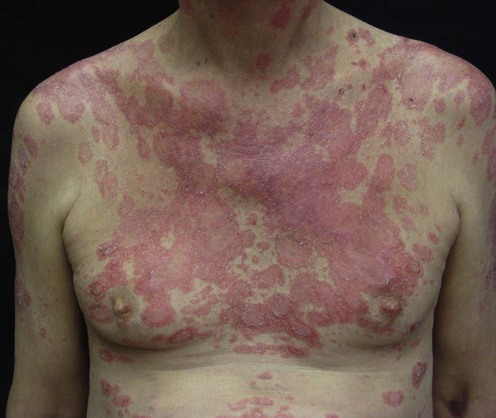
Subacute cutaneous lupus erythematosus
Specific investigations
First-line therapies
Second-line therapies
Third-line therapies
Regression of subacute cutaneous lupus erythematosus in a patient with rheumatoid arthritis treated with a biologic tumor necrosis factor alpha-blocking agent: comment on the article by Pisetsky and the letter from Aringer et al.
Fautrel B, Foltz V, Frances C, Bourgeois P, Rozenberg S. Arthritis Rheum 2002; 46: 1408–9.




 Cosmetics
Cosmetics Sunscreens and protective clothing
Sunscreens and protective clothing Corticosteroids
Corticosteroids Antimalarials
Antimalarials Topical retinoids
Topical retinoids Topical tacrolimus or pimecrolimus
Topical tacrolimus or pimecrolimus Dapsone
Dapsone Gold
Gold Thalidomide
Thalidomide Retinoids
Retinoids Immunosuppressive agents: methotrexate, azathioprine, mycophenolate mofetil
Immunosuppressive agents: methotrexate, azathioprine, mycophenolate mofetil Clofazimine
Clofazimine Phenytoin
Phenytoin High-dose intravenous immunoglobulin
High-dose intravenous immunoglobulin Cytokine therapy
Cytokine therapy Rituximab
Rituximab Ustekinumab
Ustekinumab Leflunomide
Leflunomide