CHAPTER 23 Sporadic and recurrent miscarriage
Introduction and Definitions
Threatened miscarriage is defined as uterine bleeding prior to 24 weeks of pregnancy. Inevitable miscarriage can be subdivided into complete or incomplete, depending on whether or not all fetal and placental tissues have been expelled from the uterus. Early embryonic or fetal demise is where failure of the pregnancy is identified before expulsion of the fetal and placental tissues (usually by repeated ultrasound examination). Recurrent miscarriage is defined as three or more consecutive miscarriages (Stirrat 1990). This can be further subdivided into primary recurrent miscarriage, where there have been no previous live births, and secondary recurrent miscarriage, where at least one successful pregnancy has occurred previously.
Sporadic Miscarriage
Epidemiology
Problems of definition and ascertainment
Problems of definition arise very early in pregnancy, where reliable detection of pregnancy is only possible with biochemical testing using urinary or serum β-human chorionic gonadotrophin (β-hCG), and also in late pregnancy, where the distinction between a late mid-trimester miscarriage and a stillbirth can be difficult (Chard 1991). The ideal research model for determining rates of miscarriage is a prospective longitudinal study of a representative cross-section of the population which is capable of recognizing all conceptions immediately, takes account of termination of pregnancy and follows women through the first 20 weeks of pregnancy (Regan et al 1989, Alberman 1992).
Early histological work from the 1950s (Hertig et al 1959), in which fertilized ova were directly observed in 107 hysterectomy specimens from women who had intercourse around the time of expected ovulation prior to their operation, suggests a postimplantation pregnancy rate of 58% (21 of 36 possible conceptions). In a mathematical model, total pregnancy loss rates have been estimated at 78% (Roberts and Lowe 1975).
Rate of miscarriage
Following critical review of the literature, a reasonably coherent picture is emerging (Figure 23.1). Estimates of early reproductive loss rates in the peri-implantation period of approximately 50–70% still rest largely on the early work of Hertig, although data derived from in-vitro fertilization (IVF) studies support losses of this order of magnitude (Chard 1991).
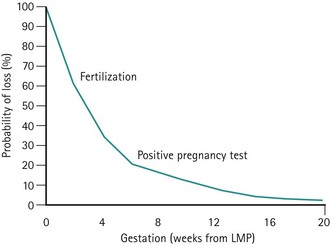
Figure 23.1 Fetal loss by gestational age.
Adapted from Kline J, Stein Z, Susser M 1989 Conception to birth — epidemiology of prenatal development. In: Kline J (ed) Monographs in Epidemiology and Biostatistics, Vol. 14. Oxford University Press, Oxford.
Postimplantation and biochemical pregnancy loss rates appear to be of the order of 30%, whereas recognized pregnancy losses after clinical recognition of pregnancy remain consistent in most studies as 10–15% (Table 23.1).
| Authors | Preclinical loss rate (%) | Clinical loss rate (%) |
|---|---|---|
| French and Bierman (1962) | – | 22 |
| Miller et al (1980) | 33 | 14 |
| Edmonds et al (1982) | 58 | 12 |
| Wilcox et al (1988) | 22 | 12 |
| Regan et al (1989) | – | 12 |
| Brambati (1990) | – | 14 |
| Nybo Andersen et al (2000) | – | 13 |
Aetiology
Chromosomal abnormalities
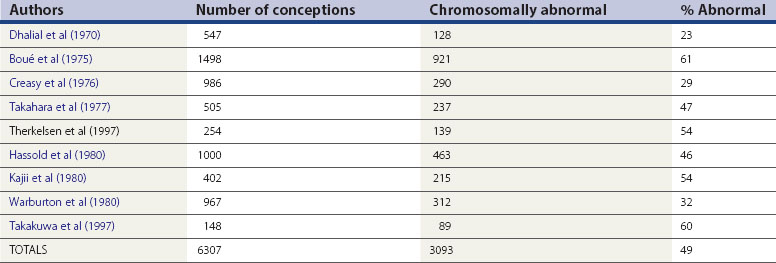
Miscarriage is a heterogeneous condition, but the single largest cause of sporadic miscarriage is fetal chromosomal abnormalities, accounting for approximately 50% of all cases (Table 23.2).
It is difficult to establish the precise contribution made by fetal chromosomal abnormalities. The figures in Table 23.2 are probably an underestimate, since miscarriages occurring as a result of fetal chromosomal anomalies are maximal at the earliest and least well-documented stages of pregnancy. Evidence from preimplantation genetic diagnosis studies of embryos created as a result of IVF suggests that at least 65% of all embryos are chromosomally abnormal (Gianaroli et al 2000). However, extrapolation of these results to miscarriage in the general population must be cautious, given the high degree of selectivity of these patients and a different microenvironment at fertilization.
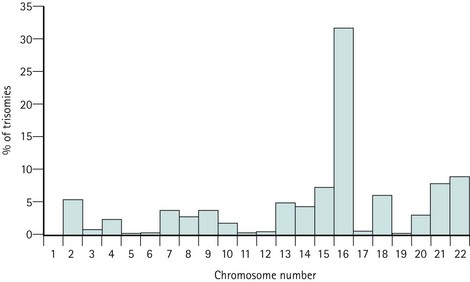
Trisomies for all chromosomes have been described, with the exception of chromosomes 1 and Y, although the relative frequencies are vastly different. Chromosome 16 and, to a lesser extent, chromosomes 2, 13, 15, 18, 21 and 22, account for the majority of trisomic abnormalities (Figure 23.2). Most trisomies are believed to be a consequence of non-disjunction during maternal meiosis. Trisomy 16 gives rise to only the most rudimentary embryonic growth with an empty sac (Edmonds 1992), and other trisomies often result in early embryonic demise.
Monosomy X (45XO) is thought to result from paternal sex chromosome loss (which can be either X or Y). It is usually associated with the presence of a fetus, although focal abnormalities, such as encephalocoele or hygromata, may occur (Edmonds 1992).
There appears to be a difference in the gestational age of pregnancy loss between different types of chromosomal abnormality, with trisomic and monosomic pregnancies miscarrying at a modal peak of 9 weeks and triploid pregnancy losses spanning 5–16 weeks of gestation (Alberman 1992).
Fetal malformations other than those caused by chromosomal anomaly
There is little doubt that the risk of miscarriage is increased with fetal malformation (Alberman 1992). However, since there has been no systematic search for malformations in fetal loss and since, in some cases, the malformation is secondary to a chromosomal anomaly, it is very difficult to assess the size of the increased risk. A single published study (Shephard et al 1989) has demonstrated an increased neural tube defect rate (3.6%) and an increased facial cleft rate (2.7%) amongst spontaneous miscarriages. Overall, the proportion of miscarriages as a result of fetal abnormalities without any associated chromosomal anomaly is small.
Placental abnormalities
Histological analysis of placental tissue from sporadic miscarriages has revealed several different patterns (Rushton 1995). These either point towards early fetal or embryonic demise with essentially normal placentation, or they have shown abnormal placental villous development with marked villous hypoplasia, reduced vascularization, enlarged intravillous spaces, often with clots and lacking significant extravillous trophoblastic infiltration, or with acute necrotic changes in the villi and associated clots which can be either patchy or global. Other reported placental changes have included inflammatory changes or a mixture of the above changes.
Amongst sporadic miscarriages, abnormalities of villous development have been reported fairly consistently at 30–40% (Hustin and Jauniaux 1997). However, the causes leading to the majority of these cases of abnormal placentation and therefore subsequent miscarriage remain unknown. Although autoimmune and infective causes can be associated with abnormal villous development, most inherently abnormal embryos probably also have abnormal placentation.
Infection
Infection has been cited as a cause of late pregnancy loss and also early pregnancy loss for the past century. However, the precise role of infection as a cause of sporadic miscarriage is poorly and inconsistently reported (Simpson et al 1996). In many cases, whether infection has actually preceded any fetal demise or merely arose afterwards remains satisfactorily unanswered.
Several organisms have been associated with miscarriage (Box 23.1). Listeria, toxoplasmosis, herpes varicella zoster and malaria (Plasmodium falciparum) appear to be the most clinically important pathogens in women with early miscarriage.
Rubella infection, although a cause of first-trimester miscarriage, is now rare. The role of cytomegalovirus is unclear, although primary infection may cause miscarriage. The role of chlamydia infection, whether via an acute primary infection or a resultant chronic endometritis, also remains unclear. An association between herpes simplex virus infection and early pregnancy loss was first reported in the 1970s (Nahmias et al 1971), although later prospective studies have failed to show any association with sporadic or recurrent miscarriage even when primary infection occurred in the first trimester (Stray-Pedersen 1993). It remains unknown whether human immunodeficiency virus (HIV) is an important cause of early pregnancy loss. Some studies have demonstrated an increased rate of early pregnancy loss with HIV (d’Ubaldo et al 1998), while others have not (Bakas et al 1996). Whether any increased risk of miscarriage is the result of the virus, the mother’s general health or her immunocompromised status remains unclear.
Syphilis (Treponema pallidum) and parvovirus B19 are more commonly a cause of late second-trimester miscarriage and stillbirth, whilst group B streptococcus has been implicated in late miscarriage and preterm labour. Bacterial vaginosis (Trichomonas vaginalis) is also associated with late second-trimester miscarriage and preterm labour, but the use of metronidazole or other antibiotics in women with demonstrable bacterial vaginosis infection does not reduce late miscarriage or preterm delivery rates (Carey et al 2000).
Fetal sex, multiple pregnancy, maternal age and parity
Most studies have reported an excess of males in miscarriages and pregnancies complicated by varying degrees of placental dysfunction (Kellokumpu-Lethtinen and Pelliniemi 1984, Edwards et al 2000). However, the sex ratio of most early conceptions still remains unknown.
Multiple pregnancy is associated with an increased risk of fetal loss, either via early resorption, postimplantation loss or second-trimester miscarriage. In early pregnancy, the risk of miscarriage is twice that of singleton pregnancies (Sebire et al 1997a). The rate of late pregnancy loss is also increased, particularly in monochorionic twin pregnancies, where late miscarriage rates can approach 12% (Sebire et al 1997b).
