Chapter 35 Stem Cells and Cellular Therapy
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
Stem cells as therapeutics to treat retinal disease
Nothing more dramatically captures the imagination of visually impaired patients or the ophthalmologist treating them than the possibility of rebuilding a damaged retina with “stem cells.” Since many retinal neuro- and vasculodegenerative diseases progress slowly, it may be possible to use stem cell-derived “replacement cells” to prevent visual loss if such therapies are performed at an early stage of disease. Defined as pluripotent, self-renewing cells capable of differentiating into a variety of cell types, stem cells can be derived from early embryos and, under appropriate conditions, will differentiate into a variety of tissues, including mesoderm (e.g., muscle), endoderm (e.g., the gastrointestinal tract), ectoderm (e.g., skin) and neuroectoderm (e.g., brain and retina). Stem cells have also been identified and isolated from adult tissues and presumably represent a pool of progenitor cells that may serve to maintain a supply of cells to maintain various tissue types as well as rescue/repair damaged tissue following injury or stress. In addition to “developmentally arrested” adult stem cells that reside in normal adult tissues, a population of pluripotent stem cells can now be derived from adult somatic tissues. These stem cells, called induced pluripotent stem cells (iPSC) represent a highly significant advance in our ability to derived autologous replacement tissues for the treatment of a variety of ocular, and other, diseases and will be discussed in greater detail below. Overall, there are now several populations of stem cells that have been described and each may have relative benefits for the treatment of different diseases1. Readers are referred to Chapter 125 (Transplantation Frontiers) for further discussion of clinical applications of retinal progenitor and adult transplantation therapies.
Definitions
Stem cells can be divided into those that are isolated from fetal transient embryonic cell populations and those from adult tissue (adult stem cells). The former are typically isolated from blastocysts and exhibit pluripotency, meaning they can give rise to virtually any adult tissue cell type under the appropriate conditions. Adult-derived stem cells typically reside in adult tissues in a quiescent, undifferentiated state and, under appropriate stimuli, will divide and differentiate into the cell type of the tissue in which they reside or, if appropriately stimulated, into other cell types. The mechanism whereby an undifferentiated, quiescent stem cell can give rise to a multitude of differentiated, postmitotic cell types is an area of active investigation, and large-scale genomic analysis2 and transcriptional profiling3 of stem cells have led to varying definitions of “stemness.”4 While truly pluripotent embryonic stem cells (ESC) have been identified that can give rise to a multitude of differentiated cell types, there is controversy as to the generality of this phenomenon with regard to the plasticity of adult stem cells; implanted adult tissue-derived “stem cells” can, indeed, contribute to tissue regeneration in a number of different systems, but it is not entirely clear whether this is a result of a stem cell differentiating into the end cell product or if this occurs by somatic cell fusion between the “stem” cell and a population of differentiated, postmitotic, tissue-specific cells that then share the stem cell’s proliferative capabilities as well as their own tissue-specific phenotypic characteristics.
There are many examples of fetal tissue-specific somatic stem cells being identified as pluripotent cells followed by studies questioning the validity of the finding. This is particularly well illustrated by the controversy surrounding the issue of whether or not fetal neural stem cells can differentiate into hematopoietic cells; several reports describe hematopoietic competency in brain-derived neural stem cells.5,6 Shortly after this, others reported that such competency was a rare event and may depend on genetic or epigenetic alterations, not true “stemness.”7 Most recently, pluripotency, including the ability to form hematopoietic cells, was once again attributed to neural stem cells.8 Given the current lack of precision in defining how cells “choose” their phenotypic fates and activate or deactivate developmental programs, it remains unclear precisely which stem cells are actually present in somatic tissues of the fetus and adult. These issues are critically discussed in reviews to which the reader is referred.9,10,10a
Embryonic stem cells
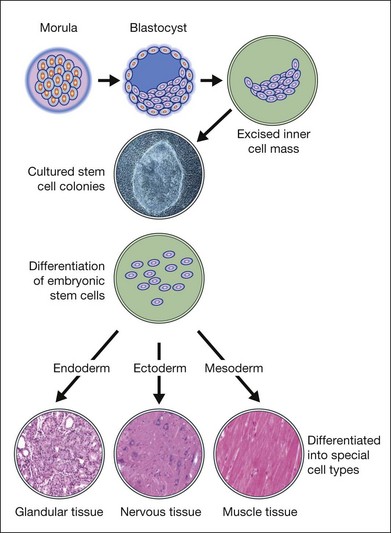
ESC are derived from a transient population of cells in the early embryo and are characterized by pluripotency (i.e., the ability to differentiate into derivatives of the three primary germ cells layers: ectoderm, endoderm, and mesoderm) and the ability to self-renew indefinitely (Fig. 35.1). Murine ESC were first derived and cultured in 1981,11,12 and have become important research tools for the creation of transgenic and knockout mice and for the study of early development in mammals (Table 35.1).13 In 1995, James Thomson’s team derived primate ESC from rhesus monkey blastocysts,14 but it was not until 1998 that human ESC (HESC) were derived and cultured from the inner cell mass of human blastocysts15 or from human primordial germ cells.16 Human blastocysts develop approximately 5 days postfertilization and the inner cell mass consists of 20–50 cells at days 5 and 6.17 In 2006, a method for culture of HESC from single blastomeres without destruction of the embryo was reported.18 Methods for the culture of murine and HESC typically utilized fibroblast feeder cells to facilitate the growth and survival of the stem cells but recently protocols have been developed for the feeder-free and serum-free culture.13
| 1981 | Mouse ESC first derived |
| 1995 | Primate ESC isolated and grown in culture |
| 1998 | Human ESC isolated and grown in culture |
| 2007 | Differentiated adult cells reprogrammed to ESC-like cells (iPSC) |
| 2009 | First human ESC clinical trial approved by US FDA for spinal cord injury |
| 2010 | FDA approval for phase I/II clinical trial to treat Stargardt disease |
| 2011 | FDA approval for phase I/II clinical trial to treat advanced dry age-related macular degeneration |
iPSC, induced pluripotent stem cells; FDA, Food and Drug Administration.
The National Institutes for Health (NIH) have developed guidelines to establish policy and procedures under which NIH will fund research using HESC, and to help ensure that NIH-funded research in this area is ethically responsible, scientifically worthy, and conducted in accordance with applicable law. These guidelines were developed in response to Executive Order 13505, were issued on March 9, 2009, and became effective on July 7, 2009. As of November 2011, there were 136 HESC lines eligible for use in NIH-supported research, as listed in the NIH Human Embryonic Stem Cell Registry (http://stemcells.nih.gov/research/registry/).
Induced pluripotent stem cells
One of the most significant advances in the field of stem cell biology came with the observation by Yamanaka and colleagues that the addition of four critical transcription factors, under appropriate conditions, to adult somatic cells (e.g. skin keratinocytes or fibroblasts) would generate an induced pluripotent state from which a number of differentiated cells types could then be derived18a. By generating such induced pluripotent stem cells (iPSC), it may now be possible to produce autologous grafts for use in treating a variety of diseases, including those of the retina. This is discussed in greater detail below.
Adult tissue stem cells
The concept that adult tissues contain pluripotent stem cells that can serve as a source of regenerative tissue useful in the repair of aging adult organ systems is an important scientific advance.19 For example, tissues traditionally thought of as not having regenerative capacity (e.g., brain and myocardium) are the ones most often touted as being the most likely to benefit from treatments with neural or myocardial “stem cells.” In fact, clinical trials using adult bone marrow-derived stem cells to regenerate infarcted myocardium have reported success in improving cardiac function,20 presumably on the basis of bone marrow stem cells differentiating into myocardium. Unfortunately, the idea that adult bone marrow-derived hematopoietic stem cells (HSC) can transdifferentiate into myocardial tissue is not supported by experimental data.21,22 While this does not invalidate the clinical observations, it does raise a cautionary note on how such results can be interpreted and emphasizes the need to establish rigorous standards by which such clinical studies are evaluated.23
While there is an extensive literature on stem cells giving rise to nervous,24 muscle,25 vascular,26,27 and hematopoietic tissue, work on retinal stem cells is more limited. Nonetheless, a literature has emerged over the past decade that strongly supports the potential for exploiting progenitor cells to maintain, and perhaps regenerate, abnormal retinal tissue. These studies describe four basic populations of cells that may contain dormant progenitor cells which, under appropriate circumstances, may have therapeutic application in the treatment of retinal disease: (1) retinal stem cells that can give rise to photoreceptors and other retinal neurons; (2) Müller/glial stem cells that can differentiate into retinal neurons; (3) retinal pigment epithelial (RPE) stem cells that can not only serve to replace diseased RPE but perhaps also be stimulated to differentiate into photoreceptors; and (4) endothelial progenitor cells (EPC) that can contribute to the retinal vasculature and exert a neurotrophic effect. Since there are a large number of reviews on retinal stem cells, this topic will not be discussed in great detail here. Glial and RPE stem cell biology are relatively new areas of investigation and, thus, there is not yet a lot of data on this potentially exciting, but understudied, area. Adult bone marrow-derived HSC containing EPC is an area of recent interest with broad-ranging potential application and will be discussed in slightly more detail given the therapeutic potential of these cells.
Retinal stem and müller/glial cells
It has long been known from classic studies in developmental biology that the retina of amphibians and chick embryos regenerates after injury and that this regenerative capacity derives from quiescent stem cells that reside in the adult retina of these species.28,29 Given that such potential exists in lower vertebrates, there have been numerous efforts to demonstrate similar regenerative capacity in the mammalian retina. For a population of retinal stem cells to exist in the adult retina, it would be necessary for such a population of progenitor cells to remain quiescent after the retina has fully differentiated. Thus, a better understanding of gene expression during retinal development and an analysis of the orderly progression of such gene expression during the establishment of the highly ordered and regulated adult retina would provide a rationale in the search for adult retinal progenitor cells. Studies using large-scale genomic analysis30 or serial analysis of gene expression in combination with in situ hybridization31 to localize gene expression temporally and spatially to individual retinal cell types has provided such a “molecular atlas.” This work can serve as the starting point for the evaluation of numerous genes and their potential role in the regulation of retinal cell developmental determination. How genes are progressively switched on and off in an orderly fashion during the generation of specific retinal cell types, and how this overlaps with gene sets utilized during the establishment of other, nonretinal neuronal cell types, will contribute significantly to our understanding of retinal progenitor biology. The regulation of cell proliferation32 and the role of various transcription factors and signaling molecules33 during this process have provided insight into putative mechanisms whereby the mammalian retina holds in reserve a subset of progenitor cells that theoretically could be used to regenerate damaged tissue in the adult. Clearly, the developmental state of the retina and the context in which a specific cell finds itself will determine how a particular retinal progenitor cell behaves; whether it will terminally differentiate or maintain a quiescent state from which it can later emerge to give rise to cells useful in the repair of a damaged retina will depend not only on its own developmental program, but the microenvironment in which it finds itself.34 Transcriptional profiling studies of retinas at different states of development coupled with in vitro studies of progenitor cell populations35 should provide the information necessary to begin such an analysis and determine what conditions help maintain quiescence and what conditions stimulate proliferation and subsequent differentiation of retinal progenitor cell populations.
