 Limousin P, Pollak P, Benazzouz A, et al. Effect on parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91-95.
8 Limousin P, Krack P, Pollak P, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 1998;339:1105-1111.
9 Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349:1925-1934.
10 Parent A, Smith Y. Organization of efferent projections of the subthalamic nucleus in the squirrel monkey as revealed by retrograde labeling methods. Brain Res. 1987;436:296-310.
11 Parent A. Carpenter’s Human Neuroanatomy, 9th ed. Baltimore: Williams & Wilkins; 1996.
12 Temel Y, Blokland A, Steinbusch HWM, et al. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol. 2005;76:393-413.
13 Parent A. Extrinsic connections of the basal ganglia. Trends Neurosci. 1990;13:254-258.
14 Bergman H, Wichmann T, Karmon B, et al. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507-520.
15 Blandini F. The role of the subthalamic nucleus in the pathophysiology of Parkinson’s disease. Funct Neurol. 2001;16:99-106.
16 Groenewegen HJ. The basal ganglia and motor control. Neural Plast. 2003;10:107-120.
17 Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002;43:111-117.
18 Papahill PA, Lozano AM. The pedunculopontine nucleus and Parkinson disease. Brain. 2000;123:1767-1783.
19 Hammond C, Shibazaki T, Rouzaire-Dubois B. Branched output neurons of the rat subthalamic nucleus: electrophysiological study of the synaptic effects on identified cells in the two main target nuclei, the entopeduncular nucleus and the substantia nigra. Neuroscience. 1983;9:511-520.
20 Breit S, Lessmann L, Unterbrink D, et al. Lesion of the pedunculopontine nucleus reverses hyperactivity of the subthalamic nucleus and substantia nigra pars reticulata in a 6-hydroxydopamine rat model. Eur J Neurosci. 2006;24:2275-2282.
21 Bezard E, Boraud T, Bioulac B, et al. Involvement of the subthalamic nucleus in glutamatergic compensatory mechanisms. Eur J Neurosci. 1999;11:2167-2170.
22 Ni ZG, Bouali-Benazzouz R, Gao DM, et al. Time-course of changes in firing rates and firing patterns of subthalamic nucleus neuronal activity after 6-OHDA–induced dopamine depletion in rats. Brain Res. 2001;899:142-147.
23 Wallace BA, Ashkan K, Heise CE, et al. Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain. 2007;130:2129-2145.
24 Temel Y, Visser-Vandewalle V, Kaplan S, et al. Protection of nigral cell death by bilateral subthalamic nucleus stimulation. Brain Res. 2006;1120:100-105.
25 Ashkan K, Wallace B, Bell BA, et al. Deep brain stimulation of the subthalamic nucleus in Parkinson’s disease 1993-2003: where are we 10 years on? Br J Neurosurg. 2004;18:19-34.
26 Benabid AL, Benazzouz A, Pollak P. Mechanisms of deep brain stimulation. Mov Disord. 2002;17(suppl 3)):S73-74.
27 Benazzouz A, Gao D, Ni Z, et al. High frequency stimulation of the STN influences the activity of dopamine neurons in the rat. Neuroreport. 2000;11:1593-1596.
28 Meissner W, Harnack D, Paul G, et al. Deep brain stimulation of subthalamic neurons increases striatal dopamine metabolism and induces contralateral circling in freely moving 6-hydroxydopamine–lesioned rats. Neurosci Lett. 2002;328:105-108.
29 Alvarez L, Macias R, Lopez G, et al. Bilateral subthalamotomy in Parkinson’s disease: initial and long-term response. Brain. 2005;128:570-583.
30 Turski L, Bressler K, Rettig KJ, et al. Protection of substantia nigra from MPP+ neurotoxicity by NMDA antagonists. Nature. 1991;349:414-418.
31 Benabid AL, Pollak P, Louveau A, et al. Combined (thalamotomy and stimulation) stereotactic surgery of the Vim thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344-346.
32 Benabid AL, Pollak P, Gao DM, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84:203-214.
33 Benabid AL, Pollak P, Gross C, et al. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Stereotact Funct Neurosurg. 1994;62:76-84.
34 Benabid AL, Ni Z, Chabardes S, et al. How are we inhibiting functional targets with high frequency stimulation? In: Kultas-Ilinsky K, Ilinsky I, editors. Basal Ganglia and Thalamus in Health and Movement Disorders. New York: Plenum Press; 2001:309-315.
35 Meissner W, Leblois A, Hansel D, et al. Subthalamic high frequency stimulation resets subthalamic firing and reduces abnormal oscillations. Brain. 2005;128:2372-2382.
36 Tai CH, Boraud T, Bezard E, et al. Electrophysiological and metabolic evidence that high-frequency stimulation of the subthalamic nucleus bridles neuronal activity in the subthalamic nucleus and the substantia nigra reticulata. FASEB J. 2003;17:1820-1830.
37 Filali M, Hutchison WD, Palter VN, et al. Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp Brain Res. 2004;156:274-281.
38 Paul G, Meissner W, Rein S, et al. Ablation of the subthalamic nucleus protects dopaminergic phenotype but not cell survival in a rat model of Parkinson’s disease. Exp Neurol. 2004;185:272-280.
