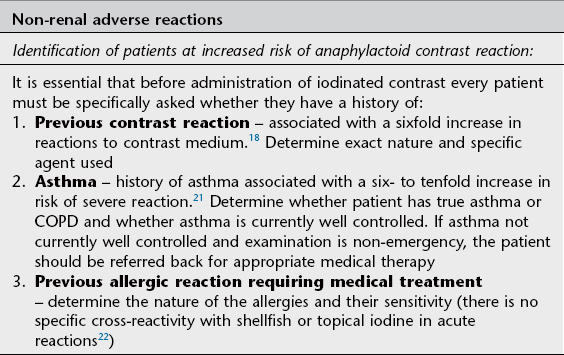
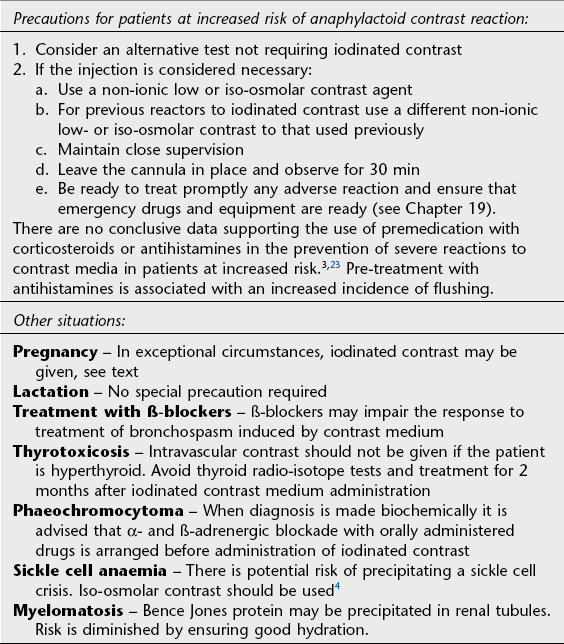
Intravascular contrast media
Historical development of radiographic agents
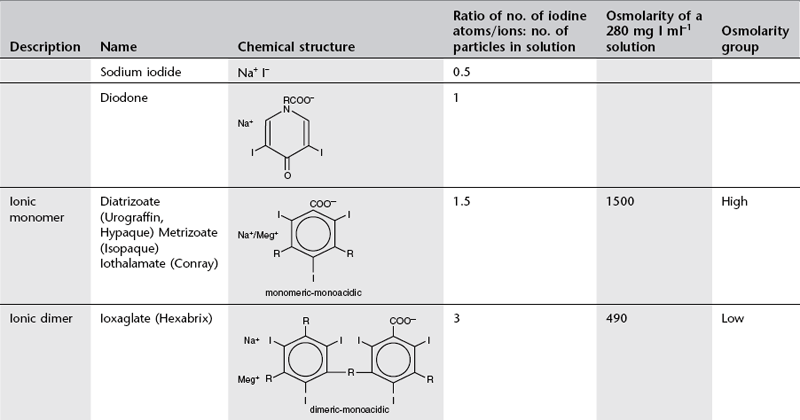
Conventional ionic contrast media had a ratio of three iodine atoms per molecule to two particles in solution, i.e. a ratio of 3 : 2 or 1.5 (Table 2.1). In order to decrease the osmolality without changing the iodine concentration, the ratio between the number of iodine atoms and the number of dissolved particles must be increased.
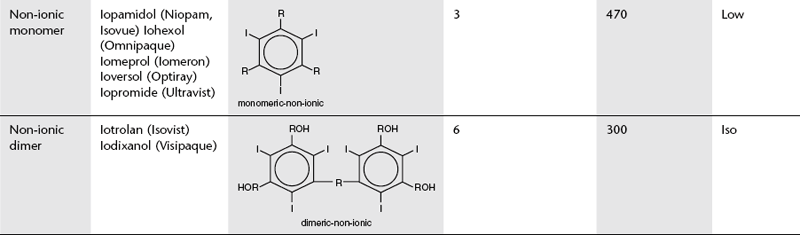
Development to produce low osmolar contrast medium (LOCM) proceeded along two separate paths (Table 2.1). The first was to combine two tri-iodinated benzene rings to produce an ionic dimer with six iodine atoms per anion; ioxaglate (Hexabrix). The alternative, more successful, approach was to produce compounds that do not ionize in solution and so do not provide radiologically useless cations. Low osmolar contrast media of this type are referred to as non-ionic. These include the non-ionic monomers iopamidol (Niopam, Iopamiron, Isovue and Solutrast), iohexol (Omnipaque), iopromide (Ultravist), iomeprol (Iomeron) and ioversol (Optiray), which are now used widely.
For both types of LOCM the ratio of iodine atoms in the molecule to the number of particles in solution is 3 : 1 and osmolality is theoretically halved in comparison with high osmolar contrast material (HOCM). However, because of aggregation of molecules in solution the measured reduction is only approximately one-third (Fig. 2.1).
