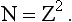STRUCTURE OF THE RESPIRATORY SYSTEM, RELATED TO FUNCTION
Introduction
We will first describe the airways of the lung and then the tissues that surround them.
The upper airways
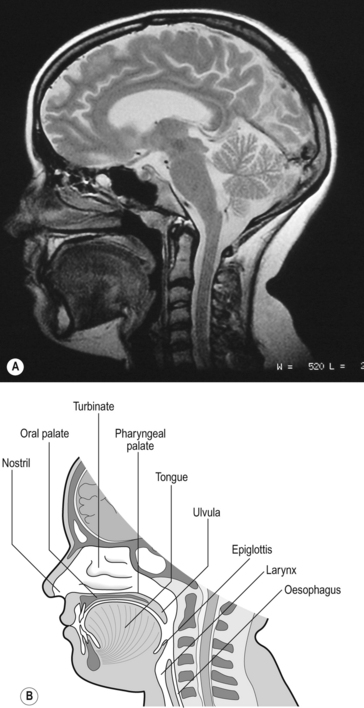
The structures of the upper airways are clearly seen in a paramedial sagittal section of the head and neck (Fig. 2.1).
Mouth and nose – rhinitis, the common cold and obstructive sleep apnoea
Much more sinister and life-threatening than rhinitis is obstructive sleep apnoea (OSA; apnoea = absence of breathing). This should not be confused with central sleep apnoea, where the patient ceases to make respiratory efforts while they are sleeping. In OSA the patient’s attempts to breathe are physically obstructed by anatomical and physiological peculiarities of the upper airways.
In Figure 2.1 the subject is breathing through his nose because the lips are closed and the tongue lies against the palate. When you breathe through the mouth – for example when you blow out a candle or suck through a straw – the soft palate is arched upward to form a seal against Passavant’s ridge at the top of the pharynx. This form of airways obstruction is a normal function. Similarly, under normal circumstances, the genioglossus muscle of the tongue has a high resting tone in conscious subjects, and this holds the tongue forward, preventing it from obstructing the airway. During sleep, and particularly in those suffering from the dangerous condition of obstructive sleep apnoea, the tongue falls against the back wall of the pharynx and obstructs breathing. The muscle tone of the pharynx itself becomes reduced, particularly during REM (rapid eye movement) sleep and in OSA the pharynx collapses under the negative pressure of inspiration. Blocking of the airways by the tongue also and almost inevitably occurs during general anaesthesia and requires immediate attention from the anaesthetist.
In humans the nose extends from the nostrils (external nares) to the choanae (internal nares), which empty into the nasal part of the pharynx. Each nostril narrows to form its nasal valve, and at this level the total cross-sectional area of the airways is narrower (3 mm2) than anywhere else in the system. This narrowing imposes the majority of the high resistance to airflow found in the nose (see Chapter 5) and, combined with the sharp turn the inspiratory air must make as it enters the wide (140 mm2) lumen of the cavum of the nose, causes turbulence. The walls of the nasal cavum are rigid bone projecting out into the airway from the lateral walls as the turbinates. These have a large surface area (150 cm2) covered by vascular mucosal erectile tissue important in the ‘air-conditioning’ activities of the nose. This mucosal tissue can swell considerably in conditions such as rhinitis (described above), and it is here that nasal decongestants such as oxymetazoline, an agonist of α adrenergic receptors on vascular smooth muscle, act to clear a blocked nose by causing the vascular smooth muscle to contract.
The major function of the upper airway is to air-condition the inspirate. It is not essential to breathe through the nose to do this, and the mouth will make a fairly good job of warming and humidifying inhaled air before it reaches the larynx. However, the mouth has not evolved for that purpose and the unpleasant consequences of using it are well known to anyone who has had to breathe through their mouth because a cold has obstructed their nasal airways.
