Chapter 101 Stereotactic Radiosurgery for Pituitary Adenomas
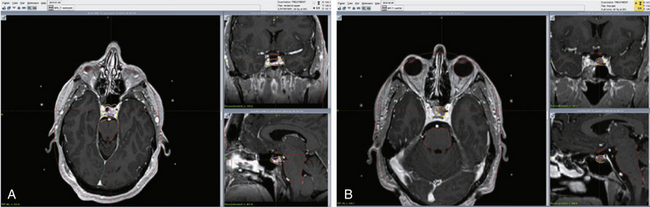
This chapter focuses on the use of gamma knife stereotactic radiosurgery (GKSRS) in the treatment of secretory and nonfunctional pituitary adenomas (NFPAs). GKSRS is most often used as an adjuvant to surgical resection and medical management to induce biochemical remission of endocrinologically active adenomas or to halt tumor progression in NFPAs (Figs. 101-1 and 101-2). Other modalities of irradiation, such as fractionated radiosurgery (FSR), have also been used and continue to play a specific role in certain clinical scenarios. In many situations, however, the exact role of GKSRS is still incompletely understood, mostly due to lack of data with long-term follow-up. Therefore, the care of patients with pituitary adenomas necessitates an interdisciplinary approach with collaboration between endocrinologists, neurosurgeons, radiation oncologists, and ophthalmologists.
Adrenocorticotropic Hormone-Producing Tumors
Cushing’s disease is associated with significant morbidity and premature death. Up to 30% of patients may experience persistent or recurrent disease after trans-sphenoidal surgery.1 Most studies define remission as normalization of 24-hour, urine-free cortisol (UFC) in the absence of medical suppression. Remission rates after radiotherapy reported in literature vary widely from 17% to 83% due to varying indications for radiosurgery, dosing, definitions of remission, and follow-up length. The time to remission also varies widely, from 2 months to 8 years,2 although the majority occur within 2 years of radiosurgery.1
Jagannathan et al.3 reviewed the GK radiosurgery experience in 90 patients with Cushing’s disease treated between 1990 and 2005, all of whom either had at least 12 months of follow-up postirradiation or experienced remission within 12 months of treatment. Patients presenting with Nelson’s syndrome were not included in this study. Indication for GK radiosurgery in all patients was persistent elevation of 24-hour UFC following surgical resection. The mean follow-up length post-GK was 45 months (range 12–132 months), and the mean marginal dose was 23 Gy (median 25 Gy). At the time of treatment, only 49 patients (54%) had tumors that were visible on magnetic resonance imaging (MRI). Remission was defined as normalized 24-hour UFC without concomitant medical therapy. Remission was achieved in 54% of patients at an average time of 13 months after radiosurgery (range 2–67 months). In patients with MRI-evident tumors, tumor volume decreased in 80%, remained constant in 14%, and increased in 6%. Of the patients who experienced biochemical remission, 20% suffered relapse at a mean time of 27 months (range, 6–60) after remission. Radiosurgical complications included hormone deficiencies in 22% of patients diagnosed at a mean time of 16 months (range, 4–36 months) after GKS and cranial nerve (CN) and visual field deficits in 5% of patients.
In the above study, no correlation between either maximum dose, marginal dose, or treatment volume and biochemical remission or tumor control was found, consistent with findings from another recent study.4 In two other studies,5,6 however, the authors found the maximum dose and prescription isodose volume to be significantly correlated with hormone response, although it is worth noting that when analyzing hormone response, these two studies lumped all functioning adenomas, and therefore the specific applicability of the conclusions to Cushing’s disease may be brought into question. Indeed, as mentioned previously, functioning adenomas show differential responses to irradiation as reflected by their differential response rates.7 To maximize the likelihood of treatment success, many authors3,5 advocate the use of more than 20 Gy at the 50% prescription isodose line when safe to do so.
Endocrinologic relapse after initial remission is an important problem in Cushing’s disease and, in some series,3,4,7 occurs in approximately 20% of patients cured by radiosurgery. In the Jagannathan et al. study,3 recurrence occurred at a mean time of 27 months after radiosurgery and 43% had their disease brought into remission again after a second radiosurgery treatment. In the study with the longest follow-up to date (mean follow-up of 94 months), Castinetti et al.4 observed a 50% cure rate in 18 patients with Cushing’s disease and recurrence in two patients (11%) at 6 and 8 years after initial treatment. Therefore, long-term endocrinologic follow-up is recommended.
