CHAPTER 5 Stem Cell Biology in the Central Nervous System
In a series of papers published in the 1960s, Joseph Altman and colleagues reported that certain regions of the rat brain contained dividing cells capable of generating progeny with a neuronal morphology.1 Evidence for cell proliferation in the rat and mouse already existed,2–4 but conventional wisdom at the time was that the adult mammalian brain was completely incapable of regeneration and that neurons were formed only during development. Because technical limitations made verifying the neuronal nature of cells difficult, Altman’s discoveries were met with great skepticism. Decades later, continued research and technical progress have led to unambiguous demonstration of adult neurogenesis. This chapter considers the nature of the neural stem cells (NSCs) responsible for the generation of new neurons and glia in the adult central nervous system (CNS), their role in certain tumors, and the potential cell-based therapies that they represent.
Neurogenesis: Location and Function
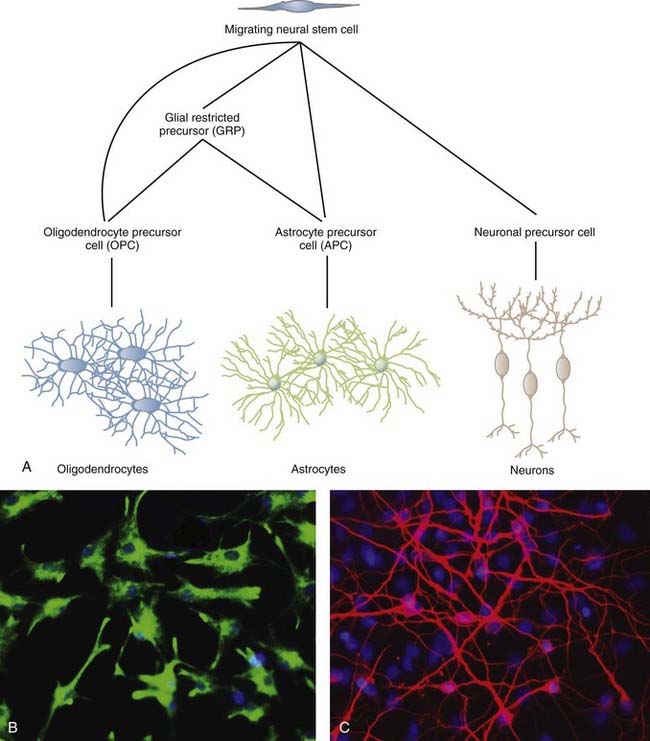
NSCs can give rise to the three major cell types of the CNS: neurons, oligodendrocytes, and astrocytes (Fig. 5-1). The two most studied sites of NSC activity are the subventricular zone (SVZ) and the dentate gyrus of the hippocampus. The bulk of our knowledge of neurogenesis comes from rodent studies, but populations of NSCs have been identified and studied in humans, as discussed later.
The Subventricular Zone
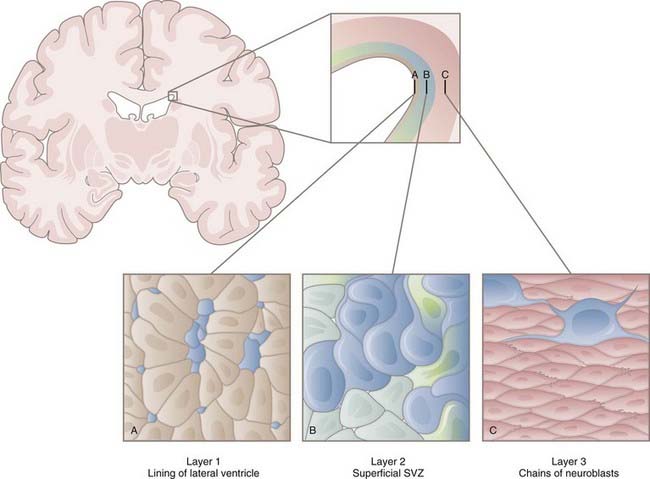
In the early 1990s, Reynolds and Weiss discovered cells in the adult mouse brain that proliferated and differentiated into neurons and astrocytes.5 These cells were also immunopositive for nestin, an intermediate filament of neuroepithelial stem cells prevalent before differentiation. These results led to identification of the SVZ as one of the neurogenic niches present in the adult brain. Within the SVZ is a layer of proliferative cells along the lateral aspect of the lateral ventricle (Fig. 5-2). These cells express glial fibrillary acidic protein (GFAP), a classic marker for astrocytes, and polysialylated neural cell adhesion molecule (PSA-NCAM). Separating the GFAP+/PSA-NCAM+ cells from the ventricular lumen is a layer of ependymal cells, with most of the astrocytes extending minute apical processes that directly contact the ventricle.6 Astrocytes from the SVZ differentiate into immature neurons called neuroblasts that travel via a migratory pathway to the olfactory bulb, where they differentiate into neurons.7–9 This pathway is known as the rostral migratory stream (RMS). The astroglial cells of the SVZ also generate astrocytes, oligodendrocyte precursor cells (OPCs, discussed later), and myelinating oligodendrocytes in response to chemical demyelinating lesions.10–13 This multipotential nature of SVZ astrocytes earns them the classification of NSCs.
Models of the cytoarchitecture of the SVZ have been in a state of flux, but the basic format has been determined. The primary precursors in vivo are the slowly dividing astrocytes mentioned earlier, called type B cells. B cells divide asymmetrically, which means that mitosis results in two different daughter cells. One is an SVZ astrocyte, just like the parent cell. The other is a short-lived transit-amplifying cell called a type C cell. C cells are antigenically distinct from B cells in that C cells express neither GFAP nor PSA-NCAM. After a brief period of increased mitotic activity, C cells ultimately give rise to migrating neuroblasts (type A cells). The neuroblasts travel from the SVZ via the RMS to the olfactory bulb, where they generate two types of inhibitory interneurons: granule cells and periglomerular neurons. C and A cells in the SVZ and subgranular zone (SGZ) can be identified by their expression of the microtubule-associated protein doublecortin14 and can be used as a marker to reflect levels of neurogenesis.15 The transcription factor Tbr2 is also expressed in early postmitotic neurons and their intermediate progenitors.16
In addition to their lineage relationships (i.e., B cells giving rise to C cells giving rise to A cells), all three types are closely associated spatially. As neuroblasts migrate toward the olfactory bulb, they coalesce to form a network of chains moving rostrally.17,18 They do so through cellular tunnels consisting mainly of type B cells with occasional C cells interspersed among them.
Newly born neurons in the adult contend with markedly different circumstances than do those of the embryonic brain. Adult neuroblasts migrate through more intricate terrain, frequently over longer distances. They do so first in a tangential fashion toward the olfactory bulb and then radially away from the RMS.19 To execute this switch, the neuroblasts must detach from the migrating chain, a process regulated by the extracellular matrix protein tenascin-R.20 Tangential and radial migration combined takes place in less than a week, after which the cells must integrate into fully functional circuits. For this reason, it is likely that the differentiation patterns do not simply recapitulate development.21 Soon after migration, the neurons display spontaneous activity and, over the course of the next 5 to 10 days, spiking activity. Overall, it takes weeks for cells to be born in the SVZ, migrate rostrally in the RMS, migrate radially after reaching the olfactory bulb, and differentiate and fully integrate into the existing neuronal circuitry. Even then, a large fraction of the cells (approximately 50%) will not survive without a sufficient level of sensory input.