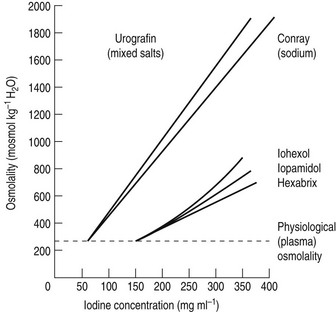
Figure 2.1 A plot of osmolality against iodine concentration for new and conventional contrast media. From Dawson et al.1 (Reproduced by courtesy of the editor of Clinical Radiology.)
Further research led to the introduction of non-ionic dimers – iotrolan (Isovist) and iodixanol (Visipaque). These have a ratio of six iodine atoms for each molecule in solution with satisfactory iodine concentrations at iso-osmolality; they are, therefore, called iso-osmolar contrast media. The safety profile of the iso-osmolar contrast agents is at least equivalent to LOCM, but any significant advantage of iso-osmolar contrast remains controversial.2
The low- and iso-osmolar contrast media are 5–10 times safer than the HOCM3 and are used routinely in clinical practice. With development having reached the stage of iso-osmolality, further research is now targeted on decreasing the chemotoxicity and viscosity of iodinated contrast media.
References
1. Dawson, P, Grainger, RG, Pitfield, J. The new low-osmolar contrast media: a simple guide. Clin Radiol. 1983; 34:221–226.
2. Morcos, SK. Contrast-induced nephropathy: are there differences between low osmolar and iso-osmolar iodinated contrast media? Clin Radiol. 2009; 64:468–472.
3. The Royal College of Radiologists. Standards for Intravenous Iodinated Contrast Administration to Adult Patients, 2nd ed. BFCR(10)4. London: The Royal College of Radiologists; 2010.
Adverse effects of intravenous water-soluble contrast media
Adverse reactions after administration of non-ionic iodinated contrast media are rare, occuring in less than 1% of all patients.1 Of these reactions, the vast majority are mild and self-limiting. The incidence of moderate or severe non-ionic contrast reactions is less than 0.001%.2
Toxic effects on specific organs
Cardiovascular toxicity
1. Intracoronary injection of contrast media may cause significant disturbance of cardiac rhythm.
2. Increased vagal activity may result in depression of the sino-atrial and atrio-ventricular nodes, causing bradycardia or asystole.
3. Injection of hypertonic contrast medium causes significant fluid and ion shifts. Immediately after injection there is a significant increase in serum osmolality. This causes an influx of water from the interstitial space into the vascular compartment, an increase in blood volume, an increase in cardiac output and a brief increase of systemic blood pressure. Peripheral dilatation may cause a more prolonged fall of blood pressure. Injection into the right heart or pulmonary artery causes transitory pulmonary hypertension and systemic hypotension; injection into the left ventricle or aorta causes brief systemic hypertension followed by a more prolonged fall.
Haematological changes
1. In the presence of a high concentration of contrast medium, such as withdrawal of blood into a syringe of contrast medium, damage to red cell walls occurs and haemolysis follows. Haemolysis and haemoglobinuria have been reported following angiocardiography. It is advisable not to re-inject blood that has been mixed with contrast medium.
2. Red cell aggregation and coagulation may occur in the presence of a high concentration of contrast medium, e.g. 350 mg I ml−1. However, disaggregation occurs easily and this is not likely to be of any clinical significance.
3. Contrast media may impair blood clotting and platelet aggregation. Low- and iso-osmolar contrast media have a minimal effect compared to HOCM.3
4. Contrast media have a potentiating effect on the action of heparin.
5. Thrombus formation occurs when blood is mixed with LOCM during injection. However, the role of the syringe is also significant; thrombus formation is maximal when blood is slowly withdrawn into a syringe so that it layers on top of the contrast medium, against the wall of the syringe, therefore this should be avoided. Activation of coagulation by the tube material probably plays a significant role.
6. Contrast media have been shown to cause HbSS blood to sickle in vitro. Iso-osmolar contrast agents have a lesser effect and their use is indicated in this disease.4 Beneficial diagnostic imaging with intravascular contrast can be performed without significant increased risk of serious complication in patients with sickle cell disease.5
7. When red blood cells are placed in a hypertonic contrast medium, water leaves the interior of the cells by osmosis and they become more rigid and less deformable. Iso-osmolar solutions also have an effect on red cell deformability, indicating that there may also be a chemical effect. Red cells that deform less easily are less able to pass through capillaries and may occlude them. This is the explanation for the transient rise in pulmonary arterial pressure during pulmonary arteriography and may also be a factor in contrast-induced nephropathy.