Patients undergoing bilateral adrenalectomy due to persistent Cushing’s disease risk the development of Nelson syndrome, characterized by aggressive growth of the residual ACTH-secreting adenoma due to a reduced feedback inhibition by cortisol. Periodic imaging and monitoring of plasma ACTH levels have allowed early detection and treatment of these tumors. Corticotroph growth is rare in radiated patients, but may occur in up to 50% of nonradiated patients within 3 years of adrenalectomy.8 Few studies have been conducted to specifically investigate the safety and efficacy of stereotactic radiosurgery for Nelson syndrome. Nonetheless, GKSRS seems to offer a favorable tumor control rate of 82% to 100% and some authors have reported endocrinologic remission rates of up to 36%.9–11
Growth Hormone–Producing Tumors
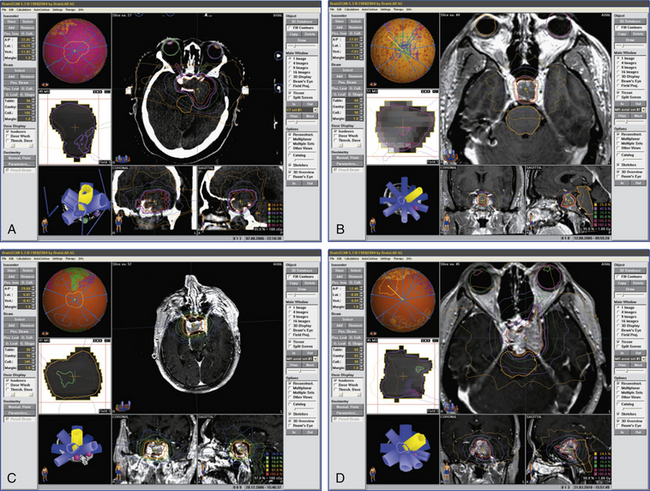
Biochemical remission for growth hormone (GH)–producing pituitary adenomas is commonly defined as normalization of age- and sex-adjusted serum level of insulin-like growth factor-1 (IGF-1) in the absence of medical therapy, as well as a basal GH level of less than 2.5 μg/L or less than 1 μg/L after glucose challenge (see Figs. 101B and 102A).1,4,12–14 In general, GH-secreting adenomas are particularly resistant to the effects of radiation. In a retrospective study13 of 83 patients with GH-secreting pituitary adenomas and acromegaly who received stereotactic radiosurgery as treatment for residual or recurrent disease following trans-sphenoidal surgery, biochemical remission was achieved in 60% of patients with a median follow-up of 69 months. The 5-year remission rate was 52%. Two patients experienced tumor progression, which occurred outside the treated volume. The median marginal dose at the 50% prescription isodose line was 21.5 Gy, no patients suffered CN toxicities, and hypopituitarism occurred in 8.5% of patients. Biochemical recurrence occurred in 1 patient and was managed medically. For patients with somatostatin analog-resistant tumors (n = 13), the authors found a much lower 16% remission rate.
In GH-secreting tumors lower basal GH levels are associated with increased rate of cure after radiosurgery. Losa et al.,13 using multivariate analysis, found increased likelihood of remission when basal GH levels were below 7.0 μg/L and basal multiples of upper limit (mUNL) IGF-1 levels were below 1.83 times UNL, with hazard ratios of 2.7 (95% confidence interval [CI] = 1.4–5.3) and 2.6 (95% CI = 1.3–5.1), respectively. This finding has since been replicated by Castinetti et al.4 in a series of 43 acromegalic patients with a mean follow-up of 102 months (42% remission). In these studies, radiation dose did not have a statistically significant impact.
