CHAPTER 281 Spondyloarthropathies (Including Ankylosing Spondylitis)
The spondyloarthropathies have an association with the major histocompatibility complex antigen HLA-B27, which presents microbial antigens to T lymphocytes. There is a racial variation in disease that parallels the variation in HLA-B27. In the general population, about 8% of white individuals, 4% of Africans, 8% of Chinese, and 1% of Japanese possess the HLA-B27 antigen.1 It is also more prevalent in the northern latitudes. In northern Scandinavia, for instance, 24% of people are HLA-B27 positive, and 1.8% are affected by AS.
Subtypes of HLA-B27 have been identified, some of which are positively associated with disease but others are negatively associated.2
Major Types of Spondyloarthropathy
Ankylosing Spondylitis
AS represents the prototypical spondyloarthropathy and is the most commonly seen of the group in the practice of a spine surgeon. Derived from Greek, the name refers to an inflammatory condition of the spine (spondylos) resulting in stiffening and angulation (angkylos).3 Besides being a source of disabling pain in both the axial and appendicular skeleton, it is a cause of major spinal deformity that often requires surgical attention.
The overall incidence of AS in the United States is 0.1% to 0.2%. Even though men are more commonly affected (3 : 1 ratio), women carry a higher risk of having an affected child.4 This pattern is also seen in inflammatory bowel disease. However, evidence does not suggest a worse prognosis or greater severity of disease in the children of affected women.5
Significant dysfunction and progressive disability are the norm, although a large percentage of patients are able to maintain employment and productivity. After a decade of disease, a clinically significant sagittal plane deformity will develop in approximately half of the patients with AS.6 Nevertheless, disability increases with disease severity, as do costs associated with advancing disease.7 Patients with AS are more likely to have never been married, more likely to be divorced, and more than twice as likely to be work-disabled than members of the general population. As would be expected, individuals with physically demanding occupations and lower educational status are at greater risk for work disability.8 Women with AS are also less likely to have had children than women in the general population.9
Psoriatic Arthritis
Genetic, environmental, and immunologic factors are believed to play a role in the development of this disease, although the specific etiology is not well understood. At least 40% of patients with psoriasis are found to have radiographic evidence of inflammatory arthritis,8 and significant disability may develop in patients with psoriatic arthritis, with up to 20% demonstrating a rapidly progressive, debilitating clinical course.10 Psoriatic arthritis is a clinical diagnosis and involves the findings of inflammatory arthropathy and psoriatic skin lesions.11 See Table 281-2 for discussion regarding the clinical features of inflammatory back pain and arthritis.
Psoriatic arthritis may be associated with obesity, hypertension, dyslipidemia, and insulin resistance because of the shared inflammatory pathway.12
Pathophysiology
The underlying inflammatory process involves T cells and cytokines, including tumor necrosis factor-α (TNF-α).13,14 Matrix metalloproteinase-3 (MMP-3) is an enzyme thought to be related to pathogenesis and is also used as a disease marker.15 AS has been found in association with conditions such as multiple sclerosis, thus raising the possibility of a common pathway to disease.16
Although the link between infection and arthritic disease is most clear in reactive arthritis, a link is suggested in the other spondyloarthropathies as well. Klebsiella IgG is found in increased levels in patients with AS.17
Muscle biopsy studies in patients with AS show evidence of abnormal, atrophied types I and II muscle fibers.18 Whether this contributes to the development of disease or is a result of the disease itself is unclear.
HLA-B27–restricted T cells have been implicated in the development of reactive arthritis as a response to bacterial infections. The HLA-B27 gene is strongly associated with the conditions, but it has a variable association with each subtype. Its strongest link is in white male patients with AS, in whom it is found 90% of the time. The prevalence of the gene is 8% in the general white population and 4% in the African American population. It is more common in regions further from the equator.19
AS develops in approximately 1% to 2% of people with HLA-B27. This risk increases to approximately 20% to 30% when a first-degree relative carries the diagnosis.20 It is likely that AS is actually more common than has been reported to date.
Overproduction of bone morphogenetic protein-2 (BMP-2) and BMP-7 has been noted in patients with AS, and serum BMP-7 levels can reflect radiographic damage.21 The processes leading to bone fusion and the links between inflammation and bone formation remain poorly defined.22 Current research into further susceptibility genes and inflammatory mediators provides hope for greater understanding and treatment options. HLA-60 is another susceptibility gene that may have a role.
Clinical Findings and Diagnosis
The spondyloarthropathies have somewhat variable clinical findings, depending on the subtype. Table 281-1 outlines some of these variations. In general, AS tends to be seen in the younger male population.
A combination of history and findings on physical examination, along with imaging and laboratory tests, is used in the diagnosis of these conditions. Although advanced disease may have obvious hallmarks, early disease may be subtle. Multiple validated systems have thus been developed to assist in the stepwise analysis of patients, such as the European Spondyloarthropathy Study Group (ESSG) criteria (Table 281-2) and the Amor criteria. The Modified New York criteria (Table 281-3) and the Rome criteria are used specifically for the diagnosis of AS. Although the ESSG criteria have been found to be the most sensitive for spondyloarthropathy and inflammatory back pain, the Modified New York criteria are the most specific for AS.23 Patients are evaluated for features of inflammatory back pain, as opposed to mechanical back pain (which may be acute in onset or associated with activity and radicular symptoms). The most specific symptoms include pain during the second half of the night, early morning stiffness lasting 30 minutes, alternating buttock pain (referred from the SI joints), and improvement with exercise, not rest (Table 281-4).
TABLE 281-2 European Spondyloarthropathy Study Group Criteria for Spondyloarthropathies
|
Entry criteria—one of the following:
|
TABLE 281-3 Modified New York Criteria for Ankylosing Spondylitis
TABLE 281-4 Inflammatory versus Mechanical Back Pain
| FEATURE | INFLAMMATORY | MECHANICAL |
|---|---|---|
| Night pain | + | − |
| Morning stiffness | + | − |
| Sacroiliac referred buttock pain | + | ± |
| Radicular pain | − | + |
| Improves with | Activity | Rest |
Appendicular skeletal disease is associated with peripheral joint involvement, most commonly in the hips and shoulders. Patients with kyphosis attempt to restore sagittal balance through hip extension, knee flexion, and ankle plantar flexion.24 Having said this, hip flexion contractures by and of themselves can be a significant source of sagittal postural imbalance. As a hip flexion contracture develops, patients adopt a forward-flexed posture that may exaggerate the appearance of the kyphosis. Evaluation of the hips is crucial in arriving at an accurate assessment of the degree of spinal deformity that may be present.
