Spine
Methods of imaging the spine
1. Plain films. These are widely available, but with low sensitivity. They are of questionable value in chronic back pain because of the prevalence of degenerative changes in both symptomatic and asymptomatic individuals of all ages beyond the second decade. They are, however, useful in suspected spinal injury, spinal deformity and postoperative assessment.
2. Myelography/radiculography. This is used when MRI is contraindicated or unacceptable to the patient. It is usually followed by CT for detailed assessment of abnormalities (CT myelography).
3. Discography. Advocates still regard it as the only technique able to verify the presence and source of discogenic pain.
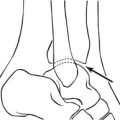
4. Facet joint arthrography. Facet joint pain origin can be confirmed if it is abolished after diagnostic injection of local anaesthetic, and treated by steroid instillation. The radiological appearances of the arthrogram are not helpful for the most part except in showing a communication with a synovial cyst. Vertical and contralateral facet joint communications can arise in the presence of pars interarticularis defects.
5. Arteriography. This is used for further study of vascular malformations shown by other methods, usually MRI, and for assessment for potential embolotherapy. It is not appropriate for the primary diagnosis of spinal vascular malformations. It may be used for pre-operative embolization of vascular vertebral tumours (e.g. renal metastasis).
6. Radionuclide imaging. This is largely performed for suspected vertebral metastases and to exclude an occult painful bone lesion (e.g. osteoid osteoma) using a technetium scintigraphic agent, for which it is a sensitive and cost-effective technique.
7. Computed tomography (CT). CT provides optimal detail of vertebral structures and is particularly useful in spinal trauma, spondylolysis, vertebral tumours, spinal deformity and postoperative states, especially if multidetector CT (MDCT) is available.
8. Magnetic resonance imaging (MRI). This is the preferred technique for virtually all spinal pathology. It is the only technique that directly images the spinal cord and nerve roots. MRI with intravenous (i.v.) gadolinium-DTPA is indicated in spinal infection, tumours and postoperative assessment.
9. Ultrasound (US). This is of use as an intra-operative method, and has uses in the infant spine.
Imaging approach to back pain and sciatica
1. Radiological investigation is essential if surgery is proposed.
2. Radiological findings should be compatible with the clinical picture before surgery can be advised.
3. It is vital for the surgeon and radiologist to identify those patients who will and who will not benefit from surgery.
4. In those patients judged to be in need of surgical intervention, success is very dependent on precise identification of the site, nature and extent of disease by the radiologist.
5. The demonstration of degenerative disease of the spine by non-invasive methods cannot be assumed to be the cause of the patient’s symptoms, as similar changes are often seen in asymptomatic individuals.
The need for radiological investigation of the lumbosacral spine is based on the results of a thorough clinical examination. A useful and basic preliminary step, which will avoid unnecessary investigations, is to determine whether the predominant symptom is back pain or leg pain. Leg pain extending to the foot is indicative of nerve root compression and imaging needs to be directed towards the demonstration of a compressive lesion, typically disc prolapse. This is most commonly seen at the L4/5 or L5/S1 levels (90–95%), and MRI should be employed as the primary mode of imaging. If the predominant symptom is back pain, with or without proximal lower limb radiation, then invasive techniques may be required, including discography and facet joint arthrography. The presence of degenerative disc and facet disease demonstrated by plain films, CT or MRI has no direct correlation with the incidence of clinical symptomatology. The annulus fibrosus of the intervertebral disc and the facet joints are richly innervated, and only direct injection can assess them as a potential pain source. However, unless there are therapeutic implications, there is no indication to go to these lengths, as many patients can be managed by physiotherapy and mild analgesics.
Conventional radiography
1. They assist in the diagnosis of conditions that can mimic mechanical or discogenic pain, e.g. infection, spondylolysis, ankylosing spondylitis and bone tumours, though in most circumstances 99mTc scintigraphy, CT and MRI are more sensitive.