39 Welter ML, Houeto JL, Bonnet AM, et al. Effects of high-frequency stimulation on subthalamic neuronal activity in parkinsonian patients. Arch Neurol. 2004;61:89-96.
40 Hammond C, Ammari R, Bioulac B, et al. Latest view on the mechanism of action of deep brain stimulation. Mov Disord. 2008;23:2111-2121.
41 Xia R, Berger F, Piallat B, et al. Alteration of hormone and neurotransmitter production in cultured cells by high and low frequency electrical stimulation. Acta Neurochir (Wien). 2007;149:67-73.
42 Lefaucheur JP, Gurruchaga JM, Pollin B, et al. Outcome of bilateral subthalamic nucleus stimulation in the treatment of Parkinson’s disease: correlation with intra-operative multi-unit recordings but not with the type of anaesthesia. Eur Neurol. 2008;60:186-199.
43 Breit S, LeBas JF, Koudsie A, et al. Targeting for the implantation of stimulation electrodes into the subthalamic nucleus: a comparative study of magnetic resonance imaging and ventriculography. Neurosurgery. 2006;58(suppl 1):ONS83-ONS95.
44 Benazzouz A, Breit S, Koudsie A, et al. Intraoperative microrecordings of the subthalamic nucleus in Parkinson’s disease. Mov Disord. 2002;17(suppl 3):S145-S149.
45 Hutchison WD, Allan RJ, Opitz H, et al. Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson’ s disease. Ann Neurol. 1998;44:622-628.
46 Pollak P, Krack P, Fraix V, et al. Intraoperative micro- and macrostimulation of the subthalamic nucleus in Parkinson’s disease. Mov Disord. 2002;17(suppl 3):S155-S161.
47 Alberts JL, Hass CJ, Vitek JL, et al. Are two leads always better than one: an emerging case for unilateral subthalamic deep brain stimulation in Parkinson’s disease. Exp Neurol. 2008;214:1-5.
48 Agostino R, Dinapoli L, Modugno N, et al. Ipsilateral sequential arm movements after unilateral subthalamic deep-brain stimulation in patients with Parkinson’s disease. Mov Disord. 2008;23:1718-1724.
49 Deuschl G, Schade-Brittinger C, Krack P, et al. for the German Parkinson Study Group, Neurostimulation Section. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896-908. Erratum in N Engl J Med. 2006;355:1289
50 Lang AE, Houeto JL, Krack P, et al. Deep brain stimulation: preoperative issues. Mov Disord. 2006;21:S171-S196.
51 Charles PD, Van Blercom N, Krack P, et al. Predictors of effective bilateral subthalamic nucleus stimulation for PD. Neurology. 2002;59:932-934.
52 Fraix V, Pollak P, Van Blercom N, et al. Effect of subthalamic nucleus stimulation on levodopa-induced dyskinesia in Parkinson’s disease. Neurology. 2000;55:1921-1923.
53 Obeso JA, Grandas F, Herrero MT, et al. The role of pulsatile versus continuous dopamine receptor stimulation for functional recovery in Parkinson’s disease. Eur J Neurosci. 1994;6:889-897.
54 Derost PP, Ouchchane L, Morand D, et al. Is DBS-STN appropriate to treat severe Parkinson disease in an elderly population? Neurology. 2007;68:1345-1355.
55 Xie J, Krack P, Benabid AL, et al. Effect of bilateral subthalamic nucleus stimulation on parkinsonian gait. J Neurol. 2001;248:1068-1072.
56 Limousin P, Pollak P, Hoffmann D, et al. Abnormal involuntary movements induced by subthalamic nucleus stimulation in parkinsonian patients. Mov Disord. 1996;11:231-235.
57 Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. Neuroreport. 2005;16:1883-1887.
58 Stefani A, Lozano AM, Peppe A, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130:1596-1607.
59 Gentil M, Chauvin P, Pinto S, et al. Effect of bilateral stimulation of the subthalamic nucleus on parkinsonian voice. Brain Lang. 2001;78:233-240.
60 Gentil M, Pinto S, Pollak P, et al. Effect of bilateral stimulation of the subthalamic nucleus on parkinsonian dysarthria. Brain Lang. 2003;85:190-196.
61 Raix V, Pollak P, Moro E, et al. Subthalamic nucleus stimulation in tremor-dominant parkinsonian patients with previous thalamic surgery. J Neurol Neurosurg Psychiatry. 2005;76:246-248.
62 Goto S, Yamada K, Ushio Y. Subthalamic nucleus stimulation in a parkinsonian patient with previous bilateral thalamotomy. J Neurol Neurosurg Psychiatry. 2004;75:164-165.
63 Anheim M, Batir A, Fraix V, et al. Improvement in Parkinson disease by subthalamic nucleus stimulation based on electrode placement: effects of reimplantation. Arch Neurol. 2008;65:612-616.
64 Deuschl G, Herzog J, Kleiner-Fisman G, et al. Deep brain stimulation: postoperative issues. Mov Disord. 2006;21(suppl 14):S219-S237.
65 Beric A, Kelly PJ, Rezai A, et al. Complications of deep brain stimulation surgery. Stereotact Funct Neurosurg. 2001;77:73-78.
66 Binder DK, Rau G, Starr PA. Hemorrhagic complications of microelectrode-guided deep brain stimulation. Stereotact Funct Neurosurg. 2003;80:28-31.