The larynx – intubation of the airways
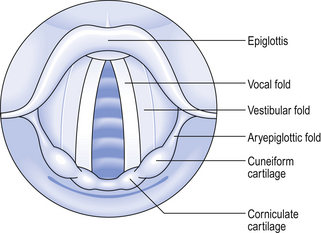
The larynx (see Fig. 2.1) is in fact a rather complicated box made up of plates of cartilage. It can be closed off by drawing together the two curtains of muscle which make up the vocal folds across the lumen of the larynx. Effective coughs depend on the closure and rapid opening of these ‘curtains’, which under less extreme circumstances are used to produce and modify the sounds that make up speech. The vocal folds can be drawn together so strongly that they are airtight against the greatest efforts to breathe the subject can make. This is clearly a ‘bad thing’ and can occur accidentally when an anaesthetist is trying to get an endotracheal tube into a patient’s trachea. This dangerous closing of the larynx is called laryngospasm. A picture of what an anaesthetist would see when approaching the larynx is shown in Fig. 2.2.
Bronchoscopy
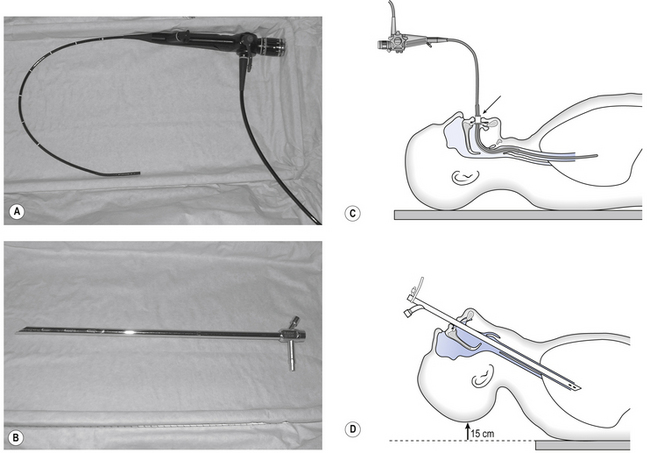
It is frequently useful to inspect the airways below the larynx. First the trachea (part of which is extrathoracic), and then the intrathoracic airways. The instrument used for this is called a bronchoscope and may be of the rigid ‘open tube’ type through which the airways are inspected, or the flexible fibreoptic variety (Fig. 2.3) which, as well as providing a view of the inside of the airways through its fibreoptic system, contains channels through which a variety of sampling and surgical instruments may be passed. Each type of bronchoscope has its advantages, but 95% of bronchoscopic procedures carried out these days are fibreoptic. Biopsy forceps, brushes and needles, balloon catheters and laser fibres can all now be passed through flexible bronchoscopes to carry out procedures after an initial inspection of even very small intrathoracic airways.
The intrathoracic airways
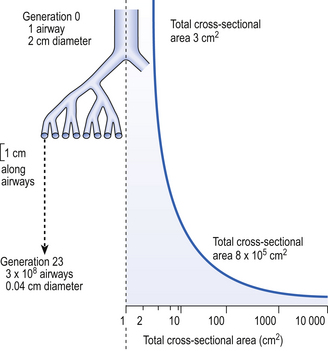
The airways of the lungs are often referred as the bronchial tree, and casts in which the airways are filled with plastic material and then the tissues dissolved away look like a deciduous tree in winter. The branches of this ‘tree’ can be represented in diagrammatic form as the ‘generations’ of a family tree (Fig. 2.4). In some bronchitic patients secretions sometimes fill small airways, solidify, and are coughed up as small ‘casts’ of part of this ‘tree’.
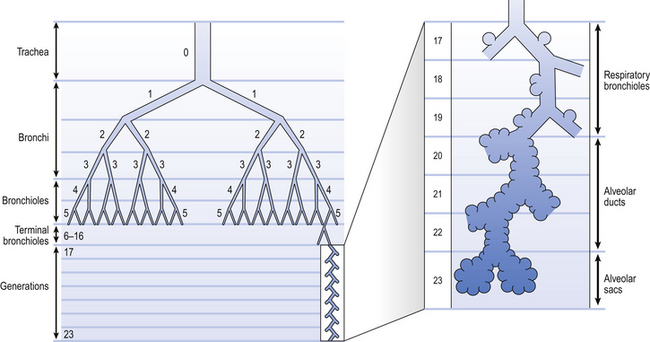
Fig. 2.4 The naming of airways. There is of course a gradual change in structure from one type of airway to another. One particular type of airway can occur at different distances into the lungs. (After Weibel, 1963)
The trachea is the first and largest of about 23 generations of airways. The airways of each generation arise from the previous one by a system of irregular dichotomous branching airways. Dichotomous because each ‘mother’ airway gives rise to two ‘daughter’ airways, and irregular because the daughters, although smaller than the mother, are not necessarily of equal size. The naming of these generations is illustrated in Figure 2.4, from which it may not be obvious that the number of airways (N) in a generation (Z), (counting the single trachea as generation 0) is:
The effect of dichotomous branching of individual airways on the total cross-sectional area (the sum of the cross-sectional areas of all the airways at that level) is remarkable and is shown in Figure 2.5. Notice that ‘Total cross-sectional area’ is measured on a log scale, and so this value increases much more than it appears to in the figure.