2. They serve as a technical aid to survey the vertebral column and spinal canal prior to myelography, CT or MRI, particularly in the sense of providing basic anatomical data regarding segmentation. Failure to do this may lead to errors in interpreting correctly the vertebral level of abnormalities prior to surgery.
3. Correlation of CT or MRI data with radiographic appearances is often helpful in interpretation.
Computed tomography and magnetic resonance imaging of the spine
In addition to the diagnosis of prolapsed intervertebral disc, CT and MRI differentiate the contained disc, where the herniated portion remains in continuity with the main body of the disc, from the sequestrated disc, where there is a free migratory disc fragment. This distinction may be crucial in the choice of conservative or surgical therapy, and of percutaneous rather than open surgical techniques. MRI studies have shown that even massive extruded disc lesions can resolve naturally with time, without intervention. Despite the presence of nerve root compression, a disc prolapse can be entirely asymptomatic. Gadolinium enhancement of compressed lumbar nerve roots is seen in symptomatic disc prolapse with a specificity of 95.9%.1
Conclusions
MRI has revolutionized the imaging of spinal disease. Advantages include non-invasiveness, multiple imaging planes and lack of radiation exposure. Its superior soft tissue contrast enables the distinction of nucleus pulposus from annulus fibrosus of the healthy disc and enables the early diagnosis of degenerative changes. However, up to 35% of asymptomatic individuals less than 40 years of age have significant intervertebral disc disease at one or more levels on MRI images. Correlation with the clinical evidence is, therefore, essential before any relevance is attached to their presence and surgery is undertaken. As MRI is, at present, not as accurate as discography in the diagnosis and delineation of annular disease, and in diagnosing the pain source, there has been a resurgence of interest in discography. MRI should be used as a predictor of the causative levels contributing to the back pain with discography having a significant role in the investigation of discogenic pain prior to surgical fusion.2
Boden, SD, Davis, DO, Dina, TS, et al. Abnormal magnetic resonance scans of the lumbar spine in asymptomatic subjects. J Bone Joint Surg Am. 1990; 72(3):403–408.
Butt, WP. Radiology for back pain. Clin Radiol. 1989; 40(1):6–10.
Cribb, GL, Jaffray, DC, Cassar-Pullicino, VN. Observations on the natural history of massive lumbar disc herniation. J Bone Joint Surg Br. 2007; 89(6):782–784.
du Boulay, GH, Hawkes, S, Lee, CC, et al. Comparing the cost of spinal MR with conventional myelography and radiculography. Neuroradiology. 1990; 32(2):124–136.
Horton, WC, Daftari, TK. Which disc as visualized by magnetic resonance imaging is actually a source of pain? A correlation between magnetic resonance imaging and discography. Spine. 1992; 17(Suppl 6):S164–S171.
Hueftle, MG, Modic, MT, Ross, JS, et al. Lumbar spine: post-operative MR imaging with gadolinium-DTPA. Radiology. 1988; 167(3):817–824.
Myelography and radiculography
Cervical myelography
Technique
1. The patient lies prone with arms at the sides and chin resting on a soft pad so that the neck is in a neutral position or in slight extension. Marked hyperextension is undesirable as it accentuates patient discomfort, particularly in those with spondylosis, who comprise the majority of patients referred for this procedure. In such cases it will further compromise a narrowed canal and may produce symptoms of cord compression. The patient must be comfortable and able to breathe easily.
2. Using lateral fluoroscopy the C1/2 space is identified. The beam should be centred at this level to minimize errors due to parallax. Head and neck adjustments may be needed to ensure a true lateral position. The aim is to puncture the subarachnoid space between the laminae of C1 and C2, at the junction of the middle and posterior thirds of the spinal canal, i.e. posterior to the spinal cord. A 20G spinal needle is used. There is better control with the relatively stiff 20G needle, and the requirement for a small needle size to minimize CSF loss does not apply in the cervical region, where CSF pressure is very low.