8. Transient eosinophilia may occur 24–72 h after administration of contrast medium.
Nephrotoxicity
Contrast-induced nephropathy (CIN) is one of the most serious adverse effects associated with the use of intravascular contrast media and is defined as an impairment of renal function (increase in serum creatinine >25% baseline value or 44 µmol l−1)6 occurring within 3 days of contrast administration in the absence of an alternative aetiology. In those affected, the serum creatinine concentration starts to rise within the first 24 h, reaches a peak by 2–3 days and usually returns to baseline by 3–7 days. In rare cases patients may need temporary or permanent dialysis. The mechanisms of CIN are summarized below:7
1. Altered vascular haemodynamics:
(a) adverse cardiotoxic effects
(b) increased peripheral vasodilatation
(c) renal vascular bed changes (increased blood flow followed by a more prolonged decrease)
2. Glomerular and tubular injury:
3. Interference with intratubular fluid volume and viscosity.
It was hoped that the iso-osmolar contrast agents might be less nephrotoxic than LOCM, however clinical trials have so far yielded conflicting results.8 CIN has a complex aetiology and the positive benefit of reduction in osmolarity achieved with iso-osmolar contrast medium may be negated by the accompanying increase in viscosity.
There are a number of predisposing factors in CIN:
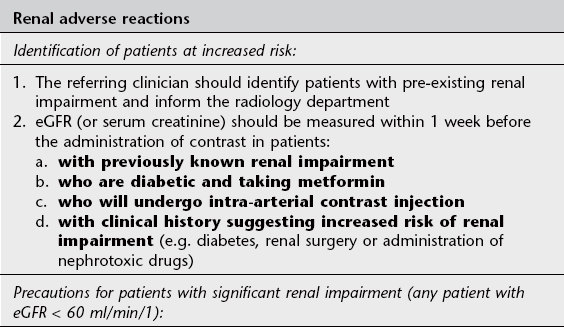
1. The single most important risk factor is pre-existing impairment of renal function; patients with normal renal function are at very low risk. A guide level of eGFR < 60 ml/min/1 is recommended to indicate renal impairment3,9 (Fig. 2.2)
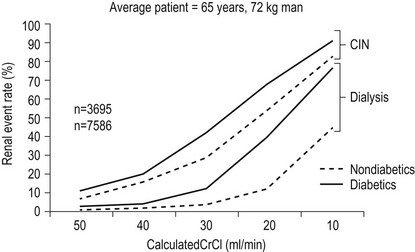
Figure 2.2 Validated risk of CIN and dialysis after diagnostic angiography and ad hoc angioplasty by creatinine clearance (CrCl) and diabetes. This assumes a mean contracts dose of 250 ml and a mean age of 65. (Adapted from Reference 9, with permission from Elsevier.)
6. Use of very large doses of contrast medium
A further hazard for patients who suffer impairment of renal function as a result of intravascular iodinated contrast is reduced renal clearance of other drugs, e.g. metformin, an oral hypoglycaemic drug which is exclusively excreted via the kidneys. The resultant accumulation of metformin may result in the development of the potentially fatal complication lactic acidosis.
See Table 2.2 for guidelines on prophylaxis of renal adverse reaction to iodinated contrast.
Neurotoxicity
Contrast medium delivered to the brain via the arterial route during angiography or neurovascular intervention may rarely cross the blood–brain barrier with resultant cerebral oedema and altered neuronal excitability. Potential clinical effects include encephalopathy, seizures, cortical blindness and focal neurological deficits which are most often temporary but infrequently persistent.10 Neurotoxicity is caused by both osmolality and chemotoxic effects.
Thyroid function
1. Iodinated contrast media may rarely cause thyroid dysfunction resulting in the development of either incident hyperthyroidism or incident overt hypothyroidism.11 Iodide administration may result in the generation of intrathyroidal iodolactones or iodolipids which inhibit thyroid peroxidise activity and disrupt normal thyroid function. Patients with no known risk factors may be affected but those with goitre or proven detectable serum antibodies to thyroperoxidase are likely at increased risk, particularly in iodine-deficient geographic regions.
2. Contrast media may interfere with the accuracy of thyroid function tests.
Idiosyncratic reactions
Adverse reactions can be classified in terms of severity as:
1. Mild: Nausea, vomiting, urticaria
2. Moderate: Mild bronchospasm, vasovagal reaction, tachycardia, diffuse erythema
3. Severe: Cardiovascular collapse, moderate or severe bronchospasm, laryngeal oedema, loss of consciousness or seizure.
Mild and moderate reactions are uncommon; major adverse reactions are very rare for both LOCM12 and iso-osmolar iodinated contrast.13 Adverse effects of low osmolar iodinated contrast are found in approximately 0.15% of all patients. In a large study of LOCM contrast doses14 the adverse effects were categorized (Table 2.3).
Table 2.3
Categories of adverse effects of LOCM contrast doses14
| Category of adverse effect14 | Proportion of adverse effect events (n = 458) | Proportion of total patient group (n = 298 000) |
| Mild | 81.6% | 0.125% |
| Moderate | 15.1% | 0.02% |
| Severe | 3.3% | 0.005% |
Fatal reactions
Deaths caused by iodinated contrast agents are very rare, occurring at a rate of 1.1–1.2 per million contrast media packages distributed. Most are attributed to renal failure, anaphylaxis or allergic reaction.15
Non-fatal reactions
1. Flushing, metallic taste in the mouth, nausea, sneezing, cough and tingling are common and related to dose and speed of injection.