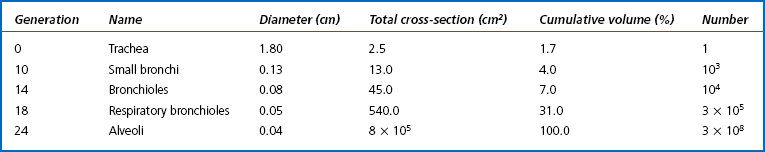
The functional consequences of this increase are profound because it causes the velocity of the air to fall rapidly as it moves into the lung. This effect is discussed in more detail in Chapter 5. The dimensions of some of the airways that make up the bronchial tree are given in Table 2.1.
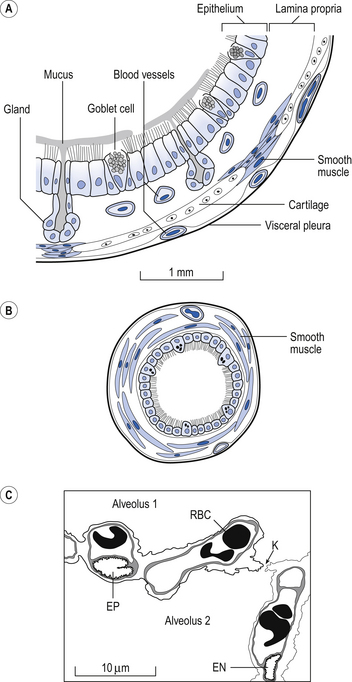
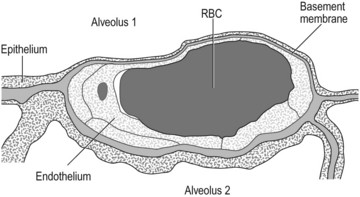
As you go deeper into the lung the transitional and respiratory generations of the airways bear more and more alveoli until the alveolar sacs are totally made up of them. Alveoli do not look like the bunches of grapes or balloons stylistically represented in many textbooks, but rather pock-marked cavities with holes (pores of Kohn, K in Fig. 2.7C) between many adjacent alveoli and with macrophages wandering over their surface ready to engulf and digest foreign particles (see Figs 2.6, 2.17).
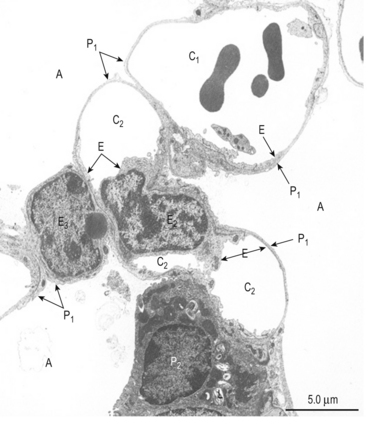
Fig. 2.6 Scanning electron micrograph of an alveolus. A, alveolus; C1, C2, C3, capillaries; E, endothelial cell; P1, type I pneumocytes; P2 type II pneumocyte; L, lamellar bodies. From Young and Heath 2000.
Histological structure of the airways
The microscopic structure of the wall of the airways changes as you go deeper into the lungs. Three ‘snapshots’ of airway wall structure are shown in Figure 2.7 but of course the structure changes gradually from generation to generation.
• The inner mucosal surface consists of ciliated epithelium and underlying mucus-secreting goblet cells. The activity of the cilia and the secretions of the globlet cells make up the mucociliary escalator (see Air-conditioning, below), which is important in removing inhaled particles in the lungs.
• Outside the mucosal layer comes a smooth muscle layer in which the fibres are in continuous bundles. This smooth muscle is found in decreasing amounts from the largest airways right down to the entrances to the alveoli.