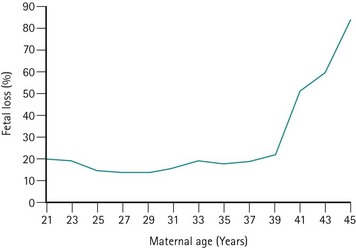
The risk of miscarriage rises with parity; however, the rise is a result of reproductive compensation (Alberman 1987). The risk of miscarriage in first pregnancies is low in young women (Regan et al 1989), but rises significantly after the age of 39 years (Figure 23.3). This rise is not only found in association with trisomic pregnancies (which rise with maternal age) but also in chromosomally normal pregnancies.
Maternal health
Virtually every maternal medical disorder has been associated with sporadic miscarriage. Women with severe medical disease rarely become pregnant, but if they do, their disease may deteriorate during pregnancy. Various mechanisms, including endocrinological, immunological or infective, have been suggested. Poorly controlled diabetes is associated with an increased risk of miscarriage (Mills et al 1988), whereas well-controlled and subclinical diabetes can rarely be considered a cause. Overall, only a small fraction of all early pregnancy losses can be considered to be attributable to severe maternal disease.
Cigarette smoking has been positively correlated with miscarriage, and a review of the effects of nicotine on ovarian, uterine and placental function suggests that cigarette smoking has an adverse effect on trophoblast invasion (Schiverick and Salafia 1999). Cocaine use has also been reported to increase the risk of miscarriage (Ness et al 1999). Alcohol consumption has been shown to be higher in women whose pregnancies ended in miscarriage compared with pregnancies which proceeded beyond 28 weeks of gestation (Harlap and Shapiro 1980, Kline et al 1980), although other studies have not confirmed this observation (Halmesmäki et al 1989, Parazzini et al 1990). There is some evidence to suggest that even moderate maternal alcohol consumption is associated with an increased risk of miscarriage (Windham et al 1997). Many studies have shown a relationship between coffee/caffeine intake and spontaneous miscarriage, although these have not controlled for other confounding variables. Only high levels of caffeine metabolites in maternal serum are associated with miscarriage, so it would appear that moderate consumption of caffeine is unlikely to increase the risk of miscarriage (Klebanoff et al 1999).
Some chemical agents including lead, ethylene oxide, solvents, pesticides, vinyl chloride and anaesthetic gases have been shown to have some association with fetal loss (Cohen et al 1971, Mur et al 1992, Rowland et al 1996, McDonald et al 1988). Although many environmental toxicologists accept these agents as proven, the evidence for low levels of exposure remains far from convincing.
Radiotherapy and chemotherapeutic agents are accepted causes of miscarriage (Zemlickis et al 1992), although they are only administered during pregnancy in seriously ill women. Although significant ionizing radiation for diagnostic purposes can lead to fetal malformation and miscarriage, a dose of more than 25 rads is associated with a 0.1% risk of abnormality (1 rad is the equivalent of eight to 10 abdominal/pelvic X-ray films).
There is a small increase in the risk of spontaneous miscarriage with general anaesthesia and incidental surgery during the first and second trimesters, although this is higher with gynaecological surgery (Duncan et al 1986).
It has been claimed that impaired psychological well-being predisposes to fetal loss (Stray-Pedersen and Stray-Pedersen 1984), although it has been difficult to exclude other confounding variables. Despite problems with recall bias, there are an increased number of negative life events in women with chromosomally normal as compared with chromosomally abnormal miscarriages (Neugebauer et al 1996), but no differences in hormonal markers of stress have been determined.
Pathophysiology
The exact pathophysiology resulting in the uterine expulsion of early pregnancy remains unknown. Abnormal placentation (either primary or itself secondary to early fetal demise) can either lead to reduced or shallow uterine invasion by the trophoblast, or can itself be caused by reduced invasion. In this situation, the usual reduction in maternal vascular tone cannot occur. It is presumed that this blood flow enters the intervillous space and dislodges the conceptus, thereby leading to embryonic demise if this has not already occurred (Rushton 1995). Nevertheless, even quite large intrauterine haematomata visualized by ultrasound have not prevented live births at term (Pedersen and Mantoni 1990), and the presence of haematomata per se does not increase the risk of miscarriage (Tower and Regan 2001).
Presentation
Threatened miscarriage
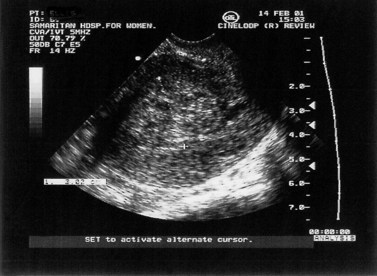
This can be defined as uterine bleeding before 24 weeks of pregnancy (the time of presumed fetal viability) with no evidence of any fetal or embryonic demise. It is usually painless. Characteristically, the bleeding is initially bright red, followed by a reducing brown loss. This can affect up to 25% of all pregnancies and is one of the most common indications for emergency early pregnancy referral. Clinical examination reveals a soft, non-tender uterus, usually of the appropriate gestational size, and a closed cervix. Transvaginal or transabdominal ultrasound scan confirms an ongoing pregnancy (Figure 23.4).
The non-specific nature of abdominal pain, vaginal bleeding and pelvic tenderness precludes their use as predictors of ultimate outcome in women with threatened miscarriage. However, continued vomiting (rather than nausea alone) in early pregnancy is associated with an increased chance of live birth (Weigel and Weigel 1989), presumably because it indicates continued placental hormone production.
Inevitable miscarriage
Miscarriage is a process rather than a single event. Of all women presenting with bleeding in early pregnancy, approximately 50% will ultimately miscarry (Stabile et al 1987). Whilst the cervix remains closed, any pain and bleeding may subside and the pregnancy may continue otherwise normally to term. However, once the cervix opens, miscarriage is inevitable.
Complete and incomplete miscarriage
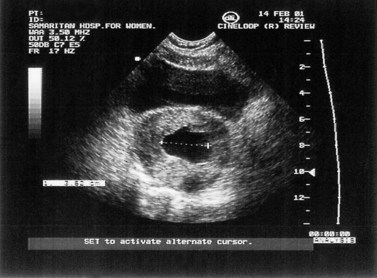
Inevitable miscarriage is either complete or incomplete depending on whether or not all fetal and placental tissues have been expelled from the uterus. The typical features of incomplete miscarriage are heavy bleeding (which may be intermittent) and abdominal cramps. The finding of a dilated cervix on examination in the presence of continued pain and bleeding is usually diagnostic. Ultrasound scan will confirm the presence of retained products of conception (Figure 23.5).
If the symptoms of incomplete miscarriage resolve spontaneously, complete miscarriage may have occurred. Nevertheless, following abdominal and pelvic examination, ultrasound may need to be perfomed to confirm that the uterus is indeed empty. Occasionally, the cervix may close despite the presence of retained products. The number of complete miscarriages is unknown. Many such early miscarriages may go unreported. Early studies of women with threatened miscarriage showed complete miscarriage rates of less than 1% (Stabile et al 1987), although more recent evidence suggests that approximately 20–30% of miscarriages are complete (Chung et al 1994, Mansur 1992). Similarly, expectant management of early fetal demise has demonstrated that, with time, up to 25% of women go on to have a complete miscarriage (Jurkovic et al 1998).
Early fetal demise (missed abortion/miscarriage)
Early fetal demise is where failure of pregnancy is identified before any expulsion of the products of conception occurs. The woman may report a disappearance of the symptoms and signs of early pregnancy, such as nausea and vomiting or breast tenderness. A brown vaginal loss may also be reported. The diagnosis may also be made in otherwise asymptomatic women at routine obstetric/dating ultrasound examination. The diagnosis is made by the lack of fetal heart activity in a pregnancy with a crown–rump length of over 5 mm (Pennell et al 1991), or when two ultrasound examinations 1 or 2 weeks apart have shown no growth and no fetal heart activity. In cases of doubt, a repeat ultrasound is always indicated. Occasionally, a collapsing gestational sac or a failed pregnancy surrounded by clot in utero may be seen (Figure 23.6). Abdominal and pelvic examination often reveals a uterus smaller than expected for the gestational age and a closed cervical os.
Sepsis
This occurs when infection complicates miscarriage or termination of pregnancy. Intrauterine sepsis rarely follows incomplete miscarriage, although the incidence is considerably higher at approximately 3.6% following termination of pregnancy (Frank 1985). The most common infecting organisms are Escherichia coli, Bacteroides, streptococci (both anaerobic and occasionally aerobic) and Clostridium welchii. Occasionally, a history of intrauterine instrumentation may be withheld. The woman usually presents with suprapubic pain, malaise, fever and, occasionally, vaginal bleeding. Findings on examination include abdominal rigidity, uterine and adnexal tenderness, and a closed cervical os. Rarely, septicaemia may ensue, leading to bacteraemic endotoxic shock and possibly maternal death.
Trophoblastic tumours
Trophoblastic tumours include complete and partial hydatidiform moles, choriocarcinoma and placental site tumours. These occasionally present as threatened miscarriage, and suggestive features are noted at ultrasound examination. Trophoblastic tumours are discussed in Chapter 43.
Investigations
The measurement of other fetoplacental hormones and proteins, such as α-fetoprotein, Schwangerschaft protein 1, human placental lactogen, pregnancy-associated plasma protein, oestrogen and progesterone, have all been reported as diagnostic tests in early pregnancy. In rare cases, they may improve the prediction of both early fetal demise and ectopic pregnancy (Grudzinskas and Chard 1992), but their routine use is not indicated.
Treatment
Threatened miscarriage
Early studies demonstrated that the presence of fetal heart activity at ultrasound examination in women who present with a history of bleeding in early pregnancy was associated with a high chance (97–98%) of a live birth (Stabile et al 1987). More recent evidence confirms livebirth rates of 90–95% for younger women, but for women over 40 years of age, miscarriage rates of 15–30% are reported even after the identification of fetal heart activity (Deaton et al 1997, Schmidt-Sarosi et al 1998). Nevertheless, for most women, reassurance and continued medical and emotional support are all that is required.