There is emerging evidence that somatostatin analogs, when present at the time of radiosurgery, have a detrimental effect on achieving biochemical remission. In the initial study by Landolt et al.,15 9 patients treated with octreotide at time of radiosurgery were compared to 22 patients who did not receive octreotide at time of radiosurgery but had baseline characteristics with respect to age, sex, GH and IGF-1 levels at time of radiosurgery, treatment volume, and radiation dose. While patients who did not receive octreotide achieved a remission rate of 60%, patients who received octreotide achieved a remission rate of only 20% at 3-year follow-up. The radioprotective effect of octreotide has since been observed in other studies as well,12,16 albeit inconsistently.4,13 It is hypothesized15 that antisecretory agents such as octreotide may exert a radioprotective effect by decreasing cell cycling and thereby render cells less vulnerable to radiation-induced DNA damage. Based on these data, it is recommended that patients undergoing stereotactic radiosurgery for GH-releasing pituitary adenomas discontinue somatostatin-analogs at least 2 months prior to, and not resume them until 6 weeks after, radiosurgery. It is important to note however, that patients in these studies were not randomized and therefore selection biases, such as patients on octreotide were likely to have more severe and intractable disease, cannot be excluded.
Prolactin-Producing Tumors
Due to prolactin-secreting tumors’ robust response to dopamine-agonists, radiosurgery is commonly the last-line treatment, reserved for tumors that fail medical management and trans-sphenoidal resection (see Fig. 101A). In a retrospective study17 of 23 patients with medically and surgically refractory prolactinomas treated by GK, biochemical remission was achieved in 26% of patients with an average time of 24.5 months. Remission was defined as normalized serum prolactin without concomitant dopamine-agonist therapy. Volumetric control of tumors was achieved in 89% of patients with shrinkage in 46%. The mean marginal dose was 18.6 Gy. Pituitary insufficiency occurred in 28% of patients with an average time to onset of 44 months. Moreover, one patient developed a 3rd CN palsy and another patient a 6th CN palsy. Both patients suffered from tumors with cavernous sinus extension and were treated with a maximum dose of 50 Gy. The remission rate reported here is consistent with other series.4,7,18,19 Among functional adenomas, prolactin-secreting tumors have been reported in a study to be the least responsive to irradiation.7 However, the study with the longest follow-up4 showed similar remission rates for secretory tumors (42%, 46%, and 50% of patients with GH-, PRL-, and ACTH-secreting adenomas, respectively). The difference was the mean time to remission (50, 24, and 28 months for GH-, PRL-, and ACTH-secreting adenomas, respectively), suggesting again that GH-secreting adenomas are more resistant to the effects of radiosurgery. Although no study has specifically addressed the issue of fertility after radiosurgery for prolactin-secreting adenomas, Landolt and Lomax18 observed that 2 out of 11 patients with normalized serum prolactin became pregnant spontaneously.
It has been found17 that biochemical remission was significantly associated with a tumor volume of less than 3 cc. Moreover, analogous to GH-secreting adenomas, dopamine-agonist therapy at the time of radiosurgery decreases the likelihood of achieving biochemical remission.17,18 The investigation of factors important to remission is hampered by the small number of patients in each series, resulting in few series with sufficient statistical power to address this issue, and also increasing the likelihood of spurious associations due to selection bias. Moreover, it has been observed2 that radiosurgery to the pituitary may cause an increase in serum prolactin, possibly via radiation-induced injury to the infundibulum, that may last several years and obscure its tumor-toxic effects.
Nonfunctional Adenomas
Stereotactic radiosurgery is a treatment option for nonfunctional pituitary adenomas (NFPAs) that exhibit parasellar extension and/or recurrent growth after surgical resection (see Figs. 101C and D and 101-2B). The primary goal of adjuvant radiosurgery is to halt tumor progression and preserve the integrity of CN function especially with respect to the optic apparatus. Losa et al.20 conducted a retrospective study analyzing the outcomes of 54 patients who had undergone GKRS for residual tumor after surgical resection. Cavernous sinus extension was present in 77.8% of patients, 29.6% of patients had visual deficits, while 3.7% had oculomotor deficits. Treatment characteristics included mean target volume of 2.3 cc and marginal dose of 16.6 Gy at the 50% isodose line. The mean follow-up length was 41 months. Tumor control was achieved in all but 2 patients during the study period, with 42.3% of patients experiencing a tumor volume decrease of 20% or more. The recurrence-free interval at 5 years was 88.2% (95% CI 72.6–100%). Recurrence in 2 patients was detected at 40 and 49 months, occurring outside the treated volume in both cases. No patient in this series experienced new or worsened neurological deficits while 23.4% suffered some form of hypopituitarism during the study period.