67 Binder DK, Rau GM, Starr PA. Risk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disorders. Neurosurgery. 2005;56:722-732.
68 Blomstedt P, Hariz MI. Are complications less common in deep brain stimulation than in ablative procedures for movement disorders? Stereotact Funct Neurosurg. 2006;84:72-81.
69 De Salles AA, Frighetto L, Behnke E, et al. Functional neurosurgery in the MRI environment. Minim Invasive Neurosurg. 2004;47:284-289.
70 Hariz MI, Fodstad H. Do microelectrode techniques increase accuracy or decrease risks in pallidotomy and deep brain stimulation? A critical review of the literature. Stereotact Funct Neurosurg. 1999;72:157-169.
71 Kleiner-Fisman G, Herzog J, Fisman DN, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21(suppl 14):S290-S304.
72 Liang GS, Chou KL, Baltuch GH, et al. Long-term outcomes of bilateral subthalamic nucleus stimulation in patients with advanced Parkinson’s disease. Stereotact Funct Neurosurg. 2006;84:221-227.
73 Lyons KE, Wilkinson SB, Overman J, et al. Surgical and hardware complications of subthalamic stimulation: a series of 160 procedures. Neurology. 2004;63:612-616.
74 Umemura A, Jaggi JL, Hurtig HI, et al. Deep brain stimulation for movement disorders: morbidity and mortality in 109 patients. J Neurosurg. 2003;98:779-784.
75 Vesper J, Haak S, Ostertag C, et al. Subthalamic nucleus deep brain stimulation in elderly patients—analysis of outcome and complication. BMC Neurol. 2007;7:7.
76 Sillay KA, Larson PS, Starr PA. Deep brain stimulation hardware-related infections: incidence and management in a large series. Neurosurgery. 2008;62:360-366.
77 Macia F, Perlemoine C, Coman I, et al. Parkinson’s disease patients with bilateral subthalamic deep brain stimulation gain weight. Mov Disord. 2004;19:206-212.
78 Guehl D, Cuny E, Benazzouz A, et al. Side-effects of subthalamic stimulation in Parkinson’s disease: clinical evolution and predictive factors. Eur J Neurol. 2006;13:963-971.
79 Kleiner-Fisman G, Fisman DN, Sime E, et al. Long-term follow up of bilateral deep brain stimulation of the subthalamic nucleus in patients with advanced Parkinson disease. J Neurosurg. 2003;99:489-495.
80 Ostergaard K, Sunde N, Dupont E. Effects of bilateral stimulation of the subthalamic nucleus in patients with severe Parkinson’s disease and motor fluctuations. Mov Disord. 2002;17:693-700.
81 Zhang JG, Zhang K, Ma Y, et al. Follow-up of bilateral subthalamic deep brain stimulation for Parkinson’s disease. Acta Neurochir Suppl. 2006;99:43-47.
82 Houeto JL, Mesnage V, Mallet L, et al. Behavioural disorders, Parkinson’s disease and subthalamic stimulation. J Neurol Neurosurg Psychiatry. 2002;72:701-707.
83 Voon V, Moro E, Saint-Cyr JA, et al. Psychiatric symptoms following surgery for Parkinson’s disease with an emphasis on subthalamic stimulation. Adv Neurol. 2005;96:130-147.
84 Krack P, Fraix V, Mendes A, et al. Postoperative management of subthalamic nucleus stimulation for Parkinson’s disease. Mov Disord. 2002;17(suppl 3):S188-S197.
85 Funkiewiez A, Ardouin C, Caputo E, et al. Long-term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:834-839.
86 Burkhard PR, Vingerhoets FJ, Berney A, et al. Suicide after successful deep brain stimulation for movement disorders. Neurology. 2004;63:2170-2172.
87 Ardouin C, Sibera-Rossignol H. [Subthalamic stimulation, Parkinson’s disease and suicide.]. Lett Neurol. 2006;10:295-298.
88 Voon V, Kubu C, Krack P, et al. Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov Disord. 2006;21(suppl 14):S305-S327.
89 Bostwick JM, Rackley SJ. Completed suicide in medical/surgical patients: who is at risk? Curr Psychiatry Rep. 2007;9:242-246.
90 Foncke EM, Schuurman PR, Speelman JD. Suicide after deep brain stimulation of the internal globus pallidus for dystonia. Neurology. 2006;66:142-143.
91 Kariminia A, Law MG, Butler TG, et al. Suicide risk among recently released prisoners in New South Wales. Australia. Med J Aust. 2007;187:387-390.
92 Lieb K, Schlaepfer TE. Deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:2256.
93 Pratt D, Piper M, Appleby L, et al. Suicide in recently released prisoners: a population-based cohort study. Lancet. 2006;368:119-123.
94 Sarwer DB. The psychological aspects of cosmetic breast augmentation. Plast Reconstr Surg. 2007;120(7 suppl 1):110S-117S.
95 Bejjani BP, Damier P, Arnulf I, et al. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med. 1999;340:1476-1480.
96 Bejjani BP, Houeto JL, Hariz M, et al. Aggressive behavior induced by intraoperative stimulation in the triangle of Sano. Neurology. 2002;59:1425-1427.