Extraskeletal disease involves multiple organ systems, with each subtype of spondyloarthropathy having a somewhat different predilection for each. Such disease includes cardiovascular disease, more specifically aortic insufficiency and conduction disturbances; pulmonary disease, such as apical pulmonary fibrosis; deposition disease, such as amyloidosis with its associated renal dysfunction; and neurological disease such as encephalitis, transverse myelitis, and peripheral neuropathy.25
Neurological deficits may arise as a result of spinal stenosis, upper cervical instability, and chronic inflammation. Ossification of the posterior longitudinal ligament (PLL) in association with AS has been noted and contributes to the acquired stenosis. Because the upper cervical spine tends to lag behind in bony ankylosis, compensatory hypermobility may develop and result in neurological complications. Cauda equina syndrome may be seen, perhaps as a result of chronic inflammation, demyelination, and fibrosis, along with the development of arachnoid diverticula.26
Imaging
Characteristic findings are noted on radiographic imaging, and the extent of such findings has been found to correlate clinically with patient spinal mobility and function.27,28
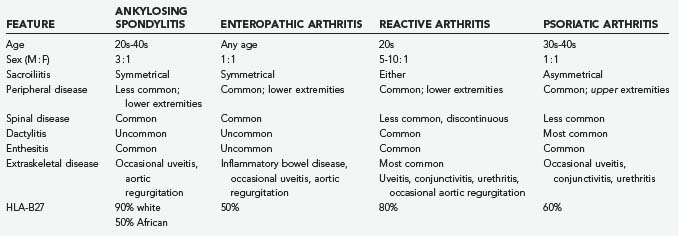
One of the hallmarks of AS is the “bamboo spine” (Fig. 281-1). As marginal syndesmophytes form along the intervertebral disks, the spine takes on the appearance of a single long bone (contributing to the loss of flexibility and increased risk for fracture). Marginal syndesmophytes are differentiated from (1) osteophytes, which do not typically bridge the disk space, and (2) nonmarginal syndesmophytes, which extend beyond the margin of the disk and spinal column, as seen in diffuse idiopathic skeletal hyperostosis. “Squaring” of the vertebral bodies occurs as the end plates lose their concavity, probably related to chronic inflammation. These spinal changes typically begin distally within the lumbar spine and progress slowly cephalad.
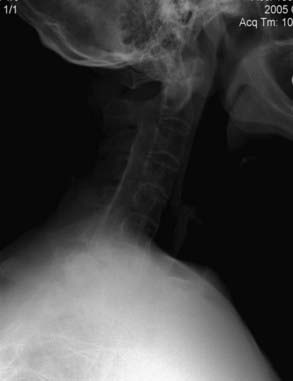
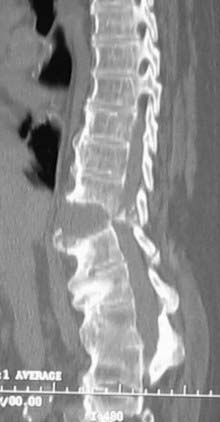
SI fusion is another hallmark and is required for diagnosis by the New York criteria (Fig. 281-2). It is typically observed before other radiographic changes. Earlier in the disease, erosions are noted at the lower end of the joint, especially over the iliac side. Erosive changes may be seen at the symphysis pubis (osteitis pubis). As ossification progresses along entheses such as the iliac crests, ischial tuberosities, and femoral trochanters, a process known as “whiskering” is observed.
Spondylodiskitis is characterized by an inflammatory, erosive process of the intervertebral disk, commonly at the thoracolumbar junction. It is unknown whether this erosion represents an area of persistent inflammation, failure of ankylosis across an area of high mechanical stress, or an area of nonunion after trauma to the already ankylosed spine.29 Approximately 5% of patients with AS have been shown to have spondylodiskitis, but only half of these patients tend to be symptomatic.30 Radiographically, spondylodiskitis appears similar to chronic infectious diskitis, with end-plate erosion and surrounding bony sclerosis. Frequently, anterior widening of the disk space is noted.
Enteropathic arthritis has radiographic features similar to those of AS. In contrast, Reiter’s syndrome and psoriatic arthritis typically result in noncontinuous spondylitis, nonmarginal syndesmophytes, and asymmetrical SI joint involvement.1
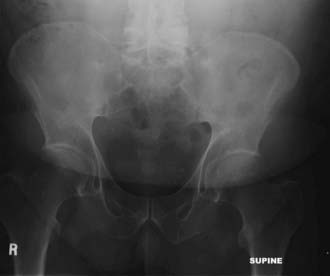
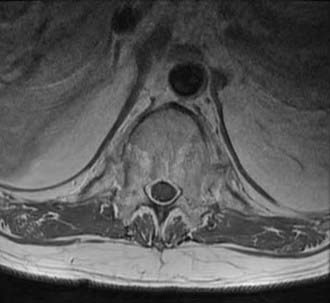
Computed tomography (CT) shows similar changes but in greater bony detail. Extensive changes may be noted at the costovertebral articulations (Fig. 281-3). Magnetic resonance imaging (MRI) may show marrow signal alterations secondary to chronic inflammation in this region (Fig. 281-4).31,32
Ossification of the PLL has been observed in association with increasing age and has variable ethnic prevalence.33
Radiologic methodologies for assessing the extent of spinal involvement have been proposed and are in use.34–36 These methods have been helpful in assessing the efficacy of treatment regimens in both the research and clinical fields, which was previously hampered by an inability to objectively gauge a patient’s progress. Such instruments include the Stoke Ankylosing Spondylitis Spine Score (SASSS) and the Bath Ankylosing Spondylitis Radiology Index (BASRI).
Methods specific to enthesitis that focus on the ligamentous and muscular insertions at the level of the Achilles tendon, femur, and humerus have been used.37
Peripheral joint involvement is seen most commonly in the hips and shoulders.
Bone mineral density is reduced because of a tremendous loss of trabecular bone. This is noted on plain x-ray imaging studies, but dual-energy x-ray absorptiometry may show a paradoxical increase in density as a result of enthesopathy and greater peripheral bone formation.38
Primary Management
Nonsteroidal anti-inflammatory drugs (NSAIDs) are used extensively for inflammatory and rheumatic disorders such as the spondyloarthropathies.39 Recent evidence suggests that NSAIDs not only treat the symptoms of inflammatory polyarthropathy but may also have more of a “disease-modifying” role by retarding radiographic progression and symptoms of arthritis.40 Celecoxib, indomethacin, naproxen, and phenylbutazone are among such medications that have specifically been used for the treatment of AS.41–44 To avoid complications associated with long-term use, medication use may be decreased during periods of relative disease inactivity.
Disease-modifying antirheumatic drugs (DMARDs) have opened up a new avenue for treatment, and work continues to be done in the pharmacology arena.45 There are several regularly used drugs that have various mechanisms of action. Sulfasalazine and methotrexate have a history of effective use in patients with rheumatoid arthritis and are used for treatment of the spondyloarthropathies as well. Nevertheless, their efficacy in treating axial spinal pathology such as seen in the spondyloarthropathies is relatively limited.46,47
Other DMARDs include adalimumab, etanercept, and infliximab. These drugs are examples of TNF-α inhibitors48–50 and are recombinant TNF-α receptors that bind the molecule in serum, thereby preventing systemic activity.51 They have been found to exert a beneficial effect on articular cartilage inasmuch as a decrease in serum markers of cartilage degradation has been observed.52 Intra-articular injection of anti-TNF agents has also been described.53 These agents may be used in combination with other medications, such as methotrexate. However, the risk for serious infection increases significantly with such therapy. Widespread use of these drugs is limited somewhat by cost54,55 and concerns regarding long-term use.56,57
The response to treatment with drugs may be monitored by following serum levels of acute-phase reactants such as the erythrocyte sedimentation rate, C-reactive protein, MMP-3,58 and various cytokines.59,60 Serum markers of bone and cartilage degradation and synthesis are also useful in this manner and are thought to be related not only to cartilage and collagen turnover but also to new bone formation through endochondral ossification, as occurs in the spine of patients with AS.61
In the case of psoriatic arthritis, TNF-α antagonists are used for both skin and joint disease, whereas efalizumab, which targets T lymphocytes, is indicated only for the skin disease.62–65
Exercise therapy is considered helpful in maintaining patient function, and there is evidence of a direct biologic effect of such therapy. Patients who regularly perform back exercises and have appropriate social support have been shown to have a greater degree of function.66 Exercises focus on strengthening of the spinal extensor muscles and maintenance of peripheral joint mobility. However, there is a need for a standardized approach to complement medical therapy.67
Tobacco abuse is also associated with poor function.53 Discontinuation of tobacco and nicotine products is thus critical not only for surgical patients but also for improved disease management in any patient with AS.