Cells with characteristics of retinal neurons have been obtained from a number of embryonic tumor cell lines, including neuroblastoma, glioblastoma, and teratocarcinoma,36 but given the malignant potential of these cells, are not likely to be useful in the treatment of retinal degenerative disorders. In subsequent studies, cultured virally transformed, immortalized neuronal precursors were evaluated for their ability to differentiate into retinal neurons after intraocular implantation.37 Attempts at inducing differentiation of ESC into retinal neuronal phenotypes were made using a variety of factors, including retinoic acid.38 It has been reported that retinal progenitor cells can, indeed, be identified in the adult retina. Such cells exist in the ciliary margin; single pigmented epithelial cells can be isolated from the ciliary margin (but not the central or peripheral pigmented epithelium) and clonally expanded in culture to give rise to a variety of retinal cell types, including rod photoreceptors, bipolar neurons, and Müller glia.39,40 The differentiation of these cells from the ciliary margin pigmented cell progenitors is not due to transdifferentiation of the ciliary margin cells, but, rather, clonal proliferation and differentiation, as observed in a true stem cell. Thus, the potential utility of these cells and their characterization have come under scrutiny and are discussed extensively in a number of excellent recent reviews.41–44
In lower vertebrates (e.g., chickens) adult, differentiated Müller glia themselves can serve as a source of stem cells that will, in response to injury or exogenously added cytokines, dedifferentiate, proliferate, and redifferentiate into additional glial cells or neurons.45 A common progenitor cell that gives rise to both Müller glia and retinal neurons was described nearly two decades ago.46 Thus, the concept that Müller glia of the adult retina can serve as a potential source of retinal stem cells is consistent with molecular profiling of developing mammalian retinas that shows a high degree of similarity between the gene expression profiles of Müller glia and mitotic retinal progenitor cells in the mouse.31 Since the Müller glia are the cells that commonly proliferate in response to retinal injury, it would not be surprising that these cells also retain the potential to differentiate along a number of pathways, some of which may lead to retinal neuronal replacement. One study has significantly expanded this concept significantly: amacrine, horizontal, and photoreceptor phenotypes were expressed by Müller glial cells following toxic injury to the adult mammalian retina in the presence of extrinsic factors (e.g., retinoic acid) or activation of intrinsic genes.47 These studies are most provocative, could provide additional insight into retinal regeneration in mammals, and provide a rationale for the targeting of Müller glia in certain inherited and acquired retinal degenerative disorders.
Müller glia are the only cells that span the entire neurosensory retina; cell processes extend anteriorly to the ganglion cell layer as well as posteriorly to the RPE and ramifications of these processes form intimate contacts with retinal blood vessels, photoreceptors, and other retinal neurons. Müller glia are activated in response to vascular changes such as those observed in a variety of retinal vascular and neurodegenerative diseases.48,49 Upon activation, these cells upregulate glial fibrillary acidic protein and in animal models of outer retinal neovascular disease their appearance and location precisely correlate, both temporally and spatially, with subretinal neovascularization and associated neuronal degeneration in the outer retina.50 Recent studies describe the use of activated Müller cell targeted adeno-associated viral vectors containing a transgene encoding a neurotrophic molecule to target the outer retina in vasculodegenerative50 and neurodegenerative disorders51 characterized by photoreceptor degeneration. This strategy using endogenous cells (e.g., activated Müller cells) to deliver gene therapy products to the outer retina could be useful clinically to avoid the need for subretinal injections of the viral vector, a procedure that can have deleterious effects on already diseased retinas.
Differentiation of HESC into photoreceptors
Studies evaluating the in vivo efficacy of HESC-derived photoreceptors are at an early stage of development. Differentiation of HESC into retinal progenitor cells has been achieved, as has further in vitro differentiation into photoreceptor-like cells.52 As compared to RPE, photoreceptor transplants would require integration into the existing outer nuclear layer and formation of functional synapses to act as a replacement therapy. Conversely, there may be some potential therapeutic effect by providing neurotrophic support to adjacent cells without true synaptic integration. When HESC-derived retinal progenitor cells were cocultured with explants from retinal degeneration mice (Aipl1–/–), they showed incorporation into the retinas, had morphologic characteristics of photoreceptor cells, and were immunoreactive for recoverin – a finding that was only rarely found when cells were cocultured with wild-type retinas.52 Subsequent experiments showed that when these cells were injected into the subretinal space of adult Crx–/– mice (a model of Leber congenital amaurosis), the HESC-derived retinal cells differentiate into photoreceptor-like cells that were immunoreactive for recoverin and rhodopsin and restored light responses with an electroretinogram-like signal in the transplanted eye.53 While the integration of HESC-derived photoreceptors may be a relatively rare event, these results demonstrate in principle that HESC can be used as a source of cells for photoreceptor replacement therapy.
Studies have shown that Noggin (inhibitor of BMP pathway) or Dickkopf (Dkk)-1 (an antagonist of wnt signaling pathway) promote anterior neural identity and then insulin-like growth factor (IGF)-1 promotes the formation of retinal progenitor cells.54 Tom Reh’s lab reported derivation of retinal and photoreceptor-like cells from HESC-derived embryoid bodies grown in suspension culture, through addition of Noggin, Dkk-1, and IGF-1 into neuronal differentiation medium in adherent culture.52,55 After 21 days of induction in the differentiation medium, they found the expression of retinal progenitor cell-related transcription factors such as Rx, Otx2, Pax6, Chx10, and Crx was significantly increased.55 When further cultured, these cells formed neural rosettes that were picked and, allowed to self-aggregate, they formed rosettes with retinal progenitors in the center, differentiated cells showing photoreceptor markers such as recoverin, and RPE differentiation at the periphery.55 Microarray analysis of HESC-derived retinal cells showed a very high correlation between genes expressed in human fetal retina and HESC-derived retinal cells.56
Investigators from the RIKEN Research Institute reported the successful derivation of retinal progenitor cell and photoreceptors from either mouse or HESC using an even more highly defined stepwise method also using suspension culture followed by adherence culture.57,58 The final steps included use of retinoic acid and taurine to induce photoreceptor differentiation. With this induction method, they found that HESC can be differentiated into photoreceptors showing both rhodopsin and recoverin immunoreactivity in 150 days.58
Differentiation of HESC into three-dimensional embryonic and retinal tissues
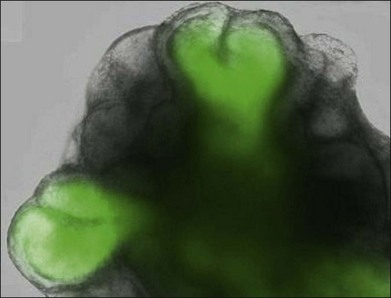
Murine ESC can be differentiated into eye-like structures with cells that have properties of the crystalline lens, neural retina, and RPE.59 Dissociated cells from these eye-like structures were shown to integrate into the retina, especially after retinal injury.60 In April 2011, investigators reported the remarkable discovery that murine ESC aggregates grown in presence of added basement membrane components formed hollow spheres that underwent evagination into vesicles and then invagination into optic cup structures. The self-organizing optic cups had RPE and neural retinal domains and generated stratified neural retinal tissues61 (Fig. 35.2). Later in 2011, it was reported that three-dimensional populations of human retinal progenitor cells can be isolated from early forebrain cultures derived from HESC, and that a transient population of optic vesicle structures arose during the time appropriate for human retinogenesis. These optic vesicle structures could be differentiated into multiple retinal cell types, including RPE and photoreceptors.62
Clearly, there would be great therapeutic value in isolating retinal stem cells from the adult mammalian eye and then using them to regenerate diseased retinas. Recent reports suggest that, if done early enough, some level of visual function may be obtained in animal eyes with retinal degeneration treated with multipotent progenitor cells isolated from retinas of neonatal mice.62a
Retinal pigmented epithelial stem cells
Transplantable RPE cell lines may serve as stem cells of sorts to replenish diseased RPE cells themselves. In a number of macular and retinal degenerative disorders there is atrophy of the RPE and associated malfunctioning in the phototransducing cellular machinery. Damaged RPE cells and associated atrophy are hallmarks of age-related macular degeneration (AMD) and heroic surgical approaches63 have been considered to provide photoreceptors in such individuals with healthier, RPE-rich regions of the retina through retinal translocation and the insertion of RPE sheets. While the former has enjoyed a limited clinical effort,64 the latter is still in the laboratory stages of development and has not yet been employed in the clinic.65,66
RPE cell-based delivery of trophic (and other) factors
Immortalized human RPE cell lines have been created to facilitate RPE cell transplantation as well as their use in cell-based therapeutic approaches. These cells enjoy an extended lifespan after being stably transfected with a plasmid encoding the simian virus 40 large T antigen and many of the factors expressed by functional RPE cells in vivo are observed to be expressed by these transformed cell lines.67 When these cells are transplanted subretinally into a rat model of retinal degeneration (the Royal College of Surgeons (RCS) rat), loss of visual function is attenuated68 and cortically dependent visual function is preserved long-term.69 These RPE cell lines can be transfected with plasmids encoding a variety of trophic factors shown to have protective effects on photoreceptors70,71 and then encapsulated into polymer devices that permit diffusion of cell products into the tissue into which they are transplanted. When transformed RPE cell lines are transfected with a plasmid encoding one such factor, ciliary neurotrophic factor (CNTF), and transplanted directly into the vitreous of dogs with retinal degeneration, photoreceptor degeneration is reduced.72 Furthermore, production of this factor and implantation of the encapsulation device into the vitreous of normal rabbits did not lead to toxic effects on either the electroretinogram or retinal histology in these animals at doses that protect photoreceptors in dogs with retinal degeneration.73 Such cell-based delivery devices may be used to provide trophic factors for the treatment of a variety of retinal degenerative diseases and, in fact, have recently been used in a human clinical trial evaluating the efficacy of CNTF in the treatment of retinal degeneration.73a In this respect, the implanted encapsulated cell devices function as a stem cell might, providing factors critical to the prevention of, or recovery from, retinal degenerative disease.