2. Perineal burning, a desire to empty the bladder or rectum and an erroneous feeling of having been incontinent of urine are more common in women.
4. Angioneurotic oedema. Most commonly affects the face. May persist for up to 3 days and its onset may be delayed.
6. Necrotizing skin lesions. Rare and mostly in patients with pre-existing renal failure (particularly those with systemic lupus erythematosus).
7. Bronchospasm. Predisposed to by a history of asthma and by therapy with ß-blockers. In most patients the bronchospasm is subclinical. Mechanisms include:
8. Non-cardiogenic pulmonary oedema. During acute anaphylaxis or hypotensive collapse and possibly due to increased capillary permeability.
10. Hypotension. Usually accompanied by tachycardia but in some patients there is vagal over-reaction with bradycardia. The latter is rapidly reversed by atropine 0.6–2 mg i.v. Hypotension is usually mild and is treatable by a change of posture. Rarely, it is severe and may be accompanied by pulmonary oedema.
11. Abdominal pain. May be a symptom in anaphylactic reactions or vagal overactivity. Should be differentiated from the loin pain that may be precipitated in patients with upper urinary tract obstruction.
12. Delayed-onset reactions – (1 h−1 week after injection) include nausea, vomiting, rashes, headaches, itching and parotid gland swelling. Late adverse reactions are commoner by a factor of three to four after iso-osmolar contrast medium injection. Risk factors for delayed reaction are previous contrast medium reaction, history of allergy and interleukin-2 treatment.16
Mechanisms of idiosyncratic contrast medium reactions
Idiosyncratic contrast medium reactions are usually labelled anaphylactoid since they have all the features of anaphylaxis but are IgE-negative in over 90% of cases. However, patients who experience a severe anaphylactoid reaction to iodinated contrast should always be investigated for possible IgE-mediated allergy including skin testing and, if available, basophil activation testing.17
Possible mechanisms of non-IgE-mediated anaphylactoid reaction include those outlined below.
Complement activation
Contrast medium may perhaps activate the complement system leading to the formation of anaphylatoxins which induce the release of histamine and other biological mediators from basophils and mast cells.18
Anxiety
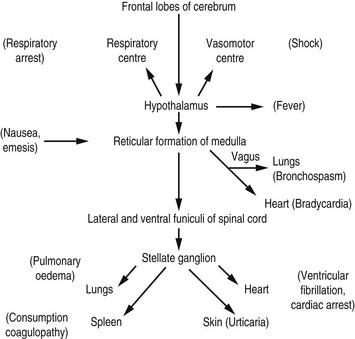
Many years ago, Lalli19 postulated that most contrast medium reactions are the result of the patient’s fear and apprehension. The high autonomic nervous system activity in an anxious patient will be stimulated further when the patient experiences the administration of contrast medium. Furthermore, contrast medium crossing the blood–brain barrier can stimulate the limbic area and hypothalamus to produce further autonomic activity. This autonomic activity is responsible for contrast medium reactions by the sequence of events illustrated in Figure 2.3.
Prophylaxis of adverse contrast medium effects
Guidelines for prophylaxis of renal and non-renal adverse contrast media reaction are given in Tables 2.2 and 2.4. Sources used in these tables include documents of the Royal College of Radiologists,3 Canadian Association of Radiologists6 and the European Society of Urogenital Radiology20 on contrast medium administration; the full text of these documents can be downloaded from the internet at www.rcr.ac.uk, www.car.ca and www.esur.org, respectively. General safety issues are:
• a doctor should be immediately available in the department to deal with any severe reaction
• facilities for treatment of acute adverse reaction should be readily available and regularly checked
• a patient must not be left alone or unsupervised for the first 5 min following injection of the contrast agent
• the patient should remain in the department for at least 15 min after the contrast injection. In patients at increased risk of reaction this should be increased to 30 min.
Iodinated contrast in pregnancy and lactation
There is no evidence of mutagenic or teratogenic effects after intravenous administration of iodinated contrast to the mother during pregnancy but there is a potential impact on the neonatal thyroid gland. The fetal thyroid develops early in pregnancy and plays an important role in the development of the nervous system. It is recommended that iodinated contrast be used in pregnant women only when (a) no alternative test is available, (b) information obtained from the study is useful to both mother and fetus during the pregnancy and (c) the referring physician considers it imprudent to delay the imaging study until after delivery.24 A single in utero exposure to low osmolar iodinated contrast is unlikely to have any effect on thyroid function at birth,25 however neonatal thyroid function should be checked during the first week of life.
Only small amounts of intravenous contrast medium reach the breast milk during lactation, approximately 0.5% of the dose received by the mother. It is considered safe for both mother and infant to continue breast feeding after iodinated contrast administration.24
References
1. Mortelé, KJ, Oliva, MR, Ondategui, S, et al. Universal use of nonionic iodinated contrast medium for CT: evaluation of safety in a large urban teaching hospital. Am J Roentgenol. 2005; 184(1):31–34.