Most series21–25 have shown tumor growth control of at least 90% using radiosurgery with up to 25% risk of new-onset pituitary insufficiency and up to 5% risk of new visual deficits. Especially worth noting is the similar rate of success in the control of tumors with parasellar extension, which would otherwise be untreatable. For instance, in a series of 61 tumors with parasellar extension treated by radiosurgery,1 volume reduction occurred in 63%, remained constant in 27%, and tumor growth occurred in 10%.
Chang et al.26 specifically examined the indications for radiotherapy by retrospectively studying the outcomes of 663 patients who received surgical resection between 1975 and 1995 for NFPAs. Although patients were not randomized, changing practice patterns allowed the authors to analyze the relationship between extent of surgical resection, radiotherapy, and tumor control. Increased recurrence was correlated with subtotal resection without adjuvant radiotherapy, while radiotherapy after gross-total resection had no effect on recurrence. Therefore, radiation seems specifically indicated as adjuvant therapy in patients with subtotal resection of NFPAs and provides excellent tumor control rates with a moderate risk of pituitary insufficiency, necessitating long-term monitoring (see Fig. 101-2D). On the other hand, the natural history of residual pituitary adenomas without extrasellar remnant seems to show that not all of them will have clinically significant growth, and therefore a watchful waiting approach with periodic MRI imaging needs to be considered particularly in young patients who may desire fertility.27
Complications
Cranial Nerve Deficits
Damage to the optic apparatus is a major morbidity associated with irradiation of the sella while targeting parasellar tumors risks damage to CNs 3, 4, and 6. The rate of visual complications, defined as visual field defect or CN 3, 4, or 6 palsy, following radiosurgery for functional or nonfunctional pituitary adenomas is 0% to 7%.3,13,17,23,24 The optic apparatus is especially sensitive to irradiation and it is recommended that the maximum dose to the chiasm not exceed 8 to 10 Gy,28,29 although some authors have advocated the use of up to 12 Gy.30 Radiation toxicity may occur at any dosage however, and has been documented after as little as 0.7 Gy (Fig. 101-1C and D).25 The tolerable dose likely varies from patient to patient, and is greatly affected by factors such as age, previous irradiation, degree of compression by the adenoma, and comorbidities such as diabetes. For adenomas located so close to the optic apparatus that a sufficient dose fall-off cannot be achieved to spare the optic chiasm, fractionated radiosurgery should be chosen to decrease the risk of visual deficits after radiosurgery. CN 3, 4, and 6 appear to be much more resistant to radiation toxicity. In a review of 35 studies involving 1621 patients, Sheehan et al.25 identified only 21 patients with CN 3, 4, or 6 neuropathies, most of which were transient.
Hypopituitarism
Radiation-induced pituitary insufficiency is a major risk for all patients undergoing sellar irradiation (see Fig. 101-1B). Currently, there is a lack of reliable data regarding the quantitative risk of developing hypopituitarism, defined as any new hormone deficiency, following radiosurgery. Most series have found a prevalence of 0%–36%3,31,32 in treated patients, although these findings have been hampered by lack of long-term follow-up, variable means of evaluation of pituitary function, and heterogeneous tumor and treatment characteristics. Despite an earlier report of up to 76%2 rate of hypopituitarism in patients followed for 17 years, Hoybye Rahn21 recently found the rate of hypopituitarism to be surprisingly low in a group of 23 patients treated with GK and followed for a median of 97 months, with no new hormone deficiencies discovered. The study population was highly selected, however, with small tumor volumes and radiation targeting parasellar extensions, thereby hampering the generalizability of the results. Pollock et al.23 found a significant association between tumor volume and the risk of developing hypopituitarism at 5 years. While 50% of patients with tumors larger than 4 cc developed hypopituitarism by 5 years, the prevalence was only 18% for patients with tumor volumes smaller than 4 cc.