Embryonic stem cells as a source of RPE
Over the past decade, a number of methods have been reported to achieve the differentiation of HESC into retinal cells.52,58,74–76 Some of these methods were developed for differentiation of HESC into one type of retinal cell such as the RPE58,74–76 or photoreceptors,58,75,76 while other methods have focused on the efficient generation of retinal progenitor cells.52
Differentiation of HESC into RPE
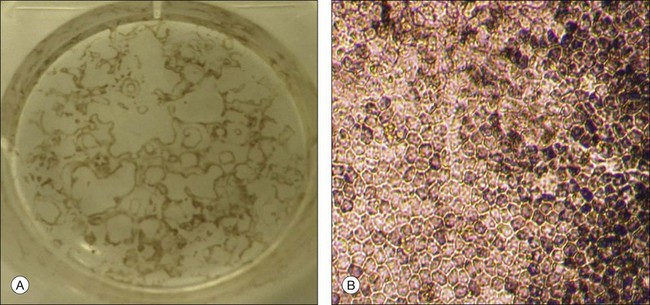
Spontaneous differentiation of HESC into RPE is the simplest and most commonly used method to produce RPE from HESC.75 HESC colonies are first allowed to overgrow in growth factor-supplemented HESC medium until the borders of the colonies contact each other. The medium is then changed to basic HESC medium without basic fibroblast growth factor supplementation and is changed every other day for several months until the RPE cells are seen as small pigmented colonies in the culture dish (Fig. 35.2). RPE can also be derived from HESC using a two-stage induction method: HESC are first differentiated towards a neuroectodermal fate in suspension culture using neural differentiation medium, and then differentiated into RPE. The neural precursors are then plated in cell culture dishes for further differentiation into RPE. During these stages of induction and differentiation, the RPE cells appear as early as 4 weeks and reach a large enough number of cells for subculture at approximately 8–10 weeks.75 Nicotinamide and Activin A (a member of the transforming growth factor-beta superfamily) can also be used to direct the induction of RPE from HESC.83
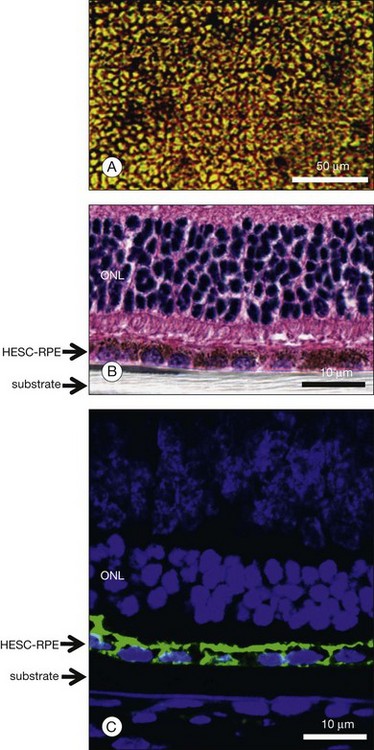
RPE cells can be easily identified and isolated from other differentiated cells in HESC cultures because of their unique pigmentation, hexagonal shape, and pattern of growth. The RPE patches can be either mechanically incised out of the cultures or enzymatically dissociated from the cultures and can be grown to confluence and passaged retaining typical pigmentation and morphology (Fig. 35.3).
Characterization of HESC-derived RPE in vitro
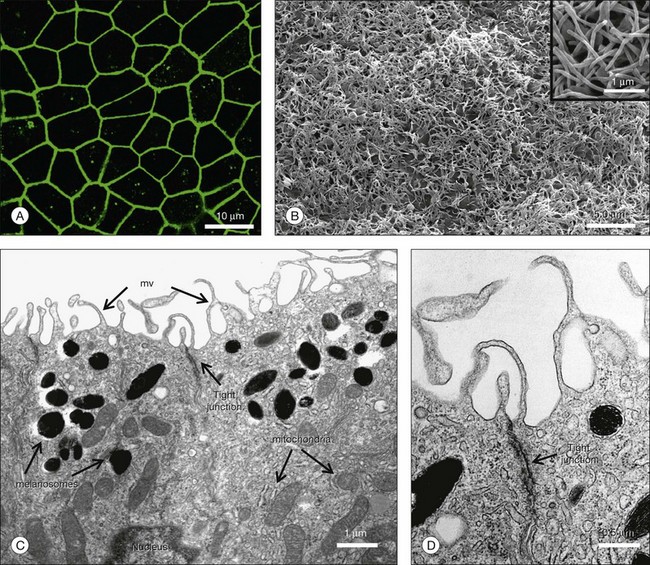
The characteristics that define human RPE cells have been outlined in Chapter 16 (Cell biology of the retinal pigment epithelium) and reviewed by others.74 HESC-derived RPE develop a typical hexagonal shape and become highly pigmented when they attain confluence (Fig. 35.3B). The cells can further differentiate and become highly polarized when grown for extended periods of time on Transwell inserts and other substrates (Fig. 35.4).77,78 Polarized HESC-derived RPE show apical microvilli, are joined in the apical regions by tight junctions, show apically distributed sodium/potassium ATPase (Na+/K+ATPase), and have a high transepithelial resistance.78 HESC-derived RPE express a host of RPE-specific and RPE highly expressed genes including visual cycle genes (RPE65, RDH 11, CRALBP); RPE membrane channel and transporter genes (BEST1, SLC); pigment biosynthesis and melanin biosynthesis genes (GPR143, TYRP1, dopachrome tautomerase (DCT), SILV); and phagocytosis-associated genes (LAMP2, VDP, Mertk, GULP1).75
Efficacy of HESC-derived RPE cells in vivo
The most commonly used model to evaluate therapeutic efficacy of HESC RPE is the RCS rat. The primary defect in the RCS rat is in the RPE; thus this model provides the ability to evaluate the effectiveness of RPE cell replacement therapy. The RCS rat has a recessively inherited mutation in the receptor tyrosine kinase gene Mertk, leading to impaired phagocytosis of shed photoreceptor outer segments with buildup of outer-segment material in the subretinal space, and subsequent secondary degeneration of photoreceptors between postnatal days 20 and 60.79 Using subretinal injections of cell suspensions of HESC RPE, several groups have shown reproducible survival of the transplanted HESC RPE in the subretinal space (>220 days in one study), and positive labeling of these cells with human-specific markers and RPE-specific genes (e.g., RPE65).80–83 The cells appear to disperse in the subretinal space and, while clumps of cells or multilayered grafts are often formed, in some cases they were arranged in an apparent monolayer. Cells showed focal rhodopsin staining, suggesting that they are phagocytosing photoreceptor outer segments.81–83 The transplanted HESC RPE were associated with histologic and functional rescue of the photoreceptors, as measured by delay in the loss of nuclei in the outer nuclear layer, and retention of electroretinogram and optomotor responses in the treated eye compared to untreated eye.80–83 Each of these studies utilized immune suppression (typically systemic cyclosporine, with or without systemic corticosteroid) to prevent immune rejection of the xenograft. Although the subretinal space is thought to be an immune-privileged site, such privilege may be compromised at the time of surgery or by the disease process.84,85
Pivotal safety studies are necessary prior to receiving approval from the Food and Drug Administration (FDA) for a clinical trial. Studies using HESC-derived RPE cell suspensions under good manufacturing practices and good laboratory practice have been performed to establish karyotypic stability in the cells, lack of infectious and adventitious agents in the product, and lack of teratoma and/or tumor formation by the cells in immune-deficient mice.81 It should be noted that G-banding karyotypic analysis may not show any significant abnormalities while high-resolution DNA analysis may show culture-induced copy number changes and loss of heterozygosity; the significance of these changes for the purpose of cell therapy is unknown.18 Importantly, none of the studies that utilized highly differentiated HESC-derived RPE in immune-deficient animals found evidence of teratoma formation.80–83 However, one study in which murine ESC differentiated toward RPE fate for only 7 days and injected in the subretinal space of rd12 (a spontaneous mutant model of Rpe65 Leber congenital amaurosis and having the same genetic background as the murine ESC) developed teratoma tumors in some animals.86
An alternative approach to the use of HESC RPE cell suspensions would be to transplant monolayer sheets of highly differentiated and polarized RPE resting on a biodegradable or nonbiodegradable membrane. The reasoning for such an approach would be that these cells would have a better chance to integrate with the host photoreceptor outer segments as an intact monolayer and thus improve the functionality of the graft.77 It has been shown that HESC RPE can be polarized in vitro to develop tight junctions with high transepithelial resistance and elaborate extensive apical microvilli (Fig. 35.4).78 It has also been observed that highly polarized HESC RPE show increased secretion of pigment epithelial-derived growth factor, a factor with neurotrophic and antiangiogenic activity.78 HESC RPE are capable of specifically phagocytosing photoreceptor outer segments.86a Polarized HESC RPE show increased phagocytosis of bovine rod outer segments in vitro compared to nonpolarized cultures.78 Studies implanting polarized HESC RPE grown on a nonbiodegradable substrate (e.g., parylene) show retention of an intact monolayer in vivo and prominent integration with host photoreceptors (Fig. 35.5).77 The relative efficacy of HESC-derived RPE cell suspensions versus polarized sheets is under active investigation by several groups.
The effect of the local tissue microenvironment on transplant survival is an important issue when considering how best to deliver stem cells and cells derived from stem cells. Cross-talk between cells and extracellular matrix is critical to maintaining the differentiated cell type as well as insuring appropriate function. Many studies, in the retina as well as other tissues, have identified a number of factors that may be critical to the successful engraftment of stem cell-derived, and other, cell types, including cardiomyocytes,87 spinal cord,88 and brain89 neurons and photoreceptors.90
The use of induced pluripotent stem cells as a source of autologous RPE (and other cell type) grafts
One of the most significant recent breakthroughs in stem cell research has been the observation that adult human somatic tissues may be induced to express pluripotency after reactivation of four transcription factors.91 These adult tissue-derived stem cells, called induced pluripotent stem cells (iPSC), can be differentiated into a variety of tissues that may be used as autologous grafts for therapeutic reimplantation in various diseases.92 The use of iPSC circumvents most of the ethical and practical problems associated with large-scale clinical use of ESC, and patients with iPSC-derived autologous transplants may not require lifelong immunosuppressive treatment to prevent graft rejection. However, safety concerns regarding iPSC include malignant transformation resulting from reactivation of the four reprogramming transcription factors (including c-MYC) randomly integrated into the genome at multiple loci following retroviral transduction. In fact, iPSC generation and transformation share many molecular mechanisms, and a high incidence of tumorigenesis is observed in iPSC-derived chimeric mice.93–96 The risk of tumorigenesis cannot be attributed solely to c-MYC reactivation: transgenic mice derived from iPS without c-MYC still form tumors.97
Exhaustive comparative analyses of multiple human (h) iPSC and HESC lines reveal that, while many hiPSC and HESC lines share very similar transcriptomic and epigenetic profiles, others are heterogeneous. The differences observed are randomly distributed and limit the differentiation capacity of the cells.98 Furthermore, recent evidence shows that, in hiPSC, reprogramming and selection pressure to obtain rapidly proliferating cell-lines may induce chromosomal aneuploidy in nonrandomly distributed loci that may further limit the differentiation capacity and promote tumorigenicity of iPSCs.99–101 Genetic instability in iPSC is correlated with higher passage numbers, so reprogramming methods that are inefficient and require multiple passages may increase the risk of tumorigenesis.99,100 Furthermore, high cytotoxicity is observed when reprogramming methods that utilize repeated transfections (reprogramming methods involving mRNA strands or recombinant proteins). Consequently, these methods could induce even higher selection pressures for highly proliferative cells with mutations; however this idea has not yet been directly tested.