2. Hunt, CH, Hartman, RP, Hesley, GK. Frequency and severity or adverse effects of iodinated and gadolinium contrast materials: retrospective review of 456,930 doses. Am J Roentgenol. 2009; 193:1124–1127.
3. Aspelin, P, Stacul, F, Thomsen, HS, et al. Effects of iodinated contrast media on blood and endothelium. Eur Radiol. 2006; 16(5):1041–1049.
4. Losco, P, Nash, G, Stone, P, et al. Comparison of the effects of radiographic contrast media on dehydration and filterability of red blood cells from donors homozygous for hemoglobin A or hemoglobin. S Am J Hematol. 2001; 68(3):149–158.
5. Campbell, KL, Hud, LM, Adams, S, et al. Safety of iodinated intravenous contrast medium administration in sickle cell disease. Am J Med. 2012; 125(1):100e11–16.
6. Canadian Association of Radiologists. Consensus Guidelines for the Prevention of Contrast Induced Nephropathy, 2nd ed. Ottowa: Ontario: Canadian Association of Radiologists; 2011.
7. Seeliger, E, Sendeski, M, Rihal, CS, et al. Contrast induced kidney injury: mechanisms, risk factors and prevention. Eur Heart J. 2012; 33(16):2007–2015.
8. Heinrich, MC, Häberle, L, Müller, V, et al. Nephrotoxicity of iso-osmolar iodixanol compared with non-ionic low-osmolar contrast media: Meta-analysis of randomized controlled trials. Radiology. 2009; 250(1):68–86.
9. McCullough, PA, Soman, SS. Contrast-induced neuropathy. Crit Care Clin. 2005; 21(2):261–280.
10. Leong, S, Fanning, NF. Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling. A case report and review of the literature. Interv Neuroradiol. 2012; 18(1):33–41.
11. Rhee, CM, Bhan, I, Alexander, EK, et al. Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med. 2012; 172(2):153–159.
12. Kopp, AF, Mortele, KJ, Cho, YD, et al. Prevalence of acute reactions to iopramide: postmarketing surveillance study of 74,717 patients. Acta Radiol. 2008; 49(8):902–911.
13. Haussler, MD. Safety and patient comfort with iodixanol: a postmarketing surveillance study in 9515 patients undergoing diagnostic CT examinations. Acta Radiol. 2010; 51(8):924–933.
14. Hunt, CH, Hartman, RP, Hesley, GK. Frequency and severity of adverse effects of iodinated and gadolinium contrast materials: retrospective review of 456,930 doses. Am J Radiol. 2009; 193(4):1124–1127.
15. Wysowski, DK, Nourjah, P. Deaths attributed to X-ray contrast media on U. S. death certificates. Am J Roentgenol. 2006; 186:613–615.
16. Bellin, MF, Webb, JA, Thomsen, HS, et al. Late adverse reactions to intravascular iodine based contrast media: an update. Eur Radiol. 2011; 21(11):2305–2310.
17. Trcka, J, Schmidt, C, Seitz, CS, et al. Anaphylaxis to iodinated contrast material: nonallergic hypersensitivity or IgE-mediated allergy? Am J Radiol. 2008; 190(3):666–670.
18. Morcos, SK. Acute serious and fatal reactions to contrast media: our current understanding. Br J Radiol. 2005; 78(932):686–693.
19. Lalli, AF. Urographic contrast media reactions and anxiety. Radiology. 1974; 112(2):267–271.
20. European Society of Urogenital Radiology Contrast Media Safety Committee. ESUR Guidelines on Contrast Media, Version 7. 0. www. esur. org, 2008.
21. Morcos, SK, Thomsen, HS. Adverse reactions to iodinated contrast media. Eur Radiol. 2001; 11(7):1267–1275.
22. Schabelman, E, Witting, M. The relationship of radiocontrast, iodine and seafood allergies: a medical myth exposed. J Emerg Med. 2010; 39(5):701–707.
23. Davenport, MS, Cohan, RH, Caolli, EM, et al. Repeat contrast medium reactions in premedicated patients: frequency and severity. Radiology. 2009; 253(2):372–379.
24. American College of Radiology. ACR Manual on Contrast Media, 7th ed. Reston, VA: American College of Radiology; 2010.
25. Bourjeily, G, Chalhoub, M, Phornphutkl, C, et al. Neonatal thyroid function: effect of single exposure to iodinated contrast medium in utero. Radiology. 2010; 256(3):744–750.
Contrast agents in magnetic resonance imaging
Historical development
Shortly after the introduction of clinical MRI, the first contrast-enhanced human MRI studies were reported in 1981 using ferric chloride as a contrast agent in the gastrointestinal tract. In 1984, Carr et al. first demonstrated the use of a gadolinium compound as a diagnostic intravascular MRI contrast agent.1 Currently, around one-quarter of all MRI examinations are performed with contrast agents.