Trauma Management
It has long been recognized that ankylosing spondylitis places a patient at higher risk for spinal fractures, even with minimal trauma.68–70 The ossified, nonpliable spine is prone to failure, and the spine acts as a single long bone with high forces of moment at either end of the fracture. Associated osteopenia further predisposes the patient to injury. Patients are at risk for multiple fractures on repeated occasions.71
In the trauma setting, it is important to place patients close to their preinjury alignment rather than a normal neutral one. This typically requires building pillows behind their neck and thoracic spine and even elevating the head of the bed for a thoracolumbar injury.72,73 Patients may self-osteotomize after a fracture and fall into a deceptively normal alignment. This is at the peril of the underlying neurological elements, which are acutely angulated at the fracture site (Fig. 281-5).
Subtle fractures may be missed either through a lack of appropriate suspicion for injury or through inadequate imaging studies. CT should be performed to rule out minimal fractures that would be missed on plain radiography.74,75 MRI can be of benefit in detecting subtle fracture patterns.76 Flexible multipurpose coils help in positioning patients unable to fit in standard coils because of deformity.
The spectrum of injuries ranges from three-column extension injuries, in which the bamboo spine essentially snaps, to relatively innocuous-appearing anterior vertebral end-plate lesions. Fractures are most common in the cervical spine.77 Healing of cervical fractures can occur reliably with the use of a halo vest in the absence of a globally destabilizing three-column injury. Surgery is indicated for unstable injuries demonstrating 360-degree instability and distraction, frequently at the thoracolumbar level. Epidural hematoma formation is common and must be evaluated with MRI in the event of neurological injury or deterioration.78,79 The integrity of a fourth column involving the costovertebral joint complex is a factor in the stability of thoracic-level fractures.80
These injuries commonly involve an extension mechanism with an anterior “fish-mouthing” deformity that is amenable to anterior stabilization and closure of the fracture site. However, a high rate of failure has been noted with isolated anterior procedures, and therefore circumferential surgical stabilization of cervical injuries is often performed.81–83 Isolated posterior treatment can be effective.84–86 Controversy exists with respect to restoring the patient’s preoperative alignment versus fixing the spine in a somewhat improved alignment in the acute setting.
Surgical Management
Preoperative Evaluation
A stepwise, systematic approach to these patients is mandatory and includes assessment of their imbalance clinically. Various postural tests can help in identifying the level of maximal deformity, including observation of the spine’s curvature in the standing, seated, and supine positions.87
Preoperative Planning
Consideration may be given to staged surgery, whether combined anterior-posterior or posterior-alone intervention is planned.88
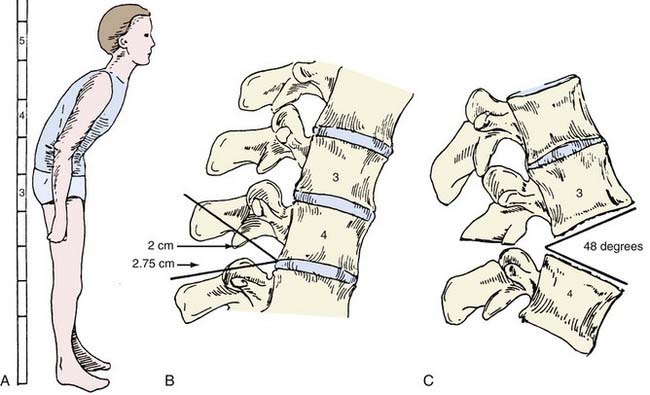
Radiographic evaluation includes assessment of full-length standing anteroposterior and lateral radiographs of the entire spine. Anteroposterior radiographs are often difficult to interpret because of the flexion deformity. Angulation of the x-ray beam from caudad to cephalad may allow better visualization. The C7 coronal and sagittal plumb lines are assessed, and the deformity is measured in centimeters from these lines. Normally, the coronal plumb line is measured from the spinous process of C7 and should fall in the midline of the sacrum and symphysis pubis. The sagittal plumb line, measured from the center of the C7 body, normally descends to the posterosuperior corner of S1. Cervical and lumbar lordosis and thoracic kyphosis are measured by the Cobb method.89 The sacral slope, inclination, and pelvic incidence is measured. Finally, the chin-brow vertical angle is measured.90,91
The chin-brow vertical angle has been of great utility in assessing the degree of sagittal imbalance and in correlating it with the degree of bone resection required for surgical correction.92 This angle is measured on standing lateral radiographs and clinical photographs of the patient to provide direct measurement of the deformity and assessment of the correction needed to restore forward gaze.
Various techniques have been used for measuring the degree of deformity and correlating it with the degree of correction required.93 Van Royen and colleagues devised a mathematical equation that factors in the variables of the degree of kyphosis and the correction needed and the level of osteotomy. A computer program is offered that may be used via the Internet.94
The whole-body kyphosis angle, measured on clinical photographs with the patient in the standing position, has also been used.95
Approaches
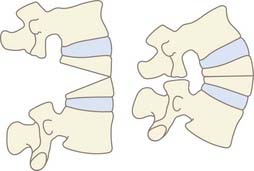
Opening Wedge Osteotomy
An opening wedge osteotomy involves resection of the posterior bony elements with subsequent hinging of the osteotomy at the posterior vertebral body and PLL level. This results in distraction, or opening, of the anterior column as the deformity is corrected (Fig. 281-6).
Closing Wedge Osteotomy
Closing wedge osteotomies involve greater resection of the posterior elements with extension through the pedicles into the vertebral body. Closure is performed while hinging on the anterior body and anterior longitudinal ligament to avoid distraction of the anterior vascular structures and allow direct bone apposition for improved healing (Fig. 281-7).
Closing wedge osteotomies are often performed at the thoracolumbar junction and opening wedge osteotomies at the cervicothoracic level.96 However, both techniques have been used with favorable results at all levels of the spine, as will be discussed further.