To generate RPE from hiPSC for treating diseases such as AMD in which the onset occurs in 55+-year-old humans, the source material (likely fibroblasts or keratinocytes) will be older and more difficult to reprogram. The RPE derivation process is long and all clones will need to be carefully screened before transplantation. Consequently, this treatment strategy could be very economically taxing to patients and healthcare systems. Therefore, to generate any tissue of interest, including RPE from hiPSC, one or two specific reprogramming protocols may need to be adopted and optimized to insure reliable and safe derivations of that cell type. Furthermore, experimental animals with implants should be monitored over extended periods of time to ensure that no tumors form. We have recently reported the generation of iPSCs reprogrammed using two or one factors (OCT4/KLF4 or OCT4 only) and small molecules.102,103 Recent advances in stem cell biology have demonstrated that iPSC can be derived from somatic cells such as skin fibroblasts by using retroviral vectors to introduce reprogramming factors (Oct4, Sox2, Nanog, Lin28) into somatic cells. However, random viral integration and spontaneous reactivation of reprogramming factors during differentiation pose a cancer risk and limit the potential use of iPSC derived in this manner in human therapy. In addition to this risk, therapeutic application of current technology is also limited by its low efficiency and slow kinetics. Finally, there is the requirement for numerous growth factors and animal cell feeder layers to obtain even limited numbers of iPSC. To overcome these hurdles, increase the efficiency of generating iPSC-derived RPE, and avoid using lentiviral vectors, it may be possible to use an episomal, or nonintegrating, vector to deliver the required transcription factors or eliminate the need to deliver such factors by using small molecules to induce pluripotency.
Potential problems associated with the use of iPSC to generate RPE grafts
Current human iPSC technology requires retroviral vectors for delivering reprogramming factors into somatic cells.90,91,104–107 Random viral integration and spontaneous reactivation of reprogramming factors pose cancer risk and impair differentiation, limiting the potential use of iPSC in human therapy.108 While recent reports have shown that adenoviral109 and plasmid110 vectors can reprogram mouse embryonic fibroblasts (MEFs) at very low efficiency, there is no evidence that these methods can be successfully applied to reprogram human somatic cells. To overcome this bottleneck, episomal expression vectors that encode the four human reprogramming factors (Oct4, Sox2, Nanog, Lin28) have been developed.111–113 Transfection of MEFs and human fibroblasts with this vector produced iPSC in which the exogenous factors are eliminated by LoxP/Cre-mediated deletion.113
The recent development of the iPSC reprogramming technology allows, for the first time, the generation of autologous cellular central nervous system grafts from readily accessible cell sources such as skin biopsies.90,91 AMD may represent an ideal target for autologous iPSC RPE transplantation therapy as RPE cell dysfunction in this disease is caused by cellular aging processes rather than a monogenic defect. While several genetic risk alleles for AMD have been identified,114 manifestation age of AMD even in the presence of these risk alleles is above 55 years. Hence, iPSC RPE cell grafts from AMD patients would be expected to represent younger, and presumably healthier, RPE that have not yet themselves become damaged by the aging processes. Furthermore, RPE cell grafts, unlike neuronal cell grafts, would not require synaptic integration into the retinal neuronal network to resume rescue activity and can be delivered locally into the subretinal space, thus avoiding the need for graft cell migration to their final localization.
Recent studies using transcriptomic analyses demonstrated significant differences between gene transcription in HESC/hiPSC-derived RPE and hRPE.115 While useful for monitoring the differentiation status of cells, variations in gene expression at the transcriptional level do not necessarily provide insight into cellular activities. Functional consequences of transcriptional activity can be analyzed with novel metabolomic-based analyses that quantitatively measure the activities of endogenous biochemical pathways.116–118 Application of sophisticated in vivo imaging techniques such as scanning laser ophthalmoscopy, optical coherence tomography, and adaptive optics, coupled with focal electroretinography, will permit the detailed evaluation of therapeutic stem cell treatments.
Synthetic small molecules to enhance iPSC and RPE production
Identification and optimization of chemical tools for HESC iPSC derivation and long-term self-renewal, as well as their differentiation to a lineage-specific functional cell type (e.g., RPE) in chemically defined conditions will provide higher consistency/robustness in HESC iPSC culture, facilitate the derivation and manufacturing of clinical-grade human stem cells for therapy, speed up understanding of the self-renewal and differentiation mechanisms, and help identify and control signaling inputs that direct self-renewal or differentiation of HESCs/iPSCs. The discovery and mechanistic characterization of synthetic small molecules that support self-renewal of ESCs in the absence of feeder cells, serum products, and conventional growth factors119,120 have been described. From reprogramming screens, small molecules that can substitute for reprogramming transcription factors and enhance reprogramming efficiency and kinetics in the generation of iPSCs have also been described.121 These data demonstrate proof of concept that rationally designed, unbiased screens may provide new tools and reveal novel mechanisms and that a single small molecule can be identified from a functional screen to achieve a desired complex phenotype by modulating one or multiple targets. While it has been demonstrated that a single small molecule can have significant positive effects on HESC self-renewal/survival, it is more likely that a combination of several small molecules (each with different mechanisms of action) will generate a more robust/efficient condition.
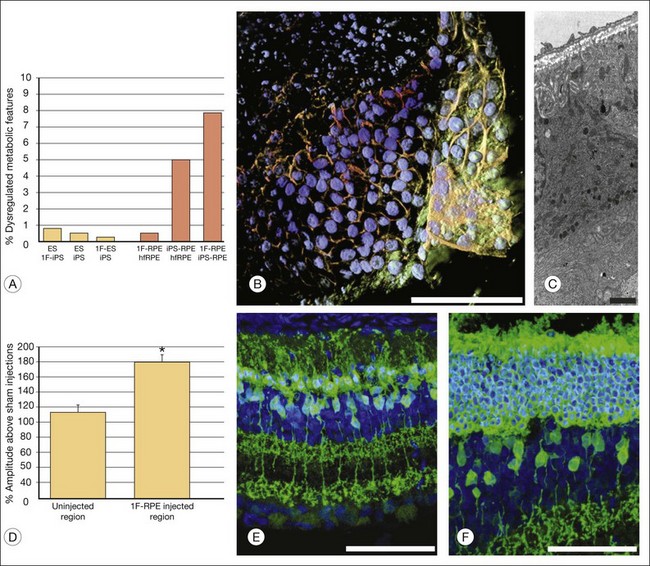
Recently, four, two, and one factor-derived iPSC (4F-, 2F-, and 1F-iPSCs, respectively) were differentiated into fully functional cuboidal-shaped pigmented cells in polarized monolayers that express RPE-specific markers.122 1F-iPS RPE strongly resemble primary human fetal RPE (hfRPE) based on proteomic and untargeted metabolomic analyses, and, utilizing novel in vivo imaging technology coupled with electroretinography, it has been demonstrated that 1F-iPS RPE mediate anatomical and functional rescue of photoreceptors after transplantation in an animal model of RPE-mediated retinal degeneration (Fig. 35.6). 1F-iPS RPE cells were injected subretinally as a suspension and formed a monolayer dispersed between host RPE cells. Furthermore, 1F-iPS-RPE do not simply provide trophic support to rescue photoreceptors as previously speculated, but actually phagocytose photoreceptor outer segments in vivo and restore visual cycling (based on high-resolution mass spectrometry-based detection of recycled photoreceptor protein and lipid endproducts and electron microscopic analysis). Thus, 1F-iPS-RPE grafts may be superior to conventional iPS-RPE for clinical use since 1F-iPS-RPE closely resemble hfRPE, mediate anatomical and functional photoreceptor rescue in vivo, and are generated using a reduced number of potentially oncogenic reprogramming factors.
Adult bone marrow-derived endothelial progenitor cells
The vast majority of diseases that lead to vision loss in industrialized nations do so as a result of abnormalities in the retinal or choroidal vasculature; macula edema, retinal and vitreous hemorrhage, and fibrovascular scarring commonly contribute to visual loss in diseases such as AMD, diabetic retinopathy, retinopathy of prematurity, and neovascular glaucoma. Inherited retinal degenerations such as retinitis pigmentosa are commonly thought of as neuronal degenerations, but most also exhibit vascular abnormalities traditionally attributed to the loss of neuronal elements and accompanying decreased metabolic demand, leading to vascular atrophy. Newly emerging paradigms describe the existence of trophic “cross-talk” between local vascular networks and the tissues they supply and such interactions almost certainly help maintain a functional differentiated state in a variety of organ systems.123–125 In fact, endothelial cells are also now known to provide trophic substances that greatly stimulate self-renewal and expand neural differentiation of neural stem cells.126 Given such interdependence of vascular endothelial cells and surrounding tissues, it may be possible to use one cell type to rescue the other in the face of severe stress such as hypoxia or genetically encoded cell-specific degenerations. Under such conditions it would be desirable to have available populations of progenitor cells useful for such protection.
Adult bone marrow contains a population of endothelial progenitor (stem) cells
Adult bone marrow is known to contain a population of HSCs that can be divided into lineage-positive (Lin+) and lineage-negative (Lin–) subpopulations with regard to their potential to differentiate into formed elements of the blood. The Lin– population contains a variety of progenitor cells, including those capable of becoming vascular endothelial cells.127 These EPC mobilize from the bone marrow in response to a variety of signaling molecules128,129 and can target sites of angiogenesis in ischemic peripheral vasculature,127 myocardium,130 or induced ocular injury,131 where they can incorporate into forming blood vessels and potentially help relieve ischemia. While there is strong evidence supporting the concept that bone marrow contains progenitor cells capable of participating in the repair of a variety of injured tissues,26 there is significant controversy as to how commonly such developmental plasticity is observed in adult HSC132 and even whether these EPC are derived from HSC or, in fact, are derived from an entirely distinct population of bone marrow-derived stem cells. While many reports in the literature demonstrate that HSC can differentiate into a variety of cell types other than hematopoietic cells, including neurons, glia, and muscle, depending on their microenvironment,133 the precise identity of the precursor cell remains unclear. While there is a large literature clearly describing the presence of EPC in the bone marrow of adult mammals, recent work has challenged whether it is actually the HSC fraction that contains the EPC and that the EPC exists as a distinct cell type residing in the bone marrow stroma and simply copurifies with Lin– HSC. A less controversial, but not necessarily more appropriate, name for this progenitor might be “bone marrow-derived EPC.” While the lineage from which these cells are derived remains controversial, their existence is not, and is well documented. Controversy in this area is not reserved for the origin of the EPC: while the ability of bone marrow-derived HSC to differentiate into neural cells remains controversial, just as much confusion surrounds the issue of whether or not neural stem cells can transdifferentiate into endothelial cells. While earlier studies suggested that this was a rare property of HSC,7 more recent studies have demonstrated that neural stem cells can differentiate into an endothelial cell lineage via a cell fusion-independent mechanism.8
The potential clinical utility of these cells is significant26 and falls into three broad categories. If circulating bone marrow-derived EPC target sites of ischemia and, thus, contribute to pathological neovascularization, it seems reasonable to inhibit their targeting or differentiation, thereby inhibiting angiopathies of the type seen in retinal and choroidal neovascularization. Alternatively, enhancing their participation in functional, ischemia-relieving angiogenesis may be of benefit in ischemic retinopathies such as diabetes. Finally, if EPC do, indeed, target to sites of neovascularization, it should be possible first to transfect these cells ex vivo with plasmids encoding angiostatic or angio/neurotrophic proteins and, thus, inhibit abnormal angiogenesis or enhance trophic activity of EPC through a form of cell-based therapy. Each of these approaches will be discussed.