• The outermost layer is of connective tissue, which in the large bronchi contains supporting cartilage. As the airways penetrate the lung they first lose their cartilage support and smooth muscle occupies a greater percentage of the airway wall. Then the ciliated epithelium becomes the squamous type, finally forming the respiratory region of the lung.
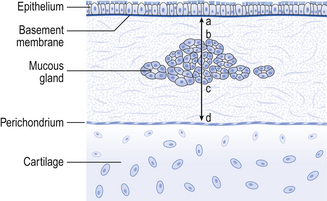
Bronchitis and the Reid Index
The arrangement representing bronchial structure illustrated in Figure 2.7 and described above is modified in chronic bronchitis in a way that provides a histopathological quantitative diagnosis of the disease. The Reid Index provides a measure of proportion of bronchial glands to total wall thickness (Fig. 2.8). In normal lungs mucous glands occupy less than 40% of total wall thickness. In chronic bronchitis this proportion is altered by hyperplasia of the glands. A characteristic of chronic bronchitis is an increase in the products of these glands.
The respiratory region
One of the characteristics of the respiratory surface of any animal is that it should be thin, offering minimal separation between the outside medium (air or water) and the blood. This is beautifully demonstrated in the lungs, which are the only place in our body where blood capillaries come into direct contact with the outside air, as a result of the fusion of the type I epithelial cells (which make up about 95% of the lining of the respiratory zone; Fig. 2.6) with the pulmonary capillary endothelium. This fusion results in an ultrathin layer ideal for the diffusion of gas but not much good for support. Evolution has resulted in this thinning occurring on only one side of the pulmonary capillaries, whereas the cells on the other side remain separate and more robust, supporting the capillary in its place (Fig. 2.9).
The rounded type II cells, much less numerous than type I and found at the junctions of alveolar septa, are the stem cells from which type II epithelial cells are formed. They are also important in producing lung surfactant (see Chapter 3).
Blood vessels
The pulmonary circulation only offers one-sixth of the resistance to blood flow that the systemic circulation offers. It is therefore a low-pressure system and this is reflected in the thin walls of its arteries. These arteries follow the airways through the lungs in connective tissue sheaths. The pulmonary arterioles are also very different from systemic arterioles, having very little smooth muscle in their walls. This absence of smooth muscle in the arterioles, and of course the capillaries and venules, persuades many scientists to consider the microcirculation of the lungs as a whole, rather than making a special case of the capillaries, which snake along several alveolar walls, one after the other, before reaching the venules. Venules join to form veins which, unlike the arteries, do not travel with the airways but make their own way along the septa that separate the segments of the lung. The airways and pulmonary blood vessels down as far as the terminal bronchioles receive their nutrition from the bronchial circulation which, as part of the systemic circulation, is distinct from the pulmonary circulation of the lungs. Part of the bronchial circulation returns to the systemic venous system in the normal way, but part drains into the pulmonary veins, ‘contaminating’ their oxygenated blood with deoxygenated blood. This situation constitutes a ‘shunt’ (see Chapter 7, p. 97).
Pulmonary hypertension
The clinical features of pulmonary hypertension are mainly the result of the increased pressure, producing oedema in the lung and imposing a pumping load on the right heart which it has not evolved to cope with. The patient complains of chest pain, dyspnoea and fatigue. Heart sounds are modified and the ECG demonstrates right ventricular hypertrophy.
The lymphatics
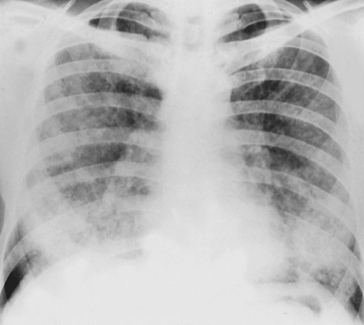
The perivascular spaces of the alveolar wall are drained by lymph vessels. The lymph system of the lungs begins as tiny blind-ended vessels just above the alveoli. These join to form lymphatics in close approximation to the blood vessels and airways. They are an important feature in the control of fluid balance in the lung and can contain considerable amounts of lymph, particularly during pulmonary oedema, when they produce the characteristic ‘butterfly shadow’ on the chest X-ray (Fig. 2.10).