Bed rest and avoiding penetrative intercourse have historically been advised. If the vascular hypothesis for the pathophysiology of miscarriage is accepted (Hustin and Jauniaux 1997), bed rest may improve pressure variation and flow changes, and therefore improve the outcome. However, there is a paucity of clinical evidence to support this hypothesis. Initial presentation is usually in primary care and, currently, over 96% of general practitioners still advise bed rest and the avoidance of intercourse, although many believe that it does not improve the eventual outcome.
Progesterone supplementation in early pregnancy has been prescribed for over 30 years for women presenting with threatened (and also recurrent) miscarriage. The historical rationale was that a progesterone deficiency would lead to miscarriage. Obviously, the converse may also be true; a failed pregnancy may lead to a progesterone deficiency. There is a wealth of published data, mostly from uncontrolled treatment trials. However, several meta-analyses (Daya 1989, Goldstein et al 1989) have been unable to demonstrate a beneficial effect for progesterone treatment. The routine use of progesterone in threatened miscarriage cannot be justified.
Incomplete miscarriage
Surgical evacuation of the uterus after cervical dilatation, if necessary, has remained the cornerstone of management of incomplete miscarriage in the industrialized world since the 1940s (Hertig and Livingstone 1944). Evacuation and curettage have been regarded as essential to ensure that the uterine cavity is empty, otherwise haemorrhage, infection and later complications such as Asherman’s syndrome may result. Early studies showed a maternal mortality rate of 1.6% in women who did not undergo surgical treatment (Russell 1947). In cases of haemodynamic compromise of cervical shock, appropriate resuscitation and urgent surgical evacuation are indicated. Most cases are performed under general anaesthesia, although effective outpatient curettage has been reported (Fawcus et al 1997). Suction evacuation is generally regarded as a safer technique than sharp curettage, with lower rates of perforation, blood loss and subsequent intrauterine adhesion formation (Edmonds 1992, Verkuyl and Crowther 1993).
The routine use of syntocinon or ergometrine has shown no benefit in reducing blood loss during the surgical treatment of first-trimester incomplete miscarriage (Beeby and Morgan Hughes 1984). Due to the risk of ascending infection and its sequelae, whenever uterine instrumentation is performed, screening for chlamydia infection is recommended, although the use of prophylactic doxycycline is only likely to be of benefit in areas of relatively high prevalence (Prieto et al 1995).
Although a minor procedure, surgical evacuation is associated with rare but serious morbidity. Complications include tearing or lacerations to the cervix, perforation of the uterus, which may also lead to bowel perforation, bladder perforation, damage to the broad ligament, infection, Asherman’s syndrome (intrauterine adhesions) and haemorrhage (Ratnam and Prasad 1990). The incidence of serious morbidity has been estimated at 2.1% (Lawson et al 1994, Royal College of General Practitioners/Royal College of Obstetricians and Gynaecologists 1985), of which the most common problem is infection. This can lead to later sequelae including secondary infertility, ectopic pregnancy and Asherman’s syndrome. The incidence of mortality associated with surgical evacuation of the uterus has been estimated at 0.5 per 100,000 (Lawson et al 1994).
Recent studies demonstrated the efficacy of expectant management or ‘observation alone’ in women with incomplete miscarriage (Nielsen and Hahlin 1995, Hurd et al 1997). In cases where there was no haemodynamic compromise or maternal anaemia, spontaneous resolution occurred within 3 days in up to 80% of cases with minimal retained products (15–50 ml). Therefore, in women with minimal intrauterine tissue (after ectopic pregnancy has been excluded), expectant management is safe. However, large volumes of retained products are associated with an increase in complications, primarily infection and prolonged bleeding (Hurd et al 1997). There is no evidence that future fertility is impaired following expectant management (Kaplan et al 1996).
Herbal remedies have been used in the past to encourage the uterus to expel its contents, but an effective non-surgical alternative to termination of pregnancy and miscarriage had to await the development of the antiprogesterone mifepristone and the prostaglandin analogues gemeprost and misoprostol. Their efficacy has been demonstrated in the treatment of incomplete miscarriage (Henshaw et al 1993, Ashok et al 1998), leading to complete miscarriage rates of approximately 95%.
The use of medical treatment or the adoption of an expectant approach in appropriately selected cases may have many medical and economic benefits. However, many women continue to express a preference for surgical treatment (Hamilton-Fairley and Donaghy 1997), citing fears regarding pain, bleeding and the length of time to resolution in the non-surgical group.
Early fetal demise
The treatment options for early fetal demise are essentially the same as for incomplete miscarriage. Surgical treatment should be preceded by cervical ripening agents (mifepristone or prostaglandins) in order to reduce the risks of cervical trauma or uterine perforation associated with forced cervical dilatation. Gemeprost, mifepristone, and oral or vaginal misoprostol seem to be equally effective (Gupta and Johnson 1992, Platz-Christiansen et al 1995, Ayres de Campos et al 2000). Expectant management is feasible, although less effective than in cases of incomplete miscarriage, with only 25% proceeding to complete miscarriage (Jurkovic et al 1998). The efficacy of medical treatment is also less when compared with incomplete miscarriage, although complete miscarriage rates of up to 90% have been reported with higher doses of mifepristone and misoprostol and a longer surveillance period (El-Refaey et al 1992).
Psychological aspects
Psychological consequences of early pregnancy loss differ widely among different women, different families and even different pregnancies in the same woman. Nevertheless, most women, irrespective of their attitude towards the pregnancy at the time, experience feelings of depression and anxiety following miscarriage (Seibel and Graves 1980), and up to 36% of women are ‘highly symptomatic’ in terms of clinical depression 4 weeks after miscarriage (Neugebauer et al 1992).
All workers caring for women with miscarriage and their families need to understand the increased psychiatric morbidity associated with pregnancy loss, and be able to offer appropriate support both at the time of diagnosis and also after treatment. Good communication with providers of primary care for the whole family and access to counselling are important, since ‘miscarriages do not occur in a uterus but in a woman, and miscarriages do not occur solely in a woman but in a family’ (Cain et al 1964). For some couples, the use of ritual can help them through the bereavement process (Hopper 1997).
In subsequent pregnancies, many women will need considerable reassurance and support (Hamilton 1989), and early access to ultrasound and hospital services may be required.
Recurrent Miscarriage
In contrast to sporadic miscarriage, recurrent miscarriage is relatively uncommon. A history of three or more consecutive miscarriages occurs in 0.5–2% of women (Stirrat 1990, Daya 1993, Katz and Kuller 1994). Recurrent miscarriage is obviously distressing and frustrating for both the couple concerned and those treating them. In many cases, the cause may not be apparent despite intensive and expensive clinical and laboratory testing, and there remains only a limited understanding of the causes of recurrent miscarriage.
The aetiologies are outlined below and summarized in Box 23.2. The management of recurrent miscarriage, including unexplained recurrent miscarriage, will be discussed below under specific headings. Unexplained miscarriage occurs in approximately 50% of women attending specialist recurrent miscarriage clinics (Clifford et al 1994, Stephenson 1996, Li 1998).
Genetic factors
Parental chromosomal abnormalities
Parental chromosomal abnormalities are the most important genetic anomalies currently detectable amongst couples with recurrent miscarriage. Most studies report an incidence of 3–5% (Stray-Pedersen and Stray-Pedersen 1984, Clifford et al 1994, Stephenson 1996, Li 1998), compared with an incidence of 0.5% in the general population.
Balanced or reciprocal translocations are the most frequently detected parental chromosomal anomaly in couples with recurrent miscarriage. The male:female ratio is approximately 1:2. A portion of one chromosome is exchanged with a portion of another, resulting in two abnormal chromosomes but, overall, a normal chromosomal complement. Translocations have been reported for all chromosomes in many different combinations. At gametogenesis, there is a 50% chance of a chromosomally abnormal gamete being produced. However, there appears to be a lower fertilization or implantation rate with abnormal gametes, since chorionic villus sampling and amniocentesis have demonstrated a 40% and 11% risk, respectively, of a chromosomally unbalanced fetus (Mikkelson 1985).
Robertsonian translocations are less common, occurring in approximately 1% of couples with recurrent miscarriage. Here, two chromosomes adhere to each other at either the centromere or the short arms of the chromosome. This leads to a total chromosome count of 45 but a normal chromosome complement overall. The risks of recurrence and miscarriage vary in different Robertsonian translocations. The most common translocation (involving chromosomes 14 and 21) results in 10–15% of pregnancies with trisomy 21 (Boué and Gallano 1984). If homologous chromosomes are involved, the risk of a chromosomally abnormal conceptus is 100%.
It is important to note that over 35% of couples with a significant parental chromosomal abnormality have already achieved a successful pregnancy in addition to their miscarriages (Clifford et al 1994).
Recurrent aneuploidy
Some couples have an increased risk of miscarriage because they produce recurrent aneuploid fetuses. This may be the result of an increased tendency to non-disjunction, either inherited or induced environmentally. Chromosomal abnormalities are seen with a higher frequency amongst preimplantation embryos created during IVF from couples with a history of recurrent miscarriage (Vidal et al 2000). Presumably, these are the result of errors in gametogenesis, and much current research is focused on the role of sperm chromosome abnormalities in recurrent miscarriage.
Other genetic factors
The karyotype of many recurrent miscarriage conceptions may be euploid (46XX or 46XY), and in the absence of any other identifiable cause or association, these unexplained recurrent early pregnancy losses may be the result of molecular mutations or single gene defects. Chromosome analysis itself is a very crude tool to determine genetic abnormalities. So far, there has only been one report of a specific single gene locus abnormality associated with recurrent miscarriage (Pegoraro et al 1997), but much work remains to be done. The impact of other genetic factors on recurrent miscarriage is confirmed by the increased incidence amongst consanguinous couples (Hendrick 1988).