Bone marrow-derived EPC can contribute to retinal and choroidal neovascularization
The observation that HSCs contain a pool of EPC capable of incorporating into retinal vasculature has recently been demonstrated by several groups. The study by Grant and colleagues was the first direct demonstration that systemically administered HSC can function as hemangioblasts during hypoxia-stimulated retinal neovascularization.134 In these studies, single HSCs isolated from bone marrow of adult transgenic mice expressing green fluorescent protein (GFP) were injected intravenously into mice that had been sublethally irradiated to destroy host bone marrow. Serial long-term transplants were performed to be certain that the reconstituted bone marrow arose from the injected stem cells. After recovering from the radiation treatment and demonstrating that engraftment was established, the retina in these mice was treated with thermal laser to stimulate retinal neovascularization and it was demonstrated that the engrafted GFP bone marrow-derived stem cells contributed to the neovascularization. This model demonstrated that circulating, undifferentiated precursor cells can be recruited to sites of retinal neovascularization and, along with proliferation of local endothelial cells, can contribute to new blood vessel growth and development. The relative contribution of circulating precursor cells and endogenous retinal vascular endothelial cells to newly forming vasculature in human disease remains unknown; the experiments of Grant and colleagues131 demonstrate that circulating cells can incorporate into laser-stimulated retinal neovascularization, but the role of these cells in nonirradiated hosts where the proliferation of local inflammatory, precursor, and endothelial cells is not impaired by lethal irradiation remains unclear. Studies from several groups have demonstrated, using the same irradiation/bone marrow reconstitution model, that circulating stem cells can also contribute to laser-stimulated choroidal neovascularization.135–137
If, indeed, circulating EPC contribute to pathological neovascularization in ischemic and inflammatory retinopathies such as diabetic retinopathy and AMD, would inhibition of their targeting to these sites reduce abnormal angiogenesis? In order to evaluate this potential therapeutic intervention better, it is necessary to know what factors are responsible for appropriate targeting of EPC. One study138 has demonstrated a role for the adhesion molecule, R-cadherin, in the targeting of HSC to the retinal vasculature. When small-molecule antagonists or function-blocking antibodies to R-cadherin are used to pretreat Lin– HSC prior to intravitreal injection, the cells no longer target sites of angiogenesis and participate in the formation of new retinal blood vessels. Other studies suggest that certain adhesion molecules, such as the integrin alpha 4 beta 1, may play a role in targeting of circulating EPC to sites of abnormal angiogenesis during vascularization of tumors and these same integrins may be potential therapeutic targets if, indeed, circulating EPC contribute to pathological ocular angiogenesis. Interfering with the function of such targeting molecules used by EPC to target sites of pathological neovascularization in combination with cell-based therapies to produce angiostatic molecules locally could significantly reduce abnormal angiogenesis. Unfortunately, inhibition of neovascularization under such ischemic conditions may serve to promote ongoing ischemia: would it be better to coax the newly forming vessels into functional ones that could alleviate hypoxia or make the endogenous vasculature and neurons more resistant to hypoxic damage?
Bone marrow-derived EPC can exert a vasculotrophic rescue
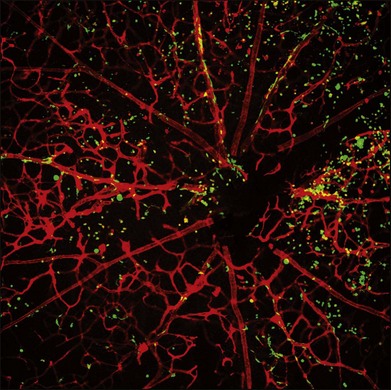
In order to address the potential utility of these cells in relieving hypoxia and help rebuild a damaged retinal vasculature, Lin– HSCs have been injected directly into the eyes of newborn mice while they were forming their retinal vasculature (Fig. 35.7); in this environment, these cells can target activated astrocytes, a hallmark of many ocular vascular and degenerative diseases. Once targeted to this template of activated astrocytes, the Lin– HSCs participate in normal developmental angiogenesis in both neonatal mice and injury-induced neovascularization in the adult.139
Is there a population of adult bone marrow-derived progenitor cells that can be used to target, incorporate into, and “stabilize” a degenerative, abnormal retinal vasculature? Studies139 suggest that this, in fact, may be possible. The HSC fraction used in these studies not only inhibited angiogenesis when engineered to express an antiangiogenic, but also rescued and stabilized (e.g., “matures”) degenerating vessels. More surprisingly, it was also observed that by preventing vascular degeneration there is a trophic rescue effect on the photoreceptors themselves,124 suggesting that autologous bone marrow grafts of HSC fractions containing EPC may provide trophic effects on associated neural tissue that goes beyond simple nutrition. Such observations could provide a rationale for the use of HSC in the treatment of a variety of inherited retinal degenerations such as retinitis pigmentosa.
The potential use of HSC for cell-based therapies in the eye presents a technical challenge; stem cells are notoriously difficult to transfect and, thus, it will be necessary to improve transfection efficiency. Another enigma in the circulating stem cell field is the issue of HSC “homing”; R-cadherin is clearly involved, but all of the molecular signals have not yet been identified. Identification of these signals would be of immense benefit in terms of exploiting the potential use of HSC in therapeutic angiogenesis as well as directed cell therapy. Finally, which cell type in adult bone marrow actually adheres to astrocytes and incorporates into the developing vasculature? Many of the surface markers routinely used to identify cell populations vary from laboratory to laboratory and, certainly, expression of these markers depends on the environment into which the cells are placed.140 Thus, sorting cells based on cell surface antigen expression too early in their lineage may lead to cases of mistaken identity or loss of potentially useful cells. Functional definition of stem cell populations, as done in the Otani139 study, surely provides an assessable endpoint but warrants further investigation. Finally, the use of stem cells as drug delivery vehicles has the potential to deliver drugs selectively and potently to the back of the eye in physiologically meaningful doses. Since this is where most vision-threatening pathologies occur, the use of these cells, as trophic cellular devices or drug delivery vehicles, holds great promise for millions of patients suffering from currently untreatable diseases.
Bone marrow-derived EPC can exert a neurotrophic rescue in retinal degeneration
Inherited degenerations of the retina affect as many as 1 in 3500 individuals and are characterized by progressive night blindness, visual field loss, optic nerve atrophy, arteriolar attenuation, altered vascular permeability, and central loss of vision, often progressing to complete blindness. Molecular genetic analysis of these diseases has identified mutations in over 110 different genes, accounting for only a relatively small percentage of the known affected individuals141,142; many of these mutations are associated with enzymatic and structural components of the phototransduction machinery, including rhodopsin,143 cGMP phosphodiesterase,144 rds peripherin,145 and RPE65.146 Despite these observations, there are still no effective treatments to slow or reverse the progression of these diseases. Recent advances in gene therapy have led to successful reversal of the rds147 and rd148 phenotypes in mice and the RPE65 phenotype in dogs149 when the wild-type transgene is delivered to photoreceptors or the RPE in animals with a specific mutation. The potential use of calcium channel blockers,150 trophic factors,151 and dietary supplements152 has also been explored in recent studies. Most inherited human retinal degenerations specifically affect rod photoreceptors but there is concomitant loss of cones, the principal cellular component of the macula. Cone-specific survival factors have been described153 and may facilitate cone survival in mouse models of retinal degeneration.
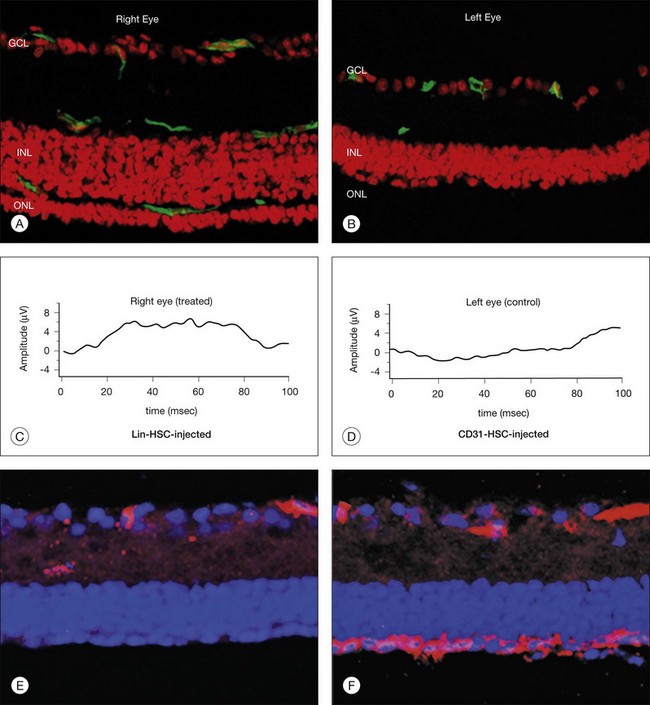
In addition to the vasculotrophic properties described above, these cells have also recently been reported to prevent completely retinal vascular degeneration, ordinarily observed in mouse models of retinal degeneration, and that the vascular rescue correlates with neuronal rescue (Fig. 35.8). The inner nuclear layer remains nearly normal and the outer nuclear layer containing photoreceptors is significantly preserved, with the rescued cells being predominantly cones. Detectable, albeit severely abnormal, electroretinogram recordings are observed in rescued mice at times when they are never observed in control-treated, or untreated, rd/rd eyes. This rescue effect is also observed when human bone marrow-derived Lin– HSCs are used to treat severe combined immunodeficient mice with retinal degeneration. Large-scale genomic analysis of rescued and nonrescued eyes revealed significant upregulation of antiapoptotic genes. It is important to note that the injected bone marrow-derived progenitor cells are never observed anywhere but in or near blood vessels; since these cells are derived from GFP transgenic mice in which all the cells are green, it is easy to determine which structures in the retina are derived from the injected progenitor cells. Green donor cells are never observed in the nuclear layers or the rescued retinas. These findings demonstrate that this newly described neurotrophic effect correlates with preservation of the vasculature and suggest that autologous bone marrow-derived EPCs may be useful in the treatment of currently untreatable retinal degenerative diseases as well as ones in which abnormal angiogenesis cause the vision loss.