Radiation-Induced Neoplasms
The study of radiation-induced neoplasms is hampered by the long time scale over which lesions develop and the difficulty in causally linking any specific lesion to previous radiation. Therefore, although radiation-induced neoplasms are exceedingly rare, their true incidence is unknown. In order for a tumor to be considered causally linked to radiosurgery, the tumor must (1) occur within the previous radiation field, (2) not be present prior to radiosurgery, (3) be histologically distinct from any primary tumor, (4) not develop in the setting of a genetic predisposition in the host, and (5) develop during an expected latency period of roughly 5 years.25 Only three cases33–35 have been reported in the radiosurgery literature comprised of more than 200,000 patients that meet the above criteria. In one patient,33 a malignant transformation of a vestibular schwannoma occurred 6 months following adjuvant GKSRS to treat residual tumor. The dose at the 50% isodose line was 15 Gy. In another patient with vestibular schwannoma treated by GKSRS (11 Gy at the 40% isodose line), a glioblastoma multiforme (GBM) occurred in the radiation scatter field 7.5 years after GKSRS.35 In the third patient, a GBM occurred following GKSRS (20 Gy peripheral dose) for an arteriovenous malformation 6.5 years after radiosurgery.34
Summary
Gamma knife SRS is a safe and effective adjuvant treatment for patients with secretory or non-functional pituitary adenomas. Secretory adenomas show differential response to GKSRS based on tumor type.7 The biochemical remission rates on long term follow-up are similar for patients with ACTH-, GH- or prolactin secreting adenomas, however the length of time to remission varies, with prolcatinomas having the longest time to remission. GKSRS is indicated for patients with NFPAs and subtotal surgical resection or parasellar extension. It offers an excellent tumor control rate of greater than 90%. Major complications from GKSRS include radiation-induced CN deficits and hypopituitarism. Up to 7% of patients may suffer visual field deficits due to radiation injury to the optic apparatus while CN 3, 4, and 6 appear to be much more resistant to irradiation. Hypopituitarism has been observed in approximately 36% of patients, although there is a lack of long-term data. Other complications, such as damage to the internal carotid artery in the cavernous sinus, brain parenchyma, and radiation-induced neoplasms have been shown to be extremely rare.1
Castinetti F., Nagai M., Morange I., et al. Long-term results of stereotactic radiosurgery in secretory pituitary adenomas. J Clin Endocrinol Metab. 2009;94:3400-3407.
Chang E.F., Zada G., Kim S., et al. Long-term recurrence and mortality after surgery and adjuvant radiotherapy for nonfunctional pituitary adenomas. J Neurosurg. 2008;108:736-745.
Girkin C., Comey C., Lunsford L., et al. Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology. 1997;104:1634-1643.
Höybye C., Rähn T. Adjuvant gamma knife radiosurgery in non-functioning pituitary adenomas; low risk of long-term complications in selected patients. Pituitary. 2009;12:211-216.
Höybye C., Grenbäck E., Rähn T., et al. Adrenocorticotropic hormone-producing pituitary tumors: 12- to 22-year follow-up after treatment with stereotactic radiosurgery. Neurosurgery. 2001;49:284-292.
Jagannathan J., Sheehan J., Pouratian N., et al. Gamma knife radiosurgery for acromegaly: outcomes after failed transsphenoidal surgery. Neurosurgery. 2008;62:1262-1269.
Jagannathan J., Sheehan J.P., Pouratian N., et al. Gamma knife surgery for Cushing’s disease. J Neurosurg. 2007;106:980.
Jagannathan J., Yen C.-P., Pouratian N., et al. Stereotactic radiosurgery for pituitary adenomas: a comprehensive review of indications, techniques and long-term results using the gamma knife. J Neurooncol. 2009;92:345-356.
Kim S., Huh R., Chang J., et al. Gamma knife radiosurgery for functioning pituitary adenomas. Stereotact Funct Neurosurg. 1999;72:101-110.
Landolt A., Lomax N. Gamma knife radiosurgery for prolactinomas. J Neurosurg. 2000;93:14-18.
Landolt A.M., Haller D., Lomax N., et al. Octreotide may act as a radioprotective agent in acromegaly. J Clin Endocrinol Metab. 2000;85:1287-1289.
Losa M., Gioia L., Picozzi P., et al. The role of stereotactic radiotherapy in patients with growth hormone-secreting pituitary adenoma. J Clin Endocrinol Metab. 2008;93:2546-2552.
Mokry M., Ramschak-Schwarzer S., Simbrunner J., et al. A six year experience with the postoperative radiosurgical management of pituitary adenomas. Stereotact Funct Neurosurg. 1999;72:88-100.