3. Using aseptic technique, the skin and subcutaneous tissues are anaesthetized with 1% lidocaine. The spinal needle is introduced with the stilette bevel parallel to the long axis of the spine, i.e. to split rather than cut the fibres of the interlaminar ligaments. Lateral fluoroscopy is used to adjust the direction of the needle, and ensure the maintenance of a perfect lateral position as the needle is advanced. It is very helpful if a nurse steadies the patient’s head.
4. The sensation of the needle penetrating the dura is similar to that experienced during a lumbar puncture and the patient may experience slight discomfort at this stage. A feature that indicates that the needle tip is close to the dura is the appearance of venous blood at the needle hub as the epidural space is traversed. If the needle trajectory is too far posterior, tenting of the dura may occur, with failure to puncture the CSF space, even though anterior-posterior (AP) screening may show that the needle tip has crossed the midline. Repositioning may be needed in such cases. Severe acute neck or radicular pain indicates that the needle has been directed too far anteriorly and has come into contact with an exiting nerve root. Clumsy technique is known to have caused cord puncture, but permanent neurological damage as a result is unlikely.
5. Following removal of the stilette, CSF will drip from the end of the needle, and a sample may be collected if required.
6. Under fluoroscopy a small amount of contrast medium is injected to verify correct needle-tip placement. This will flow away from the needle tip and gravitate anteriorly to layer behind the vertebral bodies. Transient visualization of the dentate ligaments is obtained.
7. Injection is continued slowly until the required amount has been delivered. The cervical canal should be opacified anteriorly from the foramen magnum to C7/T1. If contrast tends to flow into the head before filling the lower cervical canal, tilt the table feet down slightly, and vice versa if contrast is flowing into the thoracic region without filling the upper cervical canal.
Lumbar radiculography
Technique
1. The lumbar thecal sac is punctured at L2/3, L3/4 or L4/5. The higher levels tend to be away from the most common sites of disc herniation and stenosis, and puncture may, therefore, be easier.
2. Lumbar puncture can technically be performed in the lateral decubitus position, in the sitting position, or even in the prone position. In the prone position the needle is guided under fluoroscopy, usually at the L2/L3 interspinous space with the patient lying on a folded pillow. This is important, because in the prone position the spinous processes are approximated due to lordosis, rendering puncture more difficult. In addition, spinal extension produces a relatively narrow thecal sac. The sitting position allows easy lumbar puncture, but is unsatisfactory for two reasons. First, the injected contrast medium drops through a large volume of CSF to accumulate in the sacral sac and becomes diluted as it descends. Second, patients may faint in this position, a complication that can be very dangerous, since the radiologist is on the wrong side of the table to help in preventing the patient from coming to any harm.
3. If lumbar puncture is carried out in the lateral decubitus position (described below) moderate spinal flexion is desirable, but there is no need for the extreme flexion sometimes demanded of the patient. A small pillow is placed under the dependent lumbar angle to keep the spine straight. The relevant interspace is one or two spinous processes above the plane of the iliac crest (L4/L5). If the spinous process cannot be felt, lateral fluoroscopy may help.
4. In obese patients the apparent soft-tissue midline gravitates below the spinal midline. The midline position may be verified while introducing local anaesthetic (1% lidocaine) into the skin and subcutaneous tissue, though there is no need to infiltrate the interspinous ligament. A 22G spinal needle should be used. It should be introduced with only a 10–15° cranial angulation. The passage of the needle through the interspinous ligament has a very characteristic sensation of moderate smooth resistance. Lack of resistance implies passage in the fatty tissues lateral to the midline, and a gritty sensation means impingement on the bone of the spinous process.
5. There is a tendency in inexperienced operators to introduce the needle at too steep a cranial angle, which a casual inspection of the spinous process anatomy can show to be incorrect. While traversing the interspinous ligament, the bevel should be in the coronal plane to avoid deflection to one side or the other, but once well established in the ligament may be turned to penetrate the thecal sac parallel to its long axis.