The concept that tissue-specific vasculature has trophic effects that go beyond that expected from simply providing vascular “nourishment” is supported in numerous recent reports in the literature. For example, liver endothelial cells can be induced to produce, after vascular endothelial cell growth factor receptor-1 activation, growth factors critical to hepatocyte regeneration and maintenance in the face of hepatic injury.123 Similar interactions between vascular endothelial cells and adjacent hepatic parenchymal cells are necessary for liver organogenesis, well before the formation of functional blood vessels.125 In individuals with retinal degeneration, the presence of endothelial progenitors derived from bone marrow HSC populations may make the vasculature more resistant to degeneration and at the same time facilitate retinal neuronal survival. In humans with this disease, slowing the rate of degeneration even to a limited extent may provide years of additional sight. While the animal studies discussed above had significant, but not dramatic, preservation of electroretinogram signals, even such minimal signs of electrical function in the retina may be sufficient to support vision.
Adult bone marrow-derived stem cells may have wide utility in the treatment of retinal vascular diseases and even inherited retinal degenerations. While the use of these cells to target neovasculature and contribute to the stabilization of otherwise friable vessels in ischemic retinopathies may seem intuitive, the neurotrophic effect observed is surprising but reasonable given the “protective umbrella” effect afforded by these heat shock protein-laden cells. Thus, potential applications of these cells include not only as cell-based therapeutic delivery vehicles, but also as possible stabilizing elements in an otherwise unstable neovasculature of the type observed in ischemic retinopathies. In fact, these cells will selectively target sites of hypoxia-driven neovascularization, as demonstrated in mouse models of these diseases (Fig. 35.9). While current retinal vascular disease therapy is based largely on ablating the new vessels with angiostatics or thermal and nonthermal lasers, a novel paradigm would include the use of these stem cells to target, incorporate into, and stabilize neovasculature.
Cord blood-derived stem cells
Umbilical cord blood (UCB) is a rich source of HSCs, including high numbers of early and late myeloid progenitor cells.154 Compared to adult bone marrow and peripheral blood, UCB-derived progenitor cells exhibit increased capacity to proliferate and subsequently differentiate into colony-forming unit granulocyte macrophages.155 In addition, UCB-derived cells are less mature relative to adult stem cells; they have not been exposed to immunologic challenge and are unable to activate cytotoxic T lymphocytes to synthesize proinflammatory cytokines.156
Reports from several groups have shown that local transplantation or intravenously injected UCB-derived mononuclear cells improves functionality in areas of ischemia.154,157 This rescue function is associated with the CD14+ monocyte fraction since depletion of these cells from the UCB led to loss of the rescue function in a rat model of middle cerebral artery occlusion.158 In vitro, the UCB-derived CD14+ cells can differentiate into endothelial,159,160, neuronal,161,162 and mature myeloid (e.g., monocytes, macrophages, and dendritic) cells. UCB-derived neural progenitors have also been observed to be neuroprotective in an in vitro model of ischemia.163 Recent reports suggest that different tissue microenvironments expose myeloid cells to multiple stimuli, influencing their fate into pro- and anti-inflammatory macrophages (M1 and M2, respectively), endothelial, or dendritic cells.164–168 Thus, different subpopulations of myeloid cells could exert opposite effects during angiogenesis: one population may contribute to plaque formation in experimental models of arterial occlusion169 whereas another may promote collateral growth to alleviate ischemia.170 The retina provides a good model system in which to study these discordant results; tissue-resident and recruited macrophage populations have been implicated in both developmental171 and pathologic angiogenesis,172 processes that may occur simultaneously in the same tissue during response to ischemic injury.
A recent study173 has examined the role of macrophages in the establishment of vascular networks. These cells act as “bridge cells” by establishing tip cell anastomosis and releasing angiogenic factors, such as vascular endothelial growth factor, that can lead to vessel fusion and vascular network formation. Previous studies of the same cells have demonstrated that tumors or developing/regenerating tissues can actively recruit these cells from the circulation.174
Recently, hUCB has been used to obtain an enriched population of myeloid progenitor cells, CD14+ cells, which, when injected in eyes of mice with oxygen-induced retinopathy, exerted a profound rescue effect.175 Only CD14+ cells polarized to M2 macrophages were able to control the pathological neovascularization. Transcriptomic and metabolomic analyses were used to describe the molecular events involved in the regulation of the observed rescue effect. From these studies it was concluded that hUCB CD14+ cells differentiate in vivo into type 2 macrophages and initiate a series of events associated with modulation of the inflammatory response, leading to reduced oxidative stress and apoptosis and, ultimately, promoting normal angiogenesis and tissue repair in the retina.
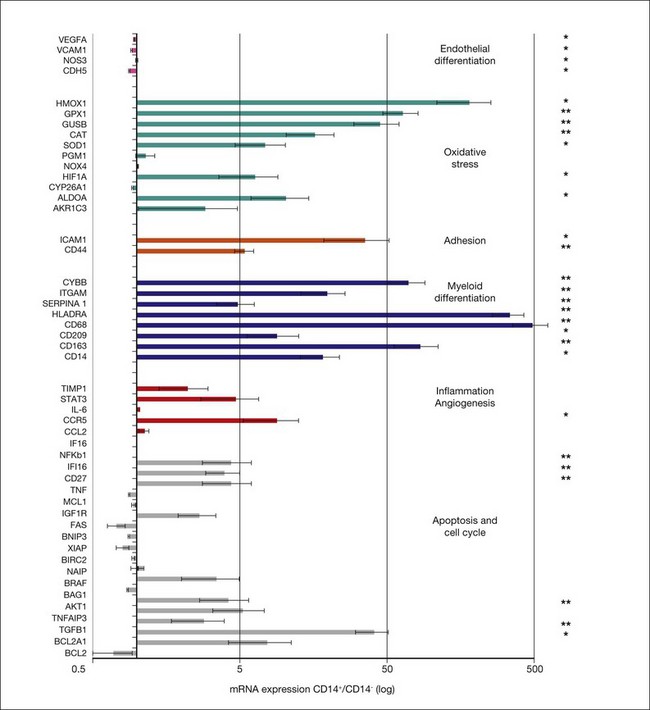
A rigorous characterization of what the progenitor/stem cell populations are before being injected into normal or diseased eyes and what they differentiate into after being placed in different microenvironments is critical to understanding how these cells achieve functional or trophic rescue. The use of new metabolomic and transcriptomic technologies to aid in these assessments has proven valuable in determining how injected stem cells interact with their environment and modulate the diseased state. For example, prior to injection into the eye, cord blood-derived CD14+ cells express mRNAs associated with the oxidative stress response, cell cycle, and macrophage cell markers. The molecular changes associated with stabilization of retinal vasculature by hUCB-derived CD14+ cells were assessed at the mRNA level by selectively detecting and quantifying the expression level of 50 human genes in the mouse retina. Specifically, the expression ratio of human genes in the mouse retina at P17 after intravitreal injection at P7 of CD14+, CD14− cells (control population), or vehicle in the oxygen-induced retinopathy model were measured (Fig. 35.10). Transcriptomic analysis of retinas treated with CD14+ cells revealed increased expression of human antigens of M2 macrophages. The molecule CD14 and other monocytic cell markers such as CD163 27, CD68 28, CD209 29, and HLA-DRA 30 were highly upregulated after the injection of CD14+ relative to CD14− (CD14+/CD14− >1.6). Importantly, we also detected increased expression of human genes (CD14+/CD14− >1.6) associated with the oxidative stress response, including SOD1, encoding superoxide dismutase 1. Interestingly, in freshly isolated cells (day 0), SOD1 expression was higher in the CD14− population compared to CD14+ cells. Overall, the transcriptomic data support histological and antibody array results, indicating that CD14+ cells differentiate into a macrophage polarized M2 type and exert rescue effects in association with the expression of genes that regulate oxidative stress, apoptosis, and control of inflammation and angiogenesis.
Human clinical trials using stem cells for the treatment of retinal diseases
In recent years, many groups176–178 have proposed using HESC- and/or iPSC-derived cells for the therapy of retinal diseases such as AMD. Human trials using ESC became a reality in 2009 (Table 35.1) with the approval by the FDA for a phase I trial (NCT01217008) sponsored by Geron Corporation. In this trial, HESC differentiated into oligodendrocyte precursor cells (GRNOPC1) are injected into the spinal cord of patients with acute, severe spinal cord injury. Approximately 2 million cells are injected. The first patient was treated in October 2010; however additional enrollment has been discontinued due to corporate restructuring at Geron.
Concluding remarks
The potential use of stem cells in the treatment of a variety of human retinal diseases remains tremendously exciting, with multiple potential approaches. Use of fetal tissue remains limited by ethical controversy, but recent changes in federal guidelines for HESC research has made it possible to move forward with this approach. Recently initiated clinical trials using HESC-derived RPE cells to treat patients with Stargardt disease and AMD is a first step in taking this technology into the clinics and will be followed very closely. Adult tissue, while significantly less controversial and more readily available, holds promise, particularly in light of recent advances in generating iPSC from adult somatic tissues. In addition to iPSC-derived tissues, populations of progenitor cells exist in adult tissues and bone marrow and each may have significant clinical utility as we seek to repair and rebuild damaged retinas. Even if such complex tissue reconstruction from stem cells could be successfully completed, re-establishing functional visual pathways will be an even greater challenge. Clearly, prevention of retinal degeneration and vascular abnormalities would preserve established visual pathways and provide a better chance of preserving vision. In this regard, vasculo- and neurotrophic rescue effects of adult bone marrow and neonatal cord blood-derived progenitor cells offer great promise. An area that warrants additional study involves the potential involvement of bone marrow-derived EPC in the development and progression of retinal neovascularization, as has been suggested by a number of animal studies. The potential utility of using levels of circulating endothelial progenitor cells as surrogate markers of risk for ischemic myocardial disease has been discussed.179,180 Whether similar surrogates could be used to predict the risk for pathological neovascularization in retinal vascular disease remains untested. If such cells are documented to participate in retinal or choroidal neovascularization it may be useful to consider preventing the targeting of such cells to sites of ocular angiogenesis, but possibly at risk to the same elderly individuals that utilize such cells positively to facilitate cardiac collateralization in the face of ischemia. The challenge remains to identify successfully the clinically useful progenitor cell types and develop techniques to diagnose disease earlier so that these cells can be successfully utilized prior to significant retinal damage.