Cervical Osteotomy
Simmons and colleagues described a 36-year experience with posterior opening wedge osteotomies.97 Surgery is performed with the patient in the upright position under local anesthesia without instrumentation and involves an opening wedge osteotomy through C7 with varying degrees of pedicle resection. Excellent results have been achieved with this technique (Figs. 281-8 and 281-9). Closing wedge osteotomies have been used through the C7 pedicle with success as well.98
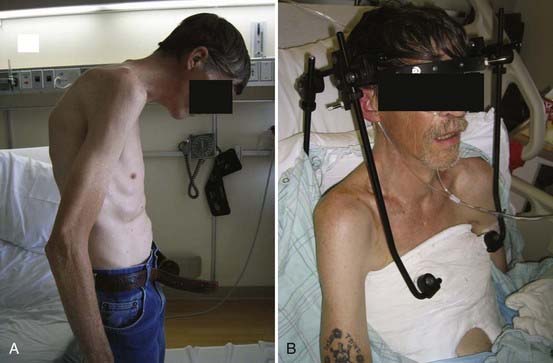
FIGURE 281-8 A and B, Preoperative and postoperative clinical photographs of a patient with a fixed cervicothoracic kyphosis.
Etame and associates reviewed the literature related to osteotomy of the cervical spine in patients with AS.99 Complications occurred frequently in six retrospective series, with rates ranging from 27% to 88%, but the major complication rate was relatively low, with a 2.6% mortality rate and a 4.3% rate of permanent neurological injury. Restoration of horizontal gaze was the norm and overall satisfaction was high.
Use of instrumentation in such cervical osteotomies has become more common, as has its use in spinal procedures in general. The advantages of instrumentation include limited need for postoperative bracing and relatively controlled motion during the osteoclasis portion of the osteotomy to prevent translational subluxation. The use of a hinged rod has been proposed to allow even further control of such translational force. The rod is temporarily fixed to screws with the hinge open at the osteotomy site, and then the hinge is locked once the desired reduction is achieved.100
Lumbar Osteotomy
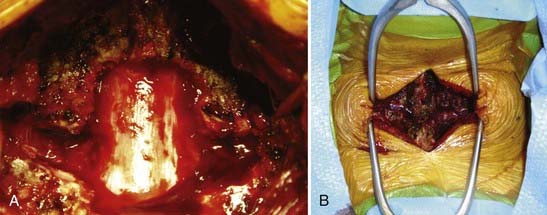
Heinig and McMaster each described a method for correction of deformity in which increased bone apposition is achieved by decancellation of the pedicle and vertebral body.101 Terms such as eggshell osteotomy, pedicle subtraction osteotomy, and closing wedge osteotomy describe similar procedures, and authors may vary in their description of the amount and technique of bone resection. Variations involving the extent of decompressive laminectomy and actual fracture of the anterior vertebral margin have been described (Figs. 281-10 and 281-11).102
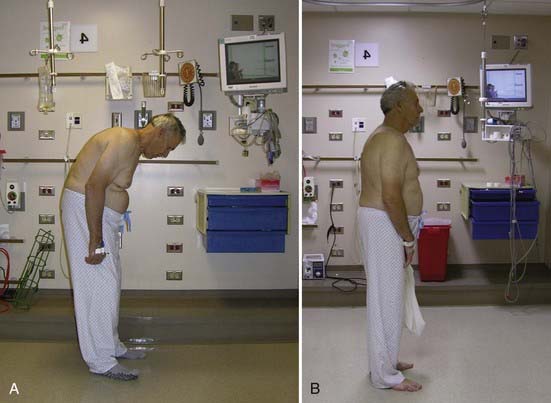
FIGURE 281-10 A and B, Preoperative and postoperative clinical photographs of a patient with fixed thoracolumbar kyphosis.
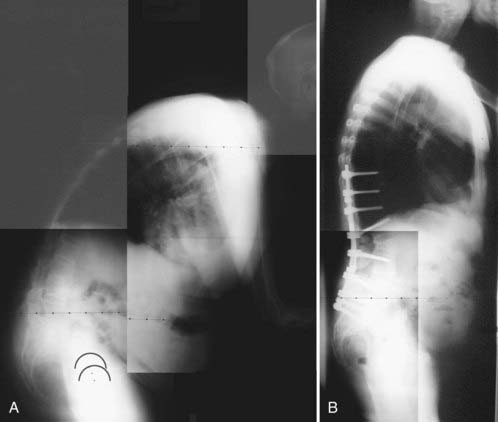
FIGURE 281-11 A and B, Preoperative and postoperative radiographs of a patient with fixed thoracolumbar kyphosis.
Combinations of closing and opening wedge osteotomies have been proposed for greater correction.7,103 When using such techniques, the first 30 degrees of correction is achieved with a closing osteotomy hinged at the anterior longitudinal ligament, followed by an additional 10 degrees by moving the hinge posterior.
Interbody cages have also been used for simultaneous closing/opening osteotomies and may afford greater support of the anterior column and decrease sagittal translation in selected cases.104
Sagittal translation at the level of osteotomy may occur with either the open or closed wedge techniques. Although such translation has been thought to afford greater correction, it is associated with higher rates of neurological injury.105
Various strategies for controlled osteotomy closure with instrumentation have been proposed, including placement of a central compression hook-rod construct through which the osteotomies are closed first, followed by secondary locking of the main pedicle screw-rod construct. This technique has been proposed to allow less direct transmission of force to the final construct itself. Meticulous attention to bone grafting and the surface area for fusion would be warranted with the placement of extra instrumentation over the planned area for fusion.106 Methods have been proposed for more controlled osteoclasis, such as placing the operating table in the prone position.107
Areas of pseudarthrosis within the otherwise ankylosed spine may take on a destructive appearance and be associated with considerable deformity.108,109 Opening wedge osteotomies with anterior interbody fusion have been used at the level of pseudarthrosis. In Kim and coworkers’ series, lumbar closing wedge osteotomies were also performed for further correction of severe deformities.108 Chang and colleagues reported performing posterior osteotomies in a series of 30 patients without concomitant anterior interbody fusion and noted no failure of treatment after nearly 5 years.
Several authors have reported their series of patients with overall favorable results.110
Chen and associates described their series of 78 patients in whom closing wedge osteotomies were performed. Good or excellent outcomes were reported in 98% of the patients, with an average correction of 34 degrees per osteotomy level, typically at L2 or L3. Severe deformity was treated with multilevel osteotomies in a staged fashion, although in one instance, 100-degree correction was obtained at a single level. No severe neurological deficits or deaths occurred.111
Weale and coauthors described their series of 50 patients. Instrumentation failures were noted with both transpedicular and other forms of fixation, but greater maintenance of correction was achieved with transpedicular constructs. A mean correction of 38 degrees was reported with two deaths and one sacral root injury.112
Kim and collaborators described a prospective series of 45 patients treated with closing wedge osteotomies.113 In their series, radiographic correction did not correlate with clinical outcome.
Chang and coauthors reported their experience with opening and closing wedge techniques.114 Correction was achieved with both techniques, and although closing osteotomies resulted in higher blood loss, a lower rate of postoperative ileus and delayed union with instrumentation failure was noted.