Anatomical factors
Uterine anomalies
The reported incidence of uterine anomalies in women with recurrent miscarriage varies widely from as low as 1% up to 27% (Regan 1997). Described anomalies vary from uterus didelphis to subseptate uteri, and some studies even include submucosal fibroids. The incidence of such anomalies in the fertile population has been reported to be 1.8–3.6% (Ashton et al 1988).
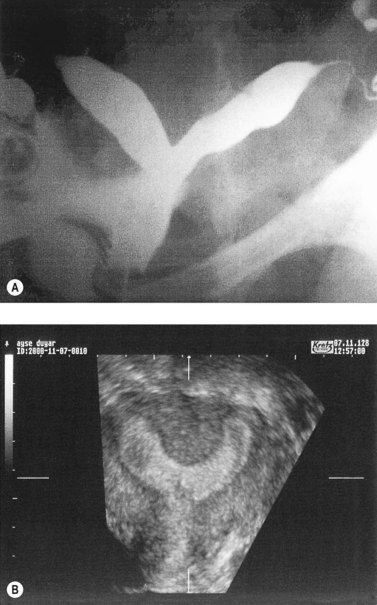
Hysterosalpingography (HSG), laparoscopy and hysteroscopy, magnetic resonance imaging, computed tomography and three-dimensional ultrasound have all been used in the diagnosis and evaluation of uterine anomalies. Although HSG has traditionally been used as the primary screening test, three-dimensional ultrasound shows promise as a less invasive screening tool (Jurkovic et al 1995). In most cases, further evaluation at laparoscopy and hysteroscopy is indicated (Figure 23.7).
Treatment of uterine anomalies in women with recurrent miscarriage remains controversial. Many reports demonstrate a benefit of hysteroscopic or conventional metroplasty in women with septate, subseptate or bicornuate uterus who have suffered mid-trimester miscarriage compared with matched controls (Candiani et al 1990, Ayhan et al 1992, Pabuccu et al 1995, Heinonen 1997). However, the likelihood of a live birth in untreated women is as high as 66% (Heinonen 1997), and a similar percentage of women presenting with recurrent miscarriage have already achieved a live birth (Clifford et al 1994). Open pelvic surgery, in particular, has been associated with subsequent infertility (Bennett 1987). The value of corrective uterine surgery in women with these anomalies and a history of early pregnancy loss is uncertain (Ben-Rafael et al 1991). Similarly, the value of transcervical myomectomy in women with submucous fibroids remains unknown, although there have been initial successful reports (Egwuatu 1989).
Cervical incompetence
The diagnosis of cervical incompetence poses a difficult problem (Medical Research Council/Royal College of Obstetricians and Gynaecologists Working Party on Cervical Cerclage 1993). It is usually based on a previous history of mid-trimester miscarriage in the absence of painful uterine contractions. Vaginal ultrasound may be useful to detect early features of cervical incompetence (shortening or funnelling) (Althuisius et al 2000), but neither ultrasound nor HSG has been found to be useful in the diagnosis of cervical incompetence before pregnancy.
Treatment of cervical incompetence is cervical cerclage in pregnancy, usually after 12–14 weeks when the toll of first-trimester miscarriages has occurred and after screening for chromosomal abnormalities has been performed. It has traditionally involved the insertion of a MacDonald suture, where a tape is inserted round the exposed vaginal cervix (MacDonald 1957), or a Shirodkar suture, where the vaginal mucosa is incised and the bladder reflected, allowing insertion of the suture at a higher level of the cervical canal (Shirodkar 1960). Modifications of this procedure have included burying the suture under the vaginal mucosa at the end of the procedure. In the authors’ experience, this is rarely accompanied by significant vaginal discharge. The MacDonald suture is more widely used and less traumatic to the cervix than the Shirodkar suture, although the exposed tape remains a possible focus for later infection. More recently, emergency cervical cerclage in women who present acutely in the mid-trimester with silent cervical dilatation and/or herniation of the membranes through the cervix has been reported with livebirth rates of 50–60% (Wong et al 1993, Aarts et al 1995). Nevertheless, there are high rates of premature delivery and infection, including chorioamnionitis, in over 30% of cases.
In some women, particularly where the diagnosis is unsure or in those who have other risk factors, regular ultrasound monitoring of the cervix can be performed. If there is funnelling or shortening of the cervix, emergency cerclage can be performed (Althuisius et al 2000). Ultrasound may also be useful in women who have already had cervical cerclage in order to warn of problems (Dijkstra et al 2000). Transabdominal cervical cerclage may be useful in a highly selected group of women with anatomical defects in the cervix and previous mid-trimester miscarriages or preterm labours following failed vaginal cervical cerclage (Gibb and Salaria 1995). Laparoscopic transabdominal cervical cerclage has also been reported (Scibetta et al 1998), but neither of these procedures have been assessed in appropriate trials.
Infective factors
Syphilis (Treponema pallidum) is a cause of recurrent late second-trimester miscarriage and stillbirth. Screening is performed routinely, and treatment with penicillins is effective. It is a rare cause of recurrent miscarriage in developed countries. Malaria infection in non-immune women is also associated with recurrent miscarriage in endemic areas (Royal College of Obstetricians and Gynaecologists 2001).
Bacterial vaginosis (Trichomonas vaginalis) is associated with recurrent late second-trimester miscarriage and preterm labour (Hay et al 1994), although there is no association with early pregnancy loss (Llahi-Camp et al 1996). Treatment with metronidazole has not been shown to be effective (Carey et al 2000).
The identification of individual organisms has proven to be disappointing in the search for causes of early or late recurrent miscarriage, which suggests that pregnancy outcome may be determined by maternal or fetal response to infection rather than the infective organism itself. Gene mutations, such as those associated with mannose-binding protein (MBP) deficiency, are an important cause of inherited immunodeficiency and increased susceptibility to infection. Early studies demonstrate a trend in association between late miscarriage and MBP genes (Baxter et al 2001). Future research regarding the role of infection in recurrent miscarriage needs to explore genetic susceptibility.
Endocrine factors
Systemic endocrine disease
As discussed previously, diabetic women with good metabolic control are no more likely to miscarry than non-diabetic women. However, diabetic women with high glycosylated haemoglobin A1c levels in the first trimester are at significantly higher risk of both miscarriage and fetal malformation (Hanson et al 1990). As the risk of miscarriage is only increased in women with poorly controlled diabetes mellitus, there is no value in screening for occult disease in asymptomatic women.
Although thyroid autoantibodies are associated with an increased risk of miscarriage, this is secondary to a generalized autoimmune abnormality rather than a specific thyroid dysfunction (Singh et al 1995). Screening asymptomatic women with a thyroid function test is unhelpful as they are usually normal (Clifford et al 1994, Rushworth et al 2000).
Luteal-phase deficiency
A functional corpus luteum is essential for the implantation and maintenance of early pregnancy, primarily through the production of progesterone, which is responsible for the conversion of a proliferative endometrium into a secretory endometrium available for embryo implantation. Disorders or removal of the corpus luteum can result in infertility and early pregnancy loss (Miller et al 1969, Csapo et al 1973). Although much controversy exists about luteal function, the success of oocyte donation with oestradiol and progesterone support in the absence of any ovarian activity would suggest that its effects are primarily progesterone determined.
The prevalence of a luteal-phase defect is reported to occur in 23–60% of women with recurrent miscarriage (Li and Cooke 1991), although it is difficult to determine the exact proportion of women with luteal-phase problems because of difficulties in diagnosis. The diagnosis is usually determined by low luteal-phase progesterone level and/or a non-secretory endometrium, by biopsy, in non-fertile, non-pregnant cycles (Jordan et al 1994, Serle et al 1994). Between 30% and 50% of cases of luteal-phase defect, as determined by endometrial histology, are found in the presence of normal circulating progesterone levels, suggesting that a primary endometrial defect is as common as deficient progesterone production (Li et al 1991). There is no reliable way of demonstrating a luteal-phase defect in conception cycles or early pregnancy as low levels of progesterone in early pregnancy are a reflection that the pregnancy has already failed because the trophoblast cannot produce sufficient progesterone. The use of colour-flow pulsed Doppler ultrasound may offer a non-invasive tool for diagnosis, and has been used in the diagnosis of luteal-phase defect, although only at a preliminary stage (Glock and Brumsted 1995).
There have been many reports of successful pregnancies following treatment with progesterone after the diagnosis of a luteal-phase defect. These studies have, for the most part, been uncontrolled. The only meta-analysis of controlled trials of progesterone treatment in women with recurrent miscarriage has not demonstrated any benefit (Daya 1989).
Treatment with hCG should stimulate progesterone production from the corpus luteum, and has been used in the treatment of luteal-phase defect. Although one small study demonstrated a beneficial effect of hCG treatment in women with oligomenorrhoea (Quenby and Farquharson 1994), meta-analysis has concluded that there is insufficient evidence at present concerning the effectiveness of hCG to recommend its use for women with unexplained recurrent miscarriage (Prendiville 1995).
Polycystic ovary syndrome and hypersecretion of luteinizing hormone
The prevalence of polycystic ovarian morphology in women with recurrent miscarriage is 40% (Rai et al 2000), and there have been numerous reports of an increased risk of miscarriage in women with hypersecretion of luteinizing hormone (LH), hyperandrogenaemia and, more recently, hyperprolactinaemia; all the classic endocrinopathies of polycystic ovary syndrome (PCOS) (Stanger and Yovich 1985, Howles et al 1987, Homburg et al 1988, Regan et al 1990, Bussen et al 1999). Nevertheless, a degree of controversy exists.
Several reports have not confirmed the relationship between hypersecretion of LH and an increased risk of miscarriage in women with recurrent miscarriage (Tulppala et al 1993, Liddell et al 1997). Whether this is due to the radioimmunoassays or a genetic variant of LH is unclear (Tulppala et al 1998). Also, suppression of high endogenous LH secretion with gonadotrophin-releasing hormone analogues in a prospective randomized placebo-controlled trial did not improve the livebirth rate (Clifford et al 1996).
The association between raised androgens and recurrent miscarriage has similarly not been confirmed with later studies (Liddell et al 1997, Rai et al 2000), although these are not universal findings and some studies have demonstrated retarded endometrial development and increased risk of miscarriage in association with raised testosterone levels (Tulppala et al 1993, Okon et al 1998).