1 Zacharias DG, Nelson TJ, Mueller PS, Hook CC. The science and ethics of induced pluripotency: what will become of embryonic stem cells? Mayo Clin Proc. 2011;86(7):634–640. PMID: 21719620
2 Ivanova NB, Dimos JT, Schaniel C, et al. A stem cell molecular signature. Science. 2002;298:601–604.
3 Ramalho-Santos M, Yoon S, Matsuzaki Y, et al. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600.
4 Cai J, Weiss ML, Rao MS. In search of “stemness”. Exp Hematol. 2004;32:585–598.
5 Bjornson CR, Rietze RL, Reynolds BA, et al. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537.
6 Shih CC, Weng Y, Mamelak A, et al. Identification of a candidate human neurohematopoietic stem-cell population. Blood. 2001;98:2412–2422.
7 Morshead CM, Benveniste P, Iscove NN, et al. Hematopoietic competence is a rare property of neural stem cells that may depend on genetic and epigenetic alterations. Nat Med. 2002;8:268–273.
8 Wurmser AE, Nakashima K, Summers RG, et al. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature. 2004;430:350–356.
9 Raff M. Adult stem cell plasticity: fact or artifact? Annu Rev Cell Dev Biol. 2003;19:1–22.
10 Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648.
10a Greenow K, Clarke AR. Controllling the stem cell compartment and regeneration in vivo: the role of pluripotency pathways. Physiol Rev. 2012;92:75–99.
11 Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156.
12 Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638.
13 Lin S, Talbot P. Methods for culturing mouse and human embryonic stem cells. Methods Mol Biol. 2011;690:31–56.
14 Thomson JA, Kalishman J, Golos TG, et al. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci U S A. 1995;92:7844–7848.
15 Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147.
16 Shamblott MJ, Axelman J, Wang S, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci U S A. 1998;95:13726–13731.
17 Hardy K, Handyside AH, Winston RM. The human blastocyst: cell number, death and allocation during late preimplantation development in vitro. Development. 1989;107:597–604.
18 Klimanskaya I, Young C, Becker S, et al. Derivation of human embryonic stem cells from single blastomeres. Nature Protocols. 2007;2:1963–1972.
18a Yamanaka S. Induced Pluripotent Stem Cells: Past, Present, and Future. Cell Stem Cell. 2012;10:678–684.
19 Korbling M, Estrov Z. Adult stem cells for tissue repair – a new therapeutic concept? N Engl J Med. 2003;349:570–582.
20 Lee MS, Makkar RR. Stem-cell transplantation in myocardial infarction: a status report. Ann Intern Med. 2004;140:729–737.
21 Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668.
22 Balsam LB, Wagers AJ, Christensen JL, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673.
23 Anderson DJ, Gage FH, Weissman IL. Can stem cells cross lineage boundaries? Nat Med. 2001;7:393–395.
24 McKay RD. Stem cell biology and neurodegenerative disease. Philos Trans R Soc Lond B Biol Sci. 2004;359:851–856.
25 Asakura A. Stem cells in adult skeletal muscle. Trends Cardiovasc Med. 2003;13:123–128.
26 Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712.
27 Yamashita JK. Differentiation and diversification of vascular cells from embryonic stem cells. Int J Hematol. 2004;80:1–6.
28 Reh TA, Levine EM. Multipotential stem cells and progenitors in the vertebrate retina. J Neurobiol. 1998;36:206–220.
29 Reh TA, Fischer AJ. Stem cells in the vertebrate retina. Brain Behav Evol. 2001;58:296–305.
30 Livesey FJ, Young TL, Cepko CL. An analysis of the gene expression program of mammalian neural progenitor cells. Proc Natl Acad Sci U S A. 2004;101:1374–1379.
31 Blackshaw S, Harpavat S, Trimarchi J, et al. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2:E247.
32 Dyer MA, Cepko CL. Regulating proliferation during retinal development. Nat Rev Neurosci. 2001;2:333–342.
33 Das AV, James J, Zhao X, et al. Identification of c-Kit receptor as a regulator of adult neural stem cells in the mammalian eye: interactions with Notch signaling. Dev Biol. 2004;273:87–105.
34 James J, Das AV, Bhattacharya S, et al. In vitro generation of early-born neurons from late retinal progenitors. J Neurosci. 2003;23:8193–8203.
35 James J, Das AV, Rahnenfuhrer J, et al. Cellular and molecular characterization of early and late retinal stem cells/progenitors: differential regulation of proliferation and context dependent role of Notch signaling. J Neurobiol. 2004;61:359–376.
36 Konobu T, Sessler F, Luo LY, et al. The hNT human neuronal cell line survives and migrates into rat retina. Cell Transplant. 1998;7:549–558.
37 Martinez-Serrano A, Rubio FJ, Navarro B, et al. Human neural stem and progenitor cells: in vitro and in vivo properties, and potential for gene therapy and cell replacement in the CNS. Curr Gene Ther. 2001;1:279–299.
38 Bain G, Kitchens D, Yao M, et al. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168:342–357.
39 Tropepe V, Coles BL, Chiasson BJ, et al. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–2036.
40 Ahmad I, Tang L, Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem Biophys Res Commun. 2000;270:517–521.
41 Ahmad I, Das AV, James J, et al. Neural stem cells in the mammalian eye: types and regulation. Semin Cell Dev Biol. 2004;15:53–62.
42 Klassen H, Sakaguchi DS, Young MJ. Stem cells and retinal repair. Prog Retin Eye Res. 2004;23:149–181.
43 Cicero SA, Johnson D, Reyntjens S, et al. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proc Natl Acad Sci U S A. 2009;106:6685–6690.
44 Gualdoni S, Baron M, Lakowski J, et al. Adult ciliary epithelial cells, previously identified as retinal stem cells with potential for retinal repair, fail to differentiate into new rod photoreceptors. Stem Cells. 2010;28:1048–1059.
45 Fischer AJ, Reh TA. Potential of Muller glia to become neurogenic retinal progenitor cells. Glia. 2003;43:70–76.
46 Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136.
47 Ooto S, Akagi T, Kageyama R, et al. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004;101:13654–13659.
48 Harada C, Guo X, Namekata K, et al. Glia- and neuron-specific functions of TrkB signalling during retinal degeneration and regeneration. Nature Commun. 2011;2:189.
49 Karl MO, Reh TA. Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends Mol Med. 2010;16:193–202.
50 Dorrell MI, Aguilar E, Jacobson R, et al. Antioxidant or neurotrophic factor treatment preserves function in a mouse model of neovascularization-associated oxidative stress. J Clin Invest. 2009;119:611–623.
51 Dalkara D, Kolstad KD, Guerin KI, et al. AAV mediated GDNF secretion from retinal glia slows down retinal degeneration in a rat model of retinitis pigmentosa. Mol Ther. 2011;19:1602–1608.
52 Lamba DA, Karl MO, Ware CB, et al. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:12769–12774.
53 Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–79.
54 Mukhopadhyay M, Gorivodsky M, Shtrom S, et al. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development. 2006;133:2149–2154.
55 Reh TA, Lamba D, Gust J. Directing human embryonic stem cells to a retinal fate. Methods Mol Biol. 2010;636:139–153.
56 Lamba DA, Reh TA. Microarray characterization of human embryonic stem cell–derived retinal cultures. Invest Ophthalmol Vis Sci. 2011;52:4897–4906.
57 Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215–224.
58 Osakada F, Ikeda H, Sasai Y, et al. Stepwise differentiation of pluripotent stem cells into retinal cells. Nature Protocols. 2009;4:811–824.
59 Hirano M, Yamamoto A, Yoshimura N, et al. Generation of structures formed by lens and retinal cells differentiating from embryonic stem cells. Dev Dynamics. 2003;228:664–671.
60 Aoki H, Hara A, Niwa M, et al. Transplantation of cells from eye-like structures differentiated from embryonic stem cells in vitro and in vivo regeneration of retinal ganglion-like cells. Graefes Arch Klin Exp Ophthalmol. 2008;246:255–265.
61 Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56.
62 Meyer JS, Howden SE, Wallace KA, et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29:1206–1218.
62a Pearson RA, Barber AC, Rizzi M, et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485:99–103.
63 Cahill MT, Freedman SF, Toth CA. Macular translocation with 360 degrees peripheral retinectomy for geographic atrophy. Arch Ophthalmol. 2003;121:132–133.
64 Lai JC, Lapolice DJ, Stinnett SS, et al. Visual outcomes following macular translocation with 360-degree peripheral retinectomy. Arch Ophthalmol. 2002;120:1317–1324.
65 Huang JC, Ishida M, Hersh P, et al. Preparation and transplantation of photoreceptor sheets. Curr Eye Res. 1998;17:573–585.
66 Ishida M, Lui GM, Yamani A, et al. Culture of human retinal pigment epithelial cells from peripheral scleral flap biopsies. Curr Eye Res. 1998;17:392–402.
67 Kanuga N, Winton HL, Beauchene L, et al. Characterization of genetically modified human retinal pigment epithelial cells developed for in vitro and transplantation studies. Invest Ophthalmol Vis Sci. 2002;43:546–555.
68 Lund RD, Adamson P, Sauve Y, et al. Subretinal transplantation of genetically modified human cell lines attenuates loss of visual function in dystrophic rats. Proc Natl Acad Sci U S A. 2001;98:9942–9947.
69 Coffey PJ, Girman S, Wang SM, et al. Long-term preservation of cortically dependent visual function in RCS rats by transplantation. Nat Neurosci. 2002;5:53–56.
70 LaVail MM, Yasumura D, Matthes MT, et al. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci. 1998;39:592–602.
71 Faktorovich EG, Steinberg RH, Yasumura D, et al. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature. 1990;347:83–86.
72 Tao W, Wen R, Goddard MB, et al. Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2002;43:3292–3298.
73 Bush RA, Lei B, Tao W, et al. Encapsulated cell-based intraocular delivery of ciliary neurotrophic factor in normal rabbit: dose-dependent effects on ERG and retinal histology. Invest Ophthalmol Vis Sci. 2004;45:2420–2430.
73a Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: Phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103:3896–3901.
74 Bharti K, Miller SS, Arnheiter H. The new paradigm: retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell Melanoma Res. 2011;24:21–34.
75 Rowland TJ, Buchholz DE, Clegg DO. Pluripotent human stem cells for the treatment of retinal disease. J Cell Physiol. 2012;227:457–466.
76 Vugler A, Lawrence J, Walsh J, et al. Embryonic stem cells and retinal repair. Mechanisms Dev. 2007;124:807–829.
77 Sonoda S, Spee C, Barron E, et al. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat Protocols. 2009;4:662–673.
78 Zhu D, Deng X, Spee C, et al. Polarized secretion of PEDF from human embryonic stem cell-derived RPE promotes retinal progenitor cell survival. Invest Ophthalmol Vis Sci. 2011;52:1573–1585.
79 D’Cruz PM, Yasumura D, Weir J, et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9:645–651.
80 Lund RD, Wang S, Klimanskaya I, et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8:189–199.
81 Lu B, Malcuit C, Wang S, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27:2126–2135.
82 Vugler A, Carr AJ, Lawrence J, et al. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp Neurol. 2008;214:347–361.
83 Idelson M, Alper R, Obolensky A, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009;5:396–408.
84 Zhou R, Caspi RR. Ocular immune privilege. F1000 Biol Rep 2(2010).
85 Masli S, Vega JL. Ocular immune privilege sites. Methods Mol Biol. 2011;677:449–458.
86 Wang NK, Tosi J, Kasanuki JM, et al. Transplantation of reprogrammed embryonic stem cells improves visual function in a mouse model for retinitis pigmentosa. Transplantation. 2010;89:911–919.