Combined Anterior and Posterior Approaches
Anterior-posterior procedures are less frequently used for the management of deformity. Mummaneni and colleagues described an anterior-posterior-anterior approach to the cervical spine.115 Placement of structural bone graft and cages has been advocated for anterior column support to prevent pseudarthrosis after an opening wedge lumbar osteotomy.116 Combined approaches are useful in treating complex multiplanar deformities with both coronal and sagittal components. Resection of the vertebral column may be performed in this manner to allow greater control of correction.117,118
Complications
Risks include neurological injury at the cord or root level. Cord injuries can occur as a result of either direct injury or uncontrolled anteroposterior translation (in addition to the extension planned). Root injuries can be decreased by performing wide resection of the pedicles at the level of the osteotomy to prevent entrapment of the exiting nerve root on closure. Dysphagia can occur as the anterior neck soft tissues are tensioned in extension. Airway issues require similar judicious monitoring. There is a greater tendency for pseudarthrosis to occur with opening wedge osteotomies than with closing wedge osteotomies, especially in the lumbar spine.119
The risk for catastrophic neurological injury has traditionally required awake surgery under local anesthesia with sedation. Even though this remains a safe and time-tested approach, advances in anesthesia and neural monitoring techniques allow surgery to be carried out with the patient in the prone position under general anesthesia.120–122 Proponents of a seated position would cite patient comfort and relative ease of judging clinical alignment, although this position is associated with a small risk for air embolism.
Iatrogenic fracture may occur intraoperatively secondary to positioning during spinal or other surgeries.123,124 Care must be taken to maintain the patient’s abnormal but native alignment during surgery.
Osteotomy at a thoracic level may result in overcorrection and upward gaze. Sengupta and coauthors reported a single-stage anterior-posterior technique for flexion osteotomy to reverse such iatrogenic deformity.125
Apple DFJr, Anson C. Spinal cord injury occurring in patients with ankylosing spondylitis. A multicenter study. Orthopaedics. 1995;18:1005-1011.
Chang KW, Chen YY, Lin CC, et al. Closing wedge osteotomy versus opening wedge osteotomy in ankylosing spondylitis with thoracolumbar kyphotic deformity. Spine. 2005;30:1584-1593.
Chen IH, Chien JT, Yu TC. Transpedicular wedge osteotomy for correction of thoracolumbar kyphosis in ankylosing spondylitis: experience with 78 patients. Spine. 2001;26:E354-E360.
Cooper C, Carbone L, Michet CJ, et al. Fracture risk in patients with ankylosing spondylitis: a population based study. J Rheumatol. 1994;21:1877-1882.
Dakwar E, Reddy J, Vale FL, et al. A review of the pathogenesis of ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E2.
Gorman JD, Sack KE, Davis JCJr. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002;346:1349-1356.
Hitchon PW, From AM, Brenton MD. Fractures of the thoracolumbar spine complicating ankylosing spondylitis. J Neurosurg. 2002;97(suppl):218-222.
Hoh DJ, Khoueir P, Wang MY. Management of cervical deformity in ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E9.
Kelleher MO, Tan G, Sarjeant R, et al. Predictive value of intraoperative neurophysiological monitoring during cervical spine surgery: a prospective analysis of 1055 consecutive patients. J Neurosurg Spine. 2008;8:215-221.
Kim KT, Suk KS, Cho YJ, et al. Clinical outcome results of pedicle subtraction osteotomy in ankylosing spondylitis with kyphotic deformity. Spine. 2002;27:612-618.
Kuntz CIV, Levin LS, Ondra SL, et al. Neutral upright sagittal spinal alignment from the occiput to the pelvis in asymptomatic adults: a review and resynthesis of the literature. J Neurosurg Spine. 2007;6:104-112.
Lee JY, Hilibrand AS, Lim MR, et al. Characterization of neurophysiologic alerts during anterior cervical spine surgery. Spine. 2006;31:1916-1922.
Moreau W, Wilcox N, Brown MF. Immobilization of spinal fractures in patients with ankylosing spondylitis. Injury. 2003;34:372-373.
Nghiem FT, Donohue JP. Rehabilitation in ankylosing spondylitis. Curr Opin Rheumatol. 2008;20:203-207.
Sansur CA, Fu KM, Oskouian RJJr, et al. Surgical management of global sagittal deformity in ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E8.
Shamji MF, Bafaquh M, Tsai E. The pathogenesis of ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E3.
Simmons ED, DiStefano RJ, Zheng Y, et al. Thirty-six years experience of cervical extension osteotomy in ankylosing spondylitis: techniques and outcomes. Spine. 2006;31:3006-3012.
Song IH, Poddubnyy DA, Rudwaleit M, et al. Benefits and risks of ankylosing spondylitis treatment with nonsteroidal antiinflammatory drugs. Arthritis Rheum. 2008;58:929-938.
Strauss EJ, Alfonso D, Baidwan G, et al. Orthopedic manifestations and management of psoriatic arthritis. Am J Orthop. 2008;37:138-147.
Suk S, Kim KT, Lee SH, et al. Significance of chin-brow vertical angle in correction of kyphotic deformity of ankylosing spondylitis patients. Spine. 2003;28:2001-2005.
Thumbikat P, Hariharan RP, Ravichandran G, et al. Spinal cord injury in patients with ankylosing spondylitis: a 10-year review. Spine. 2007;32:2989-2995.
Toussirot E, Wendling D. Current guidelines for the drug treatment of ankylosing spondylitis. Drugs. 1998;56:225-240.
Van Royen BJ, De Gast A. Lumbar osteotomy for correction of thoracolumbar kyphotic deformity in ankylosing spondylitis. A structured review of three methods of treatment. Ann Rheum Dis. 1999;58:399-406.
Ward MM. Predictors of the progression of functional disability in patients with ankylosing spondylitis. J Rheumatol. 2002;29:1420-1425.
Zochling J, Baraliakos X, Hermann KG, et al. Magnetic resonance imaging in ankylosing spondylitis. Curr Opin Rheumatol. 2007;19:346-352.
1 Feltkamp TE, Mardjuadi A, Huang F, et al. Spondyloarthropathies in eastern Asia. Curr Opin Rheumatol. 2001;13:285-290.
2 Nasution AR, Mardjuadi A, Kunmartini S, et al. HLA-B27 subtypes positively and negatively associated with spondyloarthropathy. J Rheumatol. 1997;24:1111-1114.
3 Brent LH. Ankylosing spondylitis and undifferentiated spondyloarthropathies. Available in eMedicine at www.emedicine.com, 2006.
4 Calin A, Brophy S, Blake D. Impact of sex on inheritance of ankylosing spondylitis: a cohort study. Lancet. 1999;354:1687-1690.
5 Brophy S, Taylor G, Blake D, et al. The interrelationship between sex, susceptibility factors, and outcome in ankylosing spondylitis and its associated disorders including inflammatory bowel disease, psoriasis, and iritis. J Rheumatol. 2003;30:2054-2058.
6 Vosse D, van der Heijde D, Landewé R, et al. Determinants of hyperkyphosis in patients with ankylosing spondylitis. Ann Rheum Dis. 2006;65:770-774.
7 Kobelt G, Andlin-Sobocki P, Maksymowych WP. Costs and quality of life of patients with ankylosing spondylitis in Canada. J Rheumatol. 2006;33:289-295.
8 Ward MM, Weisman MH, Davis JCJr, et al. Risk factors for functional limitations in patients with long-standing ankylosing spondylitis. Arthritis Rheum. 2005;53:710-717.
9 Ward MM, Reveille JD, Learch TJ, et al. Impact of ankylosing spondylitis on work and family life: comparisons with the US population. Arthritis Rheum. 2008;59:497-503.
10 Strauss EJ, Alfonso D, Baidwan G, et al. Orthopedic manifestations and management of psoriatic arthritis. Am J Orthop. 2008;37:138-147.