Mechanism of action
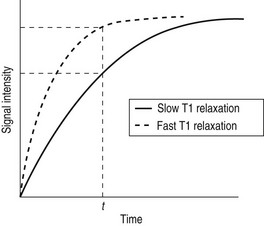
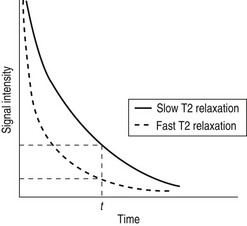
Whilst radiographic contrast agents use a direct alteration in tissue density to allow the visualization of structures, the MRI contrast agents act indirectly by altering the magnetic properties of hydrogen ions (protons) in water and lipid which form the basis of the image in MRI. To enhance the inherent contrast between tissues, MRI contrast agents must alter the rate of relaxation of protons within the tissues. The changes in relaxation vary and, therefore, different tissues produce differential enhancement of the signal (see Figs 2.4 and 2.5). These figures show that, for a given time t, if the T1 relaxation is more rapid then a larger signal is obtained (brighter images), but the opposite is true for T2 relaxation, where more rapid relaxation produces reduced signal intensity (darker images). There are different means by which these effects on protons can be produced using a range of MRI contrast agents.
MRI contrast agents must exert a large magnetic field density (a property imparted by their unpaired electrons) to interact with the magnetic moments of the protons in the tissues and so shorten their T1 relaxation time, which will produce an increase in signal intensity (see Fig. 2.4). The electron magnetic moments also cause local changes in the magnetic field, which promote more rapid proton dephasing and so shorten the T2 relaxation time. All contrast agents shorten both T1 and T2 relaxation times but some will predominantly affect T1 (longitudinal relaxation rate) and others predominantly T2 (transverse relaxation rate).
1. Ferromagnetic. These retain magnetism even when the applied field is removed. This may cause particle aggregation and interfere with cell function, making them unsafe for clinical use as MRI contrast agents.
2. Paramagnetic – e.g. gadolinium contrast agents, which are by far the most widely used MRI contrast agents. Paramagnetic agents also include liver-specific manganese-based agents such as the manganese chelate Mn-DPDP (Telescan). These paramagnetic contrast agents have magnetic moments which align to the applied field, but once the gradient field is turned off, thermal energy within the tissue is enough to overcome the alignment. Their maximum effect is on protons in the water molecule, shortening the T1 relaxation time and hence producing increased signal intensity (white) on T1 images (Fig. 2.4).
3. Superparamagnetic – e.g. particles of iron oxide (Fe3O4). These cause abrupt changes in the local magnetic field which results in rapid proton dephasing and reduction in the T2 relaxation time, and hence producing decreased signal intensity (black) on T2 images (Fig. 2.5). Superparamagnetic compounds are available as large particles in a colloid suspension for oral ingestion as gastrointestinal contrast and also as small particles of iron oxide (SPIO) agents and ultrasmall particles of iron oxide (USPIO) agents. Both SPIO and USPIO agents have submicron global particle diameters and are small enough to form a stable solution which can be injected intravenously.
Gadolinium
1. Extracellular fluid (ECF) agents: by far the most commonly used form of gadolinium. Includes:
(a) Gd-diethylenetriaminepenta-acetic acid (DTPA), Gadopentetate dimeglumine (Magnevist®)
2. Liver agents: the excretion pathway of the gadolinium chelates can be altered to produce compounds such as Gd-BOPTA, Gadobenate (Multihance®) and Gd-DTPA, Gadoxetate disodium (Primovist®) which are taken up by hepatocytes and cleared intact via the hepato-biliary system. These agents are used to improve detection of liver lesions which do not contain hepatocytes (therefore do not take up the contrast), such as liver metastases, and to characterize lesions which do take up the contrast, such as hepatocellular carcinoma. They can also be used to provide positive contrast (T1 weighted) of the hepato-biliary system.
3. Blood pool agents: these bind reversibly to human albumin resulting in higher relaxivity and longer persistence in the vascular space than ECF agents, allowing a wider range of vascular imaging, e.g. Gadofosveset trisodium (Vasovist®).
Adverse reactions
Acute adverse reactions
These are rare (Table 2.5) and are classified as:
1. Mild; e.g. nausea, vomiting, headache, dizziness, shaking, altered taste, itching, rash, facial swelling.
2. Moderate; e.g. tachycardia or bradycardia, hypertension, generalized erythema, dyspnoea, bronchospasm, wheezing, mild hypotension.
3. Severe; e.g. laryngeal oedema (severe or rapidly progressive), unresponsiveness, cardiopulmonary arrest, convulsions, clinically manifest cardiac arrhythmias.
Patients with a history of previous adverse reaction to gadolinium contrast agents have an increased likelihood of experiencing adverse reactions with a 30% recurrence rate of hypersensitivity reactions in those with previous reactions.6 Those with asthma, documented allergies or previous adverse reaction to iodinated contrast are also at increased risk of adverse reaction.7
Delayed adverse reactions
1. Renal impairment – when used at the standard doses given above, gadolinium contrast agents do not cause significant impairment of renal function.