Many immune disorders have the characteristics of asthma, whereas those causing interstitial lung disease are characterized by restrictive patterns (see Chapter 4).
The nerves
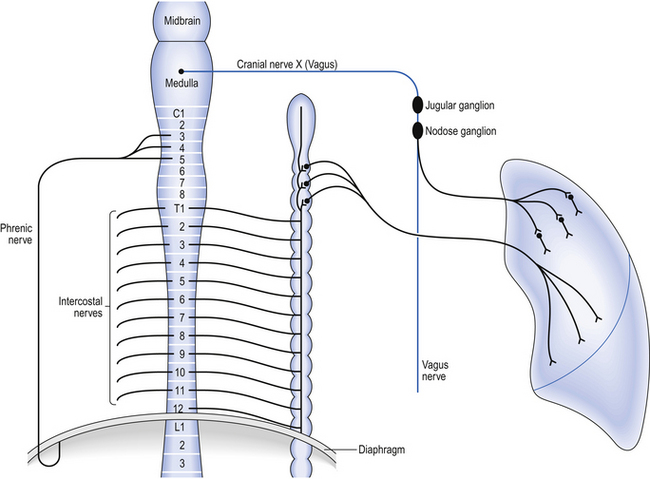
Innervation of the airways of the lungs is separate from that which brings about breathing (see below) and consists of afferent and efferent parts. The most dramatic efferent (motor) effects produced are on bronchomotor tone. The parasympathetic efferent supply is of most importance in this, and arrives at bronchial smooth muscle via preganglionic fibres which course through the jugular and then the nodose ganglia of the vagus nerves. As this is a parasympathetic outflow the fibres synapse in ganglia on the bronchi before sending short postganglionic fibres to the bronchial smooth muscle where they release acetylcholine to produce bronchoconstriction (Fig. 2.11).
Afferent nerves from receptors near the alveoli (J receptors), in the smooth muscle of airways (stretch receptors), and free nerve endings between the epithelial cells of airways (rapidly adapting, irritant, receptors) conduct sensation and sensory reflex information from the lungs to the brain, where it influences patterns of breathing (see Chapter 11) and bronchomotor tone.
Gross structure of the respiratory system
As with other organs the general name for the functional tissue of the lungs is parenchyma. The vast majority of the volume of what we see as the lungs when the chest is opened is in fact alveolar tissue surrounding air spaces (see Table 2.1). These air spaces make the lungs so insubstantial and light that they are the only organ that floats when placed in water, hence the Middle English name for lungs: lights.
Each lung is anatomically divided into lobes, made up of segments which are subdivided into lobules (Fig. 2.12).
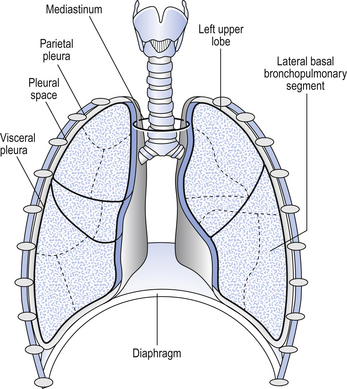
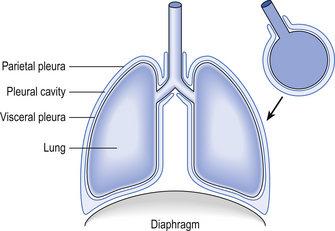
The lungs lie on both sides of the mediastinum which contains the trachea, heart, major blood vessels, nerves and oesophagus. The trachea divides into the right and left main bronchi at the carina, which is close to the aortic arch and the division of the pulmonary artery into its left and right branches. The main bronchi, pulmonary arteries and veins penetrate each lung at the hila. The lobes of the lungs are covered, except at their ‘roots’ at the medial surface, by a thin layer of tissue called the visceral pleura. The mediastinum and chest wall are lined by the parietal pleura. It helps some students to visualize the arrangement of the pleurae by thinking of a plastic bag, full of lungs, inside a second plastic bag, the two bags being the visceral and parietal pleurae, respectively (Fig. 2.13).