6. There is a characteristic sudden loss of resistance as the needle enters the thecal sac, and at this time the stilette should be withdrawn to verify CSF flow out of the needle. In the prone position suction using a 2-ml syringe may be necessary to obtain CSF. It should then be reintroduced and the needle advanced about 2 mm to ensure that the whole of the bevel has entered the thecal sac. A flexible connector is attached, taking care not to disturb the needle position.
7. Most difficulties are technical, arising from non-midline positioning of the needle, but in the presence of canal stenosis it may be difficult to find a position among the crowded roots where good CSF flow will take place. In most cases, radiologists will prefer to observe the entry of contrast medium into the thecal sac on the fluoroscopic screen, and this is especially important if there has been any difficulty in achieving a good needle position.
8. After the contrast medium has been injected, the patient turns to lie prone, and a series of films is obtained. Before taking films ensure that the relevant segment of the spinal canal is adequately filled with contrast medium. This usually requires some degree of feet-down tilt of the table, and a footrest should be in place to support the patient.
Radiographic views
1. AP and oblique views are obtained. (About 25° of obliquity is usual, but tailored in the individual case to profile the exit sleeves of the nerve roots of the cauda equina.)
2. A lateral view with a horizontal beam is useful, but further laterals in the erect or semi-erect position on flexion and extension add a dynamic dimension to the study.
3. Some radiologists advocate lateral decubitus frontal oblique views, to allow full entry of contrast medium into the dependent root sleeves of the whole lumbar and lower thoracic thecal sac. When turning patients from one side to the other ensure that they turn through the prone position, as turning supine will produce irretrievable contrast medium dilution.
At this stage the study will be complete as far as the question of root compression is concerned. The films should be carefully reviewed at this stage to ensure that all areas are fully covered, as the next manoeuvre will make any return to this area impossible. If the study is good, the patient should be screened in the lateral decubitus position, and tilted level or slightly head down until the contrast medium flows up to lie across the thoraco-lumbar junction. The patient may then be turned to lie supine and films of the conus and lower thoracic area obtained in the AP projection.
Paediatric myelography
A few points need to be borne in mind when carrying out myelography in the paediatric age group:
1. General anaesthetic is essential for all children aged 6 years or younger, and for many children up to the age of 12 years.
2. Lumbar puncture in cases of spinal dysraphism carries the risk of damaging a low-lying cord due to tethering. The thecal sac is usually wide in these conditions and the needle should be placed laterally in the thecal sac. In addition, as low a puncture as possible will minimize the risk, though in practice spinal cord injury is very uncommon or masked by the neurologic deficit already present.
3. In dysraphism, the frequent association of cerebellar tonsillar herniation precludes lateral C1/2 puncture.
Complications
1. Headache occurs in about 25% of cases, slightly more frequent in females.
2. Nausea and vomiting occur in about 5%.
3. Subdural injection of contrast medium. This occurs when only part of the needle bevel is within the subarachnoid space. Contrast medium initially remains loculated near the end of the needle, but can track freely in the subdural space to simulate intrathecal flow. When in doubt, the injection should be stopped, and AP and lateral views obtained with the needle in situ. The temptation of interpreting such an examination should be resisted and the patient re-booked.
4. Extradural injection of contrast medium outlines the nerve roots well beyond the exit foramina.
5. Intramedullary injection of contrast medium. This is a complication of lateral cervical puncture or in a low-lying spinal cord, and is recognized as a slit-like collection of contrast medium in the spinal canal. Small collections are without clinical significance.
Lumbar discography
Technique
1. There are two possible needle approaches:
(a) The posterior approach, which traverses the spinal canal
(b) The lateral oblique extradural approach, which avoids puncture of the dura and the vulnerable part of the posterior annulus. This is the preferred approach and can be carried out with the patient in the prone or left lateral decubitus position. The left lateral decubitus position is preferred, with the patient’s head resting on a pillow and a pad placed in the lumbar angle to maintain a straight spine. Moderate spinal flexion is useful, especially at L5/S1.