2. Nephrogenic systemic fibrosis (NSF) – this rare systemic disorder, first described in 2000, is characterized by increased deposition of collagen with thickening and hardening of the skin, contractures and, in some patients, clinical involvement of other tissues.8 Most cases are mild but an estimated 5% have a progressive debilitating course.9 NSF occurs in patients with renal disease and almost all patients with NSF have been exposed to gadolinium-based contrast agents within 2–3 months prior to the onset of the disease. The mechanism by which renal failure and gadolinium-based contrast agents trigger NSF is not known.
In 2009 the European Medicines Agency Committee for Medicinal Products for Human Use Scientific Advisory Group categorized gadolinium products according to risk of causing nephrogenic systemic fibrosis:
1. Low risk: macrocyclic chelates including gadoterate meglumine (Dotarem®), gadoteridol (Prohance®), gadobuterol (Gadovist®).
2. Medium risk: linear ionic chelates including gadoxetic acid (Primovist®), gadobenate dimeglumine (Multilane®).
3. High risk: linear non-ionic chelates including gadodiamide (Omniscan®) and linear ionic chelates including gadopentetate dimeglumine (Magnevist®) and made recommendations to minimize risk of NSF, which can be found at info@ema.europa.eu. Preventative guidelines have been shown to be extremely effective in protection of patients from NSF.10
Precautions for prevention of adverse reactions
Detailed guidelines are available from the American College of Radiology,7 Royal College of Radiologists11 and the European Society of Urogenital Radiology.12 These form the basis for the following advice.
Acute adverse reactions
1. Identify patients at increased risk of reaction because of previous gadolinium reaction, asthma, allergies or previous adverse reaction to iodinated contrast.
2. For those at increased risk consider an alternative test not requiring a gadolinium agent.
3. If proceeding with i.v. gadolinium contrast:
(a) patients who have previously reacted to one gadolinium-based contrast agent should be injected with a different agent if they are re-studied
(b) although there is no clinical evidence of the effectiveness of premedication, it is suggested that consideration be given to the use of premedication such as oral prednisolone 30 mg orally 12 and 2 hours before contrast medium
(c) those with an increased risk of adverse reaction should be monitored more closely after injection.
Delayed adverse reactions
1. Identify patients at risk of NSF; particularly patients with renal impairment, patients in the perioperative period after liver transplant, infants, neonates and the elderly.
2. For patients at increased risk consider an alternative test not requiring a gadolinium agent.
3. If proceeding with gadolinium contrast, the use of high-risk gadolinium contrast medium is contraindicated (see above). The lowest possible dose of low- or medium-risk gadolinium-based contrast agent should be used.
Iron-oxide magnetic resonance imaging contrast agents (SPIO and USPIO)
After i.v. injection, the superparamagnetic SPIO contrast agents are taken up via the reticulo-endothelial system into the liver and spleen resulting in decreased signal intensity in normal liver and spleen on T2-weighted images. Malignant tumour tissue or other lesions which do not contain reticulo-endothelial cells will not take up the contrast and, therefore, retain their high MRI signal. Iron-oxide contrast agents are useful for detection of liver tumour, particularly hepatocellular carcinoma.13
The USPIO contrast agents have greater T1 shortening effects and a longer plasma circulation time than the SPIO agents. In addition, they have greater uptake in bone marrow and lymph nodes and are used for imaging bone marrow and lymph node metastases.14
Gastrointestinal contrast agents
MR enteroclysis is increasingly widely used in the diagnosis of small bowel disease. During this examination, 1–3 litres of 0.5% methylcellulose solution in water, infused via a nasogastric tube (see Chapter 3) is used as the gastrointestinal tract contrast agent.
MRI contrast agents in pregnancy and lactation
MRI contrast agents should not be routinely given to pregnant patients.7 Although there are no reports of teratogenic or mutagenic effects in humans, there are no case-controlled prospective studies in large numbers of patients and the precise risk to the fetus is not known. Gadolinium chelates may accumulate in the amniotic fluid and remain there for an indefinite period of time with potential dissociation of toxic free gadolinium ion. The decision to administer an MRI contrast agent to a pregnant patient should be made on a case-by-case basis and accompanied by a thoughtful and well-documented risk–benefit analysis.
Only tiny amounts of gadolinium-based contrast medium given intravenously to a lactating mother reach the milk and a minute proportion entering the baby’s gut is absorbed. The use of gadolinium contrast agents is considered safe during lactation.15
References
1. Carr, DH, Brown, J, Bydder, GM, et al. Intravenous chelated gadolinium as a contrast agent in NMR imaging of cerebral tumours. Lancet. 1984; 1(8375):484–486.
2. Morgan, DE, Spann, JS, Lockhart, ME, et al. Assessment of adverse reaction rates during gadoteridol-enhanced MRI imaging in 28,078 patients. Radiology. 2011; 259(1):109–116.
3. Abujudeh, HH, Kosaraju, VK, Kaewlai, R. Acute adverse reactions to gadopentetate dimeglumine and gadobenate dimeglumine: experience with 32,659 injections. Am J Roentgenol. 2010; 194(2):430–434.
4. Dillman, JR, Ellis, JH, Ellis, JH, et al. Frequency and severity of acute allergic-like reactions to gadolinium-containing i. v. contrast media in children and adults. Am J Roentgenol. 2007; 189(6):1533–1538.