Hyperprolactinaemia is a common finding in women with PCOS, and has also been associated with recurrent miscarriage (Hirahara et al 1998, Bussen et al 1999). Although one study has demonstrated a beneficial effect of bromocriptine treatment, this has not been corroborated elsewhere (Hirahara et al 1998).
Autoimmune and thrombophilic factors
Autoimmune disease
Sporadic and recurrent miscarriage are recognized complications of systemic lupus erythematosus, the most common autoimmune disorder in women of reproductive age. Since the 1980s, it has been recognized that recurrent miscarriage is associated with an increase in the detection of many autoantibodies, even in asymptomatic women. The two most common groups of autoantibodies detected have been the antiphospholipid antibodies (aPL), which in conjunction with a history of recurrent miscarriage is now known as the ‘antiphospholipid syndrome’ (APS) (Harris 1987), and thyroid autoantibodies (Bussen and Steck 1995). However, with the exception of APS, the mechanisms by which a generalized increase in the autoantibody pool leads to miscarriage remain unclear. Although intravenous immunoglobulin (IVIG) therapy has been used in women with recurrent miscarriage associated with non-specific autoimmunity, at present the collective evidence indicates that IVIG does not have a therapeutic effect that is clinically meaningful (Daya et al 1999).
Antiphospholipid syndrome
APS refers to the relationship between aPL, namely lupus anticoagulant (LA) and anticardiolipin antibodies (aCL), and recurrent miscarriage, thrombosis or thrombocytopenia (Harris 1987). Since this original description, it has become apparent that the three defining clinical features are too limiting, and aPL are implicated in a wide range of clinical conditions (Box 23.3).
In women with recurrent miscarriage, a previous personal or family history of thrombosis, cardiovascular disease, epilepsy and migraine is strongly predictive of a positive aPL status (Regan 1997). The overall prevalence of aPL in women with recurrent miscarriage is approximately 15% (Li 1998).
Women with APS without treatment have a miscarriage risk of 85–90%, the majority of which occur in the first trimester after establishment of a fetal heart (Rai et al 1995a,b). Pregnancy loss associated with APS is initially attributed to defective embryonic implantation and later to thrombosis of the uteroplacental vasculature and placental infarction (Rai and Regan 1998).
Diagnosis of APS and detection of aPL are subject to widespread interlaboratory variation and the fluctuating nature of the antibodies themselves. Testing for LA should follow internationally agreed guidelines (Lupus Anticoagulant Working Party on behalf of the BSCH Homeostasis and Thrombosis Task Force 1991), and the test of choice is the dilute Russell’s viper venom time which is more sensitive than the activated partial thromboplastin time and the kaolin clotting time. Testing for aCL is by standardized enzyme-linked immunosorbent assay. Testing must be repeated on at least two occasions 8 weeks apart in order to make a diagnosis of APS. Testing for aPL other than LA and aCL is of no clinical use in women with recurrent miscarriage.
Although a variety of treatments have been described for APS, including corticosteroids (Lubbe et al 1983), low-dose aspirin (Silver et al 1993), heparin alone (Rosove et al 1990) and IVIG therapy (Carreras et al 1988), treatment with subcutaneous heparin and low-dose aspirin until 34 weeks of gestation has been shown to be the most effective treatment for women with APS and recurrent miscarriage (Rai et al 1997). In cases where there are additional medical complications, such as thrombocytopenia, IVIG may be appropriate (Cowchock 1998, Piette et al 2000).
Other thrombophilic abnormalities
While APS is an acquired autoimmune thrombophilic state, recurrent miscarriage is also associated with other inherited and acquired causes of thrombophilia (Blumenfeld and Brenner 1999). Most studies are retrospective and interpretation must be cautious because of problems with acquisition and ascertainment.
Activated protein C resistance, usually but not always inherited via factor V Leiden mutation, is an important cause of acquired venous thrombosis and thrombophilia (Bertina et al 1994). It is also associated with recurrent fetal loss (Brenner et al 1997). Successful treatment with low-dose aspirin and heparin has been reported, although there are no prospective controlled studies to date.
Hyperhomocysteinaemia, as a result of congenital enzyme deficiencies or vitamin B6, B9 and B12 deficiencies, is associated with thrombosis and premature vascular disease (Boers et al 1985). It has also been reported in association with recurrent pregnancy loss (Wouters et al 1993). Thromboprophylaxis and vitamin supplementation have been reported (Aubard et al 2000), but no prospective data are yet available.
Other inherited thrombophilias, including protein S and protein C deficiency and antithrombin III deficiency, are associated with recurrent miscarriage as well as late pregnancy complications (Girling and de Swiet 1998). These need to be managed with appropriate haematological experience because of the increased risk of thromboembolism during pregnancy.
Screening women who have a history of recurrent miscarriage has shown increased incidence of thrombin generation — a global marker of a prothrombotic state — even when not pregnant (Vincent et al 1998). Further research is needed to determine whether thromboprophylaxis is appropriate for these women.
Alloimmune factors
The possibility that maternal alloimmune abnormalities lead to recurrent miscarriage has been proposed but remains contentious, and there is no reliable test. Treatments involving immunization with trophoblast or paternal leukocytes or third-party leukocytes have been successfully used in women with recurrent miscarriage. However, results of prospective randomized trials and meta-analyses have only shown minimal benefit or no benefit with treatment (Daya and Gunby 1994, Recurrent Miscarriage Immunotherapy Trialists Group 1994, Ober et al 1999). Any potential benefit must be balanced against the risk of the treatment, and at the present time, allogenic immunization should only be offered in the context of a clinical trial. Current recommendations, both in the UK and the USA, are against treatment.
Investigations
The known causes and management of recurrent miscarriage have been detailed above. Necessary investigations to identify such causes are indicated in women with recurrent miscarriage (Box 23.4). Testing thyroid function, random glucose, autoantibody screen and TORCH screen are no longer appropriate (Li 1998).
Treatment of unexplained recurrent miscarriage
Treatment of recurrent miscarriage where a potential cause has been identified has been discussed above. In approximately 50% of recurrent miscarriages, no cause is determined. The prognosis for this group is usually good. The value of continued reassurance and psychological support has been demonstrated (Stray-Pedersen and Stray-Pedersen 1984), with a 75% chance of a live birth in unexplained recurrent miscarriage. This support should include care in a specialist clinic, psychological support, easy access to a named contact, close monitoring including ultrasonography, appropriate reassurance, and helpful and caring staff.
Treatment of unproven value should not be offered. Any empirical treatment or treatment in clinical trials needs to have a sound scientific and statistical basis, and should include careful counselling and informed consent (Clifford et al 1994, Liddell et al 1997).
Counselling
Counselling should be offered to all patients attending a recurrent miscarriage clinic. It should include an explanation of the possible underlying causes and prognoses. After three consecutive early pregnancy losses, there remains a 60–70% chance that the next pregnancy will be successful (Edmonds 1992, Li 1998). This chance decreases with each subsequent miscarriage, although even after six miscarriages, the chance of a successful pregnancy is still over 45%.
Conclusion
KEY POINTS
Aarts JM, Brons JT, Bruinse HW. Emergency cerclage: a review. Obstetrical and Gynecological Survey. 1995;50:459-469.
Alberman E. Maternal age and spontaneous abortion. In: Bennett MJ, editor. Spontaneous and Recurrent Abortion. Oxford: Blackwell Science, 1987.
Alberman E. Spontaneous abortions: epidemiology. In: Stabile I, Grudzinskas G, Chard T, editors. Spontaneous Abortion — Diagnosis and Treatment. London: Springer-Verlag, 1992.
Althuisius SM, Dekker GA, van Geijn HP, Bekedam DJ, Hummel P. Cervical incompetence prevention randomized cerclage trial (CIPRACT): study design and preliminary results. American Journal of Obstetrics and Gynecology. 2000;183:823-829.
Ashok PW, Penney GC, Flett GM, Templeton A. An effective regimen for early medical abortion: a report of 2000 consecutive cases. Human Reproduction. 1998;13:2962-2965.
Ashton D, Amin HK, Richart RM, Neuwirth RS. The incidence of asymptomatic uterine anomalies in women undergoing transcervical tubal sterilization. Obstetrics and Gynecology. 1988;72:28-30.
Aubard Y, Darodes N, Cantaloube M, Aubard V, Diallo D, Teissier MP. Hyperhomocysteinemia and pregnancy: a dangerous association. Journal of Obstetrics, Gynecology and Reproduction (Paris). 2000;29:363-372.
Ayhan A, Yücel I, Tuncer ZS, Kisnisçi HA. Reproductive performance after conventional metroplasty: an evaluation of 102 cases. Fertility and Sterility. 1992;57:1194-1196.
Ayres-de-Campos D, Teixeira-da-Silva J, Campos I, Patrício B. Vaginal misoprostol in the management of first trimester missed abortions. International Journal of Gynecology and Obstetrics. 2000;71:53-57.
Bakas C, Zarou DM, de Caprariis PJ. First-trimester spontaneous abortions and the incidence of human immunodeficiency virus seropositivity. Journal of Reproductive Medicine. 1996;41:15-18.
Baxter N, Sumiya M, Cheng S, et al. Recurrent miscarriage and variant alleles of mannose binding lectin and tumour necrosis factor genes. Clinical and Experimental Immunology. 2001;126:529-534.
Beeby D, Morgan Hughes JO. Oxytocic drugs and anaesthesia. A controlled clinical trial of ergometrine, syntocinon and normal saline during evacuation of the uterus after spontaneous abortion. Anaesthesia. 1984;39:764-767.
Bennett MJ. Congenital abnormalities of the fundus. In: Bennett MJ, Edmonds DK, editors. Spontaneous and Recurrent Abortion. Oxford: Blackwell Science, 1987.
Ben-Rafael Z, Seidman DS, Recabi K, Bider D, Mashiach S. Uterine anomalies: a retrospective matched-control study. Journal of Reproductive Medicine. 1991;36:723-727.
Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369:64-67.
Blumenfeld Z, Brenner B. Thrombophilic-associated pregnancy wastage. Fertility and Sterility. 1999;72:765-774.
Boers GH, Smals AG, Trijbels FJ, et al. Heterozygosity for homocystinuria in premature peripheral and cerebral occlusive arterial disease. New England Journal of Medicine. 1985;313:709-715.
Boué A, Gallano P. A collaborative study of the segregation of inherited structural arrangements in 1356 prenatal diagnoses. Prenatal Diagnosis. 1984;4:45-67.
Boué J, Boué A, Lazar P. Retrospective and prospective epidemiological studies of 1500 karyotyped spontaneous human abortions. Teratology. 1975;12:11-26.
Brambati B. Fate of human pregnancies. In: Edwards RG, editor. Serono Symposia: Establishing a Successful Human Pregnancy, Vol. 66. New York: Raven Press; 1990.
Brenner B, Mandel H, Lanir N, et al. Activated protein C resistance can be associated with recurrent fetal loss. British Journal of Haematology. 1997;97:551-554.
Bussen S, Steck T. Thyroid autoantibodies in euthyroid non-pregnant women with recurrent spontaneous abortions. Human Reproduction. 1995;10:2938-2940.
Bussen S, Sutterlin M, Steck T. Endocrine abnormalities during the follicular phase in women with recurrent spontaneous abortion. Human Reproduction. 1999;14:18-20.
Cain AC, Erikson ME, Fast I, et al. Children’s disturbed reaction to their mothers’ miscarriage. Psychosomatic Medicine. 1964;26:58-66.
Candiani GB, Fedele L, Parazzini F, Zamberletti D. Reproductive prognosis after abdominal metroplasty in bicornuate or septate uterus: a life-table analysis. British Journal of Obstetrics and Gynaecology. 1990;97:613-617.
Carey JC, Klebanoff MA, Hauth JC, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development of Maternal-Fetal Medicine Units. New England Journal of Medicine. 2000;342:534-540.
Carreras LO, Perez GN, Vega HR, et al. Lupus anticoagulant and recurrent fetal loss: successful treatment with gammaglobulin. Fertility and Sterility. 1988;54:991-994.
Chard T. Frequency of implantation and early pregnancy loss in natural cycles. Baillière’s Clinical Obstetrics and Gynaecology. 1991;5:179-189.
Chung TK, Cheung LP, Lau WC, Haines CJ, Chang AM. Spontaneous abortion: a medical approach to management. Australia and New Zealand Journal of Obstetrics and Gynaecology. 1994;34:432-436.
Clifford K, Rai R, Watson H, Regan L. An informative protocol for the investigation of recurrent miscarriage: preliminary experience of 500 cases. Human Reproduction. 1994;9:1328-1332.
Clifford K, Rai R, Watson H, Franks S, Regan L. Does suppressing luteinising hormone secretion reduce the miscarriage rate? Results of a randomised controlled trial. BMJ. 1996;312:1508-1511. (Clinical Research Ed.)
Cohen EN, Belville JW, Brown BW. Anesthesia, pregnancy and miscarriage: a study of operating room nurses and anesthetists. Anesthesiology. 1971;35:343-347.
Cowchock S. Treatment of antiphospholipid syndrome in pregnancy. Lupus. 1998;7(Suppl 2):S95-S97.
Creasy MR, Crolla JA, AlbermanAlberman ED. A cytogenetic study of human spontaneous abortions using banding techniques. Human Genetics. 1976;31:177-196.
Csapo AI, Pulkinnen MO, Wiest WG. Effects of lutectomy and progesterone replacement therapy in early pregnant patients. American Journal of Obstetrics and Gynecology. 1973;115:759-765.
Daya S. Efficacy of progesterone support for pregnancy in women with recurrent miscarriage. A meta-analysis of controlled trials. British Journal of Obstetrics and Gynaecology. 1989;96:275-280.
Daya S. Evaluation and management of recurrent spontaneous abortion. Current Opinion in Obstetrics and Gynecology. 1993;8:188-192.
Daya S, Gunby J. The effectiveness of allogeneic leukocyte immunization in unexplained primary recurrent spontaneous abortion. Recurrent Miscarriage Immunotherapy Trialists Group. American Journal of Reproductive Immunology. 1994;32:294-302.
Daya S, Gunby J, Porter F, Scott J, Clark DA. Critical analysis of intravenous immunoglobulins therapy for recurrent miscarriage. Human Reproduction. 1999;5:475-482.
Deaton JL, Honoré GM, Huffman CS, Bauguess P. Early transvaginal ultrasound following an accurately dated pregnancy: the importance of finding a yolk sac or fetal heart motion. Human Reproduction. 1997;12:2820-2823.
Dhalial RK, Machin AM, Tait SM. Chromosomal anomalies in spontaneously aborted human fetuses. Lancet. 1970;2:20-21.
Dijkstra K, Funai EF, O’Neill L, Rebarber A, Paidas MJ, Young BK. Change in cervical length as a predictor of preterm delivery. Obstetrics and Gynecology. 2000;96:346-350.
D’Ubaldo C, Pezzotti P, Rezza G, Branca M, Ippolito G. Association between HIV-1 infection and miscarriage: a group retrospective study. DIANAIDS Collaborative Study Group. AIDS. 1998;12:1087-1093.
Duncan PG, Pope WD, Cohen MM, Greer N. Fetal risk of anesthesia and surgery during pregnancy. Anesthesiology. 1986;64:790-794.
Edmonds DK. Spontaneous and recurrent abortion. In Shaw R, Soutter P, Stanton S, editors: Gynaecology, 2nd edn, Edinburgh: Churchill Livingstone, 1992.
Edmonds DK, Lindsay KS, Miller JF, Williamson E, Wood PJ. Early embryonic mortality in women. Fertility and Sterility. 1982;38:447-453.
Edwards A, Megens A, Peek M, Wallace EM. Sexual origins of placental dysfunction. The Lancet. 2000;355:203-204.
Egwuatu VE. Fertility and fetal salvage among women with uterine leiomyomas in a Nigerian teaching hospital. International Journal of Fertility. 1989;34:341-346.
el-Refaey H, Hinshaw K, Henshaw R, Smith N, Templeton A. Medical management of missed abortion and anembryonic pregnancy. BMJ. 1992;305:1399. (Clinical Research Ed.)
Fawcus S, McIntyre J, Jewkes RK, et al. Management of incomplete abortions at South African public hospitals. National Incomplete Abortion Study Reference Group. South African Medical Journal. 1997;87:438-442.
Frank P. Sequelae of induced abortion. In: Abortion: Medical and Social Implications. London: Pitman; 1985.
French FE, Bierman JM. Probabilities of fetal mortality. Public Health Report. 1962;77:835-847.
Gianaroli L, Magli MC, Ferraretti AP, Fortini D, Tabanelli C, Gergolet M. Gonadal activity and chromosomal constitution of in vitro generated embryos. Molecular and Cellular Endocrinology. 2000;161:111-116.
Gibb DM, Salaria DA. Transabdominal cervicoisthmic cerclage in the management of recurrent second trimester miscarriage and preterm delivery. British Journal of Obstetrics and Gynaecology. 1995;102:802-806.
Girling J, de Swiet M. Inherited thrombophilia and pregnancy. Current Opinion in Obstetrics and Gynecology. 1998;10:135-144.
Glock JL, Brumsted JR. Color-flow pulsed Doppler ultrasound in diagnosing luteal phase defect. Fertility and Sterility. 1995;64:500-504.
Goldstein P, Berrier J, Rosen S, et al. Hormone administration for the maintenance of pregnancy. In: Chalmers I, Enkin M, Kierse MJNC, editors. Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1989.
Grudzinskas JG, Chard T. Assessment of early pregnancy: measurement of fetoplacental hormones and proteins. In: Stabile I, Grudzinskas G, Chard T, editors. Spontaneous Abortion. Berlin: Springer-Verlag, 1992.
Gupta JK, Johnson N. Should we use prostaglandins, tents, or progesterone antagonists for cervical ripening before first trimester abortion? Contraception. 1992;46:489-497.
Halmesmäki E, Valimaki M, Roine R, Ylikahri R, Ylikorkala O. Maternal and paternal alcohol consumption and miscarriage. British Journal of Obstetrics and Gynaecology. 1989;96:188-191.
Hamilton SM. Should follow-up be provided after miscarriage? British Journal of Obstetrics and Gynaecology. 1989;96:743-745.
Hamilton-Fairley D, Donaghy J. Surgical versus expectant management of first trimester miscarriage: a prospective observational study. In: Grudzinskas JG, O’Brien PMS, editors. Problems in early pregnancy. London: RCOG Press, 1997.
Hanson U, Persson B, Thunell S. Relationship between haemoglobin A1C in early type (insulin dependent) diabetic pregnancy and the occurrence of spontaneous abortion and fetal malformation in Sweden. Diabetologia. 1990;33:100-104.
Harlap S, Shapiro PH. Alcohol, smoking and incidence of spontaneous abortions in the first and second trimester. The Lancet. 1980;2:2.
Harris EN. Syndrome of the black swan. British Journal of Rheumatology. 1987;26:324-326.
Hassold T, Chen N, Funkhouser J, et al. A cytogenetic study of 1000 spontaneous abortions. Annals of Human Genetics. 1980;44:151-178.
Hay PE, Lamont RF, Taylor-Robinson D. Abnormal bacterial colonisation of the genital tract and subsequent pre-term delivery and late miscarriage. BMJ. 1994;308:295-299. (Clinical Research Ed.)
Heinonen PK. Reproductive performance of women with uterine anomalies after abdominal or hysteroscopic metroplasty or no surgical treatment. Journal of the American Association of Gynecological Laparoscopists. 1997;4:311-317.
Hendrick PW. HLA-sharing, recurrent spontaneous abortion, and the genetic hypothesis. Genetics. 1988;119:199-204.