O’Sullivan E., Woods C., Glynn N., et al. The natural history of surgically treated but radiotherapy-naïve nonfunctioning pituitary adenomas. Clin Endocrinol (Oxf). 2009;71:709-714.
Pollock B., Brown P., Nippoldt T., Young W.J. Pituitary tumor type affects the chance of biochemical remission after radiosurgery of hormone-secreting pituitary adenomas. Neurosurgery. 2008;62:1271-1276.
Pollock B., Nippoldt T., Stafford S., et al. Results of stereotactic radiosurgery in patients with hormone-producing pituitary adenomas: factors associated with endocrine normalization. J Neurosurg. 2002;97:525-530.
Pollock B.E., Cochran J., Natt N., et al. Gamma knife radiosurgery for patients with nonfunctioning pituitary adenomas: results from a 15-year experience. Int J Radiat Oncol Biol Phys. 2008;70:1325-1329.
Pollock B.E., Jacob J.T., Brown P.D., Nippoldt T.B. Radiosurgery of growth hormone–producing pituitary adenomas: factors associated with biochemical remission. J Neurosurg. 2007;106:833.
Pollock B.E., Young W.F. Stereotactic radiosurgery for patients with ACTH-producing pituitary adenomas after prior adrenalectomy. Int J Radiat Oncol Biol Phys. 2002;54:839-841.
Pouratian N., Sheehan J., Jagannathan J., et al. Gamma knife radiosurgery for medically and surgically refractory prolactinomas. Neurosurgery. 2006;59:255-266.
Sheehan J.P., Kondziolka D., Flickinger J., Lunsford L.D. Radiosurgery for residual or recurrent nonfunctioning pituitary adenoma. J Neurosurg. 2002;97:408.
Sheehan J.P., Niranjan A., Sheehan J.M., et al. Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J Neurosurg. 2005;102:678.
Stafford S., Pollock B., Leavitt J., et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55:1177-1181.
Tishler R., Loeffler J., Lunsford L., et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27:215-221.
1. Jagannathan J., Yen C.-P., Pouratian N., et al. Stereotactic radiosurgery for pituitary adenomas: a comprehensive review of indications, techniques and long-term results using the gamma knife. J Neurooncol. 2009;92:345-356.
2. Höybye C., Grenbäck E., Rähn T., et al. Adrenocorticotropic hormone-producing pituitary tumors: 12- to 22-year follow-up after treatment with stereotactic radiosurgery. Neurosurgery. 2001;49:284-292.
3. Jagannathan J., Sheehan J.P., Pouratian N., et al. Gamma knife surgery for Cushing’s disease. J Neurosurg. 2007;106:980.
4. Castinetti F., Nagai M., Morange I., et al. Long-term results of stereotactic radiosurgery in secretory pituitary adenomas. J Clin Endocrinol Metab. 2009;94:3400-3407.
5. Kim S., Huh R., Chang J., et al. Gamma knife radiosurgery for functioning pituitary adenomas. Stereotact Funct Neurosurg. 1999;72:101-110.
6. Pollock B., Nippoldt T., Stafford S., et al. Results of stereotactic radiosurgery in patients with hormone-producing pituitary adenomas: factors associated with endocrine normalization. J Neurosurg. 2002;97:525-530.
7. Pollock B., Brown P., Nippoldt T., Young W.J. Pituitary tumor type affects the chance of biochemical remission after radiosurgery of hormone-secreting pituitary adenomas. Neurosurgery. 2008;62:1271-1276.
8. Assie G., Bahurel H., Coste J., et al. corticotroph tumor progression after adrenalectomy in Cushing’s disease: a reappraisal of Nelson’s syndrome. J Clin Endocrinol Metab. 2007;92:172-179.
9. Jenkins P., Trainer P., Plowman P., et al. The long-term outcome after adrenalectomy and prophylactic pituitary radiotherapy in adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 1995;80:165-171.
10. Pereira M., Halpern A., Salgado L., et al. A study of patients with Nelson’s syndrome. Clin Endocrinol. 1998;49:533-539.
11. Pollock B.E., Young W.F. Stereotactic radiosurgery for patients with ACTH-producing pituitary adenomas after prior adrenalectomy. Int J Radiat Oncol Biol Phys. 2002;54:839-841.