86a Carr AJ, Vugler A, Lawrence J, et al. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol Vis. 2009;15:283–295.
87 Li TS, Cheng K, Lee ST, et al. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell–matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–2098.
88 Salazar DL, Uchida N, Hamers FP, et al. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS ONE. 2010;5:e12272.
89 Monnier PP, Sierra A, Schwab JM, et al. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci. 2003;22:319–330.
90 Tucker B, Klassen H, Yang L, et al. Elevated MMP expression in the MRL mouse retina creates a permissive environment for retinal regeneration. Invest Ophthalmol Vis Sci. 2008;49:1686–1695.
91 Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872.
92 Koch P, Kokaia Z, Lindvall O, et al. Emerging concepts in neural stem cell research: autologous repair and cell-based disease modelling. Lancet Neurol. 2009;8:819–829.
93 Aoi T, Yae K, Nakagawa M, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702.
94 Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317.
95 Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476.
96 Markoulaki S, Hanna J, Beard C, et al. Transgenic mice with defined combinations of drug-inducible reprogramming factors. Nat Biotechnol. 2009;27:169–171.
97 Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745.
98 Bock C, Kiskinis E, Verstappen G, et al. Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144:439–452.
99 Hussein SM, Batada NN, Vuoristo S, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–62.
100 Laurent LC, Ulitsky I, Slavin I, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8:106–118.
101 Mayshar Y, Ben-David U, Lavon N, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7:521–531.
102 Li W, Zhou H, Abujarour R, et al. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells. 2009;27:2992–3000.
103 Zhu S, Li W, Zhu H, et al. Reprogramming of human primary keratinocytes by Oct4 with chemical compounds. Cell Stem Cell. 2010;7:651–655.
104 Lowry WE, Richter L, Yachechko R, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105:2883–2888.
105 Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146.
106 Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676.
107 Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324.
108 Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9:725–729.
109 Stadtfeld M, Nagaya M, Utikal J, et al. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949.
110 Okita K, Nakagawa M, Hyenjong H, et al. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953.
111 Sidhu KS. New approaches for the generation of induced pluripotent stem cells. Expert opin biol ther. 2011;11:569–579.
112 Hu K, Yu J, Suknuntha K, et al. Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood. 2011;117:e109–e119.
113 Zhao T, Zhang ZN, Rong Z, et al. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215.
114 Seddon JM, Reynolds R, Maller J, et al. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest Ophthalmol Vis Sci. 2009;50:2044–2053.
115 Liao J-L, Yu J, Huang K, et al. Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Hum Mol Genet. 2010;19:4229–4238.
116 Baker M. Metabolomics: from small molecules to big ideas. Nat Meth. 2011;8:117–121.
117 Wikoff WR, Kalisak E, Trauger S, et al. Response and recovery in the plasma metabolome tracks the acute LCMV-induced immune response. J Proteome Res. 2009;8:3578–3587.
118 Yanes O, Clark J, Wong DM, et al. Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol. 2010;6:411–417.
119 Chen S, Do JT, Zhang Q, et al. Self-renewal of embryonic stem cells by a small molecule. Proc Natl Acad Sci U S A. 2006;103:17266–17271.
120 Yao S, Chen S, Clark J, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103:6907–6912.
121 Shi Y, Do JT, Desponts C, et al. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2:525–528.
122 Krohne TU, Westenskow PD, Kurihara T, et al. Generation of retinal pigment epithelial cells from small molecules and OCT4-reprogrammed human induced pluripotent stem cells. Stem Cells Transl Med. 2012;1:96–109.
123 LeCouter J, Moritz DR, Li B, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–893.
124 Otani A, Dorrell MI, Kinder K, et al. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage negative hematopoietic stem cells. J Clin Invest. 2004;114:765–774.
125 Matsumoto K, Yoshitomi H, Rossant J, et al. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563.
126 Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340.
127 Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967.
128 Kalka C, Tehrani H, Laudenberg B, et al. VEGF gene transfer mobilizes endothelial progenitor cells in patients with inoperable coronary disease. Ann Thorac Surg. 2000;70:829–834.
129 Gill M, Dias S, Hattori K, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88:167–174.
130 Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436.
131 Grant MB, May WS, Caballero S, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–612.
132 Wagers AJ, Sherwood RI, Christensen JL, et al. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259.
133 Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377.
134 Grant MB, Caballero S, Brown GA, et al. The contribution of adult hematopoietic stem cells to retinal neovascularization. Adv Exp Med Biol. 2003;522:37–45.
135 Espinosa-Heidmann DG, Caicedo A, Hernandez EP, et al. Bone marrow-derived progenitor cells contribute to experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:4914–4919.
136 Sengupta N, Caballero S, Mames RN, et al. The role of adult bone marrow-derived stem cells in choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:4908–4913.
137 Csaky KG, Baffi JZ, Byrnes GA, et al. Recruitment of marrow-derived endothelial cells to experimental choroidal neovascularization by local expression of vascular endothelial growth factor. Exp Eye Res. 2004;78:1107–1116.
138 Dorrell MI, Otani A, Aguilar E, et al. Adult bone marrow-derived stem cells use R-cadherin to target sites of neovascularization in the developing retina. Blood. 2004;103:3420–3427.
139 Otani A, Kinder K, Ewalt K, et al. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med. 2002;8:1004–1010.
140 Filip S, Mokrý J, Karbanová J, et al. Local environmental factors determine hematopoietic differentiation of neural stem cells. Stem Cells Dev. 2004;13:113–120.
141 Humphries P, Kenna P, Farrar GJ. On the molecular genetics of retinitis pigmentosa. Science. 1992;256:804–808.
142 Farrar GJ, Kenna PF, Humphries P. On the genetics of retinitis pigmentosa and on mutation-independent approaches to therapeutic intervention. EMBO J. 2002;21:857–864.
143 Dryja TP, McGee TL, Reichel E, et al. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343:364–366.
144 Bowes C, Li T, Danciger M, et al. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680.
145 Kajiwara K, Hahn LB, Mukai S, et al. Mutations in the human retinal degeneration slow gene in autosomal dominant retinitis pigmentosa. Nature. 1991;354:480–483.
146 Gu SM, Thompson DA, Srikumari CR, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197.
147 Ali RR, Sarra GM, Stephens C, et al. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat Genet. 2000;25:306–310.
148 Takahashi M, Miyoshi H, Verma IM, et al. Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector-mediated gene transfer. J Virol. 1999;73:7812–7816.
149 Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95.
150 Frasson M, Sahel JA, Fabre M, et al. Retinitis pigmentosa: rod photoreceptor rescue by a calcium-channel blocker in the rd mouse. Nat Med. 1999;5:1183–1187.
151 Frasson M, Picaud S, Leveillard T, et al. Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Invest Ophthalmol Vis Sci. 1999;40:2724–2734.
152 Berson EL, Rosner B, Sandberg GA, et al. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111:761–772.
153 Mohand-Said S, et al. Normal retina releases a diffusible factor stimulating cone survival in the retinal degeneration mouse. Proc Natl Acad Sci U S A. 1998;95:8357–8362.
154 Newcomb JD, Sanberg PR, Klasko SK, et al. Umbilical cord blood research: current and future perspectives. Cell Transplant. 2007;16:151–158.
155 Broxmeyer HE, Hangoc G, Cooper S, et al. Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation in adults. Proc Natl Acad Sci U S A. 1992;89:4109–4113.
156 Roncarolo MG, Bigler M, Ciuti E, et al. Immune responses by cord blood cells. Blood Cells. 1994;20:573–585. discussion 585–6
157 Henning RJ, Burgos JD, Vasko M, et al. Human cord blood cells and myocardial infarction: effect of dose and route of administration on infarct size. Cell Transplant. 2007;16:907–917.
158 Womble TA, Green S, Sanberg PR, et al. CD14(+) human umbilical cord blood cells are essential for neurological recovery following MCAO. Cell Transplant. 2008;17:485–486.
159 Kuwana M, Okazaki Y, Kodama H, et al. Endothelial differentiation potential of human monocyte-derived multipotential cells. Stem Cells. 2006;24:2733–2743.
160 Romagnani P, Annunziato F, Liotta F, et al. CD14+CD34 low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res. 2005;97:314–322.
161 Kodama H, Inoue T, Watanabe R, et al. Neurogenic potential of progenitors derived from human circulating CD14+ monocytes. Immunol Cell Biol. 2006;84:209–217.
162 Chua SJ, Bielecki R, Wong CJ, et al. Neural progenitors, neurons and oligodendrocytes from human umbilical cord blood cells in a serum-free, feeder-free cell culture. Biochem Biophys Res Commun. 2009;379:217–221.
163 Arien-Zakay H, Lecht S, Bercu MM, et al. Neuroprotection by cord blood neural progenitors involves antioxidants, neurotrophic and angiogenic factors. Exp Neurol. 2009;216:83–94.
164 Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999;66:698–704.
165 Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618.
166 Sanberg PR, Park DH, Kuzmin-Nichols N, et al. Monocyte transplantation for neural and cardiovascular ischemia repair. J Cell Mol Med. 2010;14:553–563.
167 Park DH, Borlongan CV, Willing AE, et al. Human umbilical cord blood cell grafts for brain ischemia. Cell Transplant. 2009;18:985–998.
168 Stout RD, Jiang C, Matta B, et al. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349.
169 Luttun A, Tjwa M, Moons L, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840.
170 Heil M, Ziegelhoeffer T, Pipp F, et al. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002;283:H2411–H2419.
171 Checchin D, Sennlaub F, Levavasseur E, et al. Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci. 2006;47:3595–3602.
172 Sakurai E, Anand A, Ambati BK, et al. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3578–3585.
173 Fantin A, Vieira JM, Gestri G, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840.
174 Pucci F, Venneri MA, Biziato D, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–914.
175 Marchetti V, Yanes O, Aguilar E, et al. Differential macrophage polarization promotes tissue remodeling and repair in a model of ischemic retinopathy. Sci Rep. 2011;1:1–12.
176 Singh MS, MacLaren RE. Stem cells as a therapeutic tool for the blind: biology and future prospects. Proc Biol Sci. 2011;278:3009–3016.
177 Stern JH, Temple S. Stem cells for retinal replacement therapy. Neurotherapeutics.. 2011;8:736–743.
178 Marchetti V, Krohne TU, Friedlander DF, et al. Stemming vision loss with stem cells. J Clin Invest. 2010;120:3012–3021.
179 Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600.
180 Rosenzweig A. Endothelial progenitor cells. N Engl J Med. 2003;348:581–582.