Henshaw RC, Cooper K, el-Refaey H, Smith NC, Templeton AA. Medical management of miscarriage: non-surgical uterine evacuation of incomplete and inevitable spontaneous abortion. BMJ. 1993:894-895. (Clinical Research Ed.)
Hertig AT, Livingstone RG. Spontaneous, threatened and habitual abortion: their pathogenesis and treatment. New England Journal of Medicine. 1944;230:797-806.
Hertig AT, Rock J, Adams EC, Menkin MC. Thirty-four fertilised human ova, good, bad and indifferent, recovered from 210 women of known fertility. A study of biologic wastage in early human pregnancy. Pediatrics. 1959;23:202-211.
Hirahara F, Andoh N, Sawai K, Hirabuki T, Uemura T, Minaguchi H. Hyperprolactinaemic recurrent miscarriage and results of randomised bromocriptine treatment trials. Fertility and Sterility. 1998;70:246-252.
Homburg R, Armar NA, Eshel A, Adams J, Jacobs HS. Influence of serum luteinising hormone concentrations on ovulation, conception, and early pregnancy loss in polycystic ovary syndrome. BMJ. 1988;297:1024-1026. (Clinical Research Ed.)
Hopper E. Psychological consequences of early pregnancy loss. In: Grudzinskas JG, O’Brien PMS, editors. Problems in Early Pregnancy. London: RCOG Press, 1997.
Howles CM, Macnamee MC, Edwards RG. Follicular development and early luteal function of conception and non-conception cycles after in-vitro fertilization: endocrine correlates. Human Reproduction. 1987;2:17-21.
Hurd WW, Whitfield RR, Randolph JFJr, Kercher ML. Expectant management versus elective curettage for the treatment of spontaneous abortion. Fertility and Sterility. 1997;68:601-606.
Hustin J, Jauniaux E. Mechanisms and pathology of miscarriage. In: Grudzinskas JG, O’Brien PMS, editors. Problems in Early Pregnancy. London: RCOG Press, 1997.
Jordan J, Craig K, Clifton DK, Soules MR. Luteal phase defect: the sensitivity and specificity of diagnostic methods in common clinical use. Fertility and Sterility. 1994;62:54-62.
Jurkovic D, Geipel A, Gruboeck K, Jauniaux E, Natucci M, Campbell S 1995 Three-dimensional ultrasound for the assessment of uterine anatomy and detection of congenital anomalies: a comparison with hysterosalpingography and two-dimensional ultrasound. Obstetrics and Gynecology 5: 233–237.
Jurkovic D, Ross JA, Nicolaides KH. Expectant management of missed miscarriage. British Journal of Obstetrics and Gynaecology. 1998;105:670-671.
Kajii T, Ferrier A, Niikawa N, Takahara H, Ohama K, Avirachan S. Anatomic and chromosomal anomalies in 639 spontaneous abortuses. Human Genetics. 1980;55:87-98.
Kaplan B, Pardo J, Rabinerson D, Fisch B, Neri A. Future fertility following conservative management of abortion. Human Reproduction. 1996;11:92-94.
Katz VL, Kuller JA. Recurrent miscarriage. American Journal of Perinatology. 1994;11:386-397.
Kellokumpu-Lethtinen P, Pelliniemei LJ. Sex ratio of human conceptuses. Obstetrics and Gynecology. 1984;64:220-222.
Klebanoff MA, Levine RJ, DerSimonian R, Clemens JD, Wilkins DG. Maternal serum paraxanthine, a caffeine metabolite, and the risk of spontaneous abortion. New England Journal of Medicine. 1999;341:1639-1644.
Kline J, Shrout P, Stein Z, Susser M, Warburton D. Drinking during pregnancy and spontaneous abortion. The Lancet. 1980;2:176-180.
Kline J, Stein Z, Susser M. Conception to birth — epidemiology of prenatal development. In: Kline J, editor. Monographs in Epidemiology and Biostatistics, Vol. 14. Oxford: Oxford University Press; 1989.
Lawson HW, Frye A, Atrash HK, Smith JC, Shulman HB, Ramick M. Abortion mortality, United States, 1972 through 1987. American Journal of Obstetrics and Gynecology. 1994;171:1365-1372.
Li TC. Recurrent miscarriage: principles of management. Human Reproduction. 1998;13:478-482.
Li TC, Cooke ID. Evaluation of the luteal phase: a review. Human Reproduction. 1991;6:484-499.
Li TC, Dockery P, Cooke ID. Endometrial development in the luteal phase of women with various types of infertility: comparison of women with normal fertility. Human Reproduction. 1991;6:325-330.
Liddell HS, Sowden K, Farquhar CM. Recurrent miscarriage: screening for polycystic ovaries and subsequent pregnancy outcome. Australia and New Zealand Journal of Obstetrics and Gynaecology. 1997;37:402-406.
Llahi-Camp JM, Rai R, Ison C, Regan L, Taylor-Robinson D. Association of bacterial vaginosis and a history of second trimester miscarriage. Human Reproduction. 1996;11:1575-1578.
Lubbe WF, Butler WS, Palmer SJ, Liggins GC. Fetal survival after prednisone suppression of maternal lupus anticoagulant. The Lancet. 1983;1:1361-1363.
Lupus Anticoagulant Working Party on behalf of the BSCH Homeostasis and Thrombosis Task Force. Guidelines on testing for the lupus anticoagulant. Journal of Clinical Pathology. 1991;44:885-889.
MacDonald IA. Suture of the cervix for inevitable miscarriage. Journal of Obstetrics and Gynecology of the British Empire. 1957;64:731-735.
McDonald AD, McDonald JC, Armstrong B, et al. Fetal death and work in pregnancy. British Journal of Industrial Medicine. 1988;45:148-157.
Mansur MM. Ultrasound diagnosis of complete abortion can reduce need for curettage. European Journal of Obstetrics, Gynecology and Reproductive Biology. 1992;44:65-69.
Medical Research Council/Royal College of Obstetricians and Gynaecologists Working Party on Cervical Cerclage. Final report of the Medical Research Council/Royal College of Obstetricians and Gynaecologists multicentre randomised trial of cervical cerclage. British Journal of Obstetrics and Gynaecology. 1993;100:516-523.
Mikkelson M. Cytogenetic findings in first trimester chorionic villus sampling. In: Fraccaro G, Simoni G, Brambati B, editors. First Trimester Fetal Diagnosis. Berlin: Springer-Verlag, 1985.
Miller H, Durant JA, Ross DM, O’Connell FJ. Corpus luteum deficiency as a cause of early recurrent abortion: a case history. Fertility and Sterility. 1969;20:433-438.
Miller JF, Williamson E, Glue J, Gordon YB, Grudzinskas JG, Sykes A. Fetal loss after implantation: a prospective study. The Lancet. 1980;2:554-556.
Mills JL, Simpson JL, Driscoll SG, et al. Incidence of spontaneous abortion amongst normal women and insulin dependent diabetic women whose pregnancies were identified within 21 days of conception. New England Journal of Medicine. 1988;319:1617-1623.
Mur JM, Mandereau L, Deplan F, Paris A, Richard A, Hemon D. Spontaneous abortion and exposure to vinyl chloride. The Lancet. 1992;339:127-128.
Nahmias AJ, Josey WE, Naib ZM, Freeman MG, Fernandez RJ, Wheeler JH. Perinatal risk associated with maternal genital herpes simplex virus infection. American Journal of Obstetrics and Gynecology. 1971;110:825-837.
Ness RB, Grisso JA, Hirschinger N, et al. Cocaine and tobacco use and the risk of spontaneous abortion. New England Journal of Medicine. 1999;340:333-339.
Neugebauer R, Kline J, O’Connor P, et al. Depressive symptoms in women in the six months following miscarriage. American Journal of Obstetrics and Gynecology. 1992;166:104-109.
Neugebauer R, Kline J, Stein Z, et al. Association of stressful life events with chromosomally normal spontaneous abortion. American Journal of Epidemiology. 1996;143:588-596.
Nielsen S, Hahlin M. Expectant management of spontaneous first trimester abortion. The Lancet. 1995;345:84-86.
Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage. BMJ. 2000;320:1708-1712. (Clinical Research Ed.)
Ober C, Karrison T, Odem RR, et al. Mononuclear-cell immunisation in prevention of recurrent miscarriages: a randomised trial. The Lancet. 1999;354:365-369.
Okon MA, Laird SM, Tuckerman EM, Li TC. Serum androgen levels in women who have recurrent miscarriages and their correlation with markers of endometrial function. Fertility and Sterility. 1998;69:682-690.
Pabuccu R, Atay V, Urman B, Ergun A, Orhon E. Hysteroscopic treatment of septate uterus. Gynaecological Endoscopy. 1995;4:213-215.
Parazzini F, Bocciolone L, La Vecchia C, Negri E, Fedele L. Maternal and paternal moderate alcohol consumption and unexplained miscarriages. British Journal of Obstetrics and Gynaecology. 1990;97:618-622.
Pedersen JF, Mantoni M. Large intrauterine haematomata in threatened miscarriage. Frequency and clinical consequences. British Journal of Obstetrics and Gynaecology. 1990;97:75-77.
Pegoraro E, Whitaker J, Mowery-Rushton P, Surti U, Lanasa M, Hoffman EP. Familial skewed X inactivation: a molecular trait associated with high spontaneous abortion rate maps to Xq28. American Journal of Human Genetics. 1997;61:160-170.
Pennell RG, Needleman L, Pajak T, et al. Prospective comparison of vaginal and abdominal sonography in normal early pregnancy. Journal of Ultrasound in Medicine. 1991;10:63-67.
Piette JC, Le Thi Huong D, Wechsler B. Therapeutic use of intravenous immunoglobulins in the antiphospholipid syndrome. Annales de Médicine Interne (Paris). 2000;151(suppl 1):S51-S54.
Platz-Christiansen JJ, Nielsen S, Hamberger L. Is misoprostol the drug of choice for induced cervical ripening in early pregnancy termination? Acta Obstetrica et Gynaecologica Scandinavica. 1995;74:809-812.