In early studies of adult neurogenesis, some researchers suggested that the ependymal cells in the periventricular region represent the NSC population.22 Indeed, NSCs have been shown to contain a prominent cilium, although smaller and more specialized than that of the motile cilia characteristic of brain ependyma. However, studies involving antimitotic drugs and viral labeling, among others, strongly suggest that it is in fact the type B cells that represent the NSC population, and this is the generally accepted view.23 Some work suggests that one of the roles of ependymal cells in adult neurogenesis is to produce noggin, an antagonist of bone morphogenetic proteins (BMPs).24 Because BMPs are known to inhibit neurogenesis, as well as induce astrogliogenesis, noggin production by ependymal cells may be a means to maintain the neurogenic niche. Although the presence of the SVZ in humans is clear (reviewed later), existence of the RMS is controversial.25,26
The Subgranular Zone
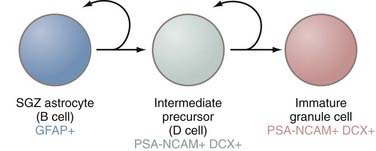
The second principal site of adult neurogenesis is located in the hippocampal formation in a region known as the SGZ (Fig. 5-3). The SGZ lies between the dentate gyrus and the hilum of the hippocampus. In this region of the brain, the primary precursors of new neurons are, as in the SVZ, astrocytes.27 These astrocytes are also referred to as B cells, but much of the rest of the terminology for the SGZ cytoarchitecture differs from that of the SVZ. In the hippocampus, B cells give rise to a set of immature D cells that divide less frequently and are smaller and more differentiated than the C cells of the SVZ. Because D cells are less mitotic than transit-amplifying cells, the increase in production of these intermediates is probably restricted. The D cells then generate the excitatory granule neurons that migrate to the granule cell layer of the hippocampus. These newly formed neurons integrate into a circuitry that is relatively close to their astrocytic forebears, in contrast to the distances that cells born in the SVZ must migrate to reach their ultimate destination in the olfactory bulb. These fully differentiated neurons then extend axonal projections called mossy fibers to hippocampal region CA3, a subfield implicated in learning and memory processes.28,29 The newly formed neurons are integrated into the hippocampal circuitry within a week and form excitatory synaptic connections with both interneurons and CA3 pyramidal cells.30 As in the SVZ, the various cell types can be distinguished on the basis of expression of certain molecular markers. Although SGZ astrocytes express GFAP, D cells and their progeny (i.e., neurons) do not and instead express PSA-NCAM.
The rate at which the hippocampus produces new neurons varies considerably, depending on age, internal factors such as neurotransmitter levels, and external factors such as exercise, stress, and sleep.31 Thousands of new cells may be produced each day, but it is only a very small percentage that ultimately survive, fully differentiate, and integrate into neural networks.
Regulation of Neurogenesis
Several factors have been shown to regulate the birth and integration of new neurons in rodents, including environmental cues, learning-related stimuli, and neuronal activity. For example, postnatal unilateral olfactory deprivation results in a significant reduction in bulb volume, an effect that is reversed with sufficient restoration of stimulation.32 Furthermore, exposure of a mouse to a complex odor environment results in both an increase in incorporation of newborn neurons and enhancement of short-term olfactory memory.33,34 Other physiologic states such as pheromone stimulation and pregnancy also modulate olfactory bulb neurogenesis.35,36 Additional stimuli regulating neurogenesis include exercise, learning, memory, environment, and stress.37 Although most of these stimuli are positive regulators, stress and the associated changes in corticosteroid levels result in a decline in neurogenesis and memory.38 For instance, stress in adult marmoset monkeys results in a decrease in the number of proliferating granule cell precursors in the dentate gyrus.39
Neurogenesis in the hippocampus is also subject to activity-dependent regulation. Because of the diversity of input to and complexity of connections in the dentate gyrus,40,41 this control may be more elaborate in the SGZ than in the SVZ. One of the key functions of the dentate gyrus is the formation of distinct representations of contexts, places, and episodes,42 a role that may render the region sensitive to the environment or cortical activity (or to both). The dentate gyrus, as part of the limbic system, also modulates emotional processes such as stress and depression.43 Consistent with these roles, hippocampal neurogenesis is regulated by the environment, cognitive and emotional processes, and exercise.44–46
On the cellular and molecular levels, γ-aminobutyric acid and glutamate exert numerous effects on neurogenesis, including effects in the realms of proliferation, survival, and integration.47 Additionally, a plethora of growth factors and extracellular matrix components influence the birth and maturation of new neurons in an activity-dependent manner.
Astrocytes as Stem Cells
Current evidence suggests that B cells are derived from radial glial cells, the principal NSC in the embryonic mammalian forebrain.48 Both are NSCs and occupy the same periventricular region of the brain, and both contact the ventricle proper via an apical process. Radial glia also possess a basal process that extends to the pial surface, and some adult SVZ GFAP+ cells also have such a process.
On closer inspection, it turns out that astrocytes are in some ways ideally suited to fulfill the role of primary progenitor.49 Their numerous processes contact many cell types and the basal lamina of blood vessels, and interastrocyte connections exist in the form of gap junctions. These structural features poise astrocytes to integrate signals from a variety of sources to effectively regulate the stem cell niche. Astrocytes are also able to produce signals that drive neurogenesis in vitro.50,51
Parenchymal astrocytes in regions other than the SVZ and SGZ do not appear to be neurogenic in vivo, although astrocytes derived from the cortex, cerebellum, and spinal cord in the first two postnatal weeks can all give rise to neurospheres in vitro.52
Functional Significance of Neurogenesis
A growing body of evidence points to neurogenesis as a critical element in an array of adult brain functions. NCAM-deficient mice show a 40% reduction in bulb size that is restricted to the granule cell layer and results in impaired odor discrimination.53 Antidepressants increase neurogenesis in the hippocampus, but only after chronic, not acute treatment.54 An abundance of evidence suggests that adult neurogenesis is directly involved in learning and memory processes and demonstrates plasticity distinct from that occurring at the level of the synapse.
Gliogenesis
Although much of the focus thus far has been on the generation of new neurons, adult gliogenesis merits attention as well. Cells capable of differentiating into astrocytes, oligodendrocytes, or both are termed glial progenitors and are widespread; they constitute anywhere from 3% to 9% of all cells in the adult CNS.55 Indeed, although only select regions of the adult brain are neurogenic, it appears that much of the brain and spinal cord are gliogenic. The transcription factor Olig2 is an important molecular signature for gliogenesis inasmuch as this marker is found to oppose neurogenesis in the SVZ56 but is an important determinant of glial cell fate.57
Recent data have established that significant myelin replacement occurs and is particularly associated with aging.58,59 Hence, at a minimum, there exist glial progenitor cells that serve a homeostatic function for myelin. However, the designation “glial progenitor” is somewhat broad and includes several cell populations identified by potentiality or expression of surface markers (or both), the most common of which are the chondroitin sulfate proteoglycan NG2, the platelet-derived growth factor receptor PDGFR-α, and the cell surface ganglioside A2B5.55,60 The fact that cells can acquire, lose, and reacquire expression of each marker complicates determining whether cells expressing these individual markers represent identical, partially overlapping, or distinct populations.