2. Full aseptic technique is mandatory; there should be no compromise on this point. The operator and any assistant should be gowned, masked, capped and gloved, and the patient should be draped. The level to be examined is determined by fluoroscopy, and the skin is anaesthetized, usually a hand’s breadth from the spinous processes.
3. The outer 21G needle is then directed towards the posterior aspect of the disc under intermittent fluoroscopic control, at an angle of 45–60° to the vertical. An additional caudal tilt may be necessary for the L5/S1 level. This needle should reach but not traverse the annulus fibrosus. This point is recognized by a distinct feeling of resistance when the outer fibres are encountered. The 26G needle is then introduced through the 21G needle and the entry of its tip into the nucleus pulposus confirmed in two planes with the aid of the image intensifier prior to contrast medium injection.
4. Contrast medium is injected slowly using a 1-ml syringe. This is done under intermittent fluoroscopic control, while the pain response, the disc volume and its radiographic morphology are monitored. The resistance to flow will gradually increase in a normal disc during the 0.5–1.0-ml stage.
Complications
1. Discitis is the most important complication. Pain with or without pyrexia after a few days indicates this development. Narrowing of the disc space with a variable degree of end-plate sclerosis is seen after a few weeks. Most cases are due to low-grade infection and require treatment with antibiotics.
2. With the transdural approach, post-lumbar puncture headache and/or vomiting may occur.
Buenaventura, RM, Shah, RV, Patel, V, et al. Systematic review of discography as a diagnostic test for spinal pain: an update. Pain Physician. 2007; 10(1):147–164.
Colhoun, E, McCall, IW, Williams, L, et al. Provocation discography as a guide to planning operations on the spine. J Bone Joint Surg Br. 1988; 70(2):267–271.
McCulloch, JA, Waddell, G. Lateral lumbar discography. Br J Radiol. 1978; 51(607):498–502.
Tehranzadeh, J. Discography 2000. Radiol Clin North Am. 1998; 36(3):463–495.
Facet joint arthrography
Technique
1. This is an outpatient procedure which involves the simultaneous injection of both joints at each level to be studied. More than one level should not be examined in each session to avoid diagnostic confusion. Needle placement can be done using fluoroscopy or CT guidance.
2. Using fluoroscopy the joint space is profiled by slowly rotating the patient from a prone position into a prone oblique orientation with the relevant side raised.
3. Sterile procedures are required.
4. The spinal needle is inserted and advanced perpendicularly to the facet joint, under fluoroscopic control. Caudal needle angulation is sometimes needed if the iliac crest overlies the L5/S1 facet joints. Alternatively the needles can be placed by simultaneous advancement into the joints using CT guidance.
5. In the majority of cases a noticeable ‘give’ indicates that the capsule is penetrated.
6. Contrast medium injection confirms correct needle placement by demonstrating immediate opacification of a superior and inferior recess.
7. Correct needle placement is documented showing intra-articular injection. The arthrographic appearances are not of any diagnostic consequence.
8. For diagnostic purposes up to 1 ml of 0.5% bupivacaine hydrochloride (Marcaine) is injected in the facet joint and the response over the ensuing 24-h period documented. For therapeutic injection, 0.5 ml of 0.5% Marcaine mixed with 0.5 ml of Depo-Medrone (methylprednisolone 40 mg ml−1) is injected after arthrography.
Fairbank, JC, Park, WM, McCall, IW, et al. Apophyseal injection of local anaesthetic as a diagnostic aid in primary low back syndromes. Spine. 1981; 6:598–605.
McCall, IW, Park, WM, O’Brien, JP. Induced pain referral from posterior lumbar elements in normal subjects. Spine. 1979; 4(5):441–446.
Maldague, B, Mathurin, P, Malghem, J. Facet joint arthrography in lumbar spondylolysis. Radiology. 1981; 140(1):29–36.