11 Mease P, Goffe BS. Diagnosis and treatment of psoriatic arthritis. J Am Acad Dermatol. 2005;52:1-19.
12 Tam LS, Tomlinson B, Chu TT, et al. Cardiovascular risk profile of patients with psoriatic arthritis compared to controls—the role of inflammation. Rheumatology (Oxford). 2008;47:718-723.
13 Shamji MF, Bafaquh M, Tsai E. The pathogenesis of ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E3.
14 Dakwar E, Reddy J, Vale FL, et al. A review of the pathogenesis of ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E2.
15 Yang CH, Huang F, Deng XH, et al. Effects of infliximab and etanercept, two types of anti–tumor necrosis factor-alpha inhibitor on serum level of matrix metalloproteinase 3 expression in patients with ankylosing spondylitis. Zhonghua Yi Xue Za Zhi. 2006;86:2451-2454.
16 Wendling D, Flipo RM, Breban M, et al. Coexistence of spondyloarthropathy and multiple sclerosis: a series of 21 cases. Ann Rheum Dis. 2008;67:901-903.
17 Ahmadi K, Wilson C, Tiwana H, et al. Antibodies to Klebsiella pneumoniae lipopolysaccharide in patients with ankylosing spondylitis. Br J Rheumatol. 1998;37:1330-1333.
18 Simmons EH, Graziano GP, Heffner RJr. Muscle disease as a cause of kyphotic deformity in ankylosing spondylitis. Spine. 1991;16(suppl 8):S351-S360.
19 Pedersen OB, Svendsen AJ, Ejstrup L, et al. Ankylosing spondylitis in Danish and Norwegian twins: occurrence and the relative importance of genetic vs. environmental effectors in disease causation. Scand J Rheumatol. 2008;37:120-126.
20 Shah BC, Khan MA. Review of ankylosing spondylitis. Compr Ther. 1987;13:52-59.
21 Park MC, Park YB, Lee SK. Relationship of bone morphogenetic proteins to disease activity and radiographic damage in patients with ankylosing spondylitis. Scand J Rheumatol. 2008;37:200-204.
22 Ward MM. Prospects for disease modification in ankylosing spondylitis: do nonsteroidal antiinflammatory drugs do more than treat symptoms? Arthritis Rheum. 2005;52:1634-1636.
23 Heuft-Dorenbosch L, Landewe R, Weijers R, et al. Performance of various criteria sets in patients with inflammatory back pain of short duration; the Maastricht early spondyloarthritis clinic. Ann Rheum Dis. 2007;66:92-98.
24 Bot SD, Caspers M, Van Royen BJ, et al. Biomechanical analysis of posture in patients with spinal kyphosis due to ankylosing spondylitis: a pilot study. Rheumatology (Oxford). 2002;38:441-443.
25 Rosenow E, Strimlan CV, Muhm JR, et al. Pleuropulmonary manifestations of ankylosing spondylitis. Mayo Clin Proc. 1977;52:641-649.
26 Fox MW, Onofrio BM, Kilgore JE. Neurological complications of ankylosing spondylitis. J Neurosurg. 1993;78:871-878.
27 Chandran V, O’Shea FD, Schentag CT, et al. Relationship between spinal mobility and radiographic damage in ankylosing spondylitis and psoriatic spondylitis: a comparative analysis. J Rheumatol. 2007;34:2463-2465.
28 Kaya T, Gelal F, Gunaydin R. The relationship between severity and extent of spinal involvement and spinal mobility and physical functioning in patients with ankylosing spondylitis. Clin Rheumatol. 2006;25:835-839.
29 Rasker J, Prevo R, Lanting P. Spondylodiscitis in ankylosing spondylitis: inflammation or trauma? Scand J Rheumatol. 1996;25:52-57.
30 Little H, Urowitz MD, Smythe HA, et al. Asymptomatic spondylodiscitis: an unusual feature of ankylosing spondylitis. Arthritis Rheum. 1974;17:487-492.
31 Zochling J, Baraliakos X, Hermann KG, et al. Magnetic resonance imaging in ankylosing spondylitis. Curr Opin Rheumatol. 2007;19:346-352.
32 Weber U, Pfirrmann CW, Kissling RO, et al. Whole body MR imaging in ankylosing spondylitis: a descriptive pilot study in patients with suspected early and active confirmed ankylosing spondylitis. BMC Musculoskelet Disord. 2007;8:20.
33 Kim TJ, Kim TH, Jun JB, et al. Prevalence of ossification of posterior longitudinal ligament in patients with ankylosing spondylitis. J Rheumatol. 2007;34:2460-2462.
34 MacKay K, Mack C, Brophy S, et al. The Bath Ankylosing Spondylitis Radiology Index (BASRI): a new, validated approach to disease assessment. Arthritis Rheum. 1998;41:2263-2270.
35 Wanders AJ, Landewé RB, Spoorenberg A, et al. What is the most appropriate radiologic scoring method for ankylosing spondylitis? A comparison of the available methods based on the Outcome Measures in Rheumatology Clinical Trials filter. Arthritis Rheum. 2004;50:2622-2632.
36 Spoorenberg A, de Vlam K, van der Linden S. Radiological scoring methods in ankylosing spondylitis. Reliability and change over 1 and 2 years. J Rheumatol. 2004;31:125-132.
37 Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum. 2008;59:686-691.
38 Devogalaer JP, Maldague B, Malghem J, et al. Appendicular and vertebral bone mass in ankylosing spondylitis: a comparison of plain radiographs with single and dual photon absorptiometry and with quantitative computed tomography. Arthritis Rheum. 1992;35:1062-1067.
39 Song IH, Poddubnyy DA, Rudwaleit M, et al. Benefits and risks of ankylosing spondylitis treatment with nonsteroidal antiinflammatory drugs. Arthritis Rheum. 2008;58:929-938.
40 Wanders A, Heijde D, Landewé R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005;52:1756-1765.
41 Toussirot E, Wendling D. Current guidelines for the drug treatment of ankylosing spondylitis. Drugs. 1998;56:225-240.
42 Dougados M, Béhier JM, Jolchine I, et al. Efficacy of celecoxib, a cyclooxygenase 2–specific inhibitor, in the treatment of ankylosing spondylitis: a six-week controlled study with comparison against placebo and against a conventional nonsteroidal antiinflammatory drug. Arthritis Rheum. 2001;44:180-185.
43 Sieper J, Klopsch T, Richter M, et al. Comparison of two different dosages of celecoxib with diclofenac for the treatment of active ankylosing spondylitis: results of a 12-week randomised, double-blind, controlled study. Ann Rheum Dis. 2008;67:323-329.
44 Gran JT, Husby G. Ankylosing spondylitis: current drug treatment. Drugs. 1992;44:585-603.
45 Woolacott N, Bravo Vergel Y, Hawkins N. Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation. Health Technol Assess. 2006;10:iii-iv. xiii-xvi, 1-239
46 Maksymowych WP, Breban M, Barun J. Ankylosing spondylitis and current disease-controlling agents: do they work? Best Pract Res Clin Rheumatol. 2002;16:619-630.
47 Clegg DO, Reda DJ, Weisman MH, et al. Comparison of sulfasalazine and placebo in the treatment of ankylosing spondylitis: a Department of Veterans Affairs Cooperative Study. Arthritis Rheum. 1996;39:2004-2012.