The diaphragm and chest wall
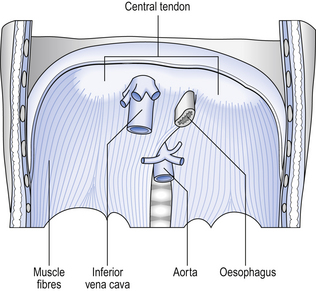
The base of the roughly cylindrical container which is the thorax is formed by the diaphragm. This is a sheet of muscle surrounding a large central tendon (Fig. 2.14).
The diaphragm lies surprisingly high in the thorax, the central tendon being about level with the eighth thoracic vertebra. Muscle fibres attached to the tendon run down obliquely to originate at the xiphisternum (see above), the lower margins of the ribcage and the upper lumbar vertebrae. Innervation of the diaphragm is by the right and left phrenic nerves, each of which serves its half of the diaphragm. The phrenic nerves originate from cervical spinal cord segments C3–C5 (‘C3, 4 and 5 keep the diaphragm alive’), with the major contribution being made by C4. Both nerves run through the thorax in contact with the mediastinum, penetrate the diaphragm, and innervate it from its inferior surface (see Fig. 2.11).
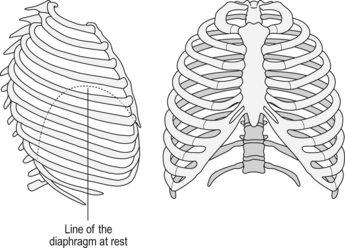
The walls of the thorax are made up of the ribcage (Fig. 2.15), which consists of the sternum anteriorly, to which ribs 1–6 are joined at about 45° by the costal cartilages. At the spinal column the ribs articulate by costovertebral joints which may involve more than one vertebra. Ribs 7–10 are joined by costal cartilage to the ribs above, and ribs 11 and 12 are free-‘floating’ at their anterior end.
Between the ribs are the three layers of the intercostal muscles:
How breathing is brought about
1. The lungs do not have muscles that contribute to breathing: the small amount of muscle they contain controls the diameter of the airways.
2. Air will only flow from a region of high pressure to a region of low pressure. In inspiration the pressure in the elastic alveoli is made low by stretching them by reducing the pressure around them by expanding the chest. Air is thus sucked into the lungs. During expiration pressure in the lungs is increased by decreasing the size of the chest, thereby compressing the gas in the lungs.
If we liken the diaphragm to the plunger of a syringe, the ribs can be likened to its walls. The action of the intercostal muscles on the ribs (mainly the second to the tenth) can, however, alter the diameter of the chest and so actively draw air into and expel it from the lungs. This is largely because the ribs are set at an angle, sloping down from the horizontal, and are capable of being raised and lowered (see Fig. 2.14).
The external intercostal muscles cause two types of movement during inspiration:
1. ‘Pump-handle’ movements, in which the anterior end of each rib is elevated like the action of an old-fashioned water pump.
2. ‘Bucket-handle’ movements, in which the diameter of the chest increases, each rib on either side acting like the raising of the handle of a bucket from the horizontal position.
Although expiration is largely passive during quiet breathing (resulting from the elastic recoil of the lungs–like a balloon collapsing) expiratory muscles can contract actively during high levels of breathing or if the airways are obstructed by disease. Under these conditions the abdominal muscles are the most important muscles of expiration. By squeezing the contents of the abdomen up against the diaphragm they force it up into the chest, thereby expelling air from the lungs. These abdominal muscles are especially active during a cough or a sneeze, as will be apparent if you press your fingers into your abdomen and cough. The internal and innermost intercostal muscles, like the external intercostals, occupy the spaces between the ribs and are innervated by segmental nerves. They pull the ribs down, reduce the diameter of the chest, and so contribute to expiration. Like the external intercostals, they reinforce the spaces between the ribs and prevent the chest from bulging out during expiration. The changes in size and shape of the chest brought about by the activity of the diaphragm, intercostals and accessory muscles are transmitted to the outer surface of the lungs. Because the lungs are so flexible, any change in pressure on their surface is rapidly transmitted to the air within the alveoli. This does not mean that the actual pressure in the fluid between the layers of pleura that form the covering of the lungs and the lining of the chest is the same as the pressure in the alveoli (see Chapter 5): in fact, it is important for the student to realize that they are very different.