Mooney, V, Robertson, J. The facet syndrome. Clin Orthop Relat Res. 1976; 115:149–156.
Percutaneous vertebral biopsy
Contraindications
1. Biopsy should not be attempted under any circumstances in the presence of abnormal and uncorrected bleeding or clotting time or if there is a low platelet count.
2. Lesions suspected of being highly vascular, e.g. aneurysmal bone cyst or renal tumour metastasis are relatively dangerous, and a fine-needle aspiration should be used instead of a trephine needle for these cases.
Technique
1. For a fluoroscopy-guided procedure the lateral decubitus position is used. For CT control the prone position is preferable.
2. The skin entry point distance from the mid-line is about 8 cm for the lumbar region and 5 cm in the thoracic region.
3. The aim of the procedure is to enter the vertebral body at about 4 o’clock or 8 o’clock (visualizing the body with the spinous process at 6 o’clock). Local anaesthetic may be injected via a 21G spinal needle to allow deep infiltration of the soft tissues into close proximity to the periosteum.
4. The biopsy needle is advanced at between 30 and 45° to the sagittal plane in the thoracic and lumbar spine, respectively.
5. When the biopsy needle impinges on the cortex of the vertebral body its position is confirmed fluoroscopically in both AP and lateral planes, or on a single CT section. The trocar and cannula are then advanced through the cortex and the trocar is then withdrawn.
6. Using alternate clockwise and anticlockwise rotation the biopsy cannula is advanced approximately 1 cm.
7. By twisting the needle firmly several times in the same direction the specimen is severed.
8. At least two cores of bone may be obtained by withdrawing the needle back to the cortex, angulating and re-entering the vertebral body.
9. The needle is then withdrawn while simultaneous suction is applied by a syringe attached to the hub.
10. To remove the specimen, the plunger is inserted at the sharp end of the needle and the tissue is pushed out. Any blood clot should be included as part of the specimen.
11. In suspected infection the end-plate rather than the disc is biopsied. Most cases are due to osteomyelitis extending to the disc space. If there is a paravertebral abscess, aspiration and culture of its contents is preferable to vertebral biopsy.
Babu, NV, Titus, VT, Chittaranjan, S, et al. Computed tomographically guided biopsy of the spine. Spine. 1994; 19(21):2436–2442.
Shaltot, A, Michell, PA, Betts, JA, et al. Jamshidi needle biopsy of bone lesions. Clin Radiol. 1982; 33:193–196.
Stoker, DJ, Kissin, CM. Percutaneous vertebral biopsy: a review of 135 cases. Clin Radiol. 1985; 36:569–577.
Tehranzadeh, J, Tao, C, Browning, CA. Percutaneous needle biopsy of the spine. Acta Radiol. 2007; 48(8):860–868.
Nerve root blocks
Blankenbaker, DG, De Smet, AA, Stanczak, JD, et al. Lumbar radiculopathy: treatment with selective lumbar nerve blocks. Comparison of effectiveness of triamcinolone and betamethasone injectable suspensions. Radiology. 2005; 237(2):738–741.
Herron, LD. Selective nerve root block in patient selection for lumbar surgery: surgical results. J Spinal Disord. 1989; 2(2):75–79.
Schellhas, KP, Pollei, SR, Johnson, BA, et al. Selective cervical nerve root blockade: experience with a safe and reliable technique using an anterolateral approach for needle placement. Am J Neuroradiol. 2007; 28(10):1909–1914.
Wagner, AL. Selective lumbar nerve root blocks with CT fluoroscopic guidance: technique, results, procedure time, and radiation dose. Am J Neuroradiol. 2004; 25(9):1592–1594.
Wagner, AL. CT fluoroscopic-guided cervical nerve root blocks. Am J Neuroradiol. 2005; 26(1):43–44.
Weiner, BK, Fraser, RD. Foraminal injection for lateral lumbar disc herniation. J Bone Joint Surg Br. 1997; 79(5):804–807.