Regardless of which marker (NG2, PDGFR-α, or A2B5) is chosen to identify them, OPCs have repeatedly been identified in the adult human CNS.61–64 OPCs are as abundant as other glial cells in both white and gray matter and account for up to 3% of cells in adult human subcortical white matter. In addition, OPCs give rise to multipotent neurospheres and can differentiate into oligodendrocytes in vitro. However, although glial progenitors are multipotent, they appear to lack the self-renewal characteristics of adult NSCs and thus have limited capacity to divide. The true parent or stem cell of the widespread glial progenitor remains to be established and is an important avenue of research with considerable clinical potential.
NG2+ Cells
The proteoglycan NG2 is expressed by a population of cells in the adult CNS that are often referred to as “oligodendrocyte progenitor cells” and sometimes as “polydendrocytes” or “synantocytes.”65 It is likely that NG2 marks multiple cell populations, one with characteristic progenitor qualities and the other a distinct form of differentiated glial cells. For example, cells isolated by NG2 can be induced to form not only oligodendrocytes but also astrocytes and neurons in vivo.66–68 However, when analyzed by electron microscopy, NG2+ cells superficially resemble astrocytes but lack intermediate filaments and possess other cytologic features that distinguish them from astrocytes.69 These cells also lack markers for mature oligodendrocytes, astrocytes, neurons, and microglia,55,60,70–75 which has led some to refer to NG2+ cells as a fourth type of neuroglial cell.69
Aside from their potential role in maintaining myelination during aging, the role of the highly prevalent OPCs in the intact adult CNS is unclear. NG2+ cells, particularly the polydendrocyte morphotype, probably have physiologic roles unrelated to myelination because their processes contact both the nodes of Ranvier76 and blood vessels.77 A subpopulation of NG2+ cells also forms synaptic junctions with multiple types of neurons,78,79 thus representing a putative mechanism for neurons to influence the behavior of progenitors so that they can be adapted to neuronal needs. Finally, OPCs mount an impressive proliferative response, change morphologies, and differentiate into myelinating oligodendrocytes whenever demyelination or inflammation occurs in the CNS.60,80–84
Platelet-Derived Growth Factor Receptor-α
PDGFR-α is also frequently used to identify cells with OPC features.85,86 Mice null for PDGF-A demonstrate the importance of this polypeptide in that knockout results in a reduction or even elimination (depending on the region) of PDGFR-α+ progenitors and oligodendrocytes.87 Depletion of these cells also results in severe hypomyelination and a tremor phenotype. Conversely, overexpression of PDGF results in excessive and ectopic oligodendrocyte production, although these redundant oligodendrocytes are eliminated via apoptosis.88 Furthermore, adult OPCs generally divide slowly in culture unless given PDGF, levels of which are upregulated after injury.89 Thus, OPCs may be in a relatively quiescent state until triggered by signals generated by injury, inflammation, or aging.
Because PDGFR-α and NG2 are used to mark the same cell (i.e., the OPC), one might well wonder whether the two are functionally related in any way. In fact, NG2 is required for effective signal transduction of PDGF at the level of the receptor.90,91 The two molecules are highly colocalized in certain populations, are upregulated in response to treatment with basic fibroblast growth factor (bFGF), and can form a molecular complex.90 PDGFR-α+/NG2+ cells can also generate progenitors expressing a third OPC marker, A2B5.
A2B5
A2B5 is a cell surface ganglioside that is also sometimes used to identify glial progenitors. Like the markers already discussed, A2B5 is expressed by oligodendrocytes early in their differentiation and is then downregulated as the cell matures. Recent work suggests that A2B5+ cells arise directly from NG2+ cells.75 A2B5 expression in and of itself is not sufficient for the identification of oligodendrocyte-restricted precursors because this marker is also found on neuronal progenitors and certain types of astrocytes.65 In fact, A2B5 is frequently used to identify a bipotential cell population called O2-A cells, so named for their capability of forming both oligodendrocytes and type 2 astrocytes (although the latter has been demonstrated only in vitro).75,90,92–94
Astrocyte Precursor Cells
In general, astrocyte precursor cells (APCs) are less well characterized than OPCs. This is probably attributable to the fact that until recently, most scientists thought that astrocyte differentiation occurred “by default.”95,96 Studies demonstrating enhanced formation of astrocytes via inhibition of neurogenesis97 or simply absence of proneural transcription factors98 implied that unless a stem cell receives signals otherwise, its fate is astrocytic. This suggests a uniform differentiation pathway. However, given the wide range of functions attributed to astrocytes and the heterogeneity with which they perform these tasks, it now seems simplistic to think that all astrocytes are created equal.
A number of studies have reported astrocyte-restricted precursors, all of which are negative for GFAP expression and are A2B5+ (or derived from A2B5+ cells).99–103 Another common feature of these cells is that nearly all of them require FGF for survival. One putative APC marker, CD44, has been detected in human fetal tissue100 and in certain gliomas (see later). Although early oligodendrocytes and radial glial cells are CD44 negative,102,104 the specificity of this marker for APCs is not quite definite.105
Neural Stem Cells in the Spinal Cord
Self-renewing and multipotent NSCs also exist in the adult spinal cord.22,73,106,107 There is evidence supporting their residence near the central canal,22,108,109 as well as in the white matter parenchyma.73,110,111 Interestingly, NSCs in the spinal cord behave differently from those in the SVZ or SGZ in that they are glial restricted (i.e., they do not give rise to neurons). However, NSCs isolated from the spinal cord are able to generate neurons in vitro111 and when transplanted into the neurogenic dentate gyrus,106 thus indicating that in addition to intrinsic factors, the niche in which NSCs are located is a critical determinant of cell fate. The essential elements of the neurogenic microenvironment are both molecular (cytokines, growth factors, other)112 and cellular, including endothelial cells113,114 and astrocytes.51,115,116
Stem Cell and Progenitor Response to Injury
After brain injury (be it traumatic, ischemic, or chemical), endogenous repair occurs, albeit in a limited fashion.117–119 The neurogenic regions of the brain (i.e., the SVZ and hippocampus) respond to ischemic and kainite insults by increasing proliferation and the number of new neurons that do survive and integrate.120–124 After an ischemic lesion in the striatum, these neurons acquire the phenotype of striatal neurons and their production continues for months after the initial injury,125,126 thus demonstrating the potential for a sustained and specific response.
However, the proliferative response to CNS injury occurs predominantly in glial precursors in the parenchyma.119,127–131 OPCs proliferate, migrate to the site of the lesion, and myelinate in response to a wide range of pathologic states, including trauma,132–136 ischemia,137–140 and demyelination.80,81,141–144 A number of growth factors are upregulated after injury, some of which (e.g., PDGF, FGF) are mitogenic for OPCs. Interestingly, because these factors seem to inhibit terminal differentiation and myelin production,88,145–149 their effects must be counteracted for myelination to occur. Neuronal differentiation in regions other than the SVZ and SGZ is rare and, when it does occur, abortive.150,151 Endogenous NSCs alone and unmodulated are therefore incapable of complete repair, in part because of the microenvironment, as discussed previously. These limitations must be considered when developing therapeutic interventions for CNS injury.
Evidence for Adult Human Neurogenesis
NSCs reside in the adult human hippocampus,152 SVZ,153–155 and even the cortex156 and subcortical white matter62 and can generate neuronal and glial progeny in vitro. As in rodents, adult human NSCs (ahNSCs) respond to certain CNS insults by proliferating and differentiating. Ischemic injury induces neurogenesis in the human brain,157–159 and when transplanted into a demyelinated rodent spinal cord, human ahNSCs may contribute to remyelination.155 Research on adult human neurogenesis and gliogenesis is still in its infancy, and the relative paucity of data necessitates significant further characterization before ahNSCs can be seriously considered for treatment of disease and injury.
Stem Cells and Cancer
Similarities abound between stem cells and tumor cells. Self-renewal is an essential feature of each, and the signaling pathways regulating this process have been implicated in cancers from a variety of organs. The Sonic Hedgehog (Shh)160 and Notch161 pathways are involved in NSC maintenance, and both have been implicated in the formation of tumors such as medulloblastoma162–164 and gliomas, including glioblastoma multiforme.165–168 These findings can be interpreted in two ways: (1) that cancer cells appropriate mechanisms for self-renewal characteristic of stem cells or (2) that stem cells are in a way predisposed to becoming tumorigenic themselves in that they have this machinery already activated. Because it is possible for transformation of cells anywhere along a given lineage (from stem cell to progenitor to mature cell), it is likely that both processes occur and contribute to cancer.
Other general similarities between stem cells and cancer cells besides proliferation include the ability to generate new (although not necessarily normal) tissues, as well as the ability of both cells to give rise to phenotypically diverse progeny, as manifested by the heterogeneity of cells composing these tissues.169–172
It is in part from these similarities that the cancer stem cell hypothesis was born. There is some confusion, however, regarding exactly what the phrase means. At face value, the term “cancer stem cell” seems to imply that there is a stem cell that in and of itself is carcinogenic; that is, a stem cell or progenitor is the cell of origin. Although this may be the case in many tumors (see later), what the term “cancer stem cell” really refers to is that certain tumors contain within their population a self-renewing cell that is capable of renewing not only itself but the tumor as well and thus shares features with stem cells. One demonstrates this by isolating a cell or cells from a tumor and engrafting them into a new host, where they proliferate and form a new lesion.173 Thus, the “stemness” of the cell is not that it can renew itself and also differentiate into mature cell types (the classic criteria for stem cells) but rather that it is capable of perpetuating the cancer-forming ability of the growth. Researchers also hypothesize that cancer stem cells are required for growth and metastasis of the tumor and that elimination of this population is necessary for cure.174
There remains a question whether the presence of progenitors in gliomas necessarily implicates them in the neoplastic process. A comparison of ahNSCs and cells from grade II astrocytoma or glioblastoma multiforme revealed significant differences with respect to proliferation, differentiation properties, and tumorigenic capacity.175 An alternative explanation is that NSCs have been drawn into the lesion microenvironment by growth factors, cytokines, chemokines, and other substances and are able to thrive there. These genetically normal progenitors, although not tumorigenic per se, once recruited will then proliferate and contribute significantly to growth of the tumor and thus enhance whatever mass effect the neoplasm may have.
Cells displaying classic OPC features, including NG2, PDGFR-α, or A2B5 expression (or any combination of these markers), have been found in a wide range of neoplasms, most commonly gliomas such as astrocytomas, mixed astrocytomas, and oligodendrogliomas.176–180 There are probably two events that would need to occur for a stem cell to become tumorigenic: (1) loss of cell cycle control, frequently because of mutation in a gene such as Pten or p53, and (2) acquired insensitivity to differentiation signals such that stem cells and progenitors proliferate excessively but never fully mature.
Stem Cell–Based Therapies
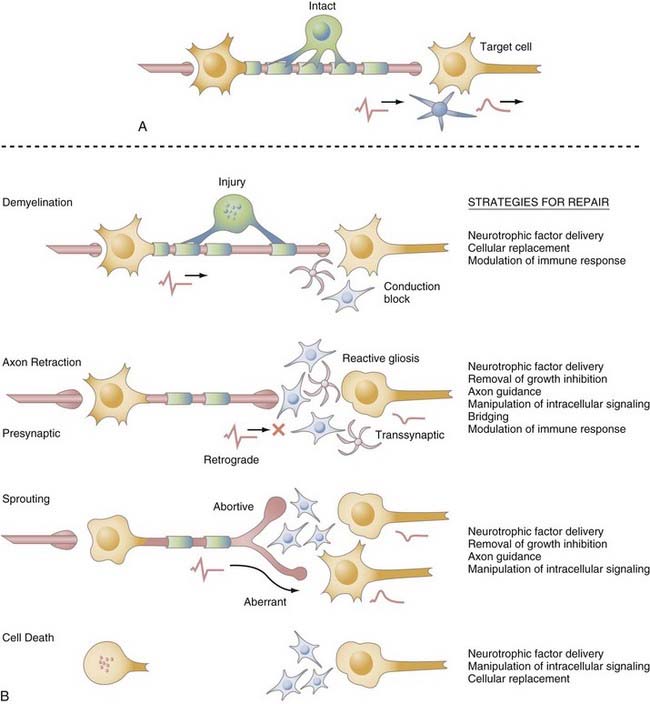
Stem cells constitute a potential resource for the treatment of a wide variety of conditions seen by neurosurgeons. Putative therapies include treatments that modulate the behavior of endogenous stem cell behavior, as well as transplantation of exogenous cells (Fig. 5-4).
Stimulation of Endogenous Mechanisms of Repair
Although some spontaneous neurogenesis does occur after brain injury, it is often insufficient to generate functional recovery. Thus, approaches are being developed to augment endogenous repair. There are many steps in neurogenesis that can be targeted, including survival, proliferation, migration, and differentiation. Candidate molecules include bFGF, EGF, bone-derived neurotrophic factor (BDNF), noggin, erythropoietin (EPO), vascular endothelial growth factor (VEGF), and many more. Some single cytokines can target multiple components of functional neurogenesis. For example, investigators have used transforming growth factor-α (TGF-α) to induce neural stem cells to proliferate, migrate, and differentiate into neurons in an animal model of Parkinson’s disease.181 In other cases, combinatorial approaches consisting of cocktails of two or more cytokines, either simultaneously or sequentially, may be used.182,183 It is worth noting that many of these compounds will have effects independent from those on NSCs, and parsing the multiple functions of molecules used for cytokine-mediated regeneration is a continuing challenge.
An important consideration when modulating endogenous regenerative responses is the age of the subject. Neurogenesis in both the SVZ and the hippocampus decreases dramatically with age. However, this may be attributable more to changes in the neurogenic niche than to NSCs themselves.184 The levels of many cytokines important for NSC function (e.g., FGF2, insulin-like growth factor I [IGF-I], VEGF, and EGF) decline with age.185,186
Transplantation of Stem Cells
Many studies have shown that NSCs strongly trend toward differentiation into glial cells, especially astrocytes, after transplantation into the CNS despite proficient capacity to generate neurons in vitro and in neurogenic regions in vivo.187–189 This lineage restriction has significant implications for the therapeutic outcome with stem cell therapy. In keeping with the theme of the importance of the microenvironment in NSC behavior, modulation of the site into which cells are transplanted may be important. In fact, artificial niches for the transplantation of ahNSCs have been developed for this purpose.190
Stem cells can also be engineered to deliver therapeutic compounds (e.g., growth factors, immune system modulators, axon guidance molecules) to a targeted area. Strategies to do so take advantage of the inherent tropism of stem cells to injured or diseased regions of the brain. For example, NSCs show a strong selective tumor tropism that is probably mediated by VEGF.191 A human NSC line has been engineered to express cytosine deaminase, which converts the prodrug 5-fluorocytosine into the anticancer agent 5-fluorouracil. These cells have been used in animal models to target and reduce volumes of breast cancer and melanoma brain metastases.192,193 Similar studies have been done in models of medulloblastoma, glioblastoma, and metastatic neuroblastoma.194–196
Stem Cell Imaging
To better understand the behavior of NSCs and the mechanisms of potential therapeutic benefit, in vivo imaging is required. Contemporary modalities include magnetic resonance imaging (MRI), positron emission tomography (PET), and optical imaging. MRI is often used after labeling cells with iron oxide particles and then tracking their migration in the CNS.197–200 Optical imaging techniques such as bioluminescence imaging involve genetically engineering NSCs to express a luciferase reporter gene that causes the cells to emit photons. These photons can be collected and analyzed to gather information about cell survival, tumorigenicity, and immunogenicity.201–204 PET has also been used to monitor stem cell behavior and metabolism, although frequently in combination with MRI.205,206
Aboody K, Najbauer J, Schmidt NO, et al. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro Oncol. 2006;8:119-126.
Belachew S, Chittajallu R, Aguirre AA, et al. Postnatal NG2 proteoglycan–expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169-186.
Burns CT, Verfaille CM, Low WC. Stem cells for ischemic brain injury: a critical review. J Comp Neurol. 2009;515:125-144.
Carleton A, Petreanu L, Lansford R, et al. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507-518.
Chojnacki AK, Mak GK, Weiss S. Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both? Nat Rev Neurosci. 2009;10:152-163.
Curtis MA, Kam M, Nannmark U, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243-1249.
Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;11:1127-1134.
Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313-1317.
Guzman R, Uchida N, Bliss TM, et al. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci U S A. 2007;104:10211-10216.
Horner PJ, Gage FH. Regeneration in the adult and aging brain. Arch Neurol. 2002;59:1717-1720.
Horner PJ, Power AE, Kempermann G, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218-2228.
Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493-495.
Lasiene J, Matsui A, Sawa Y, et al. Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell. 2009;8:201-213.
Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39-47.
Liu CY, Westerlund U, Svensson M, et al. Artificial niches for human adult neural stem cells: possibility for autologous transplantation therapy. J Hematother Stem Cell Res. 2003;12:689-699.
Merkle FT, Tramontin AD, Garcia-Verdugo JM, et al. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528-17532.
Nunes MC, Roy NS, Keyoung HM, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439-447.
Obermair FJ, Schroter A, Thallmair M. Endogenous neural progenitor cells as therapeutic target after spinal cord injury. Physiology (Bethesda). 2008;23:296-304.
Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6106-6113.
Reier PJ. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx. 2004;1:424-451.
Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian nervous system. Science. 1992;255:1707-1710.
Seri B, Garcia-Verdugo JM, McEwen BS, et al. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153-7160.
Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338-1340.
Shors TJ. From stem cells to grandmother cells: how neurogenesis relates to learning and memory. Cell Stem Cell. 2008;3:253-258.
Varghese M, Olstorn H, Sandberg C, et al. A comparison between stem cells from the adult human brain and from brain tumors. Neurosurgery. 2008;63:1022-1033.
Zai LJ, Wrathall JR. Cell proliferation and replacement following contusive spinal cord injury. Glia. 2005;50:247-257.
1 Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319-335.
2 Allen E. The cessation of mitosis in the central nervous system of the albino rat. J Comp Neurol. 1912;22:547-568.
3 Messier B, Leblond CP, Smart I. Presence of DNA synthesis and mitosis in the brain of young adult mice. Exp Cell Res. 1958;14:224-226.
4 Smart I. The subependymal layer of the mouse brain and its cell production as shown by radioautography after thymidine-H3 injection. J Comp Neurol. 1961;116:325-338.
5 Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian nervous system. Science. 1992;255:1707-1710.
6 Mirzadeh Z, Merkle FT, Soriano-Navarro M, et al. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265-278.
7 Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953-956.
8 Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscope analysis of light radioautographs. Science. 1977;197:1092-1094.
9 Corotto FS, Henegar JA, Maruniak JA. Neurogenesis persists in the subependymal layer of the adult mouse brain. Neurosci Lett. 1993;149:111-114.
10 Levinson SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201-212.
11 Menn B, Garcia-Verdugo JM, Yaschine C, et al. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907-7918.
12 Nait-Oumesmar B, Decker L, Lachapelle F, et al. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357-4366.
13 Picard-Riera N, Decker L, Delarasse C, et al. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A. 2002;99:13211-13216.
14 Brown JP, Couillard-Despres S, Cooper-Kuhn CM, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1-10.
15 Couillard-Despres S, Winner B, Schaubeck S, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1-14.
16 Hevner RF, Hodge RD, Daza RA, et al. Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci Res. 2006;55:223-233.
17 Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996;93:14895-14900.
18 Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978-981.
19 Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6106-6113.
20 Saghatelyan A, de Chevigny A, Schachner M, et al. Tenascin-R mediates activity-dependent recruitment of neuroblasts in the adult mouse forebrain. Nat Neurosci. 2004;7:347-356.
21 Carleton A, Petreanu L, Lansford R, et al. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507-518.
22 Johansson CB, Momma S, Clarke DL, et al. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25-34.
23 Chojnacki AK, Mak GK, Weiss S. Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both? Nat Rev Neurosci. 2009;10:152-163.
24 Lim DA, Tramontin AD, Trevejo JM, et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713-726.
25 Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740-744.
26 Curtis MA, Kam M, Nannmark U, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243-1249.
27 Seri B, Garcia-Verdugo JM, McEwen BS, et al. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153-7160.
28 Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449-460.
29 Stanfield BB, Trice JE. Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp Brain Res. 1988;72:399-406.
30 Ide Y, Fujiyama F, Okamoto-Furuta K, et al. Rapid integration of young newborn dentate gyrus granule cells in the adult hippocampal circuitry. Eur J Neurosci. 2008;28:2381-2392.
31 Shors TJ. From stem cells to grandmother cells: how neurogenesis relates to learning and memory. Cell Stem Cell. 2008;3:253-258.
32 Cummings DM, Henning HE, Brunjes PC. Olfactory bulb recovery after early sensory deprivation. J Neurosci. 1997;17:7433-7440.
33 Rochefort C, Gheusi G, Vincent JD, et al. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679-2689.
34 Rochefort C, Lledo PM. Short-term survival of newborn neurons in the adult olfactory bulb after exposure to a complex odor environment. Eur J Neurosci. 2005;22:2863-2870.
35 Mak GK, Enwere EK, Gregg C, et al. Male pheromone–stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat Neurosci. 2007;10:1003-1011.
36 Shingo T, Gregg C, Enwere E, et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117-120.
37 Prickaerts J, Koopmans G, Blokland A, et al. Learning and adult neurogenesis: survival with or without proliferation? Neurobiol Learn Mem. 2004;81:1-11.
38 Montaron MF, Petry KG, Rodriguez JJ, et al. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol Aging. 2006;27:645-654.
39 Gould E, Tanapat P, McEwen BS, et al. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168-3171.
40 Acsady L, Kali S. Models, structure, function: the transformation of cortical signals in the dentate gyrus. Prog Brain Res. 2007;163:577-599.
41 Houser CR. Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity. Prog Brain Res. 2007;163:217-232.
42 McHugh TJ, Jones MW, Quinn JJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94-99.
43 Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110-1115.
44 Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493-495.
45 Gould E, Beylin A, Tanapat P, et al. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260-265.
46 van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266-270.
47 Ma DK, Kim WR, Ming GL, et al. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann N Y Acad Sci. 2009;1170:664-673.
48 Merkle FT, Tramontin AD, Garcia-Verdugo JM, et al. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528-17532.
49 Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543-550.
50 Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci U S A. 1999;96:7526-7531.
51 Song H, Stevens CF, Gage FH. Astroglia induced neurogenesis from adult neural stem cells. Nature. 2002;417:39-44.
52 Laywell ED, Rakic P, Kukekov VG, et al. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci U S A. 2000;97:13883-13888.
53 Gheusi G, Cremer H, McLean H, et al. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci U S A. 2000;97:1823-1828.
54 Malberg JE, Eisch AJ, Nestler EJ, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104-9110.
55 Dawson MR, Polito A, Levine JM, et al. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476-488.
56 Hack MA, Saghatelyan A, de Chevigny A, et al. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865-872.
57 Zhao JW, Raha-Chowdhury R, Fawcett JW, et al. Astrocytes and oligodendrocytes can be generated from NG2+ progenitors after acute brain injury: intracellular localization of oligodendrocyte transcription factor 2 is associated with their fate choice. Eur J Neurosci. 2009;29:1853-1869.
58 Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol. 2002;31:581-593.
59 Lasiene J, Matsui A, Sawa Y, et al. Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell. 2009;8:201-213.
60 Nishiyama A, Chang A, Trapp BD. NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol. 1999;58:1113-1124.
61 Chang A, Nishiyama A, Peterson J, et al. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404-6412.
62 Nunes MC, Roy NS, Keyoung HM, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439-447.
63 Scolding N, Franklin R, Stevens S, et al. Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain. 1998;121:2221-2228.
64 Roy NS, Wang S, Harrison-Restelli C, et al. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci. 1999;19:9986-9995.
65 Canoll P, Goldman JE. The interface between glial progenitors and gliomas. Acta Neuropathol. 2008;116:465-477.
66 Belachew S, Chittajallu R, Aguirre AA, et al. Postnatal NG2 proteoglycan–expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169-186.
67 Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145-157.
68 Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754-1757.
69 Peters A. A fourth type of neuroglial cell in the adult central nervous system. J Neurocytol. 2004;33:345-357.
70 Nishiyama A, Yu M, Drazba JA, et al. Normal and reactive NG2+ glial cells are distinct from resting and activated microglia. J Neurosci Res. 1997;48:299-312.
71 Reynolds R, Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res. 1997;47:455-470.
72 Dawson MR, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61:471-479.
73 Horner PJ, Power AE, Kempermann G, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218-2228.
74 Mason JL, Goldman JE. A2B5+ and O4+ cycling progenitors in the adult forebrain white matter respond differently to PDGF-AA, FGF-2, and IGF-1. Mol Cell Neurosci. 2002;20:30-42.
75 Baracskay KL, Kidd GJ, Miller RH, et al. NG2-positive cells generate A2B5-positive oligodendrocyte precursor cells. Glia. 2007;55:1001-1010.
76 Butt AM, Duncan A, Hornby MF, et al. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84-91.
77 Trapp BD, Nishiyama A, Cheng D, et al. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol. 1997;137:459-468.
78 Paukert M, Bergles DE. Synaptic communication between neurons and NG2+ cells. Curr Opin Neurobiol. 2006;16:515-521.
79 Lin SC, Bergles DE. Synaptic signaling between neurons and glia. Glia. 2004;47:290-298.
80 Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labeling) to demyelination of the adult spinal cord. Glia. 1998;22:161-170.
81 Levine JM, Reynolds R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide–induced demyelination. Exp Neurol. 1999;160:333-347.
82 Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69:826-836.
83 Chari DM, Blakemore WF. Efficient recolonisation of progenitor-depleted areas of the CNS by adult oligodendrocyte progenitor cells. Glia. 2002;37:307-313.
84 Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197-203.
85 Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39-47.
86 Nishiyama A, Lin XH, Giese N, et al. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299-314.
87 Fruttiger M, Karlsson L, Hall AC, et al. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457-467.
88 Calver AR, Hall AC, Yu WP, et al. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20:869-882.
89 Wolswijk G, Noble M. Cooperation between PDGF and FGF converts slowly dividing O-2A adult progenitor cells to rapidly dividing cells with characteristics of O-2A perinatal progenitor cells. J Cell Biol. 1992;118:889-900.
90 Nishiyama A, Lin XH, Giese N, et al. Interaction between NG2 proteoglycan and PDGH alpha-receptor on O2A cells is required for optimal response to PDGF. J Neurosci Res. 1996;43:315-330.
91 Grako KA, Ochiya T, Barritt D, et al. PDGF (alpha)-receptor is unresponsive to PDGF-AA in aortic smooth muscle cells from the NG2 knockout mouse. J Cell Sci. 1999;112:905-915.
92 Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390-396.
93 Rogers SW, Gregori NZ, Carlson N, et al. Neuronal nicotinic acetylcholine receptor expression by O2A/oligodendrocyte precursor cells. Glia. 2001;33:306-313.
94 Grinspan JB, Stern JL, Pustilnik SM, et al. Cerebral white matter contains PDGF-responsive precursors to O2A cells. J Neurosci. 1990;10:1866-1873.
95 Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;11:1127-1134.
96 Ross SE, Greenberg ME, Stiles CD. Basic helix-loop-helix factors in cortical development. Neuron. 2003;39:13-25.
97 Nakashima K, Takizawa T, Ochiai W, et al. BMP2-mediated alteration in the developmental pathway of fetal mouse brain cells from neurogenesis to astrocytogenesis. Proc Natl Acad Sci U S A. 2001;98:5868-5873.
98 Nieto M, Schuurmans C, Britz O, et al. Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron. 2001;29:401-413.
99 Lin G, Goldman JE. An FGF-responsive astrocyte precursor isolated from the neonatal forebrain. Glia. 2009;57:592-603.
100 Liu Y, Han SS, Wu Y, et al. CD44 expression identifies astrocyte-restricted precursor cells. Dev Biol. 2004;276:31-46.
101 Mi H, Barres BA. Purification and characterization of astrocyte precursor cells in the developing rat optic nerve. J Neurosci. 1999;19:1049-1061.
102 Alfei L, Aita M, Caronti B, et al. Hyaluronate receptor CD44 is expressed by astrocytes in the adult chicken and in astrocyte cell precursors in early development of the chick spinal cord. Eur J Histochem. 1999;43:29-38.
103 Seidman KJ, Teng AL, Rosenkopf R, et al. Isolation, cloning and characterization of a putative type-1 astrocyte cell line. Brain Res. 1997;753:18-26.
104 Moretto G, Xu RY, Kim SU. CD44 expression in human astrocytes and oligodendrocytes in culture. J Neuropathol Exp Neurol. 1993;52:419-423.
105 Bouvier-Labit C, Liprandi A, Monti G, et al. CD44H is expressed by cells of the oligodendrocyte lineage and by oligodendrogliomas in humans. J Neurooncol. 2002;60:127-134.
106 Shihabuddin LS, Ray J, Gage FH. FGF-2 is sufficient to isolate progenitors found in the adult mammalian spinal cord. Exp Neurol. 1997;148:577-586.
107 Weiss S, Dunne C, Hewson J, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599-7609.
108 Martens DJ, Seaberg RM, van der Kooy D. In vivo infusions of exogenous growth factors into the fourth ventricle of the adult mouse brain increase the proliferation of neural progenitors around the fourth ventricle and central canal of the spinal cord. Eur J Neurosci. 2002;16:1045-1047.
109 Meletis K, Barnabe-Heider F, Carlen M, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182.
110 Ohori Y, Yamamoto S, Nagao M, et al. Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. J Neurosci. 2006;26:11948-11960.
111 Yamamoto S, Yamamoto N, Kitamura T, et al. Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp Neurol. 2001;172:115-127.
112 Obermair FJ, Schroter A, Thallmair M. Endogenous neural progenitor cells as therapeutic target after spinal cord injury. Physiology (Bethesda). 2008;23:296-304.
113 Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479-494.
114 Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338-1340.
115 Barkho BZ, Song H, Aimone JB, et al. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15:407-421.
116 Jiao J, Chen DF. Induction of neurogenesis in non-conventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals. Stem Cells. 2008;26:1221-1230.
117 Liu Y, Rao MS. Glial progenitors in the CNS and possible lineage relationships among them. Biol Cell. 2004;96:279-290.
118 Chen S, Pickard JD, Harris NG. Time course of cellular pathology after controlled cortical impact injury. Exp Neurol. 2003;182:87-102.
119 Han SS, Liu Y, Tyler-Polsz C, et al. Transplantation of glial-restricted precursor cells into the adult spinal cord: survival, glial-specific differentiation and preferential migration in white matter. Glia. 2004;45:1-16.
120 Liu J, Solway K, Messing RO, et al. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768-7778.
121 Arvidsson A, Kokaia Z, Lindvall O. N-Methyl-D-aspartate receptor–mediated increase of neurogenesis in adult rat dentate gyrus following stroke. Eur J Neurosci. 2001;14:10-18.
122 Jin K, Sun Y, Xie L, et al. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171-189.
123 Jin K, Minami M, Lan JQ, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710-4715.
124 Zhang RL, Zhang ZG, Zhang L, et al. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33-41.
125 Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963-970.
126 Thored P, Arvidsson A, Cacci E, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739-747.
127 Horner PJ, Gage FH. Regeneration in the adult and aging brain. Arch Neurol. 2002;59:1717-1720.
128 Kipnis J, Mizrahi T, Hauben E, et al. Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci U S A. 2002;99:15620-15625.
129 Ray SK, Dixon CE, Banik NL. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol Histopathol. 2002;17:1137-1152.
130 Schwartz M, Hauben E. T cell–based therapeutic vaccination for spinal cord injury. Prog Brain Res. 2002;137:401-406.
131 Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002;513:87-113.
132 Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci. 1994;14:4716-4730.
133 Amat JA, Farooq M, Ishiguro H, et al. Cells of the oligodendrocyte lineage proliferate following cortical stab wounds: an in vitro analysis. Glia. 1998;22:64-71.
134 McTigue DM, Wei P, Stoke BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21:3392-3400.
135 Hampton DW, Rhodes KE, Zhao C, et al. The responses of oligodendrocyte precursor cells, astrocytes and microglia to a cortical stab injury, in the brain. Neuroscience. 2004;127:813-820.
136 Zai LJ, Wrathall JR. Cell proliferation and replacement following contusive spinal cord injury. Glia. 2005;50:247-257.
137 Mandai K, Matsumoto M, Kitagawa K, et al. Ischemic damage and subsequent proliferation of oligodendrocytes in focal cerebral ischemia. Neuroscience. 1997;77:849-861.
138 Tanaka K, Nogawa S, Ito D, et al. Activation of NG2-positive oligodendrocyte progenitor cells during post-ischemic reperfusion in the rat brain. Neuroreport. 2001;12:2169-2174.
139 Gotts JE, Chesselet MF. Migration and fate of newly born cells after focal cortical ischemia in adult rats. J Neurosci Res. 2005;80:160-171.
140 Komitova M, Perfilieva E, Mattsson B, et al. Enriched environment after focal cortical ischemia enhances the generation of astroglia and NG2 positive polydendrocytes in adult rat neocortex. Exp Neurol. 2006;199:113-121.
141 Carroll WM, Jennings AR, Ironside LJ. Identification of the adult resting progenitor cell by autoradiographic tracking of oligodendrocyte precursors in experimental CNS demyelination. Brain. 1998;121:293-302.
142 Redwine JM, Armstrong RC. In vivo proliferation of oligodendrocyte progenitors expressing PDGFαR during early remyelination. J Neurobiol. 1998;37:413-428.
143 Reynolds R, Dawson M, Papadopoulos D, et al. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol. 2002;31:523-536.
144 Bu J, Banki A, Wu Q, et al. Increased NG2+ glial cell proliferation and oligodendrocyte generation in the hypomyelinating mutant shiverer. Glia. 2004;48:51-63.
145 Noble M, Murray K, Stroobant P, et al. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988;333:560-562.
146 Richardson WD, Pringle N, Mosley MJ, et al. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988;53:309-319.
147 Engel U, Wolswijk G. Oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells derived from adult rat spinal cord: in vitro characteristics and response to PDGF, bFGF and NT-3. Glia. 1996;16:16-26.
148 Gard AL, Pfeiffer SE. Two proliferative stage of the oligodendrocyte lineage (A2B5+ O4− and O4+ GalC−) under different mitogenic control. Neuron. 1990;5:615-625.
149 Wang Z, Colognato H, French-Constant C. Contrasting effects of mitogenic growth factors on myelination in neuron-oligodendrocyte co-cultures. Glia. 2007;55:537-545.
150 Shechter R, Ziv Y, Schwartz M. New GABAergic interneurons supported by myelin-specific T cells are formed in intact adult spinal cord. Stem Cells. 2007;25:2277-2282.
151 Vessal M, Aycock A, Garton MT, et al. Adult neurogenesis in primate and rodent spinal cord: comparing a cervical dorsal rhizotomy with a dorsal column transection. Eur J Neurosci. 2007;26:2777-2794.
152 Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313-1317.
153 Kukekov VG, Laywell ED, Suslov O, et al. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333-344.
154 Roy NS, Benraiss A, Wang S, et al. Promoter-targeted selection and isolation of neural progenitor cells from the adult human ventricular zone. J Neurosci Res. 2000;59:321-331.
155 Akiyama Y, Honmou O, Kato T, et al. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol. 2001;167:27-39.
156 Arsenijevic Y, Villemure JG, Brunet JF, et al. Isolation of multipotent neural precursors residing in the cortex of the adult human brain. Exp Neurol. 2001;170:48-62.
157 Jin K, Wang X, Xie L, et al. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103:13198-13202.
158 Minger SL, Ekonomou A, Carta EM, et al. Endogenous neurogenesis in the human brain following cerebral infarction. Regen Med. 2007;2:69-74.
159 Marti-Fabregas J, Romaguera-Ros M, Gomez-Pinedo U, et al. Proliferation in the human ipsilateral subventricular zone after ischemic stroke. Neurology. 2010;74:357-365.
160 Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103-114.
161 Henrique D, Hirsinger E, Adam J, et al. Maintenance of neuroepithelial progenitor cells by Delta-Notch signaling in the embryonic chick retina. Curr Biol. 1997;7:661-670.
162 Goodrich LV, Milenkovic L, Higgins KM, et al. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;227:1109-1113.
163 Yang ZJ, Ellis T, Markant SL, et al. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell. 2008;14:135-145.
164 Dakubo GD, Mazerolle CJ, Wallace VA. Expression of Notch and Wnt pathway components and activation of Notch signaling in medulloblastomas from heterozygous patched mice. J Neurooncol. 2006;79:221-227.
165 Becher OJ, Hambardzumyan D, Fomchenko EI, et al. Gli activity correlates with tumor grade in platelet-derived growth factor–induced gliomas. Cancer Res. 2008;68:2241-2249.
166 Bar EE, Chaudhry A, Lin A, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524-2533.
167 Ehtesham M, Sarangi A, Valadez JG, et al. Ligand-dependent activation of the hedgehog pathway in glioma progenitor cells. Oncogene. 2007;26:5752-5761.
168 Kanamori M, Kawaguchi T, Nigro JM, et al. Contribution of Notch signaling activation to human glioblastoma multiforme. J Neurosurg. 2007;106:417-427.
169 Heppner GH. Tumor heterogeneity. Cancer Res. 1984;44:2259-2265.
170 Nowell PC. Mechanisms of tumor progression. Cancer Res. 1987;46:2203-2207.
171 Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893-895.
172 Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982;217:998-1003.
173 Park CY, Tseng D, Weissman IL. Cancer stem cell–directed therapies: recent data from the laboratory and clinic. Mol Ther. 2009;17:219-230.
174 Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-111.
175 Varghese M, Olstorn H, Sandberg C, et al. A comparison between stem cells from the adult human brain and from brain tumors. Neurosurgery. 2008;63:1022-1033.
176 Colin C, Baeza N, Tong S, et al. In vitro identification and functional characterization of glial precursor cells in human gliomas. Neuropathol Appl Neurobiol. 2006;32:189-202.
177 Ligon KL, Alberta JA, Kho AT, et al. The oligodendroglial marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. 2004;63:499-509.
178 Noble M, Mayer-Proschel M. Growth factors, glia and gliomas. J Neurooncol. 1997;35:193-209.
179 Ogden AT, Waziri AE, Lochhead RA, et al. Identification of A2B5+CD133− tumor-initiating cell in adult human gliomas. Neurosurgery. 2008;62:505-514.
180 Knott JC, Pilkington GJ. A2B5 surface ganglioside binding distinguishes between two GFAP-positive clones from a human glioma-derived cell line. Neurosci Lett. 1990;118:52-56.
181 Fallon J, Reid S, Kinyamu R, et al. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc Natl Acad Sci U S A. 2000;97:14686-14691.
182 Kolb B, Morshead C, Gonzalez C, et al. Growth factor–stimulated generation of new cortical tissue and functional recovery after stroke damage to the motor cortex of rats. J Cereb Blood Flow Metab. 2007;27:983-997.
183 Nakatomi H, Kuriu T, Okabe S, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429-441.
184 Burns CT, Verfaille CM, Low WC. Stem cells for ischemic brain injury: a critical review. J Comp Neurol. 2009;515:125-144.
185 Enwere E, Shingo T, Gregg C, et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354-8365.
186 Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173-186.
187 Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Adult-derived neural precursors transplanted into multiple regions in the adult brain. Ann Neurol. 1999;46:867-877.
188 Cao QL, Zhang YP, Howard RM, et al. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. 2001;167:48-58.
189 Reier PJ. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx. 2004;1:424-451.
190 Liu CY, Westerlund U, Svensson M, et al. Artificial niches for human adult neural stem cells: possibility for autologous transplantation therapy. J Hematother Stem Cell Res. 2003;12:689-699.
191 Schmidt NO, Przylecki W, Yang W, et al. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005;7:623-629.
192 Joo KM, Park IH, Shin JY, et al. Human neural stem cells can target and deliver therapeutic genes to breast cancer brain metastases. Mol Ther. 2009;17:570-575.
193 Aboody K, Najbauer J, Schmidt NO, et al. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro Oncol. 2006;8:119-126.
194 Shimato S, Natsume A, Takeuchi H, et al. Human neural stem cells target and deliver therapeutic gene to experimental leptomeningeal medulloblastoma. Gene Ther. 2007;15:1132-1142.
195 Aboody K, Bush R, Garcia E, et al. Development of a tumor-selective approach to treat metastatic cancer. PLoS One. 2006;1:e23.
196 Dickson PV, Hamner JB, Burger RA, et al. Intravascular administration of tumor tropic neural progenitor cells permits targeted delivery of interferon-beta and restricts tumor growth in a murine model of disseminated neuroblastoma. J Pediatr Surg. 2007;42:48-53.
197 Yang J, Liu J, Niu G, et al. In vivo MRI of endogenous stem/progenitor cell migration from subventricular zone in normal and injured developing brains. Neuroimage. 2009;48:319-328.
198 Zhang ZG, Jiang Q, Zhang R, et al. Magnetic resonance imaging and neurosphere therapy of stroke in rat. Ann Neurol. 2003;53:259-263.
199 Hoehn M, Kustermann E, Blunk J, et al. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci U S A. 2002;99:16267-16272.
200 Guzman R, Uchida N, Bliss TM, et al. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci U S A. 2007;104:10211-10216.
201 Daadi MM, Li Z, Arac A, et al. Molecular and magnetic resonance imaging of human embryonic stem cell–derived neural stem cell grafts in ischemic rat brain. Mol Ther. 2009;17:1282-1291.
202 Swijnenburg RJ, Schrepfer S, Govaert JA, et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci U S A. 2008;105:12991-12996.
203 Cao F, Lin S, Xie X, et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005-1014.
204 Sher F, van Dam G, Boddeke E, et al. Bioluminescence imaging of Olig2-neural stem cells reveals improved engraftment in a demyelination mouse model. Stem Cells. 2009;27:1582-1591.
205 Waerzeggers Y, Klein M, Miletic H, et al. Multimodal imaging of neural progenitor cell fate in rodents. Mol Imaging. 2008;7:77-91.
206 Cicchetti F, Gross RE, Bulte JW, et al. Dual-modality in vivo monitoring of subventricular zone stem cell migration and metabolism. Contrast Media Mol Imaging. 2007;2:130-138.