48 Brandt J, Khariouzov A, Listing J, et al. Six-month results of a double-blind, placebo-controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum. 2003;48:1667-1675.
49 Gorman JD, Sack KE, Davis JCJr. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002;346:1349-1356.
50 Maksymowych WP, Jhangri GS, Lambert RG. Infliximab in ankylosing spondylitis: a prospective observational inception cohort analysis of efficacy and safety. J Rheumatol. 2002;29:959-965.
51 Davis JCJr, Van Der Heijde D, Braun J, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 2003;48:3230-3236.
52 Maksymowych WP, Poole AR, Hiebert L, et al. Etanercept exerts beneficial effects on articular cartilage biomarkers of degradation and turnover in patients with ankylosing spondylitis. J Rheumatol. 2005;32:1911-1917.
53 Alstergren P, Larsson PT, Kopp S. Successful treatment with multiple intra-articular injections of infliximab in a patient with psoriatic arthritis. Scand J Rheumatol. 2008;37:155-157.
54 Chen YF, Jobanputra P, Barton P, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10:1-229. iii-iv, xi-xiii
55 McLeod C, Bagust A, Boland A, et al. Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1-158. iii-iv
56 Baraliakos X, Brandt J, Listing J, et al. Outcome of patients with active ankylosing spondylitis after two years of therapy with etanercept: clinical and magnetic resonance imaging data. Arthritis Rheum. 2005;53:856-863.
57 Brandt J, Listing J, Haibel H, et al. Long-term efficacy and safety of etanercept after readministration in patients with active ankylosing spondylitis. Rheumatology (Oxford). 2005;44:342-348.
58 Chen CH, Lin KC, Yu DT. Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in ankylosing spondylitis: MMP-3 is a reproducibly sensitive and specific biomarker of disease activity. Rheumatology (Oxford). 2006;45:414-420.
59 Romero-Sánchez C, Robinson WH, Tomooka BH, et al. Identification of acute phase reactants and cytokines useful for monitoring infliximab therapy in ankylosing spondylitis. Clin Rheumatol. 2008;27:1429-1435.
60 Woo JH, Lee HJ, Sung IH, et al. Changes of clinical response and bone biochemical markers in patients with ankylosing spondylitis taking etanercept. Rheumatology. 2007;34:1753-1759.
61 Wendling D, Toussirot E. Bone and matrix remodeling markers: a new tool for assessment of treatment efficacy in ankylosing spondylitis? J Rheumatol. 2007;34:1647-1649.
62 Papp KA, Caro I, Leung HM, et al. Efalizumab for the treatment of psoriatic arthritis. J Cutan Med Surg. 2007;11:57-66.
63 Viguier M, Richette P, Aubin F, et al. Onset of psoriatic arthritis in patients treated with efalizumab for moderate to severe psoriasis. Arthritis Rheum. 2008;58:1796-1802.
64 Kragballe K, Deleuran B. Biological treatment of psoriasis and psoriatic arthritis. Ugeskr Laeger. 2008;170:2148-2150.
65 Mease PJ, Goffe BS, Metz J, et al. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000;356:385-390.
66 Ward MM. Predictors of the progression of functional disability in patients with ankylosing spondylitis. J Rheumatol. 2002;29:1420-1425.
67 Nghiem FT, Donohue JP. Rehabilitation in ankylosing spondylitis. Curr Opin Rheumatol. 2008;20:203-207.
68 Hanson JA, Mirza S. Predisposition for spinal fracture in ankylosing spondylitis. Am J Roentgenol. 2000;174:150-153.
69 Cooper C, Carbone L, Michet CJ, et al. Fracture risk in patients with ankylosing spondylitis: a population based study. J Rheumatol. 1994;21:1877-1882.
70 Markel DC, Graziano GP. Fracture of the S1 vertebral body in a patient with ankylosing spondylitis. J Spinal Disord. 1992;5:222-226.
71 Samartzis D, Anderson DG, Shen FH. Multiple and simultaneous spine fractures in ankylosing spondylitis: case report. Spine. 2005;30:E711-E715.
72 Moreau W, Wilcox N, Brown MF. Immobilization of spinal fractures in patients with ankylosing spondylitis. Injury. 2003;34:372-373.
73 Thumbikat P, Hariharan RP, Ravichandran G, et al. Spinal cord injury in patients with ankylosing spondylitis: a 10-year review. Spine. 2007;32:2989-2995.
74 Koivikko MP, Kiuru MJ, Koskinen SK. Multidetector computed tomography of cervical spine fractures in ankylosing spondylitis. Acta Radiol. 2004;45:751-759.
75 Harrop JS, Sharan A, Anderson G, et al. Failure of standard imaging to detect a cervical fracture in a patient with ankylosing spondylitis. Spine. 2005;30:E417-E419.
76 Koivikko MP, Koskinen SK. MRI of cervical spine injuries complicating ankylosing spondylitis. Skeletal Radiol. 2008;37:813-819.
77 Karasick D, Schweitzer M, Abidi N, et al. Fractures of the vertebrae with spinal cord injuries in patients with ankylosing spondylitis: Imaging findings. Am J Radiol. 1995;165:1205-1208.
78 Apple DFJr, Anson C. Spinal cord injury occurring in patients with ankylosing spondylitis. A multicenter study. Orthopaedics. 1995;18:1005-1011.
79 Hitchon PW, From AM, Brenton MD. Fractures of the thoracolumbar spine complicating ankylosing spondylitis. J Neurosurg. 2002;97(suppl):218-222.
80 Shen FH, Samartzis D. Successful nonoperative treatment of a three-column thoracic fracture in a patient with ankylosing spondylitis: existence and clinical significance of the fourth column of the spine. Spine. 2007;32:E423-E427.
81 Einsiedel T, Schmelz A, Arand M, et al. Injuries of the cervical spine in patients with ankylosing spondylitis: experience at two trauma centers. J Neurosurg Spine. 2006;5:33-45.
82 Payer M. Surgical management of cervical fractures in ankylosing spondylitis using a combined posterior-anterior approach. J Clin Neurosci. 2006;13:73-77.
83 Payer M. Surgical management of cervical fractures in ankylosing spondylitis using a combined posterior-anterior approach. J Clin Neurosci. 2006;13:73-77.
84 Taggard DA, Traynelis VC. Management of cervical spinal fractures in ankylosing spondylitis with posterior fixation. Spine. 2000;25:2035-2039.
85 Cornefjord M, Alemany M, Olerud C. Posterior fixation of subaxial cervical spine fractures in patients with ankylosing spondylitis. Eur Spine J. 2005;14:401-408.
86 Kanter AS, Wang MY, Mummaneni PV. A treatment algorithm for the management of cervical spine fractures and deformity in patients with ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E11.
87 Hu SS, Ananthakrishnan A. Ankylosing spondylitis. In: Herkowitz H, editor. Rothman-Simeone The Spine. 5th Ed. Philadelphia: Elsevier; 2006:763-771.
88 Rhee JM, Bridwell KH, Lenke LG, et al. Staged posterior surgery for severe adult spinal deformity. Spine. 2003;28:2116-2121.
89 Kuntz CIV, Levin LS, Ondra SL, et al. Neutral upright sagittal spinal alignment from the occiput to the pelvis in asymptomatic adults: a review and resynthesis of the literature. J Neurosurg Spine. 2007;6:104-112.