97 Krack P, Kumar R, Ardouin C, et al. Mirthful laughter induced by subthalamic nucleus stimulation. Mov Disord. 2001;16:867-875.
98 Herzog J, Reiff J, Krack P, et al. Manic episode with psychotic symptoms induced by subthalamic nucleus stimulation in a patient with Parkinson’s disease. Mov Disord. 2003;18:1382-1384.
99 Romito LM, Raja M, Daniele A, et al. Transient mania with hypersexuality after surgery for high frequency stimulation of the subthalamic nucleus in Parkinson’s disease. Mov Disord. 2002;17:1371-1374.
100 Funkiewiez A, Ardouin C, Krack P, et al. Acute psychotropic effects of bilateral subthalamic nucleus stimulation and levodopa in Parkinson’s disease. Mov Disord. 2003;18:524-530.
101 Czernecki V, Pillon B, Houeto JL, et al. Does bilateral stimulation of the subthalamic nucleus aggravate apathy in Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2005;76:775-779.
102 Krack P, Pollak P, Limousin P, et al. Subthalamic nucleus or internal pallidal stimulation in young onset Parkinson’s disease. Brain. 1998;121:451-457.
103 Benabid AL, Chabardes S, Seigneuret E. Deep-brain stimulation in Parkinson’s disease: long-term efficacy and safety—What happened this year? Curr Opin Neurol. 2005;18:623-630.
104 Saint-Cyr JA, Trépanier LL, Kumar R, et al. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson’s disease. Brain. 2000;123:2091-2108.
105 Parsons TD, Rogers SA, Braaten AJ, et al. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson’s disease: a meta-analysis. Lancet Neurol. 2006;5:578-588.
106 Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309-1312.
107 Ardouin C, Pillon B, Peiffer E, et al. Bilateral subthalamic or pallidal stimulation for Parkinson’s disease affects neither memory nor executive functions: a consecutive series of 62 patients. Ann Neurol. 1999;46:217-223.
108 Dujardin K, Defebvre L, Krystkowiak P, et al. Influence of chronic bilateral stimulation of the subthalamic nucleus on cognitive function in Parkinson’s disease. J Neurol. 2001;248:603-611.
109 Pillon B, Ardouin C, Damier P, et al. Neuropsychological changes between “off” and “on” STN or GPi stimulation in Parkinson’s disease. Neurology. 2000;55:411-418.
110 Woods SP, Fields JA, Troster AI. Neuropsychological sequelae of subthalamic nucleus deep brain stimulation in Parkinson’s disease: a critical review. Neuropsychol Rev. 2002;12:111-126.
111 Castelli L, Perozzo P, Caglio M, et al. Does subthalamic stimulation induce personality modifications in Parkinson’s disease? A Rorschach Test explorative study. Acta Neurol Belg. 2008;108:5-8.
112 Rodriguez-Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow up. Brain. 2005;128:2240-2249.
113 Videnovic A, Verhagen Metman L. Deep brain stimulation for Parkinson’s disease: prevalence of adverse events and need for standardized reporting. Mov Disorders. 2008;23:343-349.
114 Rothlind JC, Cockshott RW, Starr PA, et al. Neuropsychological performance following staged bilateral pallidal or subthalamic nucleus deep brain stimulation for Parkinson’s disease. J Int Neuropsychol Soc. 2007;13:68-79.
115 Deep-Brain Stimulation for Parkinson’s Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345:956-963.
116 Alegret M, Junque C, Valldeoriola F, et al. Effects of bilateral subthalamic stimulation on cognitive function in Parkinson disease. Arch Neurol. 2001;58:1223-1227.
117 Aybek S, Gronchi-Perrin A, Verney A, et al. Long-term cognitive profile and incidence of dementia after STN-DBS in Parkinson’s disease. Mov Disord. 2007;22:974-981.
118 Castelli L, Perozzo P, Zibetti M, et al. Chronic deep brain stimulation of the subthalamic nucleus for Parkinson’s disease: effects on cognition, mood, anxiety and personality traits. Eur Neurol. 2006;55:136-144.
119 Contarino MF, Daniele A, Sibilia AH, et al. Cognitive outcome 5 years after bilateral chronic stimulation of subthalamic nucleus in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:248-252.
120 Fields JA, Troster AI. Cognitive outcomes after deep brain stimulation for Parkinson’s disease: a review of initial studies and recommendations for future research. Brain Cogn. 2000;42:268-293.
121 Hariz MI. Complications of deep brain stimulation surgery. Mov Disord. 2002;17(suppl 3):S162-166.
122 Miyawaki E, Perlmutter JS, Tröster AI, et al. The behavioral complications of pallidal stimulation: a case report. Brain Cogn. 2000;42:417-434.
123 Morrison CE, Borod JC, Perrine K, et al. Neuropsychological functioning following bilateral subthalamic nucleus stimulation in Parkinson’s disease. Arch Clin Neuropsychol. 2004;19:165-181.
124 Pillon B, Ardouin C, Dujardin K, et al. Preservation of cognitive function in dystonia treated by pallidal stimulation. Neurology. 2006;66:1556-1558.
125 Trépanier LL, Kumar R, Lozano AM, et al. Neuropsychological outcome of GPi pallidotomy and GPi or STN deep brain stimulation in Parkinson’s disease. Brain Cogn. 2000;42:324-347.