Embryology
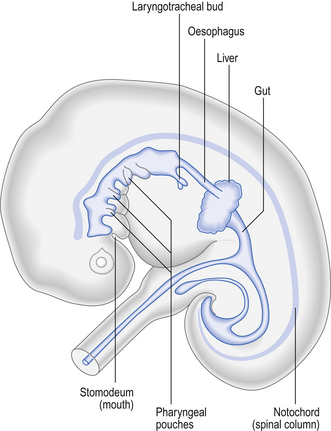
In the 4-week-old human embryo the beginnings of the respiratory system are first seen as an outpouching, the laryngotracheal bud, on the ventral surface of the endoderm of the digestive tract (Fig. 2.16). As the bud elongates the proximal portion forms the trachea and the distal end bifurcates to form first the two main bronchi and then the more distal parts of the bronchial tree, eventually forming a limited number of alveoli. The whole of the epithelium lining the entire respiratory tract is therefore derived from endoderm. The cartilage, muscle and connective tissue which make up much of the structure of the lungs develop from embryonic mesoderm that becomes associated with the laryngotracheal bud.
Air-conditioning
Heat and water
Because temperature and water vapour gradients between mucosa and inspired air are greatest in the nose and upper airways, these regions carry a large portion of the air-conditioning burden. This burden is, however, shared with the lower airways. During nose breathing at rest air transit through the nose takes < 0.1 s. During that time temperature is raised (if in comfortable room air) from 20°C to 31°C by the time the air leaves the internal nares and to 35°C by the time it reaches the mid trachea. Humidification takes place equally rapidly, inspired air being close to saturation by the time it reaches the pharynx. Humidification of inspired air places a thermal demand on the body because of the high latent heat of vaporization of water. Five times as much heat is used to vaporize water to saturate inspired air than is used to warm that air. The air-conditioning process is a metabolic ‘expense’, and up to 40% of this cost is recovered from the expired air which warms and moistens the nasal mucosa as we breathe out. Desert animals such as camels and gerbils have highly developed turbinate systems in their noses which recover more heat and water than do ours.
Particles and vapours
The smallest particles of all (<0.1 mm) are deposited by diffusion of gas molecules producing Brownian motion. The particles are ‘jostled’ until they bump into the wall of a small airway or alveolus. In this region particles are stuck to the walls by surface tension because there is no secretion of mucus. They are also beyond the end of the ciliary escalator. In the alveolar region amoeboid macrophages (Fig. 2.17) engulf particles and carry them to the escalator, or take them into the blood or lymph. When the dust load is large the macrophages dump their load around the respiratory, bronchioles, and any pathologist from a coal-mining area will have seen the black ‘halos’ so formed. Bacteria are particularly susceptible to the attentions of macrophages, which kill them with enzymes and oxygen-based free radicals (see Metabolic activity, p. 26) or transport them out of the lungs. The activities of these phagocytic cells ensure that the alveolar region of the lung is effectively sterile.
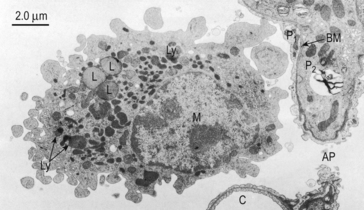
Fig. 2.17 Alveolar macrophage. Formed from monocytes produced in the bone marrow, these phagocytic cells contain enzymes destructive to microorganisms. These enzymes can produce emphysema in patients deficient in the protective protein α1-antitrypsin. M, macrophage; C, septal capillary; P1, type 1 pneumocyte; AP, alveolar pore; BM, basement membrane; Ly, lysosomes; L, lipid droplets. (Source: Young and Heath 2000.)
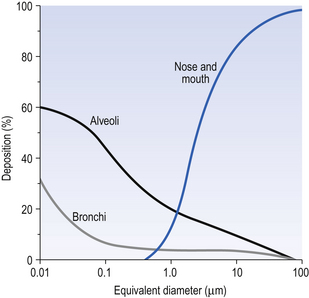
The influence of impaction, sedimentation and diffusion on particles of different aerodynamic diameters is illustrated in Figure 2.18.
The majority of particles of 0.5 mm aerodynamic diameter are not deposited: they ride the airflow into the lungs and back out again with expiration. Figure 2.18 represents the case during quiet breathing. Treatment of disease with therapeutic aerosols requires slow deep breathing to ensure deep penetration and sufficient time for diffusion. The increased ventilation of exercise enhances impaction and the danger of heavy work in dusty environments.
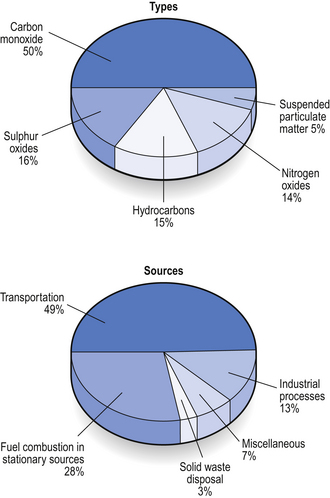
Particles account for only a small fraction by weight of the pollutants we breathe (Fig. 2.19).
Many gases and vapours also pose a serious threat, augmented by the self-abuse of tobacco smoking. Oxides of sulphur and nitrogen, hydrocarbons and chemicals produced by the action of sunlight on these substances inflame the respiratory tract. More than 1000 harmful constituents are inhaled in tobacco smoke. Smoking was identified by no less an authority than King James (VI of Scotland) I of England (1603–1625) as ‘a custom loathsome to the eye, hateful to the nose, harmful to the brain and dangerous to the lungs’. Little wonder he was known as ‘the wisest fool in Christendom’. Many harmful substances are produced by internal combustion engines, but the introduction of catalytic converters has significantly reduced production of carbon monoxide which has a particularly deleterious effect on the carriage of oxygen by the blood (see Chapter 8).
Metabolic activity
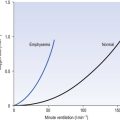
The metabolism of the tissues of the lung itself is unremarkable, with a metabolic rate only slightly higher than average for the whole body. Although it is the major extrahepatic site for mixed function oxidation by the cytochrome P450 systems, gram for gram it is much less active than the liver and much less tissue is involved. The major role of the P450 system in the lungs may therefore be in detoxification of inhaled foreign substances. Bloodborne toxic substances are extensively sequestered or detoxified in the lungs, with basic substances being particularly well processed. This protective activity on the part of the lung can be ‘heroic’ to a degree that causes fatal local damage: for example, the accumulation of oxygen-derived free radicals (useful in moderate concentrations to attack bacteria) is enhanced by the weedkiller paraquat (Weedol or Gramoxone), a dose of 1.5 g of which may be fatal because of its selective uptake by the lung. Although the initial clinical features of paraquat poisoning include dramatic ulceration of the mouth and oesophagus, diarrhoea and vomiting, it is usually the diffuse pulmonary fibrosis produced by the excess of free radicals that causes death. As well as free radicals the proteases, particularly elastase and trypsin, released by phagocytes in their normal defensive roles have to be neutralized or removed after they have carried out their function or they will attack the lung itself. Any of these substances caught up in the mucus of the mucociliary escalator will be carried out of the lung. In addition, their activity is terminated by conjugation with α1-antitrypsin in the plasma. The importance of this mechanism is demonstrated by the high incidence and severity of pulmonary emphysema in people who lack antitrypsin because of a genetic deficiency.
Metabolism of circulating biologically active substances
The production of surfactant by type II pneumocytes is discussed on p. 36.
Non-respiratory functions
Blood capacity
Pulmonary blood volume is about 500 mL in a recumbent man. This volume can be halved by increases in pressure within the chest, such as forced expiration against a closed larynx. On the other hand, the volume of blood in the chest can be doubled by a forced inspiration. This phenomenon allows the pulmonary circulation to act as a reservoir, for example at the start of exercise, when the output of the left ventricle rapidly increases. Activity of the sympathetic nervous system may influence the capacity of the system by triggering contraction of smooth muscle in the blood vessel walls.
Behaviour
Breathing is unique among the major functions of the body in that it is both voluntarily and involuntarily controlled. For example, our hearts and kidneys pump and filter our blood without our being aware of it. We cannot, however, consciously control the rate at which they work. Breathing goes on unconsciously for most of the time (except for those unfortunate individuals suffering from Ondin’s Curse, see p. 135), but in an instant we can take control of our breathing, for example to allow us to speak.