90 Van Royen BJ, De Gast A. Lumbar osteotomy for correction of thoracolumbar kyphotic deformity in ankylosing spondylitis. A structured review of three methods of treatment. Ann Rheum Dis. 1999;58:399-406.
91 Pigge RR, Scheerder FJ, Smit TH, et al. Effectiveness of preoperative planning in the restoration of balance and view in ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E7.
92 Suk S, Kim KT, Lee SH, et al. Significance of chin-brow vertical angle in correction of kyphotic deformity of ankylosing spondylitis patients. Spine. 2003;28:2001-2005.
93 Pigge RR, Scheerder FJ, Smit TH, et al. Effectiveness of preoperative planning in the restoration of balance and view in ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E7.
94 van Royen BJ, Scheerder FJ, Jansen E, et al. ASKyphoplan: a program for deformity planning in ankylosing spondylitis. Eur Spine J. 2007;16:1445-1449.
95 Min K, Hahn F, Leonardi M. Lumbar spinal osteotomy for kyphosis in ankylosing spondylitis: the significance of the whole body kyphosis angle. J Spinal Disord Tech. 2007;20:149-153.
96 Sansur CA, Fu KM, Oskouian RJJr, et al. Surgical management of global sagittal deformity in ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E8.
97 Simmons ED, DiStefano RJ, Zheng Y, et al. Thirty-six years experience of cervical extension osteotomy in ankylosing spondylitis: techniques and outcomes. Spine. 2006;31:3006-3012.
98 Tokala DP, Lam KS, Freeman BJ, et al. C7 decancellisation closing wedge osteotomy for the correction of fixed cervico-thoracic kyphosis. Eur Spine J. 2007:1471-1478. 2007
99 Etame AB, Than KD, Wang AC, et al. Surgical management of symptomatic cervical or cervicothoracic kyphosis due to ankylosing spondylitis. Spine. 2008;33:E559-E564.
100 Khoueir P, Hoh DJ, Wang MY. Use of hinged rods for controlled osteoclastic correction of a fixed cervical kyphotic deformity in ankylosing spondylitis. J Neurosurg Spine. 2008;6:579-583.
101 McMaster MJ. A technique for lumbar spinal osteotomy in ankylosing spondylitis. J Bone Joint Surg Br. 1985;67:204-210.
102 Li F, Sagi HC, Liu B, et al. Comparative evaluation of single-level closing-wedge vertebral osteotomies for the correction of fixed kyphotic deformity of the lumbar spine: a cadaveric study. Spine. 2001;26:2385-2391.
103 Kawahara N, Tomita K, Baba H, et al. Closing-opening wedge osteotomy to correct angular kyphotic deformity by a single posterior approach. Spine. 2001;26:391-402.
104 Qi Q, Chen ZQ, Guo ZQ, et al. [New type spinal osteotomy with cage inserting anteriorly and closing posteriorly to correct thoracolumbar kyphosis by a single posterior approach.]. Zhonghua Wai Ke Za Zhi. 2006;44:551-555.
105 Chang KW, Chen HC, Chen YY, et al. Sagittal translation in opening wedge osteotomy for the correction of thoracolumbar kyphotic deformity in ankylosing spondylitis. Spine. 2006;31:1137-1142.
106 Watanabe K, Lenke LG, Daubs MD, et al. A central hook-rod construct for osteotomy closure: a technical note. Spine. 2008;33:1149-1155.
107 Chin KR, Ahn J. Controlled cervical extension osteotomy for ankylosing spondylitis utilizing the Jackson operating table: technical note. Spine. 2007;32:1926-1929.
108 Kim KT, Lee SH, Suk KS, et al. Spinal pseudarthrosis in advanced ankylosing spondylitis with sagittal plane deformity: clinical characteristics and outcome analysis. Spine. 2007;32:1641-1647.
109 Geusens P, Vosse D, van der Heijde D, et al. High prevalence of thoracic vertebral deformities and discal wedging in ankylosing spondylitis patients with hyperkyphosis. J Rheumatol. 2001;28:1856-1861.
110 Thiranont N, Netrawichien P. Transpedicular decancellation closed wedge vertebral osteotomy for treatment of fixed flexion deformity of spine in ankylosing spondylitis. Spine. 1993;18:2517-2522.
111 Chen IH, Chien JT, Yu TC. Transpedicular wedge osteotomy for correction of thoracolumbar kyphosis in ankylosing spondylitis: experience with 78 patients. Spine. 2001;26:E354-E360.
112 Weale AE, Marsh CH, Yeoman PM. Secure fixation of lumbar osteotomy. Surgical experience with 50 patients. Clin Orthop Relat Res. 1995;321:216-222.
113 Kim KT, Suk KS, Cho YJ, et al. Clinical outcome results of pedicle subtraction osteotomy in ankylosing spondylitis with kyphotic deformity. Spine. 2002;27:612-618.
114 Chang KW, Chen YY, Lin CC, et al. Closing wedge osteotomy versus opening wedge osteotomy in ankylosing spondylitis with thoracolumbar kyphotic deformity. Spine. 2005;30:1584-1593.
115 Mummaneni PV, Mummaneni VP, Haid RWJr, et al. Cervical osteotomy for the correction of chin-on-chest deformity in ankylosing spondylitis. Technical note. Neurosurg Focus. 2003;14(1):E9.
116 Saraph VJ, Bach CM, Krismer M, et al. Evaluation of spinal fusion using autologous anterior strut grafts and posterior instrumentation for thoracic/thoracolumbar kyphosis. Spine. 2005;30:1594-1601.
117 Boachie-Adjei O. Role and technique of eggshell osteotomies and vertebral column resections in the treatment of fixed sagittal imbalance. Instr Course Lect. 2006;55:583-589.
118 Liew SM, Simmons EDJr. Cervical deformity: rationale for selecting the appropriate fusion technique (anterior, posterior, and 360 degree). Orthop Clin North Am. 1998;29:779-786.
119 Hoh DJ, Khoueir P, Wang MY. Management of cervical deformity in ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E9.
120 Lee JY, Hilibrand AS, Lim MR, et al. Characterization of neurophysiologic alerts during anterior cervical spine surgery. Spine. 2006;31:1916-1922.
121 Schwartz DM, Sestokas AK, Hilibrand AS, et al. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20:437-444.
122 Kelleher MO, Tan G, Sarjeant R, et al. Predictive value of intraoperative neurophysiological monitoring during cervical spine surgery: a prospective analysis of 1055 consecutive patients. J Neurosurg Spine. 2008;8:215-221.
123 Danish SF, Wilden JA, Schuster J, et al. Iatrogenic paraplegia in 2 morbidly obese patients with ankylosing spondylitis undergoing total hip arthroplasty. J Neurosurg Spine. 2008;8:80-83.
124 Ruf M, Rehm S, Poeckler-Schoeniger C, et al. Iatrogenic fractures in ankylosing spondylitis—a report of two cases. Eur Spine J. 2006;15:100-104.
125 Sengupta DK, Khazim R, Grevitt MP, et al. Flexion osteotomy of the cervical spine: a new technique for correction of iatrogenic extension deformity in ankylosing spondylitis. Spine. 2001;26:1068-1072.