Prendiville WG. HCG for recurrent miscarriage. In: Enkin MW, Keirse MJNC, Renfrew MJ, et al, editors. Pregnancy and childbirth module (CD-ROM). Oxford: Cochrane Database. Update Software, 1995.
Prieto JA, Eriksen NL, Blanco JD. A randomised trial of prophylactic doxycycline for curettage in incomplete abortion. Obstetrics and Gynecology. 1995;85:692-696.
Quenby SM, Farquharson RG. Human chorionic gonadotrophin supplementation in recurring pregnancy loss: a controlled trial. Fertility and Sterility. 1994;62:708-710.
Rai RS, Clifford K, Cohen H, Regan L. High prospective fetal loss rate in untreated pregnancies of women with recurrent miscarriage and antiphospholipid antibodies. Human Reproduction. 1995;10:3301-3304.
Rai RS, Regan L, Clifford K, et al. Antiphospholipid antibodies and beta2-glycoprotein-I in 500 women with recurrent miscarriage: results of a comprehensive screening approach. Human Reproduction. 1995;10:2001-2005.
Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies). BMJ. 1997;314:253-257. (Clinical Research Ed.)
Rai R, Regan L. Antiphospholipid syndrome and pregnancy loss. Hospital Medicine. 1998;59:637-639.
Rai R, Backos M, Rushworth F, Regan L. Polycystic ovaries and recurrent miscarriage — a reappraisal. Human Reproduction. 2000;15:612-615.
Ratnam SS, Prasad RNV. Medical management of abnormal pregnancy. Baillière’s Clinical Obstetrics and Gynaecology. 1990;4:361-374.
Recurrent Miscarriage Immunotherapy Trialists Group. Worldwide collaborative observational study and meta-analysis on allogenic leukocyte immunotherapy for recurrent spontaneous abortion. American Journal of Reproductive Immunology. 1994;32:55-72.
Regan L. Sporadic and recurrent miscarriage. In: Grudizinskas JG, O’Brien PMS, editors. Problems in Early Pregnancy — Advances in Diagnosis and Management. London: RCOG Press, 1997.
Regan L, Braude P, Trembath PL. Influence of past reproductive performance on risk of spontaneous abortion. BMJ. 1989;299:541-545. (Clinical Research Ed.)
Regan L, Owen EJ, Jacobs HS. Hypersecretion of luteinising hormone, infertility, and miscarriage. The Lancet. 1990;336:1141-1144.
Roberts CJ, Lowe DB. Where have all the conceptions gone? The Lancet. 1975;1:498.
Rosove MH, Tabsh K, Wasserstrum N, Howard P, Hahn BH, Kalunian KC. Heparin therapy for pregnant women with lupus anticoagulant or anticardiolipin antibodies. Obstetrics and Gynecology. 1990;75:630-634.
Rowland AS, Baird DD, Shore DL, Darden B, Wilcox AJ. Ethylene oxide exposure may increase the risk of spontaneous abortion, preterm birth and postterm birth. Epidemiology. 1996;7:363-368.
Royal College of General Practitioners/Royal College of Obstetricians and Gynaecologists. Induced abortion operations and their early sequelae. Journal of the Royal College of General Practitioners. 1985;35:175-180.
Royal College of Obstetricians and Gynaecologists. Infection and Pregnancy. London: RCOG Press; 2001.
Rushton DI. Pathology of abortion. In: Fox H, editor. Haines and Taylor Obstetrical and Gynecological Pathology. Edinburgh: Churchill Livingstone, 1995.
Rushworth FH, Backos M, Rai R, Chilcott IT, Baxter N, Regan L. Prospective pregnancy outcome in untreated recurrent miscarriers with thyroid autoantibodies. Human Reproduction. 2000;15:1637-1639.
Russell PB. Abortions treated conservatively: a twelve year study covering 3739 cases. Southern Medical Journal. 1947;40:314-324.
Schiverick KT, Salafia C. Cigarette smoking and pregnancy I: ovarian, uterine and placental effects. Placenta. 1999;20:265-272.
Schmidt-Sarosi C, Schwartz LB, Lublin J, Kaplan-Grazi D, Sarosi P, Perle MA. Chromosomal analysis of early fetal losses in relation to transvaginal ultrasonographic detection of fetal heart motion after infertility. Fertility and Sterility. 1998;69:274-277.
Scibetta JJ, Sanko SR, Phipps WR. Laparoscopic transabdominal cervicoisthmic cerclage. Fertility and Sterility. 1998;69:161-163.
Sebire NJ, Thornton S, Hughes K, Snijders RJ, Nicolaides KH. The prevalence and consequences of missed abortion in twin pregnancies at 10 and 14 weeks of gestation. British Journal of Obstetrics and Gynaecology. 1997;104:847-848.
Sebire NJ, Snijders RJ, Hughes K, Sepulveda W, Nicolaides KH. The hidden mortality of monochorionic twin pregnancies. British Journal of Obstetrics and Gynaecology. 1997;104:1203-1207.
Seibel M, Graves WL. The psychological implication of spontaneous abortion. Journal of Reproductive Medicine. 1980;25:161-172.
Serle E, Aplin JD, Li TC, et al. Endometrial differentiation in the preimplantation phase of women with recurrent miscarriage: a morphological and immunohistochemical study. Fertility and Sterility. 1994;62:989-996.
Shephard TH, Fantel AG, Fitzsimmons J. Congenital defect rates among spontaneous abortuses: twenty years of monitoring. Teratology. 1989;39:325-331.
Shirodkar VM. Contributions to Obstetrics and Gynaecology. Edinburgh: Churchill Livingstone; 1960.
Silver RK, MacGregor SN, Sholl JS, Hobart JM, Neerhof MG, Ragin A. Comparative trial of prednisone plus aspirin versus aspirin alone in the treatment of anticardiolipin antibody-positive obstetric patients. American Journal of Obstetrics and Gynecology. 1993;169:1411-1417.
Simpson JL, Gray RH, Queenan JT, et al. Further evidence that infection is an infrequent cause of first trimester spontaneous abortion. Human Reproduction. 1996;11:2058-2060.
Singh A, Dantas ZN, Stone SC, Asch RH. Presence of thyroid antibodies in early reproductive failure: biochemical versus clinical pregnancies. Fertility and Sterility. 1995;63:277-281.
Stabile I, Campbell S, Grudzinskas JG. Ultrasound assessment in complications of first trimester pregnancy. The Lancet. 1987;2:1237-1242.
Stanger JD, Yovich JL. Reduced in vitro fertilisation of human oocytes from patients with raised basal luteinising hormone levels during the follicular phase. British Journal of Obstetrics and Gynaecology. 1985;92:385-393.
Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertility and Sterility. 1996;66:27-29.
Stirrat GM. Recurrent miscarriage: I. Definitions and epidemiology. The Lancet. 1990;336:673-675.
Stray-Pedersen B. New aspects of perinatal infections. Annals of Medicine. 1993;25:295.
Stray-Pedersen B, Stray-Pedersen S. Etiological factors and subsequent reproductive performance in 195 couples with a prior history of habitual abortion. American Journal of Obstetrics and Gynecology. 1984;148:140-146.
Takahara H, Ohama K, Fukiwara A. Cytogenetic study in early spontaneous abortion. Hiroshima Journal of Medical Science. 1977;26:291-296.
Takakuwa K, Asano K, Arakawa M, Yasuda M, Hasegawa I, Tanaka K. Chromosome analysis of aborted conceptuses of recurrent aborters positive for anticardiolipin antibody. Fertility and Sterility. 1997;68:54-58.
Therkelsen AJ, Grunnet N, Hjort T, et al. Studies in spontaneous abortion. In: Boué A, Thibault C, editors. Chromosomal Errors in Relation to Reproductive Failure. Paris: INSERM, 1977.
Tower CL, Regan L. Intrauterine haematomas in a recurrent miscarriage population. Human Reproduction. 2001;16:2005-2007.
Tulppala M, Stenman UH, Cacciatore B, Ylikorkala O. Polycystic ovaries and levels of gonadotrophins and androgens in recurrent miscarriage: a prospective study of 50 women. British Journal of Obstetrics and Gynaecology. 100, 1993. 438–352
Tulppala M, Huhtaniemi I, Ylikorkala O. Genetic variant of luteinizing hormone in women with a history of recurrent miscarriage. Human Reproduction. 1998;13:2699-2702.
Verkuyl DA, Crowther CA. Suction v. conventional curettage in incomplete abortion. A randomised controlled trial. South African Medical Journal. 1993;83:13-15.
Vidal F, Rubio C, Simón C, et al. Is there a place for preimplantation genetic diagnosis screening in recurrent miscarriage patients? Journal of Reproduction and Fertility. 2000;55:143-146.
Vincent T, Rai R, Regan L, Cohen H. Increased thrombin generation in women with recurrent miscarriage. The Lancet. 1998;352:116.
Warbuton D, Stein Z, Kline J, et al. Chromosome abnormalities in spontaneous abortions. In: Porter IH, Hook EB, editors. Human Embryonic and Fetal Deaths. New York: Academic Press, 1980.
Weigel MM, Weigel RM. Nausea and vomiting of early pregnancy and pregnancy outcome. An epidemiological study. British Journal of Obstetrics and Gynaecology. 1989;96:1304-1311.
Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss in pregnancy. New England Journal of Medicine. 1988;319:189-194.
Windham GC, Von Behren J, Fenster L, Schaefer C, Swan SH. Moderate maternal alcohol consumption and the risk of spontaneous abortion. Epidemiology. 1997;8:509-514.
Wong GP, Farquharson DF, Dansereau J. Emergency cervical cerclage: a retrospective review of 51 cases. American Journal of Perinatology. 1993;10:341-347.
Wouters MG, Boers GH, Blom HJ, et al. Hyperhomocysteinemia: a risk factor in women with unexplained recurrent early pregnancy loss. Fertility and Sterility. 1993;60:820.
Zemlickis D, Lishner M, Degendorfer P, Panzarella T, Sutcliffe SB, Koren G. Fetal outcome after in utero exposure to cancer chemotherapy. Archives of Internal Medicine. 1992;152:573-576.