5. Hunt, CH, Hartman, RP, Hesley, GK. Frequency and severity or adverse effects of iodinated and gadolinium contrast materials: retrospective review of 456,930 doses. Am J Roentgenol. 2009; 193:1124–1127.
6. Jung, JW, Kang, HR, Kim, MH, et al. Immediate hypersensitivity reaction to gadolinium based MR contrast media. Radiology. 2012; 264(2):414–422.
7. American College of Radiology. ACR Manual on Contrast Media, 7th ed. Reston, VA: American College of Radiology; 2010.
8. Cowper, SE, Robin, HS, Steinberg, SM, et al. Scleromyxoedema-like cutaneous diseases in renal dialysis patients. Lancet. 2000; 356(9234):1000–1001.
9. Reiter, T, Ritter, O, Prince, MR, et al. Minimising risk of nephrogenic systemic fibrosis in cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012; 14(1):31.
10. Wang, Y, Alkasab, TK, Narin, O, et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium based contrast guidelines. Radiology. 2011; 260(1):105–111.
11. The Royal College of Radiologists. Gadolinium-based Contrast Media and Nephrogenic Systemic Fibrosis. BFCR(07)14. London: The Royal College of Radiologists; 2007.
12. European Society of Urogenital Radiology Contrast Media Safety Committee. ESUR Guidelines on Contrast Media, Version 7. 0. www. esur. org, 2008.
13. Gandhi, SN, Brown, MA, Wong, JG, et al. MR contrast agents for liver imaging: what, when, how. Radiographics. 2006; 26(6):1621–1636.
14. We, L, Cao, Y, Liao, C, et al. Diagnostic performance of USPIO enhanced MRI for lymph node metastases in different body regions: a meta-analysis. Eur J Radiol. 2011; 80(2):582–589.
15. Tremblay, E, Thérasse, E, Thomassin-Naggara, I, et al. Quality initiatives: guidelines for use of medical imaging during pregnancy and lactation. RadioGraphics. 2012; 32(3):897–911.
Contrast agents in ultrasonography
During the late 1960s, the first reported use of ultrasound (US) contrast was published describing the intracardiac injection of agents during echocardiography. Considerable progress has now been made in the development and clinical application of US contrast agents and these are now used more widely to assess vascularity and tissue perfusion. US contrast agents contain microbubbles of air, nitrogen or fluorocarbon gas coated with a thin shell of material such as albumin, galactose or lipid. The contrast is usually injected intravenously, but in order to cross the pulmonary filter and reach the arterial circulation the microbubbles must be 3–5 µm diameter, approximately the size of a red blood cell. Bubbles of this size only remain intact for a very short time in blood. The bubbles do not pass through the vascular endothelium therefore they provide pure intravascular contrast. The effect of a bolus injection is to increase the echo signal from blood by a factor of 500–1000. After about 5 minutes the gas from the bubbles had diffused into the blood and the very small mass of shell material is then metabolized.1
Clinical applications of US contrast agents include the following:2,3
1. Identification and characterization of solid lesions, particularly in the liver,4 but also in the spleen, pancreas, kidney, prostate, ovary and breast.
2. To assist US-guided interventions such as biopsy.
3. Voiding urosonography can be used to detect vesico-ureteric reflux in children; here US contrast is administered directly into the bladder.
4. Assessment of fallopian tubal patency at hysterosonosalpinography.
5. Determination of disease activity in patients with inflammatory bowel disease.
There are a number of different microbubble contrast agents available. Levovist® is one of the most widely used; it consists of microbubbles of air enclosed by a thin layer of palmitic acid in a galactose solution and is stable in blood for 1–4 min. SonoVue®, another microbubble contrast agent, is an aqueous suspension of stabilized sulphur hexafluoride microbubbles.
The US agents in clinical use are well tolerated and serious adverse reactions are rarely observed. These agents are not nephrotoxic and may be used in patients with any level of renal function.5 Allergy-like reactions occur rarely and adverse events are usually mild and self-resolving.6
References
1. Wilson, S, Burns, PN. Microbubble-enhanced US in body imaging: What role? Radiology. 2010; 257:24–39.
2. Quaia, E. Microbubble ultrasound contrast agents: an update. Eur Radiol. 2007; 17(8):1995–2008.
3. Furlow, B. Contrast-enhanced ultrasound. Radiol Technol. 2009; 80(6):547–561.
4. Piscaglia, F, Lencioni, R, Sagrini, E, et al. Characterization of focal liver lesions with contrast enhanced ultrasound. Ultrasound Med Biol. 2010; 36(4):531–550.
5. Wilson, S, Greenbaum, L, Goldberg, BB. Contrast enhanced ultrasound; what is the evidence and what are the obstacles? Am J Roentgen. 2009; 193(1):55–60.
6. Jakobsen, JA, Oyen, R, Thomsen, HS, et al. Safety of ultrasound contrast agents. Eur Radiol. 2005; 15(5):941–945.