12. Jagannathan J., Sheehan J., Pouratian N., et al. Gamma knife radiosurgery for acromegaly: outcomes after failed transsphenoidal surgery. Neurosurgery. 2008;62:1262-1269.
13. Losa M., Gioia L., Picozzi P., et al. The role of stereotactic radiotherapy in patients with growth hormone-secreting pituitary adenoma. J Clin Endocrinol Metab. 2008;93:2546-2552.
14. Sheehan J., Niranjan A., Sheehan J., et al. Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J Neurosurg. 2005;102:678-691.
15. Landolt A.M., Haller D., Lomax N., et al. Octreotide may act as a radioprotective agent in acromegaly. J Clin Endocrinol Metab. 2000;85:1287-1289.
16. Pollock B.E., Jacob J.T., Brown P.D., Nippoldt T.B. Radiosurgery of growth hormone–producing pituitary adenomas: factors associated with biochemical remission. J Neurosurg. 2007;106:833.
17. Pouratian N., Sheehan J., Jagannathan J., et al. Gamma knife radiosurgery for medically and surgically refractory prolactinomas. Neurosurgery. 2006;59:255-266.
18. Landolt A., Lomax N. Gamma knife radiosurgery for prolactinomas. J Neurosurg. 2000;93:14-18.
19. Mokry M., Ramschak-Schwarzer S., Simbrunner J., et al. A six year experience with the postoperative radiosurgical management of pituitary adenomas. Stereotact Funct Neurosurg. 1999;72:88-100.
20. Losa M., Valle M., Mortini P., et al. Gamma knife surgery for treatment of residual nonfunctioning pituitary adenomas after surgical debulking. J Neurosurg. 2004;100:438.
21. Höybye C., Rähn T. Adjuvant gamma knife radiosurgery in non-functioning pituitary adenomas; low risk of long-term complications in selected patients. Pituitary. 2009;12:211-216.
22. Liscak R., Vladyka V., Marek J., et al. Gamma knife radiosurgery for endocrine-inactive pituitary adenomas. Acta Neurochir (Wien). 2007;149:999-1006.
23. Pollock B.E., Cochran J., Natt N., et al. Gamma knife radiosurgery for patients with nonfunctioning pituitary adenomas: results from a 15-year experience. Int J Radiat Oncol Biol Phys. 2008;70:1325-1329.
24. Sheehan J.P., Kondziolka D., Flickinger J., Lunsford L.D. Radiosurgery for residual or recurrent nonfunctioning pituitary adenoma. J Neurosurg. 2002;97:408.
25. Sheehan J.P., Niranjan A., Sheehan J.M., et al. Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J Neurosurg. 2005;102:678.
26. Chang E.F., Zada G., Kim S., et al. Long-term recurrence and mortality after surgery and adjuvant radiotherapy for nonfunctional pituitary adenomas. J Neurosurg. 2008;108:736-745.
27. O’Sullivan E., Woods C., Glynn N., et al. The natural history of surgically treated but radiotherapy-naïve nonfunctioning pituitary adenomas. Clin Endocrinol (Oxf). 2009;71:709-714.
28. Girkin C., Comey C., Lunsford L., et al. Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology. 1997;104:1634-1643.
29. Tishler R., Loeffler J., Lunsford L., et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27:215-221.
30. Stafford S., Pollock B., Leavitt J., et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55:1177-1181.
31. Jane J.A., Vance M.L., Woodburn C.J., Laws E.R. Stereotactic radiosurgery for hypersecreting pituitary tumors: part of a multimodality approach. Neurosurg Focus. 2003;14:1.
32. Sheehan J., Jagannathan J., Pouratian N., Steiner L. Stereotactic radiosurgery for pituitary adenomas: a review of the literature and our experience. Front Horm Res. 2006;34:185-205.
33. Hanabusa K., Morikawa A., Murata T., Taki W. Acoustic neuroma with malignant transformation. J Neurosurg. 2001;95:518-521.
34. Kaido T., Hoshida T., Uranishi R., et al. Radiosurgery-induced brain tumor. J Neurosurg. 2001;95:710-713.
35. Shamisa A., Bance M., Nag S., et al. Glioblastoma multiforme occurring in a patient treated with gamma knife surgery. J Neurosurg. 2001;94:816-821.