126 Hamel W, Schrader B, Weinert D, et al. Technical complication in deep brain stimulation. Zentralbl Neurochir. 2002;63:124-127.
127 Okun MS, Tagliati M, Pourfar M, et al. Management of referred deep brain stimulation failures: a retrospective analysis from 2 movement disorders centers. Arch Neurol. 2005;62:1250-1255.
128 Amirnovin R, Williams ZM, Cosgrove GR, et al. Experience with microelectrode guided subthalamic nucleus deep brain stimulation. Neurosurgery. 2006;58(suppl):S96-102.
129 Blomstedt P, Hariz MI. Hardware-related complications of deep brain stimulation: a ten year experience. Acta Neurochir (Wien). 2005;147:1061-1064.
130 Lezcano E, Gomez-Esteban JC, Zarranz JJ, et al. Improvement in quality of life in patients with advanced Parkinson’s disease following bilateral deep-brain stimulation in subthalamic nucleus. Eur J Neurol. 2004;11:451-454.
131 Constantoyannis C, Berk C, Honey CR, et al. Reducing hardware-related complications of deep brain stimulation. Can J Neurol Sci. 2005;32:194-200.
132 Hariz MI, Johansson F. Hardware failure in parkinsonian patients with chronic subthalamic nucleus stimulation is a medical emergency. Mov Disord. 2001;16:166-168.
133 Machado AG, Hiremath GK, Salazar F, et al. Fracture of subthalamic nucleus deep brain stimulation hardware as a result of compulsive manipulation: case report. Neurosurgery. 2005;57:E1318.
134 Paluzzi A, Belli A, Bain P, et al. Operative and hardware complications of deep brain stimulation for movement disorders. Br J Neurosurg. 2006;20:290-295.
135 Coubes P, Vayssiere N, El Fertit H, et al. Deep brain stimulation for dystonia. Surgical technique. Stereotact Funct Neurosurg. 2002;78:183-191.
136 Gill CE, Konrad PE, Davis TL, et al. Deep brain stimulation for Parkinson’s disease: the Vanderbilt University Medical Center experience, 1998-2004. Tenn Med. 2007;100:45-47.
137 Gorgulho A, De Salles AA, Frighetto L, et al. Incidence of hemorrhage associated with electrophysiological studies performed using macroelectrodes and microelectrodes in functional neurosurgery. J Neurosurg. 2005;102:888-896.
138 Gross RE, Krack P, Rodriguez-Oroz MC, et al. Electrophysiological mapping for the implantation of deep brain stimulators for Parkinson’s disease and tremor. Mov Disord. 2006;21(suppl 14):S259-S283.
139 Kenney C, Simpson R, Hunter C, et al. Short-term and long-term safety of deep brain stimulation in the treatment of movement disorders. J Neurosurg. 2007;106:621-625.
140 Kupsch A, Benecke R, Müller J, et al. for the Deep Brain Stimulation for Dystonia Study Group. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978-1990.
141 Novak KE, Nenonene EK, Bernstein LP, et al. Two cases of ischemia associated with subthalamic nucleus stimulator implantation for advanced Parkinson’s disease. Mov Disord. 2006;21:1477-1483.
142 Oh MY, Abosch A, Kim SH, et al. Long-term hardware-related complications of deep brain stimulation. Neurosurgery. 2002;50:1268-1276.
143 Temel Y, Ackermans L, Celik H, et al. Management of hardware infections following deep brain stimulation. Acta Neurochir (Wien). 2004;146:355-361.
144 Tir M, Devos D, Blond S, et al. Exhaustive, one year follow up of subthalamic nucleus deep brain stimulation in a large, single-center cohort of parkinsonian patients. Neurosurgery. 2007;61:297-304.
145 Voges J, Waerzeggers Y, Maarouf M, et al. Deep-brain stimulation: long-term analysis of complications caused by hardware and surgery experiences from a single centre. J Neurol Neurosurg Psychiatry. 2006;77:868-872.
146 Voges J, Hilker R, Bötzel K, et al. Thirty days complication rate following surgery performed for deep-brain-stimulation. Mov Disord. 2007;22:1486-1489.
147 Seijo FJ, Alvarez-Vega MA, Gutierrez JC, et al. Complications in subthalamic nucleus stimulation surgery for treatment of Parkinson’s disease. Review of 272 procedures. Acta Neurochir (Wien). 2007;149:867-875.
148 Alesch F. Sudden failure of dual channel pulse generators. Mov Disord. 2005;20:64-66.
149 Hamani C, Lozano AM. Hardware-related complications of deep brain stimulation: a review of the published literature. Stereotact Funct Neurosurg. 2006;84:248-251.
150 Joint C, Nandi D, Parkin S, et al. Hardware-related problems of deep brain stimulation. Mov Disord. 2002;17(suppl 3):S175-S180.
151 Rodriguez-Oroz MC, Zamarbide I, Guridi J, et al. Efficacy of deep brain stimulation of the subthalamic nucleus in Parkinson’s disease 4 years after surgery: double blind and open label evaluation. J Neurol Neurosurg Psychiatry. 2004;75:1382-1385.
152 Hamani C, Richter E, Schwalb JM, et al. Bilateral subthalamic nucleus stimulation for Parkinson’s disease: a systematic review of the clinical literature. Neurosurgery. 2005;56:1313-1321.
153 Tommasi G, Krack P, Fraix V, et al. Pyramidal tract side effects induced by deep brain stimulation of the subthalamic nucleus. J Neurol Neurosurg Psychiatry. 2008;79:813-819.
154 Anheim M, Fraix V, Chabardès S, et al. Lifetime of Itrel II pulse generators for subthalamic nucleus stimulation in Parkinson’s disease. Mov Disord. 2007;22:2436-2439.
155 LePen C, Wait S, Moutard-Martin F, et al. Cost of illness and disease severity in a cohort of French patients with Parkinson’s disease. Pharmacoeconomics. 1999;16:59-69.
156 Lang AE, Fahn S. Assessment of Parkinson’s disease. In: Munsat TL, editor. Quantification of Neurological Deficit. Stonehame, MA: Butterworths; 1989:285-309.
157 England ACJr, Schwab RS. Postoperative medical evaluation of 26 selected patients with Parkinson’s disease. J Am Geriatr Soc. 1956;4:1219-1232.
158 Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427-442.
159 Fraix V, Houeto JL, Lagrange C, et al. Clinical and economic results of bilateral subthalamic nucleus stimulation in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:443-449.
160 Goodman RR, Kim B, McClelland S3rd, et al. Operative techniques and morbidity with subthalamic nucleus deep brain stimulation in 100 consecutive patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:12-17.
161 Houeto JL, Damier P, Bejjani PB, et al. Subthalamic stimulation in Parkinson disease: a multidisciplinary approach. Arch Neurol. 2000;57:461-465.
162 Krack P, Limousin-Dowsey P, Benabid AL, et al. Chronic stimulation of subthalamic nucleus improves levodopa-induced dyskinesias in Parkinson’s disease. Lancet. 1997;350:1676.
163 Kumar R, Lozano AM, Kim YJ, et al. Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Neurology. 1998;51:850-855.
164 Lopiano L, Rizzone M, Bergamasco B, et al. Deep brain stimulation of the subthalamic nucleus: clinical effectiveness and safety. Neurology. 2001;56:552-554.
165 Østergaard KA, Sunde N. Evolution of Parkinson’s disease during 4 years of bilateral deep brain stimulation of the subthalamic nucleus. Mov Disord. 2006;21:624-631.
166 Pahwa R, Wilkinson SB, Overman J, et al. Bilateral subthalamic stimulation in patients with Parkinson disease: long-term follow up. J Neurosurg. 2003;99:71-77.
167 Peto V, Jenkinson C, Fitzpatrick R, et al. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res. 1995;4:241-248.
168 Pinter MM, Alesch F, Murg M, et al. Deep brain stimulation of the subthalamic nucleus for control of extrapyramidal features in advanced idiopathic Parkinson’s disease: one year follow-up. J Neural Transm. 1999;106:693-709.
169 Simuni T, Jaggi JL, Mulholland H, et al. Bilateral stimulation of the subthalamic nucleus in patients with Parkinson disease: a study of efficacy and safety. J Neurosurg. 2002;96:666-672.
170 Volkmann J, Allert N, Voges J, et al. Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology. 2001;56:548-551.
171 Herzog J, Volkmann J, Krack P, et al. Two-year follow-up of subthalamic deep brain stimulation in Parkinson’s disease. Mov Disord. 2003;18:1332-1337.
172 Vingerhoets FJ, Villemure JG, Temperli P, et al. Subthalamic DBS replaces levodopa in Parkinson’s disease: two-year followup. Neurology. 2002;58:396-401.
173 Visser-Vandewalle V, van der Linden C, Temel Y, et al. Long-term effects of bilateral subthalamicnucleus stimulation in advanced Parkinson disease: a four year follow-up study. Parkinsonism Relat Disord. 2005;11:157-165.
174 Bejjani BP, Arnulf I, Demeret S, et al. Levodopa-induced dyskinesias in Parkinson’s disease: is sensitization reversible? Ann Neurol. 2000;47:655-658.
175 Krack P, Pollak P, Limousin P, et al. From off-period dystonia to peak-dose chorea. The clinical spectrum of varying subthalamic nucleus activity. Brain. 1999;122:1133-1146.
176 Moro E, Esselink RJ, Benabid AL, et al. Response to levodopa in parkinsonian patients with bilateral subthalamic nucleus stimulation. Brain. 2002;125:2408-2417.
177 Pinto S, Gentil M, Krack P, et al. Changes induced by levodopa and subthalamic nucleus stimulation on parkinsonian speech. Mov Disord. 2005;20:1507-1515.
178 Arnulf I, Bejjani BP, Garma L, et al. Improvement of sleep architecture in PD with subthalamic nucleus stimulation. Neurology. 2000;55:1732-1735.
179 Hjort N, Ostergaard K, Dupont E. Improvement of sleep quality in patients with advanced Parkinson’s disease treated with deep brain stimulation of the subthalamic nucleus. Mov Disord. 2004;19:196-199.
180 Finazzi-Agro E, Peppe A, D’Amico A, et al. Effects of subthalamic nucleus stimulation on urodynamic findings in patients with Parkinson’s disease. J Urol. 2003;169:1388-1391.
181 Seif C, Herzog J, van der Horst C, et al. Effect of subthalamic deep brain stimulation on the function of the urinary bladder. Ann Neurol. 2004;55:118-120.
182 Hilker R, Portman AT, Voges J, et al. Disease progression continues in patients with advanced Parkinson’s disease and effective subthalamic nucleus stimulation. J Neurol Neurosurg Psychiatry. 2005;76:1217-1221.
183 Diamond A, Jankovic J. The effect of deep brain stimulation on quality of life in movement disorders. J Neurol Neurosurg Psychiatry. 2005;76:1188-1193.
184 Lagrange E, Krack P, Moro E, et al. Bilateral subthalamic nucleus stimulation improves health-related quality of life in PD. Neurology. 2002;59:1976-1978.
185 Lang AE, Kleiner-Fisman G, Saint-Cyr JA, et al. Subthalamic DBS replaces levodopa in Parkinson’s disease: two-year followup. Neurology. 2003;60:154-155.
186 Bastian AJ, Kelly VE, Revilla FJ, et al. Different effects of unilateral versus bilateral subthalamic nucleus stimulation on walking and reaching in Parkinson’s disease. Mov Disord. 2003;18:1000-1007.
187 Samii A, Kelly VE, Slimp JC, et al. Staged unilateral versus bilateral subthalamic nucleus stimulator implantation in Parkinson disease. Mov Disord. 2007;22:1476-1481.
188 Chen L, Liu Z, Tian Z, et al. Prevention of neurotoxin damage of 6-OHDA to dopaminergic nigral neuron by subthalamic nucleus lesions. Stereotact Funct Neurosurg. 2000;75:66-75.
189 Maesawa S, Kaneoke Y, Kajita Y, et al. Long-term stimulation of the subthalamic nucleus in hemiparkinsonian rats: neuroprotection of dopaminergic neurons. J Neurosurg. 2004;100:679-687.
190 Nakao N, Nakai E, Nakai K, et al. Ablation of the subthalamic nucleus supports the survival of nigral dopaminergic neurons after nigrostriatal lesions induced by mitochondrial toxin 3-nitropropionic acid. Ann Neurol. 1999;45:640-651.
191 Piallat B, Benazzouz A, Benabid AL. Subthalamic nucleus lesion in rats prevents dopaminergic nigral neuron degeneration after striatal 6-OHDA injection: behavioural and immunohistochemical studies. Eur J Neurosci. 1996;8:1408-1414.
192 Piallat B, Benazzouz A, Benabid AL. Neuroprotective effect of chronic inactivation of the subthalamic nucleus in a rat model of Parkinson’s disease. J Neural Transm Suppl. 1999;55:71-77.
193 Javoy-Agid F, Hirsch EC, Dumas S, et al. Decreased tyrosine hydroxylase messenger RNA in the surviving dopamine neurons of the substantia nigra in Parkinson’s disease: an in situ hybridization study. Neuroscience. 1990;38:245-253.
194 Tass PA, Hauptmann C. Therapeutic modulation of synaptic connectivity with desynchronizing brain stimulation. Int J Psychophysiol. 2007;64:53-61.
195 Munro-Davies LE, Winter J, Aziz TZ, et al. The role of the pedunculopontine region in basal-ganglia mechanisms of akinesia. Exp Brain Res. 1999;129:511-517.
196 Mazzone P, Lozano A, Stanzione P, et al. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson’s disease. Neuroreport. 2005;16:1877-1881.
197 Yelnik J. PPN or PPD, what is the target for deep brain stimulation in Parkinson’s disease? Brain. 2007;130:e79.
198 Zrinzo L, Zrinzo LV, Hariz M. The peripeduncular nucleus: a novel target for deep brain stimulation? Neuroreport. 2007;18:1301-1302.
199 Zrinzo L, Zrinzo LV, Tisch S, et al. Stereotactic localization of the human pedunculopontine nucleus: atlas-based coordinates and validation of a magnetic resonance imaging protocol for direct localization. Brain. 2008;131:1588-1598.
200 Nandi D, Aziz TZ, Giladi N, et al. Reversal of akinesia in experimental parkinsonism by GABA antagonist microinjections in the pedunculopontine nucleus. Brain. 2002;125:2418-2430.
201 Luo J, Kaplitt MG, Fitzsimons HL, et al. Subthalamic GAD gene therapy in a Parkinson’s disease rat model. Science. 2002;298:425-429.
202 Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097-2105.
203 Marks WJJr, Ostrem JL, Verhagen L, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400-408.
204 Pollak P, Champay AS, Hommel M, et al. Subcutaneous apomorphine in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1989;52:544.
205 Stibe C, Lees A, Stern G. Subcutaneous infusion of apomorphine and lisuride in the treatment of parkinsonian on-off fluctuations. Lancet. 1987;1:871.
206 Johnston TH, Fox SH, Brotchie JM. Advances in the delivery of treatments for Parkinson’s disease. Expert Opin Drug Deliv. 2005;2:1059-1073.
207 Gill SS, Patel NK, Hotton GR, et al. Direct brain infusion of glial cell line–derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589-595.
208 Love S, Plaha P, Patel NK, et al. Glial cell line–derived neurotrophic factor induces neuronal sprouting in human brain. Nat Med. 2005;11:703-704.
209 Patel NK, Bunnage M, Plaha P, et al. Intraputamenal infusion of glial cell line–derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol. 2005;57:298-302.
210 Lang AE, Gill S, Patel NK, et al. Randomized controlled trial of intraputamenal glial cell line–derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459-466. Erratum in Ann Neurol. 2006;60:747
211 Canavero S, Bonicalzi V. Extradural cortical stimulation for movement disorders. Acta Neurochir Suppl. 2007;97:223-232.
212 Pagni CA, Albanese A, Bentivoglio A, et al. Results by motor cortex stimulation in treatment of focal dystonia, Parkinson’s disease and post-ictal spasticity. The experience of the Italian Study Group of the Italian Neurosurgical Society. Acta Neurochir Suppl. 2008;101:13-21.
213 Drouot X, Oshino S, Jarraya B, et al. Functional recovery in a primate model of Parkinson’s disease following motor cortex stimulation. Neuron. 2004;44:769-778.
214 Morizane A, Li JY, Brundin P. From bench to bed: the potential of stem cells for the treatment of Parkinson’s disease. Cell Tissue Res. 2008;331:323-336.

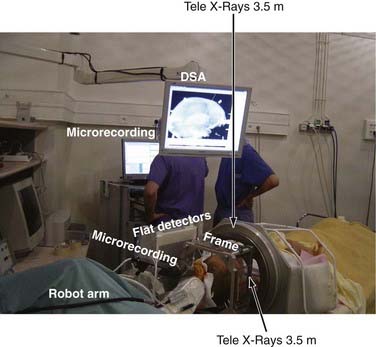
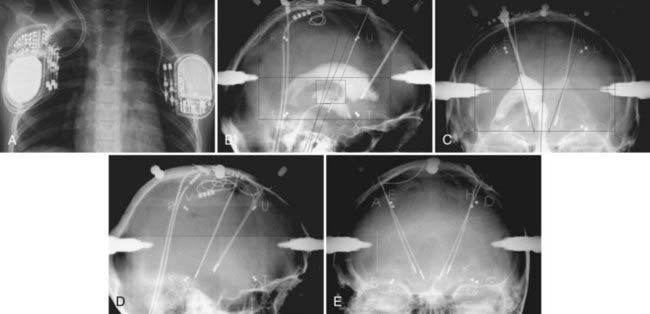
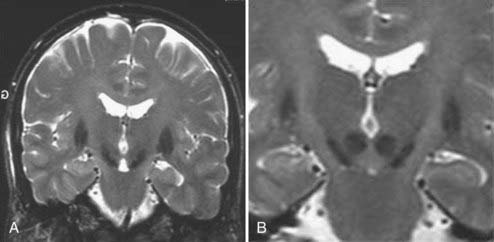
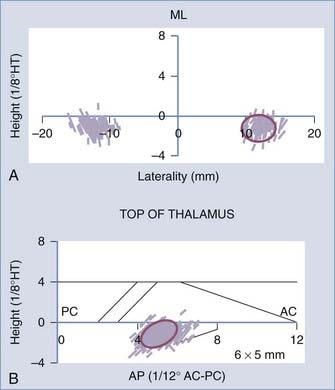
 ° the height of the thalamus. B, Lateral view. The horizontal axis represents the distance from the posterior commissure (PC) and is
° the height of the thalamus. B, Lateral view. The horizontal axis represents the distance from the posterior commissure (PC) and is  the length of anterior commissure (AC)-PC; the vertical axis units are in
the length of anterior commissure (AC)-PC; the vertical axis units are in  ° the height of the thalamus. The STN target extends over the middle third of AC-PC, below the line. On the AP view, the average laterality is 12 mm and the STN extends from 11 to 13 mm. On this scheme are projected the image of the average target (black square) and the limits of the standard deviations around the average target (rectangle). On the lateral view is represented the trajectory whose theoretical angle with the AC-PC plane is 63.57 ± 2.34 degrees according to the Guiot scheme; it is actually 63.11 ± 3.87 degrees on the right side and 62.81 ± 4.59 degrees on the left side. The globus pallidus interna target is situated in the anterior third of the AC-PC, from
° the height of the thalamus. The STN target extends over the middle third of AC-PC, below the line. On the AP view, the average laterality is 12 mm and the STN extends from 11 to 13 mm. On this scheme are projected the image of the average target (black square) and the limits of the standard deviations around the average target (rectangle). On the lateral view is represented the trajectory whose theoretical angle with the AC-PC plane is 63.57 ± 2.34 degrees according to the Guiot scheme; it is actually 63.11 ± 3.87 degrees on the right side and 62.81 ± 4.59 degrees on the left side. The globus pallidus interna target is situated in the anterior third of the AC-PC, from  + the height of the thalmus (HT) above to
+ the height of the thalmus (HT) above to  + of HT below the AC-PC plane. Laterality extends 15 to 22 mm from the midline. The ventral intermediate nucleus is 14 mm from the midline (ML) and is about
+ of HT below the AC-PC plane. Laterality extends 15 to 22 mm from the midline. The ventral intermediate nucleus is 14 mm from the midline (ML) and is about  ° to
° to  ° of the AC-PC distance ahead of the PC.
° of the AC-PC distance